核磁共振氢谱和碳谱中各溶剂的化学位移
- 格式:pdf
- 大小:160.49 KB
- 文档页数:2

核磁共振(NMR)技术是一种应用广泛的谱学技术,常用于分析有机物和生物分子的结构和性质。
在核磁共振谱中,化学位移是一个重要的参数,它与化合物中原子核周围的电子环境有关。
化学位移在碳谱和氢谱中都是十分常见的,在本文中,我们将探讨化学位移在核磁共振碳谱和氢谱中产生的原因。
1. 基本概念在核磁共振谱中,化学位移是指核磁共振信号的频率与参考物质(通常是三氯化甲烷或二甲基硅烷)信号频率之差。
化学位移通常用ppm (parts per million)表示,它是一个相对值,可以用来比较不同化合物中原子核的化学环境差异。
2. 碳谱中化学位移的影响因素碳谱中的化学位移受到多种因素的影响,其中主要包括化学环境、电子效应和磁场效应。
- 化学环境:不同化学环境下的碳原子核受到不同的化学位移影响。
芳香环上的碳原子与脂肪链上的碳原子所受的化学环境不同,因此它们的化学位移也会有所差异。
- 电子效应:分子中的电子密度分布会影响到周围原子核的化学位移。
含有电子丰富基团的碳原子通常会表现出较低的化学位移,而含有电子贫瘠基团的碳原子则会表现出较高的化学位移。
- 磁场效应:外加磁场对原子核周围的电子运动轨迹会产生影响,从而影响原子核的化学位移。
这种效应在核磁共振谱分析中是不可忽视的。
3. 氢谱中化学位移的影响因素类似于碳谱,氢谱中的化学位移也受到化学环境、电子效应和磁场效应的影响。
- 化学环境:不同化学环境下的氢原子核受到不同的化学位移影响。
α-位置上的氢原子与β-位置上的氢原子所受的化学环境不同,因此它们的化学位移也会有所差异。
- 电子效应:分子中的电子密度分布会影响到周围原子核的化学位移。
对甲苯中的甲基氢和对位氢受到的电子效应不同,因此它们的化学位移也会有所差异。
- 磁场效应:外加磁场对原子核周围的电子运动轨迹会产生影响,从而影响原子核的化学位移。
这种效应在氢谱分析中同样需要考虑。
4. 结语化学位移在核磁共振碳谱和氢谱中的产生是一个复杂而又精密的过程,受到多种因素的影响。


氘代dmso化学位移
氘代DMSO(dimethylsulfoxide)是一种有机化合物,广泛应用于化学、生物学和材料科学等领域。
氘代DMSO的主要特点是其中一个氧原子被氘原子替换,这使得它在实验研究中具有独特的优势。
氘代DMSO在化学位移中的应用非常广泛。
由于氘原子与氢原子的相互作用,氘代DMSO可以作为一种氘代溶剂,用于研究溶剂效应、氢键和分子间相互作用等。
在核磁共振氢谱(1H-NMR)和核磁共振碳谱(13C-NMR)等实验中,氘代DMSO可以作为溶剂或添加剂,提高样品的溶解度,从而获得更好的信号。
氘代DMSO的优势在于其对许多化合物的溶解度较高,能够显著提高谱图质量。
此外,氘代DMSO可以减少氢氘交换反应,降低信号干扰。
然而,氘代DMSO也存在局限性,如对某些化合物的不稳定性、可能导致样品降解等问题。
因此,在实际应用中,需要根据具体研究目标和实验条件选择合适的氘代溶剂。
在实验中使用氘代DMSO时,应注意以下几点:
1.严格遵循实验规程,确保实验安全。
氘代DMSO具有一定的毒性,应避免直接接触皮肤和眼睛,并在通风良好的环境下操作。
2.选择合适的氘代DMSO浓度。
过高或过低的浓度都可能影响实验结果,因此需要根据具体实验需求调整氘代DMSO的用量。
3.注意氘代DMSO的保存条件。
氘代DMSO应在干燥、避光的环境中储存,并注意密封防潮。
4.考虑到氘代DMSO可能与某些化合物发生相互作用,实验前应对目标化合物进行充分了解,以避免不必要的麻烦。
总之,氘代DMSO作为一种重要的氘代溶剂,在化学位移研究中具有广泛的应用。
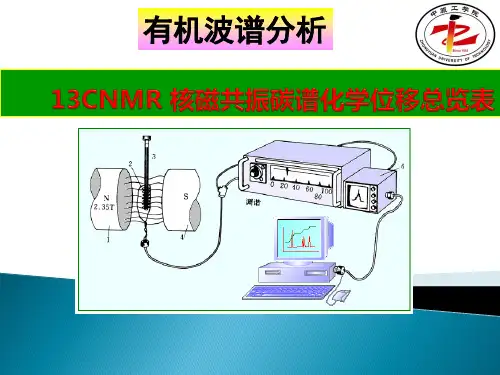

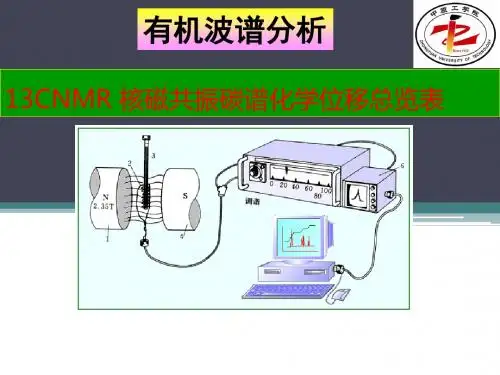
核磁共振氢谱核磁共振---NMR1945年美国斯坦福大学的 F. Block 和哈佛大学的 E. M. Purcell 同时发现了核磁共振现象,并因此荣获了1952年的 Nobel 物理奖。
核磁共振谱可为化合物鉴定提供下列信息:1.磁核的类型:由化学位移来判别,如在1HNMR 中,可判别甲基氢、芳氢、烯氢、醛氢等。
2.磁核的化学环境:由偶合常数和自旋-自旋裂分来判别,如在 1H-NMR 中可判定甲基是与-CH 2-相连,还是与苯环相连。
3.各类磁核的相对数量:氢谱中,通过积分面积或积分曲线来判断。
4 .核自旋弛豫时间:13CNMR 可提供 T 1,并用于结构归属指定,构象的测定,以及窥测体 系的运动情况。
5 .核间相对距离:通过核的 Overhause 效应可测得。
3.1核磁共振的基本原理3.1.1原子核的磁矩原子核是带正电荷的粒子,自旋将产生磁矩,但并非所有同位素的原子核有自旋,只有有自旋才有磁矩。
具有自旋运动的原子核具有一定自旋量子数(I ),I=1/2 *n ,那1,2,3···1. 核电荷数和和质量数均为偶数的原子核没自旋。
2. 核电荷数为奇数或偶数,核质量数为奇数,有自旋现象。
3. 核电荷数为奇数,核质量数为偶数,I 为整数的原子核有自旋现象。
对于自旋不为零的核来说,当其自旋时由于形成环电流,故而产生一个小磁场,这个小磁场可用核磁矩 μ 表示。
μ 是矢量,其大小由下式确定:πγγμ2)1(hI I p +==式中 γ ---核的磁旋比 p---自旋角动量不同的核有不同的 γ 值,是确定同位素核的特征常数。
3.1.2自旋核在磁场中的取向和能级对于I 不为零的核来说,如果不受外来磁场的干扰,其自旋轴的取向将是任意的。
当它们处于外加静磁场(磁场强度为H0)中时,根据量子力学理论,它们的自旋轴的取向不再是任意的,而只有(2I+1)种,这叫核自旋的空间量子化。
每一种取向可用一个磁量子数m 表示,则m=I,I-1,I-2,…-I+1,-I。

核磁氢谱中常见的官能团化学位移核磁共振(Nuclear Magnetic Resonance,简称NMR)是一种重要的分析技术,可以用于研究化合物分子结构和化学环境。
在核磁共振谱中,氢原子的化学位移是一个重要的参数,可以提供分子中不同官能团的信息。
官能团是分子中具有特定化学性质的结构单元,每个官能团都有其特有的核磁化学位移范围。
