CAC第四版(2003)食品 卫生通用规范(详解)
- 格式:pdf
- 大小:726.69 KB
- 文档页数:60

Codex Alimentarius Food Hygeine食品卫生通用规范CAC/RCP1-1969,* 注1:这一版本的《食品卫生通用规范》,包括附件《HACCP 体系及其应用指南》是由CAC 在1997 年制订的。
在1999 年进行修改。
HACCP 指南在2003 年修改。
这一法规已作为咨询版本发放至FAO 和WHO 的各成员国,并由各国政府决定将其作为指南。
前言1 目的食品卫生通用规范2 范围、应用和定义范围应用定义3 初级生产环境卫生食品原料的卫生生产搬运、贮藏和运输4 工厂:设计及设施选址厂房和车间设备设施5 操作的控制食品危害的控制卫生控制体系的关键原料接收的要求包装水管理与监督文件和记录产品回收程序6 工厂:维护和卫生维护和清洁清洁计划害虫控制体系废弃物管理监控的有效性7 工厂:个人卫生健康状况疾病和受伤个人清洁个人行为外来人员8 运输总体要求要求使用和维护9 产品信息和消费者的意识批次识别产品信息标识对消费者的教育10 培训意识和责任培训计划指导和监督培训的回顾附录:危害分析及关键控制点(HACCP)体系及其应用指南1. 导言2. 定义3. HACCP体系的原则4. HACCP体系及其应用导则介绍应用导则培训图1:HACCP应用的逻辑步骤图2:关键控制点判断树状表图3:HACCP工作表范例前言人们有权期望他们食用的食品是安全和适宜消费的,但食源性疾病导致的伤害,会令人不安,有时甚至是致命性的,这是人们最不愿看见的。
当然,食物中毒事件也会带来其他一些影响。
食源性品疾病不仅会影响到贸易和旅游业,导致收入下降、工人失业,甚至于产生法律纠纷。
食品腐败不仅会造成浪费,影响成本,还可能对贸易和消费者的信心产生不良影响。
现在,国际食品贸易和出国旅游业正在兴旺发展,带来了显着的社会和经济效益,但同时也使得疾病更易于在世界范围传播。
从新食品的生产、制作和销售技术的不断发展可以看出,在过去的20年里,许多国家人们的饮食习惯已经发生了巨大变化。


GUIDELINES ON THE JUDGEMENT OF EQUIVALENCE OF SANITARY MEASURES ASSOCIATED WITH FOOD INSPECTION AND CERTIFICATION SYSTEMS1CAC/GL 53-2003SECTION 1 – PREAMBLE1.It is often the case that importing and exporting countries operate different food inspection and certification systems. The reasons for such differences include differences in prevalence of particular food safety hazards, national choice about management of food safety risks and differences in the historical development of food control systems.2.In such circumstances, and in order to facilitate trade while protecting the health of consumers, an exporting and an importing country may work together to consider the effectiveness of sanitary measures of the exporting country in achieving the appropriate level of sanitary protection of the importing country, consistent with the principle of equivalence as provided for in the World Trade Organization Agreement on the Application of Sanitary and Phytosanitary Measures (WTO SPS Agreement).23.Application of the principle of equivalence has mutual benefits for both exporting and importing countries. While protecting the health of consumers, it serves to facilitate trade, and minimize the costs of regulation to governments, industry, producers, and consumers by allowing the exporting country to employ the most convenient means in its circumstances to achieve the appropriate level of protection of the importing country.34.Importing countries should avoid the application of unnecessary measures when they have already been carried out by the exporting country. Importing countries may be able to reduce the frequency and extent of verification measures following a judgment of equivalence of measures applied in the exporting country.SECTION 2 – SCOPE5.This document provides guidelines on the judgement of the equivalence of sanitary measures associated with food inspection and certification systems. For the purpose of determining equivalence, these measures can be broadly characterized as infrastructure; programme design, implementation and monitoring; and/or specific requirements (refer paragraph 13).1These guidelines should be read in conjunction with other relevant Codex texts, including in particular the Guidelines for the Development of Equivalence Agreements Regarding Food Import and Export Inspection and Certification Systems – CAC/GL 34-1999.2Consistent with the definition of equivalence in Section 3, measures that are equivalent (i.e., are different from the measures used by the importing country but nonetheless achieve the importing country’s appropriate level of protection) should be distinguished from measures that are the same as the measures of the importing country.3The benefits to an exporting country of application of the principle of equivalence would be offset or negated if a request for an equivalence determination were, by itself, used as a pretext for the disruption of established trade. Such action by an importing country would be contrary to the principles of international trade.SECTION 3 – DEFINITIONS6.The definitions presented in this document are derived from and consistent with those of the Codex Alimentarius Commission and the WTO SPS Agreement.Sanitary measure: Any measure applied to protect human life or health within the territoryof the country from risks arising from additives, contaminants, toxins or disease-causingorganisms in food or feedstuffs, or from risks arising from diseases carried by foods whichare animals, plants or products thereof or from risks arising from any other hazards infoods.Note: Sanitary measures include all relevant laws, decrees, regulations, requirements andprocedures including, inter alia, end product criteria; processes and production methods;testing, inspection, certification and approval procedures; provisions on relevant statisticalmethods, sampling procedures and methods of risk assessment; and packaging and labelingrequirements directly related to food safety.Hazard: A biological, chemical or physical agent in, or condition of, food with thepotential to cause an adverse health effect.4Risk: A function of the probability of an adverse health effect and the severity of thateffect, consequential to a hazard(s) in food.4Risk Assessment: A scientifically-based process consisting of the