SPE固相萃取培训
- 格式:pptx
- 大小:26.53 MB
- 文档页数:56

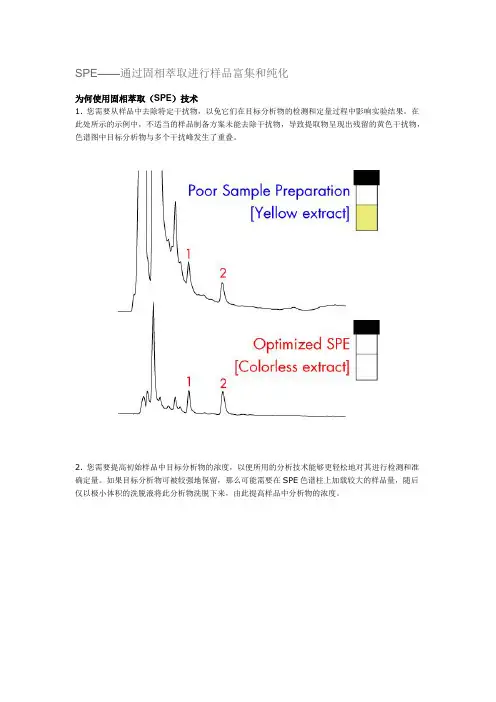
SPE——通过固相萃取进行样品富集和纯化为何使用固相萃取(SPE)技术1. 您需要从样品中去除特定干扰物,以免它们在目标分析物的检测和定量过程中影响实验结果。
在此处所示的示例中,不适当的样品制备方案未能去除干扰物,导致提取物呈现出残留的黄色干扰物,色谱图中目标分析物与多个干扰峰发生了重叠。
2. 您需要提高初始样品中目标分析物的浓度,以便所用的分析技术能够更轻松地对其进行检测和准确定量。
如果目标分析物可被较强地保留,那么可能需要在SPE色谱柱上加载较大的样品量,随后仅以极小体积的洗脱液将此分析物洗脱下来,由此提高样品中分析物的浓度。
3. 您需要去除样品中的干扰物(即使不可见),这些干扰物会在质谱检测中抑制目标分析物的信号。
在此处的示例中,蛋白沉淀法无法去除血浆提取物中的磷脂,从而造成严重的离子抑制。
优化的复合模式SPE方案可获取最纯净的提取物,并可在最大程度上降低离子抑制效应。
What is Solid-Phase Extraction (SPE)?Don't be confused by the term solid-phase extraction [SPE]. A typical SPE device has 50 times more separation power than a simple, single liquid-liquid extraction. SPE is actually column liquid-solid chromatography. Since SPE is liquid chromatography [LC], its practice isgoverned by LC principles. A sample is introduced into a column or a cartridge device containing a bed of appropriate particles, or other form, of a chromatographic packing material [stationary phase]. Solvent [mobile phase] flows through the bed. By choosing an appropriate combination of mobile and stationary phases, sample components may pass directly through the column bed, or they may be selectively retained.Individual compounds in the sample each typically appear to travel at different speeds through the device. Using a weaker solvent causes them to move slowly and/or be strongly retained. A stronger solvent speeds up their passage through the bed and elutes the analyte(s) in a more concentrated volume. Elution from an SPE device is usually done by increasing the strength of the mobile phase in a series of discrete, rather than continuous, steps during which selected analytes or interferences are either fully retained or rapidly eluted-this variation of gradient elution called a step gradient.Most commonly, SPE is practiced using miniature column or cartridge devices. An example is shown here. A mixture of three dyes is loaded onto the cartridge in a weak solvent, causing strong sample retention in a narrow band that appears black at the column inlet. Subsequent gradient steps, each with a successively stronger solvent, are used to elute the dyes individually [yellow, red, then blue].Typical SPE cartridges are low-pressure devices-constructed of solvent-resistant plastic or glass-filled with particles ≥30 µm in diameter. Suitable flow rates may be achieved by gravity or with the assistance of vacuum or low positive pressure. [The latter requires putting a cap on the open inlet of a column or using a sealed device with inlet and outlet fittings.]Importance of Sample PreparationIn the last two decades, dramatic advances in analytical instrumentation and laboratory information management systems shifted the analyst's predominant tasks from assaymeasurements to sample preparation and data processing. As the stringency of requirements for higher