下面介绍一些核磁氢谱中常见的官能团及其化学位移范围:1. 烷基(Alkyl)官能团烷基官能团是由碳和氢组成的烷烃分子链,例如甲基(CH3-)和乙基(C2H5-)等。
其化学位移范围通常在0.5~2.5 ppm之间。
2. 烯烃(Alkenyl)官能团烯烃官能团是含有碳—碳双键的分子,例如乙烯(C2H4-)和丙烯(C3H6-)等。
其化学位移范围通常在4.5~6.0 ppm之间。
3. 酮(Ketone)官能团酮官能团是由碳氧双键连接碳原子形成的结构,例如丙酮(CH3COCH3)和己酮(C5H9COCH3)等。
其化学位移范围通常在2.1~2.4 ppm之间。
4. 醇(Alcohol)官能团醇官能团是由羟基(-OH)连接到碳原子的结构,例如甲醇(CH3OH)和乙醇(C2H5OH)等。
其化学位移范围通常在0.5~5.0 ppm之间。
5. 醛(Aldehyde)官能团醛官能团是由碳氧双键和氢连接到同一个碳原子的结构,例如乙醛(CH3CHO)和丁醛(C4H9CHO)等。
其化学位移范围通常在9.0~10.0 ppm之间。
除了这些常见的官能团,核磁氢谱中还存在其他许多官能团化学位移,如羧酸、酰胺、卤代烷等。
通过对氢原子的化学位移的分析,我们可以进一步确定化合物的结构和化学环境。



NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E.Gottlieb,*Vadim Kotlyar,andAbraham Nudelman*Department of Chemistry,Bar-Ilan University,Ramat-Gan52900,IsraelReceived June27,1997In the course of the routine use of NMR as an aid for organic chemistry,a day-to-day problem is the identifica-tion of signals deriving from common contaminants (water,solvents,stabilizers,oils)in less-than-analyti-cally-pure samples.This data may be available in the literature,but the time involved in searching for it may be considerable.Another issue is the concentration dependence of chemical shifts(especially1H);results obtained two or three decades ago usually refer to much more concentrated samples,and run at lower magnetic fields,than today’s practice.We therefore decided to collect1H and13C chemical shifts of what are,in our experience,the most popular “extra peaks”in a variety of commonly used NMR solvents,in the hope that this will be of assistance to the practicing chemist.Experimental SectionNMR spectra were taken in a Bruker DPX-300instrument (300.1and75.5MHz for1H and13C,respectively).Unless otherwise indicated,all were run at room temperature(24(1°C).For the experiments in the last section of this paper,probe temperatures were measured with a calibrated Eurotherm840/T digital thermometer,connected to a thermocouple which was introduced into an NMR tube filled with mineral oil to ap-proximately the same level as a typical sample.At each temperature,the D2O samples were left to equilibrate for at least 10min before the data were collected.In order to avoid having to obtain hundreds of spectra,we prepared seven stock solutions containing approximately equal amounts of several of our entries,chosen in such a way as to prevent intermolecular interactions and possible ambiguities in assignment.Solution1:acetone,tert-butyl methyl ether,di-methylformamide,ethanol,toluene.Solution2:benzene,di-methyl sulfoxide,ethyl acetate,methanol.Solution3:acetic acid,chloroform,diethyl ether,2-propanol,tetrahydrofuran. Solution4:acetonitrile,dichloromethane,dioxane,n-hexane, HMPA.Solution5:1,2-dichloroethane,ethyl