TBP 磷酸三丁酯的检测方法
- 格式:pdf
- 大小:22.88 KB
- 文档页数:5

第43卷第2期冯勇等:气相色谱法分析检测产品中磷酸二丁酯和磷酸三丁酯225D O I: 10.13822/j.c n k i.h x sj.2021007812化学试剂,2021,43( 2),225 〜228气相色谱法分析检测产品中磷酸二丁酯和磷酸三丁酯冯勇,张少文'彭传云,赵晓洁(洛阳理工学院环境工程与化学学院,河南洛阳471023)摘要:利用毛细管色谱法建立了磷酸二丁酯(DBP)和磷酸三丁酯(TBP)的分析检测方法。
实验测得,D B P和T B P在1〜100 »xg/L浓度范围内线性关系良好(/?2多0.999 5),检出限(5/^=3)为0.04〜0.06 fxg/L,定量限(S//V= 10)为0. 16〜0. 25 pg/L,日内与日间检测的相对标准偏差分别为2. 1%〜4.3%U= 3)、2. 5%~ 5. 8%U= 3)。
采用新建方法检测企业的DBP/T B P实际产品,加标回收率为90. 1%〜108. 2%,相对标准偏差为2. 1%〜6.5%(n= 3)。
方法操作简便、快速灵敏、准确可靠,适用于D B P和T B P实际检测应用和产品质量控制。
关键词:气相色谱法;衍生化;磷酸二丁酯;磷酸三丁酯;RSD中图分类号:06文献标识码:A文章编号:0258-3283 ( 2021)02-0225-04Determination of Dibutyl Phosphate and Tributyl Phosphate in Products by Gas Chromatography FENG Yong, ZHANG Shao-wen* ,PENG Chuan-yun yZH A0 Xiao-jie(College of Environmental Engineering and Chemistry, Luoyang Institute of Science and Technology’Luoyang 471023, C h in a),Huaxue Shiji ,2021 ,43(2),225 〜228A bstract:A new determination was developed for dibutyl phosphate ( DBP) and tributyl phosphate (TB P) with gas chromatography.The linear relationship for DBP and TBP had been observed in the range of 1 ~ 100 \xg/L(R2 ^0. 999 5) with limits of de-tection ( LOD) and limits of quantification ( LOQ) of 0• 04〜0. 06 jjig/L( S//V = 3) and 0• 16〜0. 25 |ig/L( *S/yV = 10) ’respective-ly.The relative standard deviations for intra-day were 2. 4. 3% (汀=3)and that for inter-day were 2. 5% ~ 5. 8% (n = 3). Ap-plied to the real products detections, the spiked recoveries were between 90. I %and 108. 2% with the relative standard deviations (RSDs) from 2. 1%to 6. 5% ( n = 3 ) .The results showed that the method was sim ple, sensitive and specific, and applicable for quality control of DBP and TBP.Key w ord s:gas chromatography ;derivatization; DBP;TBP ; RSD磷酸三丁酯(TB P)作为金属离子萃取剂,具 有萃取能力强、选择性好、成本低等优点[|],在湿 法冶金和核燃料后处理等领域应用广泛[2]。

磷酸三丁酯-煤油-硝酸体系辐解产物的红外光谱定量测定磷酸三丁酯(TBP)与煤油混合后加入硝酸形成的辐解产物中存在着多种有机物质,这些有机物质在红外光谱中都会有不同的峰位,因此可以通过红外光谱定量测定这些物质的含量。
红外光谱是一种可以分析物质种类、分子结构和结构中的变动的分析方法。
当物质分子中的分子键振动或弯曲时,会吸收一定频率范围内的红外辐射,这个吸收峰位是与分子结构中原子间键的属性有关的。
因此,红外光谱可以通过辨别物质中特定的峰位而定量测定有机物质的含量。
在TBP-煤油-硝酸体系辐解产物中,主要包括以下几种有机物质:硝基甲酸三乙酯(TNOE)、硝基甲苯(TNT)、亚硝基甲苯(ITX)、甲基丙烯酸甲酯(MMA)和正丁醇(n-BuOH)等。
这些有机物质在红外光谱中都有各自的峰位。
硝基甲酸三乙酯的红外光谱峰位为1735 cm-1,1665 cm-1和1225 cm-1,这些峰位分别对应着CO、C=O和C-O峰位。
硝基甲苯的峰位为1515 cm-1和1345 cm-1,分别对应着NO2和C-H的振动。
亚硝基甲苯的峰位为1330 cm-1,对应着N=O的振动。
甲基丙烯酸甲酯的峰位为1725 cm-1和1420 cm-1,分别对应着C=O和C-H的振动。
正丁醇的峰位为2970 cm-1和2870 cm-1,对应着C-H的振动。
由于这些有机物质在红外光谱中有各自不同的峰位,因此可以通过计算特定峰位的吸光度来推算其浓度。
比如,对于硝基甲酸三乙酯来说,可以选择CO或C=O的峰位计算其吸光度,然后根据标准曲线来推算其浓度。
在进行红外光谱定量测定之前,需要首先制备标准曲线。
制备标准曲线的方法通常是选取不同浓度的标准品向样品中加入,制备一系列的混合物,然后分别测定其吸光度和浓度,建立吸光度和浓度之间的线性关系,得到标准曲线。
然后,测定要分析的样品吸光度,并根据标准曲线推算样品中各有机物质的浓度。
总之,红外光谱定量分析是一种简便、准确、灵敏的化学分析方法,可以用于研究不同化学物质的含量和结构,以及它们之间的化学变化。

三正丁基膦质量标准
三正丁基膦(TBP)是一种常用的磷化学试剂,主要用于磷化物、磷氧化物和磷酸盐的分离和提取。
