官能团对分子器件电输运特性的影响_夏蔡娟
- 格式:pdf
- 大小:312.32 KB
- 文档页数:7

二维材料的磁性与自旋输运自旋电子学是一门研究自旋自由度在纳米尺度下的相互作用与输运性质的新兴学科。
随着纳米科技的飞速发展,人们对自旋电子学的研究和应用也越来越多。
二维材料作为一类具有特殊结构和性质的纳米材料,不仅具有优越的电学和光学性质,而且在自旋电子学方面也具有很大的潜力。
本文将重点讨论二维材料的磁性与自旋输运的研究进展和潜力。
二维材料是近年来备受关注的研究领域,一是因为它们具有高度可调节性和可控性,可以通过层叠和合成来实现对材料性质的调控;二是因为它们独特的二维结构导致了一系列新奇的物理和化学性质,如二维晶体的光学、输运以及磁性等。
其中,磁性是二维材料最具吸引力的性质之一。
二维材料的磁性主要表现为自旋有序和自旋磁共振等现象。
在二维材料中,电子的自旋可以通过相互作用形成自旋有序,从而产生宏观的磁性。
一些研究表明,二维材料中的自旋有序可以通过控制温度、外加电场和应变等手段实现,这为自旋电子学的研究和应用提供了新的途径。
除了自旋有序,二维材料还可以通过自旋磁共振来操控自旋信息。
自旋磁共振是一种通过微波辐射作用下的自旋与磁矩的共振现象,可以通过调节磁场和频率来实现对自旋磁共振的控制。
一些二维材料具有特殊的磁矩结构和自旋耦合效应,使得它们在自旋磁共振方面具有更好的性能和应用潜力。
自旋有序和自旋磁共振是二维材料磁性研究的重点,但与此同时,二维材料的自旋输运也备受关注。
自旋输运是指自旋信息在材料中传输和操控的过程。
由于二维材料具有特殊的物理结构和电子性质,它们在自旋输运方面表现出了一些独特的特点。
比如,石墨烯作为最早被发现的二维材料之一,具有高电子迁移率和优异的热传导性能,这使得它在自旋输运领域具有重要的应用前景。
在二维材料的自旋输运研究中,除了石墨烯,其他几种二维材料也显示出了很强的自旋输运能力。
比如,过渡金属二硫化物(TMDs)具有特殊的晶体结构和能带结构,使得它们在自旋输运方面表现出了独特的特点。
一些研究表明,TMDs可以实现自旋电子的寿命延长和自旋旋转的控制,这为二维材料自旋电子学的研究和应用提供了新的途径。

《石墨炔基分子自旋电子学器件输运性质的理论研究》篇一摘要:本文通过理论计算的方法,对石墨炔基分子自旋电子学器件的输运性质进行了深入研究。
利用密度泛函理论及非平衡格林函数方法,我们对不同结构的石墨炔基分子及其与自旋电子相互作用机制进行了系统的理论模拟与分析。
本研究不仅揭示了石墨炔基分子的电子结构与自旋输运的关系,还为设计高性能的自旋电子学器件提供了理论依据。
一、引言随着纳米科技和自旋电子学的发展,分子自旋电子学器件已成为新兴的电子器件领域。
其中,石墨炔基分子以其独特的物理性质和潜在的应用价值,受到了广泛的关注。
对石墨炔基分子自旋电子学器件输运性质的研究,不仅有助于理解其微观机理,还为新型自旋电子器件的设计和优化提供了重要的理论支持。
二、理论基础与计算方法本研究采用密度泛函理论(DFT)和非平衡格林函数方法(NEGF)进行理论计算。
首先,利用DFT方法对石墨炔基分子的电子结构进行计算,获取其能带结构、态密度等关键参数。
其次,通过NEGF方法研究自旋电子在石墨炔基分子中的输运性质,分析自旋极化电流与外部条件(如磁场、电压等)之间的关系。
三、石墨炔基分子的电子结构分析我们分析了不同结构类型的石墨炔基分子的电子结构,发现这些分子具有独特的能带结构和态密度分布。
这些特性使得石墨炔基分子在自旋电子学器件中具有潜在的应用价值。
此外,我们还发现分子中某些特定位置的原子对自旋电子的输运具有重要影响。
四、自旋电子在石墨炔基分子中的输运性质通过NEGF方法,我们研究了自旋电子在石墨炔基分子中的输运过程。
结果表明,在特定条件下,石墨炔基分子可以产生明显的自旋极化电流。
此外,我们还发现外部条件如磁场和电压对自旋极化电流有显著影响。
这些结果为设计高性能的自旋电子学器件提供了重要的理论依据。
五、讨论与展望本研究表明,石墨炔基分子在自旋电子学器件中具有优异的输运性质。
我们揭示了其独特的电子结构和自旋输运机制,并发现外部条件如磁场和电压对自旋极化电流的影响。

羧基基团对导电碳骨架的影响
羧基基团对导电碳骨架的影响主要体现在以下几个方面:
1. 结构稳定性:羧基基团可以增加导电碳骨架的稳定性,因为羧基中的氧原子能够与碳骨架形成强化学键,从而提高整体结构的稳定性。
2. 电导率:羧基基团本身不具有导电性,因此会降低碳骨架的电导率。
然而,羧基可以通过与其他基团的相互作用,如氢键,影响碳骨架的电子结构和导电性能。
3. 功能性:羧基基团可以提供多种功能,如离子交换、质子传导等,这使得导电碳骨架在能源存储、传感器和生物医学等领域具有广泛的应用前景。
4. 反应性:羧基基团具有较高的反应活性,可以与其他分子或离子进行相互作用,从而影响碳骨架的反应性质。
总的来说,羧基基团对导电碳骨架的影响是复杂的,具体的影响取决于碳骨架的结构、应用领域以及与其他基团的相互作用等多个因素。

电子在石墨烯中的输运特性石墨烯是由一层厚度仅为一个原子的碳原子构成的二维晶体结构,其独特的物理性质已经引起了广泛的研究兴趣。
其中,电子在石墨烯中的输运特性尤为重要。
本文将探讨电子在石墨烯中的输运特性的一些基本特征。
首先,石墨烯具有高电子迁移率。
电子迁移率是衡量材料导电性能的重要指标之一。
在晶体材料中,通常的电子传输是通过晶格中的原子或离子之间的散射来完成的。
然而,由于石墨烯是一个二维的材料,其结构相对简单,缺乏三维结构中存在的晶格散射中心。
这使得石墨烯中的电子迁移受到极少量的散射影响,导致石墨烯的电子迁移率较高。
其次,在石墨烯中,电子呈现出线性色散关系。
色散关系是描述能带结构的关键特征之一。
在传统的三维晶体材料中,能带关系通常呈现二次曲线的形式。
然而,在石墨烯中,电子的能量与动量之间存在简单的线性关系,即E(k) = |k|vF,其中E 是电子的能量,k是电子的动量,vF是费米速度。
这种线性色散关系决定了石墨烯中的电子具有无质量的特点,被称为狄拉克费米子。
由于电子的无质量特性,石墨烯中的电子在输运过程中不会受到布洛赫波矢的限制,可以自由地在整个晶格中移动。
此外,由于石墨烯是一个二维的材料,在其边缘或缺陷处会出现一维的电子输运特性。
这种一维的输运现象被称为量子霍尔效应。
量子霍尔效应在石墨烯中表现出特殊的性质。
正常的量子霍尔效应需要材料中存在强磁场,而在石墨烯中,由于其二维性质,只需在石墨烯表面施加较弱的磁场即可观察到量子霍尔效应。
这是因为石墨烯中的电子受到边缘的限制,只能沿着边缘运动,形成了一维的输运通道。
最后,石墨烯中的电子输运受到温度和杂质的影响。
石墨烯中的电子具有高迁移率和高速度,这使得电子输运在常温下非常有效。
然而,随着温度的升高或杂质的引入,电子与杂质或热振动之间的散射会增加,导致电子迁移率的下降。
因此,控制温度和减少杂质对于石墨烯中电子输运的优化至关重要。
综上所述,电子在石墨烯中的输运特性显示出了独特的物理性质。

单分子结中负微分电阻效应全文共四篇示例,供您参考第一篇示例:单分子结中的负微分电阻效应是指当电压施加于单分子结时,电流不随着电压的增加而线性增大,而是出现电流反而随电压增大而减小的现象。
这一现象在某些特定材料的单分子结中被观察到,并且引起了学术界的广泛关注。
本文将从理论原理、实验观测和应用前景等方面详细探讨单分子结中的负微分电阻效应。
一、理论原理单分子结中的负微分电阻效应的理论基础可以从能带结构和电荷输运理论两个方面来解释。
在能带结构方面,该效应通常被认为与材料的非线性能带结构有关。
当外加电压增大时,导致能量和动量的输运发生改变,电子在能带结构中的输运速度也随之变化。
材料的非线性能带结构还可能导致电子在输运过程中与晶格振动发生耦合,从而影响电荷的迁移和散射,最终导致负微分电阻效应的出现。
在电荷输运理论方面,负微分电阻效应可以被解释为电子输运路径的变化。
随着电压增大,电子的输运路径也发生了变化,可能跳跃至不同的传输路径,导致电流随电压增大而减小。
电子在输运过程中也可能受到外界扰动的影响,如光激发、磁场等,这些扰动也会对电子的输运路径造成影响,从而引起负微分电阻效应的出现。
二、实验观测实验上,单分子结中的负微分电阻效应已经在某些材料中得到观测。
一些有机分子、金属氧化物等材料的单分子结中都发现了负微分电阻效应。
这些实验观测不仅验证了理论模型的可靠性,也为进一步研究和应用提供了基础。
通过测量电压-电流关系曲线,研究人员可以明显观察到单分子结中的负微分电阻效应。
一般情况下,当电压小于某一阈值时,电流随电压增大而增大;但当电压超过这一阈值后,电流反而随电压增大而减小,出现负微分电阻。
这一现象的观测不仅让研究人员对单分子结中的电荷输运行为有了更深入的理解,也为其在电子器件和传感器等方面的应用提供了新的思路。
三、应用前景单分子结中的负微分电阻效应具有重要的潜在应用前景。
在电子器件方面,负微分电阻效应的出现为新型电阻、开关和逻辑门等元件的设计提供了新的思路。

表面官能团对纳米球的影响
表面官能团可以在纳米球上形成化学键,从而对纳米球的物理化学性质产生影响。
以下是几个常见的表面官能团对纳米球性质的影响:
1.羧基官能团:羧基在纳米球表面形成的化学键能够增加纳米球的亲水性,使其更容易溶解在水中。
此外,羧基还能与其他物质反应,例如与氨基反应形成酰胺键,增加纳米球在生物医学领域中的应用。
2.氨基官能团:氨基官能团能够增加纳米球的亲油性,使其更容易溶解在有机溶剂中,并能够与卤代烃等电子亲吸结构反应。
