Mabselect Sure 亲和纯化
- 格式:docx
- 大小:13.91 KB
- 文档页数:2

Part 1、工艺经济性毫无疑问,亲和填料比传统填料(如离子交换或疏水相互作用层析填料)更昂贵。
它们每升的价格要高出三到十倍,因为i) 与生产其它类型的配基相比,用于生物工艺应用的亲和配基的生产更加复杂,因此成本更高,并且ii) 从公司运营的角度看,填料价格体现了AC提供的经济利益,包括减少工艺开发时间以及更高收率的生产操作。
从生产工艺的角度来看,确定填料成本的常用方法是计算每克纯化目标产品的成本。
该成本与填料可以使用的循环次数和有效结合载量(上样×步骤收率)成反比,并与填料和缓冲液成本成正比。
由于AC 填料的成本很高,它对商品总成本的影响可能很大,例如,对于特定的纯化工艺,Protein A可能占到商品总成本的60%。
但是,相比关注AC对纯化工艺总体商品成本的相对成本,如果分析的重点是实际生产成本,那么可以表明,在下游工艺中使用AC 通常会降低商品的总成本。
AC 减少了纯化步骤的数量,从而提高了整体工艺收率并缩短了工艺时间。
图 1 举例说明了这一点,该图显示了非亲和(四步)和基于亲和(三步)纯化工艺的COG 分析比较,它们都产生相同纯度的药物底物(在两个工艺中,最后两个层析步骤相同)。
与非亲和工艺相比,将AC 作为第一步的三步工艺可以将产品的总成本降低 1.5 倍,即使前两个非亲和步骤具有更高的上样载量。
由于与AC 步骤相比,第一个非亲和步骤具有低得多的步骤收率,因此与单个AC 步骤相比,两个非亲和步骤之后的整体工艺收率显著降低(30%)。
总收率越低,生产的产品质量越低,即使非亲和工艺的耗材成本与基于亲和工艺的情况相比要低得多,这也会推动COG 上升。
其他研究人员也报道了类似的结果。
显然,只有在可使整体工艺收率大幅增加时,才应考虑用AC 步骤替换多个非特异性步骤。
图1.基于亲和和非亲和捕获步骤的 DSP 工艺(工艺 I和 II)之间的 COG 分析比较结果示例。
图:A)两个工艺的简化工艺布局(相关的动态结合载量,DBC,在各自的框中给出,两个工艺的所有其它步骤相同);B) 过程 I(空心符号)和过程 II(实心符号)中每个下游加工步骤后的累积过程产量(三角形)和纯度(圆圈);C) 以过程 II 值的百分比变化表示的典型过程经济指标的变化。


ImaginationatWorkUNICORN TM 6软件控制的A ..KTA TM avant25系统,用于研发单克隆抗体(MAb )纯化的两步色谱工艺过程。
MabSelectSuRe TM ,基于蛋白A 的色谱柱料(合成树脂),用来进行初始的捕获步骤;而多模式阴离子交换剂Capto TM adhere ,用来进行第二步精制步骤,减少不纯物。
实验设计在精制步骤用来筛选上样条件。
样品的pH 、电导率和上样量有所变化。
使用1毫升预装的HiTrap TM 柱子,设计运行时间少于24小时,简化的设计实施确立了工艺过程条件的稳健性。
将功能性整合在UNICORN TM 6软件的实验设计(DoE ),与HiScreen TM 一起使用,在大约一周的时间内将所有的工艺过程进行了优化。
尽管料液研究具有挑战性,靶MAb 的高产率和纯度都达到了目的。
前言在生物药物复合物纯化工艺过程研发中,时间和灵活性是重要条件。
A ..KTA TM avant25是一款专门为全自动工艺过程研发而设计的色谱系统,使用刚性色谱柱料。
功能性整合在UNICORN TM 6软件的实验设计(DoE ),增强了产率。
实验设计(图1)有利于简便快速的工艺过程研发,是工艺过程研发实验室优化色谱条件、增加通量的有力工具。
在单克隆抗体(MAb )纯化过程中,蛋白A 是捕获步骤选择的色谱柱料,基于它的高分离性产生良好的纯度,无论大规模和小规模都可以方便地使用。
因此,蛋白A 为基础的柱料是进行MAb 纯化的平台方法的根基。
图1嵌入实验设计功能的UNICORN6软件,为简便快速的工艺过程研发而设计。
在实验功能设计中,多因子同时变化,结果数据用来生成统计模型。
模型经过验证后,用来产生系统图为判定提供帮助。
接着,下游的数据处理可以根据不同色谱技术应用指南28-9573-47AA 色谱系统使用A ..KTA TMavant25进行MAb 纯化的快速工艺过程研发模型评价使用模型进行预测和判定输入设计和技术的组合进行(尤其离子交换和疏水相互作用色谱)。
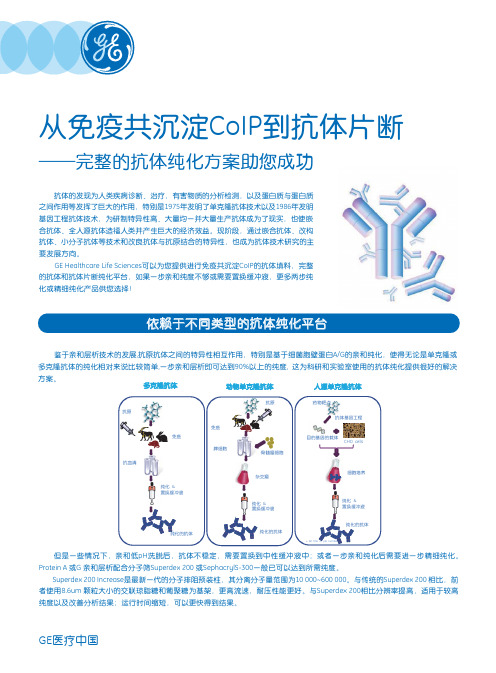

用户园地Q & A30鉴于亲和层析技术的发展,抗原抗体之间的特异性相互作用,使得抗体的纯化相对来说比较简单,一步亲和层析即可达到90%以上的纯度, 这为科研和实验室使用的抗体纯化提供很好的解决方案。
然而要得到高质量高纯度的抗体,需要我们从样品处理,纯化介质的选择以及纯化方法上悉心考虑,这里我们就这些大家常见的问题来讨论。
1,对于血清、腹水或细胞悬浮物来源的抗体样品,有哪些样品处理的方法?在纯化前,合适的样品处理不仅可以帮助我们得到高纯度高质量的抗体,还有助于保护亲和层析柱,延长使用寿命。
对于血清、腹水或细胞悬浮物来源的样品经常含有脂蛋白,酚红,小分子污染物等等。
腹水中经常含有高浓度的脂蛋白,这些脂蛋白和脂类物质会堵塞层析柱,最好能在纯化之前去除。
方法一是在二价离子存在的情况下,硫酸右旋葡糖酐能够沉淀脂蛋白,沉淀可以通过离心除去;方法二PVP能产生一个pH值依赖的沉淀效应,PVP在pH=7.0时能够沉淀b-脂蛋白和球蛋白。
很多时候,可以考虑进样前用亲和结合缓冲液稀释腹水,不仅保证样品的结合pH,还有利于降低黏度。
酚红是一种在实验室细胞培养中的pH指示剂,虽然并不直接的影响纯化,但是酚红可能结合到某些纯化介质上,应该尽可能早的被除去,可以使用脱盐柱除去酚红。
对于一些低分子污染物可以使用分级沉淀法去除,如硫酸铵沉淀,羊脂酸沉淀的方法。
最后利用脱盐柱来更换缓冲液并除盐,把样品换到合适的缓冲液中(PH和盐浓度),并除去没有用处的小分子。
更多详细的方法可以参考《抗体纯化手册》。
2,实验室需要纯化小鼠的IgG1,我是选择ProteinG还是ProteinA?首先我们要根据不同的种属亚型参考“相对结合强度”表(图1)来获取选择哪种配基的指导,针对小鼠的IgG1, ProteinA结合相对弱,可以选择ProteinG配基的介质。
但由于Protein G结合力较强,因此有时需要pH低于2.0才能有效洗脱,容易导致某些对酸敏感的抗体聚集沉淀。

