标准氧化还原电位表资料.doc
- 格式:doc
- 大小:632.50 KB
- 文档页数:18

fe3+到fe2+的氧化还原电位氧化还原反应是指物质中电子的转移和氧化态的变化。
氧化态是描述原子拥有的电子数目和电子转移方向的指标,其中正数表示失去电子的原子,负数表示获得电子的原子。
在氧化还原反应中,还原剂将电子给予氧化剂,氧化剂从中获得电子,从而完成了电子的转移。
因此,氧化还原反应也被称为电子转移反应。
氧化还原电位是用来描述氧化还原反应进行方向和剧烈程度的物理量。
在氧化还原反应中,电子从具有较低电子亲和力的物质转移到具有较高电子亲和力的物质上。
当氧化剂和还原剂在标准状态下进行氧化还原反应时,氧化剂的电子亲和力和还原剂的电子亲和力之间的差异被称为氧化还原电位。
根据氧化还原电位的差异,可以预测氧化还原反应的进行方向和剧烈程度。
对于Fe3+到Fe2+的氧化还原反应,它可以写成如下方程式:Fe3+ + e- ⇌ Fe2+这个反应是一个可逆反应,可以向前和向后进行。
在此反应中,Fe3+为氧化剂,它会接受一个电子变成Fe2+。
因此,该反应的标准电位为正值,表示Fe3+具有较高的电子亲和力。
根据文献资料,该反应的标准电位为+0.77 V。
标准电位的正负值反映了反应的方向性。
正的标准电位表示反应是自发的,即反应趋向于向前进行;负的标准电位表示反应是不自发的,即反应趋向于向后进行。
在此反应中,由于Fe3+的电子亲和力较高,因此Fe3+具有较大的驱动力向前反应。
相反,Fe2+的电子亲和力较低,因此Fe2+具有较小的驱动力向后反应。
因此,该反应的方向性是向前进行。
标准电位还可以用于计算实际反应条件下的电位。
实际反应条件下的电位可以通过标准电位和反应条件相关参数的改变计算得出。
例如,根据尼尔斯特方程,实际电位E可以由标准电位E0、反应温度T 和反应物浓度来计算:E = E0 - (0.0592/n)logQ其中,n是电子转移数目,Q是反应物浓度的比值。
通过改变反应物浓度和温度,可以改变反应的氧化还原电位。
除了标准电位,还可以通过极化曲线来研究氧化还原反应的电位。

Standard electrode potential (data page)From Wikipedia, the free encyclopedia(Redirected from Table of standard electrode potentials)Jump to: navigation, searchMain article:standard electrode potentialThe values of standard electrode potentials are given in the table below in volts relative to the standard hydrogen electrode and are for the following conditions:• A temperature of 298.15 K (25 °C);•An effective concentration of 1 mol/L for each aqueous species or a species in a mercury amalgam;• A partial pressure of 101.325 kPa (absolute) (1 atm, 1.01325 bar) for each gaseous reagent. This pressure is used because most literature data are still given for this value rather than for the current standard of 100 kPa.•An activity of unity for each pure solid, pure liquid, or for water (solvent).Legend: (s) - solid; (l) - liquid; (g) - gas; (aq) - aqueous (default for all charged species); (Hg) - amalgam.Half-reaction E° (V) [note 1] Ref.%N2(g) + H+ + e- HN3(aq) -3.09 皿Li+ + e- Li(s) -3.0401 ⑵N2(g) + 4 H2O + 2 e-「2 NH20H(aq) + 2 OH--3.04Cs+ + e- L C S(S)-3.026 121Rb+ + e- -Rb(s) -2.98 ⑵K+ + e- -K(s) -2.931 121Ba2+ + 2 e- —Ba(s) -2.912 121La(OH)3(s) + 3 e- La(s) + 3 OH--2.90 121Sr2+ + 2 e- —Sr(s) -2.899 121EU2+ + 2e-二Eu(s) -2.812Ra2+ + 2 e- —Ra(s) -2.8Na+ + e- —Na(s) -2.71 ⑵「31 Sc3+ + 3 e- Sc(s) -2.077 141 La3+ + 3 e- -La(s) -2.379 12] Y3+ + 3 e- Y⑸-2.372 ⑵Mg2+ + 2 e--Mg(s) -2.372 121 ZrO(OH)2(s) + H2O + 4 e- -Zr(s) + 4 OH--2.36Al(OH)4- + 3 e- —Al(s) + 4 OH--2.33Al(OH)3(s) + 3 e- —Al(s) + 3 OH--2.31%(g) + 2 e- 2 H--2.25Ac3+ + 3 e- —Ac(s) -2.20Be2+ + 2 e- —Be(s) -1.85U3+ + 3e-」U⑸-1.66 国AI3+ + 3e- —Al(s) -1.66 回Ti2+ + 2 e-=Ti(s) -1.63 回ZrO2(s) + 4 H+ + 4 e- Zr(s) + 2 H2O -1.553 囹Zr4+ + 4 e-」Zr(s) -1.45 囱Ti3+ + 3 e- Ti(s) -1.37 171 TiO(s) + 2 H+ + 2 e--Ti(s) + H2O -1.31Ti2O3(s) + 2 H+ + 2 e- 2 TiO(s) + H2O -1.23Zn(OH)42- + 2 e- —Zn(s) + 4 OH--1.199 囹Mn2+ + 2 e- —Mn(s) -1.185 囱Fe(CN)64- + 6 H+ + 2 e- Fe(s) + 6HCN(aq) -1.16 181 Te(s) + 2 e- -Te2--1.143 囹V2+ + 2 e- V(s) -1.13 191Nb3+ + 3e- Nb(s) -1.099Sn(s) + 4H+ + 4 e- SnH4(g) -1.07SiO2(s) + 4 H+ + 4 e--Si(s) + 2 H2O -0.91B(OH)3(aq) + 3 H+ + 3 e—B(s) + 3 H2O -0.89Fe(OH)2(s) + 2 e-,Fe(s) + 2 OH--0.89 18] Fe2O3(s) + 3 H2O + 2 e- 2Fe(OH)2(s) + 2-0.86 18] OH-TiO2+ + 2 H+ + 4 e- —Ti(s) + H2O -0.862 H2O + 2 e- H2(g) + 2 OH--0.8277 囹Bi(s) + 3 H+ + 3 e- -BiH3-0.8 囱Zn2+ + 2 e- Zn(Hg) -0.7628 囹Zn2+ + 2 e- —Zn(s) -0.7618 囹Ta2O5(s) + 10 H+ + 10 e- 2 Ta(s) + 5 H2O -0.75实用标准文案Half-reaction Cr3+ + 3e- Cr(s)[Au(CN)2]- + e- Au(s) + 2 CN-Ta3+ + 3 e- Ta(s) E° (V)[note 1] Ref. -0.74-0.60-0.6PbO(s) + H2O + 2 e- Pb(s) + 2 OH- -0.58 2 TiO2(s) + 2 H+ + 2 e- -Ti2O3(s) + H2O -0.56Ga3+ + 3 e- Ga(s) U4+ + e- -U3+ -0.53 -0.52 国H3PO2(aq) + H+ + e- —P(white)[note 2] + 2-0.508 囹H2OH3PO3(aq) + 2 H+ + 2 e——H3PO2(aq) +-0.499 囹H2OH3PO3(aq) + 3 H+ + 3 e- P(red)[note 2] + 3-0.454 回H2OFe2+ + 2 e--Fe(s) -0.44 回2CO2(g) + 2H+ + 2e- -HOOCCOOH(aq) -0.43Cr3+ + e- —Cr2+-0.42Cd2+ + 2 e- —Cd(s) -0.40 131 GeO2(s) + 2 H+ + 2 e- GeO(s) + H2O -0.37Cu2O(s) + H2O + 2 e- -2 Cu(s) + 2 OH--0.360 161 PbSO4(s) + 2 e- Pb(s) + SO42--0.3588 囹PbSO4(s) + 2 e--Pb(Hg) + SO42--0.3505 161 Eu3+ + e-「Eu2+-0.35 凶In3+ + 3 e- —In(s) -0.34 191 Tl+ + e- —Tl(s) -0.34 191 Ge(s) + 4 H+ + 4 e- GeH4(g) -0.29C02+ + 2 e- —Co(s) -0.28 161 H3PO4(aq) + 2 H+ + 2 e- H3PO3(aq) + -0.276 161H2OV3+ + e- LV2+-0.26 回Ni2+ + 2e- —Ni(s) -0.25As(s) + 3 H+ + 3 e- AsH3(g) -0.23 倒AgI(s) + e- -Ag(s) + I--0.15224 161 MoO2(s) + 4 H+ + 4 e- Mo(s) + 2 H2O -0.15Si(s) + 4 H+ + 4 e-二SiH4(g) -0.14Sn2+ + 2 e-「Sn(s) -0.13O2(g) + H+ + e- —HO2・(aq) -0.13Pb2+ + 2 e- —Pb(s) -0.13 回WO2(s) + 4 H+ + 4 e- W(s) + 2 H2O -0.12P(red) + 3 H+ + 3 e- —PH3(g) -0.111 161 CO2(g) + 2 H+ + 2 e- HCOOH(aq) -0.11Se(s) + 2H+ + 2e- -H2Se(g) -0.11CO2(g) + 2 H+ + 2 e- CO(g) + H2O -0.11SnO(s) + 2 H+ + 2 e——Sn(s) + H2O -0.10SnO2(s) + 2 H+ + 2 e- SnO(s) + H2O -0.09WO3(aq) + 6 H+ + 6 e--W(s) + 3 H2O -0.09 囹P(white) + 3 H+ + 3 e- PH3(g) -0.063 囹Fe3+ + 3 e- —Fe(s) -0.04 18] HCOOH(aq) + 2 H+ + 2 e- -HCHO(aq) +-0.03H2O2 H+ + 2 e- -H2(g)0.0000三0 AgBr(s) + e- -Ag(s) + Br-+0.07133 囹军乐+ 2e- 2型产+0.08Fe3O4(s) + 8 H+ + 8 e-「3 Fe(s) + 4 H2O +0.085 [10]N2(g) + 2H2O + 6H+ + 6 e- 2NH4OH(aq) +0.092HgO(s) + H2O + 2 e- Hg(l) + 2 OH-+0.0977Cu(NH3)42+ + e- Cu(NH3)2+ + 2 NH3+0.10 囹Ru(NH3)63+ + e- Ru(NH3)62++0.10 凶N2H4(aq) + 4 H2O + 2 e-」2 NH4+ + 4 OH-+0.11 m H2MoO4(aq) + 