3.烯基官能团:烯基是一种具有π电子的官能团,能够分别对纳米球的形态和电学性能产生影响。
烯基官能团可作为润滑剂,也可用于制造高导电性复合材料。
4.硅氧烷官能团:硅氧烷官能团能够增加纳米球的稳定性,使其在不同环境下保持不变。
这种官能团还能改善纳米材料与有机和无机材料之间的粘附性能。
总之,纳米球的表面官能团类型及其密度对其物化性能和应用都有着较大的影响。

利用第一性原理结合非平衡态格林函数方法研究分子电子传递赵健伟(南京大学化学化工学院)从分子水平研究电子传递是电化学的基础,同时也为理解分子电子学和生命过程中电子传递的现象提供参考。
为了实现真正意义上的分子水平电子传递的研究,需要制备具有纳米级曲率半径的电极,建立可靠的电极-分子界面,同时还要系统地考察分子在电场作用下,以及周围环境作用下的结构变化。
最终将这些静态的特征与动态的电子传输行为关联起来。
电子材料的动态输运是最直接的电子传递特征。
目前的理论和计算技术的发展,提供了密度泛函理论(DFT)结合非平衡格林函数(NEGF)方法的有效模拟手段,但是理论模拟的重点更应该阐述动态输运行为与静态结构之间的定量或定性关系,从而为器件设计打下基础。
上述理论方法中,电流的计算依据Landauer-Büttiker 公式积分得到:∫⋅=R L ),()),(-),((d 2R L μμb V E T V E f V E f E h e I (1)(,b T E V )代表透射谱,是特定偏压V b 下的能级E 的函数,[ L (V b ), R (V b )]是对积分有贡献的能量区域,称之为偏压窗口。
寡聚苯乙炔撑分子导线的输运行为的研究表明,由于接触金电极的作用,分子结的静态特征,如几何构型和能隙,均受到电场的显著影响。
然而输运行为的变化却很微弱。
在对二苯乙炔分子导线的研究中发现导电性对苯环之间的扭转角有直接的依赖关系,扭转角为0°时的分子结的电导是90° 时的65倍。
分子导线研究的核心内容之一是确定电子输运能力对电子传递距离的定量关系,即确定电子隧穿系数β。
宏观的金属导线的电阻随长度的增加呈线性增加,但是对于微观的分子导线,是否还遵循此种关系?通过对一系列不同结构、不同长度的分子导线的系统研究,发现分子导线的电阻随链长的增加呈指数增加,亦即电导随链长指数衰减。
)(exp 0d R R β= (2)R 0为接触电阻,d 为分子长度。

题目:以石墨烯作为电极的分子器件输运性质研究於晓明00804152导师:吴孝松研究背景:分子,是组成物质的基本单元。
对分子各类性质的研究在近几十年来的纳米科技发展中占有相当一部分比重。
由于分子结构的迥异,不同的分子往往拥有不同的性质,比如导电性、导热性、磁性、热电性等等。
不仅如此,合成的分子器件还可以实现二极管、三极管以及涉及到电化学、光电、电子自旋和分子探测等功能的混合器件。
在这里我主要研究分子的电学输运性质,因为分子器件被认为是后CMOS时代硅基器件的替代物,能够实现纳米尺度的集成电路,而另外一些器件也或多或少的与分子内部的电子运动有关,所以分子的电学输运性质就成为了分子器件研究中的核心问题。
然而这个系统是相当复杂的,器件整体表现出来的性质并不一定就是分子本身的性质,而是分子、电极、分子和电极的接触等诸多部分的综合贡献结果,将它们区分开来是比较具有挑战性的。
而且不同的分子输运性质往往用不同的理论来解释,所以一种统一的普适的理论急需被提出。
值得高兴的是,在过去几十年里,科学家们已经发展了诸多实验上的测量手段以及理论上的描述方法,并且很多已经在这两发面达成了一致,这都为我们后面的研究提供了技术和理论上的依据。
研究目的:•学习搜索与阅读文献的方法,掌握纳米器件基本的表征与测量方法•探索所研究的分子器件具有怎样的电子输运性质,这种输运现象与什么条件有关,解释I-Vsource图像随温度、门压和磁场的变化关系研究对象的特点:1、在单层石墨烯上割上锯齿形刻槽,将分子洒在上面,以石墨烯作为电极2、在衬底下面施加门电压,利用经典的场效应管研究方法测量分子在不同环境下的输运性质3、在非溶液环境测量,有效地消除了溶液中的分子运动使电路时通时断,进而测量结果带来的随机涨落器件效果图测量结果(两个器件的I-V source随温度、门压以及磁场强度的变化关系):器件一:器件二:不同T下的I-Vsource曲线不同门压下的I-Vsource曲线不同磁场下的I-Vsource曲线已有的理论模型:(一)唯象模型:A、弹道输运——电子无阻碍输运,分子不对电子进行散射B、单隧道结隧穿——电子按照一定概率隧穿,类似于单势垒经典模型C、共振隧穿——随着源漏电压之间的变化双隧道结两端能级位置发生相对移动,当能级匹配时发生共振隧穿D、库伦阻塞——库仑岛存在静电排斥能,使得充入电子变得比较困难E、跳跃俘获——当分子比较长时电子与分子耦合强度比较大,电子呈现出一种在分子的不同单元间跳跃输运的机制(二)理论计算模型A、平均场近似(自洽场方法)B、非平衡Green函数方法C、第一性原理(密度泛函)未来的方向:1、通过文献阅读来找出这种分子器件的电子究竟按照什么机制输运2、弄清楚这种输运机制与分子器件的什么因素有关(长度、电极性质、衬底和化学键的种类以及空间构型等)3、这种并联的结构对输运的结果有什么影响4、可以考虑增加拉伸系统,将石墨烯拉开,测量场效应。

ARTICLEJ. Cent. South Univ. (2019) 26: 1449−1457 DOI:https:///10.1007/s11771-019-4101-zStructural modulation of anthraquinone with different functional groups and its effect on electrochemical properties for lithium-ion batteries QIAN Su-hui(钱素惠)1, PAN Jun-xian(潘俊贤)1, ZHU Zhao-sheng(朱肇昇)1, YE Rui-tian(叶锐添)1, LIN Geng-zhong(林耿忠)1, ZHU Xiao-xing(朱潇行)1, XIONG Zhi-yong(熊志勇)2,GANESH Venkatachalam3, ZENG Rong-hua(曾荣华)1, LUO Yi-fan(罗一帆)11. Key Laboratory of Theoretical Chemistry of Environment of Ministry of Education, School of Chemistryand Environment, South China Normal University, Guangzhou 510006, China;2. School of Chemical Engineering and Materials Science, Beijing Institute of Technology Zhuhai,Zhuhai 519088, China;3. Electrodics and Electrocatalysis (EEC) Division, CSIR – Central Electrochemical Research Institute(CSIR – CECRI), Karaikudi 630003, Tamilnadu, India© Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract:Organic electrode materials have high capacity, and environmentally friendly advantages for the next generation lithium-ion batteries (LIBs). However, organic electrode materials face many challenges, such as low reduction potential as cathode materials or high reduction potential as anode materials. Here, the influence of chemical functionalities that are capable of either electron donating or electron withdrawing groups on the reduction potential and charge−discharge performance of anthraquinone (AQ) based system is studied. The cyclic voltammetry results show that the introduction of two —OH groups, two —NO2 groups and one —CH3 group on anthraquinone structure has a little impact on the reduction potential, which is found to be 2.1 V. But when three or four —OH groups are introduced on AQ structure, the reduction potential is increased to about 3.1 V. The charge−discharge