过氧化二异丙苯80-43-3
- 格式:pdf
- 大小:187.92 KB
- 文档页数:5

易制爆危险化学品名录易制爆危险化学品名录以下是易制爆危险化学品名录,包括中文名称、英文名称、主要的燃爆危险性分类和CAS号。
1.高氯酸、高氯酸盐及氯酸盐1.1 高氯酸[含酸50%-72%]英文名称:PERCHLORIC ACID主要危险性分类:氧化性液体,类别1CAS号:7601-90-31.2 氯酸钾英文名称:POTASSIUM CHLORATE主要危险性分类:氧化性固体,类别1 CAS号:3811-04-91.3 氯酸钠英文名称:SODIUM CHLORATE主要危险性分类:氧化性固体,类别1 CAS号:7775-09-91.4 高氯酸钾英文名称:POTASSIUM PERCHLORATE 主要危险性分类:氧化性固体,类别1 CAS号:7778-74-71.5 高氯酸锂英文名称:LITHIUM PERCHLORATE主要危险性分类:氧化性固体,类别1CAS号:-03-91.6 高氯酸铵英文名称:AMMONIUM PERCHLORATE主要危险性分类:爆炸物,类别1.1;氧化性固体,类别1CAS号:7790-98-91.7 高氯酸钠英文名称:SODIUM PERCHLORATE主要危险性分类:氧化性固体,类别1CAS号:7601-89-02.硝酸及硝酸盐类2.1 硝酸[含硝酸≥70%]英文名称:NITRIC ACID主要危险性分类:金属腐蚀物,类别1;氧化性液体,类别1CAS号:7697-37-22.2 硝酸钾英文名称:POTASSIUM NITRATE主要危险性分类:氧化性固体,类别3 CAS号:7757-79-12.3 硝酸钡英文名称:BARIUM NITRATE主要危险性分类:氧化性固体,类别1 CAS号:-31-82.4 硝酸锶英文名称:STRONTIUM NITRATE主要危险性分类:氧化性固体,类别1 CAS号:-76-92.5 硝酸钠英文名称:SODIUM NITRATE主要危险性分类:氧化性固体,类别3 CAS号:7631-99-42.6 硝酸银英文名称:SILVER NITRATE主要危险性分类:氧化性固体,类别2 CAS号:7761-88-82.7 硝酸铅英文名称:LEAD NITRATE主要危险性分类:氧化性固体,类别2 CAS号:-74-82.8 硝酸镍英文名称:NICKEL NITRATE主要危险性分类:氧化性固体,类别2 CAS号:-75-22.9 硝酸镁英文名称:MAGNESIUM NITRATE 主要危险性分类:氧化性固体,类别3 CAS号:-60-32.10 硝酸钙英文名称:CALCIUM NITRATE主要危险性分类:氧化性固体,类别3 CAS号:-37-52.11 硝酸锌英文名称:ZINC NITRATE主要危险性分类:氧化性固体,类别2 CAS号:7779-88-62.12 硝酸铯英文名称:CAESIUM NITRATE主要危险性分类:氧化性固体,类别3CAS号:7789-18-6本文介绍了一些常见的硝基类化合物,包括硝基甲烷、硝基乙烷和硝化纤维素等。

Designation:E755–94(Reapproved2003)Standard Test Method forDicumyl Peroxide,Assay(Liquid Chromatography)1This standard is issued under thefixed designation E755;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon(e)indicates an editorial change since the last revision or reapproval.1.Scope1.1This test method covers the assay of dicumyl peroxide2 in commercially available refined grades of dicumyl peroxide. Technical grades can also be assayed,provided that impurities that interfere chromatographically are absent(see Note1). These materials nominally contain approximately99and92% of dicumyl peroxide,respectively.N OTE1—In the assay of technical grade dicumyl peroxide,errors are possible if the product contains an impurity that elutes with the same retention time as the internal standard.A chromatogram obtained under the test conditions minus the internal standard will determine whether an interference situation exists in the assay of a particular technical grade product.If an impurity is noted,and its retention time is slightly different from that of the internal standard,the interference can often be eliminated by the use of a column known to have a high plate count.1.2Review the current material safety data sheets(MSDS) for detailed information concerning toxicity,first aid proce-dures,and safety precautions.1.3The values stated in SI units are to be regarded as the standard.1.4This standard does not purport to address all of the safety concerns,if any,associated with its use.It is the responsibility of the user of this standard to establish appro-priate safety and health practices and determine the applica-bility of regulatory limitations prior to use.Specific precau-tionary statements are