其质量标准主要包括纯度、金属杂质含量、水分含量等方面。
1. 纯度:三正丁基膦的纯度通常要求达到95%以上。
纯度的测定通常通过气相色谱或高效液相色谱进行。
2. 金属杂质含量:三正丁基膦中的金属杂质主要是指铅、镉、汞等重金属。
其含量通常要求低于一定数值,例如,铅、镉、汞的含量分别不超过10mg/kg、5mg/kg、5mg/kg。
3. 水分含量:三正丁基膦的水分含量也有一定的要求。
通常要求水分含量低于0.5%。
水分含量的测定通常通过烘干法或卡尔费休法进行。
以上只是一般的质量标准,具体的质量标准可能会根据产品的用途和制造商的要求进行调整。

实验一微量铀的测定——TBP萃取分离-偶氮胂III法一、实验目的1、了解酸溶解法铀矿分析的基本原理。
2、初步掌握铀矿分析的有关实验技术。
二、实验原理地壳中铀的平均含量约为(3~4)×10-4%。
由于铀的分布非常稀散,因此,地壳中铀矿床中铀的含量一般在百分之几到万分之几,大多数铀矿床中铀的平均含量低于1%。
自然界存在的铀矿约有200种,其组成也非常复杂。
根据铀矿的成因和产地,可把它分成原生铀矿和次生铀矿两类,前者以UO2·U n O2·mPbO形式存在,后者则以UO2·nA2O为主。
若以铀矿的化学组成来分类,大致可归纳十余种,其中包括氧化物矿、碳酸盐矿、硅酸盐、铌钽酸盐和钛铌钽酸盐矿、磷酸盐矿。
砷酸盐矿、钒酸盐矿、硫酸盐矿、钼酸盐矿以及含铀碳物质等。
铀矿石中铀的测定一般分为三个步骤:试样分解、铀与伴生杂质分离以及铀的测定。
1、试样的分解铀矿石中含铀量的准确测定,首先需要从矿石中“定量”提取铀。
把铀矿石完全溶解是一种途径,将矿石经过适当处理,把其中的铀全部“浸取”出来也是一种可取的方法。
一般的铀矿石,经研磨、过筛(180目),大部分可被盐酸—过氧化氢或氯酸钾、磷酸—过氧化氢、王水等试剂所溶解。
对于含硅量较高的矿石,可用盐酸—氢氟酸、硝酸—氢氟酸或硫酸—氢氟酸处理后,矿石中的铀都能定量溶出。
对于一些很难分解的铀矿,则必须采用熔融方法来分解。
如对含铌酸盐和钽酸盐的铀矿,既可以用氢氧化钠或者氧化钠这一类碱性熔剂来分解,也可以用焦硫酸钾或氟化氢钾等酸性溶剂来处理。
下表列出一些常见铀矿石样品及其分解方法,可供参考。
表2-1 常见铀矿样及其分解方法本实验选用盐酸-过氧化氢分解矿石,然后经硝酸处理使铀转化成硝酸铀酰。
由于矿石中的铀通常以U3O8或UO2存在,较难被盐酸直接溶解。
为此,在用盐酸或硫酸溶解U3O8或UO2时,加入H2O2可加速溶解过程,H2O2的作用是将U(Ⅳ)氧化成U (Ⅵ),反应如下:UO2+2HCl+ H2O2=UO2Cl2+2H2O (E2.1)U3O8+6HCl+ H2O2=3 UO2Cl2+4H2O (E2.2)经硝酸处理后,氯化铀酰转变成硝酸铀酰:UO2Cl2+2HNO3=3UO2(NO3)2+2HCl↑ (E2.3)2、分离提纯由于矿石中含有大量的铀的伴生元素,诸如Si、S、P、F、Fe、Al、Ca、Mg、Cu、Th、RE等,在溶矿时,某些伴生杂质全部或部分地随铀一起溶解于分解液中,其中部分杂质会妨碍或干扰铀的分析,因此,在铀的测定前必须把这些干扰成分除去。
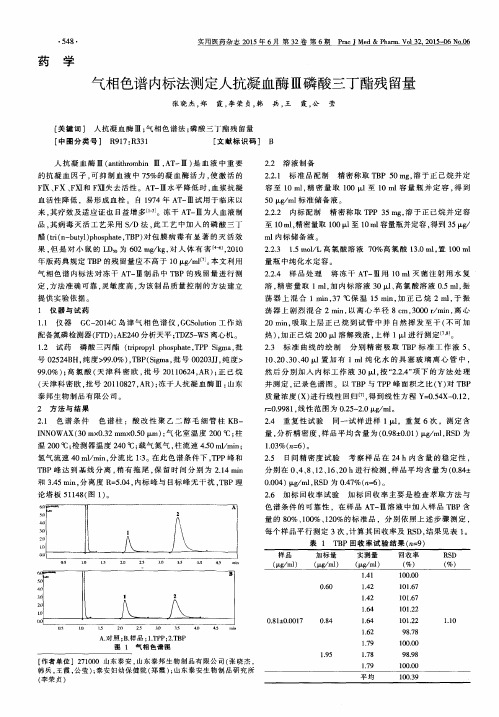

废有机溶剂磷酸三丁酯(TBP)的处理研究的开题报告一、选题背景有机溶剂是现代化学工业生产中不可或缺的一部分,其在化学反应、萃取分离、溶解催化等方面有广泛的应用,但随着大量的有机溶剂使用,有机溶剂废水的治理问题也日益突显。
其中,有机溶剂磷酸三丁酯(TBP)是一种典型的有机溶剂,在核工业、化学冶金等领域广泛使用。
然而,TBP难以被生物降解,若放任不管极易造成环境污染和健康危害。
目前,处理TBP废水的方法主要有物理法、化学法和生物法。
物理法和化学法的处理效率较高,但成本较高,同时容易产生二次污染。
生物法成本较低,但对TBP的降解效率较低,且需要较长时间,限制了其应用范围。
因此,研究 TBP废有机溶剂的处理技术及其影响因素是迫切需要的。
二、研究目的本研究旨在研究有机溶剂磷酸三丁酯(TBP)的处理技术及其影响因素,并探讨其处理效果和经济性,为TBP废水的处理提供科学依据和参考。
三、研究内容和方法本研究将重点研究以下内容:1. TBP废水的处理技术研究,包括物理法、化学法和生物法。
2. TBP废水处理的影响因素研究,包括初始pH值、处理温度、处理时间、添加剂种类等。
3. TBP废水的处理效果评估,包括COD、BOD、TOC等指标的检测和控制。
4. TBP废水处理的经济性评估,包括处理成本和处理效果之间的比较。
研究方法主要包括文献调研、实验设计和数据分析等。
四、研究意义本研究的意义在于:1. 对有机溶剂磷酸三丁酯(TBP)的处理技术和影响因素进行深入研究,为TBP 废水的治理提供科学、可行和经济的处理方案。
2. 提高有机溶剂废水管理的科学性和效率性,减少污染物对自然环境的影响,保护人类健康和生态环境安全。
3. 为推动我国有机溶剂废水治理技术的创新和提高,提供有益参考和借鉴。
TRIBUTYL PHOSPHATE5034(C4H9O)3P=O MW: 266.32 CAS: 126-73-8 RTECS: TC7700000METHOD: 5034, Issue 1 EVALUATION: PARTIAL Issue 1: 15 August 1994OSHA :0.5 ppmNIOSH:0.2 ppmACGIH:0.2 ppm(1 ppm = 10.9 mg/m3 @ NTP)PROPERTIES: liquid; boiling point 293 C; density0.98 g/mL @ 20 C; VP very low @ 20 C;vapor density 9.2 (air=1); flash point 166 C(closed cup)SYNONYMS:phosphoric acid, tributyl ester; tri-n-butyl phosphate; TBP; Celluphos 4SAMPLINGMEASUREMENTAPPLICABILITY: The working range is 0.006 to 1.4 ppm (0.06 to 15 mg/m3) for a 100-L air sample. This method may be adapted to other phosphates of relatively low volatility with appropriate changes in