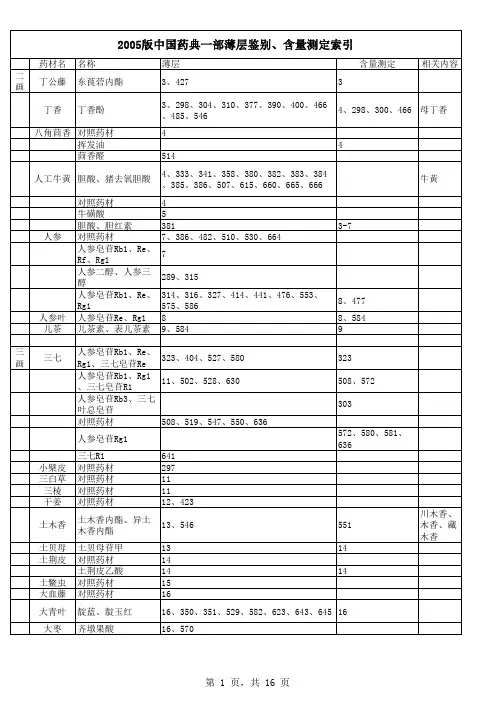
2005中国药典所需色谱柱快速检索
- 格式:xls
- 大小:255.50 KB
- 文档页数:7


![[原创]中国药典2005版与2010版“含量测定”的对比](https://uimg.taocdn.com/48c65de39b89680203d82566.webp)
中国药典2005版与2010版“含量测定”的对比一·基本规则为方便统计,简写相关名词。
列表如下。
含量测定 简写 数量化学滴定,双相滴定,电位滴定,永停滴定 滴定旋光度测定法 旋光原子吸收分光光度计 原子吸收氧瓶燃烧法 氧瓶燃烧氨基酸分析法 氨基酸气相色谱法 气相维生素A,B,D检定法 维生素A分子排阻色谱法 分子排阻紫外-可见分光光度计 紫外放射化学 放射高效液相色谱 HPLC生物检定 生物物理称重 称重薄层色谱 TLC无 无挥发油测定法 挥发油氮测定法 氮测定法荧光分析法 荧光桉油精含量测定法 桉油精含量测定法火焰光度法 火焰光度法水分测定法 水分测定法照氮测定法 照氮测定法二·药品统计2.1总数统计2005版记载 2005版实测 2010版记载 2010版实测一部 1146 1189 2165 1839 二部 1967 1982 2271 2280 合计 3113 3171 4436 4119 注:1.统计时对于复方药物的【含量测定】,采取单列成分均计算在内的方案,所以实测的理论上应大于记载的数量。
2.对于2010版一部,实际发现药典目录记载的约1700种,但药典摘要中记载为2165种,目前尚不清楚原因。
实际统计时发现时部分药物的后面会附录某一种物质的浸出物的相关规定,推测药典可能亦把该项列为一种药,目前,该猜测未得任何资料证实。
3.由于第一条的原因,该统计反映的是药典中所有涉及到【含量测定】的单列药物的不同技术方法的统计分析。
2.2各部统计2.2.1中国药典2005版一部【含量测定】统计 项目 含量测定 数量 百分比 无 无 494 41.55 高效液相色谱 HPLC 478 40.20 薄层色谱 TLC 50 4.21 化学滴定 滴定 413.45 紫外分光光度计 紫外 383.20 气相色谱法 气相 33 2.78 挥发油测定法 挥发油 33 2.78 物理称重 干燥 12 1.01 氮测定法 氮测定法 7 0.59 原子吸收分光光度计原子吸收 1 0.08 氨基酸分析法 氨基酸 1 0.08 桉油精含量测定法 桉油精含量测定法1 0.08各方法所占比例如图2.2.2中国药典2005版二部【含量测定】统计 项目 含量测定 数量 百分比 化学滴定 滴定 780 39.35 高效液相色谱 HPLC 565 28.51 紫外分光光度计 紫外 383 19.32 生物检定 生物 90 4.54 无 无 88 4.44 物理称重 干燥 201.01 维生素检定法 维生素 13 0.66 旋光度测定法 旋光 12 0.61 放射化学 放射 11 0.55 原子吸收分光光度计原子吸收 6 0.30 氮测定法 氮测定法 6 0.30 分子排阻色谱法 分子排阻 3 0.15 气相色谱法 气相 2 0.10 荧光分析法 荧光 2 0.10 氧瓶燃烧法 氧瓶燃烧 1 0.05各方法所占比例如图项目 含量测定 数量 百分比 高效液相色谱 HPLC 1206 65.58 无 无 348 18.92 气相色谱法 气相 98 5.33 化学滴定 滴定 532.88 紫外分光光度计 紫外 45 2.45 薄层色谱 TLC37 2.01 氮测定法 氮测定法 19 1.03 物理称重 干燥 16 0.87 挥发油测定法挥发油 13 0.71 原子吸收分光光度计 原子吸收 3 0.16 旋光度测定法旋光10.05各方法所占比例如图项目 含量测定 数量 百分比 高效液相色谱 HPLC 1001 43.90 化学滴定 滴定 716 31.40 紫外分光光度计 紫外 254 11.14 无 无 131 5.745614 生物检定 生物 84 3.68 气相色谱法 气相 19 0.83 物理称重 干燥 17 0.75 旋光度测定法 旋光 130.57 电位滴定 电位 12 0.53 氮测定法 氮测定法 8 0.350877 原子吸收分光光度计 原子吸收 5 0.22 离子色谱 离子色谱 4 0.175439 氧瓶燃烧法 氧瓶燃烧 3 0.13 氨基酸分析法 氨基酸 3 0.13 分子排阻色谱法 分子排阻 3 0.13 维生素A 检定法 维生素A 2 0.09 荧光分析法 荧光 2 0.087719 火焰光度法 火焰光度法 1 0.04386 水分测定法 水分测定法 1 0.04386 照氮测定法 照氮测定法 1 0.04386 各方法所占比例如图项目 2005版 2010版 无 41.55 18.92 高效液相色谱 40.20 65.58 薄层色谱 4.21 