假尿苷合酶的催化机理
- 格式:pdf
- 大小:394.56 KB
- 文档页数:4

填空一、在假尿苷中,碱基和核糖是以键相连的(1995北医)答案:杂环上的C-5与糖环的C-1′考点:tRNA分子和稀有碱基解析:要记住tRNA转录后加工各种稀有碱基的生成原理,如一般的嘧啶核苷是以杂环上N-1与糖环的C-1′连成糖苷键,这正是与假尿嘧啶核苷的区别。
二、别构酶都含有和两种结构或亚基(1995北医)答案:调节部位催化部位考点:别构酶的定义、结构解析:酶的两种最主要的活性调节方式:变构调节与共价修饰调节,要记住相应的调节原理三、可以按蛋白质的分子量、电荷及构象分离蛋白质的方法是(1995北医)答案:电泳考点:蛋白质的性质及分离纯化方法解析:蛋白质分离纯化方法是蛋白质一章中很重要的部分,最好结合蛋白质的理化性质来记忆,因为其分离纯化采用的方法是由其相应的性质决定的。
四、位于酶活性中心的必需基团有和(1995北医)答案:催化基团和结合基团考点:酶的分子结构解析:酶的活性中心结构要记住,因为这是酶发生催化作用的基础,也便于对酶促反应机制的理解。
五、蛋白质变性时,其溶液粘度溶解度(1999北医)答案:增加,降低考点:蛋白质的理化性质解析:蛋白质变性主要发生二硫键和非共价键的破坏,变性后其理化性质改变,生物活性丧失。
六、反竞争性抑制物存在时,酶反应动力学特点,Km(1999北医)答案:降低、减小考点:抑制剂对酶促反应速度的影响解析:竞争性抑制Km增大Vm不变非竞争性抑制Km不变Vm降低反竞争性抑制Km减小Vm降低七、目前常用的蛋白质序列分析法有和,可直接测量蛋白质分子空间结构的方法是(1996北医)答案:多肽链氨基酸序列分析,快速DNA序列分析,X射线晶体衍射法考点:蛋白质一级结构和空间结构的测定解析;蛋白质一级结构测定方法中,通过氨基酸的自动连续切除和鉴定是常用的方法,但不是唯一的办法,还可以先分离编码蛋白质的基因,再测定DNA序列,按照三联密码原则推断出氨基酸序列。
两项技术还可以补充互用。
八、实验测得Tyr的pK1=2.20,pK2=9.11,pKR=10.07,Tyr的pI应为。

GLP-1诱导表达的原理三磷酸鸟苷环化水解酶(GTPCH)Ⅰ是四氢生物蝶呤(BH4)合成的关键限速酶,BH4是内皮一氧化氮合酶(eNOS)的辅助因子。
糖尿病等病理条件下,BH4水平下降促使eNOS脱偶联,导致内皮功能异常。
胰高糖素样肽(GLP-1)可增强内皮eNOS的磷酸化、增加NO、发挥内皮保护作用。
该课题组前期研究显示,同型半胱氨酸可抑制内皮BH4水平、诱导eNOS脱偶联,损害患者冠脉内皮功能。
预实验显示,GLP-1受体激动剂可促进内皮细胞eNOS表达、抑制氧化应激、增加NO。
该研究分别在内皮细胞及apoE敲除小鼠整体水平,探讨GLP-1受体激动剂能否通过上调血管内皮GTPCH1水平、增加BH4、抑制氧化应激、促使eNOS复偶联等机制,发挥血管内皮保护作用,并在临床水平进一步验证。
实验结果显示GLP-1受体激动剂艾塞那肽可以改善内皮细胞氧化损伤,可能是通过激活AMPK途径增加GTPCH1表达,从而上调BH4水平,进而促进eNOS复偶联,NO产生增加,最终达到改善内皮功能的作用。
在ApoE小鼠中持续应用艾塞那肽(8周)能够显著提高主动脉中GTPCH-1的表达水平和BH4的含量,并且使得主动脉eNOS的磷酸化水平相应增高,提高eNOS的活性,使之能够保持并较好地发挥催化生成血管内皮舒张因子NO的功能,最终增加血管内NO的生成同时减少NO的损失,改善血管内皮依赖性舒张功能,从而达到保护内皮功能的作用。
临床研究显示艾塞那肽不仅能降低2型糖尿病患者的血糖,改善胰岛细胞功能,降低患者的体重、体重指数、腹围,调节血脂代谢,还可具有改善血管内皮功能,提高冠脉内血流发挥血管内皮保护作用,减少糖尿病患者心血管不良事件的发生。
GLP-1受体激动剂,通过上调内皮细胞GTPCH1表达、增加BH4水平、促使eNOS复偶联、增加NO,进而改善血管内皮功能;并可通过激活AMPK,反转被抑制的内皮细胞GTPCH1表达,增加BH4水平,促使eNOS复偶联,改善内皮依赖的血管功能,发挥血管保护作用。