纯化凝胶选择的具体方法(1)生物分子下游纯化的对象一般包括蛋白、酶、重组蛋白、单抗、抗体及抗原、肽类、病毒、核酸等。
纯化前首先需要测定生物分子的各物理和化学特性,然后通过实验选择出最有效的纯化流程。
1.测定——分子量、PI当目标蛋白的物理特性如分子量、PI等都不清楚时,可用PAGE电泳方法或层析方法加以测定。
分离范围广阔的Superose HR预装柱很适合测定未知蛋白的分子量。
用少量离子交换介质在多个含不同PH缓冲液的试管中,可简易地测出PI,并选择纯化用缓冲液的最佳PH。
2.选择——层析方法若对目标蛋白的特性或样品成分不太了解,可尝试几种不同的纯化方法:一] 使用最通用的凝胶过滤方法,选择分离范围广阔的介质如Superose、Sephacryl HR依据分子量将样品分成不同组份。
二] 用含专一配体或抗体的亲和层析介质结合目标蛋白。
亦可用各种活化偶联介质偶联目标蛋白的底物、受体等自制亲和介质,再用以结合目标蛋白。
一步即可得到高纯度样品。
三] 体积大的样品,往往使用离子交换层析加以浓缩及粗纯化。
高盐洗脱的样品,可再用疏水层析纯化。
疏水层析利用高盐吸附、低盐洗脱的原理,洗脱样品又可直接上离子交换等吸附性层析。
两种方法常被交替使用于纯化流程中。
3.纯化——大量粗品处理大量原液时,为避免堵塞柱子,一般使用sepharose big beads、sepharoseXL、sepharose fast flow等大颗粒离子交换介质。
扩张柱床吸附技术利用多种STREAmlINE介质,直接从含破碎细胞或组织萃取物的发酵液中俘获蛋白。
将离心、超滤、初纯化结合为一。
提高回收率,缩短纯化周期。
4.纯化——硫酸氨样品硫酸氨沉淀方法常被用来初步净化样品,经处理过的样本处于高盐状态下,很适合直接上疏水层析。
若作离子交换,需先用Sephadex G-25脱盐。
疏水层析是较新技术,随着介质种类不断增多,渐被融入各生产工艺中。
利用Hitrap HIC Test Kit 和RESOURCE HIC Test Kit可在八种疏水介质中选择最适合介质及最佳的纯化条件。
MabSelect SuRe™ (Superior Resistance) is a member of the MabSelect™ family* of affinity chromatography media for the capture of monoclonal antibodies (MAbs) at process scale. MabSelect SuRe is composed of a rigid, high-flow agarose matrix and alkali-stabilized protein A-derived ligand. This ligand provides greater stability than conventional protein A-based media in the alkaline conditions usedin cleaning-in-place (CIP) protocols. The enhanced alkali stability of MabSelect SuRe improves process economy; cleaning can be performed with cost-effective reagents such as sodium hydroxide, which improves process economy and product quality.Key performance characteristics of MabSelect SuRe are:• Novel, alkali-stabilized protein A ligand allows the use of 0.1–0.5 M sodium hydroxide for CIP.• Improves product quality and reduces overall costs.• Eliminates the need for expensive and corrosive CIPreagents.• High dynamic binding capacity (DBC) reduces process time and amount of medium used.• High-flow agarose matrix allows processing of large volumes of feed.High stability in alkaline conditionsThe MabSelect SuRe ligand was developed by protein engineering of one of the IgG-binding domains of Protein A. Amino acids particularly sensitive to alkali were identified and substituted with more stable ones. The final construct is a tetramer of the engineered domain with a C-terminal cysteine, which enables single-point attachment to the matrix.Fig 1. MabSelect SuRe is ideal for purification of large volumes of monoclonal antibodies and is resistant to cleaning- and sanitization-in-place using alkali.GE HealthcareData File 11-0011-65 AB Affinity chromatography MabSelect SuReAlkali-stabilized protein A-derived medium for capture of monoclonal antibodiesThe ligand is produced by validated fermentation anddownstream processes and