E171欧盟标准
- 格式:doc
- 大小:92.00 KB
- 文档页数:2

欧洲标准ICS 25. 160. 40 EN 1711 2000年二月中文版焊缝的无损检测—用涡流阻抗平面分析检验焊缝该欧洲标准由CEN于1999年11月11日批准。
CEN成员国必须遵守CEN/CENELEC内部管理规定,这些规定的内容确定了该欧洲标准的不可动摇的国家标准的地位。
至今涉及这样的国家标准的作目录和书目参考可以向中央秘书处或任何CEN成员国索取。
该欧洲标准有三种官方版本(英文,法文,德文)。
任何由成员国负责翻译成自己国家语言并通知中央秘书处的版本有官方版本同样的地位。
CEN成员是国家标准委员会:奥地利,比利时,捷克,丹麦,法国,德国,希腊,冰岛,爱尔兰,意大利,卢森堡,荷兰,挪威,葡萄牙,西班牙,瑞典,瑞士和英国。
CEN欧洲标准化委员会中央秘书处:Stassart 路,36号,B-1050布鲁塞尔,比利时©2000 CEN 世界范围内以任何形式和方法开发的所有权利均属CEN国家成员目录前言 1 1.应用范围 1 2.参考标准 1 3.名词(术语和定义) 1 4.操作(检查)人员的要求 1 5.程序 1 6.一般应用 1 6.1必要的信息 1 6.2其它信息 2 6.3表面状态 2 6.4仪器(设备) 2 6.5检验程序 5 6.6检出缺陷的能力11 6.7不合格信号的(不可接受缺陷的)评估13 6.8其它材料的焊缝检验程序17 7.(检验)报告17 附录ZA:制定根本要求的本标的条款及EU指令的其它规定前言:本欧洲标准是由CEN/TC121技术委员会,焊接介绍委员会,DS的秘书处共同制订。
最迟于2000年8月份,应签署或出版相同的版使本标准应成为国家标准,到2000年8月份,国家标准中所有与本标准冲突的部分应废弃。
本标准是在欧洲委员会和欧洲自由贸易协会委托下由CEN制订的,适合EU指令中的根本要求。
与EU指令的关系,请看附录ZA,它是本标准的整体部分。
按照CEN/CENELEC内部管理规定,以下国家标准组织应执行本标准:奥地利,比利时,捷克,丹麦,法国,德国,希腊,冰岛,爱尔兰,意大利,卢森堡,荷兰,挪威,葡萄牙,西班牙,瑞典,瑞士和英国。

喹啉黄(Quinoline Yellow,E104)为水溶性偶氮类合成色素,在英国常用于冰糕、水果、蛋糕、巧克力、面包、奶酪酱、软饮料等食品的着色。
由于该色素可能导致儿童多动症,日本、美国及挪威禁用于食品,而我国仅允许在预调酒中添加,且最大使用量为0.1g/L[1]。
酸性绿S(Green S,E142)为三芳甲烷型着色剂。
2006年,Jamal通过体外细胞培养实验发现,酸性绿S能显著增加体细胞和生殖细胞的遗传突变概率[2],表明该色素可能具有诱变作用,提高癌症发病率。
此外,一些食品安全相关研究表明,酸性绿S还会导致哮喘,皮疹和多动症。
目前,挪威、瑞士、芬兰、日本、加拿大、美国禁止在食品中使用酸性绿S。
我国国家标准GB2760中未包括该色素,也不得在食品中使用。
专利蓝V(Patent Blue V,E131)也属于三芳甲烷型着色剂。
2007年,Donna等发现该色素对儿童成长健康不利,可能造成儿童多动症[3]。
澳大利亚、挪威、日本、新西兰及美国等禁止在食品中使用该色素,在我国也不允许使用。
2010年4月欧洲食品安全局认为色素Brown HT (E 155),也可用于软饮料、烘焙产品和糖果,以及用于酱油、调料和腌制品。
专家组认为应将该色素原来的ADI值减半,变为每千克体重1.5毫克(mg/kg bw)。
理由是动物长期食用Brown HT(摄入量低于以前的ADI值)后带来轻微降低体重增加的不利影响。
E 数字为数学常数看见: E (数学常数)。
________________________________________E 数字是简易格式定义为食品添加剂和通常被发现在食物标签。
' E ' 前缀表明添加剂是批准用于欧共体。
E 被编号的添加剂的加法对食品是持续的健康忧虑的问题许多年。
许多这样添加剂应该与混乱连接包括过敏、紧张的疾病、肠混乱、癌症、心脏病和关节炎。
在最近岁月许多这些添加剂也许是基因上修改过的进一步关心提出了(GM) 起源。

EN 1714欧洲标准1997年10月+A12002年5月英文版本焊接的无损测试焊缝的超声波探伤测试(包括修正案A1)该欧洲标准是由欧洲标准化委员会(CEN)于1997年8月2日通过的,并且修正案(A1)制定于2002年5月1日。
所有CEN成员国都必须遵守CEN/CENELEC国际条例的规定,该国际条例规定了将此欧洲标准所为一项国际规章,不得有任何修改的条件。
与该欧洲标准相关的所有最新表格以及文献参考资料,都可以通过向管理中心,或任一成员组织申请索取。
该欧洲标准有三个官方语言版本,英语,法语以及德语。
其它由任一成员国负责翻译成自己国家语言的版本,一经通知管理中心后,都具有和这三种官方语言版本一样的地位和效应。
CEN的国家标准机构成员国包括有:奥地利,比利时,捷克共和国,丹麦,芬兰,法国,德国,希腊,冰岛,爱尔兰,意大利,卢森堡公国,马耳他,荷兰,挪威,葡萄牙,西班牙,瑞典,瑞士,以及英国。
CEN欧洲标准化委员会管理中心:布鲁塞尔B-1050,rue de Stassart 362002 CEN 世界范围内任何形式或以任意方式进行的使用权均CEN成员国所有。
档案编号:EN 1714:1997 +A1:2002 E目录EN1714:1997前言 (2)EN1714:1997/A1:2002前言 (2)1.范围 (3)2.参考标准 (3)3.定义以及符号说明 (3)4.概述 (4)5.测试前所需了解的信息 (4)5.1指定项目.................................................................................... (4)5.2 测试前所需要了解的信息 (4)5.3书面测试程序 (5)6.工作人员资质和设备的要求 (5)6.`1工作人员资质要求 (5)6.2设备要求 (5)6.3探测器参数 (5)6.3.1频率 (5)6.3.2入射角 (5)6.3.3曲面探伤的适配探头 (5)7.探伤范围 (6)8.探伤面的准备 (6)9.母材测试 (6)10.范围以及灵敏度的设定 (7)10.1概述 (7)10.2参考标准 (7)10.3评定标准 (8)10.4过渡修正 (8)10.5信噪比 (8)11.测试等级 (8)12.测试方法 (9)12.1概述 (9)12.2手动扫描路径 (9)12.3垂直于测试表面的缺陷测试 (9)12.4显示的位置 (9)12.5显示结果的评定 (9)12.5.1概述 (9)12.5.2最大回波幅度 (9)12.5.3显示长度 (10)12.5.4显示高度 (10)12.5.5缺陷特征 (10)13.测试报告 (10)13.1概述 (10)13.2基本参数 (10)13.3与设备有关的信息 (10)13.4与测试方法有关的信息 (10)13.5测试结果 (11)附录A:(标准的)不同种类焊缝的测试等级 (11)附录ZA:(提示附录)该欧洲标准与其它EU指令的主要要求或规定相符的条款 (25)EN1714:1997前言该欧洲标准由CEN/TC 121 “焊接”技术委员会,由DS领导的秘书办公室制定。

欧洲氢能标准
欧洲氢能标准主要由欧洲标准化委员会(CEN)和欧洲电气委员会(CENELEC)共同制定和推广。
以下是欧洲氢能标准的一些主要内容:
1.燃料氢制备和储存的标准:包括氢气的生产、储存、液化、输送和充装等方面的标准,如EN 17178:2019,EN ISO 14687-2:2019,EN 15916:2019。
2.氢供应基础设施标准:包括液态氢和压缩氢的加注站、管道和输送设施的标准,如EN 13632:2013,EN 17123:2018。
3.氢能源利用标准:包括燃料电池汽车、燃料电池公交车、燃料电池发电机等领域的标准,如EN 62282,EN 15972:2014,EN 17124:2018。
4.安全技术标准:包括氢气的可靠性和安全性方面的标准,如EN 16723-2:2017,EN 17125:2018等。
此外,欧盟还有一些支持氢能源发展的政策和计划,如“氢谷”计划和“代表未来的氢能源”研究项目等,旨在加速欧洲氢能产业的发展,并为未来提供更可持续的能源解决方案。
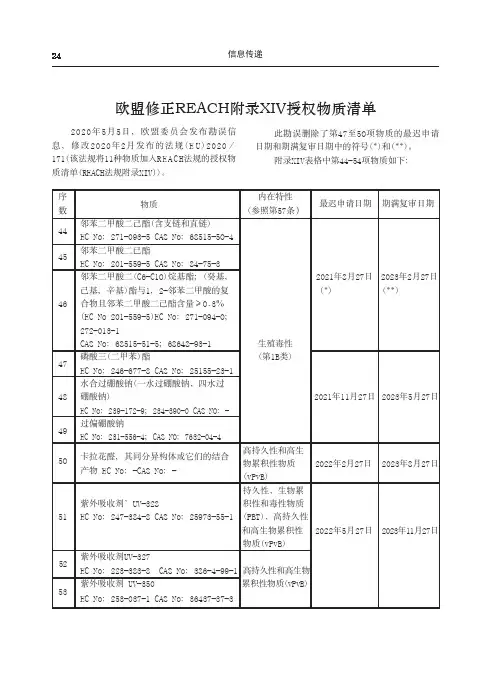
24序 物质内在特性 最迟申请日期 期满复审日期数(参照第57条)邻苯二甲酸二己酯(含支链和直链)44 EC No:271-093-5 CAS No:68515-5O-4邻苯二甲酸二已酯45 EC No:201-559-5 CAS No:84-75-3 邻苯二甲酸二(C6-C1O)烷基酯;(癸基, 2021年8月27日 2023年2月27日 己基,辛基)酯与1,2-邻苯二甲酸的复(*)(**)46 合物且邻苯二甲酸二己酯含量≥O.3%(EC No 201-559-5)EC No:271-094-O; 272-013-1CAS No:68515-51-5;68648-93-1生殖毒性47磷酸三(二甲苯)酯 (第1B类) EC No:246-677-8 CAS No:25155-23-1 水合过硼酸钠(一水过硼酸钠、四水过 48 硼酸钠)2021年11月27日 2023年5月27日 EC No:239-172-9;234-390-0 CAS NO:-49 过偏硼酸钠EC No:231-556-4;CAS NO:7632-04-45O卡拉花醛,其同分异构体或它们的结合高持久性和高生产物 EC No:-CAS No:-物累积性物质 2022年2月27日 2023年8月27日 (vPvB)持久性、生物累紫外吸收剂’UV-328 积性和毒性物质51 EC No:247-384-8 CAS No:25973-55-1 (PBT)、高持久性和高生物累积性 2022年5月27日 2023年11月27日物质(vPvB)紫外吸收剂UV-32752 EC No:223-383-8 CAS No:386-4-99-1 高持久性和高生物 紫外吸收剂 UV-350累积性物质(vPvB)53EC No:253-037-1 CAS No:36437-37-3欧盟修正REACH附录XIV授权物质清单2020年5月5日,欧盟委员会发布勘误信息,修改2020年2月发布的法规(E U)2020/171(该法规将11种物质加入R E A C H法规的授权物质清单(REACH法规附录XIV))。

欧洲标准焊缝的无损检测焊接接头的超声波检测EN 1714:1998EN 17141范围本欧洲标准规定厚度8mm以上金属材料(超声衰减特别是散射衰减较小)的熔化焊缝的手工超声检验方法。
主要用于焊缝合母材均为铁素体的全焊透焊接接头。
如有规定且合适,经签约单位同意,有关技术也可用于:(1)除上述材料以外的其他材料;(2)部分焊透焊缝;(3)自动检测设备;本标准规定的与材料有关的超声波参数,是根据纵波声速为5920±50m/s,横波声速为3255±30m/s的钢材得出的。
检测不同声速的材料时,就必须考虑到这一点。
本标准规定的四种检验等级,每种等级对应于不同的缺陷检出概率。
有关选择检验等级A、B、C的导则见附录A。
适于具体应用的检验等级要求需遵循本标准的一般要求,并征得签约单位同意。
经签约单位同意后,本标准允许采用下列方法对缺陷讯号进行评价和验收:(1)主要根据讯号的回波幅度和指示长度;(2)用探头移动法对缺陷定性、定量。
2引用标准本欧洲标准引用了其他现行标准或旧标准拟成新条文。
这些引用标准引述于文中相应处,篇名如下。
对旧标准只引用含修改条文的修订版,而对现行标准则引用最新版。
²EN 473 无损检测人员资格认证总则²EN 583—1 超声波检验(Ⅰ)总则²EN 583—2 超声波检验(Ⅱ)灵敏度和时间轴调整²EN 1330—4 超声波检验术语²EN 1712 焊缝超声波检验验收等级²EN 1713 焊缝超声波检验缺陷定性²EN 12062 金属材料焊缝NDT总则²EN 25617 钢电弧焊缝缺陷质量分等导则(ISO5817:1992)3定义与符号本标准采用²EN 12062和²EN 1330—4给出的定义。
定量参数和符号见表1。
(2)材质和产品制造形式(铸件、锻件、轧制件);(3)进行检测的制造阶段,包括热处理等;(4)焊后热处理时间、范围;(5)接头坡口和尺寸;(6)表面状态要求;(7)焊接工艺或施焊数据;(8)报告要求;(9)验收等级;(10)检测范围,包括对横向缺陷的要求;(11)检测等级;(12)人员资格等级;(13)发现不合格缺陷后的返修程序;5.3 书面检测工艺程序本标准中的规定和要求一般能满足书面工艺规程的要求。

食用二氧化钛俗称白色素,是食品级二氧化钛的简称。
它是较高分性非包膜处理的锐钛型二氧化钛,也称作食品级钛白粉,或者医药级钛白粉,作为着色剂和食品增白剂使用,无毒、无味、白色粉末状,有时候为了方便分散,也制成液体状,广泛应用于制药,食品和化妆品行业中。
基本用途医药:药物制剂中,可作为白色着色剂制备薄膜包衣混悬液,糖包衣和明胶胶囊剂,也可与其它着色剂混合使用,应用于皮肤用制剂,也可取代淀粉用做药物的赋型剂。