tests show that these materials exhibit moderate cycling stability.Key words: lithium-ion batteries; anthraquinone; electron groups; reduction potentialCite this article as: QIAN Su-hui, PAN Jun-xian, ZHU Zhao-sheng, YE Rui-tian, LIN Geng-zhong, ZHU Xiao-xing, XIONG Zhi-yong, GANESH Venkatachalam, ZENG Rong-hua, LUO Yi-fan. Structural modulation of anthraquinone with different functional groups and its effect on electrochemical properties for lithium-ion batteries [J]. Journal of Central South University, 2019, 26(6): 1449−1457. DOI: https:///10.1007/s11771-019-4101-z.1 IntroductionSecondary batteries as energy storage devices are widely used in portable electronics and electric vehicles [1−11]. The use of electrode materials for current LIBs mainly depends on depletable metal-based inorganic materials prepared from limited mineral resources. Therefore, it is an urgent demand to explore renewable electrode materials, such as organic materials, which are more abundant, low-cost, and require minimum energy consumption [12−14]. Quinone-based electrode materials (QEMs) have high theoretical capacity owing to multi-lithium ion insertion/deinsertion in quinone structure for lithium ion batteries (LIBs)Foundation item: Project(21875076) supported by the National Natural Science Foundation of China; Projects(2018A050506077, 2017A050506048) supported by the Scientific and Technological Plan of Guangdong Province, China;Project(201910574037) supported by the Undergraduatesʼ Innovating Experimentation Project of ChinaReceived date: 2018-12-12; Accepted date: 2019-01-11Corresponding authors: ZENG Rong-hua, PhD, Associate Researcher; Tel: +86-20-39310334; E-mail: zengronghua@;ORCID: 0000-0001-6009-1432; LUO Yi-fan, PhD, Professor; E-mail: luoyf2004@; ORCID:0000-0003- 1478-4275[15]. Moreover, QEMs have advantages of structure diversity, flexibility, and processability as potential candidates for either cathodes or anodes in LIBs [16−18]. However, QEMs are very restricted in practical application due to high solution, low conductivity, and low discharge platform as cathode or high discharge platform as anode [19]. In particular, discharge platform is the key to improve power of LIBs. Some electron withdrawing groups can be introduced onto QEMs, such as —F, —Cl, —N, —S and —OH groups, which can improve the discharge potentials and cycle performance of quinone based electrodes. LEE et al [20]introduced —NH groups in poly (disulfide diphenylamine) (PDTDA), and the results indicated that the discharge platform of PDTDA reached about 3.0 V, the initial discharge capacity was only 94 mA∙h/g and has been reduced to 71 mA∙h/g after ten cycles. LI et al [21] prepared poly (disulfide-3-amino- xylene) with the addition of sulfur atoms on the polymer, and the results revealed the discharge platform exhibits about 2.6 V, and the first discharge capacity was 225 mA∙h/g. NISHIDE et al [22] added methacrylate group on polymethacrylic acid (2,2,6,6-tetramethyl) piperidine-4-ester and the discharge platform of this electrode material increased to about 3.5 V. The initial discharge capacity was only 77 mA∙h/g, which was 70% of the theoretical capacity, the discharge capacity did not decrease obviously after 1000 cycles, revealing the better cycle performance. For the influence of the introduction of S element onto QEMs, for instance, the synthesized dibenzo[b,i]thianthrene-5, 7,12,14-tetrone (SPT) electrode achieved the average reduction potential about 2.6 V, which had distinctly 0.4 V inhancement compared with 5,7,12,14- pentacenetetrone (PT) by the addition of S atoms [23]. XUE et al [24] replaced oxygen on carbonyl with sulfur on anthraquinone to synthesize anthrax [1`,9`,8`-b,c,d,e] [4`,10`,5`-b`,c`,d`,e`] bis- [1,6, 6a(6a-S IV)-trithia] pentalene (ABTH), and the discharge platform reached 3.3 V. WAN et al [25] confirmed the influences of the SO3Na– groups on the reduction potential of the anthraquinone electrode, the average reduction potential increased with the increase of SO3Na– groups. Halogen as the strong electron withdrawing