the entire production processis free of components of mammalian origin. The resultinghighly purified ligand is immobilized to the agarose matrixthrough a chemically stable thio-ether linkage.MabSelect SuRe is stable under alkali conditions and hasbeen tested for up to 200 cycles of CIP using 0.1 M NaOH.The combination of low ligand leakage and high DBCtogether with the high-flow matrix makes MabSelect SuReideal for the purification of MAbs at process scale.* The MabSelect family of media for process-scale purification of monoclonal antibodies comprisesMabSelect, MabSelect Xtra™, and MabSelect SuRe. MabSelect is designed for high-throughputpurification of monoclonal antibodies from large volumes of feed. MabSelect Xtra is designed formaximum DBC, which allows capture of high expression levels of MAbs from feedstock. For moreinformation on MabSelect and MabSelect Xtra, refer to data files 18-1149-94 and 11-0011-57 respectively.imData File 11-0011-65 AB 2Rigid, highly cross-linked matrix allows high flow ratesMabSelect SuRe has been developed from the same rigid, highly cross-linked agarose matrix used for MabSelect. The matrix of MabSelect SuRe allows the use of higher flow rates in process-scale purifications of Mabs compared with conventional cross-linked agarose of similar porosity.The characteristics of MabSelect SuRe are summarized in Table 1.High dynamic binding capacity after numerous CIP cyclesCleaning-in-place is an essential step in the production of pure Mabs in industrial applications. The main drawback with using sodium hydroxide for CIP of conventional protein A-based media is the sensitivity of native and recombinant Protein A (rProtein A) to alkaline conditions. MabSelect SuRe, however, retains dynamic binding capacity after repeated CIP cycles with 0.1–0.5 M NaOH.Figure 2 shows DBC (10% breakthrough) of polyclonal human IgG (h IgG) as a function of exposure to alkaline conditions. MabSelect, with its conventional rProtein A ligand, was used for comparison. Approximately 85–90% of the initial dynamic binding capacity of MabSelect SuRe is retained after numerous CIP cycles with sodium hydroxide.The dynamic binding capacity of MabSelect SuRe remains high after CIP in conjunction with purification of humanized IgG 1 and IgG 4 from clarified cell culture (Table 2). DBC was retained to approximately 85% of the initial binding capacity. The recovery of both MAbs was consistently over 95%.The dynamic binding capacity of MabSelect SuRe remains high after CIP in conjunction with purification of humanized IgG 1 and IgG 4 from clarified cell culture (Table 2). DBC was retained to approximately 85% of the initial binding capacity. The recovery of both MAbs was consistently over 95%.Table 