食品:用于糖果包衣,凉果,果冻,口香糖,无甜味剂型固体饮料和浓缩型固体饮料中,含乳饮料,膨化食品。
蜜饯(话化类),果酱,沙拉酱,蛋黄酱等需要增白的食品中。
.化妆品:粉底,粉饼,防晒霜,眼影,口红,唇膏,牙膏,爽身粉,痱子粉,膏霜,白色香皂。
在2022年1月14日,欧盟委员会通过了一项禁令,禁止再将二氧化钛用作食品添加剂(E171)。
这项禁令将在六个月的过渡期结束后正式生效。
这意味着,从今年夏天开始,二氧化钛这种添加剂将不再允许被添加到食品中。
负责健康和食品安全的委员Stella Kyriakides说:“对于我们公民吃的食品的安全性和他们的健康是不容协商的。
这也是为什么我们需要确保执行对消费者最高安全标准的严格和持续的审查。
这项工作的基石就是确保只有安全的、有可靠科学依据支持的物质才能最终到达我们的餐桌。
通过今天的这项禁令,我们将去除一种不再被认为是安全的食品添加剂。
我希望各成员国当局共同合作,来确保食品经营者停止在食品中使用E171。
”二氧化钛是钛的天然氧化物,主要用作各种不同应用的着色剂。
几十年来,二氧化钛一直被用来给许多食物增添白色,从烘焙食品和三明治酱到汤、酱汁、沙拉酱和食品补充剂。
在2020年3月,欧盟委员会就要求欧洲食品安全局(European Food Safety Authority,简称EFSA)更新其2016年关于二氧化钛的意见(E171)。
尽管在2016年,欧洲食品安全局没有指出任何安全问题,但它发现了一些数据空白和不确定性,特别是关于颗粒大小。

LIST OF ADDITIVES CURRENTLY PERMITTED IN FOODIN THE EUROPEAN UNION AND THEIR E NUMBERS.The additives below are listed in groups for ease of reference.The groups are as follows:1. Antioxidants2. Colours3. Emulsifiers, Stabilisers, Thickeners and Gelling agents.4. Preservatives5. Sweeteners6. OthersWhen shopping you can find an additive on the list by first looking for its category, such as colour or preservative, and then looking for its name or number.This list does not in any way supplement the law in this area, nor constitute legal guidance.For information on the specifications and likely sources of these additives, please contact Mark Willis (020 7276 8559). For further information on legislation controlling the use of food additives, please contact John Caseley (020 7276 8591) for sweeteners and Andy Spencer (020 7276 8560) for the other categories.ColoursE100CurcuminE101(i) Riboflavin(ii) Riboflavin-5'-phosphateE102TartrazineE104Quinoline yellowE110Sunset Yellow FCF; Orange Yellow SE120Cochineal; Carminic acid; CarminesE122Azorubine; Carmoisine.E123 AmaranthE124Ponceau 4R; Cochineal Red AE127ErythrosineE128Red 2GE129Allura Red ACE131Patent Blue VE132lndigotine; Indigo CarmineE133Brilliant Blue FCFE141Chlorophylls and chlorophyllinsE141Copper complexes of chlorophyll and chlorophyllins E142Green SE150a Plain caramelE150b Caustic sulphite caramelE150c Ammonia caramelE150d Sulphite ammonia caramelE151Brilliant Black BN; Black PNE153Vegetable carbonE154Brown FKE155Brown HTE160a CarotenesE160b Annatto; Bixin; NorbixinE160c Paprika extract; Capsanthian; CapsorubinE160d LycopeneE160e Beta-apo-8'-carotenal (C30)E160e Ethyl ester of beta-apo-8'-carotenoic acid (C30)E161b LuteinE161g CanthaxanthinE162Beetroot Red; Betanin.E163 AnthocyaninsE170Calcium carbonateE171Titanium dioxideE172Iron oxides and hydroxidesE173AluminiumE174SilverE175GoldE180Litholrubine BK.PreservativesE200Sorbic acidE202Potassium sorbateE203Calcium sorbateE210Benzoic acidE211Sodium benzoateE212Potassium benzoateE213Calcium benzoateE214Ethyl p-hydroxybenzoateE215Sodium ethyl p-hydroxybenzoateE216Propyl p-hydroxybenzoateE217Sodium propyl p-hydroxybenzoateE218Methyl p-hydroxybenzoateE219Sodium methyl p-hydroxybenzoate E220Sulphur dioxideE221Sodium sulphiteE222Sodium hydrogen sulphiteE223Sodium metabisuiphiteE224Potassium metabisulphiteE226Calcium sulphiteE227Calcium hydrogen sulphiteE228Potassium hydrogen sulphiteE230Biphenyl; diphenylE231Orthophenyl phenolE232Sodium orthophenyl phenolE234NisinE235NatamycinE239Hexamethylene tetramine.E242Dimethyl dicarbonateE249Potassium nitriteE250Sodium nitriteE251Sodium nitrateE252Potassium nitrateE280Propionic acidE281Sodium propionateE282Calcium propionateE283Potassium propionateE284Boric acidE285Sodium tetraborate; boraxE1105Lysozyme.AntioxidantsE300Ascorbic acidE301Sodium ascorbateE302Calcium ascorbateE304Fatty acid esters of ascorbic acid E306TocopherolsE307Alpha-tocopherolE308Gamma-tocopherolE309Delta-tocopherolE310Propyl gallateE311Octyl gallateE312Dodecyl gallateE315Erythorbic acidE316Sodium erythorbateE320Butylated hydroxyanisole (BHA)E321Butylated hydroxytoluene (BHT).SweetenersE420(i) Sorbitol(ii) Sorbitol syrupE421MannitolE953lsomaltE965(i) Maltitol(ii) Maltitol syrupE966LactitolE967XylitolE950Acesulfame KE951AspartameE952Cyclamic acid and its Na and Ca saltsE954Saccharin and its Na, K and Ca saltsE957ThaumatinE959Neohesperidine DC.Emulsifiers, Stabilisers, Thickenersand Gelling AgentsE322LecithinsE400Alginic acidE401Sodium alginateE402Potassium alginateE403Ammonium alginateE404Calcium alginateE405Propane-1,2-diol alginateE406AgarE407CarrageenanE407a Processed eucheuma seaweedE410Locust bean gum; carob gumE412Guar gumE413TragacanthE414Acacia gum; gum arabicE415Xanthan gumE416Karaya gumE417Tara gumE418Gellan gumE425KonjacE432Polyoxyethylene sorbitan monolaurate; Polysorbate 20E433Polyoxyethylene sorbitan mono-oleate; Polysorbate 80E434Polyoxyethylene sorbitan monopalmitate; Polysorbate 40 E435Polyoxyethylene sorbitan monostearate; Polysorbate 60E436Polyoxyethylene sorbitan tristearate; Polysorbate 65E440Pectins.E442Ammonium phosphatidesE444Sucrose acetate isobutyrateE445Glycerol esters of wood rosinsE460CelluloseE461Methyl celluloseE463Hydroxypropyl celluloseE464Hydroxypropyl methyl celluloseE465Ethyl methyl celluloseE466Carboxy methyl cellulose Sodium arboxy methyl celluloseE468Crosslinked sodium carboxy methyl celluloseE469Enzymatically hydrolysed carboxy methyl celluloseE470a Sodium, potassium and calcium salts of fatty acidsE470b Magnesium salts of fatty acidsE471Mono- and diglycerides of fatty acidsE472a Acetic acid esters of mono- and diglycerides of fatty acidsE472b Lactic acid esters of mono- and diglycerides of fatty acidsE472c Citric acid esters of mono- and diglycerides of fatty acidsE472d Tartaric acid esters of mono- and diglycerides of fatty acidsE472e Mono- and diacetyltartaric acid esters of mono- anddiglycerides of fatty acidsE472f Mixed acetic and tartaric