methyl ketone, n-pentane,pyridine.Solution6:tert-butyl alcohol,BHT,cyclo-hexane,1,2-dimethoxyethane,nitromethane,silicone grease, triethylamine.Solution7:diglyme,dimethylacetamide,ethyl-ene glycol,“grease”(engine oil).For D2O.Solution1:acetone, tert-butyl methyl ether,dimethylformamide,ethanol,2-propanol. Solution2:dimethyl sulfoxide,ethyl acetate,ethylene glycol, methanol.Solution3:acetonitrile,diglyme,dioxane,HMPA, pyridine.Solution4:1,2-dimethoxyethane,dimethylacetamide, ethyl methyl ketone,triethylamine.Solution5:acetic acid,tert-butyl alcohol,diethyl ether,tetrahydrofuran.In D2O and CD3OD nitromethane was run separately,as the protons exchanged with deuterium in presence of triethylamine.ResultsProton Spectra(Table1).A sample of0.6mL of the solvent,containing1µL of TMS,1was first run on its own.From this spectrum we determined the chemical shifts of the solvent residual peak2and the water peak. It should be noted that the latter is quite temperature-dependent(vide infra).Also,any potential hydrogen-bond acceptor will tend to shift the water signal down-field;this is particularly true for nonpolar solvents.In contrast,in e.g.DMSO the water is already strongly hydrogen-bonded to the solvent,and solutes have only a negligible effect on its chemical shift.This is also true for D2O;the chemical shift of the residual HDO is very temperature-dependent(vide infra)but,maybe counter-intuitively,remarkably solute(and pH)independent. We then added3µL of one of our stock solutions to the NMR tube.The chemical shifts were read and are presented in Table 1.Except where indicated,the coupling constants,and therefore the peak shapes,are essentially solvent-independent and are presented only once.For D2O as a solvent,the accepted reference peak(δ)0)is the methyl signal of the sodium salt of3-(trimeth-ylsilyl)propanesulfonic acid;one crystal of this was added to each NMR tube.This material has several disadvan-tages,however:it is not volatile,so it cannot be readily eliminated if the sample has to be recovered.In addition, unless one purchases it in the relatively expensive deuterated form,it adds three more signals to the spectrum(methylenes1,2,and3appear at2.91,1.76, and0.63ppm,respectively).We suggest that the re-sidual HDO peak be used as a secondary reference;we find that if the effects of temperature are taken into account(vide infra),this is very reproducible.For D2O, we used a different set of stock solutions,since many of the less polar substrates are not significantly water-soluble(see Table1).We also ran sodium acetate and sodium formate(chemical shifts: 1.90and8.44ppm, respectively).Carbon Spectra(Table2).To each tube,50µL of the stock solution and3µL of TMS1were added.The solvent chemical shifts3were obtained from the spectra containing the solutes,and the ranges of chemical shifts(1)For recommendations on the publication of NMR data,see: IUPAC Commission on Molecular Structure and Spectroscopy.Pure Appl.Chem.1972,29,627;1976,45,217.