1. Characteristics of MabSelect SuReLigand Alkali-stabilized proteinA-derived (E. coli )Ligand coupling method Epoxy activationMatrixRigid, highly cross-linked agarose Average particle size (d 50V )* 85 µmDynamic binding capacity † At least 30 mg human IgG/mlmedium at 2.4 min residence time Recommended mobile 100–500 cm/hphase velocity ‡Chemical stability Stable in all aqueous buffers commonly used in protein Achromatography. pH working range 3–12Cleaning-in-place stability 0.1–0.5 M NaOH Delivery conditions20% ethanol* d 50V is the median particle size of the cumulative volume distribution.†Determined at 10% breakthrough by frontal analysis at a mobile phase velocity of 250 cm/h in a column with a bed height of 10 cm.‡Determined in a BPG 300 column, bed height 20 cm, operating pressure less then 2 bar.No. of CIP cyclesD B C a t 10% b r e a k t h r o u g h (p o l y c l o n a l h I g G ) [%]100806040200020 40 60 80 100 120 140 160 180 200 220Fig 2. Dynamic binding capacity of MabSelect SuRe and MabSelect for polyclonal human IgG after CIP with 0.1–0.5 M NaOH for up to 200 cycles.Table 2. Effect of CIP cycles using 0.1 M NaOH on the DBC of MabSelect SuRe in the purification of IgG 1 and IgG 4.Antibody CIP (no. ofDBC (dynamic bindingcycles × duration capacityin minutes)[% of initial DBC])IgG 1 150 × 15 min ≥ 85%IgG 4100 × 15 min≥ 85%Data File 11-0011-65 AB 3Increased residence time increases dynamic binding capacityThe already high dynamic binding capacity of MabSelect SuRe is further improved by increasing sample residence time on the medium. With a residence time of 2.4 min, the dynamic binding capacity at 10% breakthrough of humanized IgG 1, humanized IgG 4, and polyclonal h IgG is ≥ 30 mg/ml (Fig 3). Increasing the residence time to 4.8 min increases thedynamic binding capacity of the humanized immunoglobulins and h IgG to more than 38 mg/ml.h IgG 1h IgG 1454035302520151050IgG 1IgG 1IgG 4IgG 1IgG 1IgG 42.4 minutes3.0 minutes4.8 minutesFig 3. Dynamic binding capacity of MabSelect SuRe as a function of residence time of the protein on the medium.Low ligand leakageThe level of leakage of the MabSelect SuRe ligand during elution is low. A normal range of leakage is estimated to be 5–20 ppm (ng ligand/mg IgG). However, leakage is affected by chromatographic running conditions and the composition of the feedstock.Figure 4 shows the ligand leakage from MabSelect SuRe over 100 cycles of purification of a monoclonal antibody from clarified cell culture. Fractions collected from thepurification were analyzed by noncompetitive ELISA. Figure 4 confirms the low leakage of the MabSelect SuRe ligand over numerous purification/CIP cycles.Concentration of leached ligand(ng ligand/mg IgG)Purification/CIP cycles12.010.08.06.04.02.00.0Fig 