蛋⽩质的合成、转运、修饰蛋⽩质的合成蛋⽩质的种类是由基因决定的,也就是说⼈类基因组有多少个基因,⼈体就有多少种蛋⽩质,只是蛋⽩质表达的时期和部位不同.根据⼈类基因组计划分析得知:全部⼈类基因组约有2.91Gbp,约有39000多个基因;也就是说⼈体蛋⽩质的种类有39000多种蛋⽩质⽣物合成可分为五个阶段,氨基酸的活化、多肽链合成的起始、肽链的延长、肽链的终⽌和释放、蛋⽩质合成后的加⼯修饰⼀.氨基酸的活化分散在胞液中的各种氨基酸需经特异的氨基酰-tRNA合成酶催化,ATP供能,并需Mg2+或Mn2+参与在氨基酸的羧基上进⾏活化,⽣成中间复合物()后者再与相应的tRNA作⽤,将氨基酰转移到tRNA分⼦的氨基酸臂上,即3′末端腺苷酸中核糖的3′(或2′)羟基以酯键相结合形成氨基酰-tRNA【氨基酰tRNA的⽣成】tRNA各种tRNA的⼀级结构互不相同,但它们的⼆级结构都呈三叶草形三叶草形结构的主要特征是:含有四个螺旋区、三个环和⼀个附加叉四个螺旋区构成四个臂,其中含有3′末端的螺旋区称为氨基酸臂,因为此臂的3′-末端都是C-C-A-OH序列,可与氨基酸连接三个环分别⽤Ⅰ、Ⅱ、Ⅲ表⽰环Ⅰ含有5,6⼆氢尿嘧啶,称为⼆氢尿嘧啶环(DHU环)环Ⅱ顶端含有由三个碱基组成的反密码⼦,称为反密码⼦环;反密码⼦可识别mRNA分⼦上的密码⼦,在蛋⽩质⽣物合成中起重要的翻译作⽤环Ⅲ含有胸苷(T)、假尿苷(ψ)、胞苷(C),称为假尿嘧啶环(TψC环);此环可能与结合核糖体有关tRNA在⼆级结构的基础上进⼀步折叠成为倒“L”字母形的三级结构起始因⼦原核起始因⼦只有三种(IF1、IF2、IF3)真核起始因⼦(简称为eIF)种类多且复杂,已鉴定的真核起始因⼦共有12种延长因⼦原核⽣物(简称EF)由三部分组成:EF-Tu,EF-Ts,和EF-GEF-Tu它介导氨酰-tRNA进⼊核糖体的空位EF-Ts充当EF-Tu亚基的鸟嘌呤核苷酸交换因⼦,催化EF-Tu释放GDPEF-G催化tRNA的移位和多肽延伸的每个循环后期mRNA从核糖体上掉下来真核⽣物(简称eEF)真核⽣物中分为:eEF-1和eEF-2eEF-1有两个亚基,α和βγα相当于原核⽣物中的EF-Tu亚基,它介导氨酰-tRNA进⼊核糖体的空位Βγ相当于原核⽣物中EF-Ts,核苷酸交换因⼦α,催化GDP从α上释放eEF-2相当于原核⽣物的EF-G,催化tRNA的移位和多肽延伸的每个循环后期mRNA从核糖体上掉下来终⽌因⼦(释放因⼦)原核⽣物细胞的释放因⼦(简称RF):识别终⽌密码⼦引起完整的肽链和核糖体从mRNA 上释放的蛋⽩质释放因⼦1(RF1):能识别终⽌密码⼦UAA和UAG⽽终⽌蛋⽩质合成的细菌释放因⼦释放因⼦2(RF2):能识别终⽌密码⼦UAA和UGA⽽终⽌蛋⽩质合成的细菌释放因⼦释放因⼦3(RF3):与延长因⼦EF-G有关的细菌蛋⽩质合成终⽌因⼦当它终⽌蛋⽩质合成时,它使得因⼦RF1和RF2从核糖体上释放真核⽣物细胞只有⼀种终⽌因⼦(称为eRF)能识别所有的终⽌密码⼦因为它没有与GTP结合的位点,所以它不能帮助完成合成的多肽从P位点的tRNA的释放在真核⽣物内可能还存在能与eRF合作、帮组多肽从核糖体释放的蛋⽩质核糖体的活性部位单个核糖体上存在四个活性部位,在蛋⽩质合成中各有专⼀的识别作⽤1.A部位:氨基酸部位或受位:主要在⼤亚基上,是接受氨酰基-tRNA的部位2.P部位:肽基部位或供位:主要在⼩亚基上,是释放tRNA的部位3.肽基转移酶部位(肽合成酶),简称T因⼦:位于⼤亚基上,催化氨基酸间形成肽键,使肽链延长4.GTP酶部位:即转位酶(EF-G),简称G因⼦,对GTP具有活性,催化肽键从供体部位→受体部位核糖体上还有许多与起始因⼦、延长因⼦、释放因⼦以及各种酶相结合的位点核糖体的⼤⼩是以沉降系数S来表⽰,S数值越⼤、颗粒越⼤、分⼦量越⼤原核细胞与真核细胞核糖体的⼤⼩亚基是不同的⼆.核糖体循环(肽链合成)1.肽链启动阶段在蛋⽩质⽣物合成的启动阶段,核蛋⽩体的⼤、⼩亚基,mRNA与⼀种具有启动作⽤的氨基酸tRNA共同构成启动复合体。

某大学生物工程学院《普通生物化学》课程试卷(含答案)__________学年第___学期考试类型:(闭卷)考试考试时间:90 分钟年级专业_____________学号_____________ 姓名_____________1、判断题(140分,每题5分)1. DNA有多种构象,在不同的环境如温度、湿度、离子强度等的条件下可以相互转换。
()[中科院水生生物研究所2007研]答案:正确解析:DNA构象主要受湿度和盐浓度影响,受温度影响很小。
2. Rec A蛋白对单链DNA的亲和力高于对双链DNA的亲和力。
()答案:正确解析:Rec A蛋白参与DNA重组和SOS修复,这都需要它与单链DNA结合。
3. 嘌呤类化合物的分解代谢可以在核苷酸、核苷和碱基三个水平上进行。
()答案:正确解析:4. 原核生物的起始氨基酸与起始tRNA形成MettRNA后被甲酰化,甲酰化是原核生物所有蛋白质合成起始所必需的。
()答案:错误解析:MettRNAfMet的甲酰化对原核生物的蛋白质合成非常重要,不能甲酰化的突变体有严重的生长缺陷,但是甲酰化并不是原核生物所有蛋白质合成起始所必需的。
5. 淀粉和糖原的生物合成都需要有引物的存在。
()答案:正确解析:多糖合成时末端需要葡萄糖残基引物存在,以利于多糖链的延长,糖原的合成需要带有寡糖链的多肽作为引物,一般情况下引物是至少含4个葡萄糖残基的α1,4葡聚糖。
6. 大多数真核生物的mRNA和它的DNA模板是等长的。