chromatographic conditions. INTERFERENCES: Any phosphorus-containing compound that has the same retention time as the analyte is an interference.A non-polar capillary column may be used for better resolution.OTHER METHODS: This revises Method S208 [2]. Analytical methods for tributyl phosphate (TBP) have been reviewed [3]. Another packed-column GC procedure has been described recently [4]. TBP has been determined in air by capillary-column GC/NPD preceded by sampling on a glass fiber filter or XAD-7 resin [5]. GC/MS [6], LC/MS [7], and LC/TID (thermionic det ection) [8] have all been shown to be useful methods for the analysis of TBP in environmental samples. Finally, a continuous pho sphorus gas analyzer has been used to monitor TBP in air [9].REAGENTS:1.Diethyl ether*, anhydrous, reagent grade.2.Tributyl phosphate*, reagent grade.3.Hydrogen, purifiedpressed air, prefiltered5.Nitrogen, purified6.Calibration stock solution, tributyl phosphate indiethyl ether*See Special Precautions EQUIPMENT:1.Sampler: 37-mm mixed cellulose estermembrane filter (0.8-µm pore size) supportedby cellulose backup pad in three-piece filterholder.NOTE:Backup filter unit is needed whensampling at temperatures above23 °C.2.Personal sampling pump, 1 to 3 L/min, withflexible polyethylene or PTFE tubing.3.Gas chromatograph equipped with a flamephotometric detector, phosphorus filter, andcolumn (p. 5034-1).4.Electronic integrator or some other suitablemethod for measuring peak areas.5.Tweezers.6.Jars: 2 oz ointment jars for sample extraction,squat form with aluminum-lined screw caps.7.Syringes, 10-µL and other convenient sizes.8.Volumetric flasks, 10-mL and other convenientsizes.9.Pipets, 10-mL and other convenient sizes.SPECIAL PRECAUTIONS: Store diethyl ether away from heat, light, and sources of ignition in a well-ventilated area. Do not leave container open. Diethyl ether can oxidize in air to form explosive peroxides, a reaction accelerated by light. Distillation and evaporation can concentrate unstable peroxides in the residue, a potentially explosive condition [10].Avoid inhalation of tributyl phosphate vapors and contact with eyes, skin, and clothing [11,12]. Handle only in a hood.SAMPLING:1.Calibrate each personal sampling pump with the representative filter cassettes in line.2.Remove cassette plugs and connect cassette filter to the personal sampling pump with flexibletubing.NOTE:If ambient temperature is above 23 °C, use two filter cassettes connected in series witha short piece of flexible tubing for sample collection. Some tributyl phosphate may existas vapor above 23 °C.3.Sample at an accurately known flow rate of 1 to 3 L/min for a total sample size of 2 to 100 L.4.Separate front and backup filter cassettes (if two cassettes were used). Firmly seal