Audit_Checklist_for_ISO_9001_2015_en_preview
- 格式:pdf
- 大小:839.13 KB
- 文档页数:8




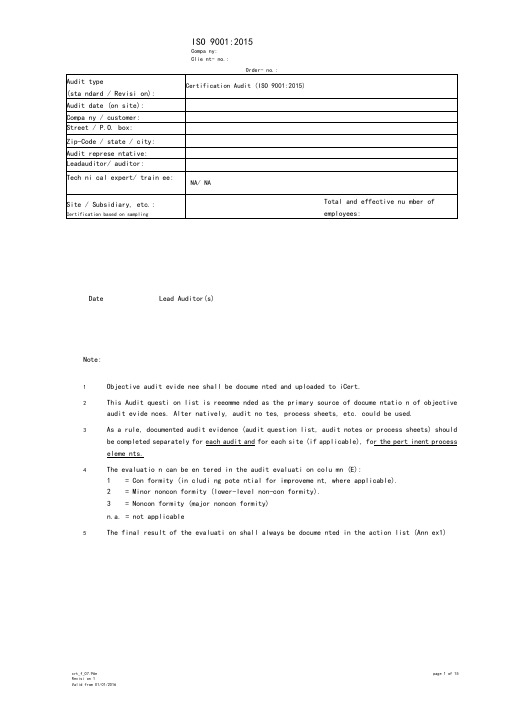
ISO 9001:2015Compa ny: Clie nt- no.:DateLead Auditor(s)Note:1 Objective audit evide nee shall be docume nted and uploaded to iCert.2This Audit questi on list is reeomme nded as the primary source of docume ntatio n of objective audit evide nces. Alter natively, audit no tes, process sheets, etc. could be used.3As a rule, documented audit evidence (audit question list, audit notes or process sheets) should be completed separately for each audit and for each site (if applicable), for the pert inent process eleme nts.4The evaluatio n can be en tered in the audit evaluati on colu mn (E): 1 = Con formity (in cludi ng pote ntial for improveme nt, where applicable). 2 = Minor noncon formity (lower-level non-con formity). 3= Noncon formity (major noncon formity)n.a. = not applicable5The final result of the evaluati on shall always be docume nted in the action list (Ann ex1)Compa ny:Clie nt- no.:Requireme nts (Audit notes identified withAudit no tes“ * ” are to be reported in the Audit Report)(Implementation / Documented Information / reviewed evidence ...)Order- no.:4 Con text of the orga ni zati on4.1Understanding the org. and its contextdetermine external and internal issues monitor and reviewinfo.4.2Understanding the needs and expectations ofinterested partiesdetermine:a)the interested parties relevant to the QMSb)the req. of these interested parties monitor and review info. about interested parties and their relevant req.4.3Determining the scope of theQMSdetermine the boundaries and applicabilityof the its scope.consider:a)the external and internal issuesb)the req. of relevant interested partiesc)the prods & servs of the org.apply all the req. of this Standard if they are applicable within the scope of its QMS. The scope shall be docu. info . state the types of prods and servs covered, and provide justification for any req. not applicable. *Min. of 1 ex.: context of the org.*Min. of 1 ex.: interested parties*Min. of 1 ex.:statutory/regulatory req.*Appropriateness of scope:4.4 QMS and its processes *Min. of 1 ex.:Order- no.:Order- no.:Order- no.:Compa ny:Clie nt- no.:Requireme nts (Audit notes identified withAudit no tes“ * ” are to be reported in the Audit Repoit)(Implementation / Documented Information / reviewed evidence ...)Order- no.:resources:a)are suitable for the specific type of monitoring andmeasurementb)are maintained to ensure their fitness retain docu. Info. of fitness for purpose of monitoring and measurement resources.measuring equipment shall be:a)calibrated or verified, or both, at specified intervals, to measurement standards; when no standards exist, the basis for calibration shall be retained as docu. Info.b)identified to determine thestatusc)safeguarded from adjustments, damage or deteriorationdetermine if the validity of previous measurement results has been adversely affected when measuring equipment is found unfit for its intended purpose, and take action .7.1・6 Organizational knowledgedetermine knowledge for the operation of its processes and conformity of prods & servs.knowledge shall be maintained and made available.When addressing changing needs and trends, consider current knowledge and determine how to acquire additional knowledge and required updates.7.2 Competencea)determine the competence of person(s)b)ensure that persons are competentc)actions to acquire the necessary competence, evaluate the effectiveness of the actionsd)retain docu. Info. of competence7.3Awarenessensure that persons are aware of:a)Q-policyb)Q-objectivesc)their contribution to the QMS, including the benefits of improved performanced)implications of not conforming with the QMS req.7.4Communicationdetermine the communications relevant to the QMS:a)whatb)whenc)with whomd)how *Evidence of fitness for purpose of monitoring and measurement resources:*Evidence of basis used for calibration or verification (if applicable):*Min. of 1 ex.:Order- no.:Order- no.:Order- no.:Order- no.:。