()答案:错误解析:真核生物DNA模板中存在大量非编码序列,因此比mRNA长。
7. 四种脂溶性维生素都是异戊二烯衍生物,属于类脂。
()答案:错误解析:四种脂溶性维生素中维生素D是环戊烷多氢菲的衍生物,其余三种都是异戊二烯的衍生物。
8. 抑制剂不与底物竞争酶结合部位,则不会表现为竞争性抑制。
()答案:正确解析:竞争性抑制作用指抑制剂与底物竞争与酶的同一活性中心结合,从而干扰了酶与底物的结合,使酶的催化活性降低的作用。


2021基础生物化学复习题一、选择题1、1分子葡萄糖在糖酵解途径中生成2分子的丙酮酸,同时净产生:A、4ATP+2NADH+2H+B、2ATP+NADH+H+C、2ATP+2NADH+2H+D、2ATP答案:C2、机体内糖、脂肪、蛋白质进行彻底氧化分解的途径只有:A、磷酸戊糖途径B、三羧酸循环C、β-氧化D、糖酵解途径答案:B3、脂肪酸β-氧化的酶促反应顺序为:A、脱氢、再脱氢、加水、硫解B、脱氢、加水、再脱氢、硫解C、脱氢、脱水、再脱氢、硫解D、加水、脱氢、硫解、再脱氢答案:B4、脂肪酸合成酶复合体催化脂肪酸从头合成的终产物是:A、丙二酸单酰CoAB、琥珀酰CoAC、硬脂酰CoAD、软脂酰CoA答案:D5、氨基酸在等电点时,应具有的特点是:A、不带正电荷B、不带负电荷C、A+BD、在电场中不泳动答案:D6、维持蛋白质二级结构的主要化学键是:A、氢键B、二硫键C、疏水键D、离子键答案:A7、核酶:A、是定位于细胞核中的酶B、是核酸酶的简称C、是核心酶的简称D、化学本质是核酸答案:D8、米式方程:A、表述了V max与K m的关系B、表述了酶反应速度与K m的线性关系C、表述了酶促反应速度与底物浓度的定量关系D、表述了K m与底物浓度的线性关系答案:C9、同工酶的:A、催化反应相同B、分子结构相同C、理化性质相同D、免疫学性质相同答案:A10、关于逆转录作用的错误叙述是:A、以RNA为模板合成DNAB、需要一个具有3′-OH末端的引物C、以5′→3′方向合成,也能3′→5′方向合成D、以dNTP为底物答案:C11、调节糖酵解途径最为关键的酶是:A、己糖激酶B、磷酸果糖激酶C、丙酮酸激酶D、磷酸丙糖异构酶答案:B12、呼吸链是机体内主要生物氧化方式,是产生ATP的主要来源,它们发生在:A、高尔基体B、线粒体C、微粒体D、溶酶体答案:B13、氰化物中毒是由于抑制了哪种细胞色素向O2传递电子:A、CytaB、CytbC、CytcD、Cytaa3答案:D14、糖酵解过程产生的NADH:A、只能进入线粒体电子传递链B、只能为某些生物化学反应提供还原力C、可以直接进入线粒体电子传递链D、可以通过穿梭系统被线粒体电子传递链所利用答案:D二、判断题1、G-C含量多的核酸Tm值大答案:正确2、所有核酸合成时,新链的延长方向都是5′→3′。

第26卷 第3期2014年3月V ol. 26, No. 3Mar., 2014生命科学Chinese Bulletin of Life Sciences文章编号:1004-0374(2014)03-0239-09DOI: 10.13376/j.cbls/2014037RNA 中的假尿苷修饰:形成、功能及鉴定李笑雨1#,孙芳芳2#,伊成器1,3*(1 北京大学生命科学学院,蛋白质与植物基因研究国家重点实验室,北京100871;2 清华大学生命科学学院,北京100084;3 北京大学合成与功能生物分子中心,北京大学-清华大学生命科学联合中心,北京100871)摘 要:假尿苷修饰是目前已知丰度最高的RNA 修饰,广泛存在于多个物种的多类RNA 中。
作为尿苷的5位异构体,假尿苷在真核生物中的形成机制主要有两种:依赖于假尿苷合酶或是依赖于H/ACA 核糖核蛋白复合体。
假尿苷修饰在多个生物学过程中发挥作用,同时不同位点的假尿苷修饰具有不同的功能。
而人为地在mRNA 终止密码子中引入假尿苷修饰则可以使其具有编码能力,这改变了传统的中心法则。
目前对于RNA 中假尿苷的研究主要是通过2D-TLC 及液质联用对其进行定量分析,使用CMCT 标记假尿苷及RNase H 和SCARLET 的方法对其进行定位。
关键词:假尿苷;形成;功能;定位中图分类号: Q525;Q528+.2;Q752 文献标志码:APseudouridines in RNA: formation, function and characterizationLI Xiao-Yu 1#, SUN Fang-Fang 2#, YI Cheng-Qi 1,3*(1 State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences, Peking University, Beijing 100871, China; 2 School of Life Sciences, Tsinghua University, Beijing 