collectedsample cassettes with plugs, and pack securely for shipment.SAMPLE PREPARATION:5.Transfer the filter and backup pad to an ointment jar using tweezers.6.Pipet 10.0 mL of diethyl ether into each jar. Seal the jar immediately to minimize evaporation.7.Allow samples to stand for at least 30 min with occasional agitation.CALIBRATION AND QUALITY CONTROL:8.Calibrate daily with at least six working standards over the range of 2 to 1000 µg per sample.a.Add known amounts of calibration stock solution to 10-mL volumetric flasks and dilute tovolume with diethyl ether.b.Analyze working standards together with samples and blanks (steps 11 and 12). This willminimize the effect of variations in FPD response with time.NOTE 1:The FPD response is very sensitive to minor variations in hydrogen flow rateand, therefore, it is recommended that calibration standards be carefullyinterspersed with the samples.NOTE 2:Use of an internal standard is recommended to minimize errors caused bysample solvent evaporation and FPD response variations.c.Prepare a calibration graph of area vs. µg of tributyl phosphate per 10 mL of sample.9.Determine recovery in the concentration range of interest for each lot of filters used forsampling. Prepare three filters at each of five levels plus three media blanks.a.Spike aliquot of calibration solution onto each filter.b.After air-drying, extract filters with 10 mL diethyl ether (steps 5 through 7).c.Analyze together with working standards (steps 11 and 12).d.Prepare graph of recovery vs. µg tributyl phosphate.10.Analyze three quality control blind spikes and three analyst spikes to ensure that the calibrationgraph and recovery graph are in control.MEASUREMENT:11.Set gas chromatograph according to manufacturer's recommendations and to conditions givenon page 5034-1. Inject 5-µL sample aliquot using solvent flush technique or with autosampler.NOTE:If peak area is above linear range of the calibration graph, dilute with diethyl ether, analyze, and apply appropriate dilution factor in calculations.12.Measure peak area.CALCULATIONS:13.Determine mass, µg (corrected for recovery), of tributyl phosphate found in the sample (W) andthe average media blank (B).14.Calculate concentration of tributyl phosphate in the actual air volume sampled, V (L):EVALUATION OF METHOD:REFERENCES:[1]Backup Data Report for Tributyl Phosphate, in Documentation of the NIOSH Validation Tests,prepared under NIOSH Contract CDC-99-74-45 (1977).[2]NIOSH Manual of Analytical Methods, 2nd. ed., V. 3, S208, U.S. Department of Health,Education, and Welfare, Publ. (NIOSH) 77-157-C (1977).[3]Davis, W., Navratil, J., Lasztity, A., and Horvath, Z., Analytical Methods [for Tributyl Phosphate],in Science and Technology of Tributyl Phosphate, Vol. 1, pp 267-327, Schulz, W. and Navratil, J., Eds., CRC, Boca Raton, FL, 1984.