ibuprofen USP39
- 格式:pdf
- 大小:178.00 KB
- 文档页数:4

最新文件---------------- 仅供参考--------------------已改成-----------word文本 --------------------- 方便更改赠人玫瑰,手留余香。
General chapters备注:蓝色字体章节为新增内容,红色字体章节为在USP38基本上修改内容。
〈1〉Injections, 53〈2〉Oral drug products—product quality tests, 66〈3〉Topical and transdermal drug products—product quality tests, 71〈5〉Inhalation and nasal drug products general information and product quality tests, 80〈11〉USP reference standards, 93〈17〉Prescription container labeling, 96〈21〉Thermometers, 98〈31〉V olumetric apparatus, 99〈41〉Weights and balances, 99〈51〉Antimicrobial effectiveness testing,100〈55〉 Biological indicators—resistance performance tests, 103〈61〉Microbiological examination of nonsterile products: microbial enumeration tests, 106〈62〉Microbiological examination of nonsterile products: tests for specified organisms, 112〈63〉Mycoplasma tests, 120〈71〉Sterility tests, 125〈81〉Antibiotics—microbial assays, 133〈85〉Bacterial endotoxins test, 151〈87〉Biological reactivity tests, in vitro, 156〈88〉Biological reactivity tests, in vivo, 158〈90〉Fetal bovine serum quality attributes and functionality tests, 167〈91〉Calcium pantothenate assay, 171〈92〉Growth factors and cytokines used in cell therapy manufacturing, 172〈111〉 Design and analysis of biological assays, 176〈115〉 Dexpanthenol assay, 191〈121〉 Insulin assays, 193〈121.1〉 Physicochemical analytical procedures for insulins, 195〈123〉 Glucagon bioidentity tests, 198〈124〉 Erythropoietin bioassays, 200〈126〉 Somatropin bioidentity tests, 202<129> Analytical Procedures for Recombinant Therapeutic Monoclonal Antibodies〈130〉 Protein A quality attributes, 204〈151〉 Pyrogen test, 211〈161〉 Transfusion and infusion assemblies and similar medical devices, 212<162> Diphtheria Antitoxin Potency Testing for Human Immune Globulins〈171〉V itamin B12activity assay, 213〈181〉 Identification—organic nitrogenous bases, 216〈191〉 Identification tests—general, 216〈193〉 Identification—tetracyclines, 219〈197〉 Spectrophotometric identification tests, 220〈201〉 Thin-layer chromatographic identification test, 221〈206〉 Aluminum, 222〈207〉 Test for 1,6-anhydro derivative for enoxaparin sodium, 223〈208〉 Anti-factor Xa and anti-factor IIa assays for unfractionated and low molecular weight heparins, 228〈211〉 Arsenic, 233〈221〉 Chloride and sulfate, 235〈223〉 Dimethylaniline, 236〈226〉 4-Epianhydrotetracycline, 236〈227〉 4-Aminophenol in acetaminophen-containing drug products, 237〈228〉 Ethylene oxide and dioxane, 238〈231〉 Heavy metals, 241〈232〉 Elemental impurities—limits, 243〈233〉 Elemental impurities—procedures,245〈241〉 Iron, 249〈251〉 Lead, 250〈261〉 Mercury, 251〈267〉 Porosimetry by mercury intrusion,253〈268〉 Porosity by nitrogen adsorption–desorption, 256〈271〉 Readily carbonizable substances test,260〈281〉 Residue on ignition, 260〈291〉 Selenium, 261〈301〉 Acid-neutralizing capacity, 261〈311〉 Alginates assay, 262〈341〉 Antimicrobial agents—content, 264〈345〉 Assay for citric acid/citrate and phosphate, 267〈351〉 Assay for steroids, 268〈361〉 Barbiturate assay, 268〈371〉 Cobalamin radiotracer assay, 268〈381〉 Elastomeric closures for injections,270〈391〉 Epinephrine assay, 275〈401〉 Fats and fixed oils, 276〈411〉 Folic acid assay, 290〈413〉 Impurities testing in medical gases,290〈415〉 Medical gases assay, 291〈425〉 Iodometric assay—antibiotics, 293〈429〉 Light diffraction measurement of particle size, 294 〈431〉 Methoxy determination, 299〈441〉 Niacin or niacinamide assay, 301〈451〉 Nitrite titration, 306〈461〉 Nitrogen determination, 306〈466〉 Ordinary impurities, 307〈467〉 Residual solvents, 309〈469〉 Ethylene glycol, diethylene glycol,and triethylene glycol in ethoxylated substances, 324〈471〉 Oxygen flask combustion, 325〈481〉 Riboflavin assay, 326〈501〉 Salts of organic nitrogenous bases,327〈503〉 Acetic acid in peptides, 327<503.1> Trifluoroacetic Acid (TFA) in Peptides〈511〉 Single-steroid assay, 328〈525〉 Sulfur dioxide, 329〈531〉 Thiamine assay, 334〈541〉 Titrimetry, 335〈551〉V itamin E assay, 338〈561〉 Articles of botanical origin, 345〈563〉 Identification of articles of botanical origin, 358〈565〉 Botanical extracts, 370〈571〉V itamin A assay, 373<580> Vitamin C Assay〈581〉V itamin D assay, 378〈591〉 Zinc determination, 387〈601〉 Inhalation and nasal drug products:aerosols, sprays, andpowders–performance quality tests, 388〈602〉 Propellants, 414〈603〉 Topical aerosols, 415〈604〉 Leak rate, 416〈610〉 Inhalation and nasal drug products:aerosols, sprays, and powders–performance quality tests, 416〈611〉 Alcohol determination, 418〈616〉 Bulk density and tapped density, 420〈621〉 Chromatography, 424〈631〉 Color and achromicity, 434〈641〉 Completeness of solution, 436〈643〉 Total organic carbon, 436〈645〉 Water conductivity, 438〈651〉 Congealing temperature, 441〈659〉 Packaging and storage requirements,443〈660〉 Containers—glass, 450〈661〉 Containers—plastics, 457<661.1> Plastic Materials of Construction<661.2> Plastic Packaging Systems for Pharmaceutical Use〈670〉 Containers—Auxiliary Components〈671〉 Containers—performance testing,465〈691〉 Cotton, 472〈695〉 Crystallinity, 474〈696〉 Crystallinity determination by solution calorimetry, 474〈698〉 Deliverable volume, 478〈699〉 Density of solids, 481〈701〉 Disintegration, 483〈705〉 Quality attributes of tablets labeled as having a functional score, 485〈711〉 Dissolution, 486〈721〉 Distilling range, 496〈724〉 Drug release, 497〈729〉 Globule size distribution in lipid injectable emulsions, 504〈730〉 Plasma spectrochemistry, 506〈731〉 Loss on drying, 513〈733〉 Loss on ignition, 514〈735〉 X-ray fluorescence spectrometry, 514〈736〉 Mass spectrometry, 519〈741〉 Melting range or temperature, 525〈751〉 Metal particles in ophthalmic ointments, 527〈755〉 Minimum fill, 527〈761〉 Nuclear magnetic resonance, 528〈771〉 Ophthalmic Products—Quality Tests〈776〉 Optical microscopy, 537〈781〉 Optical rotation, 540〈785〉 Osmolality and osmolarity, 541〈786〉 Particle size distribution estimation by analytical sieving, 543〈787〉 Subvisible particulate matter in therapeutic protein injections, 547〈788〉 Particulate matter in injections, 550〈789〉 Particulate matter in ophthalmic solutions, 553〈790〉Visible Particulates in Injections〈791〉 pH, 556〈795〉 Pharmaceutical compounding—nonsterile preparations, 559〈797〉 Pharmaceutical compounding—sterile preparations, 567〈801〉 Polarography, 611〈811〉 Powder fineness, 616〈821〉 Radioactivity, 616〈823〉 Positron emission tomography drugs for compounding, investigational, andresearch uses, 627〈831〉 Refractive index, 636〈841〉 Specific gravity, 636〈846〉 Specific surface area, 