group is also used to enhance reduction potential of QEMs for LIBs. For instance, CHEN et al [26] demonstrated the effect of chlorine groups on hydroxyl p-benzoquinone,and the electrochemical performance test displayed that the discharge platform of this material was about 2.3 V, the average reversible capacity reached to about 200 mA∙h/g. Also, the impact of fluoroalkyl groups as substituents on benzoquinone (BQ) was studied by YOKOJI et al [27]. The results showed that, compared with BQ, the average discharge platforms of 2,5-bis(trifluoromethyl)-1, 4-benzoquinone (CF3-BQ), 2,5-bis(perfluorobutyl)- 1,4-benzoquinone (Rf4-BQ) and 2,5- bis(perfluorohexyl)-3,6-dichloro-1,4-benzoquinone (Rf6-Cl-BQ) increased apparently and raised to about 3.0 V through the addition of CF3 (trifluoromethyl), Rf4 (perfluorobutyl) and Rf6 (perfluorohexyl) groups. BANDA et al [28] demonstrated the redox potential of perylene diimides (PDIs) was tuned by a simple and efficient approach as high discharge platform positive materials for sodium ion batteries (SIBs). With suitable functional groups (—Br, —CN, —S) as substituents on PDIs, the experiment shows the discharge platform has a remarkable tunability from 2.1 to 2.6 V. In addition, BACHMAN et al [15] investigated the influence of a variety of electron groups on redox potential of AQ in flow batteries by using density functional theory (DFT). The increase of methyl (electron donating groups) can decrease reduction potential of AQ. However, the C=O, carboxylic acid (COOH), and oxy-methyl dioxolane (electron withdrawing groups) can improve reduction potential of AQ. Other electron donating groups, such as tert-butyl, ethyl, phenyl, and alkoxy substituents, and electron withdrawing groups, such as alkoxy substituents, did not increase or reduce the reduction potentials.Herein, we studied the influence of the different functional (to alter the electronic structure) groups (—OH, —NO2and —CH3 groups) on the reduction potential of AQ based electrode materials in LIBs, and studied charge−discharge properties of those electrode materials.2 Experimental2.1 Physical characterizationsTo make sure high purities, anthraquinone (AQ) and its derivatives, including anthraquinones (AQ), 1,5-dihydroxy anthraquinones (2OH-AQ), 1,2,7- trihydroxy-anthraquinone (3OH-AQ) and 1,2,5,8- tetrahy-droxy-anthraquinone (4OH-AQ), 1,5-dinitroanthraquinones (2NO2-AQ), 2-methyl anthraquinones (CH3-AQ), were obtained commercially and used directly as the electrode materials for LIBs.The powder X-ray diffraction (XRD) data were collected through a Bruker AXS D8-Advanced diffractometer at 40 kV and 40 mA with Cu Kα N1 radiation (λ=1.5406 Å) at a scanning speed of 0.02 °/s. The experimental XRD patterns were refined by using Accelrys Materials Studio. The morphology was observed by using scanning electron micros-cope (SEM, JEOL JSM6700F) at an accelerating voltage of 5 kV.2.2 Electrochemical measurementsThe six electrodes were fabricated by mixing 2NO2-AQ, CH3-AQ, etc, conductive carbon blacks (Super-P), polyvinylidene fluoride (PVDF) binder at a mass ratio of 60:30:10 in N-methyl-2- pyrrolidone. Individual slurries were pasted and compressed onto Al foil, and dried in a vacuum at 100 °C for 12 h. The loaded mass of electrodes with an area of 1 cm2was about 2 mg. The electrolyte was 1 mol/L LiPF6 in EC and DMC (V EC:V DMC=1:1). The separator was a Celgard 2300 microporous membrane. Coin-type cells (size: 2025) were assembled coin-type in an Ar-filled glove box. The charge/discharge experiments were carried out at a constant current density of 100 mA/g by using LAND-CT2001A instrument. Cyclic voltammograms were performed at a scan rate of 50 µV/s by using Autolab instrument.3 Results and discussion3.1 Physical propertiesFigure 1 presents the morphology of 2NO2-AQ and CH3-AQ. As can be seen from Figures 1(a) and (b), the morphology of 2NO2-AQ shows irregular block, the diameters is in the range of 2−10 μm. However, the CH3-AQ is composed of inhomogeneous particles, the particles are 0.5−2 μm in diameters (Figures 1(c) and (d)). The structures of 2NO2-AQ and CH3-AQ are further studied through XRD (Figures 2(a) and (b)). As can be seen from Figure 2(a), after experimental XRD pattern of 2NO2-AQ was refined by using materials studio, the diffractions of 2NO2-AQ are characteristic of monoclinic system and C2c space group (lattice parameters: a=10.3279(6)Å, b=11.2263(0)Å, c= 10.7906(9)Å, β=94.5004(4)°). Also, CH3-AQFigure 1 Low and high magnification SEM images of 2NO2-AQ (a, b) and CH3-AQ (c, d)Figure 2 XRD patterns of 2NO2-AQ (a) and CH3-AQ (b)sample is a monoclinic system and P21/c space group (Figure 2(b), (PDF# 00-048-2443)), which are different from 2NO2-AQ. However, the SEM images and XRD patterns of AQ, 2OH-AQ, 3OH-AQ and 4OH-AQ have been described and analyzed in our previous work [29].3.2Relation between reduction potentials andfunctional groupsTo well comprehend the relation between the reduction potentials and functional electron groups, cyclic voltammetry (CV) testing for AQ, 2OH-AQ, 3OH-AQ, 4OH-AQ, 2NO2-AQ and CH3-AQ are performed. It should be noted that the CV results of AQ, 2OH-AQ, 3OH-AQ, 4OH-AQ have been studied by our previous work [29]. To investigate the relation between the reduction potentials and electron groups, we further analyze —OH groups in combination of —NO2 and —CH3 groups. As shown in Figures 3(a), (b), (e) and (f), during the negative sweeping, the two reduction peaks can be observed, which located at 2.1 and 2.0 V for AQ, 2.3 and 2.0 V for 2OH-AQ, 2.2 and 1.9 V for CH3-AQ, respectively. However, only one reduction peak located at 2.1 V can be observed for NO2-AQ. It is found that the reduction potential (about 2.1 V) almost not increase or decrease when two —OH groups, two —NO2groups and one —CH3group are added onto AQ structure. This means that either one or two electron groups, such as —CH3, —OH and —NO2groups, do not show any effect on the reduction potential. However, when the —OH electron withdrawing groups are continued to introduce on 2OH-AQ structure, the first reduction potential is increased to about 3.0 V for 3OH-AQ and 4OH-AQ (Figures 3(c) and (d)) [29]. The sum charge is computed on O and H in AQ, 2OH-AQ, 3OH-AQ, 4OH-AQ by using NBO analyses in our previous work [29], the sum negative charge of 3OH-AQ and 4OH-AQ is much larger than that of AQ and 2OH-AQ, indicating the added negative charge promotes the insertion of lithium ion and results in the enhancement of reduction potential in 3OH-AQ and 4OH-AQ [29]. We will continue to add the —NO2and —CH3electron groups on 2NO2-AQ and CH3-AQ structure and study the relationship between the number of the electron groups and reduction potentials in the future research work.Figure 4 shows the lithiation process of six electrode materials, and the two split reduction peaks of AQ, 2OH-AQ, 3OH-AQ, 4OH-AQ and CH3-AQ imply the electrons transfer reaction occurs step by step in the carbonyl groups, hydroxyls and lithium ions, and produces either the lithium enolate or complexes. However, only one reduction peak can be observed in 2NO2-AQ, showing two lithium ions inserted into 2NO2-AQ structure simultaneously and generate the lithium enolate [30, 31]. During the positive sweeping, one or two oxidation peaks observed represent one or two steps oxidation reaction, which are ascribed to reoxidation of lithium enolate or complexes to produce the quinonyl group [32, 33]. The lithiation mechanisms in AQ, 2OH-AQ, 3OH-AQ, 4OH-AQ are also reported in detail [29].3.3 Charge/discharge propertiesGalvanostatic charge/discharge profiles were collected for the above mentioned six electrode materials in a potential range of 1.0 to 4.5 V at a current density of 100 mA/g. Figure 5 displays the charge/discharge profiles of 2NO2-AQ and CH3-AQFigure 3Cyclic voltammograms for initial cycle at 50 μV/s for AQ (a), 2OH-AQ (b), 3OH-AQ (c), 4OH-AQ (d), 2NO2-AQ (e) and CH3-AQ (f) [29]electrode materials. The initial discharge capacities of 2NO2-AQ and CH3-AQ reach 124 and 147 mA∙h/g. After 30 cycles, the discharge capacities of NO2-AQ and CH3-AQ electrodes