USP Glycerin DEG 0.1%
- 格式:pdf
- 大小:99.72 KB
- 文档页数:3
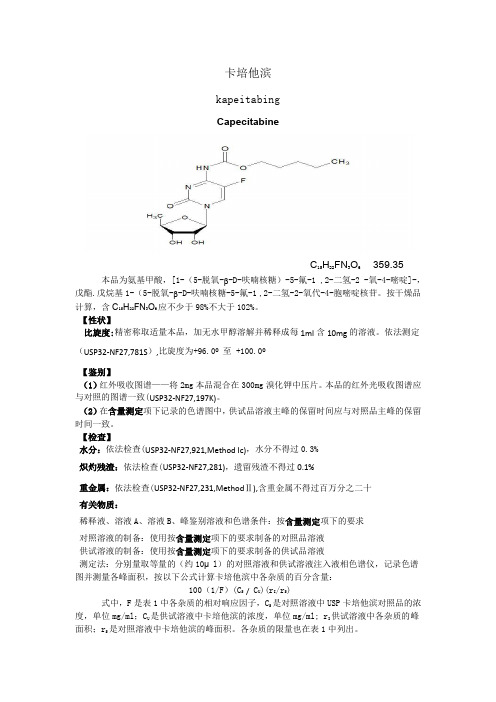
卡培他滨kapeitabingCapecitabineC15H22FN3O6 359.35本品为氨基甲酸,[1-(5-脱氧-β-D-呋喃核糖)-5-氟-1 ,2-二氢-2 -氧-4-嘧啶]-,戊酯.戊烷基1-(5-脱氧-β-D-呋喃核糖-5-氟-1 ,2-二氢-2-氧代-4-胞嘧啶核苷。
按干燥品计算,含C15H22FN3O6应不少于98%不大于102%。
【性状】比旋度:精密称取适量本品,加无水甲醇溶解并稀释成每1ml含10mg的溶液。
依法测定(USP32-NF27,781S),比旋度为+96.0⁰至 +100.0⁰【鉴别】(1)红外吸收图谱——将2mg本品混合在300mg溴化钾中压片。
本品的红外光吸收图谱应与对照的图谱一致(USP32-NF27,197K)。
(2)在含量测定项下记录的色谱图中,供试品溶液主峰的保留时间应与对照品主峰的保留时间一致。
【检查】水分:依法检查(USP32-NF27,921,Method Ic),水分不得过0.3%炽灼残渣:依法检查(USP32-NF27,281),遗留残渣不得过0.1%重金属:依法检查(USP32-NF27,231,MethodⅡ),含重金属不得过百万分之二十有关物质:稀释液、溶液A、溶液B、峰鉴别溶液和色谱条件:按含量测定项下的要求对照溶液的制备:使用按含量测定项下的要求制备的对照品溶液供试溶液的制备:使用按含量测定项下的要求制备的供试品溶液测定法:分别量取等量的(约10μl)的对照溶液和供试溶液注入液相色谱仪,记录色谱图并测量各峰面积,按以下公式计算卡培他滨中各杂质的百分含量:100(1/F)(C S / C U)(r I/r S)式中,F是表1中各杂质的相对响应因子,C S是对照溶液中USP卡培他滨对照品的浓度,单位mg/ml;C U是供试溶液中卡培他滨的浓度,单位mg/ml; r I供试溶液中各杂质的峰面积;r S是对照溶液中卡培他滨的峰面积。
各杂质的限量也在表1中列出。


附件3:化妆品用甘油原料要求为规范化妆品原料技术要求,提高化妆品卫生质量安全,参考国内外化妆品法规的变化,编写《化妆品用甘油原料要求》(以下称《要求》),本《要求》针对性地规定了甘油的安全性要求及检验方法,其他相关要求及检验方法按相应规定执行。
1. 基本信息1.1 名称甘油1.1.1 INCI名称及其ID号GLYCERINID:10771.1.2 INCI标准中文译名甘油1.1.3 化学系统命名法名称或《中国药典》中名称系统命名法:1,2,3-丙三醇(Propane-1,2,3-triol)2010年版《中国药典》(二部)中名称:甘油1.1.4 常见俗名Glycerol (INN,RIFM, EP)Glycerolum (EP)1.2 登记号1.2.1 CAS登记号56-81-51.2.2 EINECS登记号200-289-51.3 分子式、结构式及分子量分子式:C3H8O3结构式:分子量:92.091.4 性状及理化指标无色、澄清的黏稠液体;味甘甜,有引湿性,水溶液(1→10)显中性反应;本品与水或乙醇任意混溶,在丙酮中微溶,在三氯甲烷或乙醚中均不溶;相对密度在25℃时不小于1.2569。
2. 技术要求2.1 使用目的及适用范围可作保湿剂,降粘剂、变性剂等,广泛用于化妆品中。
2.2限量要求2.2.1 甘油纯度要求甘油(%)≥ 95.02.2.2甘油相关组分要求应对甘油中二甘醇含量进行必要的安全性风险评估分析,以保证产品在正常以及合理的、可预见的使用条件下,不会对人体健康产生安全危害。
3. 检验方法3.1 鉴别试验方法甘油样品的红外光吸收图谱(膜法)应与对照的图谱(2010年版《中国药典》光谱集77图)一致,图谱见下图。
3.2 含量测定方法取本品0.20g,精密称定1,加水90ml,混匀,精密加入2.14%(g/ml)高碘酸钠溶液50ml,摇匀,暗处放臵15分钟后,加50%(g/ml)乙二醇溶液10ml,摇匀,暗处放臵20分钟,加酚酞指示液2 0.5ml,用氢氧化钠滴定液(0.1mol/L)滴定至红色,30秒内不褪色,并将滴定的结果用空白试验校正3。

辅料甘油USP操作方法甘油(Glycerin),也称甘油醇,是一种无色、无味且具有粘稠性质的有机物,广泛应用于医药、食品、化妆品等行业。
本文将详细介绍甘油USP(United States Pharmacopeia)的操作方法,并给出一些使用甘油USP的常见场景和注意事项。
甘油USP操作方法:1. 准备工作:在进行任何操作之前,确保工作区域干净整洁,有充足的通风。
戴上适当的个人防护装备,如手套、护目镜等。
2. 实验室试剂准备:使用合适的容器将需要的甘油USP倒入,可以使用玻璃烧杯、瓶子或塑料容器。
3. 需要时可以准备一些蒸馏水,以便进行稀释或冲洗等操作。
4. 执行操作:将所需的量的甘油USP小心地倒入容器中。
根据需要,可以加热容器来加快甘油的溶解。
5. 如果需要稀释甘油,添加适量的蒸馏水,并轻轻搅拌混合。
6. 根据具体的应用需求,可以将甘油USP转移至专用的储存容器中,如密封瓶或仓储罐。
7. 在处理完甘油之后,清洗使用过的容器,并将残余液体妥善处理,以确保实验室环境的安全。
甘油USP的常见应用场景:1. 医药行业:甘油USP可用作许多药物、口服药物和外用药膏的基础成分,如咳嗽糖浆、缓释药片和皮肤炎症药膏等。
2. 化妆品行业:甘油USP可在护肤品和化妆品中发挥保湿、柔软皮肤、增加产品粘稠度等作用。
3. 食品行业:甘油USP可以用作甜味剂、保湿剂和防止食品干燥的添加剂,如糖果、饼干、甜点等。
4. 烟草行业:甘油USP是电子烟液中最常见的基础成分之一,提供烟雾的口感和浓度。
甘油USP的注意事项:1. 甘油USP应存放在干燥、阴凉、通风的地方,避免阳光直射和高温。
2. 使用甘油USP时应佩戴适当的个人防护装备,以避免与皮肤、眼睛或其他黏膜接触。
3. 如果意外接触到甘油USP,应立即用大量清水冲洗,必要时寻求医疗帮助。
4. 甘油USP在处理和储存过程中应避免与酸、氧化剂等有害物质接触,以免发生不可预测的反应。

Glycerin (glis' er in).C 3H 8O 392.101,2,3-Propanetriol; Glycerol [56-81-5].DEFINITIONGlycerin contains NLT 99.0% and NMT 101.0% of C 3H 8O 3, calculated on the anhydrous basis. IDENTIFICATION[N OTE —Compliance is determined by meeting the requirements for Identification tests A, B, and C . ]• A. I NFRARED A BSORPTION 197F• B. L IMIT OF D IETHYLENE G LYCOL AND E THYLENE G LYCOLStandard solution: 2.0 mg/mL of USP Glycerin RS , 0.050 mg/mL of USP Ethylene Glycol RS , 0.050 mg/mL of USP Diethylene Glycol RS , and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanolSample solution: 50 mg/mL of Glycerin and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanol Chromatographic system(See Chromatography 621, System Suitability .) Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase, and a deactivated split liner with glass wool TemperatureInjector: 220 Detector: 250Column: See the temperature program table.Carrier gas: Helium Injection size: 1.0 µLInitialTemperature ()FinalTemperature ()Flow rate: 4.5 mL/minInjection type: Split ratio, about 10:1 System suitabilitySample: Standard solution[N OTE —The relative retention times for ethylene glycol, 2,2,2-trichloroethanol, diethylene glycol, and glycerin are about 0.3, 0.6, 0.8 and 1.0, respectively. ] Suitability requirementsResolution: NLT 1.5 between diethylene glycol and glycerin AnalysisSample: Sample solutionAcceptance criteria: If a peak at the retention times for the diethylene glycol or ethylene glycol is present in the Sample solution, the peak response ratio relative to 2,2,2-trichloroethanol is NMT the peak response ratio for diethylene glycol or ethylene glycol relative to 2,2,2-trichloroethanol in the Standard solution; NMT 0.10% each for diethylene glycol and ethylene glycol is found.• C. Examine the chromatograms obtained in Identification test B . The retention time of the glycerinpeak of the Sample solution corresponds to that obtained in the Standard solution . ASSAY• P ROCEDURESodium periodate solution: Dissolve 60 g of sodium metaperiodate in sufficient water containing 120 mL of 0.1 N sulfuric acid to make 1000 mL. Do not heat to dissolve theperiodate. If the solution is not clear, pass through a sintered-glass filter. Store the solution in a glass-stoppered, light-resistant container. Test the suitability of this solution as follows. Pipet 10 mL into a 250-mL volumetric flask, and dilute with water to volume. To 550 mg of Glycerin dissolved in 50 mL of water, add 50 mL of the diluted periodate solution with a pipet. For a blank, pipet 50 mL of the solution into a flask containing 50 mL of water. Allow the solutions to stand for 30 min, then to each add 5 mL of hydrochloric acid and 10 mL of potassium iodide TS, and rotate to mix. Allow to stand for 5 min, add 100 mL of water, and titrate with 0.1 N sodium