无机物质热力学数据大全
- 格式:pdf
- 大小:111.33 KB
- 文档页数:3

化学化工物性数据手册无机卷中数据引言概述:化学化工物性数据手册是一种重要的工具,用于提供无机化学物质的相关数据。
无机卷中的数据是指无机化合物的物理和化学性质的详细描述。
本文将详细介绍化学化工物性数据手册无机卷中的数据,包括物理性质、化学性质、热力学性质、电化学性质和毒理学性质。
一、物理性质:1.1 密度:物质的密度是指单位体积的质量,通常以克/立方厘米或千克/立方米表示。
数据手册中提供了无机化合物的密度值,以帮助科学家和工程师进行实验设计和工艺开发。
1.2 熔点和沸点:熔点是指物质从固态转变为液态的温度,沸点是指物质从液态转变为气态的温度。
数据手册中列出了无机化合物的熔点和沸点,这对于合成和分离纯化过程的控制非常重要。
1.3 溶解性:溶解性是指物质在特定温度和压力下在溶剂中的溶解程度。
数据手册提供了无机化合物在不同溶剂中的溶解度数据,这对于溶解度计算和溶液的配制非常有用。
二、化学性质:2.1 酸碱性:酸碱性是指物质在水中的离子化程度,通常以pH值表示。
数据手册中提供了无机化合物的酸碱性数据,这对于酸碱中和反应和pH调节非常重要。
2.2 氧化还原性:氧化还原性是指物质在化学反应中的电子转移能力。
数据手册中提供了无机化合物的氧化还原电位和氧化还原反应的条件,这对于电化学反应和电池设计非常有用。
2.3 反应性:反应性是指物质与其他物质发生化学反应的倾向性。
数据手册中提供了无机化合物的反应类型和反应条件,这对于化学合成和反应工程的设计非常重要。
三、热力学性质:3.1 热容和焓:热容是指物质在吸热或放热过程中温度变化的响应能力,焓是指物质在恒定压力下吸热或放热的能力。
数据手册中提供了无机化合物的热容和焓值,这对于热力学计算和热力学过程的分析非常重要。
3.2 热导率:热导率是指物质传导热量的能力。
数据手册中提供了无机化合物的热导率值,这对于热传导计算和热传导过程的控制非常有用。
3.3 相变热:相变热是指物质在相变过程中吸热或放热的能力。
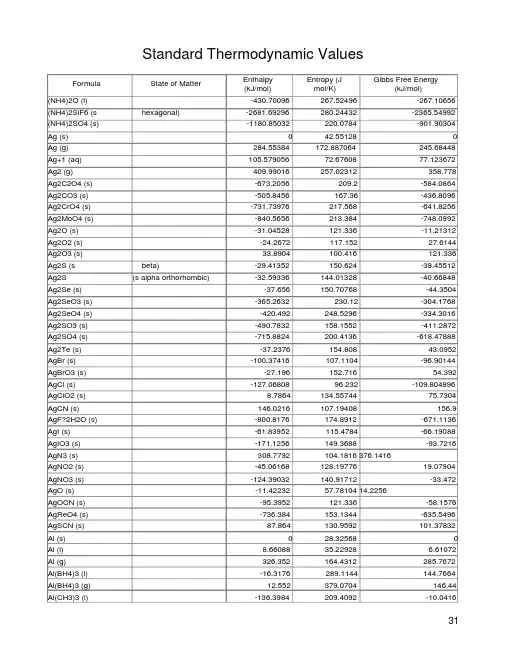
Standard Thermodynamic ValuesFormula State of Matter Enthalpy(kJ/mol)Entropy (Jmol/K)Gibbs Free Energy(kJ/mol)(NH4)2O (l) -430.70096267.52496 -267.10656 (NH4)2SiF6 (s hexagonal) -2681.69296280.24432 -2365.54992 (NH4)2SO4 (s) -1180.85032220.0784 -901.90304 Ag (s) 042.55128 0 Ag (g) 284.55384172.887064 245.68448 Ag+1 (aq) 105.57905672.67608 77.123672 Ag2 (g) 409.99016257.02312 358.778 Ag2C2O4 (s) -673.2056209.2 -584.0864 Ag2CO3 (s) -505.8456167.36 -436.8096 Ag2CrO4 (s) -731.73976217.568 -641.8256 Ag2MoO4 (s) -840.5656213.384 -748.0992 Ag2O (s) -31.04528121.336 -11.21312 Ag2O2 (s) -24.2672117.152 27.6144 Ag2O3 (s) 33.8904100.416 121.336 Ag2S (s beta) -29.41352150.624 -39.45512 Ag2S (s alpha orthorhombic) -32.59336144.01328 -40.66848 Ag2Se (s) -37.656150.70768 -44.3504 Ag2SeO3 (s) -365.2632230.12 -304.1768 Ag2SeO4 (s) -420.492248.5296 -334.3016 Ag2SO3 (s) -490.7832158.1552 -411.2872 Ag2SO4 (s) -715.8824200.4136 -618.47888 Ag2Te (s) -37.2376154.808 43.0952 AgBr (s) -100.37416107.1104 -96.90144 AgBrO3 (s) -27.196152.716 54.392 AgCl (s) -127.0680896.232 -109.804896 AgClO2 (s) 8.7864134.55744 75.7304 AgCN (s) 146.0216107.19408 156.9 AgF?2H2O (s) -800.8176174.8912 -671.1136 AgI (s) -61.83952115.4784 -66.19088 AgIO3 (s) -171.1256149.3688 -93.7216 AgN3 (s) 308.7792104.1816 376.1416AgNO2 (s) -45.06168128.19776 19.07904 AgNO3 (s) -124.39032140.91712 -33.472 AgO (s) -11.4223257.78104 14.2256AgOCN (s) -95.3952121.336 -58.1576 AgReO4 (s) -736.384153.1344 -635.5496 AgSCN (s) 87.864130.9592 101.37832 Al (s) 028.32568 0 Al (l) 8.6608835.22928 6.61072 Al (g) 326.352164.4312 285.7672 Al(BH4)3 (l) -16.3176289.1144 144.7664 Al(BH4)3 (g) 12.552379.0704 146.44 Al(CH3)3 (l) -136.3984209.4092 -10.0416Al(NO3)3?6H2O (s) -2850.47552467.7712 -2203.88016 Al(NO3)3?9H2O (s) -3757.06464569.024 -2929.6368 Al(OH)3 (s) -1284.48871.128 -1305.8264 Al+3 (aq) -531.368-321.7496 -485.344 Al2(CH3)6 (g) -230.91496524.6736 -9.79056 Al2(SO4)3 (s) -3435.064239.3248 -3506.6104 Al2Br6 (g) -1020.896547.2672 -947.2576 Al2Cl6 (g) -1295.3664475.5116 -1220.8912 Al2F6 (g) -2631.736387.02 -2539.688 Al2I6 (g) -506.264584.0864 -560.656Al2O (g) -131.3776259.408 -161.084Al2O3 (l) -1581.133689.57944 -1499.25272 Al2O3 (s gamma-corundum) -1656.86459.8312 -1562.724Al2O3 (s alpha-corundum) -1675.273650.91928 -1581.9704 Al2O3?3H2O (s gibbsite) -2562.7140.20584 -2287.3928 Al2O3?H2O (s boehmite) -1974.84896.8596 -1825.4792 Al2O3?H2O (s diaspore) -1999.95270.54224 -1840.96 Al2Si2O7?2H2O (s halloysite) -4079.8184203.3424 -3759.324 Al2Si2O7?2H2O (s kaolinite) -4098.6464202.924 -3778.152 Al2SiO5 (s andalusite) -2591.98893.3032 -2444.7112 Al2SiO5 (s kyanite) -2596.17283.80552 -2443.8744 Al2SiO5 (s sillimanite) -2593.243296.19016 -2442.6192Al4C3 (s) -207.27536104.6 -238.44616 Al4C3 (g) -215.894489.1192 -203.3424 Al6BeO10 (l) -5299.4544314.88784 -5034.1888 Al6BeO10 (s) -5624.1328175.56064 -5317.4456 Al6Si2O13 (s mullite) -6819.92274.8888 -6443.36 AlBO2 (g) -541.4096269.4496 -550.6144AlBr3 (s) -511.11744180.24672 -488.31464 AlBr3 (l) -501.20136206.4804 -486.26448 AlBr3 (g) -410.8688349.07112 -438.4832 AlC (g) 689.5232223.34192 633.0392 AlCl (g) -51.4632227.86064 -77.8224 AlCl2 (g) -288.696288.2776 -299.5744 AlCl3 (g) -584.5048314.30208 -570.07 AlCl3 (s) -705.6316109.28608 -630.06856 AlCl3 (l) -674.79552172.92472 -618.186AlCl3?6H2O (s) -2691.5672376.56 -2269.4016AlF (g) -265.2656215.0576 -290.788 AlF2 (g) -732.2263.1736 -740.568 AlF3 (s) -1510.42466.48376 -1430.928 AlF3 (g) -1209.176276.7716 -1192.8584 AlF3?3H2O (s) -2297.4344209.2 -2051.8336 AlH (g) 259.24064187.77792 231.166 AlI3 (l) -297.064219.66 -301.248 AlI3 (g) -205.016363.1712 -251.04AlI3 (s) -309.616189.5352 -305.432 AlN (s) -317.98420.16688 -287.0224 AlN (g) 435.136211.7104 410.032 AlO (g) 83.68218.27928 57.7392 AlOCl (s) -793.286454.392 -737.26264 AlOCl (g) -348.1088248.82248 -350.2008 AlOF (g) -586.5968234.26216 -587.0152 AlOH (g) -179.912216.3128 -184.096AlPO4 (s berlinite) -1692.009690.7928 -1601.2168 AlS (g) 200.832230.49656 150.2056 Ar (g) 0154.732688 0 Au (g) 366.1180.39316 326.352 Au (s) 047.40472 0 Au(CN)2-1 (aq) 242.2536171.544 285.7672 AuBr4-1 (aq) -191.6272335.9752 -167.36 AuCl4-1 (aq) -322.168266.9392 -237.31648 AuH (g) 294.972211.045144 265.684B (g) 562.748153.3436 518.816 B (s) 0 5.8576 0 B(CH3)3 (l) -143.0928238.9064 -32.2168 B(CH3)3 (g) -124.2648314.6368 -35.9824 B(OH)4-1 (aq) -1344.02632102.508 -1153.3196 B2 (g) 830.524201.79432 774.04 B2Cl4 (l) -523262.3368 -464.8424 B2H6 (g) 35.564232.0028 86.6088 B2O2 (g) -454.8008242.37912 -462.332 B2O3 (g) -843.78728279.7004 -831.9884 B2O3 (s amorphous) -1254.5305677.8224 -1182.3984 B2O3 (s) -1272.772853.9736 -1193.6952 B3N3H6 (l) -540.9912199.5768 -392.79392 B4C (s) -71.12827.11232 -71.128 B5H9 (l) 42.6768184.22152 171.66952 Ba (s) 062.3416 0 Ba (g) 179.0752169.99592 146.8584 Ba (l) 4.9789666.7348 3.84928 Ba(BrO3)2 (s) -752.65976242.672 -577.392 Ba(BrO3)2?H2O (s) -1054.7864292.4616 -824.62456 Ba(ClO3)2 (s) -680.3184196.648 -531.368 Ba(ClO4)2?3H2O (s) -1691.5912393.296 -1270.6808 Ba(IO3)2 (s) -1027.172249.3664 -864.8328 Ba(IO3)2?H2O (s) -1322.144297.064 -1104.1576 Ba(N3)2?H2O (s) -308.3608188.28 -105.0184 Ba(NO3)2 (s) -992.06824213.8024 -796.71728 Ba(OH)2?8H2O (s) -3342.1792426.768 -2793.2384 Ba(ReO4)2?4H2O (s) -3368.12376.56 -2918.34 Ba+2 (aq) -537.6449.6232 -560.73968Ba2TiO4 (s) -2243.0424196.648 -2133.0032 BaBr2 (s) -757.304146.44 -736.8024BaBr2 (g) -439.32330.536 -472.792 BaBr2?2H2O (s) -1366.076225.936 -1230.5144 BaCl2 (s) -858.1384123.67904 -810.4408 BaCl2 (l) -832.44864143.5112 -790.1484 BaCl2 (g) -498.7328325.64072 -510.69904 