[4]Kuno, Y., Hina, T., Akiyama, T., and Matsui, M., Simultaneous Determination of TributylPhosphate and Dibutyl Phosphate in Spent Fuel Reprocessing Streams by GasChromatography, J. Chromatogr., 537 (1-2): 489 (1991).[5]Haraguchi, K., Yamashita, T., and Shigemori, N., Sampling and Analysis of Phosphoric AcidTriesters in Ambient Air, Taiki Osen Gakkaishi, 20 (6): 407 (1985).[6]Ishikawa, S., Taketomi, M., and Shinohara, R., Determination of Trialkyl and Triaryl Phosphatesin Environmental Samples, Water Res., 19 (1): 119 (1985).[7]Barceló, D., Application of Thermospray Liquid Chromatography/Mass Spectrometry forDetermination of Organophosphorus Pesticides and Trialkyl and Triaryl Phosphates, Biomed.Environ. Mass Spectrom., 17 (5): 363 (1988).[8]Barceló, D., Maris, F., Frei, R., de Jong, G., and Brinkman, U., Determination of Trialkyl andTriaryl Phosphates by Narrow-Bore Liquid Chromatography with on-line Thermionic Detection,Intern. J. Environ. Anal. Chem., 30 (1-2): 95 (1987).[9]Parker, G., Continuous Quantitative Analysis of Low Concentrations of Tributyl Phosphate (TBP)Vapors in Flowing Air Streams, Am. Ind. Hyg. Assoc. J., 41 (3): 220 (1980).[10]Material Safety Data Sheet for Diethyl Ether, No. 343, General Electric Company, Schenectady,N.Y., August, 1979.[11]Material Safety Data Sheet for Tributyl Phosphate, No. 521, General Electric Company,Schenectady, N.Y., October, 1983.[12]Material Safety Data Sheet for Tributyl Phosphate, No. P1180.2, Van Waters & Rogers, Inc.(U.S.), Seattle, Washington, August 27, 1989.METHOD REVISED BY:Robert P. Streicher, Ph.D., NIOSH/DPSE.。
磷酸三丁酯棉纤维在线分离富集流动注射-火焰原子吸收光谱法测定铬(Ⅲ)和铬(Ⅵ)的量陈中兰;欧阳志刚【摘要】用TBP-棉纤维吸附实现铬(Ⅵ)与铬(Ⅲ)的在线分离富集,并用流动注射(FI)-火焰原子吸收光谱法(FAAS)分别测定其含量.将TBP棉纤维小球填入自制的锥形柱并组装在FI系统中作为分离单元.将预先调至pH 0.75的样品溶液,以4 mL·min-1流量注入FI系统中,并在锥形柱中富集分离160 s.此时铬(Ⅵ)被TBP-棉纤维吸附,而铬(Ⅲ)随流出液流出.收集流出液测定铬(Ⅲ)量.用水以2.6mL·min-1流量过锥形柱洗脱铬(Ⅵ),洗脱液引入FAAS,测定铬(Ⅵ)含量.铬质量浓度在0.100~0.900mg·L-1以内呈线性.对与0.02 μg铬(Ⅲ)共存的0.10 μg铬(Ⅵ)溶液作7次测定,计算得到铬(Ⅵ)测定值的相对标准偏差为6.4%.添加0.500 mg·L-1铬(Ⅵ)及0.100 mg·L-1铬(Ⅲ)溶液,计算得到铬(Ⅵ)及铬(Ⅲ)的平均回收率依次为119%和107%.【期刊名称】《理化检验-化学分册》【年(卷),期】2014(050)004【总页数】4页(P474-477)【关键词】流动注射-火焰原子吸收光谱法;在线分离与富集;TBP-棉纤维球;铬(Ⅵ);铬(Ⅲ)【作者】陈中兰;欧阳志刚【作者单位】西华师范大学化学化工学院化学应用与污染控制技术四川省重点实验室,南充637002;西华师范大学化学化工学院化学应用与污染控制技术四川省重点实验室,南充637002【正文语种】中文【中图分类】O657.31铬及其化合物是电镀、金属加工、冶金和制革等行业的重要原料。
由于铬组合镀层具有硬度高、耐热、耐磨、耐腐蚀、光亮及美丽等优点,因此广泛用于电镀[1],但在应用过程中,产生含铬废水的大量排放,对环境构成严重威胁。
至今,铬仍然是水体中主要金属污染物之一,也是环境监测中必测项目之一。
磷酸三酯检测标准磷酸三(2-氯乙基)酯是一种有机磷酸酯类化合物,分子式为C6H12Cl3O4P,常用作塑料软化剂、溶剂和防火剂等。
然而,它也是一种潜在的有毒物质,对人体和环境都具有较高的危害性。
因此,对磷酸三(2-氯乙基)酯的检测是非常重要的。
一、气相色谱法气相色谱法是一种常用的磷酸三(2-氯乙基)酯检测方法。
该方法首先将样品提取出来,然后用气相色谱仪测定样品中的磷酸三(2-氯乙基)酯含量。
该方法操作简便,灵敏度高,可以检测出较低浓度的磷酸三(2-氯乙基)酯。
二、高效液相色谱法高效液相色谱法是另一种常用的磷酸三(2-氯乙基)酯检测方法。
该方法首先将样品提取出来,然后以高效液相色谱法测定样品中的磷酸三(2-氯乙基)酯含量。