638〈851〉 Spectrophotometry and light-scattering, 641<855> Nephelometry, Turbidimetry, and Visual Comparison〈861〉 Sutures—diameter, 669〈871〉 Sutures—needle attachment, 670〈881〉 Tensile strength, 671〈891〉 Thermal analysis, 672〈905〉 Uniformity of dosage units, 675〈911〉V iscosity—capillar y viscometer methods, 679〈912〉 Rotational rheometer methods, 681〈913〉 Rolling ball viscometer method, 686<914> Viscosity-Pressure Driven Methods〈921〉 Water determination, 688〈941〉 Characterization of crystalline and partially crystalline solids by X-ray powder diffraction (XRPD), 692〈1005〉 Acoustic emission, 699〈1010〉 Analytical data—interpretation and treatment, 703〈1015〉 Automated radiochemical synthesis apparatus, 717〈1024〉 Bovine serum, 719〈1027〉 Flow cytometry, 732〈1030〉 Biological assay chapters—overview and glossary, 748〈1031〉 The biocompatibility of materials used in drug containers, medical devices, and implants, 759〈1032〉 Design and development of biological assays, 769〈1033〉 Biological assay validation, 787〈1034〉 Analysis of biological assays, 801〈1035〉 Biological indicators for sterilization,814〈1041〉 Biologics, 818〈1043〉 Ancillary materials for cell, gene,and tissue-engineered products, 819〈1044〉 Cryopreservation of cells, 827〈1045〉 Biotechnology-derived articles, 840〈1046〉 Cellular and tissue-based products,854〈1047〉 Gene therapy products, 883〈1048〉 Quality of biotechnological products: analysis of the expressionconstruct in cells used for production of r-DNA derived protein roducts, 911〈1049〉 Quality of biotechnological products: stability testing of biotechnological/biological products,913〈1050〉Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin, 918〈1051〉 Cleaning glass apparatus, 931〈1052〉 Biotechnology-derived articles—amino acid analysis, 931〈1053〉 Biotechnology-derived articles—capillary electrophoresis, 944〈1054〉 Biotechnology-derived articles—isoelectric focusing, 951〈1055〉 Biotechnology-derived articles—peptide mapping, 954〈1056〉 Biotechnology-derived articles—polyacrylamide gel electrophoresis, 960〈1057〉 Biotechnology-derived articles—total protein assay, 967〈1058〉 Analytical instrument qualification,971〈1059〉 Excipient performance, 977〈1061〉 Color—instrumental measurement,997〈1065〉 Ion chromatography, 1000〈1066〉 Physical environments that promote safe medication use, 1003〈1072〉 Disinfectants and antiseptics, 1010〈1074〉 Excipient biological safety evaluation guidelines, 1015〈1078〉 Good manufacturing practices for bulk pharmaceutical excipients, 1019〈1079〉 Good storage and shipping practices, 1035〈1080〉 Bulk pharmaceutical excipients—certificate of analysis, 1044〈1084〉 Glycoprotein and glycan analysis—general considerations, 1052〈1086〉 Impurities in official articles, 1063〈1087〉 Apparent intrinsic dissolution—dissolution testing procedures for rotating disk and stationary disk, 1066〈1088〉 In vitro and in vivo evaluation of dosage forms, 1070〈1090〉 Assessment of drug product performance—bioavailability, bioequivalence, and dissolution, 1081〈1091〉 Labeling of inactive ingredients,1089〈1092〉 The dissolution procedure:development and validation, 1090〈1094〉 Capsules—dissolution testing and related quality attributes, 1097〈1097〉 Bulk powder sampling procedures,1105〈1102〉 Immunological test methods—general considerations, 1118〈1103〉 Immunological test methods— enzyme-linked immunosorbent assay (ELISA), 1125〈1104〉 Immunological test methods—immunoblot analysis, 1135〈1105〉 Immunological test methods—surface plasmon resonance, 1146〈1106〉 Immunogenicity assays—design and validation of immunoassays to detect anti-drug antibodies, 1161〈1111〉 Microbiological examination of nonsterile products: acceptance criteria for pharmaceutical preparations and substances forpharmaceutical use, 1176〈1112〉 Application of water activity determination to nonsterile pharmaceutical products, 1178〈1113〉 Microbial characterization, identification, and strain typing, 1180〈1115〉 Bioburden control of nonsterile drug substances and products, 1185〈1116〉Microbiological control and monitoring of aseptic processing environments, 1191〈1117〉 Microbiological best laboratory practices, 1204〈1118〉 Monitoring devices—time, temperature, and humidity, 1210〈1119〉 Near-infrared spectrophotometry,1215〈1120〉 Raman spectroscopy, 1222〈1121〉 Nomenclature, 1230〈1125〉 Nucleic acid-based techniques—general, 1232〈1126〉 Nucleic acid-based techniques—extraction, detection, and sequencing,1237〈1127〉 Nucleic acid-based techniques—amplification, 1247〈1128〉 Nucleic acid-based techniques—microarray, 1256〈1129〉 Nucleic acid-based techniques—genotyping, 1262〈1130〉 Nucleic acid-based techniques— approaches for detecting trace nucleic acids (residual DNA testing), 1267〈1136〉 Packaging—unit-of-use, 1269〈1151〉 Pharmaceutical dosage forms, 1278〈1160〉 Pharmaceutical calculations in prescription compounding, 1303〈1163〉 Quality assurance in pharmaceutical compounding, 1317〈1171〉 Phase-solubility analysis, 1324〈1174〉 Powder flow, 1326〈1176〉 Prescription balances and volumetric apparatus, 1331〈1177〉 Good packaging practices, 1332〈1178〉 Good repackaging practices, 1335〈1180〉 Human plasma, 1337〈1181〉 Scanning electron microscopy,1360〈1184〉 Sensitization testing, 1370〈1191〉 Stability considerations in dispensing practice, 1381〈1195〉 Significant change guide for bulk pharmaceutical excipients, 1385〈1197〉 Good distribution practices for bulk pharmaceutical excipients, 1396〈1207〉 Sterile product packaging—integrity evaluation, 1418〈1208〉 Sterility testing—validation of isolator systems, 1420〈1209〉 Sterilization—chemical and physicochemical indicators and integrators, 1424〈1211〉 Sterilization and sterility assurance of compendial articles, 1427〈1216〉 Tablet friability, 1432〈1217〉 Tablet breaking, 1433〈1222〉 Terminally sterilized pharmaceutical products—parametric release, 1436〈1223〉 Validation of alternative microbiological methods, 1439〈1224〉 Transfer of analytical procedures,1443〈1225〉 Validation of compendial procedures, 1445〈1226〉 Verification of compendial procedures, 1451〈1227〉 Validation of microbial recovery from pharmacopeial articles, 1452〈1229〉 Sterilization of compendial articles,1456〈1229.1〉 Steam sterilization by direct contact, 1461〈1229.2〉 Moist heat sterilization of aqueous liquids, 1464〈1229.3〉 Monitoring of bioburden, 1468〈1229.4〉 Sterilizing filtration of liquids,1472〈1229.6〉 Liquid-phase sterilization, 1479〈1229.7〉 Gaseous sterilization, 1482〈1229.8〉 Dry heat sterilization, 1485〈1229.10〉 Radiation sterilization, 1487〈1230〉 