阿司匹林的研发历史与作用机理概述
- 格式:docx
- 大小:41.78 KB
- 文档页数:7


・506・药学学报Acta Pharmaceutica Sinica 2015, 50 (4): 506−508·新药发现与研究实例简析·新药创制是复杂的智力活动, 涉及科学研究、技术创造、产品开发和医疗效果等多维科技活动。
每个药物都有自身的研发轨迹, 而构建化学结构是最重要的环节, 因为它涵盖了药效、药代、安全性和生物药剂学等性质。
本栏目以药物化学视角, 对有代表性的药物的成功构建, 加以剖析和解读。
这是一个源自于天然产物的改构药物, 自发明一百年来, 随着生物学和医学的发展, 阐明了它的作用机制, 开拓了新的用途, 是预防心血管疾病的不可替代的全球性药物, 成为经久不衰的神奇分子。
阿司匹林是水杨酸的衍生物, 虽然与其他非甾体抗炎药的作用靶标都是环氧合酶, 但机制不同, 阿司匹林是与活性部位的丝氨酸残基发生共价键结合, 导致酶的不可逆性失活。
阿司匹林作用于双靶标 (COX-1和COX-2), 它的突出特点还在于作为超小分子 (MW<200) 且有很高的配体效率, 结构中的每个基团和片段都有正贡献, 发挥特定的结合作用, 因而是个迄今无法复制、没有后续跟踪me-only药物。
(编者按)经久不衰的阿司匹林郭宗儒(中国医学科学院药物研究所, 北京 100050)阿司匹林是乙酰水杨酸, 相对分子质量180.16, 一个非常简单的有机化合物, 诞生一百多年来, 仍然被广泛地应用, 是个“神奇”的药物。
1由天然产物水杨苷发明了阿司匹林阿司匹林(1) 的诞生起源于水杨酸的解热止痛作用, 后者可追溯到更久远的柳树皮的应用。
早在古埃及的Ebers药书就记载了柳树皮的浸液可治疗风湿痛, 到18世纪确定浸液中的有效成分是水杨苷(2, salicin), 后来证明水杨苷在体内水解成葡萄糖和水杨醇(3), 水杨醇经体内氧化成水杨酸(4) 而发挥解热止痛作用。
1897年德国拜耳药厂的化学家霍夫曼 (Felix Hoffmann) 合成了水杨酸的衍生物, 纯化后首次得到乙酰水杨酸, 其父率先用来治疗风湿性关节炎, 表明仍有止痛效果, 而对胃的刺激性低于水杨酸。
【原创】药的故事:阿司匹林[ 兰凯] 于:2008-09-30 18:20:32河里的同志们可能都吃过阿司匹林,可是它的故事可不是人人都了解的。
可以说阿司匹林是现在世界上最常用的,也是历史最悠久一种药。
这之前它的历史已经经过传奇般的一个循环,从古代的止痛药到麻风病药,经历了拿破仑的海战,到二次大战间的欧洲,到现在的又一次新的各种预防性用途。
它伴随了宇航员登月,且被记入吉尼斯世界纪录。
西班牙哲学家加赛特(Jose O. Gasset)甚至把二十世纪称作阿司匹林的世纪。
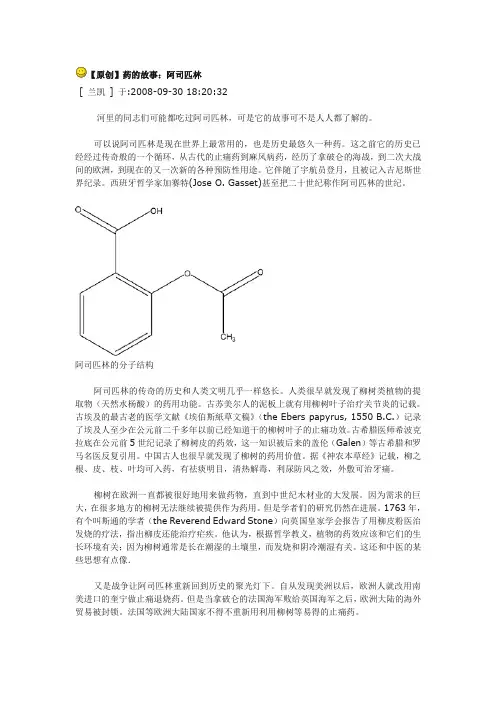
阿司匹林的分子结构阿司匹林的传奇的历史和人类文明几乎一样悠长。
人类很早就发现了柳树类植物的提取物(天然水杨酸)的药用功能。
古苏美尔人的泥板上就有用柳树叶子治疗关节炎的记载。
古埃及的最古老的医学文献《埃伯斯紙草文稿》(the Ebers papyrus, 1550 B.C.)记录了埃及人至少在公元前二千多年以前已经知道干的柳树叶子的止痛功效。
古希腊医师希波克拉底在公元前5世纪记录了柳树皮的药效,这一知识被后来的盖伦(Galen)等古希腊和罗马名医反复引用。
中国古人也很早就发现了柳树的药用价值。
据《神农本草经》记载,柳之根、皮、枝、叶均可入药,有祛痰明目,清热解毒,利尿防风之效,外敷可治牙痛。
柳树在欧洲一直都被很好地用来做药物,直到中世纪木材业的大发展。
因为需求的巨大,在很多地方的柳树无法继续被提供作为药用。
但是学者们的研究仍然在进展。
1763年,有个叫斯通的学者(the Reverend Edward Stone)向英国皇家学会报告了用柳皮粉医治发烧的疗法,指出柳皮还能治疗疟疾。
他认为,根据哲学教义,植物的药效应该和它们的生长环境有关;因为柳树通常是长在潮湿的土壤里,而发烧和阴冷潮湿有关。
这还和中医的某些思想有点像.又是战争让阿司匹林重新回到历史的聚光灯下。
自从发现美洲以后,欧洲人就改用南美进口的奎宁做止痛退烧药。
但是当拿破仑的法国海军败给英国海军之后,欧洲大陆的海外贸易被封锁。

Physicians, Fads, andPharmaceuticals:A History of AspirinAnne Adina Judith Andermann*, B.Sc., M.Phil. Cantab* To whom correspondence should be addressed: Faculty of Medicine, McGill University, 3655 Drummond St., Montreal, QC, Canada H3G 1Y6"Politics is not out there in society.Politics is down there in the laboratory."--Bruno Latour (1).Aspirin is a product of the late-nineteenth-century laboratory, pharmaceutical industry, and medical community. The prevailing scientific techniques, industrial approaches, and medical beliefs were instrumental in the development, promotion and reception of the drug. As a result, the present account does not extend further back than a few decades prior to the release of aspirin from the laboratories of Farbenfabriken vormals Friedrich Bayer & Co. in 1899. In contrast, much of the current literature on aspirin (2,3,4) attempts to trace the compound back to antiquity through the Ebers papyrus, the Hippocratic writings, and the works of Galen. Such histories tell a simple, linear tale of the numerous "discoveries" proposed to have led to the use of certain salicylate-containing plants, such as willow bark and wintergreen, or salicylate-related compounds, including salicilin and salicylic acid, as cures for a variety of ailments. Indeed, according to Mann and Plummer:Both [salicilin and salicylic acid] attacked fever and pain, and their partisans advocated the salicylates' use as antiseptics, mouthwashes, and water preservatives for ocean voyages; one important chemist further suggested (erroneously) that sodium salicylate, a chemical relative, would successfully treat scarlet fever, diphtheria, measles, syphilis, cholera, rabies and anthrax (5).However, it is difficult to establish what effect, if any, these examples of the "historical" uses of"proto-aspirin" had on the impetus for and modes of developing and using the actual drug called aspirin. As a matter of course, aspirin is usually described as the natural descendant from these salicylate forefathers. However, the history of aspirin is not as straightforward a tale as conventional histories suggest, but rather is a complex narrative of the people and circumstances involved in transforming a simple chemical compound into a popular pharmaceutical product that has remained one of the most widely consumed drugs for almost a century.Bayer began in 1863 as Friedrich Bayer & Co., a dye-manufacturing plant in Germany. When the dye industry began to wane during the late 1880s, Bayer made the transition into the more active and lucrative sector of pharmaceuticals by developing, producing, and marketing phenacetin (acetophenetidin) from a dye-making by-product. The company's switch from dyes to pharmaceuticals was so rapid that the first lots of the drug were alkylated in make-shift containers--empty beer bottles wrapped in towels--before the company decided to invest in suitable equipment and proper facilities for its production (6). However, despite the change in the products being manufactured, Bayer retained