6 H+ + 6 e- Mo(s) + 4 H2O +0.11Ge4+ + 4 e-=Ge(s) +0.12C(s) + 4 H+ + 4 e- -CH4(g) +0.13 19] HCHO(aq) + 2 H+ + 2 e—-CH30H(aq) +0.13S(s) + 2 H+ + 2 e- —H2s(g) +0.14Sn4+ + 2 e- Sn2++0.15Cu2+ + e- -Cu++0.159 囹HSO4- + 3 H+ + 2 e- —SO2(aq) + 2 H2O +0.16实用标准文案Half-reaction E° (V)[note 1]Ref. UO22+ + e- UO2++0.163 [5] SO42- + 4 H+ + 2 e-,SO2(aq) + 2 H2O +0.17TiO2+ + 2 H+ + e- —Ti3+ + H2O +0.19SbO+ + 2 H+ + 3 e- Sb(s) + H2O +0.20AgCl(s) + e--Ag(s) + Cl-+0.22233 囱H3AsO3(aq) + 3 H+ + 3 e- As(s) + 3 H2O +0.24GeO(s) + 2 H+ + 2 e-」Ge(s) + H2O +0.26UO2+ + 4 H+ + e- -U4+ + 2 H2O +0.273 凶Re3+ + 3 e- —Re(s) +0.300Bi3+ + 3 e- —Bi(s) +0.308 [6] VO2+ + 2 H+ + e- V3+ + H2O +0.34Cu2+ + 2 e- —Cu(s) +0.340 [9] [Fe(CN)6]3- + e- [Fe(CN)6]4-+0.36精彩文档O 2(g ) + 2 H 2O + 4 e - 4 OH -(aq )+0.40H 2MoO 4 + 6 H + + 3 e - —M03+ + 2 H 2O +0.43CH 3OH(aq ) + 2 H + + 2 e --CH 4(g ) + H 2O +0.50SO 2(aq ) + 4 H + + 4 e - —S(s ) + 2 H 2O +0.50Cu + + e - -Cu(s )+0.520 191CO (g ) + 2 H + + 2 e - C(s ) + H 2O +0.52I 3- + 2 e - -3 I - +0.53 13]I 2(s ) + 2 e - -2 I -+0.54回[AuI 41- + 3 e - —Au(s ) + 4 I -+0.56H 3AsO 4(aq ) + 2H + + 2e - =H 3AsO^aq ) ++0.56H 2O[AuI 21- + e - —Au(s ) + 2 I -+0.58 MnO 4- + 2 H 2O + 3 e -」MnO 2(s ) + 4 OH -+0.59精彩文档实用标准文案Half-reactionE° (V)[note 11Ref.实用标准文案Half-reaction E° (V)[note 11Ref.S2O32 - + 6 H+ + 4 e--2S⑸ + 3 H2O +0.60Fc+ + e- r^Fc(s) +0.641 [iiiH2MoO4(aq) + 2 H+ + 2 e——MoO2(s) + 2+0.65 H2O口+ 2 H+ + 2 e-」।+0.6992 囱O2(g) + 2 H+ + 2 e- H2O2(aq) +0.70TL+ + 3 e- —Tl(s) +0.72PtCl62- + 2 e- PtCl42- + 2 Cl-+0.726 15] H2SeO3(aq) + 4 H+ + 4 e- —Se(s) + 3 H2O +0.74PtCl42- + 2 e- -Pt(s) + 4 Cl-+0.758 15] Fe3+ + e- -Fe2++0.77Ag+ + e- —Ag(s) +0.7996 囹Hg22+ + 2 e- —2 Hg(l) +0.80精彩文档NO 3二(aq ) + 2H + + e _」NO 2(g ) + H 2O +0.80 2FeO 42- + 5 H 2O + 6 e - Fe 2O 3(s ) + 10 OH+0.81is1[AuBr 41- + 3 e - -Au(s ) + 4 Br - +0.85Hg 2+ + 2 e - Hg(l ) +0.85MnO 4- + H + + e - -HMnO 4- +0.902 Hg 2+ + 2 e - —Hg 22+ +0.91[91Pd 2+ + 2 e - —Pd(s )+0.915[51[AuCl 41- + 3 e - Au(s ) + 4 Cl -+0.93MnO 2(s ) + 4 H + + e - —Mn 3+ + 2 H 2O +0.95[AuBr 21- + e - -Au(s ) + 2 Br -+0.96[HXeO 6]3- + 2 H 2O + 2 e - + —[HXeO 41- + 4 +0.991121OH -H 6TeO 6(aq ) + 2 H + + 2 e - TeO 2(s ) + 4 H 2O+1.02 但精彩文档实用标准文案Half-reactionE° (V)[note 11Ref.Br2(l) + 2e- -2 Br-+ 1.066 囹Br2(aq) + 2 e-「2 Br-+ 1.0873 [6] IO3- + 5 H+ + 4 e- —HIO(aq) + 2 H2O + 1.13[AuCl2]- + e- —Au(s) + 2 Cl-+ 1.15HSeO4- + 3 H+ + 2 e- -H2SeO3(aq) + H2O + 1.15Ag2O(s) + 2 H+ + 2 e- —2 Ag(s) + H2O + 1.17ClO3- + 2 H+ + e- —ClO2(g) + H2O + 1.18[HXeO6]3- + 5 H2O + 8 e- -Xe(g) + 11 OH-+ 1.18 [121 Pt2+ + 2 e- —Pt(s) + 1.188 [5] ClO2(g) + H+ + e- —HClO2(aq) + 1.192 IO3- + 12 H+ + 10 e-」I2(s) + 6 H2O + 1.20ClO4- + 2 H+ + 2 e- —ClO3- + H2O + 1.20O2(g) + 4 H+ + 4 e- 2 H2O+ 1.229 回精彩文档MnO2(s) + 4H+ + 2e-,Mn2+ + 2 H2O + 1.23[HXeO4]- + 3 H2O + 6 e- —Xe(g) + 7 OH-+ 1.24 [12] Tl3+ + 2 e- -Tl++ 1.25Cr2O72- + 14 H+ + 6 e- —2 Cr3+ + 7 H2O + 1.33Cl2(g) + 2 e- -2 Cl-+ 1.36 回CoO2(s) + 4 H+ + e- —C03+ + 2 H2O + 1.422 NH3O H+ + H+ + 2 e—-N2H5+ + 2 H2O + 1.42 m2 HIO(aq) + 2 H+ + 2 e- I2(s) + 2 H2O + 1.44Ce4+ + e—-Ce3++ 1.44BrO3- + 5 H+ + 4 e——HBrO(aq) + 2 H2O + 1.45p-PbO2(s) + 4 H+ + 2 e- Pb2+ + 2 H2O + 1.460 囹a-PbO2(s) + 4 H+ + 2 e——Pb2+ + 2 H2O + 1.468 囹2 BrO3- + 12 H+ + 10 e——Br2(l) + 6 H2O + 1.48精彩文档实用标准文案Half-reaction E° (V) [note 11Ref. 2C1O3- + 12 H+ + 10e-」Cl2(g) + 6 H2O +1.49MnO4- + 8 H+ + 5 e- —Mn2+ + 4 H2O +1.51HO2. + H+ + e- —H2O2(aq) +1.51Au3+ + 3 e- —Au(s) +1.52NiO2(s) + 4 H+ + 2 e--Ni2+ + 2 OH- +1.592 HClO(aq) + 2 H+ + 2 e- Cl2(g) + 2 H2O +1.63Ag2O3(s) + 6 H+ + 4 e——2 Ag+ + 3 H2O +1.67HClO2(aq) + 2 H+ + 2 e- HClO(aq) + H2O +1.67Pb4+ + 2 e--Pb2+ +1.69MnO4二+ 4 H+ + 3 e——MnO2(s) + 2 H2O +1.70AgO(s) + 2 H+ + e- Ag+ + H2O +1.77H2O2(aq) + 2 H+ + 2 e- —2 H2O +1.78C03+ + e——C02+ +1.82精彩文档实用标准文案Half-reaction E° (V)[note 11Ref.Au+ + e——Au⑸+ 1.83 19] BrO4- + 2 H+ + 2e- BrO3- + H2O + 1.85Ag2+ + e- —Ag++ 1.98 囹S208M + 2 e- 2 SO42-+2.010 囹03(g) + 2 H+ + 2 e--O2(g) + H2O +2.075 15]HMnO4- + 3 H+ + 2 e——MnO2(s) + 2 H2O +2.09XeO3(aq) + 6 H+ + 6 e- Xe(g) + 3 H2O +2.12 [121 H4XeO6(aq) + 8 H+ + 8 e- -Xe(g) + 6 H2O +2.18 U2] FeO42- + 3 e- + 8 H+ —Fe3+ + 4 H2O +2.20 [141 XeF2(aq) + 2 H+ + 2 e- —Xe(g) + 2HF(aq) +2.32 [121 H4XeO6(aq) + 2 H+ + 2 e- XeO3(aq) + H2O +2.42 [121 F2(g) + 2 e- -2 F-+2.87 [91[31 F2(g) + 2 H+ + 2 e- 2 HF(aq) + 3.05 回精彩文档实用标准文案. 忡!1-11 . "ft #.沽油老油It广精彩文档实用标准文案额定电缶220V额定顺率SOH E甑定瑞率900 W内胆容积J50WL霰大装才:里150-270ML啧气舸闻均喷汽可达1。

标准氧化还原电势表氧化还原电势是描述化学反应中电子转移能力的重要物理量,它对于理解和预测化学反应的进行具有重要意义。
标准氧化还原电势表是一张记录各种物质在标准状态下的氧化还原电势的重要参考资料,它为化学研究和工业生产提供了重要的数据支持。
本文将对标准氧化还原电势表进行详细介绍,希望能为广大读者提供有益的参考信息。
标准氧化还原电势是指在标准状态下,物质发生氧化还原反应时的电势差。
标准状态是指溶液中物质的浓度为1mol/L,气体的压强为1atm,温度为25摄氏度的状态。
标准氧化还原电势表记录了各种物质在标准状态下的氧化还原电势值,通常以标准氢电极为参比电极,将其电势值定义为0。
其他物质的氧化还原电势值相对于标准氢电极进行测量和记录。
标准氧化还原电势表按照物质的性质和电位值进行排列,一般以正负号表示氧化还原性质,电位值越正表示物质越容易发生还原反应,电位值越负表示物质越容易发生氧化反应。
通过标准氧化还原电势表,可以了解各种物质的氧化还原性质,预测化学反应的进行,设计和优化电化学反应过程。
标准氧化还原电势表的应用非常广泛,它在化学研究、工业生产和环境监测等领域都具有重要作用。
在化学研究中,科学家可以通过标准氧化还原电势表预测不同物质之间的氧化还原反应,设计实验方案,探索新的化学反应途径。
在工业生产中,标准氧化还原电势表可以帮助工程师优化电化学反应过程,提高生产效率,降低能耗成本。
在环境监测中,标准氧化还原电势表可以用于分析水体、大气和土壤中的氧化还原反应,评估环境污染程度,制定环境保护措施。
总之,标准氧化还原电势表是化学领域中一份重要的参考资料,它记录了各种物质在标准状态下的氧化还原电势值,为化学研究和工业生产提供了重要的数据支持。
通过深入了解和应用标准氧化还原电势表,我们可以更好地理解和预测化学反应的进行,推动化学领域的发展和应用。
希望本文对广大读者有所帮助,谢谢阅读!。
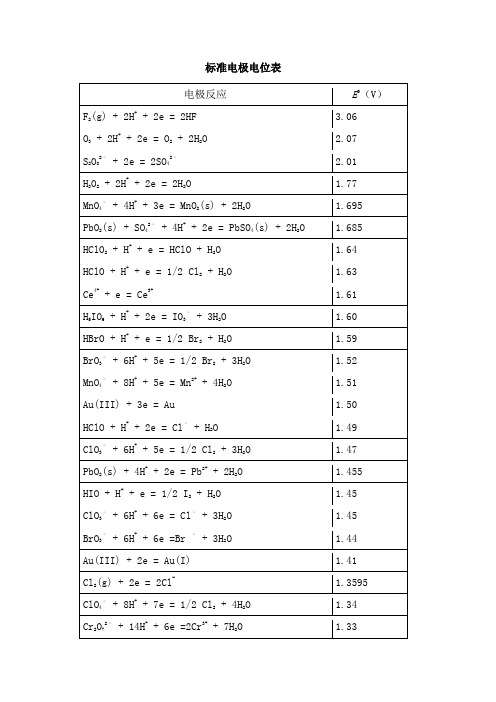
