五元素反向扩散关系PPT图表
- 格式:pptx
- 大小:1.21 MB
- 文档页数:1

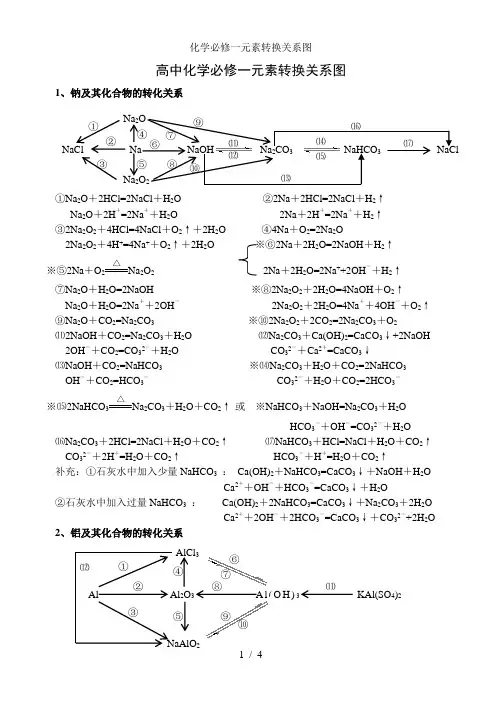
高中化学必修一元素转换关系图1、钠及其化合物的转化关系①Na 2O +2HCl=2NaCl +H 2O ②2Na +2HCl=2NaCl +H 2↑Na 2O +2H +=2Na ++H 2O 2Na +2H +=2Na ++H 2↑ ③2Na 2O 2+4HCl=4NaCl +O 2↑+2H 2O ④4Na +O 2=2Na 2O2Na 2O 2+4H +=4Na ++O 2↑+2H 2O ※⑥2Na +2H 2O=2NaOH +H 2↑ ※⑤2Na +O 2△Na 2O 2 2Na +2H 2O=2Na ++2OH -+H 2↑⑦Na 2O +H 2O=2NaOH ※⑧2Na 2O 2+2H 2O=4NaOH +O 2↑Na 2O +H 2O=2Na ++2OH - 2Na 2O 2+2H 2O=4Na ++4OH -+O 2↑ ⑨Na 2O +CO 2=Na 2CO 3 ※⑩2Na 2O 2+2CO 2=2Na 2CO 3+O 2⑾2NaOH +CO 2=Na 2CO 3+H 2O ⑿Na 2CO 3+Ca(OH)2=CaCO 3↓+2NaOH2OH -+CO 2=CO 32-+H 2O CO 32-+Ca 2+=CaCO 3↓⒀NaOH +CO 2=NaHCO 3 ※⒁Na 2CO 3+H 2O +CO 2=2NaHCO 3OH -+CO 2=HCO 3- CO 32-+H 2O +CO 2=2HCO 3-※⒂2NaHCO 3△Na 2CO 3+H 2O +CO 2↑ 或 ※NaHCO 3+NaOH=Na 2CO 3+H 2OHCO 3-+OH -=CO 32-+H 2O⒃Na 2CO 3+2HCl=2NaCl +H 2O +CO 2↑ ⒄NaHCO 3+HCl=NaCl +H 2O +CO 2↑CO 32-+2H +=H 2O +CO 2↑ HCO 3-+H +=H 2O +CO 2↑补充:①石灰水中加入少量NaHCO 3 : Ca(OH)2+NaHCO 3=CaCO 3↓+NaOH +H 2OCa 2++OH -+HCO 3-=CaCO 3↓+H 2O②石灰水中加入过量NaHCO 3 : Ca(OH)2+2NaHCO 3=CaCO 3↓+Na 2CO 3+2H 2OCa 2++2OH -+2HCO 3-=CaCO 3↓+CO 32-+2H 2O 2、铝及其化合物的转化关系NaCl Na NaOH Na 2CO 3 NaHCO 3 NaCl Na 2O Na 2O 2 ① ② ③ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩ ⑾ ⑿ ⒀ ⒁ ⒂⒃ ⒄ ④ Al Al 2O 3 A l (O H )3 KAl(SO 4)2 AlCl 3① ② ③ ④ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩ ⑾⑿①2Al +6HCl=2AlCl 3+3H 2↑ ②4Al +3O 2△2Al 2O 32Al +6H +=2Al 3++3H 2↑※③2Al +2NaOH +2H 2O=2NaAlO 2+3H 2↑ ④Al 2O 3+6HCl=2AlCl 3+3H 2O2Al +2OH -+2H 2O=2AlO 2-+3H 2↑ Al 2O 3+6H +=2Al 3++3H 2O ※⑤Al 2O 3+2NaOH=2NaAlO 2+H 2O ⑥AlCl 3+3NH 3·H 2O=Al(OH)3↓+3NH 4ClAl 2O 3+2OH -=2AlO 2-+H 2O Al 3++3NH 3·H 2O = Al(OH)3↓+3NH 4+⑦Al(OH)3+3HCl=AlCl 3+3H 2O ⑧2Al(OH)3△Al 2O 3+3H 2OAl(OH)3+3H +=Al 3++3H 2O☆⑨NaAlO 2+HCl +H 2O =Al(OH)3↓+NaCl 或NaAlO 2+2H 2O +CO 2=Al(OH)3↓+NaHCO 3AlO 2-+H ++H 2O=Al(OH)3↓ AlO 2-+2H 2O +CO 2=Al(OH)3↓+HCO 3-※⑩Al(OH)3+NaOH = NaAlO 2+2H 2O ☆⑾Al 3++3H 2O Al(OH)3胶体+3H +Al(OH)3+OH -= AlO 2-+2H 2O 明矾净水⑿AlCl 3+4NaOH = NaAlO 2+3NaCl +2H 2O Al 3++4OH -= AlO 2-+2H 2O 3、铁及其化合物的转化关系①Fe 3O 4+4CO△3Fe +4CO 2 ※② 3Fe +4H 2O(g)高温Fe 3O 4+4H 2 ③ Fe +2HCl=FeCl 2+H 2↑ 或3Fe +2O 2点燃Fe 3O 4Fe +2H +=Fe 2++H 2↑ ④2Fe +3Cl 2点燃2FeCl 3※⑤2FeCl 2+Cl 2 = 2FeCl 3 ※⑥Fe +2FeCl 3 = 3FeCl 22Fe 2++Cl 2 = 2Fe 3++2Cl - Fe +2Fe 3+= 3Fe 2+⑦FeCl 2+2NaOH = Fe(OH)2↓+2NaCl ⑧Fe(OH)2+2HCl = FeCl 2+2H 2OFe 2++2OH -= Fe(OH)2↓ Fe(OH)2+2H += Fe 2++2H 2O※⑨4Fe(OH)2+O 2+2H 2O = 4Fe(OH)3 ⑩FeCl 3+3NaOH = Fe(OH)3↓+3NaCl白色沉淀迅速变成灰绿色,最后变成红褐色 Fe 3++3OH -= Fe(OH)3↓⑾Fe(OH)3+3HCl = FeCl 3+3H 2O Fe 3++3H 2O Fe(OH)3胶体+3H +(净水) Fe(OH)3+3H += Fe 3++3H 2O ⑿2Fe(OH)3△Fe 2O 3+3H 2O⒀Fe 2O 3+6HCl = 2FeCl 3+3H 2O ※⒁FeCl 3+3KSCN = Fe(SCN)3+3KClFe 2O 3+6H += 2Fe 3++3H 2O Fe 3++3SCN -= Fe(SCN)3Fe 3O 4 Fe FeCl 2 Fe(OH)2 Fe(SCN)3 FeCl 3 Fe(OH)3 Fe O 3 ①② ③ ④⑤ ⑥ ⑦ ⑧ ⑨⑩ ⑾⑿ ⒁ ⒀①Si +O 2△SiO 2②SiO 2+2C 高温 Si +2CO ↑※③SiO 2+4HF = SiF 4↑+2H 2O (刻蚀玻璃)④Si +4HF = SiF 4↑+2H 2↑ ⑤SiO 2+CaO高温CaSiO 3※⑥SiO 2+2NaOH = Na 2SiO 3+H 2O SiO 2+CaCO 3高温CaSiO 3+CO 2↑SiO 2+2OH -= SiO 32-+H 2O ※⑦Na 2SiO 3+2HCl = H 2SiO 3↓+2NaCl SiO 2+Na 2CO 3高温Na 2SiO 3+CO 2↑ SiO 32-+2H += H 2SiO 3↓※⑦Na 2SiO 3+H 2O +CO 2=H 2SiO 3↓+Na 2CO 3或Na 2SiO 3+2H 2O +2CO 2=H 2SiO 3↓+2NaHCO 3SiO 32-+H 2O +CO 2=H 2SiO 3↓+CO 32-或SiO 32-+2H 2O +2CO 2=H 2SiO 3↓+2HCO 3-⑧H 2SiO 3+2NaOH = Na 2SiO 3+2H 2O ⑨H 2SiO 3 △H 2O +SiO 2H 2SiO 3+2OH -= SiO 32-+2H 2O 5、氯及其化合物的转化关系①2Fe +3Cl 2 点燃2FeCl 3 ②Cu +Cl 2 点燃CuCl 2 ③2FeCl 3+Cu = 2FeCl 2+CuCl 22Fe 3++Cu = 2Fe 2++Cu 2+④H 2+Cl 2 2HCl※⑤MnO 2+4HCl(浓) △MnCl 2+Cl 2↑+2H 2OMnO 2+4H ++2Cl - △ Mn 2++Cl 2↑+2H 2O ※⑥Cl 2+H 2O = HCl +HClO ※⑦2HClO 2HCl +O 2↑Cl 2+H 2O = H ++Cl -+HClO 2HClO 2H ++2Cl -+O 2↑※⑧Cl 2+2NaOH = NaCl +NaClO +H 2O ※⑨2Cl 2+2Ca(OH)2 = CaCl 2+Ca(ClO)2+2H 2OCl 2+2OH -= Cl -+ClO -+H 2O 工业制漂白粉※⑩Ca(ClO)2+H 2O +CO 2 = CaCO 3↓+2HClO 或Ca(ClO)2+2HCl = CaCl 2+2HClOCa 2++2ClO -+H 2O +CO 2= CaCO 3↓+2HClO 或ClO -+H += HClO 漂白粉的漂白原理向漂白粉溶液中通入过量的CO 2:Ca(ClO)2+2H 2O +2CO 2 = Ca(HCO 3)2+2HClOClO -+H 2O +CO 2 = HCO 3-+HClONa 2SiO 3 SiF 4 Si SiO 2 H 2SiO 3 CaSiO 3 ①②③ ④ ⑤⑥ ⑦ ⑧⑨ CuCl 2 HClO HCl Cl 2 FeCl 3NaClO Ca(ClO)2① ② ③ ④⑤ ⑥ ⑦ ⑧ ⑨ ⑩ 点燃或光照 光照光照①S +O 2 点燃SO 2②2H 2S +SO 2=3S +2H 2O※③SO 2+O 2 催化剂加热 2SO 3 ④SO 3+H 2O = H 2SO 4 ⑤SO 2+CaO△CaSO 3 或 SO 2+Ca(OH)2 = CaSO 3↓+H 2O⑥SO 3+CaO = CaSO 4 SO 2+Ca 2++2OH -=CaSO 3↓+H 2O SO 3+Ca(OH)2 = CaSO 4+H 2O ⑦2CaSO 3+O 2△2CaSO 4※⑧SO 2+Cl 2+2H 2O = H 2SO 4+2HCl ※⑨Cu +2H 2SO 4(浓) △CuSO 4+SO 2↑+2H 2OSO 2+Cl 2+2H 2O = 4H ++SO 42-+2Cl -Cu +2H 2SO 4(浓) △Cu 2++SO 42-+SO 2↑+2H 2O※⑩C +2H 2SO 4(浓)△CO 