4. Ligand leakage (ppm) of MabSelect SuRe over 100 cycles of CIP in conjunction with the purification of humanized IgG 4. Cleaning-in-place was performed with 0.1 M NaOH and the contact time was 15 min/cycle.Low risk of host cell protein contamination or carryoverRigorous CIP or sanitization-in-place with sodium hydroxide reduces the risk of both contamination from host cell proteins and microbial growth in the medium, as well as carryover in the product pools.Figure 5 is a Western blot of humanized IgG 4 fractions eluted in the ligand-leakage study described above. The Western blot confirms that no contamination from host cell proteins or carryover is detected after 50–100 purification cycles on MabSelect SuRe. Comparison of the separation of host cell protein standard in lane 4 with the purified IgG 4 in lanes 6–17 obtained from 100 affinity purifications on MabSelect SuRe indicates no presence of the host cell proteins in the bound fraction eluates. The absence of protein bands in lanes 18 (carryover after 50 cycles) and 19 (carryover after 100 cycles) indicates no carryover or cross-contamination between two consecutive purification cycles with an intermittent CIP cycle. Sample, data, and image were supplied by kind courtesy of Lonza Biologics plc, Slough, U.K.Data File 11-0011-65 AB 4Comparison of MabSelect SuRe with rProtein A-based mediaFigures 6 and 7 show the performance of MabSelect SuRe and MabSelect in the purification of humanized IgG 1 and IgG 4 from HCCF (host cell culture fluid). Selectivity and specificity of the two media were comparable.In a separate study, the purification of humanized IgG 4 expressed in cell culture fluid using MabSelect SuRe was compared to the purification performance of MabSelect and MabSelect Xtra. Purification performance of the three media was similar and contamination of host cell proteins was minimal as seen on the Western blot in Figure 8. Sample, data, and image were supplied by kind courtesy of Lonza Biologics plc, Slough, U.K.Fig 5. Western blot of humanized IgG 4 from 1–100 purification/CIP cycles on MabSelect SuRe. The Western blot was developed with two sets ofpolyclonal antibodies; one raised against the host cell proteins and another raised against the MAb. The presence of bovine serum albumin (BSA) and IgG in lane 2, as the negative and positive controls respectively, marks the specificity of the two sets of antibodies.Lane 1: HMW marker Lane 2: BSA IgG standard Lane 4: Host cell proteinLane 6: Purification/CIP cycle 1Lane 7: Cycle 5Lane 8: Cycle 10Lane 9: Cycle 20Lane 10: Cycle 30Lane 11: Cycle 40Lane 12: Cycle 50Lane 13: Cycle 60Lane 14: Cycle 70Lane 15: Cycle 80Lane 16: Cycle 90Lane 17: Cycle 100Lane 18: Carryover after 50 cycles