BaCl2?2H2O (s) -1460.13232202.924 -1296.45424 BaCO3 (s witherite) -1216.2888112.1312 -1137.6296BaCrO4 (s) -1445.9904158.5736 -1345.28152 BaF2 (s) -1208.757696.39936 -1158.5496 BaF2 (l) -1171.3108121.25232 -1128.38296 BaF2 (g) -803.7464301.16432 -814.49928 BaI2 (g) -302.9216348.1088 -353.42248 BaI2 (l) -585.88552183.6776 -587.39176 BaI2 (s) -605.4248165.14248 -601.40816 BaMoO4 (s) -1548.08138.072 -1439.7144 BaO (s) -548.10472.09032 -520.40592 BaO (l) -491.6296.56672 -471.24392 BaO (g) -123.8464235.35 -144.80824 BaS (s) -460.2478.2408 -456.056 BaSeO3 (s) -1040.5608167.36 -968.1776 BaSeO4 (s) -1146.416175.728 -1044.7448 BaSiF6 (s) -2952.2304163.176 -2794.0752 BaSiO3 (s) -1623.6012109.6208 -1540.25592 BaSO4 (s) -1473.1864132.2144 -1362.3104 BaTiO3 (s) -1659.7928107.9472 -1572.3472BaZrO3 (s) -1779.4552124.6832 -1694.52 BBr (g) 238.0696224.89 195.3928 BBr3 (g) -205.6436324.13448 -232.46304 BBr3 (l) -239.7432229.7016 -238.488BCl (g) 149.49432213.13296 120.9176 BCl2F (g) -645.1728284.512 -631.3656BCl3 (g) -403.756289.99304 -388.73544 BCl3 (l) -427.1864206.2712 -387.4384 BClF2 (g) -890.3552271.96 -876.1296Be (g) 324.26136.1892 286.604 Be (l) 12.0499216.5268 9.95792 Be (s) 09.53952 0 Be(OH)2 (s beta) -905.83646.024 -816.7168 Be+2 (aq) -382.836-129.704 -379.698Be2C (s) -117.15216.3176 -87.864 Be2SiO4 (s) -2149.320864.30808 -2032.5872 Be3N2 (s cubic) -588.270434.14144 -533.0416 BeAl2O4 (s) -2300.781666.27456 -2178.6088 BeBr2 (s) -369.8656106.2736 -353.1296BeC2 (g) 564.84218.4048 506.264 BeCl2 (s beta) -496.222475.81408 -449.52896 BeF2 (a alpha) -1026.753653.346 -979.4744 BeH (g) 326.7704170.87456 298.3192 BeI2 (s) -192.464120.4992 -209.2 BeO (s alpha) -608.353613.76536 -579.0656 BeO (g) 129.704197.52664 104.1816 BeO2-2 (aq) -790.776158.992 -640.152 BeSO4 (s alpha) -1205.201277.98976 -1093.86496 BeSO4?4H2O (s) -2423.74936232.96512 -2080.66136 BeWO4 (s) -1514.60888.36608 -1405.824 BF (g) -122.1728200.37176 -149.7872 BF3 (g) -1137.002254.01064 -1120.34968 BF4-1 (aq) -1574.8576179.912 -1486.9936 BH (g) 449.61264171.7532 419.61336 BH4-1 (aq) 48.15784110.4576 114.26504 BN (g) 647.474212.17064 614.50408 BN (s) -254.387214.81136 -228.4464 BO (g) 25.104203.42608 -4.184 BO2 (g) -300.4112229.45056 -305.8504 BO2-1 (aq) -772.3664-37.2376 -678.93768 Br (g) 111.884344174.91212 82.428984 Br-1 (aq) -121.545282.4248 -103.9724 Br2 (l) 0152.230656 0 Br2 (g) 30.907208245.353944 3.142184 Br2Cl-1 (aq) -170.2888188.6984 -128.4488 Br3-1 (aq) -130.41528215.476 -107.06856 BrCl (g) 14.644239.99424 -0.96232 BrF (g) -93.84712228.8648 -109.16056 BrF3 (l) -300.8296178.2384 -240.58 BrF3 (g) -255.60056292.41976 -229.45056 BrF5 (l) -458.5664225.0992 -351.8744 BrO (g) 125.77104237.442 108.24008 BrO-1 (aq) -94.1441.84 -33.472 BrO3-1 (aq) -83.68163.176 1.6736 C (g) 716.681544157.9865848 671.289328 C (s diamond) 1.8966072 2.376512 2.899512 C (s graphite) 0 5.694424 0 C-1 (g) 587.852151.29344 550.6144 C12H22O11 (s) -2225.4696360.2424 -1544.64912 C2 (g) 837.6368199.28392 781.5712 C2-1 (g) 443.504196.48064 393.296 C3 (g) 820.064237.2328 754.3752 C3H6 (g cyclopropane) 53.30416237.442 104.3908C3O2 (l) -117.27752181.08352 -105.0184 C3O2 (g) -93.7216276.3532 -109.83C4H10CH3(CH2)2CH(g n-butane) -126.1476310.11808 -17.1544 3C4H8 (g cyclobutane) 26.65208265.39112 110.0392C4N2 (g) 533.46289.99304 510.8664C5H10 (g cyclopentane) -77.23664292.88 38.61832 C5H10 (l cyclopentane) -105.77152204.26288 36.4008 C6H12 (g cyclohexane) -123.13512298.23552 31.75656 C6H12 (l cyclohexane) -156.23056204.34656 26.65208 C6H5CH3 (l toluene) 12.00808220.95704 113.76296 C6H5CH3 (g toluene) 49.9988320.66176 122.00544 C6H5COOH (s benzoic acid) -385.05352167.5692 -245.26608 C6H5OH (g phenol) -96.35752315.59912 -32.88624 C6H5OH (s phenol) -165.01696144.01328 -50.4172 C6H6 (l benzene) 48.99464173.25944 124.34848 C6H6 (g benzene) 82.92688269.19856 129.66216 C7H14 (l cycloheptane) -156.77448242.54648 54.05728 C8H16 (l cyclooctane) -169.78672262.00208 77.8224 Ca (s) 041.4216 0 Ca (l) 10.9202450.66824 8.20064 Ca (g) 179.2844154.76616 145.51952 Ca(ClO4)2?4H2O (s) -1948.9072433.4624 -1476.82648 Ca(H2PO4)2?H2O (s) -3409.66712259.8264 -3058.42032 Ca(IO3)2 (s) -1002.4864230.12 -839.3104 Ca(IO3)2?6H2O (s) -2780.6864451.872 -2267.728 Ca(NO3)2 (s) -938.38752193.3008 -743.20392 Ca(NO3)2?2H2O (s) -1540.758269.4496 -1229.34288 Ca(NO3)2?3H2O (s) -1838.0312319.2392 -1471.9312 Ca(NO3)2?4H2O (s) -2132.33376375.3048 -1713.47352 Ca(OH)2 (s) -986.168883.38712 -898.514 Ca[Mg(CO3)2] (s dolomite) -2326.304155.18456 -2163.5464 Ca+1 (g) 775.2952160.535896 733.4552Ca+2 (aq) -542.83216-53.1368 -553.5432 Ca10(PO4)6(OH)2 (s hydroxyapatite) -13476.664780.7344 -12677.52 Ca10(PO4)6F2 (s fluorapatite) -13744.44775.7136 -12982.952 Ca2P2O7 (s beta) -3338.832189.24232 -3132.1424 Ca2SiO4 (s beta) -2307.476127.73752 -2192.8344 Ca2SiO4 (s gamma) -2317.936120.79208 -2201.2024 Ca3(AsO4)2 (s) -3298.6656225.936 -3063.1064 Ca3(PO4)2 (s beta) -4120.8216235.9776 -3884.844 Ca3(PO4)2 (s alpha) -4109.9432240.91472 -3875.6392 CaBr2 (g) -384.928314.6368 -420.95224 CaBr2 (s) -683.2472129.704 -664.12632 CaBr2 (l) -662.99664147.86256 -649.31496 CaBr2?6H2O (s) -2506.216410.032 -2153.0864CaC2 (s) -59.831269.95648 -64.852 CaC2O4?H2O (s) -1674.8552156.4816 -1513.9804CaCl2 (s) -795.7968104.6 -748.0992CaCl2 (l) -774.04123.8464 -732.2 CaCl2 (g) -471.5368289.9512 -479.068 CaCO3 (s aragonite) -1207.1258488.7008 -1127.7972CaCO3 (s calcite) -1206.9166492.8848 -1128.8432CaCrO4 (s) -1379.0464133.888 -1277.3752 CaF2 (g) -782.408273.6336 -794.96 CaF2 (s) -1219.63668.86864 -1167.336 CaF2 (l) -1184.07292.59192 -1142.232 CaH2 (s) -186.18841.84 -147.2768 CaHPO4 (s) -1814.3916111.37808 -1681.25672CaHPO4?2H2O (s) -2403.58248189.45152 -2154.76 CaI2 (l) -500.15536178.94968 -506.51504 CaI2 (s) -536.8072145.26848 -533.12528 CaI2 (g) -258.1528327.43984 -308.7792 CaMoO4 (s) -1541.3856122.5912 -1434.6936 CaO (s) -635.131238.19992 -603.542 CaO (l) -557.3506462.29976 -532.95792 CaO?2Al2O3 (s) -3977.7288177.82 -3770.6208 CaO?2B2O3 (s) -3360.25408134.7248 -3167.12064 CaO?Al2O3 (s) -2326.304114.2232 -2208.7336 CaO?B2O3 (s) -2030.95544104.85104 -1924.09608 CaO?Fe2O3 (s) -1520.34008145.35216 -1412.81128 CaO?MgO?2SiO2 (s diopside) -3206.1992142.92544 -3032.1448 CaO?V2O5 (s) -2329.27464179.0752 -2169.69688 CaS (s) -474.88456.484 -469.8632 CaSe (s) -368.19266.944 -363.1712 CaSeO4?2H2O (s) -1706.6536221.752 -1486.9936 CaSiO3 (s pseudowollastonite) -1628.412887.36192 -1544.7328CaSiO3 (s wollastonite) -1634.9398481.92272 -1549.71176 CaSO3?H2O (s) -1752.6776184.096 -1555.1928 