IS O9001:2015内审检查表审核要点审核记录1.组织Q M S覆盖范围和过程是否有缺失?2.组织Q M S对标准条款是否剪裁?如有,所剪裁条款中过程确凿没有?4组织环境4.1理解组织及理体系预期结果的各种外部和内部因素?是否对这些相关信息其环境进行监视和评审?4.2理解相关方这些相关方制定相关要求并进行了监视和评审?4.3确定质量管了相关文件?2.组织Q M S过程是否被确定和管理?过程间顺序及关系是否被确定和管理?3.组织55.1.1审核要点5.建立实施保持改进Q M S所需资源,最高管理者如何确保提供?有否实例佐证?1.“以顾客为中心”经营理念是否在组织中得到树立?组织关注以顾客为关焦点是否放在顾客身上,特别是不满意顾客身上?注焦点5.2方针5.3组织的岗并予以沟通?6策划6.16.2Q M S策划,分析确定实现目标划审核要点6.3 1.组织针对Q M S进行变更时,是否考虑了变更的目的及潜在后7支持资源7.1.1总则7.1.2人员2.组织对资源的确定、提供、使用是否进行管理、验证,清除了不适当资源、不适当使用,提高资源利用率?1.组织各个岗位的任务、性质及要求是否确定?是否根据履行岗位职责所要求的能力安排人员?7.1.3基础设施7.1.4境审核要点7.1.52.组织已规定了哪些监视和测量活动?组织通过建立哪些过程,确保上述活动可行并与监视和测量要求相一致的方式实施?4组织是否建立了测量设备系统?所有测量设备校准均已纳入校准系统,并规定了校准或验证周期?测量是否已按规定周期或在使用前得到校准或验证?.7.测量设备调整或再调整情况、方法及要求是否明确?调整过程是否受控、防止调整使测量结果失效的措施有哪些并被实施?7.1.6审核要点7.5 1.组织所建立文件是否包括了质量方针和质量目标、质量手册、信息 2.组织是否按照标准要求建立了文件化体系?3.组织是否根据内部管理需要建立了相应程序文件?7.5.14组织是否按照标准要求建立了质量记录?总则 5.组织Q M S文件详略是否得当?是否适宜可操作?7.组织Q M S文件详略程度是否与下列因素相适应?a)组织的规模和类型;c)涉及人员所需的能力。
LEADER ISO9001:2015 最新版内部质量审核检查表Document No. Internal quality audit checklistL/Q3-PZ-030 Page 1 of25Version:A00Date:2018-03-10受审核部门 审核日期审核员审核准则 ISO9001、体系文件、适用法律法规符合说明 ○”符合;“?”观察项; “△”一般不符合; “×”重大不符合 ※不符时记入证据、事实。
涉及条款 审核内容、证据及方法审核记录审核发现范围 1. 组织 QMS 覆盖范围和过程是否有缺失?无缺失、覆盖全面 √ 2. 组织 QMS 对标准条款是否删减?如有,所删减条款中过程确无删减√凿没有?4.11. 组织是否确定与其目标和战略方向相关并影响其实现质量管最高管理者应确定与本公司质量目标和战略方向相关并影响实现质量管理体系预期结果 √ 理解组织及其 理体系预期结果的各种外部和内部因素?是否对这些相关信息的各种内部因素(公司的价值观、文化、知识、绩效等相关因素)和外部因素(国际、进行监视和评审?国家、地区和当地的各种法律法规、技术、竞争、文化和社会因素等) 。
这些因素可以包 括需要考虑的正面和负面因素或条件。
本公司定期对这些内部和外部因素的相关信息进行监视和评审,以确保其充分和适宜。
4.21. 组织是否确定了与质量管理体系有关的所有相关方?是否对公司应确定:√理解相关方的这些相关方制定相关要求并进行了监视和评审?a )与质量管理体系有关的相关方;需求和期望 b )这些相关方的要求;公司应对这些相关方及其要求的相关信息进行监视和评审,以便于理解和持续满足相关方的需求和期望。
组织应考虑以下相关方: -- 顾客;-- 最终用户或受益人; -- 法人,股东; -- 银行; -- 外部供应商;-- 雇员及其他为组织工作者; -- 法律法规及监管机关; -- 地方社区团体;-- 非政府组织;。
Contents1Control of Internal Audits _________________________________________________________________________________ 3 1.1Introduction & Purpose _______________________________________________________________________________ 31.1.1 Process Activity Map _______________________________________________________________________________ 31.1.2 References _________________________________________________________________________________________ 31.1.3 Terms & Definitions ________________________________________________________________________________ 3 1.2Application & Scope __________________________________________________________________________________ 4 1.3Responsibilities ________________________________________________________________________________________ 4 1.4Controlling Internal Audits ___________________________________________________________________________ 41.4.1 Selecting Internal Auditors ________________________________________________________________________ 41.4.2 Developing the Audit Programme _________________________________________________________________ 41.4.3 Preparing for the Audit ____________________________________________________________________________ 41.4.4 Conducting the Audit ______________________________________________________________________________ 51.4.5 Data Review & Initial Reporting ___________________________________________________________________ 51.4.6 Monitoring _________________________________________________________________________________________ 51.4.7 Final Reporting _____________________________________________________________________________________ 51.4.8 Review _____________________________________________________________________________________________ 5 1.5Conducting Audits ____________________________________________________________________________________ 51.5.1 System Audits ______________________________________________________________________________________ 51.5.2 Process Audits _____________________________________________________________________________________ 61.5.3 Supplier Audits _____________________________________________________________________________________ 