sensitivity, selectivity, accuracy, precision, and number of samples to be processed has escalated, the corresponding increases in speed and sophistication of analysis and data collection have outpaced improvements in the many traditional techniques of samplecollection and preparation. By some estimates, 75 to 80% of the work activity and operating cost in a contemporary analytical lab is spent processing and preparing samples forintroduction or injection into an analytical separation and/or measurement device. Clearly, efforts directed and products designed to streamline sample preparation protocols are essential to future progress in analytical science.Goals of Sample PreparationSuccessful sample preparation for most analytical techniques [HPLC, GC, spectrophotometry, RIA, etc.] has a threefold objective: namely, to provide the sample component of interest▪in solution▪free from interfering matrix elements▪at a concentration appropriate for detection or measurement.To accomplish these goals, a sample, or a representative portion thereof [not always easy to obtain], is prepared via traditional methods of dissolution, homogenization, extraction[liquid- or solid-phase], filtration, concentration, evaporation, separation, chemicalderivatization, standardization [internal or external], etc.Usually such methods are used in combinations of multiple steps, which form a sample prep protocol. The fewer steps and methods used in any given protocol, the simpler, moreconvenient, cost effective, and less time consuming it is. Simpler protocols lend themselves more readily to automation and also lead to increased accuracy, reliability, reproducibility, and safety.Innovation in Sample Preparation MethodsThere are many ways to combine standard tools and techniques to accomplish the goals of sample prep. However, it is best to seek innovative means to streamline sample prepprotocols:▪to combine the functions of several steps, if possible, into one operation;▪to eliminate needless sample transfers and manipulations;▪to reduce the scale as much as practicable [gaining economies of time, labor, and cost];▪to use new tools in creative ways.Benefits of Solid-Phase Extraction [SPE] CartridgesWhen compared to other sample preparation processes, solid-phase extraction using SPE cartridges offers:Lower Cost • lower solvent consumption• lower reagent consumption• less apparatusGreater Recoveries • min imal sample transferFaster Protocol • fewer stepsGreater Safety • less exposure to toxic agentsGreater Accuracy • no cross contaminationNo Emulsion Problems • less sample handling• fewer stepsNo Transporting of Samples to Lab • direct field samplingReduced Harm to Labile Samples • minimal evaporationMinimal Glass Breakage • less glassware used, less to washAchieving Sample Preparation Objectives with Solid-Phase Extraction [SPE]▪To remove sample constituents that elute after the analytes of interest or are strongly adsorbed:▪use solid-phase extraction with sorbent surface chemistry that is the same as that in the analytical HPLC column.