following steps: (i)hazard identification; (ii) hazard characterization; (iii) exposure assessment; and (iv) riskcharacterisation.4Appropriate level of sanitary protection (ALOP): The level of protection deemedappropriate by the country establishing a sanitary measure to protect human life or healthwithin its territory. (This concept may otherwise be referred to as the “acceptable level ofrisk”.)Equivalence of sanitary measures:5 Equivalence is the state wherein sanitary measures applied in an exporting country, though different from the measures applied in an importing country, achieve, as demonstrated by the exporting country, the importing country’s appropriate level of sanitary protection.SECTION 4 - GENERAL PRINCIPLES FOR THE DETERMINATION OF EQUIVALENCE 7.Determination of the equivalence of sanitary measures associated with food inspection and certification systems should be based on application of the following principles:a)An importing country has the right to set a level of sanitary protection it deems appropriate inrelation to the protection of human life and health.6The ALOP may be expressed in qualitative or quantitative terms.b)The sanitary measure7 applied in an importing country should in practice achieve the ALOP ofthe importing country and be applied consistent with article 2.3 of the SPS agreement.84Codex Alimentarius Commission: Procedural Manual (12th Edition), pages 43-44.5Equivalence is defined in CAC/GL 26-1997 as “the capability of different inspection and certification systems to meet the same objectives”.6The SPS Agreement sets out the rights and obligations of WTO Members in relation to the determination of an appropriate level of sanitary protection.7Where this guideline refers to ‘measure’ in the singular it may also be taken to refer to ‘measures’ or ‘a set of measures’, as appropriate to the circumstances.8Equivalent measures may achieve the ALOP of the importing country or, in combination with other measures, they may contribute to the achievement of the importing country’s ALOP. In the remainder of this guideline any reference to the former should be taken to include the latter possibility.c)An importing country should describe how its own sanitary measure achieves its ALOP.d)An importing country should recognize that sanitary measures different from its own may becapable of achieving its ALOP, and can therefore be found to be equivalent.e)The sanitary measure that the exporting country proposes as equivalent must be capable ofachieving the importing country’s ALOP.f)An importing country should, upon request by an exporting country, promptly enter intoconsultations with the aim of determining the equivalence of specified sanitary measures within a reasonable period of time. 9g)It is the responsibility of the exporting country to objectively demonstrate that its sanitarymeasure can achieve the importing country’s ALOP.h)The comparison of countries’ sanitary measures should be carried out in an objective manner.i)Where risk assessment is used in the demonstration of equivalence, countries should strive toachieve consistency in the techniques applied, using internationally accepted methodology where available and taking into account relevant Codex texts.j)The importing country should take into account any knowledge and past experience it has of the food inspection and certification systems in the exporting country to make the determination as efficiently and quickly as possible.k)The exporting country should provide access to enable the inspection and certification systems which are the subject of the equivalence determination to be examined and evaluated upon request of the food control authorities of the importing country.l)All judgments of equivalence should consider the means by which that equivalence will be maintained.m)Countries should ensure transparency in both the demonstration and judgment of equivalence, consulting all interested parties to the extent practicable and reasonable. The exporting and importing countries should approach an equivalence determination procedure in a cooperative way.n)An importing country should give positive consideration to a request by an exporting developing country for appropriate technical assistance that would facilitate the successful completion of an equivalency determination.SECTION 5 - THE CONTEXT OF AN EQUIVALENCE DETERMINATION8.To facilitate judgement of equivalence between countries and promote harmonisation of food safety standards, Codex members should base their sanitary measures on Codex standards and related texts.109.An equivalence determination can be sought for any sanitary measure or set of measures relevant to a food product or group of food products. Relevant sanitary measures making up a food control system in the exporting country that are not the subject of an equivalence determination should meet importing country requirements.9Guidelines for the Design, Operation, Assessment and Accreditation of Food Import and Export Inspection and Certification Systems - CAC/GL 26- 1997.10Article 3 of the WTO SPS Agreement states, inter alia, that WTO Members may introduce or maintain sanitary measures which result in a higher level of sanitary