2.01 化学滴定 3.45 2.88 紫外分光光度计 3.20 2.45 气相色谱法 2.78 5.33 挥发油测定法 2.78 0.71 物理称重 1.01 0.87 氮测定法 0.59 1.03 原子吸收分光光度计0.08 0.16 氨基酸分析法 0.080.00 桉油精含量测定法 0.08 0.00 旋光度测定法 0.00 0.05各方法所占比例如图项目 2005版 2010版 化学滴定 39.35 31.05 高效液相色谱 28.51 46.55 紫外分光光度计 19.32 11.76 生物检定 4.54 3.92 无 4.44 3.59 物理称重 1.01 0.51 维生素A检定法 0.66 0.09 旋光度测定法 0.61 0.61 放射化学 0.55 0.00 氮测定法 0.30 0.37 原子吸收分光光度计 0.30 0.23 分子排阻色谱法 0.15 0.14 气相色谱法 0.10 0.51 荧光分析法 0.10 0.05 氧瓶燃烧法 0.05 0.14 离子色谱 0.00 0.19 氨基酸分析法 0.00 0.14 火焰光度法 0.00 0.05 水分测定法 0.00 0.05 照氮测定法 0.00 0.05 各方法所占比例如图三·结论1.由2.3的数据可知,无【含量测定】的中药占的比例正在减低。

反相高效液相色谱法测定竹沥达痰丸中黄芩苷的含量(作者:___________单位: ___________邮编: ___________)【摘要】目的建立反相高效液相色谱(RP HPLC)法竹沥达痰丸中有效成分黄芩苷含量的测定方法。
方法采用HPLC 法,色谱柱为ZORBAX Extend C18柱(4.6 mm×150 mm,5 μm),流动相为甲醇水磷酸(47∶53∶0.2),检测波长为316 nm。
结果黄芩苷线性范围10.04~77.2 μg/ml,r=0.999 8 (n=7);回收率为100.5%(n=6),RSD为1.22%。
结论测定方法简便、稳定,能有效地控制该制剂的质量。
【关键词】竹沥达痰丸黄芩苷反相高效液相色谱法Abstract:Objective To establish a measurement method of baicalin, the effective component of Zhulidatan Pills. MethodsThe isolation was conducted on a ZORBAX Extend-C18 column(4.6 mm×150 mm,5μm), the mobile phase consisted of methanol-water-phosphate(47∶53∶0.2), the detection wavelength was 316 nm. Results The content of baicalin showed good linearityin the range of 10.04~77.2 μg/ml,r=0.999 8 (n=7), and the average recovery was 100.5%(n=6), RSD was 1.22%. ConclusionThe method is simple,stable, and can effectively control the quality of the preparation.Key words:Zhulidatan Pills; Baicalin; RP-HPLC竹沥达痰丸由黄芩、半夏、大黄、橘红、甘草、沉香等6味药材加工提取制得的制剂,具有豁除顽痰,清火顺气的功效,用于痰热上壅,顽痰胶结,咳喘痰多,大便干燥,烦闷癫狂等症状[1]。


下面分别是USPL1~L60的对映。
L1—Octadecyl silane chemically bonded to porous silica or ceramic micro-particles,3to 10µm in diameter.L2—Octadecyl silane chemically bonded to silica gel of a controlled surface porosity that has been bonded to a solid spherical core,30to 50µm in diameter.L3—Porous silica particles,5to 10µm in diameter.L4—Silica gel of controlled surface porosity bonded to a solid spherical core,30to 50µm in diameter.L5—Alumina of controlled surface porosity bonded to a solid spherical