many of the methods used previously in the sale of dyestuffs in highly competitive markets: sales representatives, advertisements in trade journals, and the use of patents and trade names. As McTavish, a noted medical historian, remarks:By restricting its market to the pharmaceutical and medical professions, the chemical industry avoided the unseemly trappings of the nostrum trade and established itself as a member of the 'ethical' fraternity (7).From then on, McTavish affirms, "[drug production] took place in an industrial setting. Drugs were commodities similar in most respects to any other commodity: they were manufactured for profit" (7). During the 1890's, Carl Duisberg and other key figures at Bayer were busily involved in reorganizing the company, in setting up pharmaceutical laboratories for the development and standardization of drugs, and, most importantly, in establishing links with the medical world.The late nineteenth century saw an unprecedented rise in the number of new pharmaceutical products on the market. One physician in 1889 commented: "Every week, almost every day, brings its new drug, each in turn praised as being the greatest discovery of modern therapeutics" (8). McTavish attributes this tremendous influx of new products to "the increasing industrial role of the laboratory, especially in the drug industry" (7). However, the utility of these novel therapeutic products in medical practice was a source of great debate. Certain physicians staunchlyopposed what they saw as "the growing tendency among German medical men to convert the 'Republic of Science' into a commercial oligarchy for the benefit of plutocrats at the expense of suffering humanity" (7). Others were more accepting of the new developments, but remained wary of those who hailed new compounds as milestone drugs or panaceas, "lest they bring into discredit both their own calling and that of the pharmaceutical chemist" (9). In an address on the Progress of Medicine in the Nineteenth Century, Dr. F. Roberts confessed:Out of the enormous number of medicinal agents brought under our notice by puffing advertisements in the press, medical as well as lay, by pamphlets or even large books delivered by post, or by actual 'specimens for trial' which are nowadays so liberally delivered at our residences, comparatively few hold their ground, or stand a fair and candid criticism and investigation of their vaunted merits. Still a certain proportion do and I see every reason to anticipate that, as the result of the systematic researches, scientific and practical, now carried on in so many laboratories, valuable additions will be made from time to time to the medicinal agents at our disposal for the help and comfort of our patients.I only hope that in our love for the new we will not entirely throw out old friends which have done real and effective service in the past and are today as deserving of our regard as ever (10).Therefore, for those pharmaceutical companies that had managed to establish a place for themselves within the medical community, drug production became a legitimate science-based industry, whereby manufacturers and medics engaged in a profitable producer-consumer partnership. For instance, as written in the Lancet in August 1899, many new pharmaceuticals were the product of the increased attention paid "to the toleration of drugs and to the avoiding of effects which are undesirable" (11). Furthermore:Modification of the salicylates and the introduction of new morphine derivatives [which were both activities carried out in the Bayer laboratories] occur as single examples. In these matters it is satisfactory to find that the pharmacist is guided by the medical man and not solely by a knowledge of the chemistry of the principles concerned (11).Thus, the inspiration and drive to produce aspirin can be explained in terms of a medico-industrial relationship in which the pharmaceutical companies supplied products that interested the doctors, and the doctors, in turn, maintained an active interest in what the pharmaceutical companies had to offer.During the 1880s and 1890s, when physicians became intensely interested in the possible adverse effects of fever on the human body, the use of antipyretics became one of the hottest topics in therapeutic medicine. According to one pharmacology textbook published at the beginning of the 20th century:From the highest to the lowest in the profession, the fad was to regard fever as the most deleterious factor in a case, and to treat it as if it were a part of the disease, or the disease itself, instead of a symptom of almost all infections, and one which in itself is not capable of doing harm, unless it is excessive or very prolonged. [It was believed] that not only did fever, when sufficiently high, coagulate the protoplasm of vital parts, but that the patient was having