相较于气相色谱法,高效液相色谱法更适用于分析高极性和热不稳定的样品。
三、核磁共振法核磁共振法在磷酸三(2-氯乙基)酯的检测中也有应用。
该方法可以通过分析样品的核磁共振谱图来确定样品中的磷酸三(2-氯乙基)酯含量。
核磁共振法不仅可以测定磷酸三(2-氯乙基)酯的含量,还可以分析其分子结构和化学环境等。
四、质谱法质谱法是一种常用的定性和定量分析技术,也可以用于磷酸三(2-氯乙基)酯的检测。
该方法首先将样品提取出来,然后用质谱仪进行测定,通过分析质谱图来确定样品中磷酸三(2-氯乙基)酯的含量。
除了上述的常用检测方法,还可以通过发射光谱分析、红外光谱等方法进行磷酸三(2-氯乙基)酯的检测。
这些方法可以根据需要选择,以提供精确和可靠的数据。
在磷酸三(2-氯乙基)酯的检测中,还需要参考相关的标准进行分析。
例如,美国环境保护署(EPA)颁布了许多相关的标准方法,如EPA508、EPA8015等,用于磷酸三(2-氯乙基)酯的分析。
此外,国际标准化组织(ISO)也推出了多项相关标准,如ISO6468、ISO8246等。
这些标准方法可以为磷酸三(2-氯乙基)酯的检测提供准确和统一的技术要求,保证检测结果的可比性和可靠性。
TRIBUTYL PHOSPHATE5034(C4H9O)3P=O MW: 266.32 CAS: 126-73-8 RTECS: TC7700000METHOD: 5034, Issue 1 EVALUATION: PARTIAL Issue 1: 15 August 1994OSHA :0.5 ppmNIOSH:0.2 ppmACGIH:0.2 ppm(1 ppm = 10.9 mg/m3 @ NTP)PROPERTIES: liquid; boiling point 293 C; density0.98 g/mL @ 20 C; VP very low @ 20 C;vapor density 9.2 (air=1); flash point 166 C(closed cup)SYNONYMS:phosphoric acid, tributyl ester; tri-n-butyl phosphate; TBP; Celluphos 4SAMPLINGMEASUREMENTAPPLICABILITY: The working range is 0.006 to 1.4 ppm (0.06 to 15 mg/m3) for a 100-L air sample. This method may be adapted to other phosphates of relatively low volatility with appropriate changes in chromatographic conditions. INTERFERENCES: Any phosphorus-containing compound that has the same retention time as the analyte is an interference.A non-polar capillary column may be used for better resolution.OTHER METHODS: This revises Method S208 [2]. Analytical methods for tributyl phosphate (TBP) have been reviewed [3]. Another packed-column GC procedure has been described recently [4]. TBP has been determined in air by capillary-column GC/NPD preceded by sampling on a glass fiber filter or XAD-7 resin [5]. GC/MS [6], LC/MS [7], and LC/TID (thermionic det ection) [8] have all been shown to be useful methods for the analysis of TBP in environmental samples. Finally, a continuous pho sphorus gas analyzer has been used to monitor TBP in air [9].REAGENTS:1.Diethyl ether*, anhydrous, reagent grade.2.Tributyl phosphate*, reagent grade.3.Hydrogen, purifiedpressed air, prefiltered5.Nitrogen, purified6.Calibration stock solution, tributyl phosphate indiethyl ether*See Special Precautions EQUIPMENT:1.Sampler: 37-mm mixed cellulose estermembrane filter (0.8-µm pore size) supportedby cellulose backup pad in three-piece filterholder.NOTE:Backup filter unit is needed whensampling at temperatures above23 °C.2.Personal sampling pump, 1 to 3 L/min, withflexible polyethylene or PTFE tubing.3.Gas chromatograph equipped with a flamephotometric detector, phosphorus filter, andcolumn (p. 5034-1).4.Electronic integrator or some other suitablemethod for measuring peak areas.5.Tweezers.6.Jars: 2 oz ointment jars for sample extraction,squat form with aluminum-lined screw caps.7.Syringes, 10-µL and other convenient sizes.8.Volumetric