Water for hemodialysis applications,1491〈1231〉 Water for pharmaceutical purposes,1492〈1234〉 Vaccines for human use— polysaccharide and glycoconjugate vaccines, 1518〈1235〉 Vaccines for human use—general considerations, 1534〈1237〉 Virology test methods, 1550〈1238〉 Vaccines for human use—bacterial vaccines, 1570〈1240〉 Virus testing of human plasma for further manufacture, 1582〈1241〉 Water–solid interactions in pharmaceutical systems, 1592〈1251〉 Weighing on an analytical balance,1597〈1265〉 Written prescription drug information—guidelines, 1602〈1285〉 Preparation of biological specimens for histologic and immunohistochemical analysis, 1603〈1285.1〉 Hematoxylin and eosin staining of sectioned tissue for microscopic examination, 1607〈1601〉 Products for nebulization—characterization tests, 1610〈1644〉 Theory and practice of electrical conductivity measurements of solutions,1613〈1660〉 Evaluation of the inner surface durability of glass containers, 1620<1661> Evaluation of Plastic Packaging Systems and Their Materials of Construction with Respect to Their User Safety Impact〈1724〉 Semi-solid drug products—performance tests, 1625<1730> Plasma Spectrochemistry—Theory and Practice<1735> X-Ray Fluorescence Spectrometry —Theory and Practice〈1736〉 Applications of mass spectrometry,1637〈1761〉 Applications of nuclear magnetic resonance spectroscopy, 1659<1771> Ophthalmic Products—Performance Tests〈1788〉 Methods for the determination of particulate matter in injections and ophthalmic solutions, 1693〈1911〉 Rheometry, 1742〈2021〉 Microbial enumeration tests—nutritional and dietary supplements,1751〈2022〉 Microbiological procedures for absence of specified microorganisms— nutritional and dietary supplements,1756〈2023〉 Microbiological attributes of nonsterile nutritional and dietary supplements, 1762〈2030〉 Supplemental information for articles of botanical origin, 1765<2040> Disintegration and Dissolution of Dietary Supplements〈2091〉 Weight variation of dietary supplements, 1782〈2232〉 Elemental contaminants in dietary supplements, 1783〈2250〉 Detection of irradiated dietary supplements, 1786〈2750〉 Manufacturing practices for dietary supplements, 1789最新文件---------------- 仅供参考--------------------已改成-----------word文本 --------------------- 方便更改赠人玫瑰,手留余香。

【药品名】布洛芬【英文名】Ibuprofen【别名】拔努风;波菲特;大亚芬克;芬必得;芬尼康;福尔;抗风痛;洛芬;美林;摩纯;托恩;雅维;炎痛停;异丁苯丙酸;异丁洛芬;对异丁苯丙酸;消痛停;Andran;Brufen【剂型】1.片剂:0.1g,0.2g,0.3g;2.控释片:0.2g,0.3g;3.胶囊:0.1g,0.2g,0.3g;4.缓释胶囊:0.2g;5.颗粒剂:0.1g,0.2g;6.口服液:0.1g(10ml);7.糖浆:0.2g(10ml);8.混悬液:2.0g(100ml);9.干混悬剂:34g:1.2g;10.搽剂:2.5g(5ml),2.5g(50ml);11.乳膏:5%;12.栓剂:50mg,100mg。
【药理作用】本品为苯丙酸类非甾体抗炎镇痛药。
具有较强的抗炎、抗风湿及解热镇痛作用,通过抑制前列腺素的合成,阻断炎症介质的释放而起作用。
特点为对血象及肾功能无明显影响,胃肠道刺激性小,体内无药物蓄积的趋向。
用于风湿性关节炎,其消炎、镇痛、解热作用与阿司匹林、保泰松相似,比对乙酰氨基酚好,而对胃肠道的不良反应较轻,易为患者接受。
本品治疗痛风只起消炎、镇痛作用,并不能纠正高尿酸血症。
对血小板的黏着和聚集反应有抑制作用,并延长出血时间。
其抗炎作用与阿司匹林等效。
用于类风湿性关节炎,本品每天0.8~1.6g和阿司匹林每天3~6g一样有效,而胃肠道的不良反应比阿司匹林少。
治疗骨关节炎,本品像吲哚美辛一样有效而不良反应较少。
【药动学】口服吸收快且完全,与食物同服时吸收减慢,但吸收量不减少。
服药后1~2h血药浓度可达峰值,t4~5h;血药浓度波动较小,无药物蓄积的倾向。
该药可缓慢透过滑膜腔,血药浓度降低后关节腔内仍能保持较高的浓度,并易透过胎盘和进入乳汁中,其血浆蛋白结合率为99%,主要经肝脏代谢,60%~90%经肾随尿排出,其中1%为原形物,少部分随粪便排出,进入乳汁者极少,本品可经直肠或皮肤吸收。
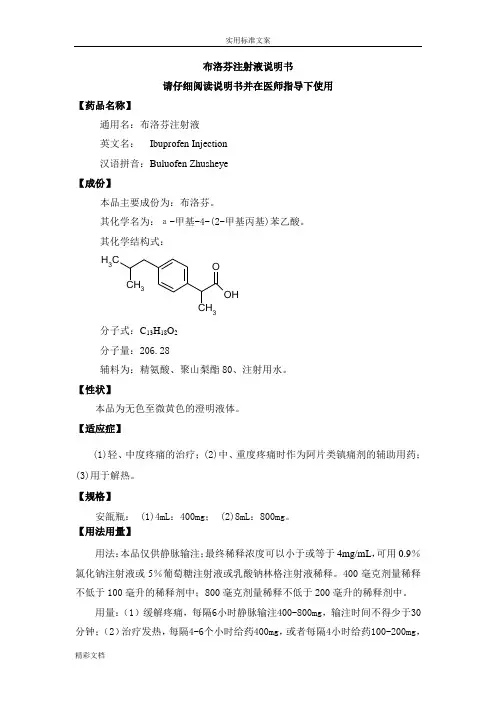
布洛芬注射液说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名:布洛芬注射液英文名: Ibuprofen Injection汉语拼音:Buluofen Zhusheye【成份】本品主要成份为:布洛芬。
其化学名为:а-甲基-4-(2-甲基丙基)苯乙酸。
其化学结构式: CH 3CH 3CH 3OH O分子式:C 13H 18O 2分子量:206.28辅料为:精氨酸、聚山梨酯80、注射用水。
【性状】本品为无色至微黄色的澄明液体。
【适应症】(1)轻、中度疼痛的治疗;(2)中、重度疼痛时作为阿片类镇痛剂的辅助用药;(3)用于解热。
【规格】安瓿瓶: (1)4mL :400mg ; (2)8mL :800mg 。
【用法用量】用法:本品仅供静脉输注;最终稀释浓度可以小于或等于4mg/mL ,可用0.9%氯化钠注射液或5%葡萄糖注射液或乳酸钠林格注射液稀释。
400毫克剂量稀释不低于100毫升的稀释剂中;800毫克剂量稀释不低于200毫升的稀释剂中。
用量:(1)缓解疼痛,每隔6小时静脉输注400-800mg ,输注时间不得少于30分钟;(2)治疗发热,每隔4-6个小时给药400mg ,或者每隔4小时给药100-200mg ,输注时间不得少于30分钟。
【不良反应】最常见的不良反应包括:恶心、肠胃气胀、呕吐、头痛、出血、头晕等。
严重胃肠道溃疡与出血可能事先没有任何警示症状,因此医生须对胃肠道出血的体征或症状加以监视。
对于长期用非甾体类抗炎药治疗的患者,应定期进行血常规检查及生化分析等。
如果出现任何临床体征或症状,包括:肝或肾疾病、全身表现(例如:嗜曙红细胞增多,皮疹等)、肝脏检查持续异常或恶化等,则需立即停用本品。
心血管风险:非甾体抗炎药(NSAIDs)会增加心血管血栓性严重不良事件、心肌梗塞及中风等病症发生的风险,这些都有可能是致命的。
持续用药可能会提高这些风险。
对于心血管疾病患者或带有心血管疾病危险因素的病人来说,其风险几率可能会更大。

芬必得的曾用名及功能主治前言芬必得是一种常见的药物,它在临床上有多种曾用名和功能主治。
本文将对芬必得的曾用名和功能主治进行详细介绍。
曾用名芬必得在不同国家和地区有不同的曾用名,以下是一些常见的芬必得曾用名:1.Ibuprofen:这是芬必得在国际上通用的曾用名,它广泛应用于世界各地的医疗机构和药店。
2.Brufen:这是芬必得在欧洲地区的常用曾用名,它是Ibuprofen的一个商标。
3.Advil:这是芬必得在美国市场的常用曾用名,也是Ibuprofen的一个商标。
4.Nurofen:这是芬必得在澳大利亚和新西兰市场的常用曾用名,同样也是Ibuprofen的一个商标。
功能主治芬必得具有多种功能主治,以下是一些常见的功能主治:1.缓解疼痛:芬必得是一种非处方药,常用于缓解各种类型的疼痛,包括头痛、牙痛、关节痛、月经痛等。
它通过抑制体内产生疼痛物质的酶的活性,从而减轻疼痛感。
2.降低体温:芬必得也常用于降低体温,特别是在感冒、流感等病情引起的发热情况下。
它通过影响体内温度调节中枢的工作,使体温保持在正常范围内。
3.减轻炎症:芬必得还具有抗炎作用,可以减轻轻度炎症引起的疼痛和不适。
它通过抑制体内炎症介质的产生和炎症反应的进行,从而减轻炎症症状。
4.缓解关节炎疼痛:芬必得可以缓解关节炎引起的疼痛和炎症,并改善患者的活动能力。
它通过减少关节内的炎症反应和抑制炎症介质的产生,从而减轻关节疼痛。
5.缓解肌肉疼痛:芬必得对肌肉疼痛也有一定的缓解作用。
它可以通过减少肌肉纤维的炎症反应和抑制炎症介质的产生,从而缓解肌肉疼痛。
注意事项在使用芬必得时,有一些注意事项需要注意:•严格按照医生或药剂师的嘱咐使用,不要超量使用。
•不要与其他非甾体抗炎药一起使用,以免增加不良反应的风险。
•长期大量使用芬必得可能会引起一些不良反应,如胃肠道溃疡、肾脏损伤等,因此要注意合理使用药物。
•孕妇、哺乳期妇女、儿童和老年人在使用芬必得时应特别注意,最好在医生的指导下使用。

精氨酸布洛芬分子量
精氨酸布洛芬(Ibuprofen L-Arginine)是一种药物,常用于缓解轻至中度疼痛和发热。
它具有镇痛、退热和抗炎作用,可以缓解头痛、牙痛、关节痛、肌肉痛和感冒引起的发热等症状,并能减轻关节炎和风湿性关节炎等疾病引起的炎症。
精氨酸布洛芬的分子量是多少呢?它的分子式为C30H39N5O7,分子量为613.66克/摩尔。
这意味着在摩尔质量为613.66克的情况下,精氨酸布洛芬中含有30个碳原子、39个氢原子、5个氮原子和7个氧原子。
这些原子通过化学键连接在一起,形成了这种药物的分子结构。
精氨酸布洛芬是一种非处方药,可以在药店或超市中购买到。
在使用精氨酸布洛芬时,我们需要根据医生或药剂师的建议,按照正确的剂量和使用方法进行使用。
一般来说,成年人每次口服200-400毫克,每天3-4次,与饮食一起服用,以减少对胃黏膜的刺激。
精氨酸布洛芬是一种常见的药物,但也存在一些注意事项。
长期服用或过量服用可能会导致胃肠道不适,如胃痛、恶心和呕吐等。
因此,在使用精氨酸布洛芬时,应遵循医生或药剂师的建议,不要超过推荐的剂量和使用频率。
精氨酸布洛芬还有一些禁忌症,如对该药物过敏、存在胃溃疡或出血倾向、正在使用其他非甾体抗炎药物等。
在这些情况下,应避免
使用精氨酸布洛芬或在医生的指导下使用。
精氨酸布洛芬是一种常用的药物,具有镇痛、退热和抗炎作用。
它的分子量为613.66克/摩尔,分子结构由碳、氢、氮和氧等原子组成。
在使用精氨酸布洛芬时,应按照正确的剂量和使用方法进行使用,并注意可能出现的副作用和禁忌症。

布洛芬的功效与作用布洛芬(Ibuprofen)是一种非处方药,属于非类固醇消炎药(NSAIDs),因其具有镇痛、退热和抗炎作用而广泛应用于临床。
本文将详细介绍布洛芬的功效与作用。
一、布洛芬的起源与发展布洛芬最早由英国的Dr. Stewart Adams在1961年发现,主要用于治疗关节炎和风湿痛。
布洛芬是通过其对环氧合酶的抑制作用,阻断了花生四烯酸的代谢,从而减少了炎症反应的发生。
布洛芬作为一种非处方药,被广泛用于缓解轻至中度的疼痛和发热,如头痛、牙痛、神经痛、痛经、感冒、关节炎等。
二、布洛芬的药物特性1. 药理学特性:布洛芬的主要作用机制是通过抑制环氧合酶,尤其是环氧合酶-2,减少花生四烯酸代谢,进而抑制前列腺素的合成,从而减少疼痛、退热和抗炎作用。
2. 药代动力学特性:布洛芬的口服吸收迅速,生物利用度高达90%以上。
其在体内的半衰期为2-4小时。
在肝脏中主要通过先行代谢,转化为草酸和邻氨基苯丙酸。
布洛芬主要经由肾脏排出,小部分通过胆汁排出。
三、布洛芬的主要功效与作用1. 镇痛作用:布洛芬具有中度至强度的镇痛作用。
其通过抑制环氧合酶,减少前列腺素的产生,进而减少痛觉传导和炎症反应。
布洛芬可用于缓解各种原因引起的头痛、牙痛、神经痛、痛经等。
2. 退热作用:布洛芬可通过抑制前列腺素的合成和释放,降低体温,起到退热作用。
它主要用于感冒、发热等情况下的退热。
3. 抗炎作用:布洛芬通过抑制环氧合酶,减少前列腺素和其他炎症介质的产生,从而抑制炎症反应。
它能减轻疼痛、肿胀和红肿等炎症症状,临床上常用于治疗关节炎、骨折、扭伤等促进炎症的疾病。
4. 抗血小板聚集作用:布洛芬可通过抑制血小板中的血小板活化因子的释放,从而减少血小板的聚集和血栓的形成,并与其他抗血小板药物共同应用以预防心脑血管疾病。
5. 预防妊娠并发症:一些研究表明,布洛芬在妊娠中的使用可能有助于预防妊娠相关的并发症,如早产、子痫前期等。
但使用布洛芬在妊娠期间需谨慎,尤其是在孕晚期,因为长期高剂量使用可能导致胎儿心脏瓣膜的早闭。

布洛芬(Ibuprofen)使用说明书您可以参考以下写作格式来撰写布洛芬(Ibuprofen)使用说明书:布洛芬(Ibuprofen)使用说明书一、药品简介布洛芬是一种非处方药,被广泛用于缓解轻至中度的疼痛、退烧和消炎等症状。