core,30to 50µm in diameter.L6—Strong cation-exchange packing–sulfonated fluorocarbon polymer coated on a solid spherical core,30to 50µm in diameter.L7—Octylsilane chemically bonded to totally porous silica particles,3to 10µm in diameter.L8—An essentially monomolecular layer of aminopropylsilane chemically bonded to totally porous silica gel support,10µm in diameter.L9—10-µm irregular or spherical,totally porous silica gel having a chemically bonded,strongly acidic cation-exchange coating.L10—Nitrile groups chemically bonded to porous silica particles,3to 10µm in diameter.L11—Phenyl groups chemically bonded to porous silica particles,5to 10µm in diameter.L12—Astrong anion-exchange packing made by chemically bonding a quaternary amine to a solid silica spherical core,30to 50µm in diameter.L13—Trimethylsilane chemically bonded to porous silica particles,3to 10µm in diameter.L14—Silica gel 10µm in diameter having a chemically bonded,strongly basic quaternary ammonium anion-exchange coating.L15—Hexylsilane chemically bonded to totally porous silica particles,3to 10µm in diameter.L16—Dimethylsilane chemically bonded to porous silica particles,5to 10µm in diameter.L17—Strong cation-exchange resin consisting of sulfonated cross-linked styrene-divinylbenzene copolymer in the hydrogen form,7to 11µm in diameter.L18—Amino and cyano groups chemically bonded to porous silica particles,3to 10µm in diameter.L19—Strong cation-exchange resin consisting of sulfonated cross-linked styrene-divinylbenzene copolymer in the calcium form,about 9µm in diameter.L20—Dihydroxypropane groups chemically bonded to porous silica particles,5to 10µm in diameter.L21—Arigid,spherical styrene-divinylbenzene copolymer,5to 10µm in diameter.L22—Acation-exchange resin made of porous polystyrene gel with sulfonic acid groups,about 10µm in size.L23—An anion-exchange resin made of porous polymethacrylate or polyacrylate gel with quaternary ammonium groups,about 10µm in size.L24—Asemi-rigid hydrophilic gel consisting of vinyl polymers with numerous hydroxyl groups on the matrix surface,32to 63µm