thiosulfate, shaking continuously and adding 3 mL of starch TS as the endpoint is approached. The ratio of the volume of 0.1 N sodium thiosulfate required for the glycerin –periodate mixture to that required for the blank should be between 0.750 and 0.765.Analysis: Transfer 400 mg of Glycerin to a 600-mL beaker, dilute with 50 mL of water, addbromothymol blue TS, and acidify with 0.2 N sulfuric acid to a definite green or greenish yellow color. Neutralize with 0.05 N sodium hydroxide to a definite blue endpoint, free from green color. Prepare a blank containing 50 mL of water, and neutralize in the same manner. Pipet 50 mL of the Sodium periodate solution into each beaker, mix by swirling gently, cover with awatch glass, and allow to stand for 30 min at room temperature (not exceeding 35) in the dark or in subdued light. Add 10 mL of a mixture of equal volumes of ethylene glycol and water, and allow to stand for 20 min. Dilute each solution with water to 300 mL, and titrate with 0.1 N sodium hydroxide VS to a pH of 8.1 ± 0.1 for the specimen under assay and 6.5 ± 0.1 for the blank, using a pH meter. Each mL of 0.1 N sodium hydroxide, after correction for the blank, is equivalent to 9.210 mg of C 3H 8O 3.Acceptance criteria: 99.0%–101.0% on the anhydrous basis IMPURITIESInorganic Impurities• C HLORIDE AND S ULFATE , Chloride 221: A 7.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (NMT 10 ppm). • C HLORIDE AND S ULFATE , Sulfate 221: A 10-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (NMT 20 ppm). • H EAVY M ETALS 231Analysis: Mix 4.0 g with 2 mL of 0.1 N hydrochloric acid, and dilute with water to 25 mL. Acceptance criteria: NMT 5 ppm • R ESIDUE ON I GNITION 281: Heat 50 g in an open, shallow 100-mL porcelain dish until it ignites,and allow it to burn without further application of heat in a place free from drafts. Cool, moisten the residue with 0.5 mL of sulfuric acid, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.01%). Organic Impurities• P ROCEDURE 1: R ELATED C OMPOUNDSSystem suitability solution: 0.5 mg/mL each of USP Diethylene Glycol RS and USP Glycerin RSSample solution: 50 mg/mL of Glycerin Chromatographic system(See Chromatography 621, System Suitability .) Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase, and an inlet liner having an inverted cup or spiral structure TemperatureInjector: 220 Detector: 250Column: See the temperature program table below.Carrier gas: Helium Injection size: 0.5 µL Linear velocity: 38 cm/sInjection type: Split ratio, about 10:1 System suitabilitySample: System suitability solution Suitability requirementsTemperature Ramp (/min)Final Temperature ()Hold Time at FinalTemperature (min)100—100—1007.52204Resolution: NLT 7.0 between diethylene glycol and glycerin AnalysisSample: Sample solutionCalculate the percentage of each impurity, excluding any solvent peaks and diethylene glycol, in the portion of Glycerin taken:Result = (r U /r T) × 100Acceptance criteriaIndividual impurities: NMT 0.1% Total impurities: NMT 1.0%• P ROCEDURE 2: L IMIT OF C HLORINATED C OMPOUNDS Sample: 5 g of GlycerinAnalysis: Transfer the Sample into a dry, round-bottom, 100-mL flask. Add 15 mL ofmorpholine, and connect the flask by a ground joint to a reflux condenser. Reflux gently for 3 h. Rinse the condenser with 10 mL of water, receiving the washings in the flask, and cautiously acidify with nitric acid. Transfer the solution to a suitable comparison tube, add 0.50 mL of silver nitrate TS, and dilute with water to 50.0 mL.Acceptance criteria: The turbidity is not greater than that of a blank to which 0.20 mL of 0.020 N hydrochloric acid has been added, the refluxing being omitted (NMT 30 ppm of Cl). • P ROCEDURE 3: F ATTY A CIDS AND E STERSSample solution: Mix 50 g of Glycerin with freshly boiled water and 5 mL of 0.5 N sodium hydroxide VS. Boil the mixture for 5 min, cool, and add phenolphthalein TS.Analysis: Titrate the excess alkali with 0.5 N hydrochloric acid VS. Perform a blank determination (see Titrimetry 541, Residual Titrations ).Acceptance criteria: NMT 1 mL of 0.5 N sodium hydroxide is consumed.SPECIFIC TESTS• C OLOR : When viewed downward against a white surface in a 50-mL color-comparison tube, thecolor is not darker than the color of a standard made by diluting 0.40 mL of ferric chloride CS with water to 50 mL and similarly viewed in a color-comparison tube of approximately the same diameter and color as that containing the Glycerin. • S PECIFIC G RAVITY 841: NLT 1.249• W ATER D ETERMINATION , Method I 921: NMT 5.0% ADDITIONAL REQUIREMENTS• P ACKAGING AND S TORAGE : Preserve in tight containers. • USP R EFERENCE S TANDARDS 11 USP D IETHYLENE G LYCOL RS USP E THYLENE G LYCOL RS USP G LYCERIN RSr U == peak response of each individual impurityfrom the Sample solutionr T== sum of the responses of all the peaks fromthe Sample solution1,2,3-Propanetriol. C 3H 8O 392.10Auxiliary Information — Please check for your question in the FAQs before contacting USP. USP34–NF29 Page 2986Pharmacopeial Forum : Volume No. 28(4) Page 1245 Chromatographic Column — GLYCERINChromatographic columns text is not derived from, and not part of, USP 34 or NF 29.。

甘油(Glycerol)含量测定试剂盒说明书(货号:G0912W微板法96样)一、产品简介:甘油储存于脂肪细胞中是甘油三酯代谢的最终产物之一。
在生产、生活中甘油可用作溶剂,润滑剂,药剂和甜味剂。
甘油被甘油激酶(GK)的催化生成甘油-1-磷酸(G-1-P)。
G-1-P被甘油磷酸氧化酶(GPO)氧化生成过氧化氢(H2O2),H2O2与4-氨基氨替吡啉等反应生成红色醌类化合物,其在510nm处有特征吸收峰,通过检测510nm处吸光值即可得出甘油含量。
二、试剂盒的组分与配制:试剂名称规格保存要求备注提取液液体100mL×1瓶4℃保存试剂一液体μL×1支-20℃保存用前甩几下使试剂落入底部,再加4.2mL蒸馏水,充分震荡混匀溶解。