his tissues burnt up, and that this excessive combustion, or conflagration, must be arrested even though the disease spent itself unaltered in its other clinical manifestations and pathological tendencies. The discovery by numerous laboratory investigations that this group of drugs decreased heat production, and increased heat dissipation, seemed to fit them in a peculiar manner to meet the therapeutic needs of the hour, and they were tested on a scale of experimental therapeutics hardly before equaled. At first, cases of untoward effects were frequently recorded, with fortunately very few fatal cases. Often these effects were due to heroic doses; in other cases, when patients in low fevers received the drug, the fall in temperature which succeeded produced collapse; while in maladies like pneumonia, with deficient aeration of the blood, or other pulmonary affectations, cyanosis, excessive sweating, and feebleness of the circulation occurred (12).Therefore, until the fever fad ended at the turn of the century, most likely as a result of the increasing popularity of the germ theory, most physicians concentrated their efforts on treating pyrexia. The drug companies responded to the medical demands of the day by catering to, and perhaps even fueling the fires of, the antipyretic era. New antipyretics and analgesics--most drugs in this class were believed to possess more or less of both properties--were introduced monthly: "those coal-tar crystalline products which have almost deluged the market as quinine substitutes, [were] being offered from time to time as analgesics, anodynes, antipyretics, as the case may be" (13). Moreover, most of these new therapeutic compounds were commonly promoted as and subsequently referred to by catchy brand names such as malarin, pyrantin, cosaprin, phesin, eupyrine, and, of course, aspirin (14).Still, it is not exactly clear how aspirin came to be. Many give the title of "discoverer of aspirin" to Felix Hoffmann, a chemist at Bayer whose father suffered from rheumatism. According to legend, Hoffmann's fatherwas taking salicylic acid, already mass-produced, widely used, and highly profitable by the end of the 1870s, to treat his rheumatic condition. Unfortunately, the drug was terribly irritating to the stomach and was associated with other ill-effects: most notably, in addition to having an unpleasant, sometimes nauseating, taste, it was believed that salicylic acid disrupted digestion and had an enfeebling action on the heart (16,17). Therefore, the dutiful son took on the task of developing a less toxic replacement. However, acetylsalicylic acid (ASA)--the common chemical name of aspirin--may have already been produced by the French chemist Charles Frédéric Gerhardt in 1853, although he called his compound acetosalicylic anhydride, which was not necessarily the same as ASA. The compound was synthesized in a purer form by Johann Kraut in 1869. Indeed, acetylsalicylic acid was already being manufactured by the Chemische Fabrik von Heyden Company in 1897, although without a brand name. Therefore, it is difficult to determine whether Hoffmann truly developed a new chemical compound or even a novel method of producing a known one, which could then have been patented in Germany.In addition to the uncertainties regarding the chemical origins of ASA, the prevailing medical opinions concerning the widely-used salicylic acid and related compounds, including acetylsalicylic acid, were mixed. Similarly, there was a wide divergence in opinion within the Bayer pharmaceutical laboratories concerning the value of the work being done on ASA in 1897. According to Mann and Plummer, there was a certain degree of animosity between Arthur Eichengrün, who ran the research and development-based Pharmaceutical Division where Hoffmann worked, and Heinrich Dreser, who was in charge of testing and standardization in the Pharmacological Division (5). Eichengrün supported Hoffmann's chemical compound, whereas Dreser initially had no interest in even testing it as a potential new drug. Apparently, Eichengrün even went so far as to surreptitiously distribute the compound to physicians for trials. However, it was Dreser who eventually published the first article on aspirin. His change of heart regarding the value of this compound likely reflects his own financial interests, since, according to Mann and Plummer:[Hoffmann and Eichengrün] had contracts with Bayer by which they would receive a royalty on any patentable product they invented. Since there was no patent, neither of them received any royalties from the sale of aspirin in Germany. However, Heinrich Dreser had an agreement with Bayer by which he would receive a royalty on any product that he introduced. Thus he received a very substantial royalty for aspirin and was able to retire early a very rich man (5).As suggested in the July 1899 