protection than would be achieved based on Codex standards, if there is a scientific justification, or as a consequence of the member’s chosen level of protection. Such measures must be based on a risk assessment appropriate to the circumstances.10.The extent of the equivalence determination will depend on the prior experience, knowledge, and confidence that the importing country has regarding the food control measures of the exporting country.11.When an importing country has prior experience, knowledge, and confidence in food control measures relevant to those being evaluated for equivalence and the countries agree that import requirements are being fully met, e.g. where trade experience exists, determination of the equivalence of sanitary measures may be made without further consideration of those other relevant measures making up the food control system.12.When an importing country does not have prior experience, knowledge, and confidence in food control measures relevant to those being evaluated for equivalence and the countries have not determined that import requirements are being fully met, e.g., where trade in a food product or group of food products is being proposed for the first time, determination of the equivalence of sanitary measures will require further consideration of those other relevant measures making up the food control system.13.For the purposes of determining equivalence, the sanitary measures associated with a food inspection and certification system can be broadly categorised as:a)infrastructure; including the legislative base (e.g., food and enforcement law), andadministrative systems (e.g., organization of national and regional authorities, enforcement systems, etc.);b)programme design, implementation and monitoring; including documentation of systems,monitoring, performance, decision criteria and action, laboratory capability, transportation infrastructure and provisions for certification and audit; and/orc)specific requirements; including requirements applicable to individual facilities (e.g., premisesdesign), equipment (e.g., design of food contact machinery), processes (e.g., HACCP plans), procedures (e.g., ante- and post-mortem inspection), tests (e.g., laboratory tests for microbiological and chemical hazards) and methods of sampling and inspection.14.Categorization in this manner is likely to facilitate agreement between countries on the basis for comparison of sanitary measures subject to an equivalence determination (see section 6). Further, allocation of measures to a particular category may assist countries in simplifying the extent of the equivalence determination relative to other sanitary measures making up the food control system. SECTION 6 - OBJECTIVE BASIS OF COMPARISON15.Since the sanitary measures applied by an importing country have the purpose of achieving its ALOP, an exporting country may demonstrate achievement of the importing country’s ALOP by demonstrating that the measures it proposes as equivalent have the same effect, relative to the achievement of the importing country’s ALOP, as the corresponding sanitary measures applied by the importing country by using an objective basis of comparison.16.The importing country should, at the request of the exporting country, specify as precisely as possible an objective basis for comparison of the sanitary measures proposed by the exporting country and its own measures.11 Dialogue between the exporting and importing country will assist in the development of understanding and, desirably, agreement on the objective basis for comparison. Supporting information to be provided by the importing country may include:a)the reason/purpose for the sanitary measure, including identification of the specific risks thatthe measure is intended to address;b)the relationship of the sanitary measure to the ALOP, i.e., how the sanitary measure achievesthe ALOP;c)where appropriate, an expression of the level of control of the hazard in a food that is achievedby the sanitary measure;d)the scientific basis for the sanitary measure under consideration, including risk assessmentwhere appropriate;e)any additional information that may assist the exporting country in presenting an objectivedemonstration of equivalence.SECTION 7 - PROCEDURE FOR THE DETERMINATION OF EQUIVALENCE17.The importing country should make available details of its sanitary measures to the exporting country on request. The exporting country should review all applicable sanitary measures of the importing country for the food involved and identify those it will meet and those for which it seeks determination of equivalence. The importing and exporting countries should then use an agreed process for exchange of the relevant information to facilitate the determination of equivalence. This information should be limited to that which is necessary for this purpose.18.The determination of equivalence is facilitated by both exporting and importing countries following a sequence of steps, such as those described below and illustrated in Figure 1. The parties should work through these steps in a cooperative manner with the aim of reaching agreement:a)The exporting country identifies the sanitary