Lane 19: Carryover after 100 cycles Lane 20: Start materialFig 6. Similar desorption characteristics of MabSelect SuRe and MabSelect in the purification of humanized IgG 1 from HCCF.Column: Tricorn ™ 5/100Sample:8 mg humanized IgG 1 in HCCFEquilibration buffer: 20 mM phosphate, 150 mM NaCl, pH 7.4Elution buffer: 100 mM citrate, pH 3.0System: ÄKTAexplorer ™ 10Fig 7. Similar desorption characteristics of MabSelect SuRe and MabSelect in the purification of humanized IgG 4 from HCCF.Column: Tricorn 5/100Sample:5 mg humanized IgG4 in HCCFEquilibration buffer: 20 mM phosphate, 150 mM NaCl, pH 7.4Elution buffer: 100 mM citrate, pH 3.0System:ÄKTAexplorer 10M r x 1032001169766453121146Lane124678 9 10 11 12 13 14 15 16 17 18 1920mAU 300025002000150********005.010.015.020.025.030.0mlMabSelect SuRe MabSelectmAU 1500100050005.010.015.0 20.0 25.0 30.0 35.0 mlMabSelect SuRe MabSelectpHData File 11-0011-65 AB 5Fig 8. Western blot confirming the purity of humanized IgG 4 purified on MabSelect SuRe, MabSelect, and MabSelect Xtra.Fig 9. Pressure/flow profile for MabSelect SuRe in a packed bed (bed height 20 cm) in a BPG 300 column (i.d. 300 mm).OperationPurificationMabSelect SuRe offers high selectivity, which renders efficiency related parameters such as sample load, flow rate, bead size and bed height less important for resolution.The primary aim of method optimization is to establish the conditions that will bind the highest amount of target molecule, in the shortest time, and with the highest product recovery. The degree to which IgG binds to protein A varies with respect to both origin and antibody subclass. In general, however, all human or humanized antibodies, except for subclass 3, have a high affinity for protein A.Typically, the clarified feedstock is loaded onto the column directly. After sufficient washing, the Mab is normally eluted at pH 3–4.Cleaning and sanitizationUse of 0.1–0.5 M NaOH is recommended for cleaning and sanitization. Optimization of contact time, concentration, and frequency of CIP cycles is required to achieve the best possible results.StorageRecommended storage solutions for MabSelect SuRe are 20% ethanol or solutions containing 2% benzyl alcohol. The recommended storage temperature is 4–8 °C.Recommendations for column packing, cleaning andsanitization, method design, and optimization can be found in the instructions delivered with each pack of medium.Scale-upAfter optimizing the antibody purification at laboratory scale, the process can be scaled up by keeping the residence time constant in order to maintain capacity. This can be achieved by increasing the column diameter, and keeping the mobile phase velocity and sample-to-bed volume ratio constant.MabSelect SuRe is based on the