它属于非甾体抗炎药(NSAIDs)类药物,能有效抑制炎症反应和疼痛传导。
二、适应症布洛芬一般适用于以下症状和疾病:1. 头痛,牙痛,关节痛等不适疼痛;2. 发热、发冷症状;3. 过敏引起的鼻窦症状、鼻过敏。
三、禁忌症以下情况下患者应避免使用布洛芬:1. 对布洛芬或其他NSAIDs过敏的患者;2. 有胃溃疡、消化道出血等胃肠道问题的患者;3. 孕妇、哺乳期妇女在医生指导下使用。
四、使用方法1. 用药剂型:布洛芬常见的剂型有片剂、混悬剂等,根据患者实际情况选择适宜的剂型;2. 剂量:成人常见剂量为每次200-400毫克,每日最大剂量不超过1200毫克。
儿童用药需按照年龄和体重确定剂量;3. 用药频率:根据疼痛或发热情况,一般每4-6小时可重复服用一次;4. 用药时间:根据症状严重程度,使用时间一般不超过3-5天。
若症状加重或持续,请咨询医生。
五、注意事项1. 需咨询医生:儿童、老年人、孕妇、哺乳期妇女、有慢性疾病或服用其他药物的患者需咨询医生后再使用;2. 饭后使用:为减少胃肠道不良反应,建议在饭后使用布洛芬;3. 不建议滥用:请遵循医生指示使用,不要超剂量或长期使用;4. 需密封保存:保持药品密封,放置在阴凉干燥的地方,远离阳光直射。
六、可能的副作用布洛芬使用过程中可能出现以下副作用:1. 胃肠道不适:如胃痛、消化不良、恶心等;2. 皮肤过敏:如皮疹、荨麻疹等;3. 呼吸系统不适:如哮喘、气促等;如果出现以上副作用,请停药并咨询医生。
七、与其他药物的相互作用通常与以下药物同时使用时可能会发生相互作用:1. 抗凝药物:如华法林等;2. 利尿剂:如氢氯噻嗪等;3. 处方药:如阿司匹林等。

布洛芬的安全性评估布洛芬(Ibuprofen)是一种非处方药,属于非甾体抗炎药(NSAIDs)的一种。
它具有镇痛、退热和抗炎作用,被广泛应用于缓解轻至中度疼痛和发热症状。
然而,任何药物的使用都应该谨慎,并且布洛芬也不例外。
本文将对布洛芬的安全性进行评估,以帮助人们正确使用该药物。
首先,布洛芬的安全性在很大程度上取决于剂量和使用时间。
在推荐剂量范围内使用,布洛芬通常是安全的。
然而,长期或过量使用可能导致一些不良反应。
因此,使用布洛芬时应遵循医生或药师的建议,不要超过推荐剂量和使用时间。
其次,布洛芬的主要不良反应包括胃肠道问题和心脏问题。
胃肠道问题可能包括胃痛、消化不良、恶心、呕吐和溃疡等。
这些问题的发生率在长期或过量使用时更高。
为了减少胃肠道问题的风险,可以在服用布洛芬时同时进食,或者选择一些胃肠保护剂来减轻不良反应。
心脏问题是布洛芬使用的另一个潜在风险。
长期或高剂量使用布洛芬可能增加心脏事件(如心脏病发作)的风险。
这主要是由于布洛芬的抗炎作用可能干扰血小板聚集和血管扩张等生理过程。
因此,对于有心脏疾病或高危因素的患者,应在医生的指导下使用布洛芬,并且避免长期或高剂量使用。
除了胃肠道问题和心脏问题外,布洛芬还可能引起其他不良反应,如头痛、眩晕、皮疹、水肿等。
这些反应通常是暂时的,停药后会自行消失。
然而,如果不良反应严重或持续,应及时咨询医生。
此外,布洛芬还有一些禁忌症和注意事项。
对于孕妇、哺乳期妇女、儿童、老年人和某些特定疾病患者,如肾脏疾病、胃溃疡、哮喘等,使用布洛芬时需要格外小心,并在医生的指导下使用。
此外,与其他药物的相互作用也需要注意,特别是与抗凝血药、降压药和利尿药等药物的联合使用。
总的来说,布洛芬是一种常用的非处方药,具有镇痛、退热和抗炎作用。
在正确使用的情况下,它是相对安全的。
然而,为了最大程度地减少不良反应的风险,使用布洛芬时需要遵循医生或药师的建议,并且注意剂量、使用时间和禁忌症等因素。

布洛芬的药物临床试验进展布洛芬(Ibuprofen)是一种非处方药,属于非甾体抗炎药(NSAIDs)的一种。
它被广泛用于缓解轻至中度的疼痛、退烧和消炎。
布洛芬的药物临床试验一直在不断进展,以探索其更广泛的应用领域和潜在的治疗效果。
布洛芬的主要作用机制是通过抑制体内的前列腺素合成,从而减轻疼痛、退烧和消炎。
它能够选择性地抑制COX-2酶,这是一种在炎症过程中产生的酶。
相比于其他NSAIDs,布洛芬的选择性COX-2抑制作用相对较弱,这使得它的抗炎效果更为温和,同时减少了不良反应的风险。
布洛芬的药物临床试验主要集中在以下几个方面:1. 疼痛缓解:布洛芬作为一种常用的非处方药,被广泛用于缓解各种类型的疼痛,包括头痛、牙痛、关节痛等。
临床试验研究了布洛芬在不同类型疼痛中的疗效和安全性,以确定最佳的剂量和用药方式。
2. 关节炎治疗:布洛芬在治疗关节炎方面也有一定的研究进展。
早期的研究表明,布洛芬可以减轻关节炎引起的疼痛和炎症,改善患者的生活质量。
近年来,一些临床试验还研究了布洛芬与其他药物的联合应用,以提高治疗效果。
3. 预防心血管疾病:一些研究表明,长期使用布洛芬可能与心血管疾病的风险相关。
然而,最新的临床试验并未明确证实这种关联。
目前,研究人员正在进行更多的试验来深入了解布洛芬对心血管系统的影响,并评估其风险和益处。
4. 抗肿瘤作用:布洛芬在抗肿瘤治疗方面也引起了研究人员的兴趣。
一些实验室和动物研究表明,布洛芬可能具有抑制肿瘤生长和转移的潜力。
然而,目前的临床试验结果尚不一致,需要进一步的研究来确认其抗肿瘤效果和安全性。
总的来说,布洛芬的药物临床试验进展仍在持续进行中。
研究人员致力于进一步探索其潜在的治疗效果和安全性,以便更好地指导其在临床实践中的应用。
然而,需要注意的是,布洛芬作为一种药物,仍存在一定的不良反应和禁忌症,患者在使用之前应咨询医生,并按照医嘱正确使用。
此外,临床试验的结果需要进一步的验证和评估,以确保其科学性和可靠性。

布洛芬擦剂(波菲特)的说明书治疗风湿跌打等疾病一直都是中医非常擅长的,西医对于这一类的疾病往往都没有太好的治疗效果。
风湿跌打主要人群是中老年人,这一类人对于药物的辨别能力比较差,因此患者家属一定要辨别好药进行治疗。
选择布洛芬擦剂(波菲特)进行风湿跌打的治疗效果非常显著,下面我们来看看关于布洛芬擦剂(波菲特)的各种介绍吧。
【药品名称】通用名称:布洛芬擦剂商品名称:布洛芬擦剂(波菲特)英文名称:Ibuprofen Liniment拼音全码:BuLuoFenBaJi(BoFeiTe)【主要成份】主要成分为每毫升含布诺芬50毫克,辅料为乙醇,聚维酮K30,氢氧化钠,香精。
【成份】化学名:2-(4-异丁基苯基)丙酸分子式:C13H18O2分子量:206.28【性状】本品为无色澄明的溶液。
【适应症/功能主治】用于缓解局部疼痛,如肌肉痛、关节痛以及拉伤、扭伤和运动损伤引起的疼痛和肿胀,也可用于骨关节炎的对症治疗。
【规格型号】2.5g:50ml【用法用量】外用,按照痛处大小,使用本品适量轻轻揉搓,一日3-4次,儿童用量请咨询医师或药师。
【不良反应】本品具有良好耐受性,个别患者有轻微的局部反应,及时停药可自行消失。
【禁忌】对其他非甾体抗炎药过敏者禁用。
【注意事项】1.该药品仅供外用,切忌口服。
避免接触眼睛及黏膜(如口、鼻黏膜)。
2.用药部位如有烧灼感、瘙痒、红肿等情况应停药,并将局部药物洗净,必要时向医师咨询。
3.孕妇、哺乳期妇女应在医师指导下使用。
4.不得用于皮肤破损处及感染性创口,且不宜大面积使用。
5.对该药品过敏者禁用,过敏体质者慎用。
6.该药品性状发生改变时禁止使用。
7.请将该药品放在儿童不能接触的地方。
8.儿童必须在成人监护下使用。
9.如正在使用其他其他药品,使用该药品前请咨询医师或药师。
【儿童用药】尚不明确。
【老年患者用药】尚不明确。
【孕妇及哺乳期妇女用药】孕妇慎用。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。

布洛芬 (Ibuprofen) 非处方止痛和抗炎药布洛芬(Ibuprofen)非处方止痛和抗炎药布洛芬(Ibuprofen)是一种非处方药,被广泛用于缓解疼痛和减轻炎症。
它属于非甾体抗炎药(NSAIDs)类药物,具有镇痛、抗炎和退热等多种作用。
本文将介绍布洛芬的药理特性、适应症、用法用量、副作用以及注意事项等内容。
一、药理特性布洛芬通过抑制环氧酶活性,减少前列腺素的合成,从而发挥止痛和抗炎作用。
它主要作用于环氧酶1和环氧酶2,抑制炎症反应中的前列腺素合成。
此外,布洛芬还可增加组织内吞噬细胞的活性,促进炎症标志物的清除。
二、适应症布洛芬常用于缓解轻至中度的头痛、牙痛、肌肉骨骼疼痛和痛经等。
它还可以用于缓解关节痛和风湿性关节炎等炎症性疾病的症状。
布洛芬还可用于减轻普通感冒和流感引起的身体不适和发热。
三、用法用量布洛芬一般以口服片剂或胶囊剂形式出售,也可作为外用药物,以乳膏或凝胶的形式涂抹在患部。
剂量的选择应根据患者的年龄、体重和症状的严重程度来确定。
一般成人剂量为每次200-400毫克,每天3-4次,不超过总剂量1200毫克。
儿童剂量需根据体重和年龄确定,建议遵循医生的指导。
四、副作用使用布洛芬可能会引起一些不良反应,包括胃部不适、消化不良、头晕、皮疹等。
长期或超量使用布洛芬可能导致消化道溃疡和出血等严重副作用。
对于存在胃肠道问题(如溃疡、出血)的患者、孕妇、哺乳期妇女以及年老体弱者,应在医生的指导下谨慎使用。
五、注意事项在使用布洛芬时,应遵循以下注意事项:1. 请不要超过推荐的剂量和疗程。
2. 如果症状未缓解或持续时间超过3天,请咨询医生。
3. 布洛芬不适用于12岁以下的儿童。
4. 孕妇和哺乳期妇女应在医生的指导下使用。
5. 与其他药物的相互作用可能发生,请在使用其他处方药物或非处方药物之前咨询医生或药师。
6. 如果发生严重不良反应,如呼吸困难、肿胀或皮疹,请立即就医。
结论:布洛芬是一种非处方止痛和抗炎药,常用于缓解头痛、肌肉骨骼疼痛、关节痛和痛经等症状。
异舒吉的注意事项异舒吉(Ibuprofen)是一种非处方药,用于缓解疼痛、减轻发热和消炎。
它是一种非甾体抗炎药(NSAID),对于许多人来说是一种常用的药物。
然而,在使用异舒吉之前,有一些注意事项是需要了解和遵守的。
首先,重要的是要明确药物的使用目的和适应症。
异舒吉主要用于缓解轻至中度的疼痛和发热,例如头痛、牙痛、感冒和发烧等。
然而,对于严重疼痛或其他严重症状,应该咨询医生以获取更合适的治疗方案。
其次,正确的剂量和使用方法至关重要。
异舒吉的剂量应根据个体情况和症状来确定。
一般推荐的剂量是每次200-400毫克,每4-6小时一次,但不应超过每日最大剂量。
在使用异舒吉之前,请务必阅读药品包装上的说明,并遵循医生或药师的建议。
除此之外,特别要注意的是不要与其他含有异舒吉成分的药物同时使用,以避免药物的过量或相互作用,造成不良反应。
同时,避免同时使用其他NSAIDs,如阿司匹林或布洛芬等。
如果必须同时使用其他药物,请咨询医生或药师的指导。
另外,异舒吉也有一些潜在的副作用和风险需要注意。
一些常见的副作用包括胃部不适、恶心、呕吐、胃溃疡等。
长期或过量使用异舒吉还可能导致肾脏损伤、高血压、心脏问题等。
特别是对于有消化道问题、肝脏疾病、心血管疾病或过敏史的人,应该在使用异舒吉之前咨询医生。
此外,对于特定的人群,如孕妇、哺乳期妇女和儿童,使用异舒吉时要特别小心。
在这些人群中,药物可能对胎儿、婴儿或儿童的发育和健康产生不良影响。
因此,对于这些人群,一定要咨询医生以了解是否安全使用异舒吉。
最后,不要滥用异舒吉。
长期和过量使用异舒吉可能会导致身体对药物的依赖,甚至药物滥用和成瘾。
在使用异舒吉期间,应按照指示使用,并避免超过建议的剂量和使用时间。
总之,异舒吉在正确使用的情况下是一种有效和安全的药物,可以缓解疼痛和发热。
然而,在使用该药物之前,应了解其注意事项,并严格按照医生或药师的建议使用。
如果出现不良反应或疑问,请及时咨询医生。
布洛芬注射液Ibuprofen Injection【警示语】请仔细阅读说明书并在医师指导下使用。
警示语:严重心血管事件和胃肠道事件风险。
非甾体抗炎药(NSAIDs)可能增加严重心血管血栓事件风险,包括心肌梗死和卒中风险,其可能是致命的。
该风险可能出现在治疗早期并且随药物使用时间的延长而增加。
本品禁用于冠状动脉旁路搭桥术(CABG)围手术期的治疗。
非甾体抗炎药(NSAIDs)可能增加严重胃肠道不良事件风险,包括:出血、溃疡、胃或肠穿孔,其可能是致命的,这些事件可能发生在使用的任何时间,且无预兆症状。
老年患者和有消化性溃疡和/或胃肠道出血疾病史患者发生严重胃肠道事件的风险更高。
【成份】本品主要成份为布洛芬,辅料为精氨酸、注射用水。
化学名称:α-甲基-4-(2-甲基丙基)苯乙酸。
化学结构式:【性状】本品为无色或几乎无色的澄明液体。
【适应症】用于成人治疗轻至中度疼痛,作为阿片类镇痛药的辅助用于治疗中至重度疼痛。
用于成人发热的退热治疗。
【规格】(1)4ml:0.4g;(2)8ml:0.8g【用法用量】根据患者个体化治疗目标,在最短用药周期使用最低有效剂量。
根据患者对本品起始治疗的反应,剂量和用药频率应以患者个体化的需求进行调整,成人最大日剂量不超过3.2g,为减少肾脏不良反应风险,患者在用药前需补充足够水分。
配制说明:本品在使用前必须稀释。
稀释后最终使用浓度不应超过4mg/mL,稀释溶液只为0.9%氯化钠注射液,不可采用葡萄糖注射液。
0.1g剂量:将本品1ml加入不少于100ml的稀释液中。
0.2g剂量:将本品2ml加入不少于100ml的稀释液中。
0.4g剂量:将本品4ml加入不少于100ml的稀释液中。
0.8g剂量:将本品8ml加入不少于200ml的稀释液中。
使用前应通过肉眼观察原溶液和稀释后溶液的悬浮微粒和变色情况,如发现乳光、不透明微粒、变色或其它外源性微粒,不得使用。
稀释后的注射液在室温条件(20~25℃)和室内光照条件下可保持24小时稳定。
布洛芬布洛芬是世界卫生组织、美国FDA唯一共同推荐的儿童退烧药,是公认的儿童首选抗炎药。
布洛芬具有抗炎、镇痛、解热作用。
治疗风湿和类风湿关节炎的疗效稍逊于乙酰水杨酸和保泰松。
适用于治疗风湿性关节炎、类风湿性关节炎、骨关节炎、强直性脊椎炎和神经炎等。
基本情况汉语拼音:buluofen中文名称:布洛芬英文名称:Ibuprofe化学名称:2-(4-一定基本集)丙酸【中文别名】2-(-4-异丁基苯基)丙酸;异丁苯丙酸,异丁洛芬,芬必得,α-甲基-4-(2-甲基丙基)苯乙酸【英文别名】2-(4-Isobutylphenyl)propanoic Acid;α-Methyl-4-(isobutyl)phenylacetic acid;(±)-2-(4-Isobutylphenyl)propanoic acid;4-Isobutyl-alpha-methylphenylacetic Acid结构式:分子式:C13H18O2分子量: 206化学反应过程生产流程图1. 在三氯化铝的催化下,乙酰氯与异丁基苯发生傅克酰化反应。
由于异丁基是体积较大的邻对位定位基,乙酰基主要进入其对位,生成4—异丁基苯乙酮。
反应无水操作。
2、由Darzen缩合,产物经水解、脱羧和重排得到2—(4—异丁基苯基)丙醛3、由2-(4-异丁基苯基)丙醛用氧化法制的布洛芬。
工艺流程:①将计量好的石油醚、三氯化铝加入反应釜内,搅拌降温至5°C以下,加入计量的异丁基苯,其间控制釜内温度<5°C。