标准电极电位表 电极反应E 0(V )F 2(g) + 2H ++ 2e = 2HF 3.06 O 3 + 2H ++ 2e = O 2 + 2H 2O 2.07 S 2O 82– + 2e = 2SO 42– 2.01 H 2O 2 + 2H + + 2e = 2H 2O1.77 MnO 4– + 4H + + 3e = MnO 2(s) + 2H 2O1.695 PbO 2(s) + SO 42– + 4H + + 2e = PbSO 4(s) + 2H 2O 1.685 HClO 2 + H + + e = HClO + H 2O 1.64 HClO + H + + e = 1/2 Cl 2 + H 2O 1.63 Ce 4+ + e = Ce 3+1.61 H 5IO 6 + H + + 2e = IO 3– + 3H 2O 1.60 HBrO + H + + e = 1/2 Br 2 + H 2O 1.59 BrO 3– + 6H + + 5e = 1/2 Br 2 + 3H 2O 1.52 MnO 4– + 8H + + 5e = Mn 2+ + 4H 2O 1.51 Au(III) + 3e = Au1.50 HClO + H + + 2e = Cl – + H 2O 1.49 ClO 3– + 6H + + 5e = 1/2 Cl 2 + 3H 2O 1.47 PbO 2(s) + 4H + + 2e = Pb 2+ + 2H 2O 1.455 HIO + H + + e = 1/2 I 2 + H 2O 1.45 ClO 3–+ 6H ++ 6e = Cl –+ 3H 2O 1.45 BrO 3– + 6H + + 6e =Br – + 3H 2O 1.44 Au(III) + 2e = Au(I) 1.41 Cl 2(g) + 2e = 2Cl -1.3595 ClO 4– + 8H + + 7e = 1/2 Cl 2 + 4H 2O 1.34 Cr 2O 72– + 14H + + 6e =2Cr 3+ + 7H 2O1.33MnO 2(s) + 4H + + 2e = Mn 2+ + 2H 2O 1.23 O 2(g) + 4H ++ 4e = 2H 2O 1.229 IO 3– + 6H + + 5e = 1/2 I 2 + 3H 2O 1.20 ClO 4–+ 2H ++ 2e = ClO 3–+ H 2O 1.19 Br 2(aq) + 2e = 2Br – 1.087 NO 2 + H + + e = HNO 2 1.07 Br 3– + 2e = 3Br –1.05 HNO 2 + H + + e = NO(g) + H 2O 1.00 VO 2+ + 2H + + e = VO 2+ + H 2O 1.00 HIO + H + + 2e = I – + H 2O 0.99 NO 3– + 3H + + 2e = HNO 2 + H 2O 0.94 ClO – + H 2O + 2e = Cl – + 2OH – 0.89 H 2O 2 + 2e = 2OH – 0.88 Cu 2+ + I – + e = CuI(s) 0.86 Hg 2+ + 2e = Hg0.845 NO 3– + 2H + + e = NO 2 + H 2O 0.80 Ag + + e = Ag 0.7995 Hg 22+ + 2e = 2Hg 0.793 Fe 3+ + e = Fe 2+0.771 BrO – + H 2O + 2e = Br – + 2OH – 0.76 O 2(g) + 2H + + 2e = H 2O 2 0.682 AsO 8– + 2H 2O + 3e = As + 4OH – 0.68 2HgCl 2 + 2e = Hg 2Cl 2(s) + 2Cl – 0.63 Hg 2SO 4(s) + 2e = 2Hg + SO 42– 0.6151 MnO 4– + 2H 2O + 3e = MnO 2 + 4OH – 0.588 MnO 4– + e = MnO 42–0.564H 3AsO 4 + 2H + + 2e = HAsO 2 + 2H 2O 0.559 I 3–+ 2e = 3I –0.545 I 2(s) + 2e = 2I – 0.5345 Mo(VI) + e = Mo(V) 0.53 Cu + + e = Cu0.52 4SO 2(aq) + 4H + + 6e = S 4O 62– + 2H 2O 0.51 HgCI 42– + 2e = Hg + 4Cl –0.48 2SO 2(aq) + 2H + + 4e = S 2O 32– + H 2O 0.40 Fe(CN)63– + e = Fe(CN)64– 0.36 Cu 2+ + 2e = Cu0.337 VO 2+ + 2H + + 2e = V 3+ + H 2O 0.337 BiO + + 2H + + 3e = Bi + H 2O 0.32 Hg 2CI 2(s)+ 2e = 2Hg + 2Cl – 0.2676 HAsO 2 + 3H + + 3e = As + 2H 2O 0.248 AgCI(s)+ e = Ag + Cl – 0.2223 SbO + + 2H + + 3e = Sb + H 2O 0.212 SO 42– + 4H + + 2e = SO 2(aq) + H 2O 0.17 Cu 2+ + e = Cu – 0.519 Sn 4+ + 2e = Sn 2+ 0.154 S + 2H + + 2e = H 2S(g) 0.141 Hg 2Br 2 + 2e = 2Hg + 2Br – 0.1395 TiO 2+ + 2H + + e = Ti 3+ + H 2O 0.1 S 4O 62– + 2e = 2S 2O 32– 0.08 AgBr(s) + e = Ag + Br – 0.071 2H + + 2e = H 20.000 O 2 + H 2O + 2e = HO 2– + OH –-0.067TiOCl + + 2H + + 3Cl – + e = TiCl 4– + H 2O -0.09 Pb 2++ 2e = Pb -0.126 Sn 2+ + 2e = Sn -0.136 AgI(s) + e = Ag + I –-0.152 Ni 2+ + 2e = Ni-0.246 H 3PO 4 + 2H + + 2e = H 3PO 3 + H 2O -0.276 Co 2+ + 2e = Co -0.277 Tl + + e = Tl -0.3360 In 3+ + 3e = In-0.345 PbSO 4(s) + 2e = Pb + SO 42– 0.3553 SeO 32– + 3H 2O + 4e = Se + 6OH – -0.366 As + 3H + + 3e = AsH 3 -0.38 Se + 2H + + 2e = H 2Se -0.40 Cd 2+ + 2e = Cd -0.403 Cr 3+ + e = Cr 2+ -0.41 Fe 2+ + 2e = Fe -0.440 S + 2e = S 2–-0.48 2CO 2 + 2H + + 2e = H 2C 2O 4 -0.49 H 3PO 3 + 2H + + 2e = H 3PO 2 + H 2O -0.50 Sb + 3H + + 3e = SbH 3-0.51 HPbO 2– + H 2O + 2e = Pb + 3OH – -0.54 Ga 3+ + 3e = Ga-0.56 TeO 32– + 3H 2O + 4e = Te + 6OH – -0.57 2SO 32– + 3H 2O + 4e = S 2O 32– + 6OH – -0.58 SO 32– + 3H 2O + 4e = S + 6OH – -0.66 AsO 43– + 2H 2O + 2e = AsO 2– + 4OH –-0.67Ag 2S(s) + 2e = 2Ag + S 2– -0.69 Cr 3++ 3e = Cr -0.744 Zn 2+ + 2e = Zn -0.7628 2H 2O + 2e = H 2 + 2OH –-8.28 Cr 2+ + 2e = Cr-0.91 HSnO 2– + H 2O + 2e = Sn – + 3OH – ->0.91 Se + 2e = Se 2–-0.92 Sn(OH)62– + 2e = HSnO 2– + H 2O + 3OH – -0.93 CNO – + H 2O + 2e = Cn – + 2OH – -0.97 WO 42- + 4H 2O + 6e = W + 8OH - -1.05 Mn 2+ + 2e = Mn -1.182 V 2+ + 2e = V-1.186 ZnO 22– + 2H 2O + 2e = Zn + 4OH – -1.216 Zr 4+ + 4e = Zr -1.529 Ti 2+ + 2e = Ti -1.628 Al 3+ + 3e = Al-1.662 HfO 2 + 4H + + 4e = Hf + 2H 2O -1.7 Be 2+ + 2e = Be-1.847 H 2AlO 3– + H 2O + 3e = Al + 4OH – -2.35 Mg 2+ + 2e = Mg -2.37 La 3+ + 3e = La -2.522 Na + + e = Na -2.714 Ca 2+ + 2e = Ca -2.866 Sr 2+ + 2e = Sr -2.89 Ba 2+ + 2e = Ba -2.906 Ra 2+ + 2e = Ra-2.916。


Standard electrode potential (data page)From Wikipedia, the free encyclopedia(Redirected from Table of standard electrode potentials)Jump to: navigation, searchMain article: standard electrode potentialThe values of standard electrode potentials are given in the table below in volts relative to the standard hydrogen electrode and are for the following conditions:• A temperature of 298.15 K (25 °C);•An effective concentration of 1 mol/L for each aqueous species ora species in a mercury amalgam;• A partial pressure of 101.325 kPa (absolute) (1 atm, 1.01325 bar) for each gaseous reagent. This pressure is used because mostliterature data are still given for this value rather than for thecurrent standard of 100 kPa.