in diameter.5L25—Packing having the capacity to separate compounds with a molecular weight range from 100–5000(as determined by polyethylene oxide),applied to neutral,anionic,and cationic water-soluble polymers.Apolymethacrylate resin base,cross-linked with polyhydroxylated ether (surface contained some residual carboxyl functional groups)was found suitable.L26—Butyl silane chemically bonded to totally porous silica particles,5to 10µm in diameter.L27—Porous silica particles,30to 50µm in diameter.L28—Amultifunctional support,which consists of a high purity,100Å,spherical silica substrate that has been bonded with anionic exchanger,amine functionality in addition to a conventional reversed phase C8functionality.L29—Gamma alumina,reverse-phase,low carbon percentage by weight,alumina-based polybutadiene spherical particles,5µm in diameter with a pore volume of 80Å.L30—Ethyl silane chemically bonded to totally porous silica particles,3to 10µm in diameter.L31—Astrong anion-exchange resin-quaternary amine bonded on latex particles attached toa core of 8.5-µm macroporous particles having a pore size of 2000Åand consisting of ethylvinylbenzene cross-linked with 55%divinylbenzene.L32—Achiral ligand-exchange packing–L-proline copper complex covalently bonded to irregularly shaped silica particles,5to 10µm in diameter.L33—Packing having the capacity to separate dextrans by molecular size over a range of 4,000to 500,000Da.It is spherical,silica-based,and processed to provide pHstability.6L34—Strong cation-exchange resin consisting of sulfonated cross-linked styrene-divinylbenzene copolymer in the lead form,about 9µm in diameter.L35—Azirconium-stabilized spherical silica packing with a hydrophilic (diol-type)molecular monolayer bonded phase having a pore size of 150Å.L36—A3,5-dinitrobenzoyl derivative of L-phenylglycine covalently bonded to 5-µm aminopropyl silica.L37—Packing having the capacity to separate proteins by molecular size over a range of 2,000to 40,000Da.It is a polymethacrylate gel.L38—Amethacrylate-based size-exclusion packing for water-soluble