measure of the importing country for which itwishes to apply a different measure, and requests the reason/purpose for the measure.b)The importing country provides the reason/purpose for the identified sanitary measure andother relevant information in accordance with section 6.c)In accordance with section 6 the importing country should specify as precisely as possible anobjective basis for comparison of the sanitary measures proposed by the exporting country and its own measures. On the initiative of the exporting country, the importing and exporting countries should enter into a dialogue concerning this objective basis for comparison with a view to reaching agreement.d)The exporting country develops a submission using risk assessment or other relevantmethodology as appropriate, to demonstrate that the application of the different sanitary measure achieves the ALOP of the importing country, and presents it to the importing country.e)The importing country reviews the submission and, if adequate, uses the submission todetermine whether the exporting country’s measure achieves the importing country’s ALOP. 11 The objective basis for comparison of sanitary measures categorized as “Infrastructure” is likely to be of aqualitative nature, e.g., the ability of food control legislation to achieve broad food safety goals. The objective basis of comparison of sanitary measures categorized as “Specific Requirements” is likely to be quantitative in nature e.g., a comparison of levels of hazard control achieved by the measure. The objective basis of comparison of sanitary measures categorized as “Programme” is likely to contain a mixture of qualitative and quantitative elements e.g., correct application of principles, and establishment of appropriate critical limits, in HACCP food control systems.f)If the importing country has any concerns with the submission as presented, it should notifythem to the exporting country at the earliest opportunity and should detail the reasons for concern. If possible, the importing country should suggest how the concerns might be addressed.g)The exporting country should respond to such concerns by providing further information,modifying its proposal or taking other action as appropriate.h)The importing country notifies the exporting country of its judgement within a reasonableperiod of time and provides the reasoning for its decision, should the judgement be that the sanitary measure is not equivalent, i.e., does not achieve the importing country’s ALOP.i)An attempt should be made to resolve any differences of opinion over judgement of asubmission, either interim or final.SECTION 8 – JUDGEMENT19.Judgement of equivalence by the importing country should be based on a transparent analytical process that is objective and consistent, and includes consultation with all interested parties to the extent practicable and reasonable.20.Judgement of the equivalence of sanitary measures should take into account:a)experience, knowledge and confidence of an exporting country’s food inspection andcertification systems (see section 5);b)supporting data submitted by the exporting country;c)analysis of the strength of the relationship between the exporting country’s specified sanitarymeasure, and the achievement of the ALOP of the importing country as reflected in the objective basis for comparison (see section 6);d)that parameters should be stated in quantitative terms to the extent possible;e)adequacy of qualitative descriptions where the level of control of hazards in foods in is notquantified;f)consideration of variability and other sources of uncertainty in data;g)consideration of all expected human health outcomes of the exporting country’s identifiedsanitary measure;h)those Codex texts relevant to the food safety matters under consideration.21.Following any judgment of equivalence, exporting and importing countries should promptly advise each other of significant changes in their supporting programmes and infrastructure that may affect the original determination of equivalence.Figure I: Simplified flow chart for the determination of equivalence(individual steps may be iterated)(18.g)NoYes。
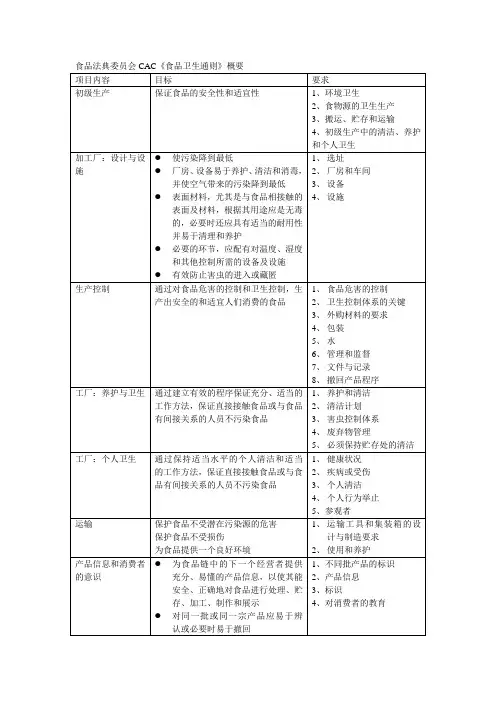



食品卫生通用规范CAC/RCP1-1969, Rev.4-2003 1前言1目的食品卫生通用规范2范围、应用和定义2.1范围2.2应用2.3定义3初级生产3.1环境卫生3.2食品原料的卫生生产3.3搬运、贮藏和运输4工厂:设计及设施4.1 选址4.2厂房和车间* 注1:这一版本的《食品卫生通用规范》,包括附件《HACCP体系及其应用指南》是由CAC在1997年制订的。
在1999年进行修改。
HACCP指南在2003年修改。
这一法规已作为咨询版本发放至FAO和WHO的各成员国,并由各国政府决定将其作为指南。
4.3设备4.4设施5操作的控制5.1食品危害的控制5.2卫生控制体系的关键5.3原料接收的要求5.4包装5.5水5.6管理与监督5.7文件和记录5.8产品回收程序6工厂:维护和卫生6.1维护和清洁6.2清洁计划6.3害虫控制体系6.4废弃物管理6.5监控的有效性7工厂:个人卫生7.1健康状况7.2疾病和受伤7.3个人清洁7.4个人行为7.5外来人员8运输8.1总体要求8.2要求8.3使用和维护9产品信息和消费者的意识9.1批次识别9.2产品信息9.3标识9.4对消费者的教育10培训10.1意识和责任10.2培训计划10.3指导和监督10.4培训的回顾附录:危害分析及关键控制点(HACCP)体系及其应用指南1.导言2.定义3.HACCP体系的原则4.HACCP体系及其应用导则4.1 介绍4.2 应用导则4.3培训图1:HACCP应用的逻辑步骤图2:关键控制点判断树状表图3:HACCP工作表范例前言人们有权期望他们食用的食品是安全和适宜消费的,但食源性疾病导致的伤害,会令人不安,有时甚至是致命性的,这是人们最不愿看见的。
当然,食物中毒事件也会带来其他一些影响。
食源性品疾病不仅会影响到贸易和旅游业,导致收入下降、工人失业,甚至于产生法律纠纷。
食品腐败不仅会造成浪费,影响成本,还可能对贸易和消费者的信心产生不良影响。
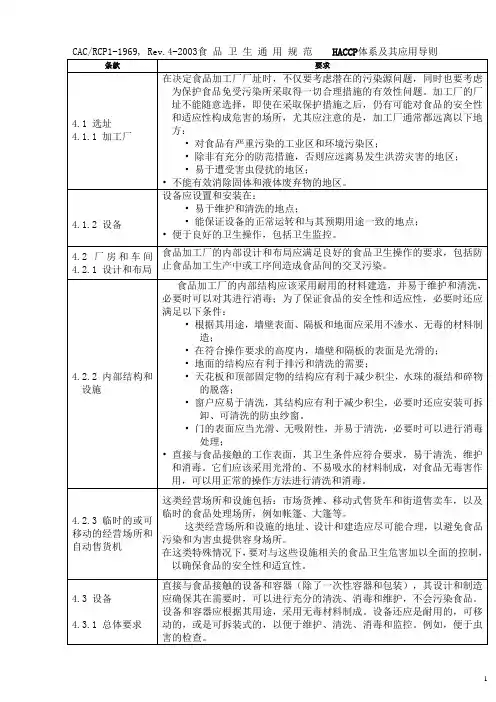

食品卫生通用规范CAC/RCP1-1969, Rev.4-2003 1前言1目的食品卫生通用规范2范围、应用和定义2.1范围2.2应用2.3定义3初级生产3.1环境卫生3.2食品原料的卫生生产3.3搬运、贮藏和运输4工厂:设计及设施4.1 选址4.2厂房和车间* 注1:这一版本的《食品卫生通用规范》,包括附件《HACCP体系及其应用指南》是由CAC在1997年制订的。