•An activity of unity for each pure solid, pure liquid, or for water (solvent).Legend: (s) –solid; (l) –liquid; (g) –gas; (aq) –aqueous (default for all charged species); (Hg) –amalgam.Half-reaction E°(V)[note 1]Ref.3⁄2 N2(g) + H+ + e−HN3(aq) −3.09 [1][2]Li+ + e−Li(s) −3.0401 [2]N2(g) + 4 H2O + 2 e−2 NH2OH(aq) + 2 OH−−3.04 [1]Cs+ + e−Cs(s) −3.026 [2]Rb+ + e−Rb(s) −2.98 [2]K+ + e−K(s) −2.931 [2]Ba2+ + 2 e−Ba(s) −2.912 [2]La(OH)3(s) + 3 e−La(s) + 3 OH−−2.90 [2]Sr2+ + 2 e−Sr(s) −2.899 [2]Ca2+ + 2 e−Ca(s) −2.868 [2]Half-reaction E°(V)[note 1]Ref. Eu2+ + 2 e−Eu(s) −2.812 [2] Ra2+ + 2 e−Ra(s) −2.8 [2] Na+ + e−Na(s) −2.71 [2][3] Sc3+ + 3 e−Sc(s) −2.077 [4] La3+ + 3 e−La(s) −2.379 [2]Y3+ + 3 e−Y(s) −2.372 [2] Mg2+ + 2 e−Mg(s) −2.372 [2] ZrO(OH)2(s) + H2O + 4 e−Zr(s) + 4 OH−−2.36 [2] Al(OH)4−+ 3 e−Al(s) + 4 OH−−2.33Al(OH)3(s) + 3 e−Al(s) + 3 OH−−2.31H2(g) + 2 e−2 H−−2.25Ac3+ + 3 e−Ac(s) −2.20Be2+ + 2 e−Be(s) −1.85Half-reaction E°(V)[note 1]Ref. U3+ + 3 e−U(s) −1.66 [5] Al3+ + 3 e−Al(s) −1.66 [3]Ti2+ + 2 e−Ti(s) −1.63 [3] ZrO2(s) + 4 H+ + 4 e−Zr(s) + 2 H2O −1.553 [6] Zr4+ + 4 e−Zr(s) −1.45 [6]Ti3+ + 3 e−Ti(s) −1.37 [7] TiO(s) + 2 H+ + 2 e−Ti(s) + H2O −1.31Ti2O3(s) + 2 H+ + 2 e−2 TiO(s) + H2O −1.23Zn(OH)42−+ 2 e−Zn(s) + 4 OH−−1.199 [6] Mn2+ + 2 e−Mn(s) −1.185 [6] Fe(CN)64−+ 6 H+ + 2 e−Fe(s) + 6HCN(aq) −1.16 [8] Te(s) + 2 e−Te2−−1.143 [9]V2+ + 2 e−V(s) −1.13 [9]Half-reaction E°(V)[note 1]Ref.Nb3+ + 3 e−Nb(s) −1.099Sn(s) + 4 H+ + 4 e−SnH4(g) −1.07SiO2(s) + 4 H+ + 4 e−Si(s) + 2 H2O −0.91B(OH)3(aq) + 3 H+ + 3 e−B(s) + 3 H2O −0.89Fe(OH)2(s) + 2 e−Fe(s) + 2 OH−−0.89 [8]Fe2O3(s) + 3 H2O + 2 e−2Fe(OH)2(s) + 2 −0.86 [8] OH−TiO2+ + 2 H+ + 4 e−Ti(s) + H2O −0.862 H2O + 2 e−H2(g) + 2 OH−−0.8277 [6]Bi(s) + 3 H+ + 3 e−BiH3−0.8 [6]Zn2+ + 2 e−Zn(Hg) −0.7628 [6]Zn2+ + 2 e−Zn(s) −0.7618 [6]Ta2O5(s) + 10 H+ + 10 e−2 Ta(s) + 5 H2O −0.75Half-reaction E°(V)[note 1]Ref. Cr3+ + 3 e−Cr(s) −0.74[Au(CN)2]−+ e−Au(s) + 2 CN−−0.60Ta3+ + 3 e−Ta(s) −0.6PbO(s) + H2O + 2 e−Pb(s) + 2 OH−−0.582 TiO2(s) + 2 H+ + 2 e−Ti2O3(s) + H2O −0.56Ga3+ + 3 e−Ga(s) −0.53U4+ + e−U3+−0.52 [5]H3PO2(aq) + H+ + e−P(white)[note 2] + 2 −0.508 [6]H2OH3PO3(aq) + 2 H+ + 2 e−H3PO2(aq) + −0.499 [6]H2OH3PO3(aq) + 3 H+ + 3 e−P(red)[note 2] + 3 −0.454 [6]H2OFe2+ + 2 e−Fe(s) −0.44 [3]Half-reaction E°(V)[note 1]Ref. 2 CO2(g) + 2 H+ + 2 e−HOOCCOOH(aq) −0.43Cr3+ + e−Cr2+−0.42Cd2+ + 2 e−Cd(s) −0.40 [3] GeO2(s) + 2 H+ + 2 e−GeO(s) + H2O −0.37Cu2O(s) + H2O + 2 e−2 Cu(s) + 2 OH−−0.360 [6] PbSO4(s) + 2 e−Pb(s) + SO42−−0.3588 [6] PbSO4(s) + 2 e−Pb(Hg) + SO42−−0.3505 [6] Eu3+ + e−Eu2+−0.35 [5]In3+ + 3 e−In(s) −0.34 [9]Tl+ + e−Tl(s) −0.34 [9] Ge(s) + 4 H+ + 4 e−GeH4(g) −0.29Co2+ + 2 e−Co(s) −0.28 [6]H3PO4(aq) + 2 H+ + 2 e−H3PO3(aq) + −0.276 [6]Half-reaction E°(V)[note 1]Ref. H2OV3+ + e−V2+−0.26 [3] Ni2+ + 2 e−Ni(s) −0.25As(s) + 3 H+ + 3 e−AsH3(g) −0.23 [9] AgI(s) + e−Ag(s) + I−−0.15224 [6] MoO2(s) + 4 H+ + 4 e−Mo(s) + 2 H2O −0.15Si(s) + 4 H+ + 4 e−SiH4(g) −0.14Sn2+ + 2 e−Sn(s) −0.13O2(g) + H+ + e−HO2•(aq) −0.13Pb2+ + 2 e−Pb(s) −0.13 [3] WO2(s) + 4 H+ + 4 e−W(s) + 2 H2O −0.12P(red) + 3 H+ + 3 e−PH3(g) −0.111 [6] CO2(g) + 2 H+ + 2 e−HCOOH(aq) −0.11Half-reaction E°(V)[note 1]Ref. Se(s) + 2 H+ + 2 e−H2Se(g) −0.11CO2(g) + 2 H+ + 2 e−CO(g) + H2O −0.11SnO(s) + 2 H+ + 2 e−Sn(s) + H2O −0.10SnO2(s) + 2 H+ + 2 e−SnO(s) + H2O −0.09WO3(aq) + 6 H+ + 6 e−W(s) + 3 H2O −0.09 [9]P(white) + 3 H+ + 3 e−PH3(g) −0.063 [6] Fe3+ + 3 e−Fe(s) −0.04 [8] HCOOH(aq) + 2 H+ + 2 e−HCHO(aq) + −0.03H2O2 H+ + 2 e−H2(g) 0.0000 ≡0 AgBr(s) + e−Ag(s) + Br−+0.07133 [6]S4O62−+ 2 e−2 S2O32−+0.08Fe3O4(s) + 8 H+ + 8 e−3 Fe(s) + 4 H2O +0.085 [10]Half-reaction E°(V)[note 1]Ref. N2(g) + 2 H2O + 6 H+ + 6 e−2 NH4OH(aq) +0.092HgO(s) + H2O + 2 e−Hg(l) + 2 OH−+0.0977Cu(NH3)42+ + e−Cu(NH3)2+ + 2 NH3+0.10 [9] Ru(NH3)63+ + e−Ru(NH3)62++0.10 [5]N2H4(aq) + 4 H2O + 2 e−2 NH4+ + 4 OH−+0.11 [1]H2MoO4(aq) + 6 H+ + 6 e−Mo(s) + 4 H2O +0.11Ge4+ + 4 e−Ge(s) +0.12C(s) + 4 H+ + 4 e−CH4(g) +0.13 [9] HCHO(aq) + 2 H+ + 2 e−CH3OH(aq) +0.13S(s) + 2 H+ + 2 e−H2S(g) +0.14Sn4+ + 2 e−Sn2++0.15Cu2+ + e−Cu++0.159 [9] HSO4−+ 3 H+ + 2 e−SO2(aq) + 2 H2O +0.16Half-reaction E°(V)[note 1]Ref. UO22+ + e−UO2++0.163 [5] SO42−+ 4 H+ + 2 e−SO2(aq) + 2 H2O +0.17TiO2+ + 2 H+ + e−Ti3+ + H2O +0.19SbO+ + 2 H+ + 3 e−Sb(s) + H2O +0.20AgCl(s) + e−Ag(s) + Cl−+0.22233 [6]H3AsO3(aq) + 3 H+ + 3 e−As(s) + 3 H2O +0.24GeO(s) + 2 H+ + 2 e−Ge(s) + H2O +0.26UO2+ + 4 H+ + e−U4+ + 2 H2O +0.273 [5] Re3+ + 3 e−Re(s) +0.300Bi3+ + 3 e−Bi(s) +0.308 [6] VO2+ + 2 H+ + e−V3+ + H2O +0.34Cu2+ + 2 e−Cu(s) +0.340 [9] [Fe(CN)6]3−+ e−[Fe(CN)6]4−+0.36Half-reaction E°(V)[note 1]Ref. O2(g) + 2 H2O + 4 e−4 OH−(aq) +0.40 [3]H2MoO4 + 6 H+ + 3 e−Mo3+ + 2 H2O +0.43CH3OH(aq) + 2 H+ + 2 e−CH4(g) + H2O +0.50SO2(aq) + 4 H+ + 4 e−S(s) + 2 H2O +0.50Cu+ + e−Cu(s) +0.520 [9] CO(g) + 2 H+ + 2 e−C(s) + H2O +0.52I3−+ 2 e−3 I−+0.53 [3]I2(s) + 2 e−2 I−+0.54 [3] [AuI4]−+ 3 e−Au(s) + 4 I−+0.56H3AsO4(aq) + 2 H+ + 2 e−H3AsO3(aq) + +0.56H2O[AuI2]−+ e−Au(s) + 2 I−+0.58MnO4−+ 2 H2O + 3 e−MnO2(s) + 4 OH−+0.59Half-reaction E°(V)[note 1]Ref. S2O32 −+ 6 H+ + 4 e−2 S(s) + 3 H2O +0.60Fc+ + e−Fc(s) +0.641 [11] H2MoO4(aq) + 2 H+ + 2 e−MoO2(s) + 2 +0.65H2O+0.6992 [6] + 2 H+ + 2 e−O2(g) + 2 H+ + 2 e− H2O2(aq) +0.70Tl3+ + 3 e−Tl(s) +0.72PtCl62−+ 2 e−PtCl42−+ 2 Cl−+0.726 [5]H2SeO3(aq) + 4 H+ + 4 e−Se(s) + 3 H2O +0.74PtCl42−+ 2 e−Pt(s) + 4 Cl−+0.758 [5] Fe3+ + e−Fe2++0.77Ag+ + e−Ag(s) +0.7996 [6] Hg22+ + 2 e−2 Hg(l) +0.80Half-reaction E°(V)[note 1]Ref. NO3−(aq) + 2 H+ + e−NO2(g) + H2O +0.802FeO42−+ 5 H2O + 6 e−Fe2O3(s) + 10 OH+0.81 [8]−[AuBr4]−+ 3 e−Au(s) + 4 Br−+0.85Hg2+ + 2 e−Hg(l) +0.85MnO4−+ H+ + e−HMnO4−+0.902 Hg2+ + 2 e−Hg22++0.91 [9] Pd2+ + 2 e−Pd(s) +0.915 [5] [AuCl4]−+ 