100084, China; 3 Peking-Tsinghua Joint Center for LifeScience, Synthetic and Functional Biomolecules Center, Peking University, Beijing 100871, China)Abstract: Pseudouridine is the most abundant RNA modification and it is widely spread in various RNAs of many organisms. As a rotation isomer of uridine, it is formed mainly through two mechanisms: stand-alone pseudouridine synthases and Box H/ACA Ribonucleoprotein. Pseudouridylation affects many cellular processes, with distinct收稿日期:2013-11-21基金项目:国家自然科学基金面上项目(31270838)*通信作者:E-mail: chengqi.yi@p #共同第一作者伊成器 教授伊成器实验室主要通过化学生物学、结构生物学、高通量测序等手段,对核酸修饰的生物功能及其生理调控机制进行研究。

tRNA的结构、功能及合成简介2008年第43卷第1期生物学通报19tRNA的结构,功能及合成简介俎鲁霞徐国恒(北京大学医学部生理系北京100083)中国图书分类号:Q342文献标识码:A组成的不同的核苷酸的排列顺序携带着不同的遗传信息.DNA分子所储存的遗传信息,必须通过转录传递给信使RNA分子(mRNA),才能到达蛋白质合成的场所——核糖体,然后合成蛋白质的酶系再把mRNA所带来的信息翻译成蛋白质.在合成蛋白质的过程中,必须要有能转运氨基酸的tRNA和构成核糖体的的tRNA的结构,功能及合成作一简单介绍.在所有原核和真核机体中存在3类主要的RNA分子:mRNA,tRNA,rRNA.mRNA分子量大小不均,一基酸掺人顺序的信息,是蛋白质生物合成的模板.rRNA是胞浆蛋白质合成机器——核蛋白体的组成成分.参与蛋白质的合成.tRNA分子较小,长度在74~95个核苷酸之问变化.tRNA的种类很多,一种细胞内约有60~80种所有的tRNA分子都具有三j:叶草型的二级结构.这种三叶草形结构的主要特征是,含有4个臂,其中含有3末端的螺旋区称为氨基酸臂,因为此臂的3末端都是C—C—A—OH序列,可与氨基酸连接.从5末端起的第1个环以含有二氢尿嘧啶为特征,称为二氢尿嘧啶环fDHU环1;第2个环为反密码子环, 其环中部的3个碱基可以与mRNA的i联体密码子形成碱基互补配对,称为反密码子:第3个环含有胸苷(T),假尿苷(),胞苷(C),称为TCC环;此环可能图1通过对tRNA结构的了解,可以知道每个tRNA分子只有1个由3个核苷酸组成的反密码子,只能结合1种氨基酸,将其转运到核蛋白体上,供蛋白质合成使参与.这一点与mRNA不同,合成一段肽链只需要一个各有其特定的tRNA,而且一种氨基酸常有几种tRNA. tRNA上的反密码子,只有与mRNA上的密码子相对应时,才能结合,否则格格不入.据此,在翻译时,带着不同氨基酸的各个tRNA就能较为准确地在mRNA分子上对号入座.核酸对氨基酸特异的功能基并没有亲和力, 那么tRNA与氨基酸的相互识别是如何实现的呢?原来氨基酸的活化及活化后与相应tRNA的结合过程都是由同一类酶所催化,此类酶称为氨基酰一tRNA合成酶,20种这样的酶才能使20种氨基酸能恰当地连接到各NA合成酶准确识别相应的tRNA的重要部分之一. tRNA是如何合成的?首先了解一下RNA合成的基本知识.以DNA为模板,在依赖于DNA的RNA聚起始于DNA模板的一个特定起点,并在另一终点处终称为模板链;另一条链的脱氧核苷酸序列正好与转录出的RNA的核苷酸序列相同(只是T与U的区别1,叫做编码链.在多基因的双链DNA分子中,每个基因的模板并不是全在同一条单链上,一个双链DNA分子的某条链既可作为某些基因的模板链,也可作为其他基的原理就是人为合成以编码链为模板的核酸f反义核酸),这些核酸的序列与模板链一致,与对应的mRNA结合形成双链杂交体.从而抑制mRNA的表达.中细胞核内有3种RNA聚合酶即I.II和III,转录不同需要在聚合酶III催化下才能转录,因此属于III类基生物学通报2008年第43卷第1期因.那么tRNA基因有什么特征呢?tRNA基因长约140原核生物大很多,真核生物众多的tRNA基因是分散在不同的基因组上,即使是携带同一种氨基酸的不同tR—NA的同功受体tRNA基因也通常位于一条以上的染色列,结构基因部分(氨基酸的编码区,又称外显子)常被间单位都要含有启动子和终止子2个必须元件.tRNA的合成过程包括起始,延长和终止3个阶段.