(2)I.e.,the signal of the proton for the isotopomer with one less deuterium than the perdeuterated material,e.g.,C H Cl3in CDCl3or C6D5H in C6D6.Except for CHCl3,the splitting due to J HD is typically observed(to a good approximation,it is1/6.5of the value of the corresponding J HH).For CHD2groups(deuterated acetone,DMSO, acetonitrile),this signal is a1:2:3:2:1quintet with a splitting of ca.2 Hz.(3)In contrast to what was said in note2,in the13C spectra the solvent signal is due to the perdeuterated isotopomer,and the one-bond couplings to deuterium are always observable(ca.20-30Hz). Figure1.Chemical shift of H DO as a function of tempera-ture..Chem.1997,62,7512-7515S0022-3263(97)01176-6CCC:$14.00©1997American Chemical Societyshow their degree of variability.Occasionally,in order to distinguish between peaks whose assignment was ambiguous,a further1-2µL of a specific substrate were added and the spectra run again.Table1.1H NMR Dataproton mult CDCl3(CD3)2CO(CD3)2SO C6D6CD3CN CD3OD D2O solvent residual peak7.26 2.05 2.507.16 1.94 3.31 4.79 H2O s 1.56 2.84a 3.33a0.40 2.13 4.87acetic acid CH3s 2.10 1.96 1.91 1.55 1.96 1.99 2.08 acetone CH3s 2.17 2.09 2.09 1.55 2.08 2.15 2.22 acetonitrile CH3s 2.10 2.05 2.07 1.55 1.96 2.03 2.06 benzene CH s7.367.367.377.157.377.33tert-butyl alcohol CH3s 1.28 1.18 1.11 1.05 1.16 1.40 1.24 OH c s 4.19 1.55 2.18tert-butyl methyl ether CCH3s 1.19 1.13 1.11 1.07 1.14 1.15 1.21 OCH3s 3.22 3.13 3.08 3.04 3.13 3.20 3.22 BHT b ArH s 6.98 6.96 6.877.05 6.97 6.92OH c s 5.01 6.65 4.79 5.20ArCH3s 2.27 2.22 2.18 2.24 2.22 2.21ArC(CH3)3s 1.43 1.41 1.36 1.38 1.39 1.40chloroform CH s7.268.028.32 6.157.587.90 cyclohexane CH2s 1.43 1.43 1.40 1.40 1.44 1.451,2-dichloroethane CH2s 3.73 3.87 3.90 2.90 3.81 3.78 dichloromethane CH2s 5.30 5.63 5.76 4.27 5.44 5.49diethyl ether CH3t,7 1.21 1.11 1.09 1.11 1.12 1.18 1.17 CH2q,7 3.48 3.41 3.38 3.26 3.42 3.49 3.56 diglyme CH2m 3.65 3.56 3.51 3.46 3.53 3.61 3.67 CH2m 3.57 3.47 3.38 3.34 3.45 3.58 3.61OCH3s 3.39 3.28 3.24 3.11 3.29 3.35 3.37 1,2-dimethoxyethane CH3s 3.40 3.28 3.24 3.12 3.28 3.35 3.37 CH2s 3.55 3.46 3.43 3.33 3.45 3.52 3.60 dimethylacetamide CH3CO s 2.09 1.97 1.96 1.60 1.97 2.07 2.08 NCH3s 3.02 3.00 2.94 2.57 2.96 3.31 3.06NCH3s 2.94 2.83 2.78 2.05 2.83 2.92 2.90 dimethylformamide CH s8.027.967.957.637.927.977.92 CH3s 2.96 2.94 2.89 2.36 