2↑+2SO 2↑+2H 2O7、氮及其化合物的转化关系 ※①N 2+O 22NO※②2NO +O 2 = 2NO 2※③3NO 2+H 2O = 2HNO 3+NO 3NO 2+H 2O =2H ++2NO 3-+NO以上三个反应为“雷雨发庄稼”原理 扩展反应有:4NO 2+O 2+2H 2O = 4HNO 3 4NO +3O 2+2H 2O = 4HNO 3 ※④Cu +4HNO 3(浓) = Cu(NO 3)2+2NO 2↑+2H 2O 上面两个反应主要用于气体溶于水时的计算Cu +4H ++2NO 3-=Cu 2++2NO 2↑+2H 2O 或 4HNO 3 4NO 2↑+ O 2↑+ 2H 2OC +4HNO 3(浓)△CO 2↑+2NO 2↑+2H 2O 浓硝酸见光易变黄的原因※⑤3Cu +8HNO 3(稀) = 3Cu(NO 3)2+2NO ↑+4H 2O ⑥N 2+3H 2催化剂 高温高压2NH 33Cu +8H ++2NO 3-=3Cu 2++2NO ↑+4H 2O 合成氨反应是人工固氮的主要途径 ⑦NH 3+HCl = NH 4Cl ⑧NH 4Cl△NH 3↑+HCl ↑NH 3+H += NH 4+(水溶液中) 补充:NH 4HCO 3 △NH 3↑+H 2O+CO 2↑氨气与酸均能反应生成铵盐,且与挥发性酸 铵盐受热都易分解,但并不是所有的铵盐 (如浓HCl 、浓HNO 3)相遇时空气中有白烟 都分解出氨气,如NH 4NO 3、(NH 4)2SO 4※⑧NH 4Cl +NaOH△NaCl +NH 3↑+H 2O NH 4++OH-△NH 3↑+H 2O所有的铵盐都能与碱作用放出氨气,可利用此反应鉴别铵离子。

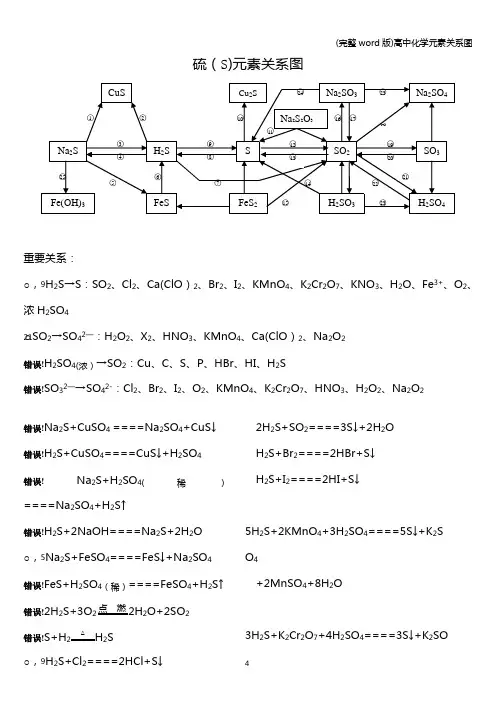
(完整word版)高中化学元素关系图硫(S)元素关系图重要关系:○,9H2S→S:SO2、Cl2、Ca(ClO)2、Br2、I2、KMnO4、K2Cr2O7、KNO3、H2O、Fe3+、O2、浓H2SO4○21SO2→SO42—:H2O2、X2、HNO3、KMnO4、Ca(ClO)2、Na2O2错误!H2SO4(浓)→SO2:Cu、C、S、P、HBr、HI、H2S错误!SO32—→SO42-:Cl2、Br2、I2、O2、KMnO4、K2Cr2O7、HNO3、H2O2、Na2O2错误!Na2S+CuSO4====Na2SO4+CuS↓错误!H2S+CuSO4====CuS↓+H2SO4错误!Na2S+H2SO4(稀)====Na2SO4+H2S↑错误!H2S+2NaOH====Na2S+2H2O○,5Na2S+FeSO4====FeS↓+Na2SO4错误!FeS+H2SO4(稀)====FeSO4+H2S↑错误!2H2S+3O2点燃2H2O+2SO2错误!S+H2△ H2S○,9H2S+Cl2====2HCl+S↓2H2S+SO2====3S↓+2H2OH2S+Br2====2HBr+S↓H2S+I2====2HI+S↓5H2S+2KMnO4+3H2SO4====5S↓+K2S O4+2MnSO4+8H2O3H2S+K2Cr2O7+4H2SO4====3S↓+K2SO 