下面分别介绍:在原核生物中,当RNA聚合酶的8亚基发现其识别位点时,全酶就与启动子的特异序列结合形成一个封闭的启动子复合物.真核生物转录起始十分复杂,需要一类叫做转录因子的蛋白质分子的协助,它们与RNA聚合酶形成转录起始复合物,共同参与转录起始的过程.tRNA基因的启动子位于转录启始的下游,称为下游启动子或内部启动子;Yc类启动子包括:A盒,B起始复合物的装配步骤如下:TFIIIC结合于A盒和B 盒:TFIIIB结合于A框的上游约50位置,并和TFIIIC —TFIIIC—DNA复合物.随后,TFIIIC离开该复合物,此时复合物为转录起始复合物,只需存在4种NTP,就可以转录生成tRNA.装配过程见图2.tRNA链的延长靠RNA聚合酶III的催化,在起始复合物上第1个GTP的核糖3一OH上与DNA模板能配对的第2个三磷酸核苷起反应形成磷酸二酯键. 聚合进去的核苷酸又有核糖3一OH游离,这样就可按链的合成方向也是5一3.c二画—r———'——————三三三三三三三≥:=一一一=...一一一—一TFIllBo一1Ir(,)——◆上在RNA延长进程中,当RNA聚合酶行进到DNA模板的特定部位——终止信号时,RNA聚合酶就不再组成的反向重复序列,在转录生成的mRNA中有相应的发卡结构.此发卡结构可阻碍RNA聚合酶的行进,富集区,其转录生成的mRNA3末端有多个U残基.通过以上过程生成的是tRNA的前体,即未成熟的tRNA,尚需要在酶的作用下加工修饰后才能成为有不含CCA序列,成熟tRNA的CCA序列是由核苷酰转工还有化学修饰作用,包括碱基的甲基化反应,脱氨反应及还原反应等.综上所述,RNA基因尤其是真核生物tRNA基因的基因的不对称转录合成的tRNA前体经过加工后成为用下结合一种氨基酸,将其转运到核蛋白体上,参与蛋白质的合成.主要参考文献1MurrayR.K.,GrannerD.K.,MayesP.A.,Rodwe11V.W..HarpersU—lustratedBiochemistry.26thed.McGraw-Hill,2003.2周爱儒.生物化学(第5版).北京:人民卫生出版社,2003.3张遒蘅.生物化学(第2版).北京:北京医科大学出版社,2000. 4王金胜.基础生物化学(第1版).北京:中国林业出版社,2003. 5敖世洲.真核生物基因的结构与功能.北京:科学出版社.1989. (E—mail:Xng@.CB)(BH)人类正在加速进化人认为的已经停止或维持一个恒定的速度,而是正处在一个加关论文12月10日发表于美国《国家科学院院刊》(PNAS)上. 领导此次研究的是美国犹他大学的人类学教授Henry过仔细检测,研究人员发现,有7%的人类基因正经历着新近的,快速的变化.更进一步的研究表明,进化速度并不是一直恒定的,新近存在着一个进化的加速Harpending说:"不同人种正在朝着彼此远离的方向进化,我们正变得越来越不同,而且不同大陆上族群的情况也互不相同."他认为这种现象的原因在于,人类在大约4万年前从非洲他同时相信,这种人类进化的加速只是一种暂时性的现象,因为人类自4万年前分散以来,新的环境特别是农业的发展,改变了我们的食物结构和社会体系,而人类至今还在适应这一变化. 摘自《科学时报))2007年12月10日。
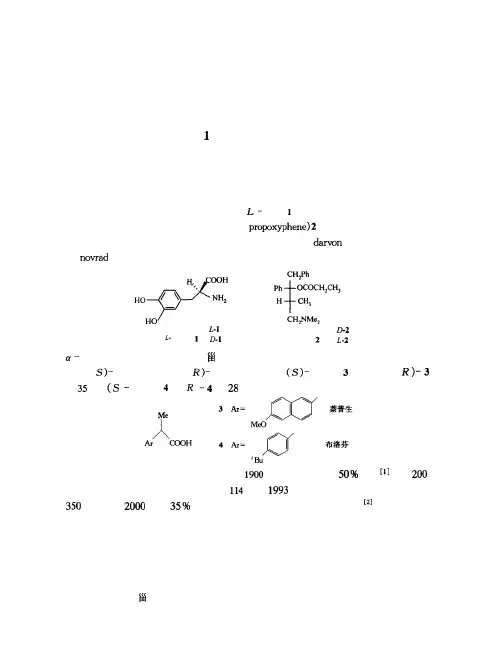
第章 导言多巴手性是指物质的一种不对称性,好比人的左手和右手的关系。
手性是自然界的特征之一,也是一切生命的基础,生命现象依赖于手性的存在和手性的识别。
因此,一切动植物以及人体对药物等都具有精确的手性识别能力。
手性药物的构型不同,它们的生理活性和毒性也不相同。
的对映体却有严重的毒性作用。
丙氧芬(体(似的镇咳活性,但只是右旋体有镇痛作用,所以右旋体()作为镇咳药已分别上市。
目前世界上使用的药物总数大约为种,手性药物占种常见的临床药物中,手性药物多达的合成药物将以单一旋光异构体上市亿美元。
年有倍;芳基丙酸类药物是重要的非的药效是(异构体高得多。
体消炎镇痛药,虽然它们的两种对映体都有药萘普生效,但(的倍。
异构体的药效比(布洛芬是(的))以上。
在种。
年光学活性药物的销售额超过。
旋光纯化合物因其所具有的特殊性质和非凡功能,不仅在药物中,而且在农药、香料、食品添加剂和昆虫信息素等领域中均获得了广泛的应用。
此外,在分子电子学、分子光学以及特殊材料中也引起了人们的普遍关注。
液晶材料在光信号的记录、储存和显示方面有重要用途。
研究结果显示:胆甾型液晶都是由手性分子组成,向列型液晶向列型液晶向胆却是由内消旋体或非手性化合物组成,若向向列型液晶中加入手性分子,就会促使型液晶转变。