2.89 2.99 3.01CH3s 2.88 2.78 2.73 1.86 2.77 2.86 2.85 dimethyl sulfoxide CH3s 2.62 2.52 2.54 1.68 2.50 2.65 2.71 dioxane CH2s 3.71 3.59 3.57 3.35 3.60 3.66 3.75 ethanol CH3t,7 1.25 1.12 1.060.96 1.12 1.19 1.17 CH2q,7d 3.72 3.57 3.44 3.34 3.54 3.60 3.65OH s c,d 1.32 3.39 4.63 2.47ethyl acetate CH3CO s 2.05 1.97 1.99 1.65 1.97 2.01 2.07C H2CH3q,7 4.12 4.05 4.03 3.89 4.06 4.09 4.14CH2C H3t,7 1.26 1.20 1.170.92 1.20 1.24 1.24 ethyl methyl ketone CH3CO s 2.14 2.07 2.07 1.58 2.06 2.12 2.19C H2CH3q,7 2.46 2.45 2.43 1.81 2.43 2.50 3.18CH2C H3t,7 1.060.960.910.850.96 1.01 1.26 ethylene glycol CH s e 3.76 3.28 3.34 3.41 3.51 3.59 3.65“grease”f CH3m0.860.870.920.860.88CH2br s 1.26 1.29 1.36 1.27 1.29n-hexane CH3t0.880.880.860.890.890.90CH2m 1.26 1.28 1.25 1.24 1.28 1.29HMPA g CH3d,9.5 2.65 2.59 2.53 2.40 2.57 2.64 2.61 methanol CH3s h 3.49 3.31 3.16 3.07 3.28 3.34 3.34 OH s c,h 1.09 3.12 4.01 2.16nitromethane CH3s 4.33 4.43 4.42 2.94 4.31 4.34 4.40 n-pentane CH3t,70.880.880.860.870.890.90CH2m 1.27 1.27 1.27 1.23 1.29 1.292-propanol CH3d,6 1.22 1.10 1.040.95 1.09 1.50 1.17 CH sep,6 4.04 3.90 3.78 3.67 3.87 3.92 4.02 pyridine CH(2)m8.628.588.588.538.578.538.52 CH(3)m7.297.357.39 6.667.337.447.45CH(4)m7.687.767.79 6.987.737.857.87 silicone grease i CH3s0.070.130.290.080.10 tetrahydrofuran CH2m 1.85 1.79 1.76 1.40 1.80 1.87 1.88 CH2O m 3.76 3.63 3.60 3.57 3.64 3.71 3.74 toluene CH3s 2.36 2.32 2.30 2.11 2.33 2.32CH(o/p)m7.177.1-7.27.187.027.1-7.37.16CH(m)m7.257.1-7.27.257.137.1-7.37.16 triethylamine CH3t,7 1.030.960.930.960.96 1.050.99 CH2q,7 2.53 2.45 2.43 2.40 2.45 2.58 2.57a In these solvents the intermolecular rate of exchange is slow enough that a peak due to HDO is usually also observed;it appears at2.81and3.30ppm in acetone and DMSO,respectively.In the former solvent,it is often seen as a1:1:1triplet,with2J H,D)1Hz. b2,6-Dimethyl-4-tert-butylphenol.c The signals from exchangeable protons were not always identified.d In some cases(see note a),the coupling interaction between the CH2and the OH protons may be observed(J)5Hz).e In CD3CN,the OH proton was seen as a multiplet atδ2.69,and extra coupling was also apparent on the methylene peak.f Long-chain,linear aliphatic hydrocarbons.Their solubility in DMSO was too low to give visible peaks.g Hexamethylphosphoramide.h In some cases(see notes a,d),the coupling interaction between the CH3and the OH protons may be observed(J)5.5Hz).i Poly(dimethylsiloxane).Its solubility in DMSO was too low to give visible peaks.Notes .Chem.,Vol.62,No.21,19977513.Chem.,Vol.62,No.21,1997NotesTable2.13C NMR Data aCDCl3(CD3)2CO(CD3)2SO C6D6CD3CN CD3OD D2O solvent signals77.16(0.0629.84(0.0139.52(0.06128.06(0.02 