▪tailor the gradient steps to elute analytes selectively.▪To remove sample constituents that coelute with an analyte of interest:▪use solid-phase extraction with sorbent surface chemistry and/or separation mode different from that in the analytical column.▪tailor the gradient steps to elute analytes selectively.▪To enrich sample components present in low concentration:▪tailor the gradient steps to elute analytes selectively.▪use "large" sample volumes in adsorption-promoting solvent.▪use "small" collection volume in desorption-promoting solvent.▪use sorbent chemistry tailored to the analyte, independent of that in analytical column.▪carefully choose chemistry of solid-phase extraction column so further sample prep will be unnecessary.▪To desalt samples:▪first, adsorb analytes on reversed-phase sorbent while salt breaks through unretained.▪then, after using water to wash away residual salt, desorb analytes using water-miscible organic solvent.▪To exchange solvents:▪adsorb the sample completely onto a strongly retentive sorbent and flush away the original solvent with a weaker eluent.▪elute the analyte with the desired solvent.▪To fractionate classes of compounds:▪use a step-gradient sequence to divide a sample-on the basis of hydrophobicity, polarity, or charge-into fractions containing groups of analytes that share common properties.▪To derivatize analytes using solid-phase reagents:▪adsorb a derivatization reagent on the surface of the sorbent; then, collect the sample (usually a gas) under conditions that favor complete adsorption of the analyte; wait for the reaction to occur and then selectively elute the derivative.SPE is ChromatographyKeep in mind that solid-phase extraction has the same fundamental basis as HPLC. Any knowledge of the chromatographic behavior of the analytes of interest, and of other matrix components, can help in choosing the proper sorbent and eluents. If, for example, you know that certain chromatographic conditions provide excellent separation of your analyte from interferences, then you may choose a similar SPE sorbent and solvent combination. Similarly, if you are trying to remove an interference that coelutes in HPLC, then you know a priori that similar SPE conditions will not be successful.General Elution ProtocolsThere are two general strategies for isolating and cleaning up sample components of interest:▪adsorb matrix interferences while components of interest pass through the cartridge unretained.