试剂二液体9mL×1瓶4℃保存试剂三液体6mL×1瓶4℃保存标准品液体mL×1支4℃保存用前用水稀释10倍即成4mM甘油标准品待检测液。
三、所需的仪器和用品:酶标仪、96孔板、可调式移液枪、离心机、研钵、蒸馏水。
四、甘油含量测定:建议正式实验前选取2个样本做预测定,了解本批样品情况,熟悉实验流程,避免实验样本和试剂浪费!1、样本制备:①组织样本:称取约0.1g组织样本加入研钵中,加入1mL提取液,在冰上进行匀浆,12000rpm,4℃或室温离心10min,取上清液待测。
【注】:若增加样本量,可按照组织质量(g):提取液(mL)为1:5~10的比例进行提取。
②细菌/细胞样本:先收集细菌或细胞到离心管内,离心后弃上清;取约500万细菌或细胞加入1mL 提取液,超声波破碎细菌或细胞(冰浴,功率200W,超声3s,间隔10s,重复30次);12000rpm4℃离心10min,取上清,置冰上待测。
【注】:若增加样本量,可按照细菌/细胞数量(104):提取液(mL)为500~1000:1的比例进行提取。
③液体样本:澄清的液体样本直接测定,若浑浊则离心后取上清检测。
那格列奈[105816-04-4]1、定义:分子式C19H27NO3,分子量:317.42.那格列奈为D-苯丙氨酸,N-{[反式-4-(1-甲基乙基)环己基]羰基};(-)-N-[(反式-4-异丙基环己基)羰基]-D-苯丙氨酸。
限度规定:按干燥品计算,含C19H27NO3为98.0%~102.0%。
性状与溶解度:白色粉末,易溶于甲醇和乙醇,溶于乙醚,略溶于乙腈和辛醇,几乎不溶于水。
2、鉴别:A、红外吸收分光光度法(197K)B、含量测定项下,样品溶液主峰的保留时间应与标准溶液的一致。
3、含量3.1配制溶液缓冲盐:无水磷酸氢二钠溶于水制成8.5g/L的溶液,并用磷酸调节pH值至7.5。
流动相:甲醇和溶液A的混合液(1:1)。
标准品溶液:称取那格列奈标准品到容量瓶,加甲醇(50%V)使其溶解并用缓冲盐稀释至定容刻度,得到1.0mg/ml的溶液。
系统适用性贮备液:那格列奈杂质C标准品和DL-苯丙氨酸溶于甲醇中制成0.2mg/ml的溶液。
(若需要,进行超声处理)系统适用性溶液:称取那格列奈标准品适量到容量瓶,加甲醇(45%V)溶解和系统适用性贮备液(5%V),并用缓冲盐定容至刻度,制成含那格列奈 1.0mg/ml、杂质C标准品0.01mg/ml和DL-苯丙氨酸0.01mg/ml的溶液。
供试品溶液:称取100mg那格列奈至100ml容量瓶中,加50ml甲醇溶解并用缓冲盐溶液定容。
3.2色谱条件(色谱分析法<621>),系统适用性)液相色谱仪检测波长:UV210nm柱长0.15m、内径6mm;填充物为6μm的L71(刚性聚甲基丙烯酸酯微球)【L71:A rigid ,spherical,polymethacrylate,4 to 6 μm indiameter.Manufacturer:Shodex Brand Name:RSpak DE-613】流速:1.0ml/min;柱温:30℃;进样量:20μl。
GlycerinC 3H 8O 3 92.101,2,3-Propanetriol.Glycerol [56-81-5].» Glycerin contains not less than 99.0 percent and not more than 101.0 percent of C 3H 8O 3, calculated on the anhydrous basis. The amount of any individual impurity, excluding diethylene glycol and ethylene glycol, if detected, meets the requirements under Other Impurities (NMT 0.1%) and the amount of total impurities, including diethylene glycol and ethylene glycol, is NMT 1.0%. Packaging and storage— Preserve in tight containers.USP Reference standards 11— USP Diethylene Glycol RS . USP Ethylene Glycol RS . USP Glycerin RS .Color— Its color, when viewed downward against a white surface in a 50-mL color-comparison tube, is not darker than the color of a standard made by diluting 0.40 mL of ferric chloride CS with water to 50 mL and similarly viewed in a color-comparison tube of approximately the same diameter and color as that containing the Glycerin.Identification— [NOTE—Compliance is determined by meeting the requirements for both Identification A and B .]A: Infrared Absorption 197F .B: Standard stock solution 1—Transfer 50 mg of USP Diethylene Glycol RS , accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix. Standard stock solution 2— Transfer 50 mg of USP Ethylene Glycol RS , accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 3— Transfer 50 mg of USP Glycerin RS , accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Resolution solution— Transfer 5.0 mL each of Standard stock solution 1, Standard stock solution 2, and Standard stock solution 3, to a 100-mL volumetric flask, dilute withmethanol to volume, and mix.Test solution— Transfer about 5 g of Glycerin to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Chromatographic system (see Chromatography 621)— The gas chromatograph is equipped with a flame-ionization detector, a 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase. The injection port temperature is maintained at 220 and the detector temperature is maintained at 250. The carrier gas is helium with a flow rate of about 4.5 mL per minute. The split flow ratio is about 10:1. The chromatograph is programmed as follows: Initially, the column temperature is equilibratedat 100 for 4 minutes, then increased at a rate of 50 per minute to 120, and is maintained at 120 for 10 minutes. It is then increased at a rate of 50 per minute to 220, and is maintained at 220 for 6 minutes. Chromatograph the Resolution solution, and record the peak responses and retention times as directed for Procedure: the relative retention times are about 0.3 for ethylene glycol, 0.8 for diethylene glycol, and 1.0 for glycerin; and the relative standard deviation for replicate injections for the diethylene glycol is not more than 10%.Procedure— Separately inject equal volumes (about 1 µL) of the Resolution solution and the Test solution into the chromatograph, and record the chromatograms. If a peak at the relative retention time for the diethylene glycol or ethylene glycol is present in the Test solution, the peak must be identifed and quantified as directed in the test for Diethylene glycol and ethylene glycol impurities.Specific gravity 841: not less than 1.249.Residue on ignition 281—Heat 50 g in an open, shallow 100-mL porcelain dish until it ignites, and allow it to burn without further application of heat in a place free from drafts. Cool, moisten the residue with 0.5 mL of sulfuric acid, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.01%).Water, Method I 921: not more than 5.0%.Chloride 221—A 7.