samples.L39—Ahydrophilic polyhydroxymethacrylate gel of totally porous spherical resin.L40—Cellulose tris-3,5-dimethylphenylcarbamate coated porous silica particles,5to 20µm in diameter.L41—Immobilized a1-acid glycoprotein on spherical silica particles,5µm in diameter.L42—Octylsilane and octadecylsilane groups chemically bonded to porous silica particles,5µm in diameter.L43—Pentafluorophenyl groups chemically bonded to silica particles by a propyl spacer,5to 10µm in diameter.L44—Amultifunctional support,which consists of a high purity,60Å,spherical silica substrate that has been bonded with a cationic exchanger,sulfonic acid functionality in addition to a conventional reversed phase C8functionality.L45—Beta cyclodextrin bonded to porous silica particles,5to 10µm in diameter.L46—Polystyrene/divinylbenzene substrate agglomerated with quaternary amine functionalized latex beads,10µm in diameter.L47—High-capacity anion-exchange microporous substrate,fully functionalized with trimethlyamine groups,8µm in diameter.7L48—Sulfonated,cross-linked polystyrene with an outer layer of submicron,porous,anion-exchange microbeads,15µm in diameter.L49—Areversed-phase packing made by coating a thin layer of polybutadiene onto spherical porous zirconia particles,3to 10µm in diameter.8L50—Multifunction resin with reversed-phase retention and strong anion-exchange functionalities.The resin consists of ethylvinylbenzene,55%cross-linked with divinylbenzene copolymer,3to 15µm in diameter,and a surface area not less than 350m2per g.Substrate is coated with quaternary ammonium functionalized latex particles consisting of styrene cross-linked with divinylbenzene.9L51—Amylose tris-3,5-dimethylphenylcarbamate-coated,porous,spherical,silica particles,5to 10µm in diameter.10L52—Astrong cation exchange resin made of porous silica with sulfopropyl groups,5to 10µm in diameter.11L53—Weak cation-exchange resin consisting of ethylvinylbenzene,55%cross-linked with divinylbenzene copolymer,3to 15µm diameter.Substrate is surface grafted with carboxylic acid and/or phosphoric acid functionalized