▪adsorb components of interest while matrix interferences pass through the cartridge unretained.The first strategy is usually chosen when the desired sample component is present in high concentration. When components of interest are present at low levels, or multiplecomponents of widely differing polarities need to be isolated, then the second strategy is generally employed. Trace enrichment of compounds present at extremely low levels and concentration of dilute samples are also achieved by the second strategy.Steps of a Solid-Phase Extraction ProcedureThe following section describes the steps involved in a complete solid-phase extraction procedure. In many applications, one or more of the steps, listed below and subsequently described by general examples, can be omitted, thereby simplifying the procedure. The procedures illustrated here use samples containing dyes so that separations may be easily visualized. Keep in mind that most samples contain colorless components that require some type of detector or test to locate them in the collected fractions. Use the following information as a guideline in the development of your own procedure or when modifying procedures published in the literature.1.Pretreatment of the sample2.Conditioning of the cartridge3.Loading the sample4.Elution of the fractionsPrincipal Separation Modes in Solid-Phase Extraction [SPE]Normal-Phase ChromatographyThis mode is classically used to separate neutral organic compounds whose chemical nature ranges from hydrophobic to moderately polar.To perform normal-phase chromatography with SPE cartridges, use a step gradient of nonpolar solvents with a polar packing material.1.Condition the cartridge with six to ten hold-up volumes of non-polar solvent, usually thesame solvent in which the sample is dissolved.2.Load the sample solution onto the cartridge bed.3.Elute unwanted components with a non-polar solvent.4.Elute the first component of interest with a more polar solvent.5.Elute remaining components of interest with progressively more polar [stronger]solvents.6.When you recover all of your components, discard the used cartridge in a safe andappropriate manner.This procedure is illustrated in the figure below for a sample containing a mixture of three neutral, relatively non-polar organic dyes [yellow, red, and blue] that appears black when initially loaded onto the cartridge bed.Illustration of a General Elution Protocol for Normal-Phase Chromatography on SPECartridges(Silica, Florisil, Alumina, Diol, CN, NH2)Reversed-Phase ChromatographyBecause of the multiplicity of aqueous samples spanning a breadth of applications from environmental water to fruits and vegetables, from beverages to biological fluids, reversed-phase chromatography has become the predominant mode of SPE.To perform reversed-phase chromatography with SPE cartridges, use a gradient of strongly to weakly polar solvents [from weak to strong solvent elution strength] with a non-polar packing material.1.Solvate the silica-bonded phase or polymer packing with six to ten hold-up volumes ofmethanol or acetonitrile. Flush the cartridge with six to ten hold-up volumes of water or buffer. Do not