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.001%).Sulfate 221—A 10-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (about 0.002%).Heavy metals 231—Mix 4.0 g with 2 mL of 0.1 N hydrochloric acid, and dilute with water to 25 mL: the limit is 5 µg per g.Limit of chlorinated compounds— Accurately weigh 5 g of Glycerin into a dry, round-bottom, 100-mL flask, add 15 mL of morpholine, and connect the flask by a ground joint to a reflux condenser. Reflux gently for 3 hours. Rinse the condenser with 10 mL of water,receiving the washing in the flask, and cautiously acidify with nitric acid. Transfer the solution to a suitable comparison tube, add 0.50 mL of silver nitrate TS, dilute with water to 50.0 mL, and mix: the turbidity is not greater than that of a blank to which 0.20 mL of 0.020 N hydrochloric acid has been added, the refluxing being omitted (0.003% of Cl).Fatty acids and esters— Mix 50 g of Glycerin with 50 mL of freshly boiled water and 5 mL of 0.5 N sodium hydroxide VS, boil the mixture for 5 minutes, cool, add phenolphthalein TS, and titrate the excess alkali with 0.5 N hydrochloric acid VS. Performa blank determination (see Residual Titrations under Titrimetry 541): not more than 1 mL of 0.5 N sodium hydroxide is consumed.Diethylene glycol and ethylene glycol impurities—Internal standard solution— Transfer 100 mg of 2,2,2-trichloroethanol (internal standard), accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 1— Transfer 50 mg of USP Diethylene Glycol RS, accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 2— Transfer 50 mg of USP Ethylene Glycol RS, accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 3— Transfer 50 mg of USP Glycerin RS, accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard solution— Transfer 5.0 mL each of Standard stock solution 1, Standard stock solution 2, and the Internal standard solution to a 100-mL volumetric flask, and dilute with methanol to volume, and mix.Resolution solution— Transfer 500 mg of USP Glycerin RS to a 10-mL volumetric flask, add 0.5 mL each of Standard stock solution 1 and Standard stock solution 2, dilute with methanol to volume, and mix.Test solution— Transfer about 5 g of Glycerin to a 100-mL volumetric flask, add 5.0 mL of Internal standard solution, dilute with methanol to volume, and mix.Chromatographic system (see Chromatography 621)— The gas chromatograph is equipped with a flame-ionization detector, a 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase. The injection port temperature is maintained at 220 and the detector temperature is maintained at 250. The carrier gas is helium, flowing at a rate of about 4.5 mL per minute. The split flow ratio is about 10:1. The chromatograph is programmed as follows. Initially, the column temperature is equilibratedat 100 for 4 minutes, then increased at a rate of 50 per minute to 120, and is maintained at 120 for 10 minutes then increased at a rate of 50 per minute to 220, andis maintained at 220for 6 minutes. Chromatograph the Standard solution, and record thepeak response ratio and retention times as directed for Procedure: the relative retention times are about 0.3 for ethylene glycol, 0.8 for diethylene glycol, and 1.0 for glycerin; the relative standard deviation for replicate injections for the diethylene glycol is not more than 10%; and the limit of quantitation of diethylene glycol and ethylene glycol is not more than 0.025%.Procedure— Separately inject equal volumes (about 1 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the ethylene glycol and diethylene glycol peaks. Calculate the percentage of diethylene glycol and ethylene glycol in the portion of Glycerin taken by the formula:100(CS / CU)(RU/ RS)in which CSis the concentration, in mg per mL, of diethylene glycol (or ethylene glycol) inthe Standard solution; CUis the concentration, in mg per mL, of Glycerin in the Testsolution; and RU and RSare the peak response ratios for diethylene glycol (or ethyleneglycol) to the internal standard peak obtained from the Test solution and the Standard solution: NMT the limit of quantitation for each, is found (0.025%).Assay—Sodium periodate solution— Dissolve 60 g of sodium metaperiodate in sufficient water containing 120 mL of 0.1 N sulfuric acid to make 1000 mL. Do not heat to dissolve the periodate. If the solution is not clear, pass through a sintered-glass filter. Store the solution in a glass-stoppered, light-resistant container. Test the suitability of this solution as follows. Pipet 10 mL into a 250-mL volumetric flask, dilute with water to volume, and mix. To about 550 mg of Glycerin dissolved in 50 mL of water add 50 mL of the diluted periodate solution with a pipet. For a blank, pipet 50 mL of the solution into a flask containing 50 mL of water. Allow the solutions to stand for 30 minutes, then to each add 5 mL of hydrochloric acid and 10 mL of potassium iodide TS, and rotate to mix. Allow to stand for 5 minutes, add 100 mL of water, and titrate with 0.1 N sodium thiosulfate, shaking continuously and adding 3 mL of starch TS as the endpoint is approached. The ratio of the volume of 0.1 N sodium thiosulfate required for the glycerin–periodate mixture to that required for the blank should be between 0.750 and 0.765.Procedure— Transfer about 400 mg of Glycerin, accurately weighed, to a 600-mL beaker, dilute with 50 mL of water, add bromothymol blue TS, and acidify with 0.2 N sulfuric acid to a definite green or greenish yellow color. Neutralize with 0.05 N sodium hydroxide to a definite blue endpoint, free from green color. Prepare a blank containing 50 mL of water, and neutralize in the same manner. Pipet 50 mL of the Sodium periodate solution into each beaker, mix by swirling gently, cover with a watch glass, and allow to stand for 30minutes at room temperature (not exceeding 35) in the dark or in subdued light. Add 10 mL of a mixture of equal volumes of ethylene glycol and water, and allow to stand for 20 minutes. Dilute each solution with water to about 300 mL, and titrate with 0.1 N sodiumhydroxide VS to a pH of 8.1 ± 0.1 for the specimen under assay and 6.5 ± 0.1 for the blank, using a pH meter. Each mL of 0.1 N sodium hydroxide, after correction for the blank, is equivalent to 9.210 mg of C 3H 8O 3.Auxiliary Information— Please check for your question in the FAQs before contacting USP.USP32–NF27 Page 2513Pharmacopeial Forum : Volume No. 28(4) Page 1245Chromatographic Column— GLYCERINChromatographic columns text is not derived from, and not part of, USP 32 or NF 27. Topic/Question Contact Expert Committee Monograph Kevin T. Moore, Ph.D.Scientist1-301-816-8369(EM105) Excipient Monographs 1Reference StandardsLili Wang, Technical ServicesScientist1-301-816-8129RSTech@。