acid esters of mono- anddiglycerides of fatty acidsE473Sucrose esters of fatty acidsE474SucroglyceridesE475Polyglycerol esters of fatty acidsE476Polyglycerol polyricinoleate.E477 Propane-1,2-diol esters offatty acidsE481Sodium stearoyl-2-lactylateE482Calcium stearoyl-2-lactylateE483Stearyl tartrateE491Sorbitan monostearateE492Sorbitan tristearateE493Sorbitan monolaurateE494Sorbitan monooleateE495Sorbitan monopalmitateE1103Invertase.OthersAcid, acidity regulators, anti-caking agents, anti-foaming agents, bulking agents, carriers and carrier solvents, emulsifying salts, firming agents, flavour enhancers, flour treatment agents, foaming agents,glazing agents, humectants, modifiedstarches, packaging gases, propellants, raising agents and sequestrants. E170Calcium carbonatesE260Acetic acidE261Potassium acetateE262Sodium acetateE263Calcium acetateE270Lactic acidE290Carbon dioxideE296Malic acidE297Fumaric acidE325Sodium lactateE326Potassium lactateE327Calcium lactateE330Citric acidE331Sodium citratesE332Potassium citratesE333Calcium citratesE334Tartaric acid (L-(+))E335Sodium tartratesE336Potassium tartratesE337Sodium potassium tartrateE338Phosphoric acidE339Sodium phosphates.E340Potassium phosphatesE341Calcium phosphatesE343Magnesium phosphatesE350Sodium malatesE351Potassium malateE352Calcium malatesE353Metatartaric acidE354Calcium tartrateE355Adipic acidE356Sodium adipateE357Potassium adipateE363Succinic acidE380Triammonium citrateE385Calcium disodium ethylene diamine tetra-acetate;calcium disodium EDTAE422GlycerolE431Polyoxyethylene (40) stearateE450DiphosphatesE451TriphosphatesE452PolyphosphatesE459Beta-cyclodextrinE479b Thermally oxidised soya bean oil interacted withmono and diglycerides of fatty acidsE500Sodium carbonatesE501Potassium carbonatesE503Ammonium carbonatesE504Magnesium carbonatesE507Hydrochloric acidE508Potassium chlorideE509Calcium chloride.E511Magnesium chlorideE512Stannous chlorideE513Sulphuric acidE514Sodium sulphatesE515Potassium sulphatesE516Calcium sulphateE517Ammonium sulphateE520Aluminium sulphateE521Aluminium sodium sulphateE522Aluminium potassium sulphateE523Aluminium ammonium sulphateE524Sodium hydroxideE525Potassium hydroxideE526Calcium hydroxideE527Ammonium hydroxideE528Magnesium hydroxideE529Calcium oxideE530Magnesium oxideE535Sodium ferrocyanideE536Potassium ferrocyanideE538Calcium ferrocyanideE541Sodium aluminium phosphateE551Silicon dioxideE 552Calcium silicateE553a(i) Magnesium silicate(ii) Magnesium trisilicateE553b TalcE554Sodium aluminium silicate.E555Potassium aluminium silicateE556Aluminium calcium silicateE558BentoniteE559Aluminium silicate; KaolinE570Fatty acidsE574Gluconic acidE575Glucono delta-lactoneE576Sodium gluconateE577Potassium gluconateE578Calcium gluconateE579Ferrous gluconateE585Ferrous lactateE620Glutamic acidE621Monosodium glutamateE622Monopotassium glutamateE623Calcium diglutamateE624Monoammonium glutamate E625Magnesium diglutamateE626Guanylic acidE627Disodium guanylateE628Dipotassium guanylateE629Calcium guanylateE630lnosinic acidE631Disodium inosinateE632Dipotassium inosinateE633Calcium inosinateE634Calcium 5'-ribonucleotidesE635Disodium 5'-ribonucieotides. E640Glycine and its sodium salt E650Zinc acetateE900DimethylpolysiloxaneE901Beeswax, white and yellowE902Candelilla waxE903Carnauba waxE904ShellacE905Microcrystalline waxE912Montan acid estersE914Oxidised Polyethylene waxE920L-CysteineE927b CarbamideE938ArgonE939HeliumE941NitrogenE942Nitrous oxideE943a ButaneE943b Iso-butaneE944PropaneE948OxygenE949HydrogenE999Quillaia extractE1200PolydextroseE1201PolyvinylpyrrolidoneE1202PolyvinylpolypyrrolidoneE1404Oxidised starchE1410Monostarch phosphateE1412Distarch phosphate.E1413Phosphated distarch phosphateE1414Acetylated starchE1420Acetylated StarchE1422 Acetylated distarch adipateE1440 Hydroxyl propyl starchE1442 Hydroxy propyl distarch phosphate E1450 Starch sodium octenyl succinateE1451Acetylated oxidised starchPolyethylene glycol 6000E1505Triethyl citrateE1518Glyceryl triacetate; triacetinE1520Propan-1,2-diol; propylene glycol。

食品中禁止使用二氧化钛2022年8月7日,欧盟全面禁用二氧化钛作为食品添加剂的法规将正式生效。
这意味着,含有二氧化钛的食品将不再获批生产并投放于欧盟市场。
作为常见的食品添加物之一,二氧化钛已沿用多年并被广泛应用于食物(如糖果及糕点)及日用品(如口罩、化妆品)的增白。
早在2021年10月,经过EFSA(欧洲食品安全局)评估后,欧盟决定批准禁止二氧化钛作为食品添加物的提案。
此前,EFSA曾表示综合不同的研究证据不排除二氧化钛颗粒具有遗传毒性,因此二氧化钛作为食物添加剂的安全性存疑。
“被欧盟剔除食品添加剂许可名单”、“成为消费者一纸诉状上的元凶”“冲上热搜榜掀起舆论风波”,二氧化钛瞬间成为众矢之的。
但目前,在中国、英国、美国、加拿大等地,二氧化钛仍被视作合法的食品添加剂。
那么,二氧化钛究竟有何妙用,又可能产生怎样的风险?在天然健康成为主旋律的消费大趋势下,二氧化钛的替代品纷纷涌现,能否加速食品添加剂迭代步伐?全球各国面对食品添加剂取态不一的安全监管,又将给整个行业带来怎样的思考?01二氧化钛浮沉史:增白数十载,质疑声不断二氧化钛作为一种无机物,性质稳定,拥有不透明性以及优异的白度、光亮度表现,被认为是最佳的白色颜料。
二氧化钛(用作食物色素时,被称作E171)作为色素在食品行业应用已有50余年的历史,主要作为增白剂和增亮剂使用。
由于添加适度的二氧化钛会让食物的白色部分看起来更加明亮,常见的食品例如香口胶、糖果、糕饼装饰、蛋黄酱、雪糕、咖啡伴侣等都有机会找到二氧化钛的踪影。
在中国《食品安全国家标准-食品添加剂使用标准》(GB2760-2014)中,二氧化钛是唯一被允许的白色着色剂。
过去数十年间,二氧化钛与食品间的联系密切而普遍,但人们始终对其安全性怀有疑虑。
2014年,美国食品药品监督管理局(FDA)Tao Chen博士团队研究认为,基于动物实验,纳米级二氧化钛存在“遗传毒性”,可能会造成细胞基因损伤。
2019年,法国食品、环境、职业健康与安全署(Agency for Food, Environmental and Occupational Health & Safety)就二氧化钛的安全性做出判断:尽管二氧化钛早已得到使用批准,但缺乏足够证据证明它的食用安全性。
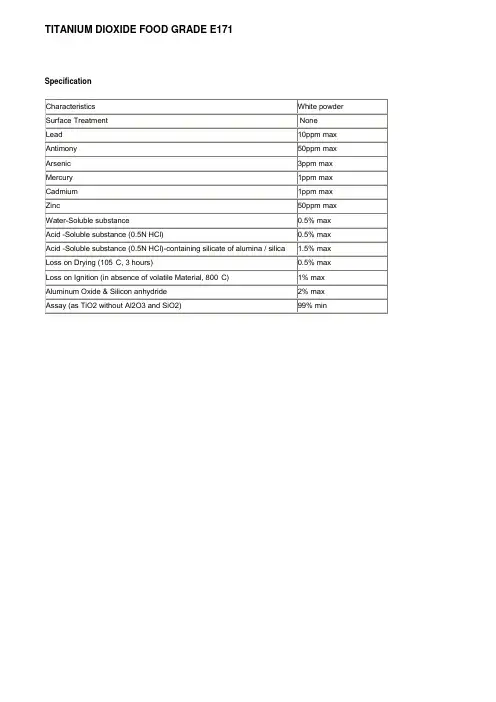
TITANIUM DIOXIDE FOOD GRADE E171 Specification
二氧化钛(钛白粉)
食品、化妆品级
Titanium Dioxide Food Grade
(欧洲食品E171)
牌号Brand :
化学名称Chemical name:二氧化钛Titanium Dioxide
分子式Molecular formula:TiO2
分子量Molecular weight:79.88(按1987年国际原子量international atomic weight in
1987')
用途Uses:本品为食用白色颜料,具有颜料性能好,有害杂质含量低等优点,是化妆品和食品着色的添加剂,同时也是制造油漆、油墨的优良材料。
It is white
pigment for food, with good pigment performance and low toxic
impurity content; used as additive of coloring of cosmetics and food, also as good material to make printing oil and paint.
优点Advantages:具有粒度均匀,分散性好的优点。
Even particle size and good
dispersion.