allow the cartridge to dry out [unless using HLB].2.Load the sample dissolved in a strongly polar [weak] solvent [typically water].3.Elute unwanted components with a strongly polar solvent.4.Elute weakly retained components of interest with a less polar solvent.5.Elute more tightly bound components with progressively more non-polar [stronger]solvents.6.When you recover all the components of interest, discard the used cartridge in a safe andappropriate manner.This procedure is illustrated in the figure below for a sample of an aqueous grape drink containing two polar food dyes [red and blue], as well as sugar and artificial flavor [but no real grape juice!]. As prepared, this drink appears light purple in a glass, since the dye concentration is dilute. When a portion is loaded onto a prepared SPE cartridge, the strongly retained dyes become concentrated near the inlet in a dark purple band.Illustration of a General Elution Protocol for Reversed-Phase Chromatography on SPECartridges(C18, tC18, C8, CN, Diol, HLB, Porapak RDX, NH2)Ion-Exchange ChromatographyCompounds that are ionic or ionizable are often best isolated using some form ofion-exchange chromatography. This separation mode is orthogonal to the more widely used normal-phase and reversed-phase modes and provides a powerful, selective second dimension to sample preparation protocols.Illustration of the Two Major Types of Phases—Anion and Cation Exchange—and How They Selectively Attract and Retain Molecules of Opposite ChargeTo perform ion-exchange chromatography with SPE cartridges, use a gradient of pH or ionic strength with an ion exchange packing material.1.Condition the cartridge with six to ten hold-up volumes of deionized water or weak buffer.2.Load the sample dissolved in a solution of deionized water or buffer.3.Elute unwanted, weakly bound components with a weak buffer.4.Elute the first component of interest with a stronger buffer (change the pH or ionicstrength).5.Elute other components with progressively stronger buffers.6.When you recover all of your components, discard the used cartridge in an appropriatemanner.This procedure is illustrated in the figure below for a sample of an aqueous mixture of two ionic dyes with different pK a values. When loaded onto the cartridge, both are strongly retained, and the combination of blue and yellow components appears as a green band near the inlet.Illustration of General Elution Protocol for Ion-Exchange Chromatography on SPE Cartridges (NH2, Accell™ Plus QMA, Accell Plus CM, SCX, SAX, WCX, WAX)Cation and anion exchangers are further categorized as either weak or strong exchangers, depending upon the type of ionic group on their surface. Strong cation exchangers possess an acidic surface moiety such as a sulfonic acid that is always ionized [negatively charged] over the whole pH range. Weak cation exchangers possess an acidic surface moiety