issue of the Lancet:No one [in the pharmaceutical industry] would undertake the irksome task of making new products known to the medical profession without being, whether rightly or wrongly, convinced of their superior properties (17).Therefore, once Dreser finally chose to promote Hoffmann's chemical compound as aspirin, he certainly built up a strong case for its superiority over other available remedies.In his article published in the Archiv fur die Gesamte Physiologie in 1899, Dreser begins by describing the unsatisfactory nature of the drugs then available, thereby creating the need for new alternatives:In many diseases related to common cold, the use of sodium salicylate would be definitely much more popular if it would not provoke strong rejection by its disgusting sweet taste which can be corrected only to some extent (18).Dreser then suggests:Pharmacological chemistry should develop synthetically a new preparation which would avoid in addition to the disgusting sweet taste other undesirable characteristics such as the overloading of the stomach. After resorption, the active salicylate should be rapidly split off from the new product.These improvements are precisely what Dreser claims to have achieved through the synthesis of aspirin. First, the taste was refined by masking the free phenolic hydroxyl group of salicylic acid through substitution of the hydrogen atom with a methyl group. To prove that aspirin is reabsorbed and cleaved into salicylic acid, Dreser cites the work of the German scientist Lesnik published in the Archiv fur Experimentelle Pathologie und Pharmakologie to maintain that the increase of nitrogen in the urine "could be due only to the nitrogen-containing metabolic product of salicylic acid . . . also clearly shown by aspirin."Dreser then carried out comparative studies of aspirin and other salicylates to demonstrate that the former was less noxious and more beneficial than the latter. For instance, he tested the sodium salt of aspirin and sodium salicylate on normal rabbits and on cold-blooded animals, which, to his mind, "showed clearly that aspirin is less poisonous than salicylic acid." Dreser also tested aspirin on the most fine and delicate tissues, such as the gills of fish, to further demonstrate the gentleness of the compound. Finally, to put to rest any fears that aspirin might depress the heart, he conducted experiments toshow that sodium salicylate decreased cardiac output, whereas the sodium salt of aspirin increased it. Dreser concludes his article as follows:Summing up the most important pharmacologic characteristics of aspirin we may suggest the following: The aspirin has a more pleasant harsh acidic taste than sodium salicylate before resorption. It is also more protective to the stomach wall according to the above experiments. It is very advantageous, furthermore, that aspirin is split by the gastric hydrochloric acid only to a small extent (0.2%). Differences are evident between aspirin and sodium salicylate also after resorption... (18).By publishing these findings in a physiological journal, Dreser was able to provide a "scientific" and "objective" account of this new compound as a potentially powerful pharmaceutical product with few side-effects. At the same time, he was one of the top employees at Bayer, and would therefore benefit personally from the success that his pharmacological analysis had brought upon aspirin.In concert with Dreser's efforts, physicians were co-opted into supporting the effectiveness and harmlessness of aspirin. Two such doctors cited in Dreser's article were Dr. C. Witthauer, who published a paper on his experiences with aspirin in Die Heilkunde in April 1899, and Dr. Julius Wohlgemuth, who had his results published in Therapeutische Monatshefte in May of the same year. Both Witthauer's and Wohlgemuth's articles (19,20) provide a general introduction to the novel powder, corroborate Dreser's findings, and describe the results of clinical trials with aspirin. Unequivocally, they conclude that the new drug is superior to the other pharmaceutical products then available.The elegance of the early medical and pharmaceutical reports lies in their ability to ally aspirin with the already widely accepted salicylic compounds, whilst concurrently presenting aspirin as distinct from them. Thus, the new drug possessed a certain familiarity, and more importantly, the manufacturers could then claim the proven medicinal properties of salicylic acid and related compounds by association. However, it was equally important to disassociate aspirin from the negative qualities that had been attributed to these products through the development of scientific truths in the laboratory which attested to such differences. In this way, a white powder that had spent many years collecting dust on a shelf along with hundreds of other chemical