3 e−Au(s) + 4 Cl−+0.93MnO2(s) + 4 H+ + e−Mn3+ + 2 H2O +0.95[AuBr2]−+ e−Au(s) + 2 Br−+0.96[HXeO6]3−+ 2 H2O + 2 e−+ [HXeO4]−+ 4+0.99 [12] OH−H6TeO6(aq) + 2 H+ + 2 e−TeO2(s) + 4 H2O +1.02 [13]Half-reaction E°(V)[note 1]Ref. Br2(l) + 2 e−2 Br−+1.066 [6] Br2(aq) + 2 e−2 Br−+1.0873 [6] IO3−+ 5 H+ + 4 e−HIO(aq) + 2 H2O +1.13[AuCl2]−+ e−Au(s) + 2 Cl−+1.15HSeO4−+ 3 H+ + 2 e−H2SeO3(aq) + H2O +1.15Ag2O(s) + 2 H+ + 2 e−2 Ag(s) + H2O +1.17ClO3−+ 2 H+ + e−ClO2(g) + H2O +1.18[HXeO6]3−+ 5 H2O + 8 e−Xe(g) + 11 OH−+1.18 [12] Pt2+ + 2 e−Pt(s) +1.188 [5] ClO2(g) + H+ + e−HClO2(aq) +1.192 IO3−+ 12 H+ + 10 e−I2(s) + 6 H2O +1.20ClO4−+ 2 H+ + 2 e−ClO3−+ H2O +1.20O2(g) + 4 H+ + 4 e−2 H2O+1.229 [3]Half-reaction E°(V)[note 1]Ref. MnO2(s) + 4 H+ + 2 e−Mn2+ + 2 H2O +1.23[HXeO4]−+ 3 H2O + 6 e−Xe(g) + 7 OH−+1.24 [12] Tl3+ + 2 e−Tl++1.25Cr2O72−+ 14 H+ + 6 e−2 Cr3+ + 7 H2O +1.33Cl2(g) + 2 e−2 Cl−+1.36 [3] CoO2(s) + 4 H+ + e−Co3+ + 2 H2O +1.422 NH3O H+ + H+ + 2 e−N2H5+ + 2 H2O +1.42 [1]2 HIO(aq) + 2 H+ + 2 e−I2(s) + 2 H2O +1.44Ce4+ + e−Ce3++1.44BrO3−+ 5 H+ + 4 e−HBrO(aq) + 2 H2O +1.45β-PbO2(s) + 4 H+ + 2 e−Pb2+ + 2 H2O +1.460 [9]α-PbO2(s) + 4 H+ + 2 e−Pb2+ + 2 H2O +1.468 [9]2 BrO3−+ 12 H+ + 10 e−Br2(l) + 6 H2O +1.48Half-reaction E°(V)[note 1]Ref. 2ClO3−+ 12 H+ + 10 e−Cl2(g) + 6 H2O +1.49MnO4−+ 8 H+ + 5 e−Mn2+ + 4 H2O +1.51HO2•+ H+ + e− H2O2(aq) +1.51Au3+ + 3 e−Au(s) +1.52NiO2(s) + 4 H+ + 2 e−Ni2+ + 2 OH−+1.592 HClO(aq) + 2 H+ + 2 e−Cl2(g) + 2 H2O +1.63Ag2O3(s) + 6 H+ + 4 e−2 Ag+ + 3 H2O +1.67HClO2(aq) + 2 H+ + 2 e−HClO(aq) + H2O +1.67Pb4+ + 2 e−Pb2++1.69 [9] MnO4−+ 4 H+ + 3 e−MnO2(s) + 2 H2O +1.70AgO(s) + 2 H+ + e−Ag+ + H2O +1.77 H2O2(aq) + 2 H+ + 2 e−2 H2O +1.78Co3+ + e−Co2++1.82Half-reaction E°(V)[note 1]Ref. Au+ + e−Au(s) +1.83 [9] BrO4−+ 2 H+ + 2 e−BrO3−+ H2O +1.85Ag2+ + e−Ag++1.98 [9]S2O82−+ 2 e−2 SO42−+2.010 [6]O3(g) + 2 H+ + 2 e−O2(g) + H2O +2.075 [5] HMnO4−+ 3 H+ + 2 e−MnO2(s) + 2 H2O +2.09XeO3(aq) + 6 H+ + 6 e−Xe(g) + 3 H2O +2.12 [12] H4XeO6(aq) + 8 H+ + 8 e−Xe(g) + 6 H2O +2.18 [12] FeO42−+ 3 e−+ 8 H+Fe3+ + 4 H2O +2.20 [14] XeF2(aq) + 2 H+ + 2 e−Xe(g) + 2HF(aq) +2.32 [12] H4XeO6(aq) + 2 H+ + 2 e−XeO3(aq) + H2O +2.42 [12] F2(g) + 2 e−2 F−+2.87 [9][3] F2(g) + 2 H+ + 2 e−2 HF(aq) +3.05 [9]实用标准文案精彩文档。
标准氧化还原电势表标准氧化还原电势表。
氧化还原电势是描述氧化还原反应进行方向和程度的物理量,通常用E表示。
在标准状态下,氧化还原电势的测定是指在标准气压下,溶液中各离子的活度均为1的情况下,电极与标准氢电极之间的电势差。
标准氧化还原电势表是根据实验测定得到的一张表格,记录了各种物质在标准状态下的氧化还原电势数值。
标准氧化还原电势表的编制是通过实验测定得到的。
实验测定氧化还原电势的方法有很多种,其中比较常用的是电动势法和电位滴定法。
通过这些实验方法,可以得到不同物质在标准状态下的氧化还原电势数值,然后整理成一张表格,就是标准氧化还原电势表。
标准氧化还原电势表的作用主要有两个方面。
首先,它可以帮助我们了解物质的氧化还原性质。
通过标准氧化还原电势表,我们可以知道不同物质的氧化还原电势数值,从而判断它们的氧化还原性质。
其次,标准氧化还原电势表也可以用来预测氧化还原反应的进行方向和程度。
根据反应物和生成物在标准氧化还原电势表中的位置,可以判断氧化还原反应是进行的方向和程度。
标准氧化还原电势表中的数值是以标准氢电极为基准的。
标准氢电极的氧化还原电势被定义为0,其他物质的氧化还原电势则是相对于标准氢电极的。
在标准氧化还原电势表中,数值为正的表示具有氧化性,数值越大,氧化性越强;数值为负的表示具有还原性,数值越小,还原性越强。
除了标准氧化还原电势表,还有一些其他氧化还原电势表,如非标准状态下的氧化还原电势表和pH值对氧化还原电势的影响等。
这些电势表在特定条件下也具有重要的应用价值。
总之,标准氧化还原电势表是描述物质氧化还原性质的重要工具,它通过实验测定得到不同物质在标准状态下的氧化还原电势数值,并记录在表格中。
标准氧化还原电势表的编制和应用对于化学研究和工业生产都具有重要的意义。
通过对标准氧化还原电势表的研究和应用,可以更好地理解和预测氧化还原反应的进行方向和程度,为相关领域的研究和实践提供重要参考依据。
Standard electrode potential (data page)From Wikipedia, the free encyclopedia(Redirected from Table of standard electrode potentials)Jump to: navigation, searchMain article: standard electrode potentialThe values of standard electrode potentials are given in the table below in volts relative to the standard hydrogen electrode and are for the following conditions:• A temperature of 298.15 K (25 °C);•An effective concentration of 1 mol/L for each aqueous species or a species in a mercury amalgam;• A partial pressure of 101.325 kPa (absolute) (1 atm, 1.01325 bar) for each gaseous reagent. This pressure is used because most literature data are stillgiven for this value rather than for the current standard of 100 kPa.•An activity of unity for each pure solid, pure liquid, or for water (solvent). Legend: (s) – solid; (l) – liquid; (g) – gas; (aq) – aqueous (default for all charged species); (Hg) –amalgam.Half-reaction E° (V)[note 1]Ref.3⁄2 N2(g) + H++ e−HN3(aq)−3.09[1][2]Li++ e−Li(s)−3.0401[2]N2(g) + 4 H2O + 2 e−2 N H2OH(aq) + 2 O H−−3.04[1]Cs++ e−Cs(s)−3.026[2]Rb++ e−Rb(s)−2.98[2]K++ e−K(s)−2.931[2] Ba2++ 2 e−Ba(s)−2.912[2] La(OH)3(s) + 3 e−La(s) + 3 O H−−2.90[2] Sr2++ 2 e−Sr(s)−2.899[2] Ca2++ 2 e−Ca(s)−2.868[2] Eu2++ 2 e−Eu(s)−2.812[2] Ra2++ 2 e−Ra(s)−2.8[2] Na++ e−Na(s)−2.71[2][3] Sc3++ 3 e−Sc(s)−2.077[4] La3++ 3 e−La(s)−2.379[2] Y3++ 3 e−Y(s)−2.372[2] Mg2++ 2 e−Mg(s)−2.372[2] ZrO(OH)2(s) + H2O + 4 e−Zr(s) + 4 O H−−2.36[2] Al(OH)4−+ 3 e−Al(s) + 4 O H−−2.33Al(OH)3(s) + 3 e−Al(s) + 3 O H−−2.31H2(g) + 2 e−2 H−−2.25Ac3++ 3 e−Ac(s)−2.20Be2++ 2 e−Be(s)−1.85U3++ 3 e−U(s)−1.66[5] Al3++ 3 e−Al(s)−1.66[3]Ti2++ 2 e−Ti(s)−1.63[3]ZrO2(s) + 4 H++ 4 e−Zr(s) + 2 H2O−1.553[6] Zr4++ 4 e−Zr(s)−1.45[6] Ti3++ 3 e−Ti(s)−1.37[7] TiO(s) + 2 H++ 2 e−Ti(s) + H2O−1.31Ti2O3(s) + 2 H++ 2 e−2 T iO(s) + H2O−1.23Zn(OH)42−+ 2 e−Zn(s) + 4 O H−−1.199[6] Mn2++ 2 e−Mn(s)−1.185[6] Fe(CN)64−+ 6 H++ 2 e−Fe(s) + 6HCN(aq)−1.16[8] Te(s) + 2 e−Te2−−1.143[9] V2++ 2 e−V(s)−1.13[9] Nb3++ 3 e−Nb(s)−1.099Sn(s) + 4 H++ 4 e−SnH4(g)−1.07SiO2(s) + 4 H++ 4 e−Si(s) + 2 H2O−0.91B(OH)3(aq) + 3 H++ 3 e−B(s) + 3 H2O−0.89Fe(OH)2(s) + 2 e−Fe(s) + 2 O H−−0.89[8] Fe2O3(s) + 3 H2O + 2 e−2Fe(OH)2(s) + 2 O H−−0.86[8] TiO2++ 