such as a carboxylic acid that is negatively charged at high pH but neutral at low pH. Similarly, strong anion exchangers typically bear quaternary ammonium groups that are always positively charged, while weak anion exchangers possess primary, secondary, or tertiary amine groups that may be positively charged at low pH but neutral at high pH.Use the following table as a guideline to choose the appropriate SPE ion-exchange cartridgetype for your particular analyte.Mixed-mode ion exchange chromatography combines the use of reversed-phase andion-exchange modes into a single protocol on a single SPE cartridge. It can be used to isolate and separate neutral, acidic, and basic compounds from a single complex matrix. An ideal mixed-mode SPE sorbent substrate remains water-wettable while exhibiting strong reversed-phase retention of hydrophobic compounds. On its surface are ion-exchange functionalities of one of the four general types just described above. Intermediate washes with organic solvent mixtures of appropriate elution strength may be used to isolate neutral compounds [including ionizable analytes in their neutral state]. Selective elution of ionically bound analytes may be attained by manipulating the charge of either the analyte [when bound to strong ion exchangers] or of the sorbent [for analytes bound to weak ion exchangers].下表汇总了各种SPE模式,为方法开发工作的开展提供了一个良好的起点:固相萃取选择色谱模式及吸附剂分析物中-低极性低-高极性/中性带电荷、可电离分离机制基于疏水性的分离基于极性的分离基于电荷的分离样品基质水溶液非极性有机溶剂水溶液/低离子强度SPE吸附剂的活化/平衡1. 用极性有机溶剂得到的溶剂化物2. 水非极性有机溶剂低离子强度缓冲液初步冲洗步骤水溶液/缓冲液非极性有机溶剂低离子强度缓冲液洗脱步骤增加极性有机溶剂的含量增加混合有机溶剂的洗脱强度更强的缓冲液——通过调节离子强度或pH值而中和电荷AX[阴离子交换]CX[阳离子交换]吸附剂官能团C18, tC18, C8, tC2,CN, NH2, HLB, RDX,Rxn RPSilica, Alumina,Florisil, Diol, CN,NH2Accell Plus QMA,NH2, SAX, MAX,WAXAccell Plus CM,SCX, MCX, WCX,Rxn CX吸附剂表面极性低至中等高至中等高高典型溶剂的极性范围高至中等低至中等高高典型的上样溶剂水、低强度缓冲液正己烷、氯仿、二氯甲烷水、低强度缓冲液水、低强度缓冲液典型的洗脱溶剂MeOH/水、CH3CN/水乙酸乙酯、丙酮、CH3CN缓冲液、高离子强度的盐类,提高pH缓冲液、高离子强度的盐类,降低pH样品洗脱顺序最大极性样品组分最先洗脱出来最弱极性样品组分最先洗脱出来最弱电离样品组分最先洗脱出来最弱电离样品组分最先洗脱出来为洗脱化合物而做的流动相溶剂改变减弱溶剂极性增强溶剂极性增加离子强度或提高pH值增加离子强度或降低pH值This has been a brief introduction to sample enrichment and purification using solid-phase extraction [SPE]. The best way to start using SPE is to first learn what others have done with analytes and/or matrices similar to those of interest to you. You will find > 7,700 references to the use of SPE in the Resource Library on . Fill in the blank with a partial compound or matrix name in the following search phrase:“Sep-Pak” OR “Oasis” AND ______*NOTE: Rather than risk a spelling error, use an asterisk [*] with a root name for best results. Using this same search string, even more references [> 60,000] may be found on GOOGLE Scholar.Further reading:J.C. Arsenault and P.D. McDonald, Beginners Guide to Liquid Chromatography, Waters [2007]; Order P/N 715001531on P.D. McDonald and E.S.P. Bouvier, A Sample Preparation Primer and Guide to Solid-Phase Extraction Methods Development, Waters [2001] Search for WA20300 on Waters, Purity by SPE [2008]; Search for 720001692en on U.D. Neue, P.D. McDonald, Topics in Solid-Phase Extraction. Part 1. Ion Suppression inLC/MS Analysis: A Review. Strategies for its elimination by well-designed, multidimensional solid-phase extraction [SPE] protocols and methods for its quantitative assessment [2005]; Search for 720001273en on 。