1.32(0.0249.00(0.01206.26(0.13118.26(0.02acetic acid CO175.99172.31171.93175.82173.21175.11177.21 CH320.8120.5120.9520.3720.7320.5621.03 acetone CO207.07205.87206.31204.43207.43209.67215.94 CH330.9230.6030.5630.1430.9130.6730.89 acetonitrile CN116.43117.60117.91116.02118.26118.06119.68 CH3 1.89 1.12 1.030.20 1.790.85 1.47 benzene CH128.37129.15128.30128.62129.32129.34tert-butyl alcohol C69.1568.1366.8868.1968.7469.4070.36 CH331.2530.7230.3830.4730.6830.9130.29 tert-butyl methyl ether OCH349.4549.3548.7049.1949.5249.6649.37 C72.8772.8172.0472.4073.1774.3275.62C C H326.9927.2426.7927.0927.2827.2226.60 BHT C(1)151.55152.51151.47152.05152.42152.85C(2)135.87138.19139.12136.08138.13139.09CH(3)125.55129.05127.97128.52129.61129.49C(4)128.27126.03124.85125.83126.38126.11CH3Ar21.2021.3120.9721.4021.2321.38C H3C30.3331.6131.2531.3431.5031.15C34.2535.0034.3334.3535.0535.36chloroform CH77.3679.1979.1677.7979.1779.44cyclohexane CH226.9427.5126.3327.2327.6327.961,2-dichloroethane CH243.5045.2545.0243.5945.5445.11 dichloromethane CH253.5254.9554.8453.4655.3254.78diethyl ether CH315.2015.7815.1215.4615.6315.4614.77 CH265.9166.1262.0565.9466.3266.8866.42 diglyme CH359.0158.7757.9858.6658.9059.0658.67 CH270.5171.0369.5470.8770.9971.3370.05CH271.9072.6371.2572.3572.6372.9271.63 1,2-dimethoxyethane CH359.0858.4558.0158.6858.8959.0658.67 CH271.8472.4717.0772.2172.4772.7271.49 dimethylacetamide CH321.5321.5121.2921.1621.7621.3221.09 CO171.07170.61169.54169.95171.31173.32174.57NCH335.2834.8937.3834.6735.1735.5035.03NCH338.1337.9234.4237.0338.2638.4338.76 dimethylformamide CH162.62162.79162.29162.13163.31164.73165.53 CH336.5036.1535.7335.2536.5736.8937.54CH331.4531.0330.7330.7231.3231.6132.03 dimethyl sulfoxide CH340.7641.2340.4540.0341.3140.4539.39 dioxane CH267.1467.6066.3667.1667.7268.1167.19 ethanol CH318.4118.8918.5118.7218.8018.4017.47 CH258.2857.7256.0757.8657.9658.2658.05 ethyl acetate C H3CO21.0420.8320.6820.5621.1620.8821.15 CO171.36170.96170.31170.44171.68172.89175.26CH260.4960.5659.7460.2160.9861.5062.32CH314.1914.5014.4014.1914.5414.4913.92 ethyl methyl ketone C H3CO29.4929.3029.2628.5629.6029.3929.49 CO209.56208.30208.72206.55209.88212.16218.43C H2CH336.8936.7535.8336.3637.0937.3437.27CH2C H37.868.037.617.918.148.097.87 ethylene glycol CH263.7964.2662.7664.3464.2264.3063.17“grease”CH229.7630.7329.2030.2130.8631.29n-hexane CH314.1414.3413.8814.3214.4314.45CH2(2)22.7023.2822.0523.0423.4023.68CH2(3)31.6432.3030.9531.9632.3632.73HMPA b CH336.8737.0436.4236.8837.1037.0036.46 methanol CH350.4149.7748.5949.9749.9049.8649.50c nitromethane CH362.5063.2163.2861.1663.6663.0863.22 n-pentane CH314.0814.2913.2814.2514.3714.39CH2(2)22.3822.9821.7022.7223.0823.38CH2(3)34.1634.8333.4834.4534.8935.302-propanol