再加入计量的乙酰氯。
搅拌反应4小时。
将反应液在10°C下压入水解釜中,滴加稀盐酸,保持釜内温度不超过10°C,搅拌0.5小时后,静止分层。
有机层为粗酮,水洗至PH=6.减压蒸馏回收石油醚后,再收集130°C/2kpa馏分,即为4—异丁基苯乙酮。
收率80%。
②将异丙醇钠压入反应釜中,搅拌下控制温度至15°C左右,将计量的4—异丁基苯乙酮与氯乙酸异丙脂的混合物慢慢滴入,于20到25°C反应6小时,升温至75°C,回流反应1h。
布洛芬(Ibuprofen)为解热镇痛类,非甾体抗炎药。
本品通过抑制环氧化酶,减少前列腺素的合成,产生镇痛、抗炎作用;通过下丘脑体温调节中枢而起解热作用。
用于缓解轻至中度疼痛如头痛、关节痛、偏头痛、牙痛、肌肉痛、神经痛、痛经。
也用于普通感冒或流行性感冒引起的发热。
该药物含有多种杂质,菲斯生物现有多种杂质提供选择,如有需要请联系我们,我们代理EP/USP/LGC/trc/TLC/sinco等多种优质标准品对照品,资料齐全、售后保证,更多详情参阅菲斯生物官网,欢迎垂询。
了解更多,请关注菲斯生物官方微信公众号:药研杂质社
布洛芬包含如下杂质:
菲斯生物科技Co.,Ltd.。
USP 39Reference Tables / Solubilities2261 SOLUBILITIESApproximate Solubilities of USP and NF ArticlesName and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether Other Acenocoumarol67,0002801301800Acetaminophen2010 1 N sodium hydroxide, 15 Acetohexamide230210Acetylcysteine54Acetyldigitoxin610062.512>10,000Ammonium Alum70.5Aluminum Chloride0.94Aluminum Sulfate1Amantadine Hydrochloride 2.5 5.118polyethylene glycol 400, 70 Amaranth15Aminocaproic Acid3methanol, 450 Aminohippuric Acid4550 3 N hydrochloric acid, 5 Aminosalicylate Sodium2Ammonium Carbonate4Amodiaquine Hydrochloride2578>10,000>10,000benzene, 10,000Anethole12Anileridine>10,00021Anileridine Hydrochloride580>10,000>10,000Antimony Potassium Tartrate123glycerin, 15Apomorphine Hydrochloride5050water at 80°, 20 Apraclonidine Hydrochloride3474>10,000methanol, 13; ethyl acetate,>10,000; hexanes, >10,000 Ascorbic Acid340Ascorbyl Palmitate>1000125>1000>1000Aspirin30051710 to 15Atropine4602125water at 80°, 90Atropine Sulfate0.5 2.55glycerin, 2.5 Bendroflumethiazide23200Benoxinate Hydrochloride0.8 2.6 2.5>10,000Benzalkonium Chloride (anhy-100benzene, 6drous)Gamma Benzene Hexachloride 3.540dehydrated alcohol, 20 Benzethonium Chloride<1<1<16000Benzocaine2500524almond oil or olive oil, 30–50 Benzoic Acid300353Benzonatate<1<1<1<1Betadex54Betamethasone530065325warm alcohol, 15; methanol, 3 Betamethasone Acetate2000916Betamethasone Sodium Phosphate2470>10,000>10,000Betamethasone Valerate10,00016<10400Bisacodyl>10,000210 2.5275Boric Acid18418boiling alcohol, 6; glycerin, 4 Bromodiphenhydramine Hydro-<1223500isopropyl alcohol, 31 chlorideBrompheniramine Maleate51515Busulfan acetone, 45Butabarbital Sodium27700010,000Butamben7000Butylated Hydroxyanisole 4.0 2.0 1.2Butylated Hydroxytoluene 4.0 1.1 1.1Caffeine (hydrous)507566002262Solubilities / Reference Tables USP 39Approximate Solubilities of USP and NF Articles (Continued)Name and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether OtherCalcium Chloride0.70.24boiling alcohol, 2Calcium Gluconate30 (slowly)5Calcium Hydroxide6301300Calcium Lactate20Calcium Pantothenate3Calcium Sulfate375485Camphor80010.51Carbinoxamine Maleate<1 1.5 1.58300Carisoprodol2083 2.5 2.3acetone, 2.5Cephaloridine5100010,00010,000Cetylpyridinium Chloride 4.5 2.5 4.5Chloral Betaine14>10,000>10,0000.1 N hydrochloric acid, 1;0.1 N sodium hydroxide, 1 Chloral Hydrate0.25 1.32 1.5Chlorambucil acetone, 2Chloramphenicol400Chlordiazepoxide>10,000506250130Chlorobutanol1251glycerin, 10Chlorocresol2600.4Chloroprocaine Hydrochloride20100Chlorpheniramine Maleate41010Chlorpromazine323benzene, 2Chlorpromazine Hydrochloride1 1.5 1.5Chlortetracycline Hydrochloride75560Cholesterol100 (slow-dehydrated alcohol, 50ly)Citric Acid0.5230Clindamycin Palmitate Hydrochlo-3ethyl acetate, 9rideClindamycin Phosphate 2.5>1000>1000>1000Clioquinol>100,00035001204500Cocaine60071 3.5olive oil, 12; liquid petrolatum,80–100Cocaine Hydrochloride0.5 3.515Codeine12020.550Codeine Phosphate 2.5325boiling alcohol, 125; water at80°, 0.5Codeine Sulfate301300water at 80°, 6.5Colchicine25220Cortisone Acetate3504acetone, 75; dioxane, 30 Cupric Sulfate30.5500glycerin, 3Cyanocobalamin80Cyclizine Hydrochloride11511575Cyproheptadine Hydrochloride2753526methanol, 1.5Dehydrocholic Acid100352200 (15°)acetic acid at 15°, 135; ace-tone at 15°, 130; benzene at15°, 960; ethyl acetate at 15°,135Demeclocycline200methanol, 40 Demeclocycline Hydrochloride60methanol, 50Denatonium Benzoate20 2.4 2.95000Desipramine Hydrochloride1214 3.5>10,000Dexamethasone Sodium Phos-2phateDexbrompheniramine Maleate 1.2 2.523000Dexchlorpheniramine Maleate 1.12 1.72500Dextroamphetamine Sulfate10800Dextromethorphan Hydrobromide65USP 39Reference Tables / Solubilities2263Approximate Solubilities of USP and NF Articles (Continued)Name and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether OtherDextrose1100Diazepam33316239Dibucaine46000.70.5 1.4Dicyclomine Hydrochloride1352770glacial acetic acid, 2 Diethylpropion Hydrochloride0.533Digitoxin15040Dihydroergotamine Mesylate125901752600Dimercaprol20Dimethisterone30.7Diphenhydramine Hydrochloride122acetone, 50Disulfiram>50003015Docusate Calcium3300>1>1>1Docusate Sodium70 (slowly)Doxapram Hydrochloride50Doxylamine Succinate122370Droperidol10,0001404500Dyclonine Hydrochloride6024 2.3>10,000hexane, >10,000 Dydrogesterone>10,000402200Echothiophate Iodide1dehydrated alcohol, 25; meth-anol, 3Edrophonium Chloride0.55Enalaprilat200>1000dimethylformamide, 40; meth-anol, 68Ephedrine200.2Ephedrine Hydrochloride314Ephedrine Sulfate 1.390Epinephrine Bitartrate3Ergotamine Tartrate500500Erythromycin1000Erythromycin Estolate2010acetone, 15Estradiol28435150Estradiol Cypionate>10,0004072800Estrone250 (15°)110 (15°)boiling alcohol, 50; boilingchloroform, 80; acetone at50°, 50; boiling acetone, 33;boiling benzene, 145 Estropipate>2000>2000>2000>2000warm alcohol, 500Ethacrynic Acid 1.66 3.5Ether112Ethyl Vanillin100 (50°)2Ferrous Gluconate5Ferrous Sulfate 1.50.5Flumethasone Pivalate>10,000893502800Fluocinolone Acetonide>10004525350Fluorometholone>10,0002002200>10,000Fluphenazine Enanthate<1<12Fluphenazine Hydrochloride 1.4 6.7Flurandrenolide7210methanol, 25Flurazepam Hydrochloride24905000methanol, 3; isopropanol, 69;benzene, 2500; petroleumether, 5000Fluroxene220Fructose15methanol, 14Gentian Violet10glycerin, 15Glyceryl Monostearate10.0100.0methanol, 100; isopropyl alco-hol, 33Glycine41254water at 50°, 2.6; at 75°, 1.9;at 100°, 1.52264Solubilities / Reference Tables USP 39Approximate Solubilities of USP and NF Articles (Continued)Name and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether Other Glycopyrrolate 4.23026035,000Guaifenesin60–70Halazone>1000140>1000>2000Haloperidol>10,0006015200Heparin Sodium20Histamine Phosphate4Homatropine Hydrobromide640420Hydralazine Hydrochloride25500Hydrocortisone40acetone, 80Hydrocortisone Acetate230200Hydrocortisone Sodium Phosphate 1.5Hydroflumethiazide>500039>50002500Hydromorphone