maintained at 93 and 96 mA∙h/g with the capacities decay of 25% and 35%, respectively. The charge/discharge properties of AQ, 2OH-AQ, 3OH-AQ and 4OH-AQ were reported by us in previous work [29]. It is found the six electrode materials exhibit moderate cyclic stability, which may be attributed to the low electronic conductivity of these materials and the solution of these QEMs in organic electrolytes, leading to the sharp fading of the capacity [34]. In addition, the discharge platforms of AQ, 2OH-AQ, 2NO2 -AQ and CH3-AQ are all at about 2.1V, indicating that the introduction of two —OH groups, two —NO2groups and one —CH3group on AQ has little influence on the discharge platform, which are consistent with the cyclic voltammetry results.Figure 4 Schematic illustrations of lithiation mechanism in AQ (a), 2OH-AQ (b), 3OH-AQ (c), 4OH-AQ (d), 2NO2-AQ (e) and CH3-AQ (f) [29]Figure 5 Discharge–charge curves for initial 30 cycles of 2NO2-AQ (a), CH3-AQ (b), cycling performance of 2NO2-AQ (c), and CH3-AQ (d)4 ConclusionsIn summary, the relationships between reduction potential and chemical functional groups that are either electron donating or withdrawing in AQ molecular structure have been studied for LIBs. The experimental results reveal that the reduction potentials of these materials have not been improved by the introduction of two —OH groups, two —NO2 groups and one —CH3 group, which is similar to the reduction potential of AQ with about 2.1 V. However, the reduction potentials of 3OH-AQ and 4OH-AQ can increase to about 3.1 V after the three or four groups are added on AQ structure. Meanwhile, the charge−discharge performances of these six electrode materials have also been studied. We will continue to increase the number of —NO2 and —CH3 groups and investigate the relation between reduction potential and electron groups in the future work. References[1]YI Jin, LIU Yang, QIAO Yu, HE Ping, ZHAO Hao-sheng.Boosting the cycle life of Li–O2batteries at elevated temperature by employing a hybrid polymer–ceramic solid electrolyte [J]. ACS Energy Letters, 2017, 2(6): 1378−1384.DOI: 10.1021/acsenergy lett.7b00292.[2]QIAO Yu, YI Jin, WU Shu-chao, YANG Si-xie, HE Ping,ZHAO Hao-sheng. Li-CO2 electrochemistry: A new strategy for CO2fixation and energy storage [J]. Joule, 2017, 1(2): 359−370. DOI: 10.1016/j.joule.2017.07.001.[3]YI Jin, LIU Xiao-yu, LIANG Peng-cheng, WU Kai, XU Jie,LIU Yu-yu, ZHANG Jiu-jun. Non-noble iron group (Fe, Co, Ni)-based oxide electrocatalysts for aqueous Zinc–air batteries: Recent progress, challenges, and perspectives [J].Organometallics, 2018, 38(6): 1−14. DOI: 10.1021/acs.organomet.8b00508.[4]LEI Ping, WANG Yao, ZHANG Fang, WANG Xin, XIANGXing-de. Carbon-coated Na2.2V1.2Ti0.8(PO4)3cathode with excellent cycling performance for aqueous Sodium-ion batteries [J]. Chem Electro Chem, 2018, 5(17): 2482−2487.DOI: 10.1002/ celc.201800379.[5]LI Wan-fang, ZHANG Fang, XIANG Xing-de, ZHANGXiu-cheng. Electrochemical properties and redox mechanism of Na2Ni0.4Co0.6[Fe(CN)6] nanocrystallites as high-capacity cathode for aqueous sodium-ion batteries [J]. The Journal of Physical Chemistry C, 2017, 121(50): 27805−27812. DOI:10.1021/acs.jpcc.7b07920.[6]LIU Yang, YI Jin, QIAO Yu, WANG Di, HE Ping, LI Qi,WU Shi-chao, ZHOU Hao-shen. Solar-driven efficient Li2O2oxidation in solid-state Li-ion O2batteries [J]. Energy Storage Materials, 2018, 11: 170−175. DOI: 10.1016/ j.ensm.2017.10.002.[7]WU Shi-chao, YI Jin, ZHU Kai, BAI Song-yan, LIU Yang,QIAO Yu, ISHIDA M, ZHOU Hao-shen. A super- hydrophobic quasi-Solid electrolyte for Li-O2battery with improved safety and cycle life in humid atmosphere[J].Advanced Energy Materials, 2017, 7(4): 1601759. DOI:10.1002/aenm.201601759.[8]LEE J, URBAN A, LI Xin, SU Dong, HAUTIER G, CEDERG. Unlocking the potential of cation-disordered oxides forrechargeable lithium batteries [J]. Science, 2014, 343(6170): 519−522. DOI: 10.1126/science.1246432.[9]LI Lei, RAJI A O, TOUR J M. Graphene-wrappedMnO2–graphene nanoribbons as anode materials for high-performance lithium ion batteries [J]. Advanced Materials, 2013, 25(43): 6298−6302. DOI: 10.1002/adma.201302915.