monomers.Capacity not less than 500µEq/column.12L54—Asize exclusion medium made of covalent bonding of dextran to highly cross-linked porous agarose beads,about 13µm in diameter.13L55—Astrong cation-exchange resin made of porous silica coated with polybutadiene–maleic acid copolymer,about 5µm in diameter.14L56—Isopropyl silane chemically bonded to totally porous silica particles,3to 10µm in diameter.15L57—Achiral-recognition protein,ovomucoid,chemically bonded to silica particles,about 5µm in diameter,with a pore size of 120Å.L58—Strong cation-exchange resin consisting of sulfonated cross-linked styrene-divinylbenzene copolymer in the sodium form,about 7to 11µm in diameter.16L59—Packing having the capacity to separate proteins by molecular weight over the range of 10to 500kDa.It is spherical (10µm),silica-based,and processed to provide hydrophilic characteristics and pHstability.17USP28L60—Spherical,porous silica gel,3or 5µm in diameter,the surface of which has been covalently modified with palmitamidopropyl groups and endcapped with acetamidopropyl groups to a ligand density of about 6µmoles per m2.18USP28。


2005版中国药典附录-高效液相色谱法高效液相色谱法系采用高压输液泵将规定的流动相泵入装有填充剂的色谱柱进行分离测定的色谱方法。
注入进样阀的供试品,由流动相带入柱内,各成分在柱内被分离,并依次进入检测器,由记录仪、积分仪或数据处理系统记录色谱信号。
采用微柱液相色谱系统可以减少溶剂的消耗并达到快速分离之目的。
高效液相色谱法的主要分离机制有吸附、分配、离子交换和排阻作用。
1.对仪器的一般要求所用的仪器为高效液相色谱仪。
仪器应定期检定并符合有关规定。
(1)色谱柱最常用的色谱柱填充剂为化学键合硅胶。
反相色谱系统使用非极性填充剂,以十八烷基硅烷键合硅胶最为常用,辛基硅烷键合硅胶和其他类型的硅烷键合硅胶(如氰基硅烷键合相和氨基硅烷键合相等)也有使用。
正相色谱系统使用极性填充剂,常用的填充剂有硅胶等。
离子交换填充剂用于离子交换色谱;凝胶或高分子微球等填充剂用于分子排阻色谱等;手性键合填充剂用于对映异构体的拆分分析。
填充剂的性能(如载体的形状、粒径、孔径、表面积、键合基团的表面覆盖度、含碳量和键合类型等因素)以及色谱柱的填充,将直接影响待测物的保留行为和分离效果。
孔径在150?以下的填料适合于分析分子量小于2000的化合物,分子量大于2000的化合物则应选择孔径在300?以上的填料。
以硅胶为载体的键合固定相填充剂适用pH2~8的流动相。
当pH大于8时,可使载体硅胶溶解;当pH小于2时,与硅胶相连的化学键合相易水解脱落。
当色谱系统中需使用pH大于8的流动相时,应选用耐碱的填充剂,如采用高纯硅胶为载体并具有高表面覆盖度的键合硅胶、包覆聚合物填充剂、有机-无机杂化填充剂或非硅胶填充剂等;当需使用pH小于2的流动相时,应选用耐酸的填充剂,如具有大体积侧链能产生空间位阻保护作用的二异丙基或二异丁基取代十八烷基硅烷键合硅胶、有机-无机杂化填充剂等。
(2)检测器最常用的检测器为紫外吸收检测器,其他常见的检测器有二极管阵列检测器(DAD)、荧光检测器、示差折光检测器、蒸发光散射检测器、电化学检测器和质谱检测器等。


2005年版《中国药典》(二部)指示剂与指示液乙氧基黄叱精指示液取乙氧基黄叱精0.1g,加乙醇100ml使溶解,即得。
变色范围 pH3.5~5.5(红→黄)。