CaSO4 (s anhydrite insoluble) -1434.10784106.692 -1321.85112 CaSO4 (s alpha soluble) -1425.23776108.3656 -1313.48312 CaSO4 (s beta soluble) -1420.80272108.3656 -1309.04808 CaSO4?0.5H2O (s beta micro) -1574.6484134.3064 -1435.86512 CaSO4?0.5H2O (s alpha macro) -1576.7404130.5408 -1436.82744 CaSO4?2H2O (s) -2022.62928194.1376 -1797.4464 CaTiO3 (s perovskite) -1660.629693.63792 -1575.276 CaTiSiO5 (s sphene) -2603.2848129.20192 -2461.8656 CaWO4 (s) -1645.1488126.39864 -1538.49864 CaZrO3 (s) -1766.9032100.08128 -1681.1312 CBr (g) 510.448233.4672 464.424 CCl (g) 502.08224.30424 468.608 Cd (g) 112.00568167.636144 77.44584 Cd (s gamma) 051.75608 0 Cd (s alpha) -0.5857651.75608 -0.58576Cd(CN)4-2 (aq) 428.0232322.168 507.5192 Cd(NH3)4+2 (aq) -450.1984336.3936 -226.3544 CdBr2 (s) -316.18488137.2352 -296.31088 CdBr2?4H2O (s) -1492.55832316.3104 -1248.032808CdCl2 (s) -391.49688115.2692 -343.96664 CdCl2?2.5H2O (s) -1131.93936227.1912 -944.094496CdCl3-1 (aq) -561.0744202.924 -487.0176 CdCO3 (s) -750.609692.4664 -669.44 CdF2 (s) -700.401677.404 -647.6832 CdI2 (s) -202.924161.084 -201.37592 CdI4-2 (aq) -341.8328326.352 -315.892 CdO (s) -258.152854.8104 -228.4464 CdS (s) -161.920864.852 -156.4816 CdSb (s) -14.3929692.8848 -13.01224 CdSeO3 (s) -575.3142.256 -497.896 CdSeO4 (s) -633.0392164.4312 -531.7864 CdSiO3 (s) -1189.092897.4872 -1105.4128 CdSO4 (s) -933.28304123.038888 -822.7836 CdSO4?8/3H2O (s) -1729.37272229.630472 -1465.337216 CdSO4?H2O (s) -1239.55184154.029776 -1068.84464 CdTe (s) -92.4664100.416 -92.048 CF (g) 255.224212.92376 221.752 CF+1 (g) 1149.3448201.2504 1115.036CF2 (g) -182.004240.70552 -191.6272 CF2+1 (g) 941.8184246.6468 924.2456 CH3(CH2)2CH2OH (g 2-butanol) -274.6796362.7528 -150.79136 CH3(CH2)2CH2OH (l 1-butanol) -327.10512226.3544 -162.50656 CH3(CH2)2CH3 (l n-butane) -147.65336230.9568 -15.0624 CH3(CH2)3CH3 (g pentane) -146.44348.9456 -8.368 CH3(CH2)4CH3 (g hexane) -167.19264388.40072 -0.25104CH3(CH2)4CH3 (l hexane) -198.82368296.05984 -3.80744 CH3(CH2)5CH3 (g heptane) -187.77792427.89768 7.99144CH3(CH2)5CH3 (l heptane) -224.38792326.01728 1.75728 CH3(CH2)6CH3 (l octane) -249.95216357.732 7.40568 CH3(CH2)6CH3 (g octane) -208.44688466.7252 16.40128 CH3(CH2)7CH3 (l nonane) -275.47456393.67256 11.75704CH3(CH2)7CH3 (g nonane) -229.03216505.67824 24.81112CH3(CH2)8CH3 (l decane) -301.0388425.5128 -17.53096 CH3CH2CH2OH (l 1-propanol) -304.00944194.556 -170.62352CH3CH2CH2OH (g 1-propnaol) -256.39552324.72024 -161.79528CH3CH2CH3 (g propane) -103.84688270.20272 -23.55592CH3CH2CHOHCH3 (g 2-butanol) -292.62896358.9872 -167.61104CH3CH2CHOHCH3 (l 2-butanol) -342.58592225.0992 -177.02504 CH3CH2OCH2CH3 (l diethyl ether) -273.2152253.132 -116.64992 CH3CH2OCH2CH3 (g diethyl ether) -252.12784342.6696 -122.34016 CH3CH2OH (l ethanol) -276.9808161.04216 -174.17992CH3CH2OH (g ethanol) -234.42952282.58736 -167.90392CH3CH3 (g ethane) -84.68416229.11584 -32.80256 CH3CHOHCH3 (g 2-propanol) -272.42024309.90888 -173.38496 CH3CHOHCH3 (l 2-propanol) -317.85848180.58144 -180.28856 CH3COCH3 (l acetone) -247.60912200.4136 -155.72848CH3COCH3 (g acetone) -216.64752294.93016 -153.05072CH3COOH (l acetic acid) -484.13064159.8288 -389.9488 CH3COOH (g acetic acid) -434.84312282.50368 -376.68552CH3OCH3 (g dimethyl ether) -184.05416267.06472 -112.92616CH3OH (g methanol) -201.08304239.70136 -162.42288CH3OH (l methanol) -239.03192127.23544 -166.81608CH4 (g methane) -74.85176186.27168 -50.8356 Cl (g) 121.29416165.0588 105.31128 Cl-1 (aq) -167.150856.484 -131.25208 Cl2 (g) 0222.96536 0 Cl2F6 (g) -339.3224489.528 -237.2328 Cl2O (g) 80.3328267.85968 97.4872 ClF (g) -54.47568217.7772 -55.94008 ClF3 (g) -158.992281.49952 -118.8256 ClF3?HF (g) -450.6168359.824 -384.0912 ClF5 (g) -238.488310.62016 -146.44 ClO (g) 101.21096226.5636 97.4872 ClO-1 (aq) -107.110441.84 -36.8192 ClO2 (g) 102.508256.77208 120.33184 ClO2-1 (aq) -66.5256101.2528 17.1544 ClO3-1 (aq) -99.1608162.3392 -3.3472 ClO3F (g) -27.15416278.8636 44.85248 ClO4-1 (aq) -129.32744182.004 -8.61904 CN (g) 435.136202.54744 405.0112 CN+1 (g) 1802.8856213.34216 1763.1376 CN-1 (aq) 150.62494.14 172.3808 CN-1 (g) 60.668195.8112 38.74384 CN2 (g) 581.576231.5844 573.208CNBr (g) 181.3764247.14888 160.62376 CNCl (g) 132.2144235.47552 125.47816 CNI (g) 225.0992256.60472 196.14592 CNI (s) 160.2472128.8672 169.36832 Co (s hexagonal) 030.04112 0 CO (g) -110.54128197.9032 -137.27704 Co (s face centered cubic) 0.4602430.71056 0.25104 Co(IO3)2?2H2O (s) -1081.9824267.776 -795.7968 Co(NH3)6+3 (aq) -584.9232146.44 -157.3184 Co(OH)2 (s pink) -539.73679.496 -454.3824 Co+2 (aq) -58.1576-112.968 -54.392 Co+3 (aq) 92.048-305.432 133.888 CO2 (g) -393.5052213.67688 -394.38384CO2 (aq undissoc) -413.7976117.5704 -386.01584 CO3-2 (aq) -677.13856-56.9024 -527.89528 Co3O4 (s) -910.02114.2232 -794.96 COBr2 (g) -96.232308.9884 -110.876 CoCl2 (s) -312.5448109.16056 -269.868 COCl2 (g) -220.9152283.75888 -206.77328 CoCl2?2H2O (s) -922.9904188.28 -764.8352CoCl2?6H2O (s) -2115.4304343.088 -1725.4816CoCl3 (g) -163.5944334.0924 -154.51512 CoF2 (s) -692.033681.96456 -647.2648 COF2 (g) -640.152258.73856 -624.58752 CoF3 (s) -790.77694.5584 -719.648 CoO (s) -237.9440852.96944 -214.2208 COS (g) -138.40672231.45888 -165.64456 CoSi (s) -100.41643.0952 -98.7424 CoSO4 (s) -888.2632117.9888 -782.408 CoSO4?6H2O (s) -2683.6176367.60624 -2235.7204 CoSO4?7H2O (s) -2979.92848406.0572 -2473.83184 Cr (g) 397.48174.22176 352.58568 Cr (l) 26.10397636.23344 22.34256 Cr (s) 023.61868 0 Cr23C6 (s) -364.8448610.0272 -373.6312 Cr2N (s) -125.5264.852 -102.21512 Cr2O3 (s) -1134.700881.1696 -1053.1128 Cr2O3 (l) -1018.3856125.60368 -950.06088 Cr2O7-2 (aq) -1490.3408261.9184 -1301.224 Cr3C2 (s) -85.353685.43728 -86.31592Cr7C3 (s) -161.9208200.832 -166.9416CrCl2 (s) -395.388115.31104 -356.0584 CrCl3 (s) -556.472123.0096 -486.1808 CrF3 (s) -1158.96893.88896 -1087.84CrN (g) 505.0088230.45472 471.91336 CrN (s) -117.15237.69784 -92.80112 CrO (g) 188.28239.15744 154.5988 CrO2 (g) -75.312269.11488 -87.36192 CrO2Cl2 (l) -579.484221.752 -510.8664 CrO2Cl2 (g) -538.0624329.6992 -501.6616 CrO3 (g) -292.88266.06056 -273.46624 CrO4-2 (aq) -881.150450.208 -727.84864 Cs (g) 76.5672175.47696 49.7896 Cs (l) 2.08781692.08984 0.025104 Cs (s) 085.1444 0 CS (g) 234.304210.4552 184.096 Cs+1 (aq) 458.5664169.72396 427.1864 CS2 (g) 117.06832237.77672 66.90216 CS2 (l) 89.70496151.33528 65.2704Cs2O (g) -92.048317.984 -104.6 CsAl(SO4)2?12H2O (s) -6064.708686.176 -5098.204 CsBr (s) -405.68064113.3864 -384.928CsCl (s) -442.83456101.181672 -414.216 CsCl (l) -434.2992101.71304 -406.2664 CsCl (g) -240.1616255.97712 -257.7344 CsF (s) -554.798488.2824 -525.5104 CsF (l) -543.8363290.08152 -515.09224 CsF (g) -356.4768243.0904 -373.2128 CsH (g) 121.336214.43 101.6712 CsI (s) -336.812125.52 -333.71584 CsOH (s) -416.726498.7424 -362.3344 CsOH (g) -259.408255.14032 -259.8264 CsOH (l) -406.01536118.44904 -365.8908 Cu (g) 338.31824166.27216 298.61208 Cu (s) 033.149832 0 Cu(C2O4)2-2 (aq) -1592.012146.44 -1335.9512 Cu(IO3)2?H2O (s) -692.0336247.2744 -468.608 Cu(NH3)+2 (aq) -38.911212.1336 15.56448 Cu(NH3)2+2 (aq) -142.256111.2944 -30.45952 Cu(NH3)3+2 (aq) -245.6008199.5768 -73.13632 Cu(NH3)4+2 (aq) -348.5272273.6336 -111.2944 Cu(OH)2 (s) -450.1984108.3656 -372.7944 Cu+1 (aq) 71.6719240.5848 49.9988 Cu+2 (aq) 64.76832-99.5792 65.52144Cu2 (g) 484.17248241.45864 431.95616 Cu2O (s) -168.615293.13584 -146.0216 Cu2S (s alpha) -79.496120.9176 -86.1904 CuBr (s) -104.696.10648 -100.8344 CuCl (s) -137.235286.1904 -119.8716 CuCl2 (s) -205.8528108.07272 -161.9208CuCl2?2H2O (s) -821.3192167.36 -656.0512CuCN (s) 94.976889.99784 108.3656 CuCO3?Cu(OH)2 (s malachite) -1051.4392186.188 -893.7024 CuF (s) -192.46464.852 -171.544 CuF2 (s) -548.940868.6176 -499.1512 CuFe2O4 (s) -965.20696141.0008 -858.80784 CuFeO2 (s) -532.623288.7008 -479.9048 CuI (s) -67.780896.6504 -69.4544 CuN3 (s) 279.0728100.416 344.7616CuO (s) -157.318442.63496 -129.704 CuS (s) -53.136866.5256 -53.5552 CuSO4 (s) -771.36224108.784 -661.9088 CuSO4?3H2O (s) -1684.31104221.3336 -1400.1756 CuSO4?5H2O (s) -2279.6524300.4112 -1880.055296 CuSO4?H2O (s) -1085.83168146.0216 -918.22064F (g) 78.99392158.65728 61.9232 F-1 (g) -255.6424145.47768 -262.3368 F2 (g) 0202.7148 0 Fe (s alpha) 027.27968 0 Fe (l) 13.12939234.28788 11.049944 Fe(CN)6-3 (aq) 561.9112270.2864 729.2712 Fe(CN)6-4 (aq) 455.637694.9768 694.92056 Fe(CO)5 (l) -774.04338.0672 -705.4224 Fe(CO)5 (g) -733.8736445.1776 -697.2636 Fe(OH)+2 (aq) -290.788-142.256 -229.40872 Fe+2 (aq) -89.1192-137.6536 -78.8684Fe+3 (aq) -48.5344-315.892 -4.6024 Fe2(SO4)3 (s) -2581.528307.524 -2263.1256 Fe2O3 (s hematite) -824.24887.40376 -742.2416 Fe2SiO4 (s fayalite) -1479.8808145.1848 -1379.0464 Fe3C (s alpha-cementite) 25.104104.6 20.0832 Fe3O4 (s magnetite) -1118.3832146.44 -1015.4568 Fe3Si (s) -93.7216103.7632 -94.5584 Fe4N (s) -10.46156.0632 3.7656 Fe7S8 (s pyrrhotite) -736.384485.7624 -748.5176FeAl2O4 (s) -1966.48106.2736 -1849.328 FeAsS (s) -41.84121.336 -50.208 FeBr2 (s) -249.7848140.66608 -237.2328 FeCl2 (s) -341.79096117.94696 -302.33584 FeCl3 (s) -399.48832142.256 -334.05056 FeCO3 (s siderite) -740.56892.8848 -666.7204 FeCr2O4 (s) -1444.7352146.0216 -1343.9008 FeF2 (s) -702.91286.98536 -661.072FeF3 (s) -1041.81698.324 -970.688FeI2 (s) -104.6167.36 -112.968 FeMoO4 (s) -1075.288129.2856 -974.872 FeO (s) -271.9660.75168 -251.4584 FeOH+1 (aq) -324.6784-29.288 -277.3992 FePO4?2H2O (s strengite) -1888.2392171.25112 -1657.7008 FeS (s pyrrhotite) -99.997660.29144 -100.416 FeS2 (s pyrite) -178.238452.9276 -166.9416 FeSi (s) -73.638446.024 -73.6384 FeSi2 (s beta-lebanite) -81.169655.6472 -78.2408 FeSO4 (s) -928.4296120.9176 -825.0848 FeSO4?7H2O (s) -3014.572409.1952 -2510.27448 FeWO4 (s) -1154.784131.796 -1054.368 FNO3 (g) 10.46292.88 73.6384 Fr (s) 094.14 0 Fr (g) 72.8016181.92032 46.6516 Fr2O (s) -338.904156.9 -299.156H+1 (aq) 00 0H2 (g) 0130.586824 0 H2AsO4-1 (aq) -909.55976117.152 -753.28736 H2CS3 (l) 25.104223.0072 27.8236 H2MoO4 (g) -851.0256355.64 -787.4288 H2O (g) -241.818464188.715136 -228.588656 H2O (l) -285.8299669.91464 -237.178408 H2O2 (g) -136.10552232.88144 -105.47864 H2O2 (l) -187.77792109.6208 -120.41552 H2PO4-1 (aq) -1296.2868890.3744 -1130.39128 H2S (g) -20.16688205.76912 -33.0536 H2Se (g) 29.7064218.90688 15.8992 H2Se (g) 29.7064218.90688 15.8992 H2SiO3 (s) -1188.6744133.888 -1092.4424H2SO4 (l) -813.9972156.9 -690.06712 H2SO4 (g) -740.568289.1144 -656.0512 H2VO4-1 (aq) -1174.0304121.336 -1020.896 H2WO4 (s) -1131.772146.44 -1004.16 H2WO4 (g) -905.4176351.456 -839.7288 H3BO3 (s) -1094.325288.82632 -969.0144 H3PO4 (l) -1254.3632150.624 -1111.6888H3PO4 (s) -1266.9152110.54128 -1112.5256H4SiO4 (s) -1481.136192.464 -1333.0224HAsO4-2 (aq) -906.33808-1.6736 -714.71088 HBO2 (s orthorhombic) -788.7676850.208 -721.74 HBO2 (s monoclinic) -794.2487237.656 -723.4136 HBr (g) -36.44264198.61448 -53.51336 HCl (g) -92.29904186.77376 -95.31152 HClO (g) -92.048236.6052 -79.496 HCN (g) 135.1432201.6688 124.6832 HCN (l) 108.86768112.84248 124.93424 HCO3-1 (aq) -691.9917691.2112 -586.84784 HCrO4-1 (aq) -878.2216184.096 -764.8352 He (g) 0126.038816 0 HF (g) -271.1232173.67784 -273.2152 Hg (l) 076.02328 0 Hg (g) 61.31652174.84936 31.852792 Hg(CH3)2 (l) 59.8312209.2 140.164 Hg(CH3)2 (g) 94.39104305.432 146.0216 Hg2(N3)2 (s) 594.128205.016 746.4256 Hg2Br2 (s) -206.8988218.73952 -181.075152 Hg2Cl2 (s) -265.22376192.464 -210.777368 Hg2CO3 (s) -553.5432179.912 -468.1896 Hg2F2 (s) -485.344158.992 -426.768 Hg2I2 (s) -121.336242.672 -111.00152 Hg2SO4 (s) -743.12024200.66464 -625.880376 HgBr2 (s) -170.7072170.33064 -153.1344HgCl (g) 84.0984259.78456 62.76 HgCl2 (s) -224.2624146.0216 -178.6568HgF2 (s) -422.584116.3152 -372.376 HgH (g) 239.99424219.49264 216.01992 HgI (g) 132.38176281.41584 88.44976 HgI2 (g) -17.1544336.01704 -59.8312 HgI2 (s red) -105.4368181.1672 -101.6712 HgO (s yellow) -90.4580871.128 -58.425376 HgO (s red hexagonal) -89.537671.128 -58.24128 HgO (s red orthorhombic) -90.8346470.2912 -58.55508 HgS (s red) -58.157682.4248 -50.6264 HgS (s black) -53.555288.2824 -47.6976 HgSe (g) 75.7304267.02288 31.38 HgSe (s) -46.02494.14 -38.0744 HgTe (s) -33.8904106.692 -28.0328HI (g) 26.48472206.4804 1.71544 HN2O2-1 (aq) -39.3296142.256 76.1488 HN3 (g) 294.1352238.86456 328.0256 HNCO (g) -116.7336238.11144 -107.36144 HNCS (g) 127.612247.6928 112.968 HNO2 (g cis) -76.5672249.32456 -41.84 HNO2 (g trans) -78.6592249.1572 -43.932 HNO3 (l) -173.2176155.60296 -79.9144 HNO3 (g) -135.05952266.26976 -74.76808HOF (g) -98.324226.64728 -85.64648 HPO4-2 (aq) -1292.14472-33.472 -1089.26256 HReO4 (s) -762.3248158.1552 -664.8376 HS-1 (aq) -17.572862.76 12.04992 HSe-1 (aq) 15.899279.496 43.932 HSeO3-1 (aq) -514.54832135.1432 -411.53824 HSeO3-1 (aq) -514.54832135.1432 -411.53824 HSeO4-1 (aq) -581.576149.3688 -452.2904 HSeO4-1 (aq) -581.576149.3688 -452.2904 HSO3-1 (aq) -626.21928139.7456 -527.8116 HSO3F (g) -753.12297.064 -690.36 HVO4-2 (aq) -1158.96816.736 -974.872 I (g) 106.83844180.681856 70.282832 I-1 (aq) -55.18696111.2944 -51.58872 I2 (s) 0116.135288 0 I2 (g) 62.437832260.57952 19.359368 IBr (g) 40.83584258.663248 