compounds stored at Bayer was transformed into a substantive pharmaceutical product. Since then, each new report by members of the medical community or pharmaceutical world has expanded and altered the ever-growing narrative on aspirin.On July 22, 1899, aspirin was featured in the "Analytical Records from the Lancet Laboratory" along with several other products that had undergone the rigors of scientific analysis: an old pale cognac found to be suitable for medicinal purposes; Johannis potash water, a diuretic and alkaline treatment; Sandron's iron tonic, which was found to contain a very small quantity of iron; and finally, two specimens of Scotch whisky. The journal's announcement of The Bayer Company's latest drug resembled, in both content and intent, the articles published previously by Dreser, Witthauer, and Wohlgemuth. Within a few years, a barrage of articles singing the praises of aspirin had been published. The clinician Floeckinger even went so far as to take two large doses of aspirin himself: first 75 grains and then another 60 grains (21). After the first dose he found himself "without toxic effects, except violent headache and tinnitus" which lasted for 16 hours, until it subsided following profuse sweats. After the second dose, Floeckinger experienced "increased pulse, reduced temperature, and flashes of light before the eyes." Nonetheless, Floeckinger concludes his article as follows:[It] presents several advantages over salicylic acid. It does not irritate the stomach. There is no cardiac depression. In ordinary doses there is no tinnitus or headache...and [it] is best prescribed in wafers or sachets for acute and chronic rheumatism, polyarthritis, and pleurisy...but it is ineffective in neuralgias and pleurodynia (21).Any adverse effects experienced when taking aspirin were attributed not to this new drug, but rather to extrinsic factors, such as the medium of administration or the magnitude of the dose. Although certain physicians claimed that "some observers--Osler, for instance--recognize little or no advantage in salicylates beyond some power in relieving pain" (15), most physicians strongly supported aspirin as a valuable addition to the pharmacopoeia.Soon after its release onto the market, aspirin began to appear in the new pharmacological texts. Nonetheless, there were still many recent and reputable works that did not mention Bayer's new drug (12,22,23). Indeed, even when aspirin was included in these works, it was not always cited for use in treating ailments with which one now associate the drug. The Index of Diseases and Remedies in an American textbook on materia medica, pharmacology and therapeutics, for example, cites aspirin for the treatment of certain diseases, but does not prescribe it as a general substitute for salicylic acid and the other salicylates. The text lists salicylic acid as a drug useful for burns, eczema, ephelides (freckles), lupus vulgaris, pertussis, and ulceration. Salicilin, salol, salipyrin, and other salicylates are recommended for different disorders such as chorea, diabetes mellitus, endocarditis, fever, pharyngitis, andpleurisy, whereas aspirin is recommended, in addition to other drugs, in the treatment of influenza, neuralgia, and neuritis (24).Indeed, within the first five years after its release, aspirin was seen less and less as an antipyretic, and was increasingly prescribed for the relief of pain. By 1903, "numerous observations had been made on the analgesic effect of aspirin in neuralgias and other painful affectations," including carcinoma (25). In this way, aspirin was similar to its predecessor phenacetin, which "found its birth in what may be called the antipyretic era [of the 1880s and 1890s, and] like its relatives has come to be employed chiefly for the relief of pain" (12). The shift in interest from the antipyretic to the analgesic properties of these drugs in the early twentieth century is best summarized by the entry in the Text-book of Pharmacology and Therapeutics of 1901:As the fad for antipyresis waned by its loss of novelty, physicians began to ask each other whether these drugs which acted so well in reducing fever had any influence in shortening the course of the disease, and it was speedily determined that they did not. Simultaneously, the increasingly thorough investigations into the pathology of fever, and our increased knowledge of the life history of the organisms causing disease, made it clear that fever was a comparatively unimportant factor in a given case, unless excessive; and it begins to be apparent that fever is not only not a peculiarly harmful process, but in some cases may be actually of value... Finally, the recollection of the fact that the use of these drugs necessitates their absorption and elimination, changed or unchanged, and that in these processes they may be guilty of a