2 H++ 4 e−Ti(s) + H2O−0.862 H2O + 2 e−H2(g) + 2 O H−−0.8277[6] Bi(s) + 3 H++ 3 e−BiH3−0.8[6] Zn2++ 2 e−Zn(Hg)−0.7628[6]Zn2++ 2 e−Zn(s)−0.7618[6] Ta2O5(s) + 10 H++ 10 e−2 T a(s) + 5 H2O−0.75Cr3+ + 3 e−Cr(s)−0.74[Au(CN)2]−+ e−Au(s) + 2 C N−−0.60Ta3++ 3 e−Ta(s)−0.6PbO(s) + H2O + 2 e−Pb(s) + 2 O H−−0.582 T iO 2(s) + 2 H++ 2 e−Ti2O3(s) + H2O−0.56Ga3++ 3 e−Ga(s)−0.53U4++ e−U3+−0.52[5] H3PO2(aq) + H++ e−P(white)[note 2]+ 2 H2O−0.508[6] H3PO3(aq) + 2 H++ 2 e−H3PO2(aq) + H2O−0.499[6] H3PO3(aq) + 3 H++ 3 e−P(red)[note 2]+ 3 H2O−0.454[6] Fe2++ 2 e−Fe(s)−0.44[3] 2 C O 2(g) + 2 H++ 2 e−HOOCCOOH(aq)−0.43Cr3++ e−Cr2+−0.42Cd2++ 2 e−Cd(s)−0.40[3] GeO2(s) + 2 H++ 2 e−GeO(s) + H2O−0.37Cu2O(s) + H2O + 2 e−2 C u(s) + 2 O H−−0.360[6] PbSO4(s) + 2 e−Pb(s) + SO42−−0.3588[6] PbSO4(s) + 2 e−Pb(Hg) + SO42−−0.3505[6]Eu3++ e−Eu2+−0.35[5] In3++ 3 e−In(s)−0.34[9] Tl++ e−Tl(s)−0.34[9] Ge(s) + 4 H++ 4 e−GeH4(g)−0.29Co2++ 2 e−Co(s)−0.28[6] H3PO4(aq) + 2 H++ 2 e−H3PO3(aq) + H2O−0.276[6] V3++ e−V2+−0.26[3] Ni2++ 2 e−Ni(s)−0.25As(s) + 3 H++ 3 e−AsH3(g)−0.23[9] AgI(s) + e−Ag(s) + I−−0.15224[6] MoO2(s) + 4 H++ 4 e−Mo(s) + 2 H2O−0.15Si(s) + 4 H++ 4 e−SiH4(g)−0.14Sn2++ 2 e−Sn(s)−0.13O2(g) + H++ e−HO2•(aq)−0.13Pb2++ 2 e−Pb(s)−0.13[3] WO2(s) + 4 H++ 4 e−W(s) + 2 H2O−0.12P(red) + 3 H++ 3 e−PH3(g)−0.111[6] CO2(g) + 2 H++ 2 e−HCOOH(aq)−0.11Se(s) + 2 H++ 2 e−H2Se(g)−0.11CO2(g) + 2 H++ 2 e−CO(g) + H2O−0.11SnO(s) + 2 H++ 2 e−Sn(s) + H2O−0.10SnO2(s) + 2 H++ 2 e−SnO(s) + H2O−0.09WO3(aq) + 6 H++ 6 e−W(s) + 3 H2O−0.09[9] P(white) + 3 H++ 3 e−PH3(g)−0.063[6] Fe3++ 3 e−Fe(s)−0.04[8] HCOOH(aq) + 2 H+ + 2 e−HCHO(aq) + H2O−0.032 H++ 2 e−H2(g)0.0000≡ 0 AgBr(s) + e−Ag(s) + Br−+0.07133[6] S4O62−+ 2 e−2 S2O32−+0.08Fe3O4(s) + 8 H++ 8 e−3 F e(s) + 4 H2O+0.085[10]+0.092N2(g) + 2 H2O + 6 H++ 6 e−2 N H4OH(aq)HgO(s) + H2O + 2 e−Hg(l) + 2 O H−+0.0977Cu(NH3)42++ e−Cu(NH3)2++ 2 N H3+0.10[9] Ru(NH3)63++ e−Ru(NH3)62++0.10[5] N2H4(aq) + 4 H2O + 2 e−2 N H4++ 4 O H−+0.11[1] H2MoO4(aq) + 6 H++ 6 e−Mo(s) + 4 H2O+0.11Ge4+ + 4 e−Ge(s)+0.12C(s) + 4 H++ 4 e−CH4(g)+0.13[9] HCHO(aq) + 2 H++ 2 e−CH3OH(aq)+0.13S(s) + 2 H++ 2 e−H2S(g)+0.14Sn4++ 2 e−Sn2++0.15Cu2++ e−Cu++0.159[9]Half-reaction E° (V)[note 1]Ref. HSO4−+ 3 H++ 2 e−SO2(aq) + 2 H2O+0.16UO22++ e−UO2++0.163[5] SO42−+ 4 H++ 2 e−SO2(aq) + 2 H2O+0.17TiO2++ 2 H++ e−Ti3++ H2O+0.19SbO++ 2 H++ 3 e−Sb(s) + H2O+0.20AgCl(s) + e−Ag(s) + Cl−+0.22233[6] H3AsO3(aq) + 3 H++ 3 e−As(s) + 3 H2O+0.24GeO(s) + 2 H++ 2 e−Ge(s) + H2O+0.26UO2++ 4 H++ e−U4++ 2 H2O+0.273[5] Re3++ 3 e−Re(s)+0.300Bi3++ 3 e−Bi(s)+0.308[6] VO2++ 2 H++ e−V3++ H2O+0.34Cu2++ 2 e−Cu(s)+0.340[9] [Fe(CN)6]3−+ e−[Fe(CN)6]4−+0.36O2(g) + 2 H2O + 4 e−4 O H−(aq)+0.40[3]H2MoO4+ 6 H++ 3 e−Mo3++ 2 H2O+0.43CH3OH(aq) + 2 H++ 2 e−CH4(g) + H2O+0.50SO2(aq) + 4 H++ 4 e−S(s) + 2 H2O+0.50Cu++ e−Cu(s)+0.520[9]Half-reaction E° (V)[note 1]Ref. CO(g) + 2 H++ 2 e−C(s) + H2O+0.52I3−+ 2 e−3 I−+0.53[3] I2(s) + 2 e−2 I−+0.54[3] [AuI4]−+ 3 e−Au(s) + 4 I−+0.56H3AsO4(aq) + 2 H++ 2 e−H3AsO3(aq) + H2O+0.56[AuI2]−+ e−Au(s) + 2 I−+0.58MnO4−+ 2 H2O + 3 e−MnO2(s) + 4 O H−+0.59S2O32 −+ 6 H++ 4 e−2 S(s) + 3 H2O+0.60Fc++ e−Fc(s)+0.641[11] H2MoO4(aq) + 2 H++ 2 e−MoO2(s) + 2 H2O+0.65+0.6992[6] + 2 H++ 2 e−O2(g) + 2 H++ 2 e− H2O2(aq)+0.70Tl3++ 3 e−Tl(s)+0.72PtCl62−+ 2 e−PtCl42−+ 2 C l−+0.726[5] H2SeO3(aq) + 4 H++ 4 e−Se(s) + 3 H2O+0.74PtCl42−+ 2 e−Pt(s) + 4 C l−+0.758[5] Fe3++ e−Fe2++0.77Ag++ e−Ag(s)+0.7996[6] Hg22++ 2 e−2 H g(l)+0.80NO3−(aq) + 2 H++ e−NO2(g) + H2O+0.802FeO42−+ 5 H2O + 6 e−Fe2O3(s) + 10 O H−+0.81[8][AuBr4]−+ 3 e−Au(s) + 4 B r−+0.85Hg2++ 2 e−Hg(l)+0.85MnO4−+ H++ e−HMnO4−+0.902 H g2++ 2 e−Hg22++0.91[9] Pd2++ 2 e−Pd(s)+0.915[5] [AuCl4]−+ 3 e−Au(s) + 4 C l−+0.93MnO2(s) + 4 H++ e−Mn3++ 2 H2O+0.95[AuBr2]−+ e−Au(s) + 2 B r−+0.96[HXeO6]3−+ 2 H2O + 2 e− + [HXeO4]−+ 4 O H−+0.99[12] H6TeO6(aq) + 2 H++ 2 e−TeO2(s) + 4 H2O+1.02[13] Br2(l) + 2 e−2 B r−+1.066[6] Br2(aq) + 2 e−2 B r−+1.0873[6] IO3−+ 5 H++ 4 e−HIO(aq) + 2 H2O+1.13[AuCl2]−+ e−Au(s) + 2 C l−+1.15HSeO4−+ 3 H++ 2 e−H2SeO3(aq) + H2O+1.15Ag2O(s) + 2 H++ 2 e−2 A g(s) + H2O+1.17ClO3−+ 2 H++ e−ClO2(g) + H2O+1.18[HXeO6]3−+ 5 H2O + 8 e−Xe(g) + 11 O H−+1.18[12] Pt2++ 2 e−Pt(s)+1.188[5] ClO2(g) + H++ e−HClO2(aq)+1.192 I O3−+ 12 H++ 10 e−I2(s) + 6 H2O+1.20ClO4−+ 2 H++ 2 e−ClO3−+ H2O+1.20O2(g) + 4 H++ 4 e−2 H2O+1.229[3] MnO2(s) + 4 H++ 2 e−Mn2++ 2 H2O+1.23[HXeO4]−+ 3 H2O + 6 e−Xe(g) + 7 O H−+1.24[12] Tl3++ 2 e−Tl++1.25Cr2O72−+ 14 H++ 6 e−2 C r3++ 7 H2O+1.33Cl2(g) + 2 e−2 C l−+1.36[3] CoO2(s) + 4 H++ e−Co3++ 2 H2O+1.422 N H3O H++ H++ 2 e−N2H5++ 2 H2O+1.42[1] 2 H IO(aq) + 2 H++ 2 e−I2(s) + 2 H2O+1.44Ce4++ e−Ce3++1.44BrO3−+ 5 H++ 4 e−HBrO(aq) + 2 H2O+1.45β-PbO2(s) + 4 H++ 2 e−Pb2++ 2 H2O+1.460[9]α-PbO2(s) + 4 H++ 2 e−Pb2++ 2 H2O+1.468[9] 2 B rO3−+ 12 H++ 10 e−Br2(l) + 6 H2O+1.482ClO3−+ 12 H++ 10 e−Cl2(g) + 6 H2O+1.49MnO4−+ 8 H++ 5 e−Mn2++ 4 H2O+1.51HO2•+ H++ e− H2O2(aq)+1.51Au3++ 3 e−Au(s)+1.52NiO2(s) + 4 H++ 2 e−Ni2++ 2 O H−+1.592 H ClO(aq) + 2 H++ 2 e−Cl2(g) + 2 H2O+1.63Ag2O3(s) + 6 H++ 4 e−2 A g++ 3 H2O+1.67HClO2(aq) + 2 H++ 2 e−HClO(aq) + H2O+1.67Pb4++ 2 e−Pb2++1.69[9] MnO4−+ 4 H++ 3 e−MnO2(s) + 2 H2O+1.70AgO(s) + 2 H++ e−Ag++ H2O+1.77 H2O2(aq) + 2 H++ 2 e−2 H2O+1.78Co3++ e−Co2++1.82Au++ e−Au(s)+1.83[9] BrO4−+ 2 H++ 2 e−BrO3−+ H2O+1.85Ag2++ e−Ag++1.98[9] S2O82−+ 2 e−2 S O42−+2.010[6] O3(g) + 2 H++ 2 e−O2(g) + H2O+2.075[5] HMnO4−+ 3 H++ 2 e−MnO2(s) + 2 H2O+2.09XeO3(aq) + 6 H++ 6 e−Xe(g) + 3 H2O+2.12[12] H4XeO6(aq) + 8 H++ 8 e−Xe(g) + 6 H2O+2.18[12] FeO42−+ 3 e−+ 8 H+Fe3++ 4 H2O+2.20[14] XeF2(aq) + 2 H++ 2 e−Xe(g) + 2HF(aq)+2.32[12] H4XeO6(aq) + 2 H++ 2 e−XeO3(aq) + H2O+2.42[12] F2(g) + 2 e−2 F−+2.87[9][3]F2(g) + 2 H++ 2 e−2 H F(aq)+3.05[9] .。