产品标准Standard:欧洲食品E171
技术指标符合下列要求The technical indexes satisfy the following requirements:。

欧洲标准EN 1713:1998/A1:2002焊缝无损检测——超声波检测焊缝中缺陷的特征目录序言1范围2标准参考3种类与定义3.1概述3.2使用惯例3.3回波高度标准3.3.1低波幅(步骤1)3.3.2高波幅(步骤2)3.4定向反射特征条件(步骤3)3.5回波静态波形条件(步骤4)3.6回波动态波形条件(步骤5)3.7补充检测附录A(规范性附录)焊缝内部缺陷分类—分类流程图附录B(资料性附录)扫查入射角附录C(资料性附录)反射体的基本动态回波波形附录ZA(资料性附录)选择欧标条款的基本要求或欧盟其它规程的规定序言平面状或非平面状缺陷显示的分类应依据以下几个参数:——焊接技术:——显示的几何位置;——最大回波高度;——定向反射特性;——静态回波波形(即A显示);——动态回波波形。
分类的步骤包括检测每个参数(不同于其它参数),以得到一个正确的结论。
作为指导,附录A的流程图给出了适用于焊缝内部显示缺陷的分类方法。
流程图应结合上述的基本参数来应用。
若规范有规定,最好根据EN1712标准要求来完成这些分类。
1.范围本标准给了一个流程框图,见附录A,此流程图专用于平面状或非平面状内部显示缺陷的分类。
本标准仅适用于距焊接接头(未打磨)表面5mm以下的显示缺陷的定位,见图1。
1焊缝(定位)范围图1:显示缺陷的定位2.参考标准本欧洲标准引用了来自其它标准中注册日期或未注册日期的资料和条款。
这些标准资料引用在本文中适合的位置,篇名如下。
对于注册日期的引用标准,只适用于通过补充或修订内容并入引用标准后,才可用于本标准。
EN 1712 焊缝的无损检测—焊接接头的超声波检验—验收等级3.种类定义3.1概述通过几个不同条件的逐步应用来完成(缺陷)分类:——回波幅度;——定向反射特性;——静态回波波形(A显示);——动态回波波形。
当满足上述条件之一时,流程图流程即终止。
作为一般原则,分类时使用的探头应与检测时使用的探头相同。
II(Non-legislative acts)REGULATIONSCOMMISSION REGULATION (EU) 2022/63of 14 January 2022amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of theCouncil as regards the food additive titanium dioxide (E 171)(Text with EEA relevance)THE EUROPEAN COMMISSION,Having regard to the Treaty on the Functioning of the European Union,Having regard to Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008on food additives(1), and in particular Article 10(3) thereof,Having regard to Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings(2), and in particular Article 7(5) thereof,Whereas:(1)Annex II to Regulation (EC) No 1333/2008 lays down a Union list of food additives approved for use in foods andtheir conditions of use.(2)Annex III to Regulation (EC) No 1333/2008 lays down a Union list of food additives approved for use in foodadditives, food enzymes, food flavourings, nutrients and their conditions of use.(3)Titanium dioxide (E 171) is a substance authorised as a colour in certain foods, in accordance with Annex II toRegulation (EC) No 1333/2008.(4)Pursuant to Article 3(1) of Regulation (EC) No 1331/2008, the Union list of food additives may be updated either onthe initiative of the Commission or following an application.(5)Article 32(1) of Regulation (EC) No 1333/2008 provides that all food additives that were already permitted in theUnion before 20 January 2009are subject to a new risk assessment by the European Food Safety Authority (‘the Authority’).(6)On 14 September 2016, the Authority published a scientific opinion on the re-evaluation of the safety of titaniumdioxide (E 171) as a food additive(3)concluding that the margins of safety calculated in the opinion would not be of concern. Nevertheless, the Authority recommended additional toxicological testing, an extended 90-day study or a multigeneration or extended-one generation reproduction toxicity study according to the current OECD guidelines, in order to be able to establish a health-based guidance value (acceptable daily intake – ADI) for titanium dioxide (E 171). The Authority also recommended amendments to the Union specifications for titanium dioxide (E 171) by introducing a characterisation of the particle size distribution and the percentage of particles in the nanoscale present in titanium dioxide (E 171) used as a food additive, and revising the maximum limits for impurities of toxic elements.(1)OJ L 354, 31.12.2008, p. 16.(2)OJ L 354, 31.12.2008, p. 1.(7)On 30 January 2017, the Commission launched a public call for scientific and technological data on titaniumdioxide (E 171), targeting the data needs identified in the scientific opinion on the re-evaluation of this substance asa food additive.(8)On 2 October 2017and 29 June 2018, in view of the recommendations made by the Authority, business operatorsmade a proposal for the amendment of the specifications for titanium dioxide (E 171) and submitted the necessary data. On 7 August 2018, the Commission requested the Authority to provide a scientific opinion on whether the data provided adequately support the proposed amendment of the specifications for titanium dioxide (E 171). (9)On 12 July 2019, the Authority published a scientific opinion on the proposed amendments of the specifications fortitanium dioxide (E 171) used as a food additive. The Authority concluded on additional parameters related to particle size distribution to be included in the specifications and recommended a revision of the definition of the food additive titanium dioxide (E 171) in the Union specifications. The Authority also concluded that, based on the proposed change in the specifications, revisiting the toxicological database on titanium dioxide (E 171) as a food additive should consequently be conducted in line with the data requirements specified in the 2018 ‘Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain’(4).(10)On 6 March 2020, the Commission requested the Authority to assess the safety of the food additive titanium dioxide(E 171), taking into account the proposed amendments of the specifications, the data from an extended onegeneration reproductive study submitted by a consortium of interested business operators in reply to the public call for data launched in 2017 as well as all new relevant data available since the completion of the re-evaluation of titanium dioxide (E 171) in 2016, including the data considered to be in line with the data requirements specified in the 2018 Guidance on nanotechnology.(11)On 6 May 2021, the Authority published a scientific opinion on the safety assessment of titanium dioxide (E 171) asa food additive(5). In light of the opinion on the proposed amendments of the specifications and following the 2018Guidance on nanotechnology, the opinion also takes into account, in addition to all new relevant data, the data on the potential genotoxicity of titanium dioxide nanoparticles published before 2016, that had not previously been identified as relevant for the 2016 re-evaluation. In its opinion the Authority indicated that, based on all the evidence available, a concern for genotoxicity could not be ruled out, and given the many uncertainties, it concluded that titanium dioxide (E 171) can no longer be considered safe when used as a food additive. The Authority neither identified nor recommended any new studies that could alleviate the genotoxicity concern and other remaining uncertainties.(12)In light of the conclusion of the 2021 Authority’s opinion about the safety of titanium dioxide (E 171) when used asa food additive, it is appropriate to remove the authorisation to use titanium dioxide (E 171) in foods. Accordingly,titanium dioxide (E 171) may no longer be used in foods. As titanium dioxide (E 171) would no longer be authorised for use in foods, it is also appropriate to remove the reference to it from the entry on the use of potassium aluminium silicate (E 555) as a carrier laid down in Part 1 of Annex III to Regulation (EC) No 1333/2008.(13)However, given that the Authority did not identify an immediate health concern linked to titanium dioxide (E 171)used as a food additive and in order to allow for a smooth transition, it is appropriate that foods that contain titanium dioxide (E 171) used in accordance with the rules applicable before the date of entry into force of this Regulation may be placed on the market until six months after that date. Those foods may then continue to be marketed until their date of minimum durability or ‘use by’ date.(4)EFSA Journal 2018;16(7):5327.(14)Directive 2009/35/EC of the European Parliament and of the Council(6)restricts the use of colours in human andveterinary medicinal products to those authorised in accordance with Regulation (EC) No 1333/2008 on food additives, for which specifications are laid down in Commission Regulation (EU) No 231/2012(7). Uses of excipients other than colours in medicinal products are subject to the Union rules on medicinal products and are evaluated as part of the overall benefit risk profile of a medicinal product.(15)In response to a request from the Commission, the European Medicines Agency (EMA) provided on 8 September2021a scientific analysis on the technical purpose of the use of titanium dioxide (E 171) in medicinal products, the feasibility of replacement and possible timeframes for alternatives. In its conclusions, EMA indicated that titanium dioxide is mainly used in medicinal products as a colour and opacifier, even if it has multiple functions. It also stressed that titanium dioxide is used frequently in a number of essential medicinal products in oral-solid and oral semi-solid dosage forms. EMA also stressed that, from a technical point of view, it should be possible to find alternatives to replace titanium dioxide (E 171)-containing coatings, both as colour and for other uses. However, it also underlined that its feasibility is not confirmed at this stage, as replacing titanium dioxide (E 171) would impact in a negative manner the quality, safety and efficacy of medicinal products. EMA highlighted the need to carefully assess alternatives, notably to ensure their compatibility with the various components of individual pharmaceutical products. The replacement of titanium dioxide (E 171) in authorised medicinal products would require an individual review and assessment, possibly requiring bioequivalence studies. Furthermore, EMA concluded that it is difficult at this stage to recommend a precise transition period timeframe for the replacement of titanium dioxide (E 171) used in medicinal products, as the time needed to reformulate each individual product could take several years, depending on the complexity of reformulation and studies required. Finally, considering the scale of the use of this excipient and the volume of products impacted, and taking into account global supply chains, EMA stressed that a requirement to replace titanium dioxide (E 171) would almost certainly cause significant medicines shortages on the Union market.