given in Section7.2.Referenced Documents2.1ASTM Standards:3D1193Specification for Reagent WaterE180Practice for Determining the Precision of ASTM Methods for Analysis and Testing of Industrial Chemicals E682Practice for Liquid Chromatography Terms and Re-lationshipsE685Practice for Testing Fixed-Wavelength Photometric Detectors Used in Liquid ChromatographyE300Practice for Sampling Industrial Chemicals3.Summary of Test Method3.1A solution of sample and internal standard in methanol is chromatographed on a reversed-phase ODS column using 85/15methanol/water as the mobile phase and an ultraviolet (UV)detector at254nm.The percent of dicumyl peroxide in the sample is determined by the internal standard technique, using the peak height ratios of standard and sample chromato-grams.4.Significance and Use4.1This test method provides a means for determining the percent of dicumyl peroxide in technical and refined grades of this material.This test method is specific for dicumyl peroxide. No interference is encountered from dimethylbenzyl alcohol, acetophenone,or other minor impurities normally associated with commercial dicumyl peroxide.5.Apparatus5.1Liquid Chromatograph,equipped with a254-nm UV detector,an injection valve,and an isocratic solvent delivery system capable of operating to a gage pressure of3000psi(21 MPa).The detector should be equipped with an attenuator switch to change the sensitivity range as required.5.2Recorder,0to10-mV range,or an electronic integrator with printer-plotter,or both.5.3Chromatographic Column,reversed-phase C-18,250by 4.6-mm inside diameter,containing octadecyldimethylsilane chemically bonded to spherical,5-µm microparticulate silica.4 N OTE2—Commercial HPLC columns vary in physical dimensions, degree of substrate loading,and size and type of support material.Some modification in the operating parameters may be required to achieve optimum separation for these reasons.5.4Guard Column,reversed-phase C-18,12.5by4.0mm, with an inlinefilter.41This test method is under the jurisdiction of ASTM Committee E15on Industrial and Specialty Chemicals and is the direct responsibility of Subcommittee E15.02on Product Standards.Current edition approved Dec.15,1994.Published February1995.Originally published as E755–st previous edition E755–90.2Dicumyl Peroxide;peroxide,bis(1-methyl-1-phenyethyl)C18H22O2;CASRegistry No.80-43-3.3For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTM Standards volume information,refer to the standard’s Document Summary page on the ASTM website.4The commercial guard and analytical columns found to be most satisfactory for use with this test method are Zorbax Rx-18,Part Nos.820764.914and 880967.9002,respectively,available from Mac Mod,127Commons Court,Chadds Ford,PA19317.This column was selected for its separation characteristics and linear response of the dicumyl peroxide and internal standard.1Copyright©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA19428-2959,United States.5.5Precision Sample Injection Valve ,with a 10-µL loop and filler