二甲基黄指示液取二甲基黄0.1g。
加乙醇100ml使溶解,即得。
变色范围 pH2.9~4.0(红→黄)。
二甲基黄-亚甲蓝混合指示液取二甲基黄与亚甲蓝各15mg,加氯仿100ml,振摇使溶解(必要时微温),滤过,即得。
二甲基黄-溶剂蓝19混合指示液取二甲基黄与溶剂蓝19各15mg,加氯仿100ml使溶解,即得。
二甲酚橙指示液取二甲酚橙0.2g,加水100ml使溶解,即得。
二苯偕肼指示液取二苯偕肼1g,加乙醇100ml使溶解,即得。
儿茶酚紫指示液取儿茶酚紫0.1g,加水100ml使溶解,即得。
变色范围 pH6.0~7.0~9.0(黄→紫→紫红)。
中性红指示液取中性红0.5g,加水使溶解成100ml,滤过,即得。
变色范围 pH6.8~8.0(红→黄)。
孔雀绿指示液取孔雀绿0.3g,加冰醋酸100ml使溶解,即得。
变色范围 pH0.0~2.0(黄→绿);11.0~13.5(绿→无色)石蕊指示液取石蕊粉末10g,加乙醇40ml,回流煮沸1小时,静置,倾去上层清液,再用同一方法处理2次,每次用乙醇30ml,残渣用水10ml洗涤,倾去洗液,再加水50ml煮沸,放冷,滤过,即得。
变色范围 pH4.5~8.0(红→蓝)。
甲基红指示液取甲基红0.1g,加0.05mol/L氢氧化钠溶液7.4ml使溶解,再加水稀释至200ml,即得。
变色范围 pH4.2~6.3(红→黄)。
甲基红-亚甲蓝混合指示液取0.1%甲基红的乙醇溶液20ml,加0.2%亚甲蓝溶液8ml,摇匀,即得。
甲基红-溴甲酚绿混合指示液取0.1%甲基红的乙醇溶液20ml,加0.2%溴甲酚绿的乙醇溶液30ml,摇匀,即得。
甲基橙指示液取甲基橙0.1g,加水100ml使溶解,即得。
变色范围 pH3.2~4.4(红→黄)。
2005年版《中国药典》(二部)试药(一)本试药系指在2005年版《中国药典》(二部)中供各项试验用的试剂,不包括各种色谱用的吸附剂、载体与填充剂。
除生化试剂与指示剂外,一般常用化学试剂分为基准试剂、优级纯、分析纯与化学纯4个等级,选用时可参考下列原则:(1) 标定滴定液用基准试剂;(2) 制备滴定液可采用分析纯或化学纯试剂,但不经标定直接按称重计算浓度者,则应采用基准试剂;(3) 制备杂质限度检查用的标准溶液,采用优级纯或分析纯试剂;(4) 制备试液与缓冲液等可采用分析纯或化学纯试剂。
一水合碳酸钠 Sodium Carbonate Monohydrate [Na2CO3·H2O=124.00] 本品为白色斜方晶体;有引湿性,加热至100℃失水。
在水中易溶,在乙醇中不溶。
一氧化铅 Lead Monoxide [PbO=223.20] 本品为黄色至橙黄色粉末或结晶;加热至300~500℃时变为四氧化三铅,温度再升高时又变为一氧化铅。
在热的氢氧化钠溶液、醋酸或稀硝酸中溶解。
一氯化碘 Iodine Monochloride [ICl=162.36] 本品为棕红色油状液体或暗红色结晶;具强烈刺激性,有氯和碘的臭气;有腐蚀性和氧化性。
乙二胺四醋酸二钠 Disodium Edetate [C10H14N2Na2O8·2H2O=372.24] 本品为白色结晶性粉末。
在水中溶解,在乙醇中极微溶解。
乙氧基黄叱精 Ethoxychrysoidine Hydrochloride [C14H16N4O·HCl=292.77] 本品为深红棕色或黑褐色粉末。
在水或乙醇中溶解。
乙腈 Acetonitrile [CH3CN=41.05] 本品为无色透明液体;微有醚样臭气;易燃。
与水或乙醇能任意混合。
乙酰丙酮 Acetylacetone [CH3COCH2COCH3=100.12] 本品为无色或淡黄色液体;微有丙酮和乙酸的臭气;易燃。
中国药典2005年版中药薄层色谱鉴别技术中药的真伪优劣直接关系到人民用药的安全和有效。
在检验中药真伪方面通常采用的检测手段有形态鉴别、理化鉴别和光谱色谱分析等。
而作为色谱技术一个分支的薄层色谱由于其独具的长处而被广泛应用于中药分析。
自从《中华人民共和国药典》(1990年版一部)起,薄层色谱鉴别除有化学对照品作为鉴别药材的指标成分对照品以外,鉴于单一化学对照品不能反映药材的整体特征,一些多种植物共存的化学成分没有专属性,以及没有化学对照品鉴别就无法进行的问题,开始增加了“对照药材”,以药材对照品的色谱整体为特征进行对比鉴别,解决了上述存在的问题,并于1993年出版了《中华人民共和国药典1990年版中药薄层色谱彩色图集》,作为中药薄层鉴别的重要参考资料。
在该书的第一章中根据多年的实践经验并参考Friedrich Geiss的《Fundamentals of Thin Layer Chromatography (Planar Chromatography)》一书的内容,从实用的角度叙述了薄层色谱实际操作中的各个环节的注意事项,起到了薄层色谱操作规范化的指导作用。