same matrix as MabSelect and has similar pressure and flow characteristics. Thepressure/flow curve shown in Figure 9 is for MabSelect SuRe packed in a large-format BPG ™ column. For more details on packing in Chromaflow ™ columns, see Application Note 11-0007-52.M r x 1032001169766453121146Lane2468101112Lane 2: MabSelect eluateLane 4: MabSelect SuRe eluate Lane 6: MabSelect Xtra eluateLane 8: Antibody reference marker Lane 10: HMW markersLane 11: Host cell protein standard Lane 12:BSA/IgG standards0.00.10.20.38006004002000Pressure (MPa)M o b i l e p h a s e v e l o c i t y (c m /h )Ordering InformationProductPack sizeCode NoMabSelect SuRe 25 ml 17-5438-01 25 ml 17-5438-01 200 ml 17-5438-02 1 l 17-5438-03 5 l 17-5438-0410 l17-5438-05Lab-scale columns XK 16/20 column 18-8773-01 XK 16/40 column18-8774-01 Packing connector XK 16 18-1153-44 XK 16/20 tube 19-0315-01 AK 16 adapter 18-8778-01RK 16/26 reservoir 18-8793-01Prepacked columns MabSelect SuRe,Tricorn 10/100 GLInquireRelated literatureData FilesBPG columns18-1115-23 BioProcess columns 18-1167-76Chromaflow columns18-1138-92Application notes MabSelect SuRe - Leakage 11-0011-64 and ToxicityMabSelect - Column packing 11-0007-52All bulk media products are supplied in suspension in 20% ethanol. Foradditional information, please contact your local GE Healthcare representative.Recommended columnsMabSelect SuRe can be used together with most equipment available for chromatography from laboratory scale toprocess scale. To ensure best performance at process scale, pack MabSelect SuRe to a bed height of 10–30 cm. Recommended columns are listed in Table 3.Column* Inner diameterBed volume Bed height(mm) (cm)Lab scaleXK 16/4016 16–60 ml 8–30Production scaleBPG 100–300 0.8–21 l 10–30BioProcess 100–1200 0.8–339 l 10–30Chromaflow400–200013–942 l10–30All large-scale columns can be supplied as variable bed height columns. Note that all bed height and diameter combinations above are possible but not necessarily suitable. For example, do not choose large-diameter columns if the bed height is low. Another general recommendation is not to choose a bed height above 30 cm.Table 3. Recommended columns for MabSelect SuRe. For maximum productivity and robust performance, bed heights of 10–30 cm are normally usedGE, imagination at work, and GE monogram are trademarks of General Electric Company.ÄKTAexplorer, BioProcess, BPG, Chromaflow, MabSelect, MabSelect SuRe, MabSelect Xtra, Tricorn, and Drop design are trademarks of GE Healthcare companies. All third party trademarks are the property of their respective owners.