在1999年进行修改。
HACCP指南在2003年修改。
这一法规已作为咨询版本发放至FAO和WHO的各成员国,并由各国政府决定将其作为指南。
4.3设备4.4设施5操作的控制5.1食品危害的控制5.2卫生控制体系的关键5.3原料接收的要求5.4包装5.5水5.6管理与监督5.7文件和记录5.8产品回收程序6工厂:维护和卫生6.1维护和清洁6.2清洁计划6.3害虫控制体系6.4废弃物管理6.5监控的有效性7工厂:个人卫生7.1健康状况7.2疾病和受伤7.3个人清洁7.4个人行为7.5外来人员8运输8.1总体要求8.2要求8.3使用和维护9产品信息和消费者的意识9.1批次识别9.2产品信息9.3标识9.4对消费者的教育10培训10.1意识和责任10.2培训计划10.3指导和监督10.4培训的回顾附录:危害分析及关键控制点(HACCP)体系及其应用指南1.导言2.定义3.HACCP体系的原则4.HACCP体系及其应用导则4.1 介绍4.2 应用导则4.3培训图1:HACCP应用的逻辑步骤图2:关键控制点判断树状表图3:HACCP工作表范例前言人们有权期望他们食用的食品是安全和适宜消费的,但食源性疾病导致的伤害,会令人不安,有时甚至是致命性的,这是人们最不愿看见的。
当然,食物中毒事件也会带来其他一些影响。
食源性品疾病不仅会影响到贸易和旅游业,导致收入下降、工人失业,甚至于产生法律纠纷。
食品腐败不仅会造成浪费,影响成本,还可能对贸易和消费者的信心产生不良影响。


CodexAlimentariusFoodHygeine食品卫生通用规范CAC/RCP1-1969,Rev.4-2003*注1:这一版本的《食品卫生通用规范》,包括附件《HACCP体系及其应用指南》是由CAC在1997年制订的。
在1999年进行修改。
HACCP指南在2003年修改。
这一法规已作为咨询版本发放至FAO和WHO的各成员国,并由各国政府决定将其作为指南。
前言1目的22.12.22.333.13.23.344.14.24.34.455.15.25.35.45.5水5.65.75.866.1维护和清洁6.2清洁计划6.3害虫控制体系6.4废弃物管理6.5监控的有效性7工厂:个人卫生7.1健康状况7.2疾病和受伤7.3个人清洁7.4个人行为7.5外来人员8运输8.1总体要求8.2要求8.3使用和维护9产品信息和消费者的意识9.1批次识别9.2产品信息9.3标识9.4对消费者的教育10培训10.110.210.310.4附录:1.导言2.定义4.14.24.3图1:图2图3:前言人们有权期望他们食用的食品是安全和适宜消费的,但食源性疾病导致的伤害,会令人不安,有时甚至是致命性的,这是人们最不愿看见的。
当然,食物中毒事件也会带来其他一些影响。
食源性品疾病不仅会影响到贸易和旅游业,导致收入下降、工人失业,甚至于产生法律纠纷。
食品腐败不仅会造成浪费,影响成本,还可能对贸易和消费者的信心产生不良影响。
现在,国际食品贸易和出国旅游业正在兴旺发展,带来了显着的社会和经济效益,但同时也使得疾病更易于在世界范围传播。
从新食品的生产、制作和销售技术的不断发展可以看出,在过去的20年里,许多国家人们的饮食习惯已经发生了巨大变化。
因此,对食品卫生进行有效地控制是非常重要的,以避免由于食源性疾病,食源性伤害和食品腐败给人们身体健康带来的损害及造成经济损失。
我们每一个人,包括农场主和耕种者、加工和制制造商、食品经营者和消费者,都有责任来保证食用的食物是安全和适宜消费的。
PRINCIPLES FOR THE RISK ANALYSIS OF FOODS DERIVED FROM MODERN BIOTECHNOLOGY (CAC/GL 44-2003)SECTION 1 - INTRODUCTION1. For many foods, the level of food safety generally accepted by the society reflects the history of their safe consumption by humans. It is recognised that in many cases the knowledge required to manage the risks associated with foods has been acquired i n the course of their long history of use. Foods are generally considered safe, provided that care is taken during development, primary production, processing, storage, handling and preparation.2. The hazards associated with foods are subjected to the risk analysis process of the Codex Alimentarius Commission to assess potential risks and, if necessary, to develop approaches to manage these risks. The conduct of risk analysis is guided by general decisions of the Codex AlimentariusCommission[1] as well as the Codex Working Principles for Risk Analysis[2].3. While risk analysis has been used over a long period of time to address chemical hazards(e.g. residues of pesticides, contaminants, food additives and processing aids), and it is being increasingly used to address microbiological hazards and nutritional factors, the principles were not elaborated specifically for whole foods.4. The risk analysis approach can, in general terms, be applied to foods including foods derived from modern biotechnology. However, it is recognised that this approach must be modified when applied to a whole food rather than to a discrete hazard that may be present in food.5. The principles presented in this document should be read in conjunction with the Codex Working Principles for Risk Analysis to which these principles are supplemental.6. Where appropriate, the results of a risk assessment undertaken by other regulatory authorities may be used to assist in the risk analysis and avoid duplication of work.SECTION 2 - SCOPE AND DEFINITIONS7. The purpose of these Principles is to provide a framework for undertaking risk analysis on the safety and nutritional aspects of foods derived from modern biotechnology. This document does not address environmental, ethical, moral and socio-economic aspects of the research, development, production and marketing of these foods[3].8. The definitions below apply to these Principles:"Modern Biotechnology" means the application of:i) In vitro nucleic acid techniques, including recombinant deoxyribonucleic acid (DNA) and direct injection of nucleic acid into cells or organelles, orii) Fusion of cells beyond the taxonomic family,that overcome natural physiological reproductive or recombinant barriers and that are not techniques used in traditional breeding and selection[4]."Conventional Counterpart"means a related organism/variety, its components and/or products for which there is experience of establishing safety based on common use as food.[5]SECTION 3 - PRINCIPLES9. The risk analysis process for foods derived from modern biotechnology should be consistent with the Codex Working Principles for Risk Analysis.RISK ASSESSMENT10. Risk assessment includes a safety assessment, which is designed to identify whether a hazard, nutritional or other safety concern is present, and if present, to gather information on its nature and severity. The safety assessment should include a comparison between the food derived from modern biotechnology and its conventional counterpart focusing on determination of similarities and differences. If a new or altered hazard, nutritional or other safety concern is identified by the safety assessment, the risk associated with it should be characterized to determine its relevance to human health.11. A safety assessment is