(16)On the basis of the EMA scientific analysis, and in order to avoid shortages of medicinal products that could haveimpacts on public health, titanium dioxide (E 171) should remain provisionally on the list of authorised additives to allow its use in medicinal products as a colour, pending the development of adequate alternatives to replace it while ensuring the quality, safety and efficacy of the medicinal products concerned. During this time, titanium dioxide (E 171) should however be included in the list of colours that may not be sold directly to consumers.(17)It is of critical importance that the pharmaceutical industry makes any possible efforts to accelerate the research anddevelopment of alternatives that would be used as a replacement for titanium dioxide (E 171) in medicinal products, and to submit the necessary variation to the terms of the marketing authorisations concerned. In the absence of such efforts, competent authorities may request the concerned stakeholders to submit objective and verifiable reason explaining the non-feasibility of the replacement.(18)The Commission is committed to review the necessity to maintain titanium dioxide (E 171) or otherwise delete itfrom the Union list of food additives for exclusive use as a colour in medicinal products within three years after the date of entering into force of this Regulation. This review should be based on an updated assessment of the EMA to be performed before 1 April 2024. It should take into account the progress made during this period to develop alternatives to titanium dioxide (E 171) in medicinal products both for new products and for replacing it in authorised products, and possible impacts on quality, safety and efficacy, as well as on the availability of medicinal products. Where replacement of titanium dioxide (E 171) in medicinal products has not taken place or been initiated within this period, only objective verifiable reasons related to the lack of feasibility of its replacement should be taken into account.(19)Annexes II and III to Regulation (EC) No 1333/2008 should therefore be amended accordingly.(20)The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee onPlants, Animals, Food and Feed,(6)Directive 2009/35/EC of the European Parliament and of the Council of 23 April 2009 on the colouring matters which may be addedto medicinal products (OJ L 109, 30.4.2009, p. 10).(7)Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and IIIHAS ADOPTED THIS REGULATION:Article 1Annexes II and III to Regulation (EC) No 1333/2008 are amended in accordance with the Annex to this Regulation.Article 2Until 7 August 2022, foods produced in accordance with the rules applicable before 7 February 2022may continue to be placed on the market. After that date, they may remain on the market until their date of minimum durability or ‘use by’ date.Article 3The Commission shall, following consultation on the European Medicines Agency, review the necessity to maintain titanium dioxide (E 171) or to delete it from the Union list of food additives for the exclusive use as colour in medicinal products in Part B of Annex II to Regulation (EC) No 1333/2008 within three years after the date of entering into force of this Regulation.Article 4This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.This Regulation shall be binding in its entirety and directly applicable in all Member States.Done at Brussels, 14 January 2022.For the CommissionThe PresidentUrsula VON DER LEYENANNEX1.Annex II to Regulation (EC) No 1333/2008 is amended as follows:(a)in point 2 of Part A, point 5 is replaced by the following:‘5.The colours E 123, E 127, E 160b(i), E 160b(ii), E 161g, E 171, E 173 and E 180 and mixtures thereof may not be sold directly to the consumer.’(b)in Part B, point 1 ‘Colours’ is amended as follows:1.the entry for the food additive E 171 (Titanium dioxide) is replaced by the following:2.the following footnote (**) is added after footnote (*):‘(**)Titanium dioxide is not authorised in the food categories listed in Part D and E. The substance is in list B1 because it is used in medicinal products in accordance with Directive 2009/35/EC of the EuropeanParliament and of the Council (OJ L 109, 30.4.2009, p. 10).’;(c)in Part C, point (2) ‘Group II: Food colours authorised at quantum satis’, the entry for the food additive E 171(Titanium dioxide) is deleted;(d)Part E is amended as follows:1.In category 04.2.4.1 (Fruit and vegetable preparations excluding compote), the entry concerning the foodadditive E 171 (Titanium dioxide) is deleted;2.In category 09.2 (Processed fish and fishery products including molluscs and crustaceans), the three entriesconcerning the food additive E 171 (Titanium dioxide) are deleted;2.In Part 1 of Annex III to Regulation (EC) No 1333/2008, the entry concerning the food additive E 555 (Potassiumaluminium silicate) is replaced by the following:。
•E100 Curcumin•E101 (i) Riboflavin (ii) Riboflavin-5'-phosphate•E102 Tartrazine•E104 Quinoline Yellow•E110 Sunset Yellow FCF, Orange Yellow S•E120 Cochineal, Carminic acid, Carmines•E122 Azorubine, Carmoisine•E123 Amaranth•E124 Ponceau 4R, Cochineal Red A•E127 Erythrosine•E128 Red 2G•E129 Allura Red AC•E131 Patent Blue V•E132 Indigotine, Indigo carmine•E133 Brilliant Blue FCF•E140 Chlorophylis and Chlorophyllins: (i) Chlorophylls (ii) Chlorophyllins•E141 Copper complexes of chlorophylls and chlorophyllins (i) Copper complexes of chlorophylls (ii) Copper complexes of chlorophyllins •E142 Greens S•E150a Plain caramel•E150b Caustic sulphite caramel•E150c Ammonia caramel•E150d Sulphite ammonia caramel•E151 Brilliant Black BN, Black PN•E153 Vegetable carbon•E154 Brown FK•E155 Brown HT•E160a Carotenes: (i) Mixed carotenes (ii) Beta-carotene•E160b Annatto, bixin, norbixin•E160c Paprika extract, capsanthin, capsorubin•E160d Lycopene•E160e Beta-apo-8'-carotenal (C 30)•E160f Ethyl ester of beta-apo-8'-carotenic acid (C 30)•E161b Lutein•E161g Canthaxanthin•E162 Beetroot Red, betanin•E163 Anthocyanins•E170 Calcium carbonates•E171 Titanium dioxide•E172 Iron oxides and hydroxides•E173 Aluminium•E174 Silver•E175 Gold•E180 Latolrubine BK•E200 Sorbic acid•E202 Potassium sorbate•E203 Calcium sorbate•E210 Benzoic acid•E211 Sodium benzoate•E212 Potassium benzoate•E213 Calcium benzoate•E214 Ethyl p-hydroxybenzoate•E215 Sodium ethyl p-hydroxybenzoate•E216 Propyl p-hydroxybenzoate•E217 Sodium propyl p-hydroxybenzoate•E218 Methyl p-hydroxybenzoate•E219 Sodium methyl p-hydroxybenzoate•E220 Sulphur dioxide•E221 Sodium sulphite•E222 Sodium hydrogen sulphite•E223 Sodium metabisulphite•E224 Potassium metabisulphite•E226 Calcium sulphite•E227 Calcium hydrogen sulphite•E228 Potassium hydrogen sulphite•E230 Biphenyl, diphenyl•E231 Orthophenyl phenol•E232 Sodium orthophenyl phenol•[E233 Thiabendazole] - Item deleted by Directive 98/72/EC•E234 Nisin•E235 Natamycin•E239 Hexamethylene tetramine•E242 Dimethyl dicarbonate•E249 Potassium nitrite•E250 Sodium nitrite•E251 Sodium nitrate•E252 Potassium nitrate•E260 Acetic acid•E261 Potassium acetate•E262 Sodium acetates (i) Sodium acetate (ii) Sodium hydrogen acetate (sodium diacetate)•E263 Calcium acetate•E270 Lactic acid•E280 Propionic acid•E281 Sodium propionate•E282 Calcium propionate•E283 Potassium propionate•E284 Boric acid•E285 Sodium tetraborate (borax)•E290 Carbon dioxide•E296 Malic acid•E297 Fumaric acid•E300 Ascorbic acid•E301 Sodium ascorbate•E302 Calcium ascorbate•E304 Fatty acid esters of ascorbic acid (i) Ascorbyl palmitate (ii) Ascorbyl stearate•E306 Tocopherol-rich extract•E307 Alpha-tocopherol•E308 Gamma-tocopherol•E309 Delta-tocopherol•E310 Propyl gallate•E311 Octyl gallate•E312 Dodecyl gallate•E315 Erythorbic acid•E316 Sodium erythorbate•E320 Butylated hydroxyanisole (BHA)•E321 Butylated hydroxytoluene (BHT)•E322 Lecithins•E325 Sodium lactate•E326 Potassium lactate•E327 Calcium lactate•E330 Citric acid•E331 Sodium citrates (i) Monosodium citrate (ii) Disodium citrate (iii) Trisodium citrate•E332 Potassium citrates (i) Monopotassium citrate (ii) Tripotassium citrate•E333 Calcium citrates (i) Monocalcium citrate (ii) Dicalcium citrate (iii) Tricalcium citrate•E334 Tartaric acid (L(+)-)•E335 Sodium tartrates (i) Monosodium tartrate (ii) Disodium tartrate•E336 