SPE 固相萃取技术手册一:前言1.1 引言1.2 目的和范围1.3 目标读者二:概述2.1 SPE 固相萃取技术简介2.2 SPE 技术的应用领域2.3 SPE 技术的优势和局限性三:SPE 固相萃取原理3.1 SPE 固相萃取的基本原理3.2 SPE 固相萃取的工作流程3.2.1 样品制备和预处理3.2.2 萃取柱的选择与操作3.2.3 萃取条件的优化3.2.4 萃取物的洗脱和收集3.2.5 萃取物的后处理四:SPE 固相萃取常用技术和方法4.1 正相固相萃取4.1.1 正相固相萃取原理4.1.2 正相固相萃取方法和步骤4.2 反相固相萃取4.2.1 反相固相萃取原理4.2.2 反相固相萃取方法和步骤4.3 隐极固相萃取4.3.1 隐极固相萃取原理4.3.2 隐极固相萃取方法和步骤4.4 离子交换固相萃取4.4.1 离子交换固相萃取原理4.4.2 离子交换固相萃取方法和步骤五:示例应用和操作指南5.1 环境水样中有机污染物的分析5.2 食品中农药残留的分析5.3 药物代谢物在生物体内的测定5.4 生物样品中的蛋白质富集六:质量控制和数据分析6.1 样品前处理和质量控制6.2 数据处理和分析附录一:SPE 固相萃取操作步骤示意图附录二:常用的 SPE 固相萃取柱类型和性能参数表注释:1. SPE - 固相萃取(Solid Phase Extraction)技术是一种常用于分离和富集溶液中目标分析物的方法。
2. 正相固相萃取是指固相萃取柱中填充了亲水性材料,用于吸附疏水性的目标分析物。
3. 反相固相萃取是指固相萃取柱中填充了疏水性材料,用于吸附亲水性的目标分析物。
4. 隐极固相萃取是指固相萃取柱中填充了阳离子或阴离子交换材料,用于吸附带电目标分析物。
5. 离子交换固相萃取是指固相萃取柱中填充了具有离子交换功能的聚合物或树脂材料,用于吸附特定离子性目标分析物。
本文档涉及附件:1. 附录一:SPE 固相萃取操作步骤示意图2. 附录二:常用的 SPE 固相萃取柱类型和性能参数表本文所涉及的法律名词及注释:1. SPE - 固相萃取(Solid Phase Extraction)技术:一种常用于分离和富集溶液中目标分析物的方法。


06)SPE基础原理及应用SPE(固相萃取)技术是一种用于分离、浓缩和提取化合物的方法,其基本原理和应用非常广泛。
本文将详细介绍SPE的基础原理以及其在实际应用中的一些典型场景。
SPE的基础原理可以概括为四个步骤:样品预处理、固相萃取柱的条件设定、萃取过程以及萃取物的回收和分析。
首先是样品预处理。
样品预处理是指将待分析的样品进行预处理,包括固体样品的研磨、液体样品的稀释等。
这一步骤的目的是使样品更适合于后续的固相萃取。
其次是固相萃取柱的条件设定。
在这一步骤中,需要选择合适的固相填料,调整样品的pH、离子强度和有机溶剂的成分等因素,以使目标化合物能够与固相填料发生适当的相互作用。
不同的固相填料适用于不同类型的化合物,常见的填料有C18、C8、C2、环糊精、离子交换树脂等。
接下来是萃取过程。
样品经过预处理后,可以将其通过SPE柱进行处理。
样品溶液被加至固相柱中,随后可以使用不同的洗涤剂和淋洗溶剂,以去除干扰物或其他非目标化合物。
萃取时间、洗涤剂和淋洗溶剂的选择,对于分离和富集目标化合物都至关重要。
最后是萃取物的回收和分析。
萃取物被从柱洗脱后,可以通过吹扫、浓缩、浓缩替代剂、超滤等方法,使其适合后续的分析。
SPE方法通常与色谱、质谱等分析方法相结合,以分离和定量所需的目标化合物。
SPE技术在实际应用中有着广泛的应用场景,以下是几个典型的应用示例:1.环境污染物的分析:SPE方法可以用于水体、土壤、空气等环境样品中的有机污染物分析。
例如,可以使用SPE柱来去除样品中的溶剂残留、重金属离子或其他干扰物质,从而实现对目标污染物的富集和分析。
2.食品和饮料分析:SPE方法被广泛应用于食品和饮料行业中,以检测其中的农药残留、食品添加剂、毒素等。
该技术可以对样品中的化合物进行快速、高效、选择性的富集和分离,从而提高分析的准确性和可靠性。
3.制药和生物医学领域:SPE方法在制药和生物医学领域中也有着广泛的应用。
例如,可以使用SPE技术从血液、尿液等生物样品中提取和富集药物、代谢产物以及其他生物标志物,以支持药物代谢研究、药物监测等方面的工作。


固相萃取的培训资料xx年xx月xx日CATALOGUE目录•固相萃取简介•固相萃取的实验流程•固相萃取实验操作•固相萃取的应用案例•固相萃取的未来发展•固相萃取常见问题及解决方案01固相萃取简介固相萃取是一种样品预处理技术,通过固体吸附剂与目标分析物之间的相互作用,实现目标分析物与基体分离、富集和净化。
固相萃取可用于多种样品类型,如水样、环境空气样、生物样品等。
固相萃取主要基于吸附和解吸的原理,通过使用具有特定吸附性能的吸附剂,实现对目标分析物的吸附和与基体分离。
固相萃取的吸附剂通常包括C18硅胶、氧化铝、活性炭等。
固相萃取在样品预处理中具有广泛应用,可用于分离和富集水样中的有机物、环境空气样中的有害物质、生物样品中的药物和代谢物等。
固相萃取可与其他分析方法联用,如高效液相色谱、气相色谱等,提高分析方法的灵敏度、准确度和可靠性。
02固相萃取的实验流程收集具有代表性的样品,并进行必要的预处理,如去除杂质、破碎、溶解等,以便后续萃取过程顺利进行。
样品收集将样品通过过滤装置,以去除其中的颗粒物和大分子物质,确保萃取柱的堵塞和萃取效果。
样品过滤样品预处理萃取剂选择根据样品的性质和目标分析物,选择合适的萃取剂,如有机溶剂、离子液体等,以达到最佳的萃取效果。
萃取剂制备根据实际需要,对选择的萃取剂进行稀释或纯化,以得到适合的浓度和纯度。
萃取剂的选择和制备萃取温度通过调节温度来改善萃取效果,一般情况下,升高温度可以增加分析物的溶解度和扩散速度,但也可能导致萃取剂挥发和样品热分解。