port.5.6Syringe ,250-µL capacity.5.7Sample Filter ,consisting of a syringe and 0.2-µm filter assembly to remove microparticulate matter from the prepared sample solution.55.8Glass Bottles ,4oz,with polyethylene-lined screw caps.6.Reagents6.1Water —Prepare ASTM Type II reagent water in accor-dance with Specification D 1193,or distill deionized water.Filter through a 0.2-µm,Nylon-66filter,and store in a glass container.Alternatively,commercial multicartridge systems that produce water meeting or exceeding the requirements of Type II can be used.6.2Methanol ,chromatographic grade,distilled in glass.6.3Methanol-Water Mobile Phase ,85:15—Mix 8.5vol-umes of methanol with 1.5volumes of water.6.4Di-n-Heptyl Phthalate ,purified.66.5Dicumyl Peroxide,Recrystallized —Transfer 25.0g of commercial refined dicumyl peroxide into a 100-mL Erlenm-eyer flask.Add 8.0mL of methanol,and gently warm the solution in a water bath while swirling to effect complete solution.Cool to 0°C in an ice bath.Transfer the contents to a medium-porosity sintered glass crucible and vacuum filter.Allow air to pass through the filter for 10to 15min to dry the peroxide.Repeat the crystallization twice using approximately 1mL of methanol solvent for every 3g of peroxide.Place the recrystallized dicumyl peroxide in a tightly capped bottle,and store in a refrigerator (see Section 7).7.Safety Precautions7.1Small quantities of solid or molten dicumyl peroxide can be handled safely at temperatures up to 55°C.Dicumyl peroxide should not be heated above 55°C because the rate of peroxide decomposition increases rapidly with increasing tem-peratures above this point.7.2A recirculating water bath or a water bath that has been preheated to the desired temperature and removed from the heat source should be used for warming vessels containing dicumyl peroxide.Electrically heated water baths should not be used since they may cause localized hot spots.Other sources of heat considered unsafe for warming containers of dicumyl peroxide include ovens,hot plates,open flames,and direct steam.7.3Organic peroxides may ignite violently in contact with an open flame or electrical spark.These heat sources must be avoided for this reason.8.Sampling8.1Prior to sampling technical and refined grades of di-cumyl peroxide,it is essential that the sample be blended thoroughly after melting.This is best accomplished by placingthe container in a 55°C water bath.After the sample has melted completely,mix thoroughly by swirling or stirring before withdrawing the sample for analysis (see 7.1-7.3).See Note 3.N OTE 3—Refer to Practice E 300for guidelines on sampling.9.Procedure9.1Preparation of Standard Dicumyl Peroxide Solution :9.1.1To the nearest 0.1mg,weigh 0.4060.05g of recrystallized dicumyl peroxide and 0.2060.05g of di-n-heptyl phthalate (internal standard)into a tared 4-oz glass bottle.9.1.2Add 100mL of methanol,and mix well until the sample and internal standard have dissolved completely.9.1.3Filter the solution through a 0.2-µm filter,collecting the filtrate in clean 