characterized by an assessment of a whole food or a component thereof relative to the appropriate conventional counterpart:A) taking into account both intended and unintended effects;B) identifying new or altered hazards;C) identifying changes, relevant to human health, in key nutrients.12. A pre-market safety assessment should be undertaken following a structured and integrated approach and be performed on a case-by-case basis. The data and information, based on sound science, obtai ned using appropriate methods and analysed using appropriate statistical techniques, should be of a quality and, as appropriate, of quantity that would withstand scientific peer review.13. Risk assessment should apply to all relevant aspects of foods deri ved from modern biotechnology. The risk assessment approach for these foods is based on a consideration of science-based multidisciplinary data and information taking into account the factors mentioned in the accompanying Guidelines[6].14. Scientific data for risk assessment are generally obtained from a variety of sources, such as the developer of the product, scientific literature, general technical information, independent scientists, regulatory agencies, international bodies and other interested parties. Data should be assessed using appropriate science-based risk assessment methods.15. Risk assessment should take into account all available scientific data and information derived from different testing procedures, provided that the procedures are scientifically sound and the parameters being measured are comparable.RISK MANAGEMENT16. Risk management measures for foods derived from modern biotechnology should be proportional to the risk, based on the outcome of the risk assessment and, where relevant, taking into account other legitimate factors in accordance with the general decisions of the Codex AlimentariusCommission[7] as well as the Codex Working Principles for Risk Analysis.17. It should be recognised that different risk management measures may be capable of achieving the same level of protection with regard to the management of risks associated with safety and nutritional impacts on human health, and therefore would be equivalent.18. Risk managers should take into account the uncertainties identified in the risk assessment and implement appropriate measures to manage these uncertainties.19. Risk management measures may include, as appropriate, food labelling[8]conditions for marketing approvals and post-market monitoring.20. Post-market monitoring may be an appropriate risk management measure in specific circumstances. Its need and utility should be considered, on a case-by-case basis, during risk assessment and its practicability should be considered during risk management. Post-market monitoring may be undertaken for the purpose of:A) verifying conclusions about the absence or the possible occurrence, impact and significance of potential consumer health effects; andB) monitoring changes in nutrient intake levels, associated with the introduction of foods likely to significantly alter nutritional status, to determine their human health impact.21. Specific tools may be needed to facilitate the implementation and enforcement o f risk management measures. These may include appropriate analytical methods; reference materials; and, the tracing of products[9]for the purpose of facilitating withdrawal from the market when a risk to human health has been identified or to support post-market monitoring in circumstances as indicated in paragraph 20.RISK COMMUNICATION22. Effective risk communication is essential at all phases of risk assessment and risk management. It is an interactive process involving all interested parties, including government, industry, academia, media and consumers.23. Risk communication should include transparent safety assessment and risk management decision-making processes. These processes should be fully documented at all stages and open to public scrutiny, whilst respecting legitimate concerns to safeguard the confidentiality of commercial and industrial information. In particular, reports prepared on the safety assessments and other aspects of the decision-making process should be made available to all interested parties.24. Effective risk communication should include responsive consultation processes. Consultation processes should be interactive. The views of all interested parties should be sought and relevant food safety and nutritional issues that are raised during consultation should be addressed during the risk analysis process.CONSISTENCY25. A consistent approach should be adopted to characterise and manage safety and nutritional risks associated with foods derived from modern biotechnology. Unjustified differences in the level of risks presented to consumers between these foods and similar conventional foods should be avoided.26. A transparent and well-defined regulatory framework should be provided in characterising and managing the risks associated with foods derived from modern biotechnology. This should include consistency of data requirements, assessment frameworks, the acceptable level of risk, communication and consultation mechanisms and timely decision