- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Con
HOMO
disrotatory
Examples
Ground State Conrotatory
Excited State Disrotatory
Con
LUMO
HOMO
Disrotatory
Conrotatory
Activation Energy (kcal/mol) for electrocyclic ring opening
dis-out
favorable H Me R A
Three-Atom Electrocyclizations (4 electrons)
H H A H A Dis?? R C H A Con?? R H A A H
relative rate
1
4
40,000
Ψ3
Ring-fused Cyclopropyl Systems When the cis substiltutents on the cyclopropyl ring are tied together in a ring the following observsations have been made
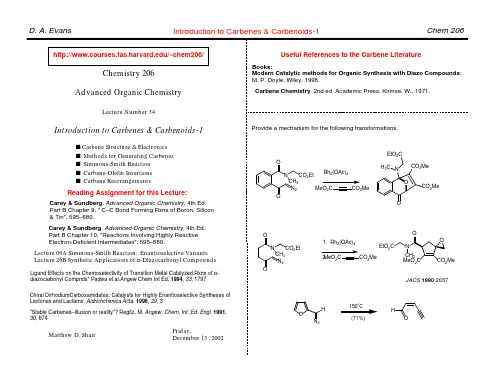
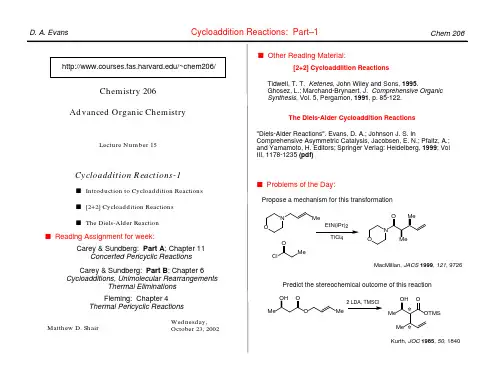
D. A. Evans
Pericyclic Reactions: Part–2
s Other Reading Material:
Chem 206
/~chem206/
Chemistry 206 Advanced Organic Chemistry
Electrocyclic Processes-1
Chem 206
Controtation and on to the indicated bonding and anti-bonding orbitals of cyclobutene:
LUMO
Excited State (Photochemical Process) disrotatory conrotatory
A H R A H Dis?? R C H A
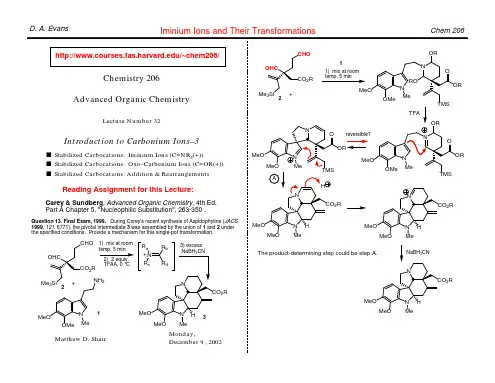
Does solvolysis proceed via cation 1 followed by rearrangement to 2 (Case 1), or does it proceed directly to 2 (Case 2)?
Ψ3 fast +X– 2 Me H H Case 2 Ψ2 nonbonding X
The Nazarov Reaction
O OH +H+ A A A A f f A –H+ A O R Con?? A A
p
Chem 206
R
p
R Dis?? A A
p
A
A
Denmark, S. E. In Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 5; pp 751. O O +H
Predict the stereochemical outcome of this reaction.
Ph O O O Ph
h
O O
s Reading Assignment for week: Carey & Sundberg: Part A; Chapter 11 Concerted Pericyclic Reactions Fleming: Chapter 4 Thermal Pericyclic Reactions
Evans, Breit
Electrocyclic Processes-2: Torquoselectivity
How do we explain?
R R R
Chem 206
Donor substituents prefer con–out mode Pi acceptor substituents prefer con–in mode
Torquoselectivilty is defined as the predisposition of a given R substituent for a given conrotatory motion Houk et al. Acc. Chem. Res 1996, 29, 471
R
con in
Houk, et. al. Acc. Chem. Res. 1996, 29, 471-477. Houk, et. al. JOC. 1996, 61, 2813-2825. Monday, Columbus Day, October 14, 2002
Ph
heat
h
Huisgen, TL, 1964, 3381.
View the 2 conrotatory modes by looking at the breaking sigma bond from this perspective
H
con
H
out
R R H H
Examples:
Donor substituents prefer con–out mode Pi acceptor substituents prefer con–in mode
HOMO Dis
C
Note that there are two disrotatory modes
R R X Dis R X R R Dis R Sterically favored R Favored for R = ring R R LUMO
X
Me H Me C C H
HOMO Dis
+
H
R
+
+X–
X
TsO Ψ1 cation anion H
TsO H
Me
TsO H
H Me
H Me
relative rate 1
LUMO
4
DePuy, Accts. Chem. Res. 1967, 1, 33
40,000
+
R
H A H A
R Dis
+
H A C H C A
HOMO
X
LUMO
H Me H C Me Me
Ph
O
Suggest a mechanism for the following reaction.
H CO2Me MeO2C CO2Me H
heat
H CO2Me
Matthew D. Shair
H
Bloomfield, TL, 1969, 3719.
Evans, Breit
Electrocyclic Reaction - Selection Rules Ground State (Thermal process) 4n π e(n = 1,2...) 4n+2 π e(n = 0,1,2...) conrotatory
27
Disrotatory
O Ph
Conrotatory
Disrotatory
Ph O
O
O O
Disrotatory
Conrotatory
Ph Ph O O O
R Con R R R R
R
Con
R
Ph O
O
O
Ph
R Sterically favored
Ph Huisgen, TL, 1964, 3381. Ph O
O Cl base O– Cl –Cl– 3-exo-tet disallowed O– O
dis-in
•• Ar N
H CO2Me
MeO2C products
•• (–)
Evans, Breit
Five-Atom Electrocyclizations (4 electrons)
Electrocyclic Processes-3
Activation Energy (kcal/mol) for electrocyclic ring opening
Conrotatory
Disrotatory
42
45
Disrotatory Conrotatory
Conrotatory
H
29
H Criegee, Chem. Ber. 1968, 101, 102.
s Woodward-Hoffmann Theory R. B. Woodward and R. Hoffmann, The Conservation of Orbital Symmetry, Verlag Chemie, Weinheim, 1970. s Frontier Molecular Orbital Theory I. Fleming, Frontier Orbitals and Organic Chemical Reactions, John-Wiley and Sons, New York, 1976. s Dewar-Zimmerman Theory T. H. Lowry and K. S. Richardson, Mechanism and Theory in Organic Chemistry, 3rd Ed., Harper & Row, New York, 1987. s General Reference R. E. Lehr and A. P. Marchand, Orbital Symmetry: A Problem Solving Approach, Academic Press, New York, 1972.
Me H
R
R
H H
Me Me
Evans, Breit