61.5.4 Legislation Audits __________________________________________________________________________________ 7 1.6Corrective Action ______________________________________________________________________________________ 7 1.7Forms & Records ______________________________________________________________________________________ 7 1.8Internal Audit Process Map ___________________________________________________________________________ 81.2Application & ScopeThe scope of this procedure is focused on assessing the effectiveness of your organization’s quality management system. Where such processes are found to be deficient, the audit will lead to improvement in those processes. By applying the principles of auditing, outlined by ISO 19011:2011, your organization ensures that all internal audits are conducted with due professional care, integrity and independence. All conclusions derived from the audit are based upon objective and traceable evidence.1.3ResponsibilitiesIt is the responsibility of the Quality Manager<amend as appropriate> to coordinate the whole internal audit programme. The Quality Manager<amend as appropriate> is required to:∙Determine the root causes of non-conformities;∙Maintain a system for reporting audit results;∙Determine conformity to planned arrangements;∙Determine proper implementation and maintenance;∙Provide the results of audits to top management;∙Review the effectiveness of corrective actions taken.1.4Controlling Internal Audits1.4.1 Selecting Internal AuditorsTo ensure impartiality and objectivity, the audit team will include personnel from departments not directly associated with the area, process or department being audited. The Internal Auditors are selected on the basis of their:∙Education: secondary or higher;∙Work Experience: more than 5 years;∙Relevant Training: provided in-house or externally;∙Audit Experience: demonstrable knowledge/skills.1.4.2 Developing the Audit ProgrammeThe Quality Manager<amend as appropriate>is required to:∙Determine the status and importance of each process;∙Establish audit frequency based on the status and importance of each process;∙Develop and communicate the audit schedule;∙Appoint audit team leader where required;∙Select audit team;∙Assign audit duties to the auditor team.1.4.3 Preparing for the AuditThe Internal Auditors <amend as appropriate>are required to:∙Review relevant management system documents and records;∙Determine their adequacy with respect to the audit criteria and with ISO 9001;∙Review and prepare the internal audit checklist;The audit team then reviews the process inputs and outputs using the Turtle Diagram at the front of this procedure. The audit is conducted using the ISO 9001-2015 Supplier Audit Checklist.1.5.4 Legislation AuditsAt least once per year, audit is conducted on the scope and applicability of the register of applicable legislation in order to verify continued compliance. Using the register of legislation, the auditor determines the most significant legislation applicable to our organization at the time of the audit, by taking a sample and seeking objective evidence that the legislation is current and is being complied with.Samples of legislation are noted and the register brought up to date as required. The samples taken are selected based on current risks but ensures that the whole register is audited at least once in each 3 year period.1.6Corrective ActionThe corrective actions are identified on the Internal Audit, Non-conformity & Correct Action Tracker along with the person responsible and the timescales for completion. The process or procedure is re-audited and the issue closed out when all corrective actions are completed. A member of the audit team will then sign off the audit report. An audit summary is prepared for management review.1.7Forms & RecordsAll documentation and records generated by the internal audit process are retained and managed in accordance with the Control of Documented Information procedure.。