processes.CAPACITY BUILDING AND INFORMATION EXCHANGE27. Efforts should be made to improve the capability of regulatory authorities, particularly those of developing countries, to assess, manage and communicate risks, including enforcement, associated with foods derived from modern biotechnology or to interpret assessments undertaken by other authorities or recognised expert bodies, including access to analytical technology. In addition capacity building for developing countries either through bilateral arrangements or with assistance of international organizations should be directed toward effective application of these principles[10].28. Regulatory authorities, international organisations and expert bodies and industry should facilitate through appropriate contact points including but not limited to Codex Contact Points and other appropriate means, the exchange of information including the information on analytical methods.REVIEW PROCESSES29. Risk analysis methodology and its application should be consistent with new scientific knowledge and other information relevant to risk analysis.30. Recognizing the rapid pace of development in the field of biotechnology, the approach to safety assessments of foods derived from modern biotechnology should be reviewed when necessary to ensure that emerging scientific information is incorporated into the risk analysis. When new scientific information relevant to a risk assessment becomes available the assessment should be reviewed to incorporate that information and, if necessary, risk management measures adapted accordingly.[1] These decisions include the Statements of principle concerning the role of science in the Codex decision-making process and the extent to which other factors are taken into account and the Statements of principle relating to the role of food safety risk assessment (Codex Alimentarius Commission Procedural Manual; Thirteenth edition).[2]"Working Principles for Risk Analysis for Application in the Framework of the Codex Alimentarius"(adopted by the 26th Session of the Codex Alimentarius Commission, 2003; Codex Alimentarius Commission Procedural Manual; Thirteenth edition)[3] This document does not address animal feed and animals fed such feed except insofar as these animals have been developed by using modern biotechnology.[4]This definition is taken from the Cartagena Biosafety Protocol under the Convention on Biological Diversity.[5] It is recognized that for the foreseeable future, foods derived from modern biotechnology will not be used as conventional counterparts.[6] Reference is made to the Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants (CAC/GL 45-2003) and the Guideline for the Conduct of Food Safety Assessment of Foods Produced using Recombinant-DNA Microorganisms (CAC/GL 46-2003).[7] See footnote 1.[8] Reference is made to the CCFL in relation to the Proposed Draft Guidelines for the Labelling of Foods and Food Ingredients obtained through certain techniques of genetic modification/genetic engineering at Step 3 of the Codex Elaboration Procedure.[9]It is recognised that there are other applications of product tracing. These applications should be consistent with the provisions of the SPS and TBT Agreements. The application of product tracing to the areas covered by both Agreements is under consideration within Codex on the basis of decisions of 49th Session of Executive Committee.[10] Reference is made to technical assistance of provisions in Article 9 of the SPS Agreement and Article 11 of the TBT Agreement.。
Codex Alimentarius Food Hygeine食品卫生通用规范CAC/RCP1-1969,* 注1:这一版本的《食品卫生通用规范》,包括附件《HACCP 体系及其应用指南》是由CAC 在1997 年制订的。
在1999 年进行修改。
HACCP 指南在2003 年修改。
这一法规已作为咨询版本发放至FAO 和WHO 的各成员国,并由各国政府决定将其作为指南。
前言1 目的食品卫生通用规范2 范围、应用和定义范围应用定义3 初级生产环境卫生食品原料的卫生生产搬运、贮藏和运输4 工厂:设计及设施选址厂房和车间设备设施5 操作的控制食品危害的控制卫生控制体系的关键原料接收的要求包装水管理与监督文件和记录产品回收程序6 工厂:维护和卫生维护和清洁清洁计划害虫控制体系废弃物管理监控的有效性7 工厂:个人卫生健康状况疾病和受伤个人清洁个人行为外来人员8 运输总体要求要求使用和维护9 产品信息和消费者的意识批次识别产品信息标识对消费者的教育10 培训意识和责任培训计划指导和监督培训的回顾附录:危害分析及关键控制点(HACCP)体系及其应用指南1. 导言2. 定义3. HACCP体系的原则4. HACCP体系及其应用导则介绍应用导则培训图1:HACCP应用的逻辑步骤图2:关键控制点判断树状表图3:HACCP工作表范例前言人们有权期望他们食用的食品是安全和适宜消费的,但食源性疾病导致的伤害,会令人不安,有时甚至是致命性的,这是人们最不愿看见的。
当然,食物中毒事件也会带来其他一些影响。
食源性品疾病不仅会影响到贸易和旅游业,导致收入下降、工人失业,甚至于产生法律纠纷。
食品腐败不仅会造成浪费,影响成本,还可能对贸易和消费者的信心产生不良影响。
现在,国际食品贸易和出国旅游业正在兴旺发展,带来了显着的社会和经济效益,但同时也使得疾病更易于在世界范围传播。
从新食品的生产、制作和销售技术的不断发展可以看出,在过去的20年里,许多国家人们的饮食习惯已经发生了巨大变化。
Codex Alimentarius FoodHygeine食品卫生通用规范CAC/RCP1-1969, Rev.4-2003* 注1:这一版本的《食品卫生通用规范》,包括附件《HACCP 体系及其应用指南》是由CAC 在1997 年制订的。
在1999 年进行修改。
HACCP 指南在2003 年修改。
这一法规已作为咨询版本发放至FAO 和WHO 的各成员国,并由各国政府决定将其作为指南。
前言1 目的食品卫生通用规范2 范围、应用和定义2.1 范围2.2 应用2.3 定义3初级生产3.1 环境卫生3.2 食品原料的卫生生产3.3 搬运、贮藏和运输4 工厂:设计及设施4.1 选址4.2 厂房和车间4.3 设备4.4 设施5 操作的控制5.1 食品危害的控制5.2 卫生控制体系的关键5.3 原料接收的要求5.4 包装5.5 水5.6 管理与监督5.7 文件和记录5.8 产品回收程序6 工厂:维护和卫生6.1 维护和清洁6.2 清洁计划6.3 害虫控制体系6.4 废弃物管理6.5 监控的有效性7 工厂:个人卫生7.1 健康状况7.2 疾病和受伤7.3 个人清洁7.4 个人行为7.5 外来人员8 运输8.1 总体要求8.2 要求8.3 使用和维护9 产品信息和消费者的意识9.1 批次识别9.2 产品信息9.3 标识9.4 对消费者的教育10 培训10.1 意识和责任10.2 培训计划10.3 指导和监督10.4 培训的回顾附录:危害分析及关键控制点(HACCP)体系及其应用指南1. 导言2. 定义3. HACCP体系的原则4. HACCP体系及其应用导则4.1 介绍4.2 应用导则4.3培训图1:HACCP应用的逻辑步骤图2:关键控制点判断树状表图3:HACCP工作表范例前言人们有权期望他们食用的食品是安全和适宜消费的,但食源性疾病导致的伤害,会令人不安,有时甚至是致命性的,这是人们最不愿看见的。