17-mL vials equipped with PTFE-lined caps.Cap the vials and store in a cool,dark location.This solution should be used within 36h,after which time a fresh solution should be prepared.Gradual peroxide decomposition will cause a change in the internal standard/dicumyl peroxide peak height ratio.9.2Preparation of Sample Solution :9.2.1To the nearest 0.1mg,weigh 0.4060.05g of melted dicumyl peroxide sample and 0.2060.05g of di-n-heptyl phthalate (internal standard)into a tared 4-oz glass bottle.9.2.2Add 100mL of methanol,and swirl until the sample and internal standard have dissolved completely.9.2.3Filter a portion of the solution through a 0.2-µm filter,collecting the filtrate in a 17-mL vial equipped with a PTFE-lined cap.Cap the vial and store in a cool,dark location.This solution should be used within 36h,after which time a fresh solution should be prepared.Gradual peroxide decomposition will cause a change in the internal standard/dicumyl peroxide peak height ratio.9.3Calibration :9.3.1Adjust the liquid chromatograph in accordance with the following parameters,and allow the instrument to equili-brate until a stable baseline is obtained.Column oven 40°CDetectorUV,254nmMobile phase methanol:water,85:15Flow rate1.0mL/min Recorder chart speed0.5cm/minN OTE 4—The parameters given above apply to a liquid chromatograph equipped with a Zorbax Rx-18C-18reversed-phase column,4.6-mm diameter by 25cm in length.Other columns may require some modifi-cation in the flow rate or mobile phase composition (see Note 2).N OTE 5—See Practice E 682for liquid chromatography terms and relationships.See Practice E 685for testing fixed-wavelength photometric detectors.9.3.2When the column is in equilibrium with the mobile phase,flush the sample loop with approximately 250µL of the standard dicumyl peroxide solution and inject a 10-µL aliquot.Typical retention times for dicumyl peroxide and di-n-heptyl phthalate are 9and 20min,respectively.9.4Analysis of Sample :9.4.1Immediately after obtaining the chromatogram of the standard solution,flush the sample loop with approximately 250µL of the prepared sample solution,and inject a 10-µL5Waters Associates Sample Clarification Kit,Catalog No.26870,has been found to be satisfactory for this purpose.6Di-n-heptyl phthalate,Lancaster Synthesis,Cat.No.5396,has been found to be satisfactory for use as an internalstandard.aliquot.A typical chromatogram of technical grade dicumyl peroxide obtained under the conditions outlined in 9.3.1is shown in Fig.1.10.Calculation10.1Measure the heights of the dicumyl peroxide and di-n-heptyl phthalate peaks in the chromatogram of the stan-dard solution.10.2Calculate the response factor,F C ,for dicumyl peroxide as follows:F C 5W C 3H ISW IS3HC(1)where:F C =response factor,W C =weight of standard dicumyl peroxide,g,see 9.1,W IS =weight of di-n-heptyl phthalate,g,see 9.1,H IS =peak height of di-n-heptyl phthalate,and H C =peak height of dicumyl peroxide in standard.10.3Measure the heights of the dicumyl peroxide and di-n-heptyl phthalate peaks in