氧化还原电位测定标准氧化还原电位(也称为电极电位)是描述化学反应的倾向性的物理量。
它是衡量溶液中氧化还原系统强弱的标准。
在测定氧化还原电位时,常用的参比电极是标准氢电极(SHE)。
标准氢电极的氧化还原电位被定义为0V。
其他电极相对于标准氢电极的氧化还原电位被称为电动势。
在标准条件下,溶液中的氧化还原反应的电动势可以用Nernst方程表示:E = E0 - (RT/nF) lnQ其中,E是电动势,E0是标准电动势(即在标准状况下的电动势),R是气体常数,T是温度,n是电子转移的数目,F是法拉第常数,Q是氧化还原反应的活度积。
在实际测定中,通常使用配对电极法来测定溶液中的氧化还原电位。
该方法涉及到两个电极,一个工作电极和一个参比电极。
电动势计测量的是工作电极相对于参比电极的电势差。
不同的氧化还原反应对应着不同的工作电极。
常见的氧化还原电极包括玻璃电极、银-氯化银电极、铜-铜离子电极、氟化银电极等。
这些电极在标准状态下的电动势值已经被测定和记录下来,并成为了氧化还原电极测定的标准。
在测定氧化还原电位时,需要注意到一些因素。
首先,温度会对电位值产生影响,所以需要在测定过程中保持恒定的温度。
其次,测定的溶液浓度也会影响电位值,因此需要在测定前将溶液稀释到一定的浓度。
最后,在测定氧化还原电位时,还需要考虑到溶液中可能存在的其他反应以及pH值的变化。
总之,氧化还原电位的测定是一种重要的分析手段,可以用于研究化学和物理过程中的氧化还原反应。
通过测定溶液中的氧化还原电位,可以了解反应的热力学性质以及反应条件的选择,为相关领域的研究提供重要的数据基础。
氧化还原电位第一节 概 述化学氧化还原是转化废水中污染物的有效方法。
废水中呈溶解状态的无机物和有机物,通过化学反应被氧化或还原为微毒、无毒的物质,或者转化成容易与水分离的形态,从而达到处理的目的。
按照污染物的净化原理,氧化还原处理方法包括药剂法、电化学法(电解)和光化学法三大类。
在选择处理药剂和方法时,应当遵循下面一些原则:①处理效果好,反应产物无毒无害,不需进行二次处理。
②处理费用合理,所需药剂与材料易得。
②操作特性好,在常温和较宽的pH 值范围内具有较快的反应速度;当提高反应温度和压力后,其处理效率和速度的提高能克服费用增加的不足;当负荷变化后,通过调整操作参数,可维持稳定的处理效果。
④与前后处理工序的目标一致,搭配方便。
与生物氧化法相比,化学氧化还原法需较高的运行费用。
因此,目前化学氧化还原法仅用于饮用水处理、特种工业用水处理、有毒工业废水处理和以回用为目的的废水深度处理等有限场合。
简单无机物的化学氧化还原过程的实质是电子转移。
失去电子的元素被氧化,是还原剂;得到电子的元素被还原,是氧化剂。
在一个化学反应中,氧化和还原是同时发生的,某一元素失去电子,必定有另一元素得到电子。
氧化剂的氧化能力和还原剂的还原能力是相对的,其强度可以用相应的氧化还原电位的数值来比较。
许多种物质的标准电极电位θE 值可以在化学书中查到(本书引用还原电位)。
θE 值愈大,物质的氧化性愈强,θE 值愈小,其还原性愈强。
例如,()V S S E 36.1//2=-θ,其氧化态Cl 2转化为Cl -时,可以作为较强的氧化剂。
相反,()V S S E 48.0//2-=-θ,其还原态S 2-转化为氧化态S 时,可以作为较强的还原剂。
两个电对的电位差愈大,氧化还原反应进行得越完全。
标准电极电位θE 是在标准状况下测定的,但在实际应用中,反应条件往往与标准状况不同,在实际的物质浓度、温度和pH 值条件下,物质的氧化还原电位可用能斯特方程来计算:[][]还原态氧化态ln nF RT E E +=θ (6-1)式中n 为反应中电子转移的数目。
Standard electrode potential (data page)From Wikipedia, the free encyclopedia(Redirected from Table of standard electrode potentials)Jump to: navigation, searchMain article: standard electrode potentialThe values of standard electrode potentials are given in the table below in volts relative to the standard hydrogen electrode and are for the following conditions:• A temperature of 298.15 K (25 °C);•An effective concentration of 1 mol/L for each aqueous species ora species in a mercury amalgam;• A partial pressure of 101.325 kPa (absolute) (1 atm, 1.01325 bar) for each gaseous reagent. This pressure is used because mostliterature data are still given for this value rather than for thecurrent standard of 100 kPa.•An activity of unity for each pure solid, pure liquid, or for water (solvent).Legend: (s) – solid; (l) – liquid; (g) – gas; (aq) – aqueous (default for all charged species); (Hg) –amalgam.Half-reaction E° (V)[note 1]Ref.3⁄2 N2(g) + H+ + e−HN3(aq)−3.09[1][2] Li+ + e−Li(s)−3.0401[2] N 2(g) + 4 H2O + 2 e−2 NH2OH(aq) + 2 OH−−3.04[1] Cs+ + e−Cs(s)−3.026[2] Rb+ + e−Rb(s)−2.98[2] K+ + e−K(s)−2.931[2] Ba2+ + 2 e−Ba(s)−2.912[2] La(OH)3(s) + 3 e−La(s) + 3 OH−−2.90[2] Sr2+ + 2 e−Sr(s)−2.899[2] Ca2+ + 2 e−Ca(s)−2.868[2] Eu2+ + 2 e−Eu(s)−2.812[2] Ra2+ + 2 e−Ra(s)−2.8[2]Half-reaction E° (V)[note 1]Ref. Na+ + e−Na(s)−2.71[2][3] Sc3+ + 3 e−Sc(s)−2.077[4] La3+ + 3 e−La(s)−2.379[2] Y3+ + 3 e−Y(s)−2.372[2] Mg2+ + 2 e−Mg(s)−2.372[2] ZrO(OH)2(s) + H2O + 4 e−Zr(s) + 4 OH−−2.36[2] Al(OH)4− + 3 e−Al(s) + 4 OH−−2.33Al(OH)3(s) + 3 e−Al(s) + 3 OH−−2.31H 2(g) + 2 e−2 H−−2.25Ac3+ + 3 e−Ac(s)−2.20Be2+ + 2 e−Be(s)−1.85U3+ + 3 e−U(s)−1.66[5] Al3+ + 3 e−Al(s)−1.66[3]Half-reaction E° (V)[note 1]Ref. Ti2+ + 2 e−Ti(s)−1.63[3] ZrO 2(s) + 4 H+ + 4 e−Zr(s) + 2 H2O−1.553[6] Zr4+ + 4 e−Zr(s)−1.45[6] Ti3+ + 3 e−Ti(s)−1.37[7] TiO(s) + 2 H+ + 2 e−Ti(s) + H 2O−1.31Ti 2O3(s) + 2 H+ + 2 e−2 TiO(s) + H2O−1.23Zn(OH)42− + 2 e−Zn(s) + 4 OH−−1.199[6] Mn2+ + 2 e−Mn(s)−1.185[6] Fe(CN)64− + 6 H+ + 2 e−Fe(s) + 6HCN(aq)−1.16[8] Te(s) + 2 e−Te2−−1.143[9] V2+ + 2 e−V(s)−1.13[9] Nb3+ + 3 e−Nb(s)−1.099Sn(s) + 4 H+ + 4 e−SnH 4(g)−1.07Half-reaction E° (V)[note 1]Ref. SiO 2(s) + 4 H+ + 4 e−Si(s) + 2 H2O−0.91B(OH)3(aq) + 3 H+ + 3 e−B(s) + 3 H2O−0.89Fe(OH)2(s) + 2 e−Fe(s) + 2 OH−−0.89[8] Fe 2O3(s) + 3 H2O + 2 e−2Fe(OH)2(s) + 2 OH−−0.86[8] TiO2+ + 2 H+ + 4 e−Ti(s) + H 2O−0.862 H 2O + 2 e−H2(g) + 2 OH−−0.8277[6] Bi(s) + 3 H+ + 3 e−BiH 3−0.8[6] Zn2+ + 2 e−Zn(Hg)−0.7628[6] Zn2+ + 2 e−Zn(s)−0.7618[6] Ta 2O5(s) + 10 H+ + 10 e−2 Ta(s) + 5 H2O−0.75Cr3+ + 3 e−Cr(s)−0.74[Au(CN)2]− + e−Au(s) + 2 CN−−0.60Ta3+ + 3 e−Ta(s)−0.6Half-reaction E° (V)[note 1]Ref. PbO(s) + H 2O + 2 e−Pb(s) + 2 OH−−0.582 TiO 2(s) + 2 H+ + 2 e−Ti2O3(s) + H2O−0.56Ga3+ + 3 e−Ga(s)−0.53U4+ + e−U3+−0.52[5] H 3PO2(aq) + H+ + e−P(white)[note 2] + 2 H2O−0.508[6] H 3PO3(aq) + 2 H+ + 2 e−H3PO2(aq) + H2O−0.499[6] H 3PO3(aq) + 3 H+ + 3 e−P(red)[note 2] + 3 H2O−0.454[6] Fe2+ + 2 e−Fe(s)−0.44[3] 2 CO 2(g) + 2 H+ + 2 e−HOOCCOOH(aq)−0.43Cr3+ + e−Cr2+−0.42Cd2+ + 2 e−Cd(s)−0.40[3] GeO 2(s) + 2 H+ + 2 e−GeO(s) + H2O−0.37Cu 2O(s) + H2O + 2 e−2 Cu(s) + 2 OH−−0.360[6]Half-reaction E° (V)[note 1]Ref. PbSO 4(s) + 2 e−Pb(s) + SO42−−0.3588[6] PbSO 4(s) + 2 e−Pb(Hg) + SO42−−0.3505[6] Eu3+ + e−Eu2+−0.35[5] In3+ + 3 e−In(s)−0.34[9] Tl+ + e−Tl(s)−0.34[9] Ge(s) + 4 H+ + 4 e−GeH 