USP微生物限度及无菌检验方法1 表1. 培养基的生长促进、抑菌、指示性能定量检测—选择和再培养—将在样品的制备和预培养项下规定的制备品和/或分别包含,,和(或0.1毫升,0.01毫升,和0.001毫升)该供试品的稀释液,接种于适量的肠道菌增菌肉汤培养基Mossel。
在温度30℃至35℃下培养24至48小时。
在紫红色胆汁葡萄琼脂培养基的平皿上对培养物进行再次培养。
在温度30℃至35℃下培养18至24小时。
说明—菌落的生长构成了阳性结果。
记录产生阳性结果的最少量供试品和产生阴性结果的最大量供试品。
从表2中大概确定细菌的数量。
表2. 结果的说明大肠杆菌样品的制备和预培养—按在非无菌产品微生物学检测:微生物计数检查法<61>章节下的描述,将不少于1克的供试品以1:10稀释来制备样品,并使用10毫升或对应1克或1毫升的数量,接种于适量(按照检查方法的适用性项下的内容来确定)的大豆酪蛋白消化物肉汤培养基,混匀,并在温度30℃至35℃下培养18至24小时。
选择和再次培养—摇动容器,转移1毫升大豆酪蛋白消化物肉汤培养基至100毫升的麦康凯(MacConkey)肉汤培养基,并在温度42 ℃至44 ℃下培养24至48小时。
在温度30至35℃下,于麦康凯(MacConkey)琼脂培养基的平皿上再次培养18至72小时。
说明—菌落的生长表明了大肠杆菌存在的可能性。
这需通过鉴别检测来证明。
如果没有菌落存在或如果确认鉴别检测为阴性,则该供试品符合该检测的要求。
沙门氏杆菌样品的制备和预培养—按照在非无菌产品微生物学检测:微生物计数检查法<61>章节下的描述制备供试品,并使用对应不少于10克或10毫升的数量接种于适量(按照检查方法的适用性项下内容来确定)的大豆酪蛋白消化物肉汤培养基,混合,在温度30℃至35℃下培养18至24小时。
选择和再次培养—大豆酪蛋白消化物肉汤培养基至10毫升氯化镁孔雀绿沙门氏菌富集肉汤培养基,并在温度30至35℃之间培养18至24小时。
Technical BulletinPROPYLENE GLYCOL - USP GENERIC NAMES 1,2 propanediol DESCRIPTION A relatively nontoxic liquid that is practically colorless, odorless, and tasteless. APPLICATIONSA solvent for flavors, extracts, drugs, an d food antioxidants: a heat transfer medium; an emollient and humectant and plasticize r f or tobacco products, baked goods, coconut, cellophane, cork, adhesives, and paper products; a l ubricant and mold inhibitor for food processing equipment; a humectant for pet food.SALES SPECIFICATIONS This product meets the requirements of the Propylene Glycol monograph listed in the United States Pharmacopoeia (USP), latest edition, European Pharmacopoeia (EP), and Food Chemicals Codex (FCC).Property Specifications Test Method * Acidity (as acetic acid), wt.% 0.002 max. ST-31.46, B Appearance Substantially free of suspended matter ST-30.1 Ash, wt.% 0.005 max. ST-31.12 Chlorides as CI, ppm 0.5 max. ST-4.44 Color, Pt-Co 10 max. ST-30.12 Heavy metals as Pb, ppm 5 max. ST-31.30 IR spectra Passes USP Propylene Glycol, area % by gas chromatography 99.5 min. ST-35.102 Specific gravity, 25/25°C1.0350 min. 1.0370 max. ST-30.31 Sulfate, ppm 60 max. USP Volatile organic impurities Passes USP-NF Water, wt.% 0.2 max. ST-31.53∗ Test methods are available upon request.QualiChem Technologies QualiChem Technologies warrants only that its products meet the spec ifications stated herein. Typical properties, where stated, are to be consi dered as representative of current production and should not be t re ated as speci f ications. W h ile al l the information pr esented i n thi s document i s bel ieved to be r e liable and t o r epresent the best av a ilable data on these pr o ducts, N O GUARANTEE, WARRANTY, OR REPRESENTATION IS MADE, INTENDED, OR IM PLIED AS T O T HE C ORRECTNESS OR SU FFICIENCY OF AN Y IN FORMATION, O R AS T O T HE SUITABILITY OF A NY CHEMICAL COMPOUNDS FOR A N Y PARTICULAR USE, OR THAT ANY CHEMICAL COMPOUNDS OR US E THEREOF ARE NOT SUBJECT TO A CLAIM BY A THIRD PART Y FOR INFRINGEMENT OF ANY PAT ENT OR OT H ER INT E LLECTUAL PROPER T Y RIGHT. EACH USER SHOU L D CONDUCT A SUFFICIENT INVEST I GATION T O ESTABLISH THE SUITABILITY OF ANY PRODUCT FOR ITS INTENDED USE. Products may be toxic and require special precautions in handling. For all products listed, user should obtain detailed information on toxicity, together with proper shipping, handling and storage procedures, and comply with all applicable safety and environmental standards.PROPYLENE GLYCOL – USPTYPICAL PROPERTIESChemical PropertiesStructure CHHOCH2CH3Molecular Weight 76Physical Properties Regulatory InformationArsenic, ppm 1 max DOT Classification not regulated Boiling range, Freight Classification propylene glycolASTM, °C, IBP 185 min.ASTM, °C, DP 189 max.HMIS Code 0-1-0 Flash point, PMCC, °F 212CAS Number 57-55-6 Freezing point, °C <-76TSCA Inventory listed on or exempt. Refractive index, 20°C 1.4310 min.WHMIS Classification not regulated1.4330 max.Canadian DSL listed on or exempt.<1European EINECS listed on or exempt. Vapor pressure, 20°C, mmHgViscosity, 20°C, cp 60Australian AICS listed on or exempt. Water solubility, % >10Japanese MITI listed on or exempt. Weight, 20°C, lb/gal 8.64PRODUCT SAFETY POLICYIt is the product safety policy of Q ualiChem Technologies to provide our cust omers with information on the safe handling and use of our products. The Material Safe t y Data Sheet (MSDS) should always be read and understood thoroughly before handling the product, and adequa te safety procedures should be followed. Information on the toxicity, environmental, and industri al hygiene aspects of our products m ay be found in the MSDS. Precautionary measures include: use only wit h adequate ventilation; avoid breat hing vapor, mist or gas; avoid contact with eyes, skin and clothing; keep container closed; wash thoroughly after handling.HANDLING, STORAGE, and SHIPPINGPropylene Glycol USP is a high-purity m aterial, which must be handled with special precautions to avoid contamination. Under ordinary conditions, m ild steel is a satisfactory materi a l of construction; however, for long term storage and where iron contamination and color a re objectionable, stainless steel or aluminum vessels are recommended. For additional information on handling and s torage of PG-USP, please contact the QualiChem Technologies. Product is available in b arges, lined tank cars and dedicated tank trucks, and 55-gallon non-returnable drums, 480 pounds net weigh t. Please contact us for other sizes.Certain government regulations may apply at the t ime of shipment.QualiChem Technologies。