the chromatogram of the sample solution.10.4Calculate the percent of dicumyl peroxide present in the sample as follows:Wt %dicumyl peroxide 5W IS 3H C 3F C 3100W S 3H IS(2)where:F C =response factor,see 10.2,W IS =weight of di-n-heptyl phthalate,g,see 9.2,W S =weight of sample,g,see 9.2,H IS =peak height of di-n-heptyl phthalate,and H C =peak height of dicumyl peroxide in sample.11.Report11.1Report the percentage of dicumyl peroxide to the nearest 0.1%.12.Precision and Bias12.1Precision —The following criteria should be used for judging the acceptability of the results (Note 6):12.1.1Repeatability (Single Analyst)—The standard devia-tion for a single determination has been estimated to be 0.418%absolute at 14DF.The 95%limit for the difference between two such runs is 1.3%absolute.12.1.2Laboratory Precision (Within-Laboratory,Between Days,Formerly Called Repeatability)—The standard deviation of results (each the average of duplicates),obtained by the same analyst on different days,has been estimated to be 0.30%absolute at 7degrees of freedom.The 95%limit for the difference between two such averages is 0.8%absolute.12.1.3Reproducibility (Multilaboratory)—The standard de-viation of results (each the average of duplicates),obtained by analysts in different laboratories,has been estimated to be 0.58%absolute at 6degrees of freedom.The 95%limit for the difference between two such averages is 1.6%absolute.N OTE 6—The above precision estimates are based on an interlaboratory study performed in 1992on one sample containing approximately 99%dicumyl peroxide.One analyst in each of seven laboratories performed duplicate determinations on each of two different days for a total of 28determinations.7Practice E 180was used in developing these precision statements.7Supporting data are available from ASTM Headquarters.Request RR:E15-1023.FIG.1Typical Chromatogram of Technical Grade DicumylPeroxide12.2Bias—The bias of this test method has not beendetermined due to the unavailability of suitable referencematerials.13.Keywords13.1assay;dicumyl peroxide;HPLC;liquid chromatogra-phy;peroxidesASTM International takes no position respecting the validity of any patent rights asserted in connection with any item mentioned in this ers of this standard are expressly advised that determination of the validity of any such patent rights,and the riskof infringement of such rights,are entirely their own responsibility.This standard is subject to revision at any time by the responsible technical committee and must be reviewed everyfive years and if not revised,either reapproved or withdrawn.Your comments are invited either for revision of this standard or for additional standardsand should be addressed to ASTM International Headquarters.Your comments will receive careful consideration at a meeting of theresponsible technical committee,which you may attend.If you feel that your comments have not received a fair hearing you shouldmake your views known to the ASTM Committee on Standards,at the address shown below.This standard is copyrighted by ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA19428-2959, United States.Individual reprints(single or multiple copies)of this standard may be obtained by contacting ASTM at the aboveaddress or at610-832-9585(phone),610-832-9555(fax),or service@(e-mail);or through the ASTM website().。