[10]HUANG Jun-da, WEI Zeng-xi, LIAO Jia-qin, NI Wei,WANG Cai-yun, MA Jian-min. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: Beyond MoS2 [J]. Journal of Energy Chemistry, 2018, 33(7): 1−25.DOI: 10.1016/j.jechem.2018.09.001.[11]LEI Kai-xiang, WANG Chen-chen, LIU Luo-jia, LUOYu-wen, MU Chao-nan, LI Fun-jun, CHEN Jun. A porous network of bismuth used as the anode material for high-energy-density Potassium-ion batteries [J]. Angewandte Chemie International Edition, 2018, 130(17): 4777−4781.DOI: 10.1002/ange.201801389.[12]GUO Zhao-wei, MA Yuan-yuan, DONG Xiao-li, HUANGJian-hang, WANG Yong-gang, XIA Yong-yao. An environmentally friendly and flexible aqueous Zinc battery using an organic cathode [J]. Angewandte Chemie International Edition, 2018, 57(36): 11737−11741. DOI:10.1002/ange.201807121.[13]YI Jin, LIANG Peng-cheng, LIU Xiao-yu, WANGYong-gang, XIA Yong-yao, ZHANG Jiu-jun. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc–air batteries [J]. Energy & Environmental Science, 2018, 11(11): 3075−3095. DOI: 10.1039/C8EE01991F.[14]LUO Zhi-qiang, LIU Luo-jia, NING Jia-xin, LEI Kai-xiang,LU Yong, LI Fun-jun, CHEN Jun. A microporous covalent organic framework with abundant accessible carbonyls for lithium-ion batteries [J]. Angewandte Chemie International Edition, 2018, 57(30): 1−6. DOI: 10.1002/anie.201805540. [15]BACHMAN J E, CURTISS L A, ASSARY R S.Investigation of the redox chemistry of anthraquinone derivatives using density functional theory [J]. Journal of Physical Chemistry A, 2014, 118(38): 8852−8860. DOI:10.1021/jp5060777.[16]HAUPLER B, WILD A, SCHUBERT U S. Carbonyls:powerful organic materials for secondary batteries [J].Advanced Energy Materials, 2015, 5(11): 1402034. DOI:10.1002/aenm.201402034.[17]WANG Heng-guo, SHUANG Yuan, MA De-long, HUANGXiao-lei. Tailored aromatic carbonyl derivative polyimides for high-power and long-cycle sodium-organic batteries [J].Advanced Energy Materials, 2014, 4: 1301651. DOI:10.1002/aenm.201301651.[18]LIANG Yan-liang, ZHANG Peng, YANG Si-qi, TAOZhan-liang. Fused heteroaromatic organic compounds for high-power electrodes of rechargeable lithium batteries [J].Advanced Energy Materials, 2013, 3(5): 600−605. DOI:10.1002/aenm.201200947.[19]XING Li-dan, ZHENG Xiong-wen, SCHROEDER M,ALV ARADO J, CRESCE A W, XU Kang, LI Qiao-shu, LI Wei-shan. Deciphering the ethylene carbonate–propylene carbonate mystery in Li-ion batteries [J]. Accounts of Chemical Research, 2018, 51(2): 282−289. DOI:10.1021/acs.accounts.7b00474.[20]LEE Y G, RYU K S, CHANG S H. Chemically synthesizedhigh molecular weight poly(2,2’-dithiodianiline) (PDTDA) as a cathode material for lithium rechargeable batteries [J].Journal of Power Sources, 2003, 119: 321−325. DOI:10.1016/S0378-7753(03)00146-0.[21]LI Jin-xia, ZHAN Hui, ZHOU Lei, DENG Shi-ren, LIZhao-ying, ZHOU Yun-hong. Aniline-based polyorganodisulfide redox system of high energy for secondary lithium batteries [J]. Electrochemistry Communications, 2004, 6(6): 515−519. DOI:10.1016/j.elecom.2004.03.010.[22]NISHIDE H, IWASA S, PU Y J, SUGA T, NAKAHARA K,SATOH M. Organic radical battery: nitroxide polymers as a cathode-active material [J]. Electrochimica Acta, 2004, 50(2): 827−831. DOI: 10.1016/j.electacta.2004.02.052.[23]YAO M, ANDO H, KIYOBAYASHI T. Polycyclic quinonefused by a sulfur-containing ring as an organic positive- electrode material for use in rechargeable lithium batteries [J]. Energy Procedia, 2016, 89: 222−230. DOI: 10.1016/ j.egypro.2016.05.029.[24]XUE Long-jian, LI Jin-xia, HU Su-qing, ZHANG Ming-xia,ZHOU Yun-hong, ZHAN Cai-mao. Anthracene based organodisulfide positive active materials for lithium secondary battery [J]. Electrochemistry Communications, 2003, 5(10): 903−906. DOI: 10.1016/j.elecom.2003.08.018.[25]WAN Wang, LEE Hung-sui, YU Xi-qian, WANG Chao.Tuning the electrochemical performances of anthraquinone organic cathode materials for Li-ion batteries through the sulfonic sodium functional group [J]. RSC Advances, 2014, 4(38): 19878−19882. DOI: 10.1039/c4ra01166j.