Hydrochloride3Hydroquinone1745116.5Hydroxocobalamin5010010,00010,000Hydroxyzine Hydrochloride1 4.513>1000Hydroxyzine Pamoate>1000700>1000>1000dimethylformamide, 10; 10 Nsodium hydroxide, 3.5 Hyoscyamine Hydrobromide 2.5 1.72300Hyoscyamine Sulfate0.55Ichthammol10Indigotindisulfonate Sodium100Indomethacin503040Iodine300013carbon disulfide, 4; glycerin,80Ipodate Sodium<12dimethylacetamide, 2; dimeth-ylformamide, 3.5; dimethyl-sulfoxide, 3.5Isoniazid850Isopropamide Iodide50105Isopropyl Alcohol<1<1<1<1Isoproterenol Hydrochloride350Isoproterenol Sulfate4>2000>2000>2000Isoxsuprine Hydrochloride500100>10,000>10,0000.1 N hydrochloric acid, 2500;0.1 N sodium hydroxide, 100 Ketamine Hydrochloride41460>10,000methanol, 6; absolute alcohol,60Lactose 5 (slowly) 2.6Levorphanol Tartrate50120Levothyroxine Sodium700300Lisinopril10>10,000>10,000>10,000methanol, 70Magnesium Hydroxide>10,000>10,000>10,000>10,000Magnesium Sulfate0.80.5glycerin, 1 (slowly)Mannitol 5.5Menadione60benzene, 10; vegetable oils, 50 Mesoridazine Besylate11136300Methacholine Chloride 1.2 1.7 2.1Methacrylic Acid Copolymer water in methanol (≥3 in 100),10; water in alcohol (≥3 in100), 10; water in isopropylalcohol (≥3 in 100), 10; waterin acetone (≥3 in 100), 10 Methacycline Hydrochloride100300>1000>10000.1 N sodium hydroxide, 25 Methdilazine Hydrochloride226>10,0000.1 N hydrochloric acid, 1;0.1 N sodium hydroxide, 1 Methenamine 1.512.510320Methenamine Mandelate1020350Methimazole55 4.5125USP 39Reference Tables / Solubilities2265Approximate Solubilities of USP and NF Articles (Continued)Name and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether Other Methocarbamol40 (20°)Methotrimeprazine10102methanol, 10 Methoxyflurane500<1<1<1Methsuximide3503<12Methyclothiazide>10,00092.5>10,0002700Methylbenzethonium Chloride0.80.9>10,0000.7Methylene Blue2565Methylergonovine Maleate10017519008400Methylparaben400310water at 80°, 50 Methylprednisolone10,000100800800Methylprednisolone Acetate15004002501500Methylprednisolone Sodium Succi-nate 1.512>10,000>10,000Methysergide Maleate2001653400>10,000Miconazole>100,0009.5215isopropyl alcohol, 4; propyleneglycol, 9; methanol, 5.3 Miconazole Nitrate625031252550,000isopropyl alcohol, 1408; pro-pylene glycol, 119; methanol,75Morphine Sulfate16570alcohol at 60°, 240; water at80°, 1Nalidixic Acid>100091029>1000Neomycin Sulfate1Niacin60Niacinamide 1.510 5.5Nifedipine>10,000acetone, 10Nitrofurazone4200590propylene glycol, 350 Nitromersol>2000>2000>2000>2000Norepinephrine Bitartrate 2.5300Norethindrone Acetate>10,00010<118dioxane, 2Nortriptyline Hydrochloride903020methanol, 10Oxandrolone520057<5860acetone, 69Oxazepam>10,0002202702200Oxtriphylline1Oxymetazoline Hydrochloride 6.7 3.6862Oxymetholone>10,00040582dioxane, 14Oxymorphone Hydrochloride4100>1000>1000methanol, 25 Oxytetracycline4150>10,0006250absolute alcohol, 66 Oxytetracycline Calcium>1000>1000>1000>10000.1 N sodium hydroxide, 15 Papaverine Hydrochloride30120Paraldehyde11017Paramethasone Acetate50methanol, 40Paromomycin Sulfate<1>10,000>10,000>10,000Pectin20Penicillin G Benzathine500065Sterile Penicillin G Procaine2503060Sterile Penicillin G Sodium40Penicillin V Benzathine320033042910acetone, 37Penicillin V Potassium150Pentazocine>100011242Pentazocine Hydrochloride30204>10,000Pentobarbital>2000 4.5 4.010Pentolinium Tartrate0.5475>1000>2000Perphenazine7acetone, 13 Phenazopyridine Hydrochloride<102059331>5000cold water, 300; glycerin, 100 Phenindamine Tartrate40350Phenmetrazine Hydrochloride0.422Solubility data for compounds that ordinarily are liquids at 25° are expressed in terms of the ratio of the volume of solute to the volume of solvent;2266Solubilities / Reference Tables USP 39Approximate Solubilities of USP and NF Articles (Continued)Name and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether Other Phenobarbital100010Phenol15mineral oil, 70Phentolamine Mesylate14700Phenylethyl Alcohol60<1<1<1alcohol solution (1 in 2), 2;diethylphthalate, <1; benzylbenzoate, <1 Phenylmercuric Acetate180225 6.8200Phenylmercuric Nitrate600Phenylpropanolamine Hydrochlo- 1.17.44100ridePhysostigmine Salicylate75166250Physostigmine Sulfate40.41200Pilocarpine Hydrochloride0.33360Pilocarpine Nitrate475Piminodine Esylate>100062>1000Pimozide>10,0001000101000acetone, 100; methanol, 1000;0.1 N hydrochloride acid,>1000Polyethylene Glycol 154013absolute alcohol, 100 Polyethylene Glycol 40004 2.52Potash, Sulfurated2Potassium Acetate0.50.23Potassium Alum70.3Potassium Benzoate275alcohol solution (9 in 10), 50 Potassium Chloride 2.82Potassium Citrate1glycerin, 2.5Potassium Gluconate3Potassium Hydroxide13glycerin, 2.5Potassium Iodide0.70.522glycerin, 2Potassium Permanganate15 3.5Potassium Sodium Tartrate1Potassium Sorbate 4.535>1000>1000Pramoxine Hydrochloride35Prednisolone30180acetone, 50Prednisolone Acetate120Prednisolone Hemisuccinate4170 6.31064248Prednisolone Sodium Phosphate4methanol, 13Prednisone150200Prilocaine Hydrochloride 3.5 4.2175Primaquine Phosphate15Primidone2000200Procaine Hydrochloride115Prochlorperazine Edisylate21500Prochlorperazine Maleate1200Procyclidine Hydrochloride359611,000Promazine Hydrochloride3Propoxycaine Hydrochloride210>10,00080Propoxyphene Napsylate10,0001510Propylhexedrine>5000.40.20.1Propylparaben2500400 1.53Protriptyline Hydrochloride2 3.5 2.5>10,000Pseudoephedrine Hydrochloride0.5 3.6917000Pyrazinamide671351000absolute alcohol, 175; metha-nol, 72Pyridoxine Hydrochloride5115Pyrilamine Maleate0.532absolute alcohol, 15 Pyrimethamine2001251Solubility data for compounds that ordinarily are liquids at 25° are expressed in terms of the ratio of the volume of solute to the volume of solvent;USP 39Reference Tables / Solubilities2267Approximate Solubilities of USP and NF Articles (Continued)Name and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether Other Pyrrocaine Hydrochloride 1.5128Quinidine Sulfate1001015Quinine Sulfate500120Reserpine18006Resorcinol11Rotoxamine Tartrate10100>10,000>10,000Saccharin2902531Saccharin Calcium 2.6 4.7Saccharin Sodium 1.550Salicylic Acid460153453benzene, 135Scopolamine Hydrobromide 1.520Secobarbital0.5 N sodium hydroxide, 8.5 Selenium Sulfide1611667Sennosides35210037006100Silver Nitrate0.40.130boiling alcohol, 6.5 Simethicone2>10,000102102benzene, 102; absolute alcohol,>10,000Sodium Acetate0.80.619Sodium Ascorbate 1.3Sodium Benzoate27590 percent alcohol, 50Sodium Bicarbonate12Sodium Bisulfite4Sodium Borate161glycerin, 1Sodium Carbonate3 1.8Sodium Chloride 2.8 2.7glycerin, 10Sodium Citrate (hydrous) 1.50.6Sodium