CH325.1425.6725.4325.1825.5525.2724.38 CH64.5063.8564.9264.2364.3064.7164.88 pyridine CH(2)149.90150.67149.58150.27150.76150.07149.18 CH(3)123.75124.57123.84123.58127.76125.53125.12CH(4)135.96136.56136.05135.28136.89138.35138.27 silicone grease CH3 1.04 1.40 1.38 2.10 tetrahydrofuran CH225.6226.1525.1425.7226.2726.4825.67 CH2O67.9768.0767.0367.8068.3368.8368.68 toluene CH321.4621.4620.9921.1021.5021.50C(i)137.89138.48137.35137.91138.90138.85CH(o)129.07129.76128.88129.33129.94129.91CH(m)128.26129.03128.18128.56129.23129.20CH(p)125.33126.12125.29125.68126.28126.29triethylamine CH311.6112.4911.7412.3512.3811.099.07 CH246.2547.0745.7446.7747.1046.9647.19a See footnotes for Table1.b2J PC)3Hz.c Reference material;see text.For D2O solutions there is no accepted reference for carbon chemical shifts.We suggest the addition of a drop of methanol,and the position of its signal to be defined as49.50ppm;on this basis,the entries in Table2were recorded.The chemical shifts thus obtained are,on the whole,very similar to those for the other solvents. Alternatively,we suggest the use of dioxane when the methanol peak is expected to fall in a crowded area of the spectrum.We also report the chemical shifts of sodium formate(171.67ppm),sodium acetate(182.02and 23.97ppm),sodium carbonate(168.88ppm),sodium bicarbonate(161.08ppm),and sodium3-(trimethylsilyl)-propanesulfonate[54.90,19.66,15.56(methylenes1,2, and3,respectively),and-2.04ppm(methyls)],in D2O. Temperature Dependence of HDO Chemical Shifts.We recorded the1H spectrum of a sample of D2O, containing a crystal of sodium3-(trimethylsilyl)propane-sulfonate as reference,as a function of temperature.The data are shown in Figure1.The solid line connecting the experimental points corresponds to the equation which reproduces the measured values to better than1 ppb.For the0-50o C range,the simplergives values correct to10ppb.For both equations,T is the temperature in°C.Acknowledgment.Generous support for this work by the Minerva Foundation and the Otto Mayerhoff Center for the Study of Drug-Receptor Interactions at Bar-Ilan University is gratefully acknowledged.JO971176Vδ)5.060-0.0122T+(2.11×10-5)T2(1)δ)5.051-0.0111T(2)Notes .Chem.,Vol.62,No.21,19977515。

碳谱、氢谱的解析分析氢谱有如下的步骤。
(1) 区分出杂质峰、溶剂峰、旋转边带。
杂质含量较低,其峰面积较样品峰小很多,样品和杂质峰面积之间也无简单的整数比关系。
据此可将杂质峰区别出来。
氘代试剂不可能100%氘代,其微量氢会有相应的峰,如CDCl3中的微量CHCl3在约7.27ppm 处出峰。
边带峰的区别请阅6.2.1。
(2) 计算不饱和度。
不饱和度即环加双键数。
当不饱和度大于等于4时,应考虑到该化合物可能存在一个苯环(或吡啶环)。
(3) 确定谱图中各峰组所对应的氢原子数目,对氢原子进行分配。
根据积分曲线,找出各峰组之间氢原子数的简单整数比,再根据分子式中氢的数目,对各峰组的氢原子数进行分配。
(4) 对每个峰的δ、J 都进行分析。
根据每个峰组氢原子数目及δ值,可对该基团进行推断,并估计其相邻基团。
对每个峰组的峰形应仔细地分析。
分析时最关键之处为寻找峰组中的等间距。