© 2007 General Electric Company—All rights reserved First published Nov. 2004Purification and preparation of fusion proteins and affinity peptides comprising at least two adjacent histidine residues may require a license under US pat 5,284,933 and US pat 5,310,663, including corresponding foreign patents (assigne: Hoffman La Roche, Inc).All goods and services are sold subject to the terms and conditions of sale ofthecompany within GE Healthcare which supplies them. A copy of these terms and conditions is available on request. Contact your local GE Healthcare representative for themost current information.GE Healthcare UK Limited, Amersham Place, Little Chalfont, Buckinghamshire, HP7 9NA, UKGE Healthcare Bio-Sciences Corp., 800 Centennial Avenue, P.O. Box 1327, Piscataway,NJ 08855-1327, USA GE Healthcare Europe GmbH, Munzinger Strasse 5, D-79111 Freiburg, GermanyGE Healthcare Bio-Sciences KK, Sanken Bldg., 3-25-1, Hyakunincho, Shinjuku-ku, Tokyo, 169-0073 JapanFor contact information for your local office, please visit, /contact /mabselect GE Healthcare Bio-Sciences AB Björkgatan 30 751 84 Uppsala Sweden11-0011-65 AB 11/2007imagination at workElanders Östervåla 2007 12345Elanders Östervåla 2007 12345 Elanders Östervåla 2007Elanders Östervåla 2007 E l a n d e r s Ös t e r v ål a 2007 12345E l a n d e r s Ös t e r v ål a 2007 12345E l a n d e r s Ös t e r v ål a 2007。
Mabselect Sure ——唯一耐强碱的蛋白A亲和层析介质
在生物药物的生产过程中,为避免细菌、内毒素或病毒的污染,需要对层析系统、介质等进行在位消毒 (SIP),以保证产品的质量。
另外,变性的蛋白、脂类和DNA等分子会不可逆的沉淀在层析柱的顶部而降低柱效和传质速度,从而影响收率和产品质量。
0.1~1N的氢氧化钠可以有效的除菌除热原以及灭活病毒,而且对于沉淀的
蛋白、脂类和核酸具有很好的溶解作用,可以有效的对层析介质进行在位清洗(CIP) 而延长使用寿命,因此使用氢氧化钠进行消毒和清洗成为生物制药生产中SIP/CIP的经典方法。
这一点对于内毒素要求极为严格的抗体生产来说尤为重要。
但天然或重组的蛋白A配基对NaOH非常敏感,强碱性条件下易变性或脱落,因此只能选用高浓度的盐酸胍或尿素进行清洗,但清洗的效果远远不如NaOH,而且清洗成本非常高。
天然或重组的蛋白A 配基具有5 个不同的抗体Fc 结合结构域,每个域均可
以和IgG的Fc段结合,但不同的域结合强度略有差异。
将其中的B domain 中对碱敏感的Asp 氨基酸突变为耐碱的氨基酸,得到新的四聚体配基SuRe Ligand,将此新配基偶联到高流速琼脂糖基质上,成为耐碱的新型高流速Mabselect SuRe 蛋白A 层析介质。
Mabselect SuRe 的优点:
a. Mabselect Sure 可以耐受0.1~0.5N的氢氧化钠:使用高达0.5N的氢氧化钠进行在位清洗/消毒大大降低了抗体产品被内毒素污染和批间交叉污染的风险,清洗效果更好,有利于延长介质使用寿命,同时也大大降低了CIP/SIP 的成本。
Mabselect SuRe 介质可使用0.1N NaOH 15min/circle 进行“纯化-清洗”循环200次以上仍保持稳定的载量,对碱的耐受性远远好于其它的蛋白A 亲和层析
介质。
R.Hahn 的研究也表明:与其他蛋白A 亲和介质相比,Mabselect SuRe 具有无可比拟的稳定性,配基脱落最少,寿命最长,而且宿主蛋白HCP的残留比Prosep A低10倍以上。
b. 更温和的洗脱,避免抗体聚集,提高收率:新的SuRe 配基是一个同型四
聚体配基,避免了不同配基与抗体Fc 段亲和性的差异,也消除了某些域对Fab 段的亲和作用,使得洗脱条件更加均一而温和。
相比以rProtein A 为配基的介质,Mabselect SuRe介质可以用更高的pH进行洗脱,有效的避免抗体在低pH 下的聚集,产品纯度和均一性更高,浊度也更低。
c. 不同抗体洗脱所需pH 差异小:由于消除了对抗体Fab 段的亲和作用,使
得同一种属亚型的不同抗体分子洗脱所需的条件更接近,有利于平台技术的建立,进一步降低了不同的抗体分离纯化工艺的研发成本。
d. SuRe 配基稳定性更好:新型的SuRe配基对碱和蛋白酶更稳定47,纯化
过程中脱落更少 (<10 ppm),有利于后期泄漏配基的进一步去除。
甚至只有1-3ppm的脱落,无需在后续步骤中增加专门去除Sure配基的步骤,即可达到FDA 的蛋白A 配基残留标准。
Mabslect SuRe 介质是市场上唯一耐碱的蛋白A 亲和层析介质,寿命最长,稳定性最好,这一点已被文献所证实。
纯化所得的抗体除蛋白A 泄漏更少、纯度更高之外,其它产品性质(如CHOP、电泳、IEF 等) 和Mabselect介质相当。
目前,已有多家大规模抗体生产厂商,将其它蛋白A亲和胶转换为寿命更长、稳定性更好的MabSelect SuRe用于抗体生产。
MabSelect SuRe 也成为市场上
销量最大的抗体生产亲和凝胶。