3.72376 ICl (l) -23.89064135.1432 -13.598 ICl (g) 17.782247.44176 -5.4392 ICl3 (s) -89.5376167.36 -22.34256 IF (g) -95.64624236.06128 -118.49088 IF5 (g) -840.1472334.72 -771.5296IF7 (g) -943.9104346.4352 -818.3904 IO (g) 175.05856245.3916 149.7872 IO-1 (aq) -107.5288-5.4392 -38.4928 IO3-1 (aq) -221.3336118.4072 -128.0304 K (g) 89.119290.03968 60.668 K (l) 2.28446471.46272 0.263592 K (s) 064.68464 0 K2B4O7 (s) -3334.2296208.3632 -3136.7448 K2CO3 (s) -1150.1816155.51928 -1064.4096 K2O (s) -363.171294.14 -322.168 K2O2 (s) -495.804112.968 -429.6968 K2SiO3 (s) -1548.08146.14712 -1455.6136K2SO4 (s) -1433.68944175.728 -1316.37008 K3AlCl6 (s) -2092376.56 -1938.4472 KAl(SO4)2 (s) -2465.38016204.5976 -2235.46936 KAl(SO4)2?12H2O (s) -6057.34416687.4312 -5137.1152 KAlCl4 (s) -1196.624196.648 -1096.208 KBF4 (s) -1886.984133.888 -1784.8944 KBH4 (s) -226.7728106.60832 -159.8288 KBO2 (s) -994.955279.99808 -978.6376 KBr (s) -392.1663296.4412 -379.19592 KBrO3 (s) -332.2096149.1596 -243.5088 KCl (s) -435.8891282.67584 -408.31656 KCl (g) -215.8944239.49216 -235.1408 KClO3 (s) -391.204142.96728 -289.90936 KClO4 (s) -430.1152151.0424 -300.4112 KCN (s) -113.47008127.77936 -102.04776 KF (s) -568.605666.56744 -538.8992 KF?2H2O (s) -1158.968150.624 -1015.4568 KH (s) -57.8228850.208 -34.05776 KH2AsO4 (s) -1135.956155.14272 -991.608 KHF2 (s) -931.3584104.26528 -863.1592 KI (s) -327.64904106.39912 -322.29352 KIO3 (s) -508.356151.4608 -425.5128 KMnO4 (s) -813.3696171.71136 -713.7904 KNO3 (s) -492.70784132.92568 -393.12864 KO2 (s) -284.512122.5912 -240.58 KOH (s) -425.8475278.8684 -379.0704 Kr (g) 0163.975144 0 Li (g) 160.6656138.65776 128.0304 Li (l) 2.38069633.93224 0.933032 Li (s) 0160.6656 0 Li2B4O7 (s) -3363.936155.6448 -3171.472 Li2BeF4 (s) -2273.5856130.5408 -2171.496 Li2CO3 (s) -1216.0377690.1652 -1132.1904Li2O (s) -598.730437.90704 -561.9112Li2O2 (s) -632.620856.484 -571.116 Li2Si2O5 (s) -2561.0264125.52 -2417.0968 Li2SiO3 (s) -1649.332880.3328 -1558.9584 Li2TiO3 (s) -1670.671291.75512 -1579.8784Li3AlF6 (s) -3383.6008187.8616 -3223.772 Li3N (s) -197.484837.656 -153.9712 LiAlF4 (g) -1853.512326.352 -1811.672LiAlH4 (s) -117.15287.864 -48.5344 LiAlO2 (s) -1189.511253.346 -1127.1696 LiBeF3 (s) -1651.843289.1192 -1576.1128 LiBH4 (s) -190.4556875.81408 -124.76688 LiBO2 (s) -1019.222451.71424 -963.1568LiBr (s) -350.9120874.0568 -341.6236 LiCl (s) -408.2747259.28728 -384.04936 LiCl?H2O (s) -712.57704103.7632 -632.6208 LiClO4 (s) -380.744125.52 -253.9688 LiF (s) -616.930835.64768 -588.6888 LiH (s) -90.6254420.04136 -68.45024 LiI (s) -270.077285.772 -269.6588 LiO (g) 83.68210.8736 60.4588 LiOH (s) -484.925642.80232 -438.9016 LiOH?H2O (s) -789.8136892.048 -689.5232 Mg (s) 032.693776 0 Mg (l) 9.0374442.50944 6.10864 Mg (g) 147.61152148.532 113.09352 Mg(ClO4)2?6H2O (s) -2445.548520.908 -1863.1352 Mg(NO3)2 (s) -790.65048164.0128 -589.5256 Mg(NO3)2?6H2O (s) -2613.28456451.872 -2080.7032 Mg(OH)2 (s) -924.66463.1784 -833.8712 Mg(VO3)2 (s) -2201.57896160.6656 -2039.40712 Mg+1 (g) 891.6104154.30592 848.9336Mg+2 (aq) -466.85072-138.072 -454.8008 Mg2Al4Si5O19 (s cordierite) -9108.568407.1032 -8598.12 Mg2Ge (s) -108.78486.48328 -105.8552 Mg2Si (s) -77.822466.944 -75.312 Mg2SiO4 (s forsterite) -2174.006495.14416 -2055.1808 Mg2TiO4 (s) -2164.3832109.32792 -2047.6496 Mg2V2O7 (s) -2835.9152200.4136 -2645.29216 Mg3(PO4)2 (s) -3780.6624189.20048 -3538.8272 Mg3N2 (s) -460.658487.864 -400.8272 Mg3Si2O5(OH)4 (s chrysotile) -4365.5856221.3336 -4037.9784 Mg3Si4O10(OH)2 (s talc) -5922.452260.6632 -5542.9632MgAl2O4 (s) -2312.915288.7008 -2190.324 MgBr2 (s) -524.2552117.152 -503.7536MgBr2?6H2O (s) -2409.984397.48 -2056.0176 MgCl2 (s) -641.616489.62128 -592.11968。

《实用无机物热力学数据手册》使用说明1 关于化学反应吸热(或放热)量的计算1.1计算公式根据《手册》P.21式(70):()()298G G G G G G T T 298T 298H H H -H H -H ⎡⎤⎡⎤∆=∆+-⎣⎦⎣⎦∑∑iiii生成物反物nn (1.1)式中:T GH ∆——应理解为实际状态(101.325kPa ,T K )下的定压化学反应热P,T Q 。
在反应前后温度T 相同时,(因压力均为101.325kPa )故也可理解为定压化学反应热效应。
化学反应热效应与反应热的区别仅仅在于:热效应是状态量(反应前后的温度、压力必须相同),而反应热是过程量(反应前后的温度、压力不一定相同)。
298G H ∆——为热化学标准状态(101.325kPa ,298K )下,生成物与反应物的标准生成焓298GH 之差。
按下式计算:()()298G G G 298298H H H ⎡⎤⎡⎤∆=-⎣⎦⎣⎦∑∑iiii生成物反物nn(1.2)()GG T298H -H ⎡⎤⎣⎦∑ii生成物n——化学反应的每个生成物,从反应温度T K 降温到298K 的焓变(放热量)之和。
()G G T298H -H ⎡⎤⎣⎦∑ii反物n——化学反应的每个反应物,从298K 升温到反应温度T K 的焓变(吸热量)之和。
()G 298H ⎡⎤⎣⎦∑ii生成物n——化学反应的每个生成物,从反应温度T K 降温到298K 的焓变(放热量)之和。
()G298H ⎡⎤⎣⎦∑ii反物n——化学反应的每个反应物,从298K 升温到反应温度T K 的焓变(吸热量)之和。
()G G T298H-H i——单个生成物从反应温度T K 降温到298K 的焓变,或单个反应物从298K 升温到反应温度T K 的焓变。
in——单个生成物(或单个反应物)的化学计量系数,即:化学反应方程式中,该物质的分子式前面的系数(也就是参与反应的该物质的摩尔数)。


无机物热力学手册
无机物热力学手册是一份关于无机物热力学性质的手册,通常包含了各种无机物的热力学数据,如热容、熵、焓、吉布斯自由能等。
这些数据对于化学工程、材料科学、环境科学等领域的研究和应用非常重要。
由于无机物的种类繁多,手册通常会按照元素周期表进行分类,并列出每种无机物的热力学数据。
这些数据通常是通过实验测量得出的,因此手册中也会提供详细的实验方法和数据来源。
除了热力学数据之外,无机物热力学手册还可能包含无机物的物理性质、化学性质、晶体结构等方面的信息。
这些数据可以帮助人们更好地了解无机物的性质和行为,从而更好地应用于各个领域。
总的来说,无机物热力学手册是一份非常实用的工具书,对于化学工程、材料科学、环境科学等领域的研究和应用非常重要。
如果您需要了解某种无机物的热力学性质或其他相关信息,可以查阅相关的手册或数据库。

纯物质热化学数据手册纯物质热化学数据手册是一份包含各种物质的热力学数据和热力学性质的参考手册。
这些数据是在标准状态下(常温常压)测定的,对于热化学计算和工程设计非常有用。
以下是一些常见物质的热力学数据:1. 水(H2O)标准状态下,水的热力学性质如下:- 摩尔质量:18.01528 g/mol- 摩尔熵(S°):69.91 J/(mol K)- 摩尔焓(H°):-285.83 kJ/mol- 摩尔自由能(G°):-237.13 kJ/mol- 热容(Cp):75.29 J/(mol K)2. 氨(NH3)标准状态下,氨的热力学性质如下:- 摩尔质量:17.03052 g/mol- 摩尔熵(S°):193.60 J/(mol K)- 摩尔焓(H°):-45.92 kJ/mol- 摩尔自由能(G°):-16.45 kJ/mol- 热容(Cp):35.63 J/(mol K)3. 二氧化碳(CO2)标准状态下,二氧化碳的热力学性质如下:- 摩尔质量:44.0095 g/mol- 摩尔熵(S°):213.79 J/(mol K)- 摩尔焓(H°):-393.52 kJ/mol- 摩尔自由能(G°):-394.36 kJ/mol- 热容(Cp):37.13 J/(mol K)4. 乙醇(C2H5OH)标准状态下,乙醇的热力学性质如下:- 摩尔质量:46.06844 g/mol- 摩尔熵(S°):160.70 J/(mol K)- 摩尔焓(H°):-277.69 kJ/mol- 摩尔自由能(G°):-174.76 kJ/mol- 热容(Cp):112.97 J/(mol K)以上仅是几个常见物质的热力学数据,不同温度和压力下的数据会有所不同,具体数据可在相关手册或数据库中查找。

氯酸钾分解的热力学数据引言氯酸钾(化学式:KClO3)是一种常见的无机化合物,它在高温下可以分解产生氧气和氯化钾。
研究氯酸钾的热力学性质对于了解其在化学反应中的行为和应用具有重要意义。
本文将介绍氯酸钾分解的热力学数据,包括其热稳定性、热分解反应方程式、标准生成焓和生成自由能等。
热稳定性氯酸钾是一种相对稳定的化合物,在室温下不会自发分解。
然而,当加热到一定温度时,它会发生分解反应,生成氧气和氯化钾。
热分解反应方程式氯酸钾的热分解反应方程式如下所示:2KClO3(s) → 2KCl(s) + 3O2(g)根据该方程式可知,在该反应中,每个摩尔的氯酸钾产生2摩尔的氯化钾和3摩尔的氧气。
标准生成焓标准生成焓(ΔH°)是指在标准状态下,形成1摩尔化合物所释放或吸收的热量。
对于氯酸钾的热分解反应,其标准生成焓可以通过实验测定得到。
实验结果显示,氯酸钾的热分解反应是一个放热反应,即生成焓为负值。
根据实验数据计算得到的标准生成焓为-391.6 kJ/mol。
标准生成自由能标准生成自由能(ΔG°)是指在标准状态下,形成1摩尔化合物时系统可用做功的最大值。
对于氯酸钾的热分解反应,其标准生成自由能可以通过以下公式计算得到:ΔG° = ΔH° - TΔS°其中,ΔH°为标准生成焓,T为温度(单位:K),ΔS°为标准摩尔熵变。
根据实验数据和计算结果可知,在常温下(298 K),氯酸钾的标准生成自由能为-456.4 kJ/mol。
其他热力学性质除了上述介绍的热稳定性、热分解反应方程式、标准生成焓和生成自由能外,还有其他热力学性质与氯酸钾的分解相关。
热分解温度热分解温度是指氯酸钾开始分解的温度。
实验结果显示,氯酸钾的热分解温度约为368°C。
热容热容是指物质在吸收或释放热量时所发生的温度变化。
对于氯酸钾的热分解反应,其热容可以通过实验测定得到。
实验结果显示,在298 K下,氯酸钾的摩尔热容为128.6 J/(mol·K)。
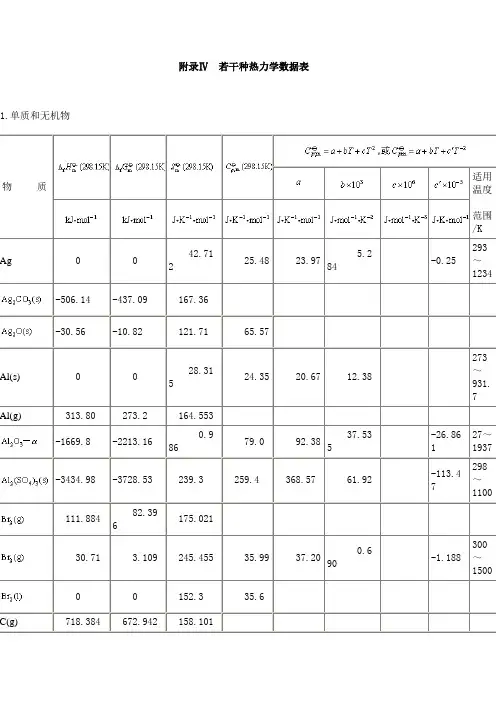