flasks, 10-mL and other convenientsizes.9.Pipets, 10-mL and other convenient sizes.SPECIAL PRECAUTIONS: Store diethyl ether away from heat, light, and sources of ignition in a well-ventilated area. Do not leave container open. Diethyl ether can oxidize in air to form explosive peroxides, a reaction accelerated by light. Distillation and evaporation can concentrate unstable peroxides in the residue, a potentially explosive condition [10].Avoid inhalation of tributyl phosphate vapors and contact with eyes, skin, and clothing [11,12]. Handle only in a hood.SAMPLING:1.Calibrate each personal sampling pump with the representative filter cassettes in line.2.Remove cassette plugs and connect cassette filter to the personal sampling pump with flexibletubing.NOTE:If ambient temperature is above 23 °C, use two filter cassettes connected in series witha short piece of flexible tubing for sample collection. Some tributyl phosphate may existas vapor above 23 °C.3.Sample at an accurately known flow rate of 1 to 3 L/min for a total sample size of 2 to 100 L.4.Separate front and backup filter cassettes (if two cassettes were used). Firmly seal collectedsample cassettes with plugs, and pack securely for shipment.SAMPLE PREPARATION:5.Transfer the filter and backup pad to an ointment jar using tweezers.6.Pipet 10.0 mL of diethyl ether into each jar. Seal the jar immediately to minimize evaporation.7.Allow samples to stand for at least 30 min with occasional agitation.CALIBRATION AND QUALITY CONTROL:8.Calibrate daily with at least six working standards over the range of 2 to 1000 µg per sample.a.Add known amounts of calibration stock solution to 10-mL volumetric flasks and dilute tovolume with diethyl ether.b.Analyze working standards together with samples and blanks (steps 11 and 12). This willminimize the effect of variations in FPD response with time.NOTE 1:The FPD response is very sensitive to minor variations in hydrogen flow rateand, therefore, it is recommended that calibration standards be carefullyinterspersed with the samples.NOTE 2:Use of an internal standard is recommended to minimize errors caused bysample solvent evaporation and FPD response variations.c.Prepare a calibration graph of area vs. µg of tributyl phosphate per 10 mL of sample.9.Determine recovery in the concentration range of interest for each lot of filters used forsampling. Prepare three filters at each of five levels plus three media blanks.a.Spike aliquot of calibration solution onto each filter.b.After air-drying, extract filters with 10 mL diethyl ether (steps 5 through 7).c.Analyze together with working standards (steps 11 and 12).d.Prepare graph of recovery vs. µg tributyl phosphate.10.Analyze three quality control blind spikes and three analyst spikes to ensure that the