3352Glyburide / Official Monographs USP 35Mode: LC AnalysisDetector: UV 230 nm Samples:Standard solution and Sample solutionColumn: 4.6-mm × 15-cm; 5-µm packing L7Calculate the percentage of each glyburide impurity in the Column temperature: 30°Tablets:Flow rate: 1.5 mL/minResult = (r U/r S) × (C S/C U) × F × 100 Injection size: 200 µLSystem suitabilityr U= peak response from the Sample solution Sample:Standard solutionr S= peak response from the Standard solution Suitability requirementsC S= concentration of USP Glyburide RS in theColumn efficiency: NLT 5000Standard solution (mg/mL) Tailing factor: 0.8–2.0C U= nominal concentration of glyburide in theRelative standard deviation: NMT 2%Sample solution (mg/mL) AnalysisF= relative response factor, use 0.8 for glyburide Samples:Standard solution and Sample solutionrelated compound A, and use 1.0 for all other Determine the percentage of C23H28ClN3O5S dissolved:impuritiesAcceptance criteriaResult = (r U/r S) × (C S/C U) × 100[N OTE—Disregard any peak less than 0.05%, and r U= peak response from the Sample solution disregard any peak obser ved in the blank.]r S= peak response from the Standard solution Glyburide related compound A: NMT 1.0%C S= concentration of the Standard solution (mg/mL)Any other individual impurities: NMT 0.2%C U= nominal concentration of glyburide in the Sample Total impurities: NMT 0.50%, excluding glyburidesolution (mg/mL)related compound ATolerances: NLT 85% (Q) of the labeled amount of•P ROCEDURE2: M ETFORMIN H YDROCHLORIDEglyburide is dissolved.Solution A, Mobile phase, Sample solution,Metformin hydrochloride Chromatographic system, and System suitability: Medium: 0.05 M phosphate buffer, pH 6.8. Prepare by Proceed as directed in the Assay for Metformindissolving 6.8 g of monobasic potassium phosphate in Hydrochloride.1000 mL of water, and adjust with 0.2 N sodium Analysishydroxide to a pH of 6.8 ± 0.1; 1000 mL.Sample:Sample solutionApparatus 2: 50 rpm Calculate the percentage of each impurity in the portion Time: 30 min of Tablets taken:Standard solution: Dissolve a quantity of USP MetforminResult = (r U/r T) × 100 Hydrochloride RS in Medium. Dilute further, if necessar y,with Medium to obtain a solution having a metforminr U= peak response for each impurity from the hydrochloride concentration, in mg/mL, of L/1000 whereSample solutionL is the label claim, in mg, of metformin hydrochloride.r T= sum of the responses of all peaks from the Sample solution: Sample per Dissolution 〈711〉. Pass aSample solutionportion of the solution under test through a 0.45-µmAcceptance criteriapolypropylene filter or a 1-µm glass fiber filter. Dilute with[N OTE—Disregard any peak less than 0.05%, and Medium, if necessar y.disregard any peak obser ved in the blank.] Spectrometric conditionsIndividual impurities: NMT 0.1% (See Spectrophotometry and Light-Scattering 〈851〉.)Total impurities: NMT 0.5 %Mode: UV-VisAnalytical wavelength: 232 nm ADDITIONAL REQUIREMENTSAnalysis•P ACKAGING AND S TORAGE: Preserve in tight, light-resistant Samples:Standard solution and Sample solution containers, and store at controlled room temperature.Calculate the percentage of C4H11N5·HCl dissolved:•USP R EFERENCE S TANDARDS〈11〉USP Glyburide RSResult = (A U/A S) × (C S/C U) × 100USP Glyburide Related Compound A RS(4-[2-(5-Chloro-2-methoxybenzamido)ethyl]A U= absorbance of the Sample solutionbenzenesulfonamide.A S= absorbance of the Standard solutionUSP Metformin Hydrochloride RSC S= concentration of USP Metformin HydrochlorideUSP Metformin Related Compound B RSRS in the Standard solution (mg/mL)1-Methylbiguanide.C U= nominal concentration of metforminC3H9N5115.14hydrochloride in the Sample solution (mg/mL)USP Metformin Related Compound C RS Tolerances: NLT 85% (Q) of the labeled amount ofDimethylmelamine, or N,N-dimethyl-[1,3,5]triazine-2,4,6-metformin hydrochloride is dissolved.triamine.•U NIFORMITY OF D OSAGE U NITS〈905〉: Meet the requirementsC5H10N6154.17for Weight Variation for metformin hydrochloride and forContent Uniformity for glyburideIMPURITIESOrganic Impurities•P ROCEDURE1: G LYBURIDE GlycerinSolution A, Mobile phase, Diluent, Sample solution,Chromatographic system, and System suitability:Proceed as directed in the Assay for Glyburide.Standard solution: Dilute 1.0 mL of the Standard solutionfrom the Assay for Glyburide with Diluent to 100 mL.C3H8O392.101,2,3-Propanetriol;Glycerol [56-81-5].USP 35Official Monographs / Glycerin3353DEFINITION date solution with a pipet. For a blank, pipet 50 mL of the Glycerin contains NLT 99.0% and NMT 101.0% of C3H8O3, cal-solution into a flask containing 50 mL of water. Allow the culated on the anhydrous basis.solutions to stand for 30 min, then to each add 5 mL ofhydrochloric acid and 10 mL of potassium iodide TS, and IDENTIFICATION rotate to mix. Allow to stand for 5 min, add 100 mL of[N OTE—Compliance is determined