Fluoride25Sodium Formaldehyde Sulfoxylate 3.4510175180Sodium Hydroxide1Sodium Iodide0.62glycerin, 1Sodium Lauryl Sulfate10Sodium Nitrite 1.5Sodium Phosphate, Dried8Sodium Propionate10.6524>10,000>10,000Sodium Thiosulfate0.5Sorbic Acid1000101530absolute alcohol, 8; methanol,8; propylene glycol, 19 Sorbitol0.45Stanozolol>10004174370Stearic Acid2023Stibophen1>10,000>10,00010,000Succinylcholine Chloride1350Sucrose0.50.2170Sucrose Octaacetate110011acetone, 0.3; benzene, 0.6; tol-uene, 0.5Sulconazole Nitrate3333100333pyridine, 10; methanol, 71; ac-etone, 130; methylene chlo-ride, 286; toluene, 2000;dioxane, 2000 Sulfacetamide Sodium 2.5Sulfadiazine13,000human serum at 37°, 620 Sulfadiazine Sodium2Sulfadimethoxine200 2 N hydrochloric acid, 50 Sulfaethidole>30007513001700methanol, 51; acetone, 13;benzene, 2277 Sulfamethizole20003819001900acetone, 13 Sulfamethoxazole34005010001000carbon disulfide, 2 (slowly andusually incompletely)2268Solubilities / Reference Tables USP 39Approximate Solubilities of USP and NF Articles (Continued)Name and Volume, in mL, of SolventBoilingSolute (1 g)Water Water Alcohol Chloroform Ether Other Sulfapyridine3500440acetone, 65Sulfasalazine>10,0002900>10,000>10,000methanol, 1500 Sulfisoxazole7700boiling alcohol, 10 Sulfisoxazole Acetyl176351064methanol, 203Precipitated Sulfur carbon disulfide, 2 (slowly andusually incompletely); oliveoil, 100Tartaric Acid0.80.53250methanol, 1.7Terpin Hydrate2003513140140boiling alcohol, 3 Testolactone4050Testosterone2100absolute alcohol, 6 Tetracaine>1000522Tetracycline250050Tetracycline Hydrochloride10100Tetrahydrozoline Hydrochloride 3.57.5>1000>1000Theophylline Sodium Glycinate6Thiamine Hydrochloride1170Thiamine Mononitrate44Thiethylperazine Maleate1700530>10,000>10,000Thimerosal112Thioguanine7700Thiotepa138.3 1.9 4.1Thiothixene>10,0002120absolute alcohol, 110 Thiothixene Hydrochloride8280>10,000absolute alcohol, 270Thymol100011 1.5olive oil, 2Tolazoline Hydrochloride<123>10,000Triamcinolone Diacetate1380methanol, 40Triamterene formic acid, 30; 2-methoxyeth-anol, 85Triazolam>10,000100025>10,0000.1 N hydrochloric acid, 600 Trichlormethiazide11004850001400dioxane, 9.1; dimethylforma-mide, 4.35 Trichloroethylene>10,000Triethylenemelamine 2.513 3.6methanol, 8; acetone, 9.5;benzene, 18Trifluoperazine Hydrochloride 3.511100Triflupromazine Hydrochloride<1<1 1.7Trimeprazine Tartrate22051800Trimethobenzamide Hydrochloride25967720Trioxsalen115084methylenedichloride, 43; 4-methyl-2-pentanone, 100 Tripelennamine Hydrochloride166acetone, 350Triprolidine Hydrochloride 2.1 1.812000Tromethamine 1.845.5>10,000Tubocurarine Chloride2045Urea 1.510boiling alcohol, 1Vanillin100glycerin, 20; water at 80°, 20 Xylometazoline Hydrochloride35Zinc Acetate 2.530Zinc Chloride0.5 1.5glycerin, 2Zinc Sulfate0.6glycerin, 2.51Solubility data for compounds that ordinarily are liquids at 25° are expressed in terms of the ratio of the volume of solute to the volume of solvent;i.e., 1 mL dissolved in mL of solvent.2Liquid phase only; silicon dioxide remains as a residue in these solvents.USP 39Reference Tables / Atomic Weights2269 ATOMIC WEIGHTSStandard Atomic Weights of the Elements, Recommended by the Commission on Atomic Weights and Isotopic Abun-dances of the International Union of Pure and Applied Chemistry (2007) (©2008 IUPAC)Standard atomic weights 2007 [In alphabetical order: scaled to A r(12C) = 12, where 12C is a neutral atom in its nuclear and electronic ground state.]The atomic weights of many elements are not invariant but depend on the origin and treatment of the material. The standard values of A r(E) and the uncertainties (in parentheses, following the last significant figure to which they are attrib-uted) apply to elements of natural terrestrial origin. The footnotes to this Table elaborate the types of variation which may occur for individual elements and which may be larger than the listed uncertainties of values of A r(E). Names of elements with atomic numbers 112, 113, 114, 115, 116, and 118 are temporary.Atomic AtomicName Symbol Number Atomic Weight Footnotes Actinium*Ac89Aluminum Al1326.9815386(8)Americium*Am95Antimony (Stibium)Sb51121.760(1)gArgon Ar1839.948(1)g, rArsenic As3374.92160(2)Astatine*At85Barium Ba56137.327(7)Berkelium*Bk97Beryllium Be49.012182(3)Bismuth Bi83208.98040(1)Bohrium*Bh107Boron B510.811(7)g, m, rBromine Br3579.904(1)Cadmium Cd48112.411(8)gCaesium (Cesium)Cs55132.9054519(2)Calcium Ca2040.078(4)g Californium*Cf98Carbon C612.0107(8)g, rCerium Ce58140.116(1)gChlorine Cl1735.453(2)mChromium Cr2451.9961(6)Cobalt Co2758.933195(5)Copper Cu2963.546(3)rCurium*Cm96Darmstadtium*Ds110Dubnium*Db105Dysprosium Dy66162.500(1)g Einsteinium*Es99Erbium Er68167.259(3)gEuropium Eu63151.964(1)gFermium*Fm100Fluorine F918.9984032(5)Francium*Fr87Gadolinium Gd64157.25(3)gGallium Ga3169.723(1)Germanium Ge3272.64(1)Gold Au79196.966569(4)Hafnium Hf72178.49(2)*Element has no stable nuclides. One or more well-known isotopes are given in the accompanying table with the appropriate relative atomic mass and half-life. However, three such elements (Th, Pa, and U) do have a characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated.†Commercially available Li materials have atomic weights that are known to range between 6.939 and 6.996; if a more accurate value is required,it must be determined for the specific material.g Geological specimens are known in which the element has an isotopic composition outside the limits for normal material. The difference between the atomic weight of the element in such specimens and that given in the Table may exceed the stated uncertainty.m Modified isotopic compositions may be found in commercially available material because it has been subjected to an undisclosed or inadvertent isotopic fractionation. Substantial deviations in atomic weight of the element from that given in the Table can occur.。
布洛芬乳膏【药品名称】通用名称:布洛芬乳膏英文名称:Ibuprofen Cream【成份】布洛芬【适应症】用于局部疼痛及炎症缓解:1局部软组织疼痛及炎症:如扭伤、拉伤、劳损以及肩周炎、腱鞘炎、滑囊炎、腰背痛等。
2类风湿性关节炎及骨关节炎。
【用法用量】依患处面积大小,用本品适量,轻轻揉搓,每日三至四次,或遵医嘱。
【不良反应】偶有皮肤瘙痒、发红、皮疹等,用药片刻后即消失,一般不影响使用;极个别病人有头昏及轻度胃肠道不适,一般可耐受,停药后即消失。
【禁忌】1 对本品及其它非甾体类抗炎药过敏者禁用。
2 对丙二醇及对羟基苯甲酸甲酯钠过敏者禁用【注意事项】1 本品仅可用于完整皮肤,不用于皮肤破损部位。
2 勿与眼睛及粘膜接触。
3 本品仅供外用,切勿入口。
4 本品不推荐孕妇、哺乳期妇女使用。
【特殊人群用药】儿童注意事项:12岁以下的儿童,不得使用布洛芬乳膏。
妊娠与哺乳期注意事项:不推荐孕妇、哺乳期妇女使用。
老人注意事项:老年人需慎用非甾体类抗炎药。
【药物相互作用】如与其他药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。
【药理作用】本品为外用非甾体类镇痛消炎药,作用机制是抑制前列腺素的合成,从而发挥镇痛消炎作用。
【贮藏】密闭保存。
有效期2年【有效期】24个月【批准文号】国药准字H20066210【生产企业】企业名称:哈药集团生物工程有限公司生产地址:哈尔滨市呼兰区利民开发区珠海路99号。