deleterious influence, has still further decreased their popularity as antipyretics, while the discovery that all of them possess pain-relieving properties has also diverted attention to their use for other purposes than antipyresis (12).Thus, the uses of aspirin changed with the changing trends in the medical profession, becoming progressively less linked to the drug's initial description and indications first marketed by the pharmaceutical company. The original experiments conducted on aspirin in the Bayer laboratory were superceded by more recent clinical findings conducted by medical men not affiliated with the pharmaceutical company. Gradually, all the stories told by those who had been instrumental in presenting and promoting acetylsalicylic acid as aspirin faded into the background. By 1903, authors no longer felt the need to include comprehensive profiles of aspirin in their articles: "the remedy is now sufficiently known to make its description unnecessary here" (15). Thus, the original narrative of aspirin had been disseminated and accepted by the medical profession to such an extent that it no longer needed repeating.Aspirin had quickly become a household name around the world, finding its way even into literary works of the early twentieth century. For instance, when the young Lady Caroline Desta of Elizabeth von Arnim's 1922 novel The Enchanted April complained of a headache during a holiday in Italy, one of her companions asked, "Do you know what aspirin is in Italian?"--to which an erudite old Englishwoman interjected that "the proper remedy for headaches...is castor oil." In a similar vein, Franz Kafka once explained to his fiancée Felice Bauer, in the course of their tormented relationship, that aspirin was one of the few things that eased the unbearable pain of being (5).Aspirin has certainly been put to many different uses throughout the twentieth century, and serves as an example of one of many products of the novel and tenuous relationship that developed during the late nineteenth century between laboratory science, the manufacturing industry, and medical humanitarianism. Indeed, the early pharmaceutical industry's establishment of a close association with the medical community and its adoption of scientific techniques, or, at the very least, a scientific veneer, were instrumental in its success, "and changed the character of medical practice as much as it did the industry itself" (26). Over the years, these medico-industrial connections have consolidated to form the modern pharmaceutical industry of today, an industry that has pervaded almost all aspects of medical science and practice.The story of aspirin--its origins, popularization, and varied uses--is rather unique:Few groups of drugs have provided the manufacturers with such fortunes, physicians with such therapeutic resources, and the laity with so many semi-proprietary remedies as have the so-called antipyretic or analgesic derivatives of coal tar. Nor is there any group which illustrates so well the close relationship between chemistry and practical therapeutics, and the relation of chemical constitution to physiological action (12).Yet, the story of aspirin to a great degree epitomizes the stories of many pharmaceutical products developed both for increased therapeutic efficacy and for profit. The histories of these products generally share certain themes. The usually vague and contentious origins of a drug soon become overshadowed by the multitude of clinical reports produced with the help of medical allies. Extensive clinical trials serve to introduce new drugs to the greater medical community, to specific patient groups, and eventually, to the population at large. As the years pass, however, many drugs are used to treat diseases different from those for which the drugs were originally intended. For example, with the advent of the "anti-coagulant era," aspirin has acquired new indications as a plateletanti-aggregant, and is already widely used in the prophylaxis and treatment of strokes and myocardial infarcts. Therefore, drugs currently being produced and prescribed remain a reflection of the ever-changing state of medical knowledge and of the pharmaceutical industry's eagerness to meet the needs of the day.As the quest for more potent and less toxic drugs continues in the age of rational therapeutics, advanced technology, and designer drugs, the treatment of disease continues to be shaped by the symbiotic relationship between physicians and pharmaceutical companies forged a century ago.ACKNOWLEDGMENTSThe author wishes to acknowledge the assistance of Harmke Kamminga in the preparation of this work. She also wishes to thank the Wellcome Trust for awarding her the Masters Scholarship, and McGill University for their generous Philip F. Vineberg Travelling Fellowship, both of which made it possible to pursue this work towards an M.Phil. at the Wellcome Unit for the History of Medicine in Cambridge, England.REFERENCES1. Latour B. "The Costly Ghastly Kitchen" in Cunningham A. and Williams P., eds., The Laboratory Revolution in Medicine. Cambridge: Cambridge University Press; 1992.2. Rainsford KD. Aspirin and the Salicylates. London: Butterworths; 1984.3. Vane JR, Botting RM. Aspirin and other Salicylates. London: Chapman and Hall Medical Publishers; 1992.4. Mann J. Murder, Magic and Medicine. Oxford: Oxford University Press; 1992.5. Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine, and 100 Years of Rampant Competition. Boston: Harvard Business School Press; 1991.6. Verg E, Plumpe G, Schultheis H. Meilensteine: The official Bayer publication in commemoration of the centenary of aspirin's release; 1989.7. McTavish J. What's in a name? Aspirin and the American Medical Association. Bulletin of the History of Medicine61: 364-365; 1987.8. Dr. Pope of the Leicester Infirmary and Fever House. The Lancet, April 13, 1889, p. 728.。

阿司匹林的调研报告
阿司匹林调研报告
1. 引言
阿司匹林是一种非处方药,广泛用于缓解轻度至中度疼痛和降低发热。
本报告旨在对阿司匹林进行综合调研,包括其的药理特性、适应症、副作用及市场销售情况等方面进行分析。
2. 药理特性
阿司匹林主要通过阻断环氧化酶活性起药效,以抑制前列腺素合成,从而产生抗炎、镇痛和退热作用。
由于其对血小板的抑制作用,阿司匹林也被广泛应用于抗血栓治疗。
3. 适应症
阿司匹林适用于缓解轻度至中度的头痛、牙痛、关节疼痛等各种疼痛,同时可用于治疗发热症状。
此外,由于其抗血栓作用,阿司匹林也被用于预防心脑血管疾病的发生。
4. 副作用
阿司匹林的主要副作用包括胃肠道不适、出血风险增加及过敏反应等。
胃肠道不适常见症状有胃灼热、恶心等,严重者可导致溃疡及出血。
出血风险增加主要表现为停经、咯血等,患有消化性溃疡、凝血功能障碍及肾功能不全者更易发生出血。
对阿司匹林过敏的患者在使用该药时可能会出现过敏反应,如皮肤瘙痒、荨麻疹等。
5. 市场销售情况
阿司匹林作为非处方药,是全球最常用的药物之一。
现在市面上有许多不同品牌和剂型的阿司匹林产品,如片剂、颗粒剂、口服液等。
阿司匹林的市场竞争激烈,价格相对较低,普遍受到消费者的青睐。
6. 结论
综上所述,阿司匹林是一种常用的非处方药,具有良好的药理特性和广泛的适应症。
然而,使用阿司匹林时需注意其副作用及适用人群,如果出现不适或严重副作用应及时咨询医生。
未来,随着科技进步和药物研发的不断推进,阿司匹林的应用前景将更为广阔。


主要抗血小板药物作用机理一:环氧化酶COX抑制剂阿司匹林可促进C0X-1活性部位第529位丝氨酸乙酰化,不可逆抑制COX- 1的活性。
C0X-1在前列腺素类生物合成的初始步骤中起着关键作用,它可催化花生四烯酸转化为前列腺素H2 PGH2,而PGH2是TXA2的直接前体。
阿司匹林抑制C0X-1的结果是导致TXA2生成减少,而TXA2是强烈的血小板致聚物,TXA2生成减少终影响到血小板的聚集和释放反应。
目前,阿司匹林是动脉粥样硕化性疾病最基础的抗血小板药物。
但阿司匹林在应用过程中亦存在如下主要问题:1胃肠道损伤;2阿司匹林哮喘;3阿司匹林抵抗等。
1胃肠道损害阿司匹林所引起的胃肠道损害包括溃疡、出血甚至穿孔等。
关于阿司匹林对胃肠黏膜损伤的机制尚不完全清楚。
目前认为阿司匹林可能主耍影响了胃肠道黏膜的防御功能。
1抑制胃肠道C0X-1:胃肠黏膜C0X-1可催化花生四烯酸形成前列腺素PG, 而PG特别是PGE2具有扩张血管、增加胃肠黏膜血流、促进黏液和碳酸氢盐分泌的作用。
阿司匹林可抑制胃肠道C0X-1,干扰PG合成,进而减弱PG对胃肠黏膜的保护作用。
2 阿司匹林可穿透胃肠黏膜上皮细胞膜,破坏黏膜屏障,对胃肠黏膜产生直接损伤。
3阿司匹林可抑制血小板聚集,削弱机体的止血机制,诱发出血。
阿司匹林的抗栓作用在较宽的剂量范围内30" 1300 mg/d没有剂效关系,这是因为血小板无核,每个血小板COX-1含量趋丁-恒定,低剂量阿司匹林对血小板COX-1的抑制已经饱和。
相反,阿司匹林的消化道不良反应存在剂效关系,这是因为上消化道黏膜为有核细胞,阿司匹林对有核细胞COX-1的抑制程度与用药剂量和给药间期相关。
研究显示,服用75 mg/d 阿nJ匹林与150mg/d相比胃肠道出血可减少30%,与300 mg/d比可减少40%。
OASIS-7研究显示:服用阿司匹林300^325 mg/d较75、100 mg/d并没有减少急性冠脉综合征ACS患者的血栓性事件,反而增加了出血事件[1]。

浅析阿司匹林的药理及其正副作用【摘要】众所周知,阿司匹林是常见的抗生素类药物,这类药物的药理作用需要探究,通过了解其药理与正副作用,从而提高该药物用药的合理性与科学性,防止一些副作用的产生。
基于此,本文主要围绕阿司匹林这类药物进行分析,先阐述阿司匹林药物的药理作用,而后探究其药物正面作用与负面作用,正面作用上,可以用于预防缺血性事件以及癌症危险等,负面作用上,受到剂量与疗程周期等因素的影响,容易出现各种副作用,希望可以为有关临床研究与应用医护人员提供参考,实现这类药物的有效应用。
【关键词】阿司匹林;正副作用;药理分析在如今阿司匹林临床应用过程中,要求医护人员加强这类药物药理的了解,实现对症治疗,减少不良反应的出现。
然而,在实际临床应用过程中,由于各种因素的影响,导致一些副作用的产生,难以发挥出该药物正面作用,大大降低患者疾病治疗效果。
对此,医护人员以及患者都需要提高对于该类药物的认识,正确认知其药物作用,明确其药物治疗症状,环节疼痛以及感冒发热病症等,达到该药物的解热镇痛的临床应用目的,从而提高阿司匹林西药的使用安全性,为医药研究以及应用等方面提供助力。
1阿司匹林的药理分析阿司匹林其原名名称为Aspirin,属于音译舶来词汇,其化学名称为2-(乙酰氧基)苯甲酸,通常也称之为乙酰水杨酸。
表现为白色结晶或者结晶粉末状,无明显臭味(会略带酸臭),能够溶于水中,易溶于乙醇中,微溶于无水乙醚或水中,能够在强碱中溶解并发生化学反应,其Mp为135℃~140℃。
阿司匹林的酸碱度分析为PK3.49,属于弱酸。
应用于解热镇痛中副作用小,效果强于水杨酸钠。
使用能能够抑制血小板中的血栓素形成,具有高效抗血小板凝聚效果。
通过相关资料研究表明,阿司匹林能够预防结肠癌,相应的副作用表现在刺激胃粘膜,甚至会引发十二指肠出血(游离羧基的作用),所以常常将阿司匹林合成使用。
一直以来,科研人员试图寻找Aspirin的替代品,却一直未果,因为镇痛处理需要大量达到活性要求的阴离子,否则,酸性减少不利于抗炎。

阿司匹林的药理和正副作用解析摘要】目的:对阿司匹林的药理和正副作用进行解析,为今后的合理用药提供可靠的参考依据。
方法:从阿司匹林的药理分析、阿司匹林临床正副作用两个角度进行分析探讨。
结果:阿司匹林又名乙酰水杨酸,具有强解热镇痛药理作用,在抗风湿、抗感染治疗中应用广泛。
结论:阿司匹林为临床常用药物,药理作用较多,临床应用广泛,存在一定副作用,临床应做到合理用药,保证用药安全。
【关键词】阿司匹林;药理特性;临床应用;正副作用;临床分析【中图分类号】R96 【文献标识码】A 【文章编号】2095-1752(2016)26-0232-02阿司匹林最早诞生于1899年3月,属于解热镇痛类药物,临床上多用于治疗头痛、压痛、感冒发热、关节风湿疾病等,阿司匹林也是良好的抗血栓药物,能够有效抑制血小板聚集,在心绞痛、心肺梗塞、心脏病、脑血栓等疾病治疗中,效果显著[1]。
本次研究中,出于对阿司匹林的药理和正副作用进行解析,为今后的合理用药提供可靠的参考依据的目的,从阿司匹林的药理分析、阿司匹林临床正副作用两个角度进行分析探讨,结果汇报如下。
1.阿司匹林的药理分析阿司匹林又名乙酰水杨酸,化学名为2-乙酰氧基。
阿司匹林呈现为白色的结晶护士白色结晶粉末,稍有醋酸臭味,也可无明显臭味,味微酸。
若是遇到湿气,则很容易出现缓慢水解,在乙醇中,或者是出现溶解后,同时在乙醚、氯仿中出现溶解。
但是,阿司匹林会在水或者是无水乙醇中发生微溶现象。
在碳酸钠溶液、氢氧化钠溶液中,会出现溶解,但很容易在这一过程中发生分解情况。
因阿司匹林为弱酸性,临床使用的过程中可以产生良好的解热镇痛效果,并且这一作用较水杨酸钠强。
与此同时,阿司匹林在临床应用的过程中,可以较好的对血小板中血栓素A2进行有效的抑制,从而实现抑制血小板聚集的效果[2]。
长期以来,科研工作者视图寻找阿司匹林的替代品,但是却抑制没有结果,因为镇痛处理需要大量达到活性要求的阴离子,不然,酸性减少,会影响到抗炎效果。

一、实验目的1. 了解阿司匹林的化学性质及其在药物中的应用。
2. 掌握阿司匹林提取的实验原理和方法。
3. 熟悉实验操作流程,提高实验技能。
二、实验原理阿司匹林,化学名为乙酰水杨酸,是一种常用的解热镇痛药。
阿司匹林在体内可以抑制前列腺素的合成,从而起到解热镇痛的作用。
本实验采用溶剂提取法,从阿司匹林片剂中提取阿司匹林,通过重结晶等方法纯化,最后测定其含量。
三、实验仪器与试剂1. 仪器:分析天平、研钵、烧杯、漏斗、抽滤瓶、烘箱、恒温水浴锅、紫外-可见分光光度计等。
2. 试剂:阿司匹林片剂、无水乙醇、蒸馏水、氢氧化钠溶液、盐酸溶液、硫酸钠溶液、氯化钠溶液等。
四、实验步骤1. 阿司匹林提取(1)取一定量的阿司匹林片剂,研细后过筛,准确称取0.1g,置于烧杯中。
(2)向烧杯中加入10mL无水乙醇,充分溶解,搅拌均匀。
(3)将烧杯置于恒温水浴锅中,加热至60℃,恒温搅拌30min,使阿司匹林充分溶解。
(4)将溶液过滤,滤液收集于烧杯中。
2. 阿司匹林纯化(1)将滤液置于烧杯中,加入适量的氢氧化钠溶液,调节pH至9.0。
(2)将溶液静置过夜,使阿司匹林沉淀。
(3)用漏斗过滤,收集沉淀。
(4)将沉淀用蒸馏水洗涤3次,每次10mL。
(5)将沉淀置于烘箱中,在60℃下烘干至恒重。
3. 阿司匹林含量测定(1)准确称取烘干后的阿司匹林0.01g,置于烧杯中。
(2)向烧杯中加入10mL无水乙醇,充分溶解,搅拌均匀。