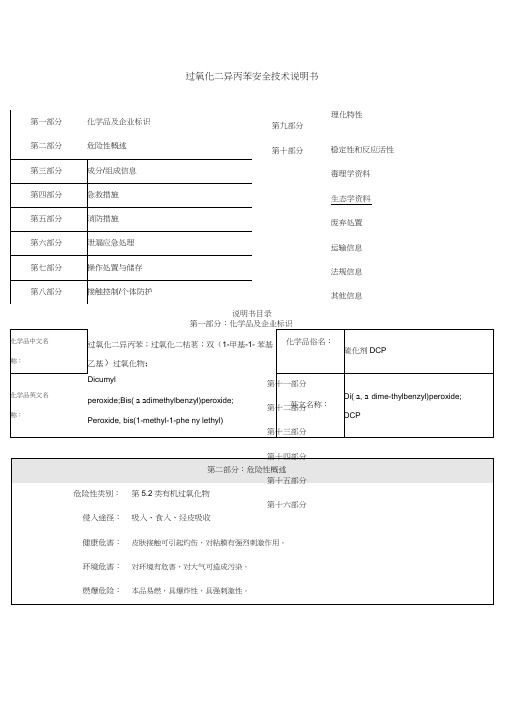
过氧化二异丙苯
一、标识
中文名:过氧化二异丙苯[工业纯][含量>42%,带有惰性固体]
别名:过氧化二枯基;硫化剂DCP
危规号:52030
UN.No.:2121
分子式:CHO 18222
相对分子质量:270.37
二、理化特性
相对密度[水=1]:1.082(20?)
熔点:42?
外观性状:白色无臭透明的菱形结晶,不溶于水,能溶于乙醇、丙酮等,见光或受热不稳定。
三、危险特性
受热、光照,猛烈撞击,或遇明火有引起燃烧爆炸的危险
四、事故处置
泄漏处置:隔离泄漏污染区,周围设警告标志,切断火源。
应急处理人员戴好防毒面具,穿化学防护
服。
用惰性的、潮湿的、不燃的材料吸收,然后收集运至废物处理场所处置。
如大量泄漏,利用围堤收
容,然后收集、转移、回收或无害处理后废弃。
消防措施:可使用的灭火剂为雾状水、砂土。
健康危害及急救措施:本品吸入、口服或经皮肤吸收对身体有害。
急救措施:迅速脱离现场至空气新
鲜处,保持呼吸道畅通,如呼吸困难,给输氧;如呼吸停止,立即进行人工呼吸,并立即就医;如果皮
肤接触,迅速脱去被污染的衣着,用大量流动清水冲洗至少15分钟;如果眼睛接触,立即翻开眼睑,用大量流动清水或生理盐水冲洗至少15分钟,严重者立即就医。
五、储运措施
应单独存放于一个仓间,避光保存,远离火种及热源,最高仓温不宜超过30?;应与还原剂、易燃
物(如硫磷、木炭等)、其他有机物分仓间存放,严禁混放混运;包装要密封,搬运时轻装轻卸,严禁
震动、撞击;防止包装损坏;储期不易过长。

目录5.1类氧化剂过氧化氢的理化性质及危险特性(表-) (1)过氧化钠的理化性质及危险特性(表-) (2)高氯酸[含酸50%~72%]的理化性质和危险特性(表-) (3)高氯酸钠的理化性质和危险特性(表-) (4)氯酸钠的理化性质和危险特性(表-) (5)氯酸钾的理化性质和危险特性(表-) (6)亚氯酸钠的理化性质及危险特性(表-) (7)高锰酸钠的理化性质及危险特性(表-) (8)高锰酸钾的理化性质及危险特性(表-) (9)硝酸钠的理化性质及危险特性(表-) (10)硝酸钾的理化性质和危险特性(表-)............. 错误!未定义书签。
硝酸钙的理化性质和危险特性(表-) . (12)硝酸锶的理化性质和危险特性(表-) (13)硝酸钡的理化性质及危险特性(表-) (14)硝酸锌的理化性质和危险特性(表-) (15)硝酸银的理化性质及危险特性(表-) (17)硝酸铅的理化性质及危险特性(表-) (18)亚硝酸钾的理化性质及危险特性(表-) (19)过(二)碳酸钠的理化性质及危险特性(表-) (20)过硫酸铵的理化性质及危险特性(表-) (21)过硫酸钾的理化性质及危险特性(表-) (23)过硼酸钠的理化性质及危险特性(表-) (24)漂白粉的理化性质及危险特性(表-) (25)溴酸钠的理化性质和危险特性(表-) (26)溴酸钾的理化性质和危险特性(表-) (27)高碘酸的理化性质和危险特性(表-) (28)高碘酸钠的理化性质和危险特性(表-) (29)高碘酸钾的理化性质和危险特性(表-) (30)碘酸钠的理化性质和危险特性(表-) (31)碘酸钾的理化性质和危险特性(表-) (32)三氧化铬[无水]的理化性质及危险特性(表-) (33)重铬酸钾的理化性质及危险特性(表-) (34)硝酸镁的理化性质和危险特性(表-) (35)硝酸铁的理化性质和危险特性(表-) (36)硝酸镍的理化性质和危险特性(表-) (37)硝酸钴的理化性质及危险特性(表-) (38)硝酸铝的理化性质和危险特性(表-) (39)硝酸锰的理化性质和危险特性(表-) (40)硝酸铜的理化性质和危险特性(表-) (41)硝酸铋的理化性质和危险特性(表-) (42)硝酸镧的理化性质和危险特性(表-) (43)硝酸铈铵的理化性质和危险特性(表-) (45)亚硝酸钠的理化性质及危险特性(表-) (46)氧化银的理化性质及危险特性(表-) (47)5.2类有机过氧化物过氧化二异丙苯[含量>42%,带有惰性固体]的理化性质和危险特性(表-)48 过氧化(二)苯甲酰[77%<含量<95%,含水]的理化性质和危险特性(表-) 49 过氧化乙酸的理化性质及危险特性(表-) (50)高氯酸[含酸50%~72%]的理化性质和危险特性(表-)高氯酸钠的理化性质和危险特性(表-)氯酸钠的理化性质和危险特性(表-)亚氯酸钠的理化性质及危险特性(表-)硝酸钠的理化性质及危险特性(表-)硝酸银的理化性质及危险特性(表-)过硼酸钠的理化性质及危险特性(表-)溴酸钠的理化性质和危险特性(表-)重铬酸钾的理化性质及危险特性(表-)硝酸钴的理化性质及危险特性(表-)氧化银的理化性质及危险特性(表-)。

过氧化二异丙苯过氧化二异丙苯过氧化二异丙苯(dicumyl peroxide)又称硫化剂DCP、过氧化二枯茗。
分子式C18H22O2,270.37。
物化性能白色结晶。
熔点41~42℃。
相对密度1.082。
分解温度120~125℃。
室温下稳定,见光逐渐变成微黄色。
不溶于水,溶于乙醇、乙醚、乙酸、苯和石油醚。
活性氧含量5.92%(纯度100%),5.62%(纯度95%)。
溶于苯中半衰期:171℃:1min;117℃:10h;101℃:100h。
是一种强氧化剂。
基本信息过氧化二异丙苯(dicumyl peroxide)又称硫化剂DCP、过氧化二枯茗。
分子式C18H22O2,270.37。
结构式:物化性能白色结晶。
熔点41~42℃。
相对密度1.082。
分解温度120~125℃。
折射率1.5360。
升华温度100℃(26.7Pa)。
活性氧含量5.9%,活化能169.99kJ/mol。
闪点133℃。
室温下稳定,见光逐渐变成微黄色。
不溶于水,溶于乙醇、乙醚、乙酸、苯和石油醚。
活性氧含量5.92%(纯度100%),5.62%(纯度95%)。
溶于苯中半衰期:171℃:1min;117℃:10h;101℃:100h。
是一种强氧化剂。
可燃。
低毒,LD50 4100mg/kg。
主要用途主要用作天然橡胶、合成橡胶的硫化剂,聚合反应的引发剂,还可用作聚乙烯树脂交联剂。
交联的聚乙烯用作电缆绝缘材料,不仅具有优良的绝缘性和加工性能,而且可提高其耐热性,100份聚乙烯使用硫化剂DCP2.4份。
硫化剂DCP可使(EVA)泡沫材料形成细微均匀的泡孔,同时提高制品的耐热性和耐候性。