[26]CHEN Hai-yan, POIZOT P, DOLHEM F, BASIR N I,MENTRE O, TARASCON J M. Electrochemical reactivity of lithium chloranilate vs Li and crystal structures of the hydrated phases [J]. Electrochemical and Solid-State Letters, 2009, 12(5): A102−A106. DOI: 10.1149/1.3082038.[27]YOKOJI T, MATSUBARA H, SATOHB M. Rechargeableorganic lithium-ion batteries using electron-deficient benzoquinones as positive-electrode materials with high discharge voltages [J]. Journal of Materials Chemistry A, 2014, 2: 19347−19354. DOI: 10.1039/C4TA02812K.[28]BANDA H, DAMIEN D, NAGRAJAN K, RAJ A,HARUARAN M, SHAIJUMON M. Sodium-ion batteries: Twisted perylene diimides with tunable redox properties for organic sodium-ion batteries [J]. Advanced Energy Materials, 2017, 7(20): 1701316. DOI: 10.1002/aenm.201770112. [29]ZENG Rong-hua, XING Li-dan, QIU Yong-cai, WANGYa-ting, HUANG Wan-na, LI Wei-shan, YANG Shi-he.Polycarbonyl(quinonyl) organic compounds as cathode materials for sustainable lithium ion batteries [J].Electrochimica Acta, 2014, 146: 447−454. DOI:10.1016/j.electacta.2014.09.082.[30]HAN Xiao-yan, CHANG Cai-xian, YUAN Liang-jie, SUNTao-lei. Aromatic carbonyl derivative polymers as high performance Li-ion storage materials [J]. Advanced Materials, 2010, 19(12): 1616−1621. DOI: 10.1002/adma.200790044.[31]XIONG Jiang-feng, CHANG Cai-xian, LI Ming, WU Si-min.A novel coordination polymer as positive electrode materialfor lithium ion battery [J]. Crystal Growth & Design, 2008, 8(1): 280−282. DOI: 10.1021/cg070386q.[32]ZHOU Qing, ZHU Zhi-qiang, CHEN Jun. Molecularengineering with organic carbonyl electrode materials for advanced stationary and redox flow rechargeable batteries [J].Advanced Materials, 2017, 29(48). DOI: 10.1002/adma.201607007.[33]WU Yi-wen, ZENG Rong-hua, NAN Jun-min, SHU Dong.Quinone electrode materials for rechargeable lithium/sodium ion batteries[J]. Advanced Energy Materials, 2017, 7(24): 1700278. DOI: 10.1002/aenm.201700278.[34]PIRNAT K, DOMINKO R, CERE-KOROSEC R, MALI G,GENORIO B, GABERSCSK M. Electrochemically stabilised quinone based electrode composites for Li-ion batteries [J]. Journal of Power Sources, 2012, 199(1): 308−314. DOI: 10.1016/j.jpowsour.2011.10.068.(Edited by FANG Jing-hua)中文导读不同官能团的蒽醌结构调控及其对锂离子电池材料电化学性能的影响摘要:有机电极材料作为下一代锂离子电池材料具有容量高,环境友好型等优势。

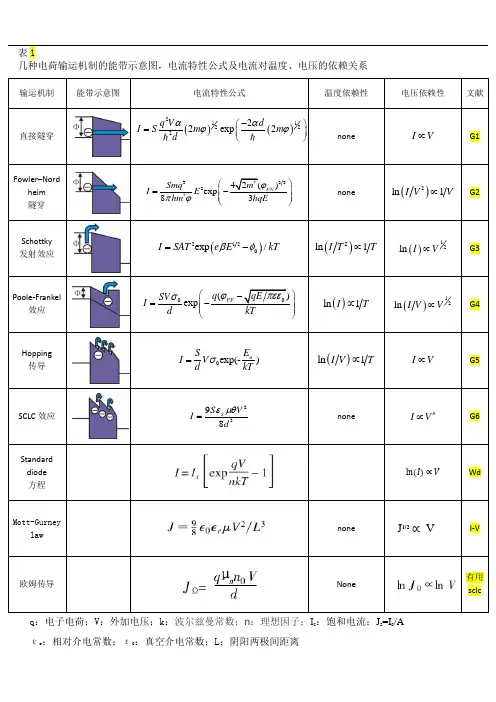
q:电子电荷;V:外加电压;k:波尔兹曼常数;n:理想因子;I s:饱和电流;J s=I s/Aεr :相对介电常数;ε:真空介电常数;L:阴阳两极间距离()()1122222exp2q V dm mdααϕϕ-⎛⎫⎪⎝⎭*3232*42()exp83FNmSmqEhm hqEϕπϕ⎛⎫-⎪⎪⎝⎭理解薄膜中电荷的输运机制对于分子电子器件的应用具有重要意义,例如分子二极管、分子晶体管和分子存储元件等。
因此,关于金属电极薄膜中电荷的输运机制的研究已成为纳米材料研究中倍受关注的热点课题。
电荷在金属电极-薄膜-金属电极结构中的输运机制主要有直接隧穿、Fowler –Nordheim 隧穿、Schottky 发射效应、Poole-Frankel 效应、跳跃传导(Hopping conduction )及空间电荷限制(SCLC )效应六种,各种输运机制的能带示意图,电流特性公式及电流对温度、电压的依赖关系如表1所示。
直接隧穿和Fowler –Nordheim 隧穿属于非共振遂穿,电流大小均和温度无关,其中直接隧穿适用于小电压范围(eV φ<),电流和电压呈线性关系;Fowler –Nordheim 隧穿适用于较高电压范围(eV φ>),()2ln I V 和1V 呈线性关系。
在小电压范围,美国耶鲁大学Reed G7研究组利用直接隧穿模型研究了饱和烷硫醇自组装薄膜器件在变温条件下的电荷输运机制,并推算出势垒高度φ及衰减系数β。
清华大学陈培毅教授G8等也对烷基硫醇饱和分子结中的电荷输运进行了研究,证实了隧穿为饱和分子结中的主要电荷输运机制。
中国科学技术大学王晓平G9研究组研究了自组装硫醇分子膜输运特征的压力依赖性,分析表明自组装硫醇分子膜输运特征的压力依赖性也主要源于电荷在分子膜中的链间隧穿过程。
在较高电压范围,韩国光州科学研究院Lee G10等观察到饱和烷硫醇自组装薄膜器件电流输运机制由直接隧穿转变为Fowler –Nordheim 隧穿,并研究了不同条件下过渡电压的变化规律。
《石墨炔基分子自旋电子学器件输运性质的理论研究》篇一摘要:本文通过理论计算的方法,对石墨炔基分子自旋电子学器件的输运性质进行了深入的研究。
文章首先对相关研究背景和现状进行了阐述,随后提出了本文的主要研究方法、内容以及取得的主要结果。
最后,总结了本文的结论和展望了未来的研究方向。
一、引言随着纳米电子学和自旋电子学的快速发展,石墨炔基分子因其独特的电子结构和物理性质,在自旋电子学器件中展现出巨大的应用潜力。
自旋电子学器件的输运性质研究对于理解其工作原理、优化器件性能具有重要意义。
因此,本文以石墨炔基分子为研究对象,对其自旋电子学器件的输运性质进行理论研究。
二、石墨炔基分子的基本性质与结构石墨炔基分子是由碳原子组成的二维网络结构,具有优异的导电性和稳定的物理化学性质。
其独特的电子结构使得它成为自旋电子学器件的理想候选材料。
本文首先对石墨炔基分子的基本性质和结构进行了概述,包括其电子能级结构、磁学性质等。
三、自旋电子学器件的工作原理与模型自旋电子学器件基于自旋极化电流的控制来实现信息处理。
本文介绍了自旋电子学器件的基本工作原理和输运模型,为后续的石墨炔基分子自旋电子学器件输运性质研究提供了理论基础。
四、研究方法与模型建立本文采用密度泛函理论(DFT)和格林函数方法(GF)相结合的方式,对石墨炔基分子自旋电子学器件的输运性质进行研究。
首先,通过DFT方法计算了石墨炔基分子的电子结构和相关物理参数。
然后,基于GF方法建立了器件的输运模型,并通过非平衡格林函数方法计算了器件的输运特性。
五、结果与讨论(一)能级结构与磁性研究通过DFT计算发现,石墨炔基分子具有合理的能级结构,适合作为自旋电子学器件的材料。
此外,其磁性研究显示,石墨炔基分子具有可调控的磁矩,为自旋极化电流的产生提供了可能。
(二)输运性质分析利用GF方法计算了石墨炔基分子自旋电子学器件的电流-电压特性,分析了不同条件下的输运机制。
结果表明,石墨炔基分子在自旋电子学器件中具有良好的输运性能,且可以通过调控外部条件如门电压、温度等来优化器件性能。