Potassium tartrates (i) Monopotassium tartrate (ii) Dipotassium tartrate•E337 Sodium potassium tartrate•E338 Phosphoric acid•E339 Sodium phosphates (i) Monosodium phosphate (ii) Disodium phosphate (iii) Trisodium phosphate•E340 Potassium phosphates (i) Monopotassium phosphate (ii) Dipotassium phosphate (iii) Tripotassium phosphate•E341 Calcium phosphates (i) Monocalcium phosphate (ii) Dicalcium phosphate (iii) Tricalcium phosphate•E343 Magnesium phosphates (i) monomagnesium phosphate (ii) Dimagnesium phosphate [Under discussion and may be included in a future amendment to the Directive on miscellaneous additives]•E350 Sodium malates (i) Sodium malate (ii) Sodium hydrogen malate •E351 Potassium malate•E352 Calcium malates (i) Calcium malate (ii) Calcium hydrogen malate•E353 Metatartaric acid•E354 Calcium tartrate•E355 Adipic acid•E356 Sodium adipate•E357 Potassium adipate•E363 Succinic acid•E380 Triammonium citrate•E385 Calcium disodium ethylene diamine tetra-acetate (Calcium disodium EDTA)•E400 Alginic acid•E401 Sodium alginate•E402 Potassium alginate•E403 Ammonium alginate•E404 Calcium alginate•E405 Propan-1,2-diol alginate•E406 Agar•E407 Carrageenan•E407a Processed eucheuma seaweed [Added in December 1996 by Directive 96/83/EC]•E410 Locust bean gum•E412 Guar gum•E413 Tragacanth•E414 Acacia gum (gum arabic)•E415 Xanthan gum•E416 Karaya gum•E417 Tara gum•E418 Gellan gum•E420 Sorbitol (i) Sorbitol (ii) Sorbitol syrup•E421 Mannitol•E422 Glycerol•E425 Konjac (i) Konjac gum (ii) Konjac glucomannane[Added in October 1998 by Directive 98/72/EC]•E426 Soybean hemicellulose [Listed in proposed amendment in COM(2004)650 published October 2004]•E431 Polyoxyethylene (40) stearate•E432 Polyoxyethylene sorbitan monolaurate (polysorbate 20)•E433 Polyoxyethylene sorbitan monooleate (polysorbate 80)•E434 Polyoxyethylene sorbitan monopalmitate (polysorbate 40) •E435 Polyoxyethylene sorbitan monostearate (polysorbate 60) •E436 Polyoxyethylene sorbitan tristearate (polysorbate 65)•E440 Pectins (i) pectin (ii) amidated pectin•E442 Ammonium phosphatides•E444 Sucrose acetate isobutyrate•E445 Glycerol esters of wood rosins•E450 Diphosphates: (i) Disodium diphosphate (Disodium dihydrogen diphosphate, Disodium dihydrogen pyrophosphate, Sodium acidpyrophosphate, Disodium pyrophosphate); (ii) Trisodiumdiphosphate (Acid trisodium pyrophosphate, Trisodium monohydrogen diphosphate); (iii) Tetrasodium diphosphate (Tetrasodiumpyrophosphate, Sodium pyrophosphate); (iv) Dipotassiumdiphosphate (v) Tetrapotassium diphosphate (Potassiumpyrophosphate, Tetrapotassium pyrophosphate); (vi) Dicalcium diphosphate (Calcium pyrophosphate); (vii) Calcium dihydrogen diphosphate (Acid calcium pyrophosphate, Monocalcium dihydrogen pyrophosphate)•E451 Triphosphates: (i) Pentasodium triphosphate (pentasodium tripolyphosphate, sodium tripolyphosphate); (ii) Pentapotassium triphosphate (Pentapotassium tripolyphosphate, Potassiumtriphosphate, Potassium tripolyphosphate)•E452 Polyphosphates: (i) Sodium polyphosphates (Sodium hexametaphosphate, Sodium tetrapolyphosphate, Graham's salt, Sodium polyphosphates, glassy, Sodium polymetaphosphate, Sodium metaphosphate, Insoluble sodium metaphosphate, Maddrell's salt, Insoluble sodium polyphosphate, IMP); (ii) Potassiumpolyphosphates (Potassium metaphosphate, Potassiumpolymetaphosphate, Kurrol salt); (iii) Sodium calciumpolyphosphate (Sodium calcium polyphosphate, glassy); (iv) Calcium polyphophates (Calcium metaphosphate, Calcium polymetaphosphate)•E459 Beta-cyclodextrine [Added in October 1998 by Directive 98/72/EC]•E460 Cellulose (i) Microcrystalline cellulose (ii) Powdered cellulose•E461 Methyl cellulose•E462 Ethyl cellulose [Listed in proposed amendment in COM(2004)650 published October 2004]•E463 Hydroxypropyl cellulose•E464 Hydroxypropyl methyl cellulose•E465 Ethyl methyl cellulose•E466 Carboxy methyl cellulose, Sodium carboxy methyl cellulose•E467 Ethyl hydroxyethyl cellulose [Number had been allocated in a proposed amendment to Directive 95/2 circulated in August 1999. However, it was not accepted and further evaluation was requested]•E468 Crosslinked sodium carboxymethyl cellulose [Added in October 1998 by Directive 98/72/EC]•E469 Enzymically hydrolysed carboxy methyl cellulose[Added in October 1998 by Directive 98/72/EC]•E470a Sodium, potassium and calcium salts of fatty acids•E470b Magnesium salts of fatty acids•E471 Mono- and diglycerides of fatty acids•E472a Acetic acid esters of mono- and diglycerides of fatty acids •E472b Lactic acid esters of mono- and diglycerides of fatty acids •E472c Citric acid esters of mono- and diglycerides of fatty acids •E472d Tartaric acid esters of mono- and diglycerides of fatty acids •E472e Mono- and diacetyl tartaric acid esters of mono- and diglycerides of fatty acids•E472f Mixed acetic and tartaric acid esters of mono- and diglycerides of fatty acids•E473 Sucrose esters of fatty acids•E474 Sucroglycerides•E475 Polyglycerol esters of fatty acids•E476 Polyglycerol polyricinoleate•E477 Propane-1,2-diol esters of fatty acids•E479b Thermally oxidized soya bean oil interacted with mono- and diglycerides of fatty acids•E481 Sodium stearoyl-2-lactylate•E482 Calcium stearoyl-2-lactylate•E483 Stearyl tartrate•E491 Sorbitan monostearate•E492 Sorbitan tristearate•E493 Sorbitan monolaurate•E494 Sorbitan monooleate•E495 Sorbitan monopalmitate•E500 Sodium carbonates (i) Sodium carbonate (ii) Sodium hydrogen carbonate (iii) Sodium sesquicarbonate•E501 Potassium carbonates (i) Potassium carbonate (ii) Potassium hydrogen carbonate•E503 Ammonium carbonates (i) Ammonium carbonate (ii) Ammonium hydrogen carbonate•E504 Magnesium carbonates (i) Magnesium carbonate (ii) Magnesium hydroxide carbonate (syn. Magnesium hydrogen carbonate)•E507 Hydrochloric acid•E508 Potassium chloride•E509 Calcium chloride•E511 Magnesium chloride•E512 Stannous chloride•E513 Sulphuric acid•E514 Sodium sulphates (i) Sodium sulphate (ii) Sodium hydrogen sulphate•E515 Potassium sulphates (i) Potassium sulphate (ii) Potassium hydrogen sulphate•E516 Calcium sulphate•E517 Ammonium sulphate•E520 Aluminium sulphate•E521 Aluminium sodium sulphate•E522 Aluminium potassium sulphate•E523 Aluminium ammonium sulphate•E524 Sodium hydroxide•E525 Potassium hydroxide•E526 Calcium hydroxide•E527 Ammonium hydroxide•E528 Magnesium hydroxide•E529 Calcium oxide•E530 Magnesium oxide•E535 Sodium ferrocyanide•E536 Potassium ferrocyanide•E538 Calcium ferrocyanide•E541 Sodium aluminium phosphate, acidic•E551 Silicon dioxide•E552 Calcium silicate•E553a (i) Magnesium silicate (ii) Magnesium trisilicate•E553b Talc•E554 Sodium aluminium silicate•E555 Potassium aluminium silicate•E556 Calcium aluminium silicate•E558 Bentonite•E559 Aluminium silicate (Kaolin)•E570 Fatty acids•E574 Gluconic acid•E575 Glucono-delta-lactone•E576 Sodium gluconate•E577 Potassium gluconate•E578 Calcium gluconate•E579 Ferrous gluconate•E585 Ferrous lactate•E586 4-hexylresorcinol [Listed in proposed amendment in COM(2004)650 published October 2004]•E620 Glutamic acid•E621 Monosodium glutamate•E622 Monopotassium glutamate•E623 Calcium diglutamate•E624 Monoammonium glutamate•E625 Magnesium diglutamate•E626 Guanylic acid•E627 Disodium guanylate•E628 Dipotassium guanylate•E629 Calcium guanylate•E630 Inosinic acid•E631 Disodium inosinate•E632 Dipotassium inosinate•E633 Calcium inosinate•E634 Calcium 5'-ribonucleotides•E635 Disodium 5'-ribonucleotides•E640 Glycine and its sodium salt•E650 Zinc acetate[Added in February 2001 by Directive 2001/5/EC]•E900 Dimethyl polysiloxane•E901 Beeswax, white and yellow•E902 Candelillla wax•E903 Carnauba wax•E904 Shellac•E905 Microcrystalline wax [Added in October 1998 by Directive 98/72/EC]•E907 Hydrogenated poly-1-decene [Added in December 2003 by Directive 2003/114/EC]•E912 Montanic acid esters•E914 Oxidized polyethylene wax•E920 L-Cysteine [Added in October 1998 by Directive 98/72/EC]•E927b Carbamide•E938 Argon•E939 Helium•E941 Nitrogen•E942 Nitrous oxide•E943a Butane[Added in February 2001 by Directive 2001/5/EC]•E943b Isobutane [Added in February 2001 by Directive 2001/5/EC]•E944 Propane [Added in February 2001 by Directive 2001/5/EC]•E948 Oxygen•E949 Hydrogen [Added in February 2001 by Directive 2001/5/EC]•E950 Acesulfame K•E951 Aspartame•E952 Cyclamic acid and its Na and Ca salts•E953 Isomalt•E954 Saccharin and its Na, K and Ca salts•E955 Sucralose [Added in December 2003 by Directive 2003/115]•E957 Thaumatin•E959 NeohesperidineDC•E962 Salt of aspartame - acesulfame [Added in December 2003 by Directive 2003/115]•E965 Maltitol (i) Maltitol (ii) Maltitol syrup•E966 Lactitol•E967 Xylitol•E968 Erythritol [Listed in proposed amendment in COM(2004)650 published October 2004]•E999 Quilllaia extract•E1103 Invertase [Added in October 1998 by Directive 98/72/EC]•E1105 Lysozyme•E1200 Polydextrose•E1201 Polyvinylpyrrolidone•E1202 Polyvinylpolypyrrolidone•E1404 Oxidized starch•E1410 Monostarch phosphate•E1412 Distarch phosphate•E1413 Phosphated distarch phosphate•E1414 Acetylated distarch phosphate•E1420 Acetylated starch•E1422 Acetylated distarch adipate•E1440 Hydroxy propyl starch•E1442 Hydroxy propyl distarch phosphate•E1451 Acetylated oxidised starch[Added in October 1998 by Directive 98/72/EC]•E1450 Starch sodium octenyl succinate•E1505 Triethyl citrate•E1517 Glyceryl diacetate (diacetin)•E1518 Glyceryl triacetate (triacetin)•E1519 Benzyl alcohol•E1520 Propan-1,2-diol (propylene glycol)[Added in February 2001 by Directive 2001/5/EC]。