另外,还用作不饱和聚酯的固化交联剂。
质量指标指标名称[1]一等品合格品外观无色或白色棱形结晶白色或略带粉红色结晶熔点/℃≥ 38.5 37.5过氧化二异丙苯的质量分数/%≥ 99.0 98.0总挥发物含量/%≤0.3 0.5实验室制法在500mL三口反应烧瓶中,加入121.6g异丙苯过氧化氢、108.8g -苯基异丙醇和36g无水草酸。
易制爆危险化学品名录(2011年版)易制爆危险化学品名录(2011年版)序号中文名称英文名称主要的燃爆危险性分类CAS号联合国危险货物编号1 高氯酸、高氯酸盐及氯酸盐1.1 高氯酸[含酸50%-72%]PERCHLORIC ACID 氧化性液体,类别1 7601-90-3 18731.2 氯酸钾POTASSIUM CHLORATE 氧化性固体,类别1 3811-04-9 1485 1.3 氯酸钠SODIUM CHLORATE 氧化性固体,类别1 7775-09-9 1495 1.4 高氯酸钾POTASSIUM PERCHLORATE 氧化性固体,类别1 7778-74-7 1489 1.5 高氯酸锂LITHIUM PERCHLORATE 氧化性固体,类别1 7791-03-91.6 高氯酸铵AMMONIUM PERCHLORATE 爆炸物,1.1项氧化性固体,类别17790-98-9 14421.7 高氯酸钠SODIUM PERCHLORATE 氧化性固体,类别1 7601-89-0 15022 硝酸及硝酸盐类2.1 硝酸[含硝酸≥70%]NITRIC ACID金属腐蚀物,类别1氧化性液体,类别17697-37-2 20312.2 硝酸钾POTASSIUM NITRATE 氧化性固体,类别3 7757-79-1 1486 2.3 硝酸钡BARIUM NITRATE 氧化性固体,类别2 10022-31-8 1446 2.4 硝酸锶STRONTIUM NITRATE 氧化性固体,类别3 10042-76-9 1507 2.5 硝酸钠SODIUM NITRATE 氧化性固体,类别3 7631-99-4 1498 2.6 硝酸银SILVER NITRATE 氧化性固体,类别2 7761-88-8 14932.7 硝酸铅LEAD NITRATE 氧化性固体,类别2 10099-74-8 1469 2.8 硝酸镍NICKEL NITRATE 氧化性固体,类别2 14216-75-2 2725 2.9 硝酸镁MAGNESIUM NITRATE 氧化性固体,类别3 10377-60-3 1474 2.10 硝酸钙CALCIUM NITRATE 氧化性固体,类别3 10124-37-5 1454 2.11 硝酸锌ZINC NITRATE 氧化性固体,类别2 7779-88-6 15142.12 硝酸铯CAESIUM NITRATE 氧化性固体,类别3 7789-18-6 14513 硝基类化合物3.1 硝基甲烷NITROMETHANE 易燃液体,类别3 75-52-5 1261 3.2 硝基乙烷NITROETHANE 易燃液体,类别3 79-24-3 2842 3.3 硝化纤维素3.3.1 硝化纤维素[干的或含水(或乙醇)<25%]NITROCELLULOSE,DRY ORWETTED WITH WATER(ORALCOHOL)爆炸物,1.1项9004-70-0 03403.3.2 硝化纤维素[含增塑剂<18%]NITROCELLULOSE WITHPLASTICIZING SUBSTANCE爆炸物,1.1项9004-70-0 03413.3.3 硝化纤维素[含乙醇≥25%]NITROCELLULOSE WITHALCOHOL爆炸物,1.3项9004-70-0 03423.3.4 硝化纤维素[含水≥25%]NITROCELLULOSE WITHWATER易燃固体,类别1 25553.3.5 硝化纤维素[含氮≤12.6%,含乙醇≥25%]NITROCELLULOSE WITHALCOHOL,NOT MORETHAN12.6% NITROGEN易燃固体,类别1 25563.3.6 硝化纤维素[含氮≤12.6%,含增塑剂≥18%]NITROCELLULOSE WITHPLASTICIZING SUBSTANCE,NOT MORETHAN 12.6%NITROGEN易燃固体,类别1 25573.4 硝基萘类化合物NITRONAPHTHALENES3.5 硝基苯类化合物NITROBENZENES3.6 硝基苯酚(邻、间、对)类化合物NITROPHENOLS(O-,M-,P-)3.7 硝基苯胺类化合物NITROANILINES3.8 2,4-二硝基甲苯2,4-DINITROTOLUENE 121-14-2 20382,6-二硝基甲苯2,6-DINITROTOLUENE 606-20-2 16003.9 二硝基(苯)酚[干的或含水<15%]DINITROPHENOL 爆炸物,1.1项25550-58-7 00763.10 二硝基(苯)酚碱金属盐[干的或含水<15%]DINITROPHENOLATES 爆炸物,1.3项00773.11 二硝基间苯二酚[干的或含水<15%]DINITRORESSORCINOL 爆炸物,1.1项519-44-8 00784 过氧化物与超氧化物4.1 过氧化氢溶液4.1.1 过氧化氢溶液[含量≥70%]HYDROGEN PEROXIDESOLUTION氧化性液体,类别1 7722-84-1 20154.1.2 过氧化氢溶液[70%﹥含量≥50%]HYDROGEN PEROXIDESOLUTION氧化性液体,类别2 7722-84-1 20144.1.3 过氧化氢溶液[50%﹥含量≥27.5%]HYDROGEN PEROXIDESOLUTION氧化性液体,类别3 7722-84-1 20144.2 过氧乙酸PEROXYACETIC ACID 易燃液体,类别3有机过氧化物D型79-21-04.3 过氧化钾POTASSIUM PEROXIDE 氧化性固体,类别1 17014-71-0 1491 4.4 过氧化钠SODIUM PEROXIDE 氧化性固体,类别1 1313-60-6 1504 4.5 过氧化锂LITHIUM PEROXIDE 氧化性固体,类别2 12031-80-0 1472 4.6 过氧化钙CALCIUM PEROXIDE 氧化性固体,类别2 1305-79-9 1457 4.7 过氧化镁MAGNESIUM PEROXIDE 氧化性固体,类别2 1335-26-8 1476 4.8 过氧化锌ZINC PEROXIDE 氧化性固体,类别2 1314-22-3 1516 4.9 过氧化钡BARIUM PEROXIDE 氧化性固体,类别2 1304-29-6 1449 4.10 过氧化锶STRONTIUM PEROXIDE 氧化性固体,类别2 1314-18-7 1509 4.11 过氧化氢尿素UREA HYDROGEN PEROXIDE 氧化性固体,类别3 124-43-6 15114.12 过氧化二异丙苯[工业纯]DICUMYL PEROXIDE 有机过氧化物F型80-43-33109液态3110固态4.13 