旋光纯高聚物与消旋体相比具有优良的性能,光治疗帕金森症有毒性作用 丙氧芬是治疗帕金森病的良药,但它的左旋体与右旋体有相)作为镇痛药,左旋镇痛药镇咳药多巴乙基丙内酯聚合物的熔点竟比相应的外消旋体聚合物苯基学活性的构型。
若用人工合成的氨)外消旋体拆分图要方法可归纳为:)手性放大[式()不对称诱导[式(高构型,而天然的糖类化合物大多是手性是生命科学中的一个关键因素,酶的高度立体专一性这一事实,就足以说明生命过程包含着极为丰富且又非常复杂的立体化学内容。
组成蛋白质的天然氨基酸都是基酸组成多肽和蛋白质,这样的多肽和蛋白质该有什么样的生理作用?探讨这些问题,无疑会在分子水平揭开生命的奥秘。


中图分类号:Q55文献标识码:A文章编号:1002-5064(2001)04-0005-04#综述与专论#端粒酶的研究进展Advance s of the Research on the Telomerase陈新荣林爱星*陈永福CH EN X in-ro ng LI N Ai-xing C H EN Y o ng-fu中国农业大学西校区农业生物技术国家重点实验室,北京100094National Laboratories for Agrbiotechnology,China Agricultural University,Beijing100094摘要:端粒酶是由R NA模板和蛋白质催化亚基组成的核糖蛋白颗粒。
它解决染色体的未端复制问题,归属于逆转录酶家族又和逆转录酶有一定的差别。
端粒酶的缺失能在细胞和机体水平引起各种疾病的发生,且端粒的过度表达和细胞的永生化和癌变直接相关,其中端粒蛋白在此过程中可能起调控作用。
端粒酶的结构和功能决定了它有广泛的应用前景。
关键词:端粒梅;端粒蛋白;永生化;肿瘤生成A bstra ct:Telomerase is a r ibonucleoprotein r esponsible in most eukaryotes for replication of the end of chromosmes.ItsR NA subunit acts as a template for the systhesis of telomeric DNA,while a protein component catalyzes t his process to make up for conventional DNA polymerases.inability to replicate completely the end of linear DNA.It belongs to the re2 verse transcr iptase family but differ s from reverse tr anscriptase.T he absence of telomerase can induce many diseases in cell and organ level.The overexpression of telomerase has close relat ionship with cell.s immortalizat ion and tumorigenesis and t he telomere associated proteins play a important role in the process.The structure and function of telomerase suggest its extensive application in the near futur e.K e y words:telomerase;telomere associated protein;immortal;tumor progression染色体的未端复制问题是指线性DNA进行复制时,DNA聚合酶既不具有从头合成的能力又不具有3c y5c方向的合成能力,它必须以RNA为引物,当5c末端的引物被切除后,剩下5c未端的引物空隙没法填补这样就导致了子代DNA中的一条链比亲代DNA短出一段(至少相当于或大于RNA引物链的长度),如果没有适当的机制来补充这一段空隙,经过连续的复制后,子代DNA将越来越短。
dmap催化酰化机理DMAP(4-二甲胺吡啶)是一种常用的有机催化剂,可以在酰化反应中起到催化作用。
酰化反应是有机合成中常见的一种反应,用于合成酯化合物。
在传统的酰化反应中,通常需要较高的反应温度和较长的反应时间,而加入DMAP可以显著提高反应速率和产率,从而减少反应时间和温度。
DMAP的催化作用是通过其碱性来实现的。
DMAP中的二甲胺基团可以与酯化反应中的酸酐形成氢键,将酸酐的羧基质子化,使其更容易被醇亲核进攻。
同时,DMAP也可以与醇形成氢键,使其亲核性增加,从而加速反应的进行。
酰化反应通常是通过将酸酐与醇反应来实现的。
在传统的酰化反应中,酸酐和醇在高温下反应,生成酯化合物和水。