by meeting the requirements water, and titrate with 0.1 N sodium thiosulfate, shakingfor Identification tests A, B, and C.]continuously and adding 3 mL of star ch TS as the endpoint •A. I NFRARED A BSORPTION〈197F〉is approached. The ratio of the volume of 0.1 N sodium •B. L IMIT OF D IETHYLENE G LYCOL AND E THYLENE G LYCOL thiosulfate required for the glycerin–periodate mixture to Standard solution: 2.0 mg/mL of USP Glycerin RS, 0.050that required for the blank should be between 0.750 and mg/mL of USP Ethylene Glycol RS, 0.050 mg/mL of USP0.765.Diethylene Glycol RS, and 0.10 mg/mL of 2,2,2-Analysis: Transfer 400 mg of Glycerin to a 600-mL beaker, trichloroethanol (internal standard) in methanol dilute with 50 mL of water, add bromothymol blue TS, and Sample solution: 50 mg/mL of Glycerin and 0.10 mg/mL of acidify with 0.2 N sulfuric acid to a definite green or green-2,2,2-trichloroethanol (internal standard) in methanol ish yellow color. Neutralize with 0.05 N sodium hydroxide Chromatographic system to a definite blue endpoint, free from green color. Prepare a (See Chromatography 〈621〉, System Suitability.)blank containing 50 mL of water, and neutralize in the same Mode: GC manner. Pipet 50 mL of the Sodium periodate solution into Detector: Flame ionization each beaker, mix by swirling gently, cover with a watch Column: 0.53-mm × 30-m fused-silica analytical column glass, and allow to stand for 30 min at room temperature coated with 3.0-µm G43 stationar y phase, and a deacti-(not exceeding 35°) in the dark or in subdued light. Add 10 vated split liner with glass wool mL of a mixture of equal volumes of ethylene glycol and Temperature water, and allow to stand for 20 min. Dilute each solution Injector: 220°with water to 300 mL, and titrate with 0.1 N sodium hy-Detector: 250°droxide VS to a pH of 8.1 ± 0.1 for the specimen under Column: See the temperature program table.assay and 6.5 ± 0.1 for the blank, using a pH meter. EachmL of 0.1 N sodium hydroxide, after correction for theblank, is equivalent to 9.210 mg of C3H8O3.Hold TimeAcceptance criteria: 99.0%–101.0% on the anhydrous basis Initial Temperature Final at FinalTemperature Ramp Temperature TemperatureIMPURITIES(°)(°/min)(°)(min)Inorganic Impurities100—1004•CHLORIDE AND S ULFATE, Chloride〈221〉: A 7.0-g portion shows 1005012010no more chloride than corresponds to 0.10 mL of 0.020 N120502206hydrochloric acid (NMT 10 ppm).•C HLORIDE AND S ULFATE, Sulfate〈221〉: A 10-g portion shows Carrier gas: Helium no more sulfate than corresponds to 0.20 mL of 0.020 N Injection size: 1.0 µL sulfuric acid (NMT 20 ppm).Flow rate: 4.5 mL/min•H EAVY M ETALS〈231〉Injection type: Split ratio, about 10:1Analysis: Mix 4.0 g with 2 mL of 0.1 N hydrochloric acid, System suitability and dilute with water to 25 mL.Sample:Standard solution Acceptance criteria: NMT 5 ppm[N OTE—The relative retention times for ethylene glycol,•R ESIDUE ON I GNITION〈281〉: Heat 50g in an open, shallow 2,2,2-trichloroethanol, diethylene glycol, and glycerin are100-mL porcelain dish until it ignites, and allow it to burn about 0.3, 0.6, 0.8 and 1.0, respectively.]without further application of heat in a place free from Suitability requirements drafts. Cool, moisten the residue with 0.5 mL of sulfuric Resolution: NLT 1.5 between diethylene glycol and acid, and ignite to constant weight: the weight of the resi-glycerin due does not exceed 5 mg (0.01%).Analysis Organic ImpuritiesSample:Sample solution•P ROCEDURE1: R ELATED C OMPOUNDSAcceptance criteria: If a peak at the retention times for the System suitability solution: 0.5 mg/mL each of USP Dieth-diethylene glycol or ethylene glycol is present in the Sample ylene Glycol RS and USP Glycerin RSsolution, the peak response ratio relative to 2,2,2-Sample solution: 50 mg/mL of Glycerintrichloroethanol is NMT the peak response ratio for diethyl-Chromatographic systemene glycol or ethylene glycol relative to 2,2,2-trichloroetha-(See Chromatography 〈621〉, System Suitability.)nol in the Standard solution; NMT 0.10% each for diethylene Mode: GCglycol and ethylene glycol is found.Detector: Flame ionization•C. Examine the chromatograms obtained in Identification test Column: 0.53-mm × 30-m fused-silica analytical columnB. The retention time of the glycerin peak of the Sample coated with 3.0-µm G43 stationar y phase, and an inletsolution corresponds to that obtained in the Standard liner having an inverted cup or spiral structuresolution.TemperatureInjector: 220°ASSAY Detector: 250°•P ROCEDURE Column: See the temperature program table below. Sodium periodate solution: Dissolve 60g of sodiummetaperiodate in sufficient water containing 120 mL of 0.1Hold Time N sulfuric acid to make 1000 mL. Do not heat to dissolveInitial Temperature Final at Final the periodate. If the solution is not clear, pass through aTemperature Ramp Temperature Temperature sintered-glass filter. Store the solution in a glass-stoppered,(°)(°/min)(°)(min) light-resistant container. Test the suitability of this solution asfollows. Pipet 10 mL into a 250-mL volumetric flask, and100—100—dilute with water to volume. T o 550 mg of Glycerin dis-1007.52204 solved in 50 mL of water, add 50 mL of the diluted perio-3354Glycerin / Official MonographsUSP 35Carrier gas: Helium contain one or more suitable antimicrobial pre-Injection size: 0.5 µL servatives. [NOTE —In the preparation of thisLinear velocity: 38 cm/sOphthalmic Solution, use Glycerin that has a low Injection type: Split