但是直至中国药典2000年版,中药薄层色谱鉴别基本是以手工点样、实验室自制薄层板为基础的较为粗放的操作进行试验,由于硅胶材料的质量不高和手工操作的个体差异较大,致使色谱质量仍然不高,分离度、重现性均难以达到满意的效果。
尽管自上世纪90年代以来,国外尤其是欧洲国家在薄层色谱技术及相应的仪器开发逐步有了很大发展,目前已经达到单元操作计算机自动化,高质量商品预制薄层板(常规板与高效板)代替了自制薄层板,进入一个高层次的仪器化和微机化的阶段。
美国药典、欧洲药典等对草药(植物药)均采用薄层色谱鉴别,并有规范化的要求,以保证结果的专属性和可重现性,这对药典的实施是非常重要的。
因而我国药典在中药的薄层色谱鉴别的应用和推广也需要在技术层面有明显的提高。
在实验室条件方面。
复方阿米三嗪片(萝巴新)(二甲硫酸阿米三嗪)33≯410≯215萝巴新3≯3≯115盐酸马普替林3≯2≯110盐酸米托蒽醌3≯3≯710盐酸异丙嗪片4 ≯215盐酸异丙嗪注射液4 ≯215盐酸雷尼替丁4 ≯215盐酸雷尼替丁片4 ≯410盐酸雷尼替丁胶囊4 ≯410盐酸雷尼替啶注射液4 ≯610盐酸萘甲唑林3 ≯210柳氮磺吡啶4 ≯410 配制对照品溶液检查已知杂质的品种有盐酸乙胺丁醇、盐酸小檗碱、盐酸左旋咪唑、盐酸吗啡、癸氟奋乃静和氯硝西洋等40余个。
此外配制自身稀释对照溶液和配制对照品溶液,用以检查未知杂质及已知杂质并控制杂质斑点数的品种有盐酸氯米帕明(杂质斑点≯4个)和贝诺酯(杂质斑点≯4个)。
本版药典在薄层色谱法中增加了系统适用性试验和测定法,上述列举的国内外药典的实例可供采用薄层色谱法作为鉴别与有关物质测定的品种正文,在修订或方法研究时参考,可根据品种自身的特点与具体情况,选择系统适用性试验项下的有关要求订入标准中,以使检测方法严谨和完善,确保方法的准确性和重现性,以利于药品质量控制。
《中国药典》2005年版(二部)残留溶剂检查法介绍胡昌勤 刘颖(中国药品生物制品检定所,北京100050)I n troduction of Residua l Solven ts Test i n Ch i nese Pharmacopoe i a2005Ed ition(Volu m e ) H u Changqin and L iu Y ing(N ational Institu te f or the Control of P har m aceu tical and B iolog ical P rod ucts,B eij ing100050) 药品中的残留溶剂系指在原料药、辅料以及制剂生产中使用的,但在工艺过程中未能完全去除的有机挥发性化合物。
I CH(人用药品注册技术要求国际协调会)对残留溶剂的这一定义,明确了药品中残留溶剂的最基本特征,同时也包含了药品残留溶剂的测定具有如下特点:(1)残留溶剂的种类相对固定(I CH规定了69种);(2)在具体样品中具有不确定性;(3)残留量相对较低,一般在痕量或微量范围;(4)同一样品中不同溶剂的残留量相差较大。
薄层色谱法附录Ⅵ B. 薄层色谱法薄层色谱法,系将适宜的吸附剂或载体涂布于玻璃板、塑料或铝基片上,成一均匀薄层。
待点样、展开后,与适宜的对照物按同法在同板上所得的色谱图对比,并可用薄层扫描仪进行扫描,用以进行药品的鉴别、杂质检查或含量测定的方法。
1.仪器与材料(1) 玻板除另有规定外,用10cm×10cm、10cm×15cm、20cm×10cm或20cm×20cm 的规格,要求光滑、平整,洗净后不附水珠,晾干。
(2) 吸附剂或载体最常用的有硅胶G、硅胶GF<[254]>、硅胶H、硅胶HF<[254]>,其次有硅藻土、硅藻土G、氧化铝、氧化铝G、微晶纤维素、微晶纤维素F<[254]>等。
其颗粒大小一般要求直径为10~40μm。
薄层涂布,除另有规定外,一般可分无黏合剂和含黏合剂两种;前者系将吸附剂或载体用水适量调成糊状,均匀涂布于玻璃板上,后者系在吸附剂或载体中加入一定量的黏合剂,一般常用10%~15%煅石膏(CaSO4·2H2O在140 ℃加热4小时),混匀后加水适量使用,或用羧甲基纤维素钠水溶液(0.2%~0.5%)适量调成糊状,均匀涂布于玻璃板上。
也有含一定改性剂如荧光剂或缓冲液等的薄层。
(3) 涂布器应能使吸附剂或载体在玻璃板上手工或自动涂成一层符合厚度要求的均匀薄层。
(4) 点样器一般采用微升毛细管或与之相应的点样器材。
(5) 展开室可用适合薄层板大小的专用玻璃缸,底部平底或有双槽,盖子须密闭。
2.操作方法(1) 薄层板制备除另有规定外,将吸附剂1份和水3份在研钵中向一方向研磨混合, 去除表面的气泡后,倒入涂布器中,在玻板上平稳地移动涂布器进行涂布(厚度为0.25 ~0.5mm),取下涂好薄层的玻板,于室温下,置水平台上晾干,在反射光及透射光下检视,表面应均匀,平整,无麻点、无气泡、无破损及污染,于110℃烘30分钟,冷却后立即使用或置干燥箱中备用。