附录Ⅳ若干种热力学数据表1.单质和无机物物质适用温度范围/KAg0 042.71225.48 23.975.284-0.25293~1234-506.14 -437.09 167.36-30.56 -10.82 121.71 65.57Al(s)0 028.31524.35 20.67 12.38273~931.7Al(g)313.80 273.2 164.553-1669.8 -2213.160.98679.0 92.3837.535-26.86127~1937-3434.98 -3728.53 239.3 259.4 368.57 61.92 -113.47298~1100111.88482.396175.02130.71 3.109 245.455 35.99 37.200.690-1.188300~1500C(金刚石) 1.896 2.8662.4396.079.1213.22 -6.19 ~1200C(石墨)0 05.6948.6617.154.27-8.79298~2300CO(g)-110.525 -137.285 198.01629.14227.6 5.0290~2500-393.511 -394.38 213.7637.1244.149.04-8.54298~2500Ca(s)0 0 41.63 26.27 21.92 14.64 273~673-62.8 -67.8 70.2 62.34 68.6 11.88 -8.66 298~720-1206.87 -1128.70 92.8 81.83 104.52 21.92 -25.94 298~1200-795.0 -750.2 113.8 72.63 71.88 12.72 -2.51 298~1055CaO(s)-635.6 -604.2 39.7 48.53 43.834.52-6.52298~1800-986.5 -896.89 76.1 84.5(硬石膏)-1432.68 -1320.24 106.7 97.65 77.49 91.92 -6.561273~1373 -167.456 -131.168 55.10Cu(s)0 0 33.32 24.47 24.564.18-1.201 ~1357CuO(s)-155.2 -127.1 43.51 44.4 38.79 20.08 298~1250-166.69 -146.33 100.8 69.8 62.34 23.85 298~12000 0 203.5 31.46 34.691.84-3.35273~2000 0 27.15 25.23 17.28 26.69 273~1041-747.68 -673.84 92.8 82.13 48.66 112.1 298~885FeO(s)-266.52 -244.3 54.0 51.1 52.806.242-3.188273~1173-822.1 -741.0 90.0 104.6 97.74 17.13 -12.887298~1100-117.1 -1014.1 146.4 143.42 167.03 78.91 -14.88 298~1100(续表)物质适用温度4 00 0130.69528.8329.08-0.842.0300~15000 0144.88429.228.5770.8791.958298~1500HBr(g)-36.24 -53.22 198.6029.1226.155.861.09298~1600HBr(aq)-120.92 -102.8080.7 1HCl(g)-92.311 -95.265186.78629.1226.534.61.90298~2000HCl(aq)-167.44 -131.1755.1 0-698.7 -623.37 191.2Hl(g)-25.94 -1.32 206.4229.1226.325.940.92298~1000-241.825 -228.577188.82333.57130.1211.30273~2000-285.838 -237.14269.94075.296-291.850 (-234.03) (39.4)-187.61 -118.04 102.2682.2 9-20.146 -33.040 205.7533.9729.2915.69273~1300-811.35 (-866.4) 156.85 137.57 -811.32-885.75 -752.99 126.8662.242 19.34 260.6036.8 70 0191.59829.1226.874.27273~2500-46.19 -16.603 192.6135.6529.7925.48-1.665273~1400NO(g)89.860 90.37210.30929.86129.583.85-0.59273~150033.85 51.86 240.5737.942.938.54-6.7481.55 103.62 220.1038.745.698.62-8.54273~5009.660 98.39 304.42 79.083.8930.7514.92.51 110.5 342.4 108.0O(g)247.521 230.095161.06321.930 0205.13829.3731.463.39-3.77273~2000142.3 163.45 237.738.1 5-229.940 -157.297-10.53 9S(单斜)0.29 0.09632.5523.6414.929.08368.6~392S(斜方)0 0 31.922.614.9826.11273~368.6(g)124.94 76.08 227.7632.5536.111.09273~2000S(g)222.80 182.27167.825-3.51-395.18 -370.40 256.3450.757.3226.86 -13.05273~900-907.51 -741.90 17.2 2.有机化合物在指定温度范围内恒压热容可用下式计算物质适用温度范围/K烃类-74.847 50.827 186.3035.71517.45160.461.117-7.205298~1500226.748 209.200200.92843.92823.4685.768-58.34215.870298~150052.283 68.157 219.56 43.564.197154.59-81.09016.815298~1500-84.667 -32.821 229.6052.654.936182.259-74.85610.799298~150020.414 62.783 267.05 63.893.305235.86-117.60022.677298~1500-103.847 -23.391 270.02 73.51-4.799307.311-160.15932.748298~1500-0.13 71.60 305.71 85.652.540344.929-191.28441.664~1500-6.99 65.96 300.94 78.918.774342.448-197.32234.271298~1500-11.17 63.07 296.59 87.828.381307.541-148.25627.284298~1500-16.90 58.17 293.70 89.127.084321.632-166.07133.497298~1500-126.15 -17.02 310.23 97.450.469385.376-198.88239.996298~1500-134.52 -20.79 294.75 96.82-6.841409.643-220.54745.739298~150082.927 129.723 269.31 81.67 -33.899471.872-298.34470.835298~150049.028 124.597 172.35 135.77 59.50 255.01 281~353-123.14 31.92 298.51 106.27 -67.664679.452-380.76178.006298~1500-167.19 -0.09 388.85 143.093.084565.786-300.36962.061298~1500-198.82 -4.08 295.89 194.9349.999 122.388 319.86 103.76 -33.882557.045-342.37379.873298~150018.995 122.207 352.86 133.26 -14.811591.136-339.59074.697~1500-24.439 110.495 246.48 187.917.238 118.977 357.80 127.57 -27.384620.87-363.89581.379298~1500-25.418 107.817 252.17 183.317.949 121.266 352.53 126.86 -25.92460.670-350.56176.877298~1500(续表)物质适用温度范围/K -24.426 110.244 247.36 183.7含氧化合物-115.90 -110.0 220.2 35.3618.8258.379-15.606291~1500-362.63 -335.69 251.1 54.4 30.67 89.20-34.539300~700-201.17 -161.83 237.8 49.4 20.42 103.68-24.64~700-238.57 -166.15 126.8 81.6-166.36 -133.67 265.8 62.831.054121.457-36.577298~1500-487.0 -392.4 159.8 123.4 54.81 230-436.4 -381.5 293.4 72.4 21.76 193.09 -76.78 300~700-277.63 -174.36 160.7 111.46 106.52 165.7 575.3 283~348-235.31 -168.54 282.1 71.120.694-205.38-99.809300~1500-248.283 -155.33 200.0 124.73 55.61 232.2 298~320-216.69 -152.2 296.00 75.322.472201.78-63.521298~1500-273.2 -116.47 253.1 170.7 290-384.55 -245.5 170.7 155.2 卤代烃-82.0 -58.6 234.29 40.7914.90396.2-31.552273~800-88 -59 270.62 51.38 33.47 65.3 273~800-131.8 -71.4 202.9 116.3-100 -67 296.48 65.8129.506148.942-90.713273~800-139.3 -68.5 214.43 131.75 97.99 111.71 273~330-106.7 -64.0 309.41 85.51116.3 -198.2 197.5 145.6含氮化合物(待续)见网页(fulu4b2s2.html)(续表)物质适用温度范围/K78.87 159.9 179.1 140.2 29335.31 153.35 191.6 199.6 338.28 -1068.6 2022.1 278~34815.90 146.36 244.3 185.4 293本附录数据主要取自Handbook of Chemistry and Physics ,70 th Ed.,1990;Editor John A.Dean ,Lange's Handbook of Chemistry ,1967。

热力学数据在无机化学中的应用引言化学热力学数据在化学领域里有着广泛的应用。
在无机化学范围内,主要讨论能量平衡和一定条件下,化学反应进行的方向及限度和反应发生条件,还有就是判断一些无机物的溶解性。
1、在化学反应中的应用1.1 判断化学反应进行的方向及发生条件综合焓变(△H)和熵变(△S)这两个能量项, 热力学用自由能(G)这个状态函数的变化量(△G)来判断反应进行的方向, 即吉布斯一亥姆霍兹提出的[1]:△G = △H - T△S (1)或△Gθ298 = △Hθ298 - T△Sθ298(2)当△G < 0 过程自发△G = 0 过程处于平衡态△G > O 过程非自发(或逆过程自发)因此, 在恒温恒压下进行的化学反应:(1 ) 体系焓减或熵增, △G < 0 , 反应自发进行。
(2 ) 当反应热很小, 熵效应对反应进行的方向起决定性作用。
(3 ) 反应熵变很小,特别在低温时, T△S此项影响不大,△H决定反应进行的方向。
(4 ) 反应熵变较大, 特别当温度变化较大时, 有可能导致△G符号的改变, 从而改变反应进行的方向。
例1 石灰窑中烧制石灰的反应为CaCO3(s)→ CO2(g) + CaO(s)试计算欲使石灰石以一定速度分解所需的最低温度是多少?解: 查得有关物质的△Gθ、△Hθ和SθCaCO3(s) →CO2(g) + CaO(s) △Gθ(KJ•mol-1) -1128.8 -604.2 -394.0△Hθ(KJ•mol-1) -1207.0 -635.1 -393.0Sθ (J•mol-1•K-1) 92.9 39.7 214.0计算得该反应的△Gθ298= 130.6 KJ•mol-1 , 则该反应在室温下不能自发进行。
再计算得该反应的△Hθ298=178.9 J•mol-1;△Sθ298=160.8J•mol-1•K-1,1根据△G θ = △H θ - T △S θ 欲使反应自发进行,必需△G θ < 0,即T △S θ > △H θ。

化学化工物性数据手册无机卷中数据引言概述:化学化工物性数据手册是一种重要的工具,它提供了无机化学和化工领域中各种物质的数据信息。
在无机卷中,这些数据包括了物质的物理性质、化学性质、热力学性质等方面的信息。
本文将分四个部份详细介绍化学化工物性数据手册无机卷中的数据内容。
一、物质的物理性质1.1 密度和相对密度:数据手册提供了无机物质在不同温度和压力下的密度和相对密度信息。
这对于物质的计量和混合物的设计非常重要。
1.2 熔点和沸点:熔点和沸点是物质的重要物理性质,它们可以用于判断物质的纯度和稳定性。
数据手册中提供了无机物质在不同条件下的熔点和沸点数据。
1.3 折射率和透明度:折射率和透明度是无机物质透明性的重要指标,数据手册中提供了不同无机物质在不同波长下的折射率和透明度数据。
二、物质的化学性质2.1 反应性:数据手册中提供了无机物质的反应性信息,包括与其他物质的反应类型、反应速率等。
这对于无机物质的合成和应用具有重要的指导意义。
2.2 溶解性:无机物质的溶解性是其在溶液中的溶解度,数据手册提供了无机物质在不同溶剂中的溶解度数据。
这对于溶液的配制和反应的进行非常重要。
2.3 氧化还原性:无机物质的氧化还原性是其与氧化剂或者还原剂反应的能力,数据手册中提供了无机物质的氧化还原电位和反应类型等信息。
三、物质的热力学性质3.1 热容和热导率:热容和热导率是无机物质的重要热力学性质,数据手册中提供了无机物质的热容和热导率数据。
这对于热传导和热平衡的计算和设计非常重要。