(3)将溶液过滤,滤液收集于容量瓶中,定容至10mL。
(4)取适量滤液,在紫外-可见分光光度计上测定吸光度。
(5)根据标准曲线计算阿司匹林含量。
五、实验结果与分析1. 阿司匹林提取结果通过实验,成功从阿司匹林片剂中提取出阿司匹林,提取率约为95%。
2. 阿司匹林纯化结果通过重结晶等方法,将提取的阿司匹林纯化,纯度达到98%以上。
3. 阿司匹林含量测定结果根据紫外-可见分光光度计测定的吸光度,计算出阿司匹林含量为99.3%。

第25卷第2期2008年3月河北工业科技HebeiJournalofIndustrialScienceandTechnologyV01.25,No.2Mar.2008文章编号:1008—1534(2008)02—0119—03阿司匹林制备研究进展张宝华1,史兰香2,牟微2,郭瑞霞2(1.石家庄学院物理系,河北石家庄050035;2.石家庄学院化工学院,河北石家庄050035)摘要:阿司匹林是一种常用的药物,从催化剂和合成技术2个方面对阿司匹林生产工艺的改进作了简要综述。
评价了各种工艺的优缺点,认为对甲苯磺酸、硫酸氢钠、苯甲酸钠和维生素C等可望成为较好的能取代液体浓硫酸并对环境友好的固体酸催化剂。
关键词:阿司匹林;催化剂;绿色合成;酯化中图分类号:TQ465.9文献标识码:ACurrentdevelopmentinthepreparationofaspirinZHANGBao-hual,SHILan-xian92,MUWei2,GUORui-xia2(1.DepartmentofPhysics,ShijiazhuangUniversity.ShijiazhuangHebei050035。
China;2.CollegeofChemicalEngineering,Shi-jiazhuangUniversity,ShijiazhuangHebei050035,China)Abstract:Aspiriniscommonlyuseddrug.Thecurrentmethodsanddevelopmentinthepreparationofaspirinreviewedbriefly,whichincludesimprovedcatalystsandsynthesistechnology.Theadvantagesanddisadvantagesofallprocessbrieflydiscussed.Itwasexpectedthatp-TsOH,NaHSO,,PhCOzNaandVCwouldbecomegoodcatalystsforthesynthesisofaspi—rin.Keywords:aspirimcatalyst;greensynthesis;esterification阿司匹林又名乙酰水杨酸,是临床常用的解热镇痛、抗炎、抗风湿、抗血栓形成的药物。
阿司匹林的研发历史与作用机理概述
赵珍
(东北师范大学城市与环境科学学院,中国长春130024)
摘要:阿司匹林从最早被研制的纯水杨酸到经霍夫曼改进的乙酰水杨酸,直至后来的阿司匹林被拜耳引入医疗领域,一路走来已有百余年的历史。
阿司匹林具有各种药用价值,具有镇痛、消炎和抑制血小板聚集的传统药理作用。
并且在人类对阿司匹林不断的认识过程中发现了其新的药效,如今,阿司匹林已经被广泛应用于临床。
本文在总结前人经验的基础上,主要针对阿司匹林的研发历史和作用机理进行简要的论述。
以便人们能够更合理的利用阿司匹林,发挥其最大的效用,为医疗领域某些疾病的防治提供依据。
关键词:阿司匹林;研发历史;作用机理;医疗
1.引言
阿司匹林,又名乙酰水杨酸,为较温和的解热镇痛药,有较强的抗炎抗风湿作用,并有促进尿酸排泄和抗血小板凝聚的作用,临床用于头痛、风湿热、风湿性关节炎、痛风症和心脑血管疾病,预防短暂性缺血、中风、缺血性心脏病等,预防心肌梗塞、减少心律失常的发病率和死亡率。
阿司匹林已应用百余年,也是评价和比较其他药物的标准制剂。
由于本药用途广泛,新的药理作用不断被发现,因此,一直受到人们的重视,随着人们对其药理作用的深入了解,临床应用范围也在逐渐扩大。
2.研发历史
2.1 历史背景
阿司匹林的问世,最早可追溯到公元前4世纪希波格拉底时期,人们开始用柳树叶煮汤以治头痛。
在中国和西方,人们自古以来就知道柳树皮具有解热镇痛的神奇功效,在缺医少药的年代里,人们常常将它作为治疗发烧的廉价“良药”,在许多偏远的地方,当产妇生育时,人们也往往让她咀嚼柳树皮,作为镇痛的药物。
早在200多年以前它们的抗高热效应就得到了公认。
人们一直无法知道柳树皮里究竟含有什么物质,以致于具有这样神奇的功效,直至1800年,人们才从柳树皮中提炼出了具有解热镇痛作用的有效成分――水杨酸,由此解开这个千年之谜。
1898年,德国化学家霍夫曼用水杨酸与醋酐反应,合成了乙酰水杨酸,1899年,德国拜耳药厂正式生产这种药品,取商品名为Aspirin,这就是医院里最常用的药物――阿斯匹林。
根据文献记载,都说阿司匹林的发明人是德国的费利克斯·霍夫曼,但这项发明中,起着非常重要作用的还有一位犹太化学家阿图尔·艾兴格林。
在阿司匹林的发明中,阿图尔·艾兴格林功不可没。
在1897年,费利克斯·霍夫曼的确第一次合成了构成阿司匹林的主要物质,但他是在他的上司——知名的化学家阿图尔·艾兴格林的指导下,并且完全采用艾兴格林提出的技术路线才获得成功的。
2.2 研发过程
公元前5世纪,古希腊人使用柳树皮提取物缓解疼痛和发烧;1763年,英国人使用柳树皮提取物缓解疟疾引起的发烧疼痛等症状;1828年,意大利和法国化学家从柳树皮中分离得到了其有效成分水杨酸;1838年,意大利化学家将结晶水杨酸加工成水杨酸;1875年,
水杨酸钠开始用于解热镇痛和关节炎以及痛风等疾病治疗;1899年,德国的拜耳公司合成乙酰水酸,阿司匹林作为商品化药物进入市场。
2.3 发展进程
1979年,美国FDA准许其作为预防脑血栓再发药物而使用。
1985年适应症扩大到预防心肌梗塞再发。
其后,随着临床数据的积累,1994年APT国际研究小组发表了一项综合统计数据,确立了应用阿司匹林等药物的抗血栓疗法作为预防和治疗动脉血栓再发的首选药地位。
自从1899年正式进入消费和医用领域之后,阿司匹林在不断的发展与进步。
1980年,FDA批准阿司匹林用于TIA或脑卒中的二级预防。
并且经过十多年的应用,在1996年,FDA 又成功推荐阿司匹林作为预防心脏事件发生的常规用药。
阿司匹林在中国的生产始于1958年。
2003年,拜阿司匹林○R100mg在中国上市,并获得了良好的反响。
中国专家在2006年达成规范使用阿司匹林的共识,以便控制阿司匹林的使用规范,避免不必要的用药危险。
2009年,SFDA批准拜阿司匹林○R100mg肠溶片用于心肌梗塞一级预防。
2.4阿司匹林的发展改造
由于阿司匹林对胃肠道的刺激作用较强,人们致力于对阿司匹林的改造研究。
国外现已研制出乙酰水杨酸钙脲,并应用于临床。
它既保留了乙酰水杨酸的抗炎作用,又具有对胃肠道刺激轻的优点。
谢湘林和周鸣旧的研究表明:乙酰水杨酸钙脲具有明显的抗炎作用。
且刺激大鼠胃溃疡的形成显著低于乙酰水杨酸。
阿司匹林可以解热、镇痛、抗炎。
锌离子则有解热、抗炎、保护胃粘膜的作用。
乙酰水杨酸锌为乙酰水杨酸的锌盐,兼具两者的作用特点。
从而使解热、镇痛作用增强。
并降低了胃粘膜的损伤。
薛淑英等刚给家兔耳静脉注射伤寒副伤寒甲乙菌苗1.0mL/kg致热,1h后分别给乙酰水杨酸锌和乙酰水杨酸各150mg/kg,2h 及3h后乙酰水杨酸锌组的体温增高值低于乙酰水杨酸组(p<0.05)。
2.5 研发工艺
常用生产工艺
经过几十年的生产实践,阿司匹林的生产形成了一套十分成熟的工艺:以苯酚为原料,经过和二氧化碳的羧化反应,生成水杨酸,经升华后得到升华水杨酸,再采用醋酐-醋酸法,将水杨酸和醋酐进行酰化反应,最终得到乙酰水杨酸也即阿司匹林。
多年来,这套生产工艺基本没有什么变化。
由于此生产工艺不复杂,收率、成本等也较为理想,几十年来,国内外生产企业基本按照这条工艺路线进行生产。
故该工艺较为成熟。
阿司匹林主要通过两步反应生产:①苯酚羧化生成水杨酸;②水杨酸乙酰化生成阿司匹林。
(1)水杨酸的合成:在苛性钠溶液中,酚形成钠盐,成为酚的负离子,再发生羧基化反应(130℃;6kg/cm2)。
(2)水杨酸生产工艺流程
(3)水杨酸乙酰化:水杨酸乙酰化比较方便的方法是用水杨酸与乙酐反应,反应式如下:
(4)水杨酸乙酰化工艺流程
3. 阿司匹林的作用
3.1 阿司匹林的药理作用
关于阿司匹林的解热镇痛抗炎和对动物生产的影响。
国内做了一些初步的研究。
阿司匹林的解热镇痛作用较强。
能降低发热者的体温,对正常体温几乎无影响,但只能缓解症状,不能治疗病因。
它有较强的抗炎功效,能治疗急性风湿热、类风湿性关节炎,多在用药后24~48h即可退热,使关节红肿、疼痛症状明显减轻,所以它一直是治疗类风湿性关节炎、骨关节炎等病的首选药物之一。
阿司匹林对抑制血小板聚集有独特功效,能阻止血栓形成,对防治脑中风、冠心病等也有一定效果。
长期以来,阿司匹林在高血压患者的一级和二级预防中扮演重要角色,而对于其在高血压的降压治疗中的作用尚存在争议。
国外有资料显示,不同时间和不同剂量服用阿司匹林可以影响血压的波动。
段玉忠等证明阿司匹林可通过影响巨噬细胞蛋白激酶一磷酸酶系统平衡,干预细胞内信号蛋白磷酸化反应发挥抗炎作用。
3.2阿司匹林的作用机理
(1)解热、镇痛、抗炎的作用机制
阿司匹林作为一种非甾体类中枢神经系统抑制药,是通过调整机体机能来产生作用的。
国外对其解热镇痛机理做了很多研究。
Guzman等于1964年研究发现,阿司匹林是通过抑制几种关键酶的活性来发挥外周镇痛作用。
阿司匹林主要通过抑制细胞环氧化酶从而抑制前列腺素(Prostaglandin,PG)的合成发挥解热、镇痛、抗炎、抗风湿作用。
然而,Rueff和ray 发现,在许多动物模型中PG即使浓度很高也并无兴奋伤害性感觉传人。
Belmonte等的实验也有与此相同的结果。
综上所述,阿司匹林解热镇痛的机制除了抑制COX外,可能还有其他的作用机理。
(2)阿司匹林抗血小板的作用机制
20世纪70年代,Vane认为阿司匹林通过抑制前列腺素(PG)的生成,特别是血栓素A2而发挥其抑制血小板激活和聚集的作用。
Roth等进一步阐明小剂量阿司匹林通过不可地乙酰化血小板环氧化酶。
主要是乙酰化环氧化酶-1(cox-1)丝氨酸530位点,抑制TXA的形成。
3.3阿司匹林药理作用的新研究
(1)防治糖尿病及其并发症
(2)预防老年性白内障
(3)可以防治中风及心肌梗塞
(4)防治恶性肿瘤
4.前景展望
阿司匹林是老药新用的典型代表,近年来在临床上应用越来越广泛,随着医学科学的发展以及对阿斯匹林药理学作用的机理认识的进一步加深,阿司匹林这一百年老药的新用途仍在不断地被发现,被人类所应用。
人们应该及时发现和总结其作用及副作用,合理吸取有益的药学效应。
避免不良反应,使之服务于临床并发挥其重要的药物价值。
参考文献
[1]王英俊赵贝贝.阿司匹林的作用及其机制研究进展. 山东:现代农业科技2009
[2]刘杰阿司匹林药理作用的研究新进展广东:中国中医药2009
[3]丁健桦郝丽乐长高.阿司匹林的合成条件研究[J].东华理工学院学报.2005
[4] 要文波.阿司匹林的药理特性及临床应用.2011
[5]马长生刘旭李毅刚郭远航.阿司匹林浙江大学出版社.2009
[6]高革王福慧索鸿勋张连洪.阿司匹林锌的研制I[J].中国现代应用药学杂志,2000。