Ibuprofen(eye'' bue proe' fen).C13H18O2 206.28Benzeneacetic acid, α-methyl-4-(2-methylpropyl), (±)-.(±)-p-Isobutylhydratropic acid.(±)-2-(p-Isobutylphenyl)propionic acid [15687-27-1].(±) Mixture [58560-75-1].» Ibuprofen contains not less than 97.0 percent and not more than 103.0 percent of C13H18O2, calculated on the anhydrous basis.Packaging and storage—Preserve in tight containers.USP Reference standards 〈11〉—USP Ibuprofen RSUSP Ibuprofen Related Compound C RSIdentification—A: Infrared Absorption 〈197M〉—Do not dry specimens.B: Ultraviolet Absorption 〈197U〉—Solution: 250 µg per mL.Medium: 0.1 N sodium hydroxide.Respective absorptivities at 264 nm and 273 nm, calculated on the anhydrous basis, do not differ by more than 3.0%.C: The chromatogram of the Assay preparation obtained as directed in the Assay exhibits a major peak for ibuprofen, the retention time of which, relative to that of the internal standard, corresponds to that exhibited in the chromatogram of the Standard preparation, obtained as directed in the Assay.Change to read:Water Determination, Method I 〈921〉(CN 1-May-2016): not more than 1.0%.Residue on ignition 〈281〉: not more than 0.5%.Delete the following:Heavy metals, Method II 〈231〉: 0.002%.(Official 1-Jan-2018)Chromatographic purity—Mobile phase—Prepare a suitable filtered mixture of water, previously adjusted with phosphoric acid to a pH of 2.5 and acetonitrile (1340:680). Make adjustments if necessary (see System Suitability under Chromatography 〈621〉).Test preparation—Prepare a solution of Ibuprofen in acetonitrile containing about 5 mg per mL.Resolution solution—Prepare a solution in acetonitrile containing in each mL about 5 mg of Ibuprofen and 5 mg of valerophenone.Chromatographic system (see Chromatography 〈621〉)—The liquid chromatograph is equipped with a 214-nm detector and a 4-mm × 15-cm column that contains 5-µm packing L1 and is maintained at 30 ± 0.5°. The flow rate is about 2 mL per minute. Chromatograph a series of 5-µL injections of the Test preparation to condition the column. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.8 for valerophenone and 1.0 for ibuprofen, and the resolution, R, between the valerophenone peak and the ibuprofen peak is not less than 2.0. Procedure—[note—Use peak areas where peak responses are indicated.] Inject about 5 µL of the Test preparation into the chromatograph, record the chromatogram, and measure the peak responses. Calculate the percentage of each impurity taken by the formula:100ri / rtin which ri is the response of an individual peak, other than the solvent peak and the main ibuprofen peak, and rt is the sum of the responses of all the peaks, excluding that of the solvent peak: not more than 0.3% of any individual impurity is found, and the sum of all the individual impurities found does notexceed 1.0%.Limit of ibuprofen related compound C—Using the chromatograms of the Assay preparation and the Ibuprofen related compound C standard solution, obtained as directed in the Assay, calculate the percentage of ibuprofen related compound C (C12H16O) in the portion of Ibuprofen taken by the formula:10,000(C / W)(RU / RS)in which C is the concentration, in mg per mL, of USP Ibuprofen Related Compound C RS in the Ibuprofen related compound C standard solution; W is the weight, in mg, of Ibuprofen taken to prepare the Assay preparation; and RU and RS are the peak response ratios of ibuprofen related compound C to valerophenone obtained from the Assay preparation and the Ibuprofen related compound C standard solution, respectively: not more than 0.1% is found.Assay—Mobile phase—Dissolve 4.0 g of chloroacetic acid in 400 mL of water, and adjust with ammonium hydroxide to a pH of 3.0. Add 600 mL of acetonitrile, filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography 〈621〉).Internal standard solution—Prepare a solution of valerophenone in Mobile phase having a concentration of about 0.35 mg per mL.Standard preparation—Dissolve an accurately weighed quantity of USP Ibuprofen RS in Internal standard solution to obtain a solution having a known concentration of about 12 mg per mL.Ibuprofen related compound C standard solution—Quantitatively dissolve an accurately weighed quantity of USP Ibuprofen Related Compound C RS inacetonitrile to obtain a solution having a known concentration of about 0.6 mg per mL. Add 2.0 mL of this stock solution to 100.0 mL of Internal standard solution, and mix to obtain a solution having a known concentration of about0.012 mg of ibuprofen related compound C per mL.Assay preparation—Transfer about 1200 mg of Ibuprofen, accurately weighed, to a 100-mL volumetric flask, dilute with Internal standard solution to volume, and mix.Chromatographic system (see Chromatography 〈621〉)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is about 2 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: therelative retention times are about 1.4 for the internal standard and 1.0 for ibuprofen; the resolution, R, between ibuprofen and the internal standard is not less than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%. Chromatograph the Ibuprofen related compound C standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 1.0 for valerophenone and 1.2 for ibuprofen relatedcompound C; the resolution, R, between valerophenone and ibuprofen related compound C is not less than 2.5; the tailing factors for the individual peaks are not more than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.Procedure—Separately inject equal volumes (about 5 µL) of the Standard preparation, the Assay preparation, and the Ibuprofen related compound C standard solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C13H18O2 in the portion of Ibuprofen taken by the formula:100C(RU / RS)in which C is the concentration, in mg per mL, of USP Ibuprofen RS in the Standard preparation; and RU and RS are the peak response ratios obtained from the Assay preparation and the Standard preparation, respectively.Auxiliary Information— Please check for your question in the FAQs before contacting USP.Topic/Question Contact Expert CommitteeMonograph Clydewyn M. Anthony,Ph.D.Senior ScientificLiaison(301) 816-8139(CHM62015) Chemical Medicines Monographs 6Reference Standards RS Technical Services 1-301-816-8129rstech@USP39–NF34 Page 4267Pharmacopeial Forum: Volume No. 34(4) Page 941Chromatographic Column—IBUPROFENChromatographic columns text is not derived from, and not part of, USP 39 or NF34.。