每一种间距相应于一个耦合关系。
一般情况下,某一峰组内的间距会在另一峰组中反映出来。
通过此途径可找出邻碳氢原子的数目。
当从裂分间距计算J 值时,应注意谱图是多少兆周的仪器作出的,有了仪器的工作频率才能从化学位移之差Δδ(ppm)算出Δν(Hz)。
当谱图显示烷基链3J 耦合裂分时,其间距(相应6-7Hz)也可以作为计算其它裂分间距所对应的赫兹数的基准。
(5) 根据对各峰组化学位移和耦合常数的分析,推出若干结构单元,最后组合为几种可能的结构式。
每一可能的结构式不能和谱图有大的矛盾。
(6) 对推出的结构进行指认。
每个官能团均应在谱图上找到相应的峰组,峰组的δ值及耦合裂分(峰形和J 值大小)都应该和结构式相符。
如存在较大矛盾,则说明所设结构式是不合理的,应予以去除。
通过指认校核所有可能的结构式,进而找出最合理的结构式。
必须强调:指认是推结构的一个必不可少的环节。
如果未知物的结构稍复杂,在推导其结构时就需应用碳谱。
在一般情况下,解析碳谱和解析氢谱应结合进行。
注:JHD为溶剂本身的其他1H对与之相对应的1H之间的耦合常数,JCD为溶剂本身1H对13C的耦合常数,H2O和交换了D的HOD上的1H产生的即水峰的化学位移氯仿:小、中小、中等极性DMSO:芳香系统(日光下自然显色、紫外荧光)。
对于酚羟基能够出峰。
芳香化合物还是芳香甙,都为首选。
吡啶:极性大的,特别是皂甙对低、中极性的样品,最常采用氘代氯仿作溶剂,因其价格远低于其它氘代试剂。
极性大的化合物可采用氘代丙酮、重水等。
针对一些特殊的样品,可采用相应的氘代试剂:如氘代苯(用于芳香化合物、芳香高聚物)、氘代二甲基亚砜(用于某些在一般溶剂中难溶的物质)、氘代吡啶(用于难溶的酸性或芳香化合物)等。
丙酮:中等极性甲醇:极性大氯仿—甲醇:石:乙 5;1小极性石:丙 2:1——1:1中等极性氯仿:甲醇6:1极性以上含有一个糖2:1 含有两个糖含有糖的三萜皂甙:一般用吡啶常见溶剂的化学位移常见溶剂的1H在不同氘代溶剂中的化学位移值常见溶剂的化学位移常见溶剂的13C在不同氘代溶剂中的化学位移值核磁知识(NMR)一:样品量的选择氢谱,氟谱,碳谱至少需要5mg. 1H-1H COSY, 1H-1H NOESY, 1H-13C HMBC, 1H-13C HSQC需要10-15mg. 碳谱需要30mg.二:如何选择氘代溶剂常用氘代溶剂: CDCl3, DMSO, D2O, CD3OD.特殊氘代溶剂: CD3COCD3, C6D6, CD3CN。
极性较大的化合物可以选择用D2O或CD3OD,如果想要观察活泼氢切记不能选择D2O和CD3OD。
CDCl3为人民币2-3元,D2O为人民币6元,DMSO为人民币10元,CD3OD为人民币30元。
Solvent 化学位移(ppm) 水峰位移(ppm)CDCl3 7.26 1.56DMSO 2.50 3.33CD3OD 3.31 4.87D2O 4.79CD3COCD3 2.05 2.84。
核磁氢谱溶剂峰化学位移表解释说明1. 引言1.1 概述核磁氢谱溶剂峰化学位移表是化学分析中非常重要的工具之一。
在核磁共振(NMR)技术中,溶剂峰是指由于溶剂中特定原子核的共振信号所引起的信号峰。
这些溶剂峰可以提供有关样品分子结构和化学环境的宝贵信息。
本篇文章将详细介绍核磁氢谱溶剂峰化学位移表的概念、意义以及构建方法,并解释如何使用该表进行核磁氢谱数据分析和解读。
1.2 文章结构本文将分为五个主要部分进行讨论。
首先,在引言部分,我们会对本文作出概述,并介绍文章内容和结构。
然后,我们将在第二部分介绍核磁氢谱溶剂峰的基本概念以及其在化学位移中的意义。
接着,我们将在第三部分详细探讨建立核磁氢谱溶剂峰化学位移表的方法。
在第四部分,我们将通过实际应用案例来说明如何分析和解读核磁氢谱溶剂峰化学位移表。
我们将介绍应用案例的背景,并阐述如何使用化学位移表来解读样品核磁氢谱数据。
最后,我们会讨论实际应用中可能遇到的挑战,并提出相应的解决方案。
最后,在结论与展望部分,我们将总结本文的研究成果,并对未来相关研究方向进行展望。
1.3 目的本文旨在全面介绍核磁氢谱溶剂峰化学位移表及其分析和解释方法,以帮助读者更好地理解和运用这一重要工具。
通过对该表的深入了解,读者可以准确地分析和解读核磁氢谱数据,并在实际应用中有效利用溶剂峰化学位移信息进行样品结构和环境的推测。
2. 核磁氢谱溶剂峰化学位移表:2.1 核磁氢谱概述:核磁共振(NMR)是一种重要的分析技术,广泛应用于化学和生物学领域。
核磁氢谱是其中一种常见的NMR实验,用于确定分子中氢原子的化学环境和相互作用。
在核磁氢谱图中,峰表示不同化学位移的氢原子信号。
2.2 溶剂峰化学位移的意义:在进行核磁氢谱测定时,需要选择一个特定的溶剂作为溶剂系统的参考标准。
这个溶剂在谱图中会产生一个固定位置的峰,称为溶剂峰。
通过与溶剂峰对比,可以精确地确定其他化合物中氢原子信号的化学位移。
溶剂峰化学位移表是记录各种常见有机溶剂在核磁共振实验中对应峰位置(通常以ppm表示)的表格。
核磁共振氢谱化学位移表
核磁共振氢谱化学位移表是一种用于确定有机分子结构的有效工具。
它是根据核磁共振(NMR)信号的频率和强度来确定有机分子中氢原子的化学位移的。
核磁共振氢谱化学位移表的基本原理是,每种类型的氢原子都有不同的化学位移,这些位移可
以通过NMR信号的频率和强度来确定。
根据这些位移,可以确定有机分子中氢原子的位置,从
而确定有机分子的结构。
核磁共振氢谱化学位移表的结构是由一系列的氢原子位移值组成的,这些位移值可以用来确定
有机分子中氢原子的位置。
核磁共振氢谱化学位移表中的氢原子位移值可以分为三类:α位移,β位移和γ位移。
α位移是指氢原子与其他原子之间的相对位移,β位移是指氢原子
与其他原子之间的绝对位移,而γ位移是指氢原子与其他原子之间的相对位移。
核磁共振氢谱化学位移表的应用非常广泛,它可以用来确定有机分子的结构,从而更好地了解
有机分子的性质。
此外,核磁共振氢谱化学位移表还可以用来确定有机分子中氢原子的位置,
从而更好地了解有机分子的结构。
总之,核磁共振氢谱化学位移表是一种有效的工具,可以用来确定有机分子的结构,从而更好
地了解有机分子的性质。
它可以帮助科学家们更好地理解有机分子的结构,从而更好地利用有
机分子的性质。