3.2 热膨胀系数:热膨胀系数是无机物质在温度变化下的膨胀程度,数据手册提供了无机物质的热膨胀系数数据。
这对于材料的热膨胀补偿和热稳定性分析具有重要意义。
3.3 燃烧热和生成热:燃烧热和生成热是无机物质燃烧或者反应时释放的热量,数据手册提供了无机物质的燃烧热和生成热数据。
这对于能量转化和反应热力学分析非常重要。
四、其他物性数据4.1 毒性和危(wei)险性:数据手册中提供了无机物质的毒性和危(wei)险性评价,包括对人体的危害、环境的影响等方面。

常见标准热力学数据(29815K)附录附录1 常见标准热力学数据(298.15K)-1-1-1-1物质状态ΔH/(kJ?mol) ΔG/(kJ?mol) S/(J?mol?K) fmfmmAg s 0 0 42.6 +Ag aq 105.6 77.1 72.7 AgBr s -100.4 -96.9 107.1 AgCl s -127.0 -109.8 96.3 AgI s -61.8 -66.2 115.5 AgNO s -124.4 -33.4 140.9 3 AgO s -31.1 -11.2 121.3 2Al s 0 0 28.3 3+Al aq -531.0 -485.0 -321.7 AlCls -704.2 -628.8 110.7 3Al(OH) s -1284 -1306 71 3Br l 0 0 152.2 2,Br aq -121.6 -104.0 82.4 C(石墨) s 0 0 5.7s 1.9 2.9 2.4 C(金刚石)Ca s 0 0 41.6 2+Ca aq -542.8 -553.6 -53.1 CaC s -59.8 -64.9 70.0 2 CaCO(方解石) s -1207.6 -1129.1 91.7 3CaCl s -795.4 -748.8 108.4 2CaO s -634.9 -603.3 38.1 Ca(OH) s -985.2 -897.5 83.4 2Cl g 0 0 223.1 2- Claq -167.2 -131.2 56.5- ClOaq -104.0 -8.0 162.3 3- ClOaq -129.3 -8.5 182.0 4CCl l -128.2 -62.6 216.2 4CH g -74.6 -50.5 186.3 4CHOH l -239.2 -166.6 126.8 3CO(NH) s -333.1 -196.8 104.6 22CHNH g -22.5 32.7 242.9 32CH g 227.4 209.9 200.9 22CH g 52.4 68.4 219.3 24CHCHO l -192.2 -127.6 160.2 3CHCOOH l -484.3 -389.9 159.8 3CH g -84.0 -32.0 229.2 26CHOH l -277.6 -174.8 160.7 25(CH)CO l -248.4 -152.7 199.8 321-1-1-1-1H/(kJ?mol) ΔG/(kJ?mol) S/(J?mol?K) 物质状态ΔfmfmmCH g -103.8 -23.4 270.3 38CH l 49.1 124.5 173.4 66g 82.9 129.7 269.2 CO g -110.5 -137.2 197.7 CO g -393.5 -394.4 213.8 22-CO aq -677.1 -527.8 -56.9 32-CrO aq -881.2 -727.8 50.2 4 CrO s -1139.7 -1058.1 81.2 23Cu s 0 0 33.2 2+Cu aq 64.8 65.5 -99.6 CuO s -157.3 -129.7 42.6 CuO s -168.6 -146.0 93.1 2CuS s -53.1 -53.6 66.5 F g 0 0 202.8 2,F aq -332.6 -278.8 -13.8 Fe s 0 0 27.3 2+Fe aq -89.1 -78.9 -137.7 3+Fe aq -48.5 -4.7 -315.9 FeO s -824.2 -742.2 87.4 23FeSO s -928.4 -820.8 107.5 4H g 0 0 130.7 2+H aq 0 0 0 HBr g -36.3 -53.4 198.7 HCl g -92.3 -95.3 186.9-HCO aq -692.0 -586.8 91.2 3HCHO g -108.6 -102.5 218.8 HCOOH l -425.0 -361.4 129.0 HF g -273.3 -275.4 173.8 HI g 26.5 1.7 206.6 HNO l -174.1 -80.7 155.6 3 HO l -285.8 -237.1 70.0 2g -241.8 -228.6 188.8 HO l -187.8 -120.4 109.6 22g -136.3 -105.6 232.7 HS g -20.6 -33.4 205.8 2HSO l -814.0 -690.0 156.9 24HgO s -90.8 -58.5 70.3 I s 0 0 116.1 2g 62.4 19.3 260.7 -I aq -55.2 -51.6 111.3 K s 0 0 64.7 +K aq -252.4 -283.3 102.52-1-1-1-1H/(kJ?mol) ΔG/(kJ?mol) S/(J?mol?K) 物质状态ΔfmfmmKCl s -436.5 -408.5 82.6 KClO s -397.7 -296.3 143.1 3+Li aq -278.5 -293.3 13.4 Mg s 0 0 32.7 2+Mg aq -466.9 -454.8 -138.1 MgCl s -641.3 -591.8 89.6 2 MgO s -601.6 -569.3 27.0 Mg(OH) s -924.5 -833.5 63.2 2MgSO s -1284.9 -1170.6 91.6 42+Mn aq -220.8 -228.1 -73.6 MnO s -520.0 -465.1 53.1 2- MnOaq -541.4 -447.2 191.2 4N g 0 0 191.6 2Na s 0 0 51.3 +Na aq -240.1 -261.9 59.0 NaCl s -411.2 -384.1 72.1 NaCO s -1130.7 -1044.4 135.0 23NaF s -576.6 -546.3 51.1 NaO s -414.2 -375.5 75.1 2NaOH s -425.6 -379.5 40.0 NH g -45.9 -16.4 192.8 3+NH aq -132.5 -79.3 113.4 4NHNO s -365.5 -183.9 151.1 43NO g 91.3 87.6 210.8 NO g 33.2 51.3 240.1 2- NOaq -207.4 -111.3 146.4 3O g 0 0 205.2 2O g 142.7 163.2 238.9 3-OH aq -230.0 -157.2 -10.8 P g 58.9 24.4 280.0 4PCl g -287.0 -267.8 311.8 3PCl g -374.9 -305.0 364.6 53-PO aq -1277.4 -1018.7 -220.5 4S(正交) s 0 0 32.1 SO g -296.8 -300.1 248.2 2SO g -395.7 -371.1 256.8 3Si s 0 0 18.8 SiCll -687.0 -619.8 239.7 4g -657.0 -617.0 330.7 SiH g 34.3 56.9 204.6 4SiO s -910.7 -856.3 41.5 2Sn(白) s 0 0 51.23-1-1-1-1H/(kJ?mol) ΔG/(kJ?mol) S/(J?mol?K) 物质状态ΔfmfmmSnO s -577.6 -515.8 49.0 2Zn s 0 0 41.6 ZnO s -350.5 -320.5 43.7附录2 常见弱电解质的标准解离常数(298.15K) 附录2.1酸,,名称化学式 K pK aa, -3砷酸HAsO K 5.50×10 2.26 134a, -7 K1.74×10 6.76 2a, -12 K 5.13×10 11.29 3a -10HAsO 9.29 亚砷酸 5.13×10 33 -10硼酸HBO 5.81×10 9.236 33, -4焦硼酸HBO K1.00×10 4.00 1247a, -9 K1.00×10 9.00 2a, -7碳酸HCO K4.47×10 6.35 123a, -11 K4.68×10 10.33 2a, -1HCrO 0.74 铬酸K1.80×10 124a, -7 K3.20×10 6.49 2a -4氢氟酸 HF 6.31×10 3.20-4亚硝酸HNO 5.62×10 3.25 2 -12过氧化氢HO 2.4×10 11.62 22, -3磷酸HPO K6.92×10 2.16 134a, -8 7.21 K6.23×10 2a, -13 K4.80×10 12.32 3a, -1焦磷酸HPO K1.23×10 0.91 1427a, -3 K7.94×10 2.10 2a, -7 K2.00×106.70 3a, -10 K4.79×10 9.32 4a, -8HS7.05 氢硫酸K8.90×10 12a, -14K1.26×10 13.9 2a, -2亚硫酸HSO K1.40×10 1.85 123a, -2 K6.31×10 7.202a, -2硫酸HSO K1.02×10 1.99 224a, -10偏硅酸 HSi O K1.70×10 9.77 123a, -12 11.80 K1.58×10 2a -4甲酸HCOOH 1.772×10 3.75-5醋酸CHCOOH 1.74×10 4.76 3, -2草酸HCO K5.9×10 1.23 1224a, -5K6.46×10 4.19 2a, -3酒石酸HOOC(CHOH)COOH K1.04×10 2.98 12a, -5 4.34K4.57×10 2a -10苯酚CHOH 1.02×10 9.99 654,, pK 名称化学式 Kaa, -5抗坏血酸 5.0×10 4.10K=1C(OH)=C(OH)OCaCHCHOHOHCH2, -10O K1.5×10 11.79 2a , -4柠檬酸 HO-C(CHCOOH)COOH K7.24×10 3.14 122 a, -5 K1.70×10 4.77 2a, -7 6.39K4.07×10 3a -5苯甲酸CHCOOH 6.45×10 4.19 65, -3邻苯二甲酸 CH(COOH)K1.30×10 2.89 1642a, -6 K3.09×10 5.51 2a附录2.2碱,,名称化学式 K pK bb-5NH?HO 4.75 氨水 1.79×10 32-4甲胺 CHNH4.20×10 3.38 32-4乙胺CHNH 4.30×10 3.37 252-4二甲胺(CH)NH5.90×10 3.23 32-4二乙胺(CH)NH6.31×10 3.2 252-10苯胺CHNH 3.98×10 9.40 652, -5HNCHCHNH 4.08 乙二胺K8.32×10 12222b, -8 K7.10×10 7.15 2b-5乙醇胺HOCHCHNH 3.2×10 4.50 222-7三乙醇胺(HOCHCH)N 5.8×10 6.24 223-9六次甲基四胺(CH)N 1.35×10 8.87 264-9吡啶CHN 1.80×10 8.70 55附录3 常见难溶电解质的溶度积(298.15K~离子强度I=0) ,,,,化学式 K pK 化学式 K pK spspspsp-13-11AgBr 5.35×10 12.27 CaF 3.45×10 10.46 2 -12-27AgCO 8.46×10 11.07 CdS 8.0×10 26.10 23-10-21AgCl 1.77×10 9.75 CoS(α) 4.0×10 20.40 -12-25AgCrO 1.12×10 11.95 CoS(β) 2.0×10 24.70 24-17-31AgI 8.52×10 16.07 Cr(OH) 6.3×10 30.20 3-8-9AgOH 2.0×10 7.71 CuBr 6.27×10 8.20 -50-7AgS 6.3×10 49.20 CuCl 1.72×10 6.76 2-33-12Al(OH)(无定形) 1.3×10 32.89 CuI 1.27×10 11.90 3-9-36BaCO 2.58×10 8.59 CuS 6.3×10 35.20 3-7-48BaCO 1.6×10 6.79 CuS 2.5×10 47.60 242-10-13BaCrO 1.17×10 9.93 CuSCN 1.77×10 12.75 4-10-7BaSO 1.08×10 9.97 FeCO,2HO 3.2×10 6.50 4242-9-17CaCO 3.36×10 8.47 Fe(OH) 4.87×10 16.31 32-9-39CaCO,HO 2.32×10 8.63 Fe(OH) 2.79×10 38.55 24235,,,, pK 化学式 K pK 化学式 Kspspspsp-18-14FeS 6.3×10 17.20 PbCO 7.40×10 13.13 3-18-10HgCl 1.43×10 17.84 PbCO 4.8×10 9.32 2224-29-13HgI 5.2×10 28.72 PbCrO 2.8×10 12.55 224-53-8HgS(红) 4.0×10 52.40 PbF 3.3×10 7.48 2-52-9HgS(黑) 1.6×10 51.80 PbI 9.8×10 8.01 2-6-20MgCO 6.82×10 5.17 Pb(OH) 1.43×10 19.84 32-6-28MgCO,2HO 4.83×10 5.32 PbS 8.0×10 27.10 242-11-8MgF 5.16×10 10.29 PbSO 2.53×10 7.60 24-13-10MgNHPO 2.5×10 12.60 SrCO 5.60×10 9.25 443-12-7Mg(OH) 5.61×10 11.25 SrSO 3.44×10 6.46 24-13-27Mn(OH) 1.9×10 12.72 Sn(OH) 5.45×10 26.26 22-13-56MnS 2.5×10 12.60 Sn(OH) 1.0×10 56.00 4-16-17Ni(OH) 5.48×10 15.26 Zn(OH)(无定形) 3×10 16.5 22-19-24NiS (α) 3.2×10 18.49 ZnS(α) 1.6×10 23.80-24-22NiS (β) 1.0×10 24.00 ZnS(β) 2.5×10 21.60, 附录4 常见氧化还原电对的标准电极电势E附录4.1在酸性溶液中电对电极反应 E/ V ++Li/ Li Li + e Li -3.0401 ++Cs/Cs Cs + e Cs -3.026 + +K/ K K + e K -2.931 2+2+Ba / Ba Ba + 2e Ba -2.912 2+2+Ca / Ca Ca + 2e Ca -2.868 ++Na / Na Na + e Na -2.71 2+2+Mg/ Mg Mg + 2e Mg -2.372 --H/ H 1/2 H+ e H -2.23 2 2 3+3+Al /Al Al + 3e Al -1.662 2+2+Mn / Mn Mn + 2e Mn -1.185 2+2+Zn / Zn Zn + 2e Zn -0.7618 3+3+Cr /Cr Cr + 3e Cr -0.744-2-AgS /Ag AgS +2e 2Ag + S -0.691 22+CO / HCO 2CO + 2H + 2e HCO -0.481 222422242+2+Fe / Fe Fe + 2e Fe -0.447 3+2+3+2+Cr/ Cr Cr + e Cr -0.407 2+2+Cd/ Cd Cd + 2e Cd -0.40302-PbSO/ Pb PbSO +2e Pb + SO -0.3588 4442+2+Co/Co Co + 2e Co -0.28 -PbCl/ Pb PbCl +2e Pb +2Cl -0.2675 222+2+Ni / Ni Ni + 2e Ni -0.2576电对电极反应 E/ V , -0.15224 AgI /Ag AgI + e Ag + I2+2+Sn / Sn Sn + 2e Sn -0.1375 2+2+Pb / Pb Pb + 2e Pb -0.1262 3+3+Fe / Fe Fe +3e Fe -0.037-AgCN / Ag AgCN + e Ag + CN -0.017 ++H / H 2H + 2e H 0.0000 22-AgBr /Ag AgBr + e Ag + Br 0.07133+S/HS S + 2H + 2e HS (aq) 0.142 224+2+4+2+Sn/Sn Sn + 2e Sn 0.1512++2++Cu/Cu Cu + e Cu 0.153 ,AgCl/Ag 0.22233 AgCl + e Ag + Cl -HgCl/Hg HgCl +2e 2Hg +2Cl 0.26808 22222+2+Cu /Cu Cu + 2e Cu 0.3419 +2-2-SO/S SO + 6H + 4e 2S + 3HO 0.5 23232++Cu /Cu Cu + e Cu0.521 ,,I/ I I+ 2e 2I 0.5355 2 2 ,,,,0.536 I/ I I+ 2e 3I 33 ,2--2-MnO/ MnO MnO + e MnO 0.558 4444+HAsO/HAsO HAsO + 2H + 2e HAsO + 2HO 0.560 34234222-AgSO/Ag AgSO +2e 2Ag + SO 0.654 24244+ O / HO O + 2H+ 2e HO0.695 2222223+2+3+2+Fe /Fe Fe + e Fe 0.771 2+2+Hg/ Hg Hg + 2e 2Hg 0.7973 22++Ag /Ag Ag + e Ag 0.7996+--NO /NO 2NO + 4H + 2e NO + 2HO 0.803 32432422+2+Hg / Hg Hg + 2e Hg 0.851 2+2+-Cu /CuI Cu + I + e CuI 0.86 2+2+2+2+Hg/Hg 2Hg + 2e Hg 0.920 22+--NO / HNO NO + 3H + 2e HNO+ HO 0.934 32 32 2+- - NO/ NO NO+ 4H + 3e NO + 2HO 0.957 332+HNO/ NO HNO+ H + e NO + HO 0.983 2 2 2,,, [AuCl]/Au [AuCl] + 3e Au + 4Cl1.002 44--Br/ Br Br(l) + 2e 2Br 1.066 2 2,,2+2+-Cu / [Cu(CN)] Cu + 2CN + e [Cu(CN)] 1.103 22+--IO / HIO IO + 5H + 4e HIO +2HO 1.14 332+--IO / I 2IO + 12H + 10e I + 6HO 1.195 323222++2+MnO/ Mn MnO + 4H +2e Mn + 2HO 1.224 222+O / HO O + 4H + 4e 2HO 1.229 2222+3+2-3+2-O + 14H + 6e 2Cr + 7HO 1.232 CrCrO /Cr 27227- - Cl/Cl Cl(g) + 2e 2Cl 1.35827 2 2+-- ClO/Cl 2ClO + 16H + 14e Cl+ 8HO 1.39 42422-+---ClO/Cl ClO + 6H + 6e Cl + 3HO 1.451 3327电对电极反应 E/ V2++2+ /Pb PbO + 4H +2e Pb + 2HO 1.455 PbO222+--ClO/Cl ClO + 6H + 5e 1/2 Cl + 3HO 1.47 32322+--BrO /Br 2BrO + 12H + 10e Br + 6HO 1.482 32322-+-HClO /Cl HClO + H + 2e Cl + HO 1.482 23+3+Au /Au Au + 3e Au 1.498 2++2+--MnO/Mn MnO + 8H + 5e Mn + 4HO 1.507 4423+2+3+2+Mn / Mn Mn + e Mn 1.5415+HBrO / Br 2HBrO + 2H + 2e Br + 2HO 1.596 222,,+HIO / IO HIO + H +2e IO + 3HO 1.601 5635632+HClO /Cl 2HClO + 2H + 2e Cl + 2HO 1.611222+HClO /HClO HClO + 2H + 2e HClO + HO 1.645 222,,+MnO/ MnO MnO + 4H + 3e MnO + 2HO 1.679 42422+2-PbO / PbSO PbO + SO + 4H +2e PbSO + 2HO1.6913 242442+HO / HO HO + 2H + 2e 2HO 1.776 2222223+2+3+2+Co / Co Co +e Co 1.92 ,2- 2-22-SO/ SO SO + 2e 2SO 2.010 284284+O / O O + 2H + 2e O + HO 2.076 32322--F / F F + 2e 2F 2.866 22+F/ HF F(g) + 2H + 2e 2HF 3.503 2 2附录4.2在碱性溶液中电对电极反应 E/ V- Mn(OH) / Mn Mn(OH) + 2e Mn + 2OH-1.56 222- 2-- [Zn(CN)]/ Zn[Zn(CN)] + 2e Zn + 4CN-1.34 44-2-2-ZnO / Zn ZnO + 2HO + 2e Zn + 4OH -1.215 2222- -2---[Sn(OH)]/ HSnO [Sn(OH)] + 2e HSnO + 3OH + HO -0.93 62622-2-2-2-2-SO/ SO SO+ HO + 2e SO+ 2OH -0.93 4 34 23 -- - HSnO/ Sn HSnO+ HO + 2e Sn + 3OH -0.909 222-HO/ H 2HO + 2e H + 2OH -0.8277 2222-Ni(OH) / Ni Ni(OH) +2e Ni + 2OH -0.72 22-3--3--AsO / AsO AsO + 2HO + 2eAsO + 4OH -0.71 42422-2-2-SO/ S SO+ 3HO + 4e S + 6OH -0.59 3 3 2-2-2-2-2-SO/ SO 2SO+ 3HO + 4e SO + 6OH -0.571 3 233 2232- 2-S /SS + 2e S -0.47627- --[Ag(CN)]/Ag [Ag(CN)] + e Ag + 2CN -0.31 22-2--2--CrO/ CrO CrO + 4HO + 3e Cr(OH) + 4OH -0.13 42424---O / HO O + HO + 2e HO + OH -0.076 22222-----NO / NO NO + HO + 2e NO + 2OH 0.01 323222-2-2-2- SO / SO SO + 2e 2SO0.08 462346233+2+3+2+[Co(NH)]/ [Co(NH)] [Co(NH)] + e [Co(NH)]0.108 363636362+-MnO/ Mn Mn(OH) +e Mn(OH)+ OH 0.15 232 -3+2- +e Co(OH) + OH 0.17 Co(OH)CrO /Cr 32278电对电极反应 E/ V- O/Ag AgO + HO + 2e 2Ag + 2OH 0.342 Ag222-- O/ OH O + 2HO + 4e 4OH 0.401 2 22---MnO/ MnO MnO + 2HO + 3e MnO + 4OH 0.595 42422,,---BrO / Br BrO + 3HO + 6e Br + 6OH 0.61 332,,---0.761 BrO / Br BrO + HO + 2e Br +2OH 2-----ClO/ Cl ClO + HO + 2e Cl + 2OH 0.81 2--HO / OH HO + 2e 2OH0.88 2222--O / OH O + HO + 2e O + 2OH 1.24 3322,附录5 一些氧化还原电对的条件电极电势E’,电极反应介质E’ / V +-1Ag(?) + e Ag 1.927 4mol?LHNO 3-11.70 Ce(?) + e Ce(?) 1mol?LHClO 4-1 1.61 1mol?LHNO 3-1 1.44 0.5mol?LHSO 24-1 1.28 1mol?LHCl 3+2+-1-1[Co(en)] + e [Co(en)] -0.20 0.1mol?LKNO + 0.1mol?L en 333+3+2--1CrO + 14H + 6e 2Cr + 7HO 1.000 1mol?LHCl 272-1 1.0301mol?LHClO 4-1 1.080 3mol?LHCl-1 1.050 2mol?LHCl-1 1.150 4mol?LHSO 24-2---1CrO + 2HO + 3e CrO + 4OH -0.1201mol?LNaOH 422-1Fe(?) + e Fe(?) 0.750 1mol?LHClO 4-1 0.670 0.5mol?LHSO 24-1 0.700 1mol?LHCl-1 0.460 2mol?LHPO 34+-1HAsO + 2H + 2e HAsO + HO 0.557 1mol?LHCl 34332+-1HSO + 4H + 4e S + 3HO 0.557 1mol?LHClO 2324-2--1Fe(EDTA) + eFe(EDTA) 0.120 0.1mol?LEDTA(pH=4~6) 3-4--1[Fe(CN)] + e [Fe(CN)] 0.480 0.01mol?LHCl 66-1 0.560 0.1mol?LHCl-1 0.720 1mol?LHClO 4--1+I(水)+ 2e 2I 0.6276 1mol?LH 2+2+--1MnO + 8H + 5e Mn + 4HO 1.450 1mol?LHClO 424-1 1.27 8mol?LHPO 342-2---1[SnCl] + 2e [SnCl] +2Cl 0.140 1mol?LHCl 642+-1Sn + 2e Sn -0.160 1mol?LHClO 4-1Sb(?) + 2e Sb(?) 0.750 3.5mol?LHCl -- --1[Sb(OH)] + 2e SbO +2OH+ 2HO -0.4283mol?LNaOH 622- --1SbO + 2HO + 3e Sb + 4OH -0.675 10mol?LKOH 22-1Ti(?) +e Ti(?) -0.010 0.2mol?LHSO 249,电极反应介质E’ / V -1 0.120 2mol?LHSO 24-1 -0.040 1mol?LHCl-1Pb(?) + 2e Pb -0.320 1mol?LNaAc -1 -0.140 1mol?LHClO 4附录6 常见配离子的稳定常数配位体金属离子n lgβ n+NH Ag 1, 2 3.24, 7..05 32+ Cu 4.31, 7.98, 11.02, 13.32 1,……, 4 2+ Ni 1,……, 6 2.80, 5.04, 6.77, 7.96, 8.71, 8.742+ Zn 1,……, 4 2.37, 4.81, 7.31, 9.46-3+F Al 1,……, 6 6.10, 11.15, 15.00, 17.75, 19.37, 19.843+ Fe 1, 2, 3 5.28, 9.30, 12.06-2+Cl Hg 6.74, 13.22, 14.07, 15.07 1,……, 4-+CN Ag 2, 3, 4 21.1, 21.7, 20.62+ Fe 6 353+ Fe 6 422+ Ni 4 31.32+ Zn 4 16.7+2-SO Ag 1, 2 8.82, 13.46 232+ Hg 2, 3, 4 29.44, 31.90, 33.24 -3+OH Al 1, 4 9.27, 33.033+ Bi 1, 2, 4 12.7, 15.8, 35.22+ Cd 1,……, 4 4.17, 8.33, 9.02, 8.622+ Cu 7.0, 13.68, 17.00, 18.5 1,……, 42+ Fe 1,……, 4 5.56, 9.77, 9.67, 8.583+ Fe 1, 2, 3 11.87, 21.17, 29.672+ Hg 1, 2, 3 10.6, 21.8, 20.92+ Mg 1 2.582+ Ni 1, 2, 3 4.97, 8.55, 11.332+ Pb 1, 2, 3, 6 7.82, 10.85, 14.58, 61.02+ Sn 1, 2, 3 10.60, 20.93, 25.382+ Zn 1,……, 4 4.40, 11.30, 14.14, 17.66+EDTA Ag 1 7.323+ Al 1 16.112+ Ba1 7.783+ Bi1 22.82+ Ca1 11.02+ Cd1 16.42+ Co 1 16.313+ Co 1 36.0010n 配位体金属离子lgβn3+EDTA Cr 1 232+ Cu 1 18.702+ Fe 1 14.333+ Fe 1 24.232+ Hg 1 21.802+ Mg 1 8.642+ Mn 1 13.82+ Ni 1 18.562+ Pb 1 18.32+ Sn 1 22.12+ Zn 1 16.4 注:表中数据为20,25?、I=0的条件下获得。