而加入DMAP后,反应速率大大增加。
DMAP可以与酸酐形成氢键,使其质子化,从而增加了酯化反应的亲核性。
同时,DMAP也可以与醇形成氢键,使其亲核性增加,加速反应进行。
酰化反应中,DMAP的催化作用是通过两个步骤实现的。
首先,DMAP与酸酐反应,形成DMAP酸酐盐。
这个盐具有较高的反应活性,能够更容易地被醇亲核进攻。
其次,DMAP与醇反应,形成DMAP醇盐。
这个盐同样具有较高的反应活性,能够更容易地亲核进攻酸酐,形成酯化产物。
DMAP催化酰化反应的机理是一个两步反应。
首先,DMAP与酸酐反应,生成DMAP酸酐盐。
在这个过程中,DMAP的二甲胺基团与酸酐的羧基形成氢键,将酸酐质子化。
这个质子化的酸酐盐更容易被醇亲核进攻,形成中间体。
然后,DMAP与醇反应,生成DMAP醇盐。
在这个过程中,DMAP的二甲胺基团与醇形成氢键,增加了醇的亲核性。
这个亲核的DMAP醇盐可以进一步与中间体反应,形成酯化产物。
DMAP催化酰化反应具有许多优点。
首先,DMAP催化反应的反应速率较快,反应时间较短。
其次,DMAP催化反应的反应温度较低,有助于反应的选择性和产率。
此外,DMAP催化反应对于含有酸敏感基团的底物也具有较好的兼容性。
因此,DMAP催化酰化反应在有机合成中得到了广泛的应用。
(19)中华人民共和国国家知识产权局(12)发明专利申请(10)申请公布号 (43)申请公布日 (21)申请号 202011421953.7(22)申请日 2020.12.08(83)生物保藏信息CGMCC No.21122 2020.11.06(71)申请人 浙江珲达生物科技有限公司地址 310000 浙江省杭州市钱塘新区白杨街道6号大街452号2幢D2014-2017号房(72)发明人 陈伟 郑玲辉 朱进伟 高祥 张敏 陈世敏 彭湘屏 汪超 (74)专利代理机构 北京领科知识产权代理事务所(特殊普通合伙) 11690代理人 张丹(51)Int.Cl.C12N 1/20(2006.01)C12P 19/38(2006.01)C12R 1/465(2006.01)(54)发明名称一种链霉菌及其发酵产假尿苷的方法(57)摘要本发明公开了一种链霉菌(Streptomycessp.)HDCC00030,保藏于中国微生物菌种保藏管理委员会普通微生物中心(CGMCC),保藏编号为CGMCC NO.21122,保藏日期为2020年11月6日。
所述链霉菌为一种全新的假尿苷产生菌,生产能力高,其产生假尿苷的能力比现有技术中其他菌种有了大幅度的提高,假尿苷的效价可达到1360mg/L以上,有利于实现工业化生产。
权利要求书1页 说明书10页序列表1页 附图2页CN 112746036 A 2021.05.04C N 112746036A1.一种链霉菌(Streptomyces sp.)HDCC00030,保藏于中国微生物菌种保藏管理委员会普通微生物中心(CGMCC),保藏编号为CGMCC NO.21122,保藏日期为2020年11月6日。
2.根据权利要求1所述的链霉菌(Streptomyces sp.)HDCC00030在制备假尿苷或者含有假尿苷的药物组合物的应用。
3.一种假尿苷的制备方法,其特征在于:包括采用如权利要求1所述的链霉菌(Streptomyces sp.)HDCC00030在含有可同化的碳源和/或氮源的营养培养基里,进行有氧发酵的步骤。
galnac递送原理
GalNAc递送原理是一种基因治疗技术,用于将药物载体有效
地递送到细胞内。
GalNAc(N-乙酰半乳糖胺)是一种能与肝细胞表面上的阴离
子交互的物质,它可以与糖蛋白(glycoprotein)结合。
在GalNAc递送原理中,将药物与GalNAc结合,形成GalNAc-conjugate(GalNAc共轭体)。
在人体内,GalNAc-conjugate通过静脉注射进入血液循环,并
被肝细胞特异性GALNT2酶识别和结合。
一旦与肝细胞结合,GalNAc-conjugate会通过内吞过程进入细胞内部。
在细胞内部,GalNAc-conjugate会与内质网膜上的受体结合,
启动内吞囊泡向细胞质运输的过程。
这种运输过程使GalNAc-conjugate与药物负载进入细胞质,并最终释放药物。
通过GalNAc递送原理,药物可以有效地递送到肝细胞内,从
而实现治疗目的。
这种技术特别适用于治疗与肝脏相关的疾病,如遗传性血脂蛋白异常病、肝癌等。
它不仅可以提高药物的生物利用度,还可以减少药物剂量和给药频率,提高治疗的效果和安全性。
关于trna的二级结构的假尿苷糖苷键转运RNA(transfer RNA,tRNA)是一类重要的非编码RNA分子,它在蛋白质合成中起着关键的作用。
tRNA的二级结构是它功能的基础,其中包括了假尿苷糖苷键(pseudo-uridine)的形成。