当然,食物中毒事件也会带来其他一些影响。
基本文本食品法典前言食品法典委员会及FAO/WHO食品标准计划食品法典委员会负责执行粮农组织/世界卫生组织联合食品标准计划,旨在保护消费者健康和促进食品贸易的公平进行。
食品法典(拉丁文指食品法律或法规)是以统一格式表述的国际已采纳的食品标准的汇编。
它还包括了以操作规范、准则和其它建议措施为形式的咨询性规定,以助于达到食品法典的目标。
委员会已表明,这些操作规范可为国家食品控制或执法机构提供需要的有用清单。
食品法典的出版旨在指导和促进各种食品的定义及其要求的详细界定和确立,以有助于其协调一致,并借此促进国际贸易。
食品卫生的基本文本 — 第三版食品法典委员会于1997年和1999年采纳了食品卫生的基本文本。
这是1997年首次出版的汇编册的第三版,包括了2003年食品法典委员会采纳的对HACCP系统应用准则的修订。
希望该版有助于食品卫生基本原则的广泛使用和理解,并将鼓励政府部门、执法机构、食品企业和所有的食品加工者和消费者使用。
有关这些文本的详细信息,或食品法典委员会的其他方面内容可从以下获得:食品法典委员会秘书处F AO/WHO联合食品标准计划,F AO, Viale delle Terme di Caracalla,00100, 意大利罗马传真: +39(6)57.05.45.93电子邮箱: codex@iii基本文本食品法典目录国际推荐操作规范食品卫生总则 -------------------------------------------------- 1 危害分析和关键控制点系统(HACCP)及其应用准则-------------------------29 制定和应用食品微生物标准的原则--------------------------------------------------41 实施微生物危险性评估的原则和准则-----------------------------------------------47 出版史--------------------------------------------------------------------------------------55 索引-----------------------------------------------------------------------------------------56v基本文本食品法典国际推荐操作规范食品卫生总则CAC/RCP 1-1969, 第4修订版(2003)引言------------------------------------------------------------------------------------- 3第一节-目标----------------------------------------------------------------------- 3 食品卫生的食典总则----------------------------------------------------------------- 3第二节-范围、应用和定义-------------------------------------------------------- 4 2.1范围----------------------------------------------------------------------------- 4 2.2应用----------------------------------------------------------------------------- 5 2.3定义----------------------------------------------------------------------------- 5第三节-初级生产-------------------------------------------------------------------- 7 3.1环境卫生-----------------------------------------------------------------------7 3.2食物来源的卫生生产--------------------------------------------------------7 3.3处理、贮藏和运输-----------------------------------------------------------8 3.4初级生产中的清洁处理、保持卫生及个人卫生------------------------8第四节-加工场所:设计与设施---------------------------------------------------- 9 4.1选址-----------------------------------------------------------------------------9 4.2加工场所和房间--------------------------------------------------------------10 4.3设备-----------------------------------------------------------------------------11 4.4设施-----------------------------------------------------------------------------12第五节-加工的卫生控制-------------------------------------------------------------14 5.1食品危害的控制----------------------------------------------------------------14 5.2卫生控制系统的关键方面----------------------------------------------------15 5.3原料的进货要求----------------------------------------------------------------16 5.4包装------------------------------------------------------------------------------17 5.5用水------------------------------------------------------------------------------17 5.6管理与监督----------------------------------------------------------------------18 5.7文件管理与记录----------------------------------------------------------------18 5.8产品召回程序-------------------------------------------------------------------181食品法典食品卫生第六节-场所:维护与清洁----------------------------------------------------------19 6.1维护与清洁----------------------------------------------------------------------19 6.2清洁方案-------------------------------------------------------------------------20 6.3害虫控制系统-------------------------------------------------------------------20 6.4废弃物的处理-------------------------------------------------------------------21 6.5监测有效性----------------------------------------------------------------------21第七节-场所:个人卫生-------------------------------------------------------------22 7.1健康状况-------------------------------------------------------------------------22 7.2疾病和损伤----------------------------------------------------------------------22 7.3个人清洁-------------------------------------------------------------------------23 7.4个人习惯-------------------------------------------------------------------------23 7.5来访者----------------------------------------------------------------------------23第八节-食品运输--------------------------------------------------------------------24 8.1基本要求-------------------------------------------------------------------------24 8.2具体要求-------------------------------------------------------------------------24 8.3使用和维护----------------------------------------------------------------------25第九节-产品信息及对消费者知情权--------------------------------------------26 9.1批号鉴别-------------------------------------------------------------------------26 9.2产品信息-------------------------------------------------------------------------27 9.3标签------------------------------------------------------------------------------27 9.4对消费者的教育----------------------------------------------------------------27第十节-培训--------------------------------------------------------------------------27 10.1知情权和责任-----------------------------------------------------------------27 10.2培训计划-----------------------------------------------------------------------27 10.3指导和监督--------------------------------------------------------------------28 10.4培训内容的更新--------------------------------------------------------------282基本文本食品法典引言人们有权要求所食用的食品是安全和适于食用的。