ECE系列标准清单ECE系列标准序号代号法规名称1 ECE R1 关于批准发射不对称近光和/或远光并装有R2/或HS1类灯丝灯泡的机动车前照灯的统一规定2 ECE R2 关于批准发射不对称近光和/或远光的前照灯用白炽电灯泡的统一规定3 ECE R3 关于批准机动车辆及其挂车回复反射装置的统一规定4 ECE R4 关于批准机动车辆(摩托车除外)及其挂车后牌照板照明装置的统一规定5 ECE R5 关于批准发射欧洲型不对称近光和/或远光机动车封闭式前照灯(SB) 的统一规定6 ECE R6 关于批准机动车及其挂车转向指示器的统一规定7 ECE R7 关于批准机动车(不含摩托车)及其挂车前后位置(侧边)灯、制动灯和示廓灯的统一规定8 ECE R8 关于批准发射不对称近光和/或远光并装有卤素灯丝灯泡(H1、H2、H3、HB3、HB4、H7、H8、H9、HIR1、HIR2和/或H11)的机动车前照灯的统一规定9 ECE R9 关于就噪声方面批准L2、L4和L5类车辆的统一规定10 ECE R10 关于就电磁兼容性方面批准车辆的统一规定11 ECE R11 关于就门锁和车门保持件方面批准车辆的统一规定12 ECE R12 关于就碰撞中防止转向机构伤害驾驶员方面批准车辆的统一规定13 ECE R13 关于就制动方面批准M类、N类和O类车辆的统一规定14 ECE R13--H 关于就制动方面批准乘用车的统一规定(欧美日协调版)15 ECE R14 关于就安全带固定点方面批准车辆的统一规定16 ECE R15 关于就发动机气体污染物排放方面批准装有点火式发动机或压燃式发动机的车辆的统一规定--点火式发动机的功率测量方法---车辆的油耗测量方法17 ECE R16 关于:1批准机动车成年乘客用安全带和约束系统;2批准装用安全带的车辆的统一规定18 ECE R17 关于就座椅、座椅固定点和头枕方面批准车辆的统一规定19 ECE R18 关于就防盗方面批准机动车的统一规定20 ECE R19 关于批准机动车前雾灯的统一规定21 ECE R20 关于批准发射非对称近光和/或远光并装有卤素灯丝灯泡(H4)的机动车前照灯的统一规定22 ECE R21 关于就内饰件方面批准车辆的统一规定23 ECE R22 关于批准摩托车轻便摩托车驾驶员及乘客用头盔和面罩的统一规定24 ECE R23 关于批准机动车辆及其挂车的倒车灯的统一规定25 ECE R24 关于1.就可见污染物排放方面批准压燃式(C.I)发动机;2.就已获得型式批准的C.I.发动机的安装方面批准机动车; 3. 就发动机可见污染物排放方面批准装用C.I.发动机的车辆; 4 .C.I.发动机的功率测量的统一规定26 ECE R25 关于批准与车辆座椅一体或非一体的头枕的统一规定27 ECE R26 关于就外部凸出物方面批准车辆的统一规定28 ECE R27 关于批准提前三角警告牌的统一规定29 ECE R28 关于就声响信号方面批准声报警装置和机动车辆的统一规定30 ECE R29 关于就商用车辆驾驶室乘员防护方面批准车辆的统一规定31 ECE R30 关于批准机动车及其挂车气压轮胎的统一规定32 ECE R31 关于批准发射非对称近光和/或远光的卤素封闭式(HSB)机动车前照灯的统一规定33 ECE R32 关于就追尾碰撞中被撞车辆的结构特性方面批准车辆的统一规定34 ECE R33 关于就正面冲撞中被撞的结构特性方面批准车辆的统一规定35 ECE R34 关于就火灾预防方面批准车辆的统一规定36 ECE R35 关于就脚控制件的布置方面批准车辆的统一规定37 ECE R36 关于就一般结构方面批准大型客车的统一规定38 ECE R37 关于批准用于已经批准的机动车和挂车灯具中的白炽灯的统一规定39 ECE R38 关于批准机动车和挂车后雾灯的统一规定40 ECE R39 关于就车速表及其安装方面批准车辆的统一规定41 ECE R40 关于就发动机气体污染物的排放方面批准装有点火式发动机的摩托车的统一规定42 ECE R41 关于就噪声方面批准摩托车的统一规定43 ECE R42 关于就车辆前、后保护装置(保险杠等)批准车辆的统一规定44 ECE R43 关于批准安全玻璃材料的统一规定45 ECE R44 关于批准机动车儿童乘客约束装置(儿童约束系统)的统一规定46 ECE R45 关于就前照灯清洗器方面批准机动车辆和批准前照灯清洗器的统一规定47 ECE R46 关于批准后视镜和就后视镜的安装方面批准机动车辆的统一规定48 ECE R47 关于就发动机的气体污染物排放方面批准装有点火发动机的轻便摩托车的统一规定49 ECE R48 关于就灯光和光信号装置的安装方面批准车辆的统一规定50 ECE R49 关于就发动机污染物排放方面批准压燃式发动机、天然气发动机和燃用液化石油气的点燃式发动机以及装有这些发动机的车辆的统一规定51 ECE R50 关于批准轻便摩托车、摩托车及其类似车辆前后位置灯、制动灯、转向信号灯和后牌照板照明装置的统一规定52 ECE R51 关于就噪声排放方面批准四轮及四轮以上机动车的统一规定53 ECE R52 关于就一般结构方面批准小型公共运输车辆(M2、M3)的统一规定54 ECE R53 关于就灯光及光信号装置的安装方面批准L3类车辆(摩托车)的统一规定55 ECE R54 关于批准商用车辆及其挂车气压轮胎的统一规定56 ECE R55 关于批准汽车列车机械联结件的统一规定57 ECE R56 关于批准轻便摩托车以及类似车辆前照灯的统一规定58 ECE R57 关于批准摩托车以及类似车辆前照灯的统一规定59 ECE R58 关于1.批准后下部防护装置; 2.就已批准的后下部防护装置的安装方面批准车辆;3.就后下部防护装置方面批准车辆的统一规定60 ECE R59 关于批准备用消声系统的统一规定61 ECE R60 关于就驾驶员操纵的控制件,包括控制件、信号装置和指示器的识别方面批准两轮摩托车的统一规定62 ECE R61 关于就驾驶室后挡板之前的外部凸出物方面批准商用车的统一规定63 ECE R62 关于就防盗方面批准带有操纵把的机动车的统一规定64 ECE R63 关于就噪声方面批准两轮轻便摩托车的统一规定65 ECE R64 关于批准装有应急备用车轮/轮胎的车辆的统一规定66 ECE R65 关于批准机动车特别警告灯的统一规定67 ECE R66 关于就上部结构强度方面批准大型客车的统一规定68 ECE R67 关于:1批准在其驱动系统中使用液化石油气的机动车辆特殊装置;2 在这一装置的安装方面批准装用使其驱动系统使用液化石油气的特殊装置的车辆的统一规定69 ECE R68 关于就最大车速的测量方面批准包括纯电动车辆在内的机动车的统一规定70 ECE R69 关于批准低速车辆及其挂车后标志牌的统一规定71 ECE R70 关于批准重,长型车辆后标志牌的统一规定72 ECE R71 关于就驾驶员视野方面批准农用拖拉机的统一规定73 ECE R72 关于批准发射非对称近光和远光并装有卤素灯(HS1灯)的摩托车前大灯统一规定74 ECE R73 关于就侧防护方面批准货车、挂车和半挂车的统一规定75 ECE R74 关于就灯光和光信号装置方面批准L1类车辆的统一规定76 ECE R75 关于批准摩托车和轻便摩托车气压轮胎的统一规定77 ECE R76 关于批准发射远光和近光的轻便摩托车前照灯的统一规定78 ECE R77 关于批准机动车驻车灯的统一规定79 ECE R78 关于就制动方面批准L类车辆的统一规定80 ECE R79 关于就转向装置方面批准车辆的统一规定81 ECE R80 关于就座椅及其固定点方面批准大型客车座椅和车辆的统一规定82 ECE R81 关于就车把上后视镜的安装方面批准后视镜及带与不带边斗的二轮机动车的统一规定83 ECE R82 关于批准装有卤素灯丝灯泡(HS2)的轻便摩托车前照灯的统一规定84 ECE R83 关于根据发动机燃油要求就污染物排放方面批准车辆的统一规定85 ECE R84 关于就油耗测量方面批准装有内燃机的机动车的统一规定86 ECE R85 关于就净功率和最大30分钟电传动系功率的测量方面批准用于驱动M和N类机动车辆的内燃机或电传动系的统一规定87 ECE R86 关于就灯光和光信号装置的安装方面批准农林拖拉机的统一规定88 ECE R87 换向批准机动车白天行车灯的统一规定89 ECE R88 关于批准摩托车反光轮胎的统一规定90 ECE R89 关于1.就最高车速限制或其可调车速限制功能方面批准车辆;2.就已批准型式的最高车速限制装置或可调车速限制装置的安装方面批准车辆;3.批准车速限制装置或可调车速限制装置的统一规定91 ECE R90 关于批准机动车辆及其挂车用可更替制动衬片总成和鼓式制动器衬片的统一规定92 ECE R91 关于批准机动车及其挂车侧标志灯的统一规定93 ECE R92 关于批准摩托车、轻便摩托车和三轮车辆非原装可更换排气消声系统的统一规定94 ECE R93 关于1.批准前下部防护装置; 2.就已批准型式的前下部防护装置的安装方面批准车辆;3.就前下部防护方面批准车辆的统一规定95 ECE R94 关于就前碰撞中乘员防护方面批准车辆的统一规定96 ECE R95 关于就侧碰撞中乘员防护方面批准车辆的统一规定97 ECE R96 关于就发动机污染物排放方面批准农林拖拉机和非道路机动机械装用的压燃式发动机的统一规定98 ECE R97 关于批准车辆报警系统和就报警系统方面批准机动车辆的统一规定99 ECE R98 关于批准装用气体放电光源的机动车前照灯的统一规定100 ECE R99 关于批准用于已获得型式批准的机动车气体放电灯具的气体放电光源的统一规定101 ECE R100 关于就结构、功能安全性和氢排放的特殊要求方面批准蓄电池电动车辆的统一规定102 ECE R101 关于就CO2排放和油耗的测量方面批准装用内燃机的乘用车和就电能消耗量和范围的测量方面批准装用电传动系的M1和N1类车辆的统一规定103 ECE R102 关于1.批准紧耦合装置;2.就已批准的紧耦合装置的安装方面批准车辆的统一规定。
食品添加剂欧盟编码•E100 Curcumin•E101 (i) Riboflavin (ii) Riboflavin-5'-phosphate•E102 Tartrazine•E104 Quinoline Yellow•E110 Sunset Yellow FCF, Orange Yellow S•E120 Cochineal, Carminic acid, Carmines•E122 Azorubine, Carmoisine•E123 Amaranth•E124 Ponceau 4R, Cochineal Red A•E127 Erythrosine•E128 Red 2G•E129 Allura Red AC•E131 Patent Blue V•E132 Indigotine, Indigo carmine•E133 Brilliant Blue FCF•E140 Chlorophylis and Chlorophyllins: (i) Chlorophylls (ii) Chlorophyllins •E141 Copper complexes of chlorophylls and chlorophyllins (i) Copper complexes of chlorophylls (ii) Copper complexes of chlorophyllins•E142 Greens S•E150a Plain caramel•E150b Caustic sulphite caramel•E150c Ammonia caramel•E150d Sulphite ammonia caramel•E151 Brilliant Black BN, Black PN•E153 Vegetable carbon•E154 Brown FK•E155 Brown HT•E160a Carotenes: (i) Mixed carotenes (ii) Beta-carotene •E160b Annatto, bixin, norbixin•E160c Paprika extract, capsanthin, capsorubin•E160d Lycopene•E160e Beta-apo-8'-carotenal (C 30)•E160f Ethyl ester of beta-apo-8'-carotenic acid (C 30) •E161b Lutein•E161g Canthaxanthin•E162 Beetroot Red, betanin•E163 Anthocyanins•E170 Calcium carbonates•E171 Titanium dioxide•E172 Iron oxides and hydroxides•E173 Aluminium•E174 Silver•E175 Gold•E180 Latolrubine BK•E200 Sorbic acid•E202 Potassium sorbate•E203 Calcium sorbate•E210 Benzoic acid•E211 Sodium benzoate•E212 Potassium benzoate•E213 Calcium benzoate•E214 Ethyl p-hydroxybenzoate•E215 Sodium ethyl p-hydroxybenzoate •E216 Propyl p-hydroxybenzoate•E217 Sodium propyl p-hydroxybenzoate •E218 Methyl p-hydroxybenzoate•E219 Sodium methyl p-hydroxybenzoate •E220 Sulphur dioxide•E221 Sodium sulphite•E222 Sodium hydrogen sulphite•E223 Sodium metabisulphite•E224 Potassium metabisulphite•E226 Calcium sulphite•E227 Calcium hydrogen sulphite•E228 Potassium hydrogen sulphite•E230 Biphenyl, diphenyl•E231 Orthophenyl phenol•E232 Sodium orthophenyl phenol•[E233 Thiabendazole] - Item deleted by Directive 98/72/EC•E234 Nisin•E235 Natamycin•E239 Hexamethylene tetramine•E242 Dimethyl dicarbonate•E249 Potassium nitrite•E250 Sodium nitrite•E251 Sodium nitrate•E252 Potassium nitrate•E260 Acetic acid•E261 Potassium acetate•E262 Sodium acetates (i) Sodium acetate (ii) Sodium hydrogen acetate (sodium diacetate)•E263 Calcium acetate•E270 Lactic acid•E280 Propionic acid•E281 Sodium propionate•E282 Calcium propionate•E283 Potassium propionate•E284 Boric acid•E285 Sodium tetraborate (borax)•E290 Carbon dioxide•E296 Malic acid•E297 Fumaric acid•E300 Ascorbic acid•E301 Sodium ascorbate•E302 Calcium ascorbate•E304 Fatty acid esters of ascorbic acid (i) Ascorbyl palmitate (ii) Ascorbyl stearate•E306 Tocopherol-rich extract•E307 Alpha-tocopherol•E308 Gamma-tocopherol•E309 Delta-tocopherol•E310 Propyl gallate•E311 Octyl gallate•E312 Dodecyl gallate•E315 Erythorbic acid•E316 Sodium erythorbate•E320 Butylated hydroxyanisole (BHA)•E321 Butylated hydroxytoluene (BHT)•E322 Lecithins•E325 Sodium lactate•E326 Potassium lactate•E327 Calcium lactate•E330 Citric acid•E331 Sodium citrates (i) Monosodium citrate (ii) Disodium citrate (iii) Trisodium citrate•E332 Potassium citrates (i) Monopotassium citrate (ii) Tripotassium citrate •E333 Calcium citrates (i) Monocalcium citrate (ii) Dicalcium citrate (iii) Tricalcium citrate•E334 Tartaric acid (L(+)-)•E335 Sodium tartrates (i) Monosodium tartrate (ii) Disodium tartrate•E336 Potassium tartrates (i) Monopotassium tartrate (ii) Dipotassium tartrate•E337 Sodium potassium tartrate•E338 Phosphoric acid•E339 Sodium phosphates (i) Monosodium phosphate (ii) Disodium phosphate (iii) Trisodium phosphate•E340 Potassium phosphates (i) Monopotassium phosphate (ii) Dipotassium phosphate (iii) Tripotassium phosphate•E341 Calcium phosphates (i) Monocalcium phosphate (ii) Dicalcium phosphate (iii) Tricalcium phosphate•E343 Magnesium phosphates (i) monomagnesium phosphate (ii) Dimagnesium phosphate [Under discussion and may be included in a futureamendment to the Directive on miscellaneous additives]•E350 Sodium malates (i) Sodium malate (ii) Sodium hydrogen malate•E351 Potassium malate•E352 Calcium malates (i) Calcium malate (ii) Calcium hydrogen malate •E353 Metatartaric acid•E354 Calcium tartrate•E355 Adipic acid•E356 Sodium adipate•E357 Potassium adipate•E363 Succinic acid•E380 Triammonium citrate•E385 Calcium disodium ethylene diamine tetra-acetate (Calcium disodium EDTA)•E400 Alginic acid•E401 Sodium alginate•E402 