本文将详细阐述tRNA二级结构以及假尿苷糖苷键的形成和功能。
tRNA的二级结构:tRNA是一种相对较短的RNA分子,一般含有70-90个核苷酸。
它具有一个特殊的L形结构,由一个发夹状的单链和多个稳定的二级结构域组成。
tRNA的二级结构可以分为四个区域:TΨC环、顶部环、折叠环和D环。
其中TΨC环含有假尿苷糖苷键,是tRNA二级结构中的一个重要组成部分。
假尿苷糖苷键的形成:假尿苷是一种位于tRNA的反器核作用中产生的伪尿苷。
在tRNA合成过程中,Uracil核苷酸(U)会在Primer和Anticodon环之间发生底物及辅助辅酶对应(substrate-assisted coenzyme A-dependent)的反式异构化。
底物即U核苷酸在底物的辅助酶调节下与Coenzyme A发生酯化反应产生糖酯。
而Phosphopantetheinyl转移酶将Pantothenic酸从Coenzyme A终端转移到底物上。
接下来磷酸作为辅助酶参与,使底物的糖酯和磷酸反应,进而生成假尿苷糖苷键。
假尿苷糖苷键在tRNA功能中的作用:假尿苷糖苷键的存在对tRNA的结构和功能有重要的影响。
首先,假尿苷糖苷键增加了tRNA的结构稳定性。
假尿苷糖苷键的形成使tRNA 具有了特殊的结构特点,提高了其基础对照编码子序列的稳定性,有助于正确识别和配对mRNA上的密码子,从而保证正确的蛋白质合成。
其次,假尿苷糖苷键的存在有助于tRNA与氨酰-tRNA合成酶的相互作用。
tRNA在蛋白质合成中起着携带氨基酸的功能,而氨酰-tRNA 合成酶则负责将正确的氨基酸与tRNA结合。
假尿苷糖苷键与氨酰-tRNA合成酶之间的相互作用可以增加tRNA与酶的结合亲和性,有助于正确的氨酰-tRNA的生成。
假尿苷的亚基和肽键
假尿苷(Pseudouridine)是一种核苷酸类似物,其结构类似于天然的尿苷,但是其中的氮原子被甲基基团所取代。
假尿苷可以通过化学合成得到,并且可以用作DNA或RNA的合成底物。
假尿苷通常由两个亚基组成,即假尿苷酸(Pseudouridine)和假尿苷酸甲酯(Pseudouridine methyl ester)。
假尿苷酸是由假尿苷的核苷部分和磷酸基团组成,而假尿苷酸甲酯则是由假尿苷酸的核苷部分和甲酯基团组成。
在DNA或RNA的合成中,假尿苷可以用作连接两个核苷酸分子的连接基团。
在这种情况下,假尿苷的两个亚基可以被分别连接到两个核苷酸分子的3'末端,从而形成一个类似于天然核苷酸的结构。
在这个过程中,假尿苷酸的两个亚基中的一个被用作连接基团,而另一个则被用作核苷酸的5'末端。
需要注意的是,由于假尿苷的化学结构与天然尿苷不同,因此在DNA或RNA合成中使用假尿苷可能会对分子的稳定性和生物学性质产生影响。
因此,在使用假尿苷时需要谨慎,并进行适当的实验验证。
假尿苷分子中的糖苷键1. 简介假尿苷(Pseudouridine)是一种嘌呤核苷酸,通常存在于RNA分子中。
它的特殊之处在于其核糖环上的一个氮原子发生了异构化,形成了一个稳定的五元环结构。
这种异构化是通过糖苷键的形成实现的。
本文将详细介绍假尿苷分子中的糖苷键,包括其结构、性质和功能。
2. 糖苷键的结构糖苷键是连接核糖或脱氧核糖与核酸碱基之间的共价键。
在假尿苷分子中,糖苷键连接着假尿苷分子的核糖部分和碱基部分。
具体来说,假尿苷分子由一个嘌呤碱基(通常是腺嘌呤)和一个核糖组成。
这两个部分通过一个骨架上的羟基(-OH)和羰基(C=O)之间的缩合反应形成了糖苷键。
3. 糖苷键的性质3.1 强度和稳定性糖苷键是相对稳定的化学键,能够在生物体内经受一定程度的酸碱和温度变化而不容易断裂。
这种稳定性使得假尿苷分子在RNA分子中能够保持其结构和功能的完整性。
3.2 氢键形成糖苷键中的羟基和羰基上的氧原子可以与其他分子中的氮或氧原子形成氢键。
这种氢键形成有助于维持假尿苷分子与其他分子之间的稳定相互作用,进而影响RNA分子的折叠和功能。
3.3 水解反应糖苷键也可以发生水解反应,即被水分子加成断裂。
这种水解反应可以由水解酶催化,也可通过酸碱条件下的非催化反应发生。
水解反应是RNA降解的主要途径之一。
4. 糖苷键在假尿苷中的功能4.1 稳定RNA结构糖苷键连接了假尿苷分子的核糖和碱基部分,使得假尿苷能够稳定地存在于RNA分子中。
这种稳定性有助于维持RNA的整体结构,从而影响RNA的折叠和功能。
4.2 调控基因表达假尿苷作为RNA的一部分,可以通过与其他分子相互作用来调控基因表达。
研究表明,假尿苷在转录和翻译过程中起着重要的调控作用,参与了多种生物学过程的调节。
4.3 影响RNA稳定性糖苷键的存在可以影响RNA的稳定性。
一些研究发现,假尿苷在某些条件下可增强RNA分子对核酸酶的抵抗能力,从而延长其寿命。
5. 结论通过对假尿苷分子中的糖苷键进行详细介绍,我们了解到糖苷键在假尿苷分子中起着至关重要的作用。