calibrationgraph and recovery graph are in control.MEASUREMENT:11.Set gas chromatograph according to manufacturer's recommendations and to conditions givenon page 5034-1. Inject 5-µL sample aliquot using solvent flush technique or with autosampler.NOTE:If peak area is above linear range of the calibration graph, dilute with diethyl ether, analyze, and apply appropriate dilution factor in calculations.12.Measure peak area.CALCULATIONS:13.Determine mass, µg (corrected for recovery), of tributyl phosphate found in the sample (W) andthe average media blank (B).14.Calculate concentration of tributyl phosphate in the actual air volume sampled, V (L):EVALUATION OF METHOD:REFERENCES:[1]Backup Data Report for Tributyl Phosphate, in Documentation of the NIOSH Validation Tests,prepared under NIOSH Contract CDC-99-74-45 (1977).[2]NIOSH Manual of Analytical Methods, 2nd. ed., V. 3, S208, U.S. Department of Health,Education, and Welfare, Publ. (NIOSH) 77-157-C (1977).[3]Davis, W., Navratil, J., Lasztity, A., and Horvath, Z., Analytical Methods [for Tributyl Phosphate],in Science and Technology of Tributyl Phosphate, Vol. 1, pp 267-327, Schulz, W. and Navratil, J., Eds., CRC, Boca Raton, FL, 1984.[4]Kuno, Y., Hina, T., Akiyama, T., and Matsui, M., Simultaneous Determination of TributylPhosphate and Dibutyl Phosphate in Spent Fuel Reprocessing Streams by GasChromatography, J. Chromatogr., 537 (1-2): 489 (1991).[5]Haraguchi, K., Yamashita, T., and Shigemori, N., Sampling and Analysis of Phosphoric AcidTriesters in Ambient Air, Taiki Osen Gakkaishi, 20 (6): 407 (1985).[6]Ishikawa, S., Taketomi, M., and Shinohara, R., Determination of Trialkyl and Triaryl Phosphatesin Environmental Samples, Water Res., 19 (1): 119 (1985).[7]Barceló, D., Application of Thermospray Liquid Chromatography/Mass Spectrometry forDetermination of Organophosphorus Pesticides and Trialkyl and Triaryl Phosphates, Biomed.Environ. Mass Spectrom., 17 (5): 363 (1988).[8]Barceló, D., Maris, F., Frei, R., de Jong, G., and Brinkman, U., Determination of Trialkyl andTriaryl Phosphates by Narrow-Bore Liquid Chromatography with on-line Thermionic Detection,Intern. J. Environ. Anal. Chem., 30 (1-2): 95 (1987).[9]Parker, G., Continuous Quantitative Analysis of Low Concentrations of Tributyl Phosphate (TBP)Vapors in Flowing Air Streams, Am. Ind. Hyg. Assoc. J., 41 (3): 220 (1980).[10]Material Safety Data Sheet for Diethyl Ether, No. 343, General Electric Company, Schenectady,N.Y., August, 1979.[11]Material Safety Data Sheet for Tributyl Phosphate, No. 521, General Electric Company,Schenectady, N.Y., October, 1983.[12]Material Safety Data Sheet for Tributyl Phosphate, No. P1180.2, Van Waters & Rogers, Inc.(U.S.), Seattle, Washington, August 27, 1989.METHOD REVISED BY:Robert P. Streicher, Ph.D., NIOSH/DPSE.。