超氧化钾POTASSIUM SUPEROXIDE 氧化性固体,类别1 12030-88-5 24664.14 超氧化钠SODIUM SUPEROXIDE 氧化性固体,类别1 12034-12-7 2547 5燃料还原剂类5.1 环六亚甲基四胺[乌洛托品]HEXAMETHYLENETETRAMINE 易燃固体,类别3 100-97-0 13285.2 甲胺[无水] METHYLAMINE 易燃气体,类别1 74-89-5 1061 5.3 乙二胺ETHYLENE DIAMINE 易燃液体,类别3 107-15-3 1604 5.4 硫磺SULPHUR 易燃固体,类别2 7704-34-9 13505.5 铝粉[未涂层的]ALUMINIUM POWDERUNCOATED遇水放出易燃气体的物质,类别37429-90-5 13965.6 金属锂LITHIUM 遇水放出易燃气体的物质,类别17439-93-2 14155.7 金属钠SODIUM 遇水放出易燃气体的物质,类别17440-23-5 14285.8 金属钾POTASSIUM 遇水放出易燃气体的物质,类别17440-09-7 22575.9 金属锆粉[干燥的]ZIRCONIUM POWDER,DRY1.发火的:自燃固体,类别1;遇水放出易燃气体的物质,类别12.非发火的:自热物质,类别17440-67-7 20085.10 锑粉ANTIMONY POWDER 7440-36-0 28715.11 镁粉(发火的)MAGNESIUM POWDER(PYROPHORIC) 自燃固体,类别1;遇水放出易燃气体的物质,类别1;7439-95-45.12 镁合金粉MAGNESIUM ALLOYS POWDER 遇水放出易燃气体的物质,类别15.13 锌粉或锌尘(发火的)ZINC POWDER or ZINC DUST(PYROPHORIC)自燃固体,类别1;遇水放出易燃气体的物质,类别17440-66-6 14365.14 硅铝粉ALUMINIUM SILICON POWDER 遇水放出易燃气体的物质,类别313985.15 硼氢化钠SODIUM BOROHYDRIDE 遇水放出易燃气体的物质,类别116940-66-2 14265.16 硼氢化锂LITHIUM BOROHYDRIDE 遇水放出易燃气体的物质,类别116949-15-8 14135.17 硼氢化钾POTASSIUM BOROHYDRIDE 遇水放出易燃气体的物质,类别113762-51-1 18706其他6.1 苦氨酸钠[含水≥20%]SODIUM PICRAMATE 易燃固体,类别1 831-52-7 13496.2 高锰酸钠SODIUM PERMANGANATE 氧化性固体,类别2 10101-50-5 1503 6.3 高锰酸钾POTASSIUM PERMANGANATE 氧化性固体,类别2 7722-64-7 1490注:1.“主要的燃爆危险性分类”栏列出的化学品分类,是根据《化学品分类、警示标签和警示性说明安全规范(GB20576~20591)》等国家标准,对某种化学品燃烧爆炸危险性进行的分类,每一类由一个或多个类别组成。
第一部分化学品及企业标识化学品中文名:过氧化二异丙苯[含量≤52%,含惰性固体≥48%]化学品英文名:bis(α,α-dimethylbenzyl)peroxide (not more than 42%,and inert solid not less than 58%)化学品别名:-CASNo.:80-43-3ECNo.:201-279-3分子式:C18H22O2第二部分危险性概述紧急情况概述固体。
对皮肤有刺激性。
对眼睛有严重刺激性。
对水生物有剧毒,使用适当的容器,以预防污染环境。
对水生环境可能会引起长期有害作用。
使用适当的容器,以预防污染环境。
GHS危险性类别根据GB30000-2013化学品分类和标签规范系列标准(参阅第十六部分),该产品分类如下:皮肤腐蚀/刺激,类别2;眼损伤/眼刺激,类别2A;危害水生环境-急性毒性,类别1;危害水生环境-慢性毒性,类别1。
标签要素象形图警示词:警告危险信息:造成皮肤刺激,造成严重眼刺激,对水生生物毒性极大,对水生生物毒性极大并具有长期持续影响。
预防措施:作业后彻底清洗。
避免释放到环境中。
戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
事故响应:收集溢出物。
如发生皮肤刺激:求医/就诊。
如仍觉眼刺激:求医/就诊。
脱去被污染的衣服,清洗后方可重新使用。
如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
安全储存:不适用。
废弃处置:按照地方/区域/国家/国际规章处置内装物/容器。
物理化学危险:无资料健康危害:吸入该物质可能会引起对健康有害的影响或呼吸道不适。
意外食入本品可能对个体健康有害。
皮肤直接接触可造成皮肤刺激。
通过割伤、擦伤或病变处进入血液,可能产生全身损伤的有害作用。
本品能造成严重眼刺激。
眼睛直接接触可能会造成严重的炎症并伴随有疼痛。
眼睛直接接触本品可导致暂时不适。
环境危害:本品对水生生物毒性极大。
本品对水生生物毒性极大并具有长期持续影响。
过氧化二异丙苯(CAS号)一、引言过氧化二异丙苯(CAS号:78-63-7),也被称为二异丙基过氧化物,是一种常用的有机过氧化物。
它具有不同于普通过氧化物的稳定性和热分解特性,被广泛应用于化学、医药和其他领域。
本文将详细介绍过氧化二异丙苯的结构、性质、制备方法以及其在不同领域的应用。
二、结构与性质1.结构过氧化二异丙苯的化学式为C9H12O2,它的分子中含有两个异丙基过氧基(-O-O-)结构。
这种结构使得过氧化二异丙苯具有较高的稳定性和抗热分解性,能够在较高温度下稳定存在。
2.物理性质过氧化二异丙苯是一种无色液体,具有特殊的芳香气味。
它的密度为1.02 g/cm³,沸点为135-145°C,闪点为70°C。
过氧化二异丙苯在空气中能够缓慢氧化,但遇到明火或高温容易引发爆炸。
3.化学性质过氧化二异丙苯在高温下可以分解放出氧气,生成相应的酮化合物。
它具有较强的氧化性,可以与许多物质发生反应。
此外,过氧化二异丙苯也是一种良好的自由基引发剂,在聚合反应等过程中具有重要作用。
三、制备方法1.工业制备方法过氧化二异丙苯可以通过异丙苯与过氧化氢反应得到。
反应条件一般为常温下,通过控制过氧化氢与异丙苯的摩尔比例和反应时间,可以得到较高纯度的过氧化二异丙苯。
2.实验室制备方法过氧化二异丙苯也可以通过将过氧化叔丁醇与亚硒酰氯反应制备得到。
具体的反应方程式为:(CH₃)₃C(O)OC(O)C(CH₃)₃ + SeOCl₂ → (CH₃)₃COOC(O)C(CH₃)₃ + SeO2该方法相对于工业制备方法来说更适合实验室小规模制备,但产物的纯度较低。
四、应用领域过氧化二异丙苯在化学、医药和其他领域中有多种应用。
1.化学领域过氧化二异丙苯是一种理想的自由基引发剂,在聚合反应中被广泛应用。
它可以用于聚合物的合成、改性以及涂料、胶黏剂、塑料等化学品的制备。
2.医药领域过氧化二异丙苯在医药领域中有一定的应用价值。