ratio, about 10:1water content, in order that the Ophthalmic So-System suitabilitySample: System suitability solution lution may comply with the Water limit. This may Suitability requirementsbe ensured by using Glycerin having a specific Resolution: NLT 7.0 between diethylene glycol and gravity of not less than 1.2607, corresponding to glycerin a concentration of 99.5 per cent.]AnalysisSample: Sample solutionNOTE —Do not use the Ophthalmic Solution if it Calculate the percentage of each impurity, excluding any contains crystals, or is cloudy or discolored, or solvent peaks and diethylene glycol, in the portion of contains a precipitate.Glycerin taken:Packaging and storage—Preserve in tight containers of glass Result = (r U /r T ) × 100or plastic, containing not more than 15 mL, protected from light. The container or individual carton is sealed and tamper-r U= peak response of each individual impurity fromproof so that sterility is ensured at time of first use.the Sample solutionr T= sum of the responses of all the peaks from the USP Reference standards 〈11〉—Sample solutionUSP Glycerin RSAcceptance criteriaIdentification—It responds to the Identification test under Individual impurities: NMT 0.1%Glycerin .Total impurities: NMT 1.0%Sterility 〈71〉: meets the requirements.•P ROCEDURE 2: L IMIT OF C HLORINATED C OMPOUNDSSample: 5g of GlycerinpH 〈791〉: between 4.5 and 7.5, determined potentiometri-Analysis: Transfer the Sample into a dr y, round-bottom,cally in a solution prepared by the addition of 5 mL of Sodium 100-mL flask. Add 15 mL of morpholine, and connect the Chloride Injection to 5 mL of Ophthalmic Solution.flask by a ground joint to a reflux condenser. Reflux gently Water, Method I 〈921〉: not more than 1.0%.for 3 h. Rinse the condenser with 10 mL of water, receiving Assay—Transfer an accurately measured volume of Ophthalmic the washings in the flask, and cautiously acidify with nitric Solution, equivalent to about 3g of glycerin, to a 500-mL volu-acid. Transfer the solution to a suitable comparison tube,metric flask, dilute with water to volume, and mix. T ransfer a 3-add 0.50 mL of silver nitrate TS, and dilute with water to mL portion to a conical flask, add 100.0 mL of a solution of 50.0 mL.potassium periodate (prepared by dissolving 3g of potassium Acceptance criteria: The turbidity is not greater than that periodate in about 500 mL of warm water, cooling to room of a blank to which 0.20 mL of 0.020 N hydrochloric acid temperature, and then diluting with water to 1000 mL), swirl,has been added, the refluxing being omitted (NMT 30and allow to stand at room temperature for 10 minutes. Add ppm of Cl).4g of sodium bicarbonate and 2g of potassium iodide, and •P ROCEDURE 3: F ATTY A CIDS AND E STERStitrate immediately with 0.1 N potassium arsenite VS, adding 3Sample solution: Mix 50g of Glycerin with freshly boiled mL of starch TS as the endpoint is approached. Per form a blank water and 5 mL of 0.5 N sodium hydroxide VS. Boil the determination, using water in place of the Ophthalmic Solution,mixture for 5 min, cool, and add phenolphthalein TS.and note the difference in volumes required. Each mL of 0.1 N Analysis: Titrate the excess alkali with 0.5 N hydrochloric potassium arsenite is equivalent to 2.303 mg of glycerin acid VS. Per form a blank determination (see Titrimetry (C 3H 8O 3).〈541〉, Residual Titrations ).Acceptance criteria: NMT 1 mL of 0.5 N sodium hydroxide is consumed.SPECIFIC TESTSGlycerin Oral Solution•C OLOR : When viewed downward against a white sur face in a 50-mL color-comparison tube, the color is not darker than » Glycerin Oral Solution contains not less than the color of a standard made by diluting 0.40 mL of ferric chloride CS with water to 50 mL and similarly viewed in a 95.0 percent and not more than 105.0 per cent color-comparison tube of approximately the same diameter of the labeled amount of glycerin (C 3H 8O 3).and color as that containing the Glycerin.•S PECIFIC G RAVITY 〈841〉: NLT 1.249Packaging and storage—Preserve in tight containers.•W ATER D ETERMINATION , Method I 〈921〉: NMT 5.0%Identification—Heat a few drops with about 500 mg of po-tassium bisulfate in a test tube: pungent vapors of acrolein are ADDITIONAL REQUIREMENTSevolved.•P ACKAGING AND S TORAGE : Preserve in tight containers.pH 〈791〉: between 5.5 and 7.5.•USP R EFERENCE S TANDARDS 〈11〉Assay—Transfer an accurately measured volume of Oral Solu-USP Diethylene Glycol RS tion, equivalent to about 3g of glycerin, to a 500-mL volumet-USP Ethylene Glycol RS ric flask, dilute with water to volume, and mix. T ransfer a 3-mL USP Glycerin RS portion to a conical flask, add 100.0 mL of a solution of potas-1,2,3-Propanetriol.sium periodate (prepared by dissolving 3g of potassium perio-C 3H 8O 392.10date in about 500 mL of warm water, cooling to room temper-ature, and then diluting with water to 1000 mL), swirl, andallow to stand at room temperature for 10 minutes. Add 4g of sodium bicarbonate and 2g of potassium iodide, and titrate immediately with 0.1 N potassium arsenite VS, adding 3 mL of Glycerin Ophthalmic Solutionstarch TS as the endpoint is approached. Per form a blank deter-mination, using water in place of the Oral Solution, and note » Glycerin Ophthalmic Solution is a sterile, anhy-the difference in volumes required. Each mL of 0.1 N potassium drous solution of Glycerin, containing not less arsenite is equivalent to 2.303 mg of glycerin (C 3H 8O 3).than 98.5 percent of glycerin (C 3H 8O 3). It may。