4(g)−0.29Co2+ + 2 e−Co(s)−0.28[6] H 3PO4(aq) + 2 H+ + 2 e−H3PO3(aq) + H2O−0.276[6] V3+ + e−V2+−0.26[3] Ni2+ + 2 e−Ni(s)−0.25As(s) + 3 H+ + 3 e−AsH 3(g)−0.23[9] AgI(s) + e−Ag(s) + I−−0.15224[6] MoO 2(s) + 4 H+ + 4 e−Mo(s) + 2 H2O−0.15Half-reaction E° (V)[note 1]Ref. Si(s) + 4 H+ + 4 e−SiH 4(g)−0.14Sn2+ + 2 e−Sn(s)−0.13O 2(g) + H+ + e−HO2•(aq)−0.13Pb2+ + 2 e−Pb(s)−0.13[3] WO 2(s) + 4 H+ + 4 e−W(s) + 2 H2O−0.12P(red) + 3 H+ + 3 e−PH 3(g)−0.111[6] CO 2(g) + 2 H+ + 2 e−HCOOH(aq)−0.11Se(s) + 2 H+ + 2 e−H 2Se(g)−0.11CO 2(g) + 2 H+ + 2 e−CO(g) + H2O−0.11SnO(s) + 2 H+ + 2 e−Sn(s) + H 2O−0.10SnO 2(s) + 2 H+ + 2 e−SnO(s) + H2O−0.09WO 3(aq) + 6 H+ + 6 e−W(s) + 3 H2O−0.09[9] P(white) + 3 H+ + 3 e−PH 3(g)−0.063[6]Half-reaction E° (V)[note 1]Ref. Fe3+ + 3 e−Fe(s)−0.04[8] HCOOH(aq) + 2 H+ + 2 e−HCHO(aq) + H 2O−0.032 H+ + 2 e−H 2(g)0.0000≡ 0 AgBr(s) + e−Ag(s) + Br−+0.07133[6] S 4O62− + 2 e−2 S2O32−+0.08Fe 3O4(s) + 8 H+ + 8 e−3 Fe(s) + 4 H2O+0.085[10]+0.092N 2(g) + 2 H2O + 6 H+ + 6 e−2 NH4OH(aq)HgO(s) + H 2O + 2 e−Hg(l) + 2 OH−+0.0977Cu(NH 3)42+ + e−Cu(NH3)2+ + 2 NH3+0.10[9] Ru(NH 3)63+ + e−Ru(NH3)62++0.10[5] N 2H4(aq) + 4 H2O + 2 e−2 NH4+ + 4 OH−+0.11[1] H 2MoO4(aq) + 6 H+ + 6 e−Mo(s) + 4 H2O+0.11Ge4+ + 4 e−Ge(s)+0.12Half-reaction E° (V)[note 1]Ref. C(s) + 4 H+ + 4 e−CH 4(g)+0.13[9] HCHO(aq) + 2 H+ + 2 e−CH 3OH(aq)+0.13S(s) + 2 H+ + 2 e−H 2S(g)+0.14Sn4+ + 2 e−Sn2++0.15Cu2+ + e−Cu++0.159[9] HSO 4− + 3 H+ + 2 e−SO2(aq) + 2 H2O+0.16UO 22+ + e−UO2++0.163[5] SO 42− + 4 H+ + 2 e−SO2(aq) + 2 H2O+0.17TiO2+ + 2 H+ + e−Ti3+ + H 2O+0.19SbO+ + 2 H+ + 3 e−Sb(s) + H 2O+0.20AgCl(s) + e−Ag(s) + Cl−+0.22233[6] H 3AsO3(aq) + 3 H+ + 3 e−As(s) + 3 H2O+0.24GeO(s) + 2 H+ + 2 e−Ge(s) + H 2O+0.26Half-reaction E° (V)[note 1]Ref. UO 2+ + 4 H+ + e−U4+ + 2 H2O+0.273[5] Re3+ + 3 e−Re(s)+0.300Bi3+ + 3 e−Bi(s)+0.308[6] VO2+ + 2 H+ + e−V3+ + H 2O+0.34Cu2+ + 2 e−Cu(s)+0.340[9] [Fe(CN)6]3− + e−[Fe(CN)6]4−+0.36O 2(g) + 2 H2O + 4 e−4 OH−(aq)+0.40[3] H 2MoO4 + 6 H+ + 3 e−Mo3+ + 2 H2O+0.43CH 3OH(aq) + 2 H+ + 2 e−CH4(g) + H2O+0.50SO 2(aq) + 4 H+ + 4 e−S(s) + 2 H2O+0.50Cu+ + e−Cu(s)+0.520[9] CO(g) + 2 H+ + 2 e−C(s) + H 2O+0.52Half-reaction E° (V)[note 1]Ref.I 3− + 2 e−3 I−+0.53[3] I 2(s) + 2 e−2 I−+0.54[3] [AuI 4]− + 3 e−Au(s) + 4 I−+0.56H 3AsO4(aq) + 2 H+ + 2 e−H3AsO3(aq) + H2O+0.56[AuI 2]− + e−Au(s) + 2 I−+0.58MnO 4− + 2 H2O + 3 e−MnO2(s) + 4 OH−+0.59S 2O32 − + 6 H+ + 4 e−2 S(s) + 3 H2O+0.60Fc+ + e−Fc(s)+0.641[11] H 2MoO4(aq) + 2 H+ + 2 e−MoO2(s) + 2 H2O+0.65+0.6992[6] + 2 H+ + 2 e−O 2(g) + 2 H+ + 2 e− H2O2(aq)+0.70Tl3+ + 3 e−Tl(s)+0.72PtCl 62− + 2 e−PtCl42− + 2 Cl−+0.726[5]Half-reaction E° (V)[note 1]Ref.H 2SeO3(aq) + 4 H+ + 4 e−Se(s) + 3 H2O+0.74PtCl 42− + 2 e−Pt(s) + 4 Cl−+0.758[5] Fe3+ + e−Fe2++0.77Ag+ + e−Ag(s)+0.7996[6] Hg 22+ + 2 e−2 Hg(l)+0.80NO 3−(aq) + 2 H+ + e−NO2(g) + H2O+0.802FeO 42− + 5 H2O + 6 e−Fe2O3(s) + 10 OH−+0.81[8] [AuBr 4]− + 3 e−Au(s) + 4 Br−+0.85Hg2+ + 2 e−Hg(l)+0.85MnO 4− + H+ + e−HMnO4−+0.902 Hg2+ + 2 e−Hg 22++0.91[9] Pd2+ + 2 e−Pd(s)+0.915[5] [AuCl 4]− + 3 e−Au(s) + 4 Cl−+0.93Half-reaction E° (V)[note 1]Ref. MnO 2(s) + 4 H+ + e−Mn3+ + 2 H2O+0.95[AuBr 2]− + e−Au(s) + 2 Br−+0.96[HXeO 6]3− + 2 H2O + 2 e− + [HXeO4]− ++0.99[12] 4 OH−H 6TeO6(aq) + 2 H+ + 2 e−TeO2(s) + 4 H2O+1.02[13] Br 2(l) + 2 e−2 Br−+1.066[6] Br 2(aq) + 2 e−2 Br−+1.0873[6] IO 3− + 5 H+ + 4 e−HIO(aq) + 2 H2O+1.13[AuCl 2]− + e−Au(s) + 2 Cl−+1.15HSeO 4− + 3 H+ + 2 e−H2SeO3(aq) + H2O+1.15Ag 2O(s) + 2 H+ + 2 e−2 Ag(s) + H2O+1.17ClO 3− + 2 H+ + e−ClO2(g) + H2O+1.18[HXeO 6]3− + 5 H2O + 8 e−Xe(g) + 11 OH−+1.18[12]Half-reaction E° (V)[note 1]Ref. Pt2+ + 2 e−Pt(s)+1.188[5] ClO 2(g) + H+ + e−HClO2(aq)+1.192 IO 3− + 12 H+ + 10 e−I2(s) + 6 H2O+1.20ClO 4− + 2 H+ + 2 e−ClO3− + H2O+1.20O 2(g) + 4 H+ + 4 e−2 H2O+1.229[3] MnO 2(s) + 4 H+ + 2 e−Mn2+ + 2 H2O+1.23[HXeO 4]− + 3 H2O + 6 e−Xe(g) + 7 OH−+1.24[12] Tl3+ + 2 e−Tl++1.25Cr 2O72− + 14 H+ + 6 e−2 Cr3+ + 7 H2O+1.33Cl 2(g) + 2 e−2 Cl−+1.36[3] CoO 2(s) + 4 H+ + e−Co3+ + 2 H2O+1.422 NH 3O H+ + H+ + 2 e−N2H5+ + 2 H2O+1.42[1] 2 HIO(aq) + 2 H+ + 2 e−I 2(s) + 2 H2O+1.44Half-reaction E° (V)[note 1]Ref. Ce4+ + e−Ce3++1.44BrO 3− + 5 H+ + 4 e−HBrO(aq) + 2 H2O+1.45β-PbO 2(s) + 4 H+ + 2 e−Pb2+ + 2 H2O+1.460[9]α-PbO 2(s) + 4 H+ + 2 e−Pb2+ + 2 H2O+1.468[9] 2 BrO 3− + 12 H+ + 10 e−Br2(l) + 6 H2O+1.482ClO 3− + 12 H+ + 10 e−Cl2(g) + 6 H2O+1.49MnO 4− + 8 H+ + 5 e−Mn2+ + 4 H2O+1.51HO 2• + H+ + e− H2O2(aq)+1.51Au3+ + 3 e−Au(s)+1.52NiO 2(s) + 4 H+ + 2 e−Ni2+ + 2 OH−+1.592 HClO(aq) + 2 H+ + 2 e−Cl 2(g) + 2 H2O+1.63Ag 2O3(s) + 6 H+ + 4 e−2 Ag+ + 3 H2O+1.67HClO 2(aq) + 2 H+ + 2 e−HClO(aq) + H2O+1.67Half-reaction E° (V)[note 1]Ref. Pb4+ + 2 e−Pb2++1.69[9] MnO 4− + 4 H+ + 3 e−MnO2(s) + 2 H2O+1.70AgO(s) + 2 H+ + e−Ag+ + H 2O+1.77 H 2O2(aq) + 2 H+ + 2 e−2 H2O+1.78Co3+ + e−Co2++1.82Au+ + e−Au(s)+1.83[9] BrO 4− + 2 H+ + 2 e−BrO3− + H2O+1.85Ag2+ + e−Ag++1.98[9] S 2O82− + 2 e−2 SO42−+2.010[6] O 3(g) + 2 H+ + 2 e−O2(g) + H2O+2.075[5] HMnO 4− + 3 H+ + 2 e−MnO2(s) + 2 H2O+2.09XeO 3(aq) + 6 H+ + 6 e−Xe(g) + 3 H2O+2.12[12] H 4XeO6(aq) + 8 H+ + 8 e−Xe(g) + 6 H2O+2.18[12]Half-reaction E° (V)[note 1]Ref. FeO 42− + 3 e− + 8 H+Fe3+ + 4 H2O+2.20[14] XeF 2(aq) + 2 H+ + 2 e−Xe(g) + 2HF(aq)+2.32[12] H 4XeO6(aq) + 2 H+ + 2 e−XeO3(aq) + H2O+2.42[12] F 2(g) + 2 e−2 F−+2.87[9][3] F 2(g) + 2 H+ + 2 e−2 HF(aq)+3.05[9]。