搅拌速度搅拌速度可以增加萃取剂和样品之间的接触面积,提高萃取效率,但搅拌速度过快可能导致设备磨损和能耗增加。
静置时间静置时间是保证萃取剂和样品充分接触和溶解的重要因素,但静置时间过长可能导致萃取剂挥发和样品分解。
萃取时间延长萃取时间可以增加分析物的溶解和扩散,但也可能导致萃取剂挥发和样品分解。
因此需要合理选择萃取时间。
萃取条件的优化1萃取产物的处理23将萃取产物进行浓缩处理,以去除大部分萃取剂,得到较为纯净的分析物溶液。

SPE入门指南(固相萃取)一种可提高样品制备水平的强有力工具作为一位分析科学家,当您在决定哪些工具最能帮助您获得所需的结果时必然面临诸多挑战。
选择使用哪些样品制备工具和方法是需要考虑的重点事项,它关乎到您的分析结果成功与否。
理想情况下,如果无需进行任何样品制备,那么您将会很高兴。
然而事实上,样品制备通常是必不可少的程序。
对于现有的样品您可能需要优化样品制备方法,以提高分析通量或降低每次分析所需的成本。
或者,您可能需要分析种类繁多的样品用以报告新型目标化合物。
每种新的样品类型都可能存在不同的分析挑战。
此外,科学家目前正面临着在不影响准确度和精确度的情况下报告前所未有更低浓度化合物检测值的严峻挑战。
我们希望帮助您探索并了解一种功能极其强大的样品制备工具:固相萃取(SPE)技术。
您将领会这项技术如何通过使用包含色谱填充材料的装置来帮助您应对分析挑战。
固相萃取的定义SPE通常是一种使用装在柱芯型装置内的固体颗粒和色谱填充材料对样品中的不同组分进行化学分离的样品制备技术。
样品几乎始终呈液态,但特殊应用项目可能使用气相中的某些样品进行。
图1显示出一份样品(图示为黑色)正在使用SPE装置进行处理,以使组成该样品的每种染色组分通过色谱分析实现分离。
图1:SPE方法示例色谱床可以用来分离一份样品中的不同组分,从而使后续的分析测试更容易成功。
例如,SPE常用来选择性地除去干扰物。
从技术角度对此项技术给出的准确命名应该是“液相-固相萃取”;这是因为色谱颗粒是固体,而样品则呈液态。
此处所用液相色谱的基本色谱原理与高效液相色谱相同,只是使用形式与使用原因存在差异。
此处使用色谱是为了在将样品提交分析测试之前对其进行较好的制备。
在样品制备方面,样品的来源非常广泛。
它们可能是生物样本(如:血浆、唾液或尿液)、环境样品(如:水、空气或土壤)、食品(如:谷物、肉和海鲜)、药品、营养添加剂食品、饮料或工业产品。
甚至蚊虫的头部也可能是样品!当科学家需要分析从蚊虫脑部提取出的神经肽时,SPE是首选的样品制备方法(沃特世应用数据库,1983年)。

一、固相萃取基本原理与操作1、固相萃取吸附剂与目标化合物之间的作用机理固相萃取主要通过目标物与吸附剂之间的以下作用力来保留/吸附的1)疏水作用力:如C18、C8、Silica、苯基柱等2)离子交换作用:SAX, SCX,COOH、NH2等3)物理吸附:Florsil、Alumina等2、p H值对固相萃取的影响pH值可以改变目标物/吸附剂的离子化或质子化程度。
对于强阳/阴离子交换柱来讲,因为吸附剂本身是完全离子化的状态,目标物必须完全离子化才可以保证其被吸附剂完全吸附保留。
而目标物的离子化程度则与pH值有关。
如对于弱碱性化合物来讲,其pH值必须小于其pKa 值两个单位才可以保证目标物完全离子化,而对于弱酸性化合物,其p H值必须大于其pKa值两个单位才能保证其完全离子化。
对于弱阴/阳离子交换柱来讲,必须要保证吸附剂完全离子化才保证目标物的完全吸附,而溶液的pH值必须满足一定的条件才能保证其完全离子化。
3、固相萃取操作步骤及注意事项针对填料保留机理的不同(填料保留目标化合物或保留杂质),操作稍有不同。
1)填料保留目标化合物固相萃取操作一般有四步(见图1):Ø 活化---- 除去小柱内的杂质并创造一定的溶剂环境。
(注意整个过程不要使小柱干涸)Ø 上样---- 将样品用一定的溶剂溶解,转移入柱并使组分保留在柱上。
(注意流速不要过快,以1ml/min为宜,最大不超过5ml/min)Ø 淋洗---- 最大程度除去干扰物。
(建议此过程结束后把小柱完全抽干)Ø 洗脱---- 用小体积的溶剂将被测物质洗脱下来并收集。
(注意流速不要过快,以1ml/min为宜)此主题相关图片如下11.jpg:2)填料保留杂质固相萃取操作一般有三步(见图2):Ø 活化--除去柱子内的杂质并创造一定的溶剂环境。
(注意整个过程不要使小柱干涸)Ø 上样--将样品转移入柱,此时大部分目标化合物会随样品基液流出,杂质被保留在柱上,故此步骤要开始收集(注意流速不要过快)Ø 洗脱---用小体积的溶剂将组分淋洗下来并收集,合并收集液。

固相萃取-高效液相色谱法测定水溶液中的腺嘌呤核苷【实验目的】1. 掌握固相萃取前处理样品的基本步骤及方法2. 熟悉高效液相色谱仪的一般使用方法【实验原理】固相萃取(SPE)分离是利用固体吸附剂将液体样品中的目标化合物吸附,使其与样品的基体或干扰化合物分离,然后再用适当的溶剂洗除杂质,最后用适当溶剂洗脱目标化合物从而达到分离和富集目标化合物的目的。
腺嘌呤核苷是生物细胞维持生命活动的基本组成元素之一,参与DNA的代谢过程,在体内许多系统和组织有着广泛的生理学作用,具有抗肿瘤、抗病毒、基因治疗等多种生物活性。
本实验利用固相萃取—高效液相色谱的方法,实现了水溶液中的腺嘌呤核苷与其结构类相似物2’-脱氧鸟嘌呤核苷,鸟嘌呤核苷,胞7嘧啶核苷,尿嘧啶核苷,胸腺嘧啶核苷的定量分离。
当样品杂质的极性>目标物的极性,用反相模式。
采用固相萃取C18柱。
洗脱剂的选择,洗杂质、目标物时极性相似。
目标物与杂质同时吸附,而样品中杂质的极性>目标物的极性时,先用极性大的溶剂,将杂质洗下来(15%甲醇),再用极性小的溶剂洗脱目标物(50%甲醇)。
必须活化,才能与溶质产生重线性。
先用强溶剂预洗,以消除小柱上在生产或储存过程中可能带入的污染物,避免在色谱图上出现与样品无关得杂质峰。
反相选甲醇。
然后再用较弱的甲醇-水,洗脱润湿小柱,从而保证样品在柱上有足够的保留与回收率。
润湿能打开卷曲在硅胶表面上的烷基链,使溶质与键合相之间充分接触。
上样溶剂:水;淋洗:5~10弱溶剂(水-缓冲液或甲醇-水);洗脱剂:溶剂强度>上样溶剂,用小体积洗目标产物。
分别接洗脱后溶液,进色谱C18柱,极性小的后出柱。
【仪器和试剂】高效液相色谱仪;ODS色谱柱;固相萃取仪;C18固相萃取柱;腺嘌呤核苷(A),2’-脱氧鸟嘌呤核苷(2’-dG),鸟嘌呤核苷(G),胞嘧啶核苷(C),尿嘧啶核苷(U),胸腺嘧啶核苷(T),甲醇,混合磷酸盐缓冲液等。
【实验步骤】1. 色谱条件色谱柱:ODS柱(250×4.6mmID, 5 m);流动相:12.5%甲醇+PH4.0磷酸盐缓冲液;检测波长:260nm;柱温:25℃;流速:1.0mL/min;进样量:20μL。