Potassium alginate•E403 Ammonium alginate•E404 Calcium alginate•E405 Propan-1,2-diol alginate•E406 Agar•E407 Carrageenan•E407a Processed eucheuma seaweed [Added in December 1996 by Directive 96/83/EC]•E410 Locust bean gum•E412 Guar gum•E413 Tragacanth•E414 Acacia gum (gum arabic)•E415 Xanthan gum•E416 Karaya gum•E417 Tara gum•E418 Gellan gum•E420 Sorbitol (i) Sorbitol (ii) Sorbitol syrup•E421 Mannitol•E422 Glycerol•E425 Konjac (i) Konjac gum (ii) Konjac glucomannane[Added in October 1998 by Directive 98/72/EC]•E426 Soybean hemicellulose [Listed in proposed amendment in COM(2004)650 published October 2004]•E431 Polyoxyethylene (40) stearate•E432 Polyoxyethylene sorbitan monolaurate (polysorbate 20)•E433 Polyoxyethylene sorbitan monooleate (polysorbate 80)•E434 Polyoxyethylene sorbitan monopalmitate (polysorbate 40)•E435 Polyoxyethylene sorbitan monostearate (polysorbate 60)•E436 Polyoxyethylene sorbitan tristearate (polysorbate 65)•E440 Pectins (i) pectin (ii) amidated pectin•E442 Ammonium phosphatides•E444 Sucrose acetate isobutyrate•E445 Glycerol esters of wood rosins•E450 Diphosphates: (i) Disodium diphosphate (Disodium dihydrogen diphosphate, Disodium dihydrogen pyrophosphate, Sodium acidpyrophosphate, Disodium pyrophosphate); (ii) Trisodium diphosphate (Acid trisodium pyrophosphate, Trisodium monohydrogen diphosphate);(iii) Tetrasodium diphosphate (Tetrasodium pyrophosphate, Sodiumpyrophosphate); (iv) Dipotassium diphosphate (v) Tetrapotassiumdiphosphate (Potassium pyrophosphate, Tetrapotassium pyrophosphate);(vi) Dicalcium diphosphate (Calcium pyrophosphate); (vii) Calciumdihydrogen diphosphate (Acid calcium pyrophosphate, Monocalciumdihydrogen pyrophosphate)•E451 Triphosphates: (i) Pentasodium triphosphate (pentasodium tripolyphosphate, sodium tripolyphosphate); (ii) Pentapotassiumtriphosphate (Pentapotassium tripolyphosphate, Potassium triphosphate, Potassium tripolyphosphate)•E452 Polyphosphates: (i) Sodium polyphosphates (Sodium hexametaphosphate, Sodium tetrapolyphosphate, Graham's salt, Sodium polyphosphates, glassy, Sodium polymetaphosphate, Sodiummetaphosphate, Insoluble sodium metaphosphate, Maddrell's salt,Insoluble sodium polyphosphate, IMP); (ii) Potassium polyphosphates(Potassium metaphosphate, Potassium polymetaphosphate, Kurrol salt);(iii) Sodium calcium polyphosphate (Sodium calcium polyphosphate,glassy); (iv) Calcium polyphophates (Calcium metaphosphate, Calcium polymetaphosphate)•E459 Beta-cyclodextrine [Added in October 1998 by Directive 98/72/EC]•E460 Cellulose (i) Microcrystalline cellulose (ii) Powdered cellulose•E461 Methyl cellulose•E462 Ethyl cellulose [Listed in proposed amendment in COM(2004)650 publishedOctober 2004]•E463 Hydroxypropyl cellulose•E464 Hydroxypropyl methyl cellulose•E465 Ethyl methyl cellulose•E466 Carboxy methyl cellulose, Sodium carboxy methyl cellulose•E467 Ethyl hydroxyethyl cellulose [Number had been allocated in a proposedamendment to Directive 95/2 circulated in August 1999. However, it was not accepted and further evaluation was requested]•E468 Crosslinked sodium carboxymethyl cellulose [Added in October 1998 by Directive 98/72/EC]•E469 Enzymically hydrolysed carboxy methyl cellulose[Added in October 1998 by Directive 98/72/EC]•E470a Sodium, potassium and calcium salts of fatty acids•E470b Magnesium salts of fatty acids•E471 Mono- and diglycerides of fatty acids•E472a Acetic acid esters of mono- and diglycerides of fatty acids•E472b Lactic acid esters of mono- and diglycerides of fatty acids•E472c Citric acid esters of mono- and diglycerides of fatty acids•E472d Tartaric acid esters of mono- and diglycerides of fatty acids•E472e Mono- and diacetyl tartaric acid esters of mono- and diglycerides of fatty acids•E472f Mixed acetic and tartaric acid esters of mono- and diglycerides of fatty acids•E473 Sucrose esters of fatty acids•E474 Sucroglycerides•E475 Polyglycerol esters of fatty acids•E476 Polyglycerol polyricinoleate•E477 Propane-1,2-diol esters of fatty acids•E479b Thermally oxidized soya bean oil interacted with mono- and diglycerides of fatty acids•E481 Sodium stearoyl-2-lactylate•E482 Calcium stearoyl-2-lactylate•E483 Stearyl tartrate•E491 Sorbitan monostearate•E492 Sorbitan tristearate•E493 Sorbitan monolaurate•E494 Sorbitan monooleate•E495 Sorbitan monopalmitate•E500 Sodium carbonates (i) Sodium carbonate (ii) Sodium hydrogen carbonate (iii) Sodium sesquicarbonate•E501 Potassium carbonates (i) Potassium carbonate (ii) Potassium hydrogen carbonate•E503 Ammonium carbonates (i) Ammonium carbonate (ii) Ammonium hydrogen carbonate•E504 Magnesium carbonates (i) Magnesium carbonate (ii) Magnesium hydroxide carbonate (syn. Magnesium hydrogen carbonate)•E507 Hydrochloric acid•E508 Potassium chloride•E509 Calcium chloride•E511 Magnesium chloride•E512 Stannous chloride•E513 Sulphuric acid•E514 Sodium sulphates (i) Sodium sulphate (ii) Sodium hydrogen sulphate •E515 Potassium sulphates (i) Potassium sulphate (ii) Potassium hydrogen sulphate•E516 Calcium sulphate•E517 Ammonium sulphate•E520 Aluminium sulphate•E521 Aluminium sodium sulphate•E522 Aluminium potassium sulphate•E523 Aluminium ammonium sulphate•E524 Sodium hydroxide•E525 Potassium hydroxide•E526 Calcium hydroxide•E527 Ammonium hydroxide•E528 Magnesium hydroxide•E529 Calcium oxide•E530 Magnesium oxide•E535 Sodium ferrocyanide•E536 Potassium ferrocyanide•E538 Calcium ferrocyanide•E541 Sodium aluminium phosphate, acidic•E551 Silicon dioxide•E552 Calcium silicate•E553a (i) Magnesium silicate (ii) Magnesium trisilicate •E553b Talc•E554 Sodium aluminium silicate•E555 Potassium aluminium silicate•E556 Calcium aluminium silicate•E558 Bentonite•E559 Aluminium silicate (Kaolin)•E570 Fatty acids•E574 Gluconic acid•E575 Glucono-delta-lactone•E576 Sodium gluconate•E577 Potassium gluconate•E578 Calcium gluconate•E579 Ferrous gluconate•E585 Ferrous lactate•E586 4-hexylresorcinol [Listed in proposed amendment in COM(2004)650 published October 2004]•E620 Glutamic acid•E621 Monosodium glutamate•E622 Monopotassium glutamate•E623 Calcium diglutamate•E624 Monoammonium glutamate•E625 Magnesium diglutamate•E626 Guanylic acid•E627 Disodium guanylate•E628 Dipotassium guanylate•E629 Calcium guanylate•E630 Inosinic acid•E631 Disodium inosinate•E632 Dipotassium inosinate•E633 Calcium inosinate•E634 Calcium 5'-ribonucleotides•E635 Disodium 5'-ribonucleotides•E640 Glycine and its sodium salt•E650 Zinc acetate[Added in February 2001 by Directive 2001/5/EC]•E900 Dimethyl polysiloxane•E901 Beeswax, white and yellow•E902 Candelillla wax•E903 Carnauba wax•E904 Shellac•E905 Microcrystalline wax [Added in October 1998 by Directive 98/72/EC]•E907 Hydrogenated poly-1-decene [Added in December 2003 by Directive 2003/114/EC]•E912 Montanic acid esters•E914 Oxidized polyethylene wax•E920 L-Cysteine [Added in October 1998 by Directive 98/72/EC]•E927b Carbamide•E938 Argon•E939 Helium•E941 Nitrogen•E942 Nitrous oxide•E943a Butane[Added in February 2001 by Directive 2001/5/EC]•E943b Isobutane [Added in February 2001 by Directive 2001/5/EC]•E944 Propane [Added in February 2001 by Directive 2001/5/EC]•E948 Oxygen•E949 Hydrogen [Added in February 2001 by Directive 2001/5/EC]•E950 Acesulfame K•E951 Aspartame•E952 Cyclamic acid and its Na and Ca salts•E953 Isomalt•E954 Saccharin and its Na, K and Ca salts•E955 Sucralose [Added in December 2003 by Directive 2003/115]•E957 Thaumatin•E959 NeohesperidineDC•E962 Salt of aspartame - acesulfame [Added in December 2003 by Directive 2003/115]•E965 Maltitol (i) Maltitol (ii) Maltitol syrup•E966 Lactitol•E967 Xylitol•E968 Erythritol [Listed in proposed amendment in COM(2004)650 published October 2004]•E999 Quilllaia extract•E1103 Invertase [Added in October 1998 by Directive 98/72/EC]•E1105 Lysozyme•E1200 Polydextrose•E1201 Polyvinylpyrrolidone•E1202 Polyvinylpolypyrrolidone•E1404 Oxidized starch•E1410 Monostarch phosphate•E1412 Distarch phosphate•E1413 Phosphated distarch phosphate•E1414 Acetylated distarch phosphate•E1420 Acetylated starch•E1422 Acetylated distarch adipate•E1440 Hydroxy propyl starch•E1442 Hydroxy propyl distarch phosphate•E1451 Acetylated oxidised starch[Added in October 1998 by Directive 98/72/EC]•E1450 Starch sodium octenyl succinate•E1505 Triethyl citrate•E1517 Glyceryl diacetate (diacetin)•E1518 Glyceryl triacetate (triacetin)•E1519 Benzyl alcohol•E1520 Propan-1,2-diol (propylene glycol)[Added in February 2001 by Directive 2001/5/EC]。
TITANIUM DIOXIDE FOOD GRADE E171 Specification
二氧化钛(钛白粉)
食品、化妆品级
Titanium Dioxide Food Grade
(欧洲食品E171)
牌号Brand :
化学名称Chemical name:二氧化钛Titanium Dioxide
分子式Molecular formula:TiO2
分子量Molecular weight:79.88(按1987年国际原子量international atomic weight in
1987')
用途Uses:本品为食用白色颜料,具有颜料性能好,有害杂质含量低等优点,是化妆品和食品着色的添加剂,同时也是制造油漆、油墨的优良材料。
It is white
pigment for food, with good pigment performance and low toxic
impurity content; used as additive of coloring of cosmetics and food, also as good material to make printing oil and paint.
优点Advantages:具有粒度均匀,分散性好的优点。
Even particle size and good
dispersion.
产品标准Standard:欧洲食品E171
技术指标符合下列要求The technical indexes satisfy the following requirements:。