4(完整word版)高中化学元素关系图+Cr2(SO4)3+7H2O3H2S+2HNO3(稀)====3S↓+2NO↑+2H2OH2S+H2O2====S↓+2H2OH2S+2FeCl3====S↓+2FeCl2+2HCl2H2S+O2====2S↓+2H2OH2S+H2SO4(浓)====SO2↑+S↓+2H2O2H2S+Ca(ClO)2====2S↓+CaCl2+2HCl○10S+2Cu△Cu2S错误!Na2S2O3+2HCl====2NaCl+S↓+SO2↑+H2O错误!S+O 点燃SO2错误!3SO2+2Na2S====3S↓+2Na2SO3错误!H2SO3+2H2S====3H2O+3S↓错误!4FeS2+11O高温2Fe2O3+8SO2○,16SO2+2NaOH====Na2SO3+H2O H2O+2Na2CO3+SO2(少量)====Na2SO3+2NaHCO3Na2CO3+SO2(适量)====Ma2SO3+CO2错误!Na2SO3(s)+H2SO4(浓)====Na2SO4+SO2↑+H2O 错误!SO2+Na2O2====Na2SO4SO2+2NaClO+H2O====Na2SO4+2HCl错误!2SO2+O2催化剂450℃2SO3○,205SO3+2P△P2O5+5SO2错误!SO2+H2O2====H2SO4SO2+X2+2H2O====H2SO4+2HX(X=Cl、Br、I)2SO2+O2+2H2O====2H2SO43SO2+2HNO3(稀)+2H2O====2NO+3H2SO45SO2+2KMnO4+2H2O====K2SO4+2M nSO4+2H2SO4错误!2H2SO4(浓)+Cu====CuSO4+SO2↑+2H2O2P+5H2SO4(浓)△2H3PO4+5SO2↑+2H2OC+2H2SO4(浓)△CO2↑+2SO2↑+2H2OS+2H2SO4(浓)△3SO2↑+2H2O2HBr+H2SO4(浓)====Br2+SO2+2H2O 2HI+H2SO4(浓)====I2+SO2+2H2O错误!SO32—+Cl2+H2O====SO42—+2Cl-+2H+2H2SO3+O2====2H2SO42Na2SO3+O2====2Na2SO4错误!Na2SO3+2Na2S+3H2SO4====3S↓+3Na 2SO4+3H2O错误!3Na2S+2FeCl3+H2O====6NaCl+3H2S↑+2Fe(OH)3↓氯(Cl)元素关系图重要关系:错误!HCl→Cl2:MnO2、KMnO4、K2Cr2O7、KClO3、Ca(ClO)2 Cl2→Cl-:H2S、SO2、SO42—、Br-、I-、Fe2+○1Cl2+2FeCl2====2FeCl32Fe+3Cl点燃2FeCl3(冒棕褐色烟)错误!Cl2+Cu点燃CuCl2(冒棕黄色烟)○,3Cl2+2I-====I2+2Cl-错误!Cl2+2Br-====Br2+2Cl—错误!Cl2+SO2+2H2O====2HCl+H2SO4错误!3Cl2+6KOH△KClO3+5KCl+H2O ○,7Cl2+2NaOH====NaCl+NaClO+H2O ○8NaClO+HCl(浓)====Cl2↑+NaOHNaClO+NaCl+H2SO4====Na2SO4+Cl2↑+H2O○9Cl2+H2O====HCl+HClO○102NaCl+2H2O电解Cl2↑+H2↑+2NaOH 2NaCl+MnO2+3H2SO4△2NaHSO4+MnSO4+Cl2↑+2H2O错误!2Na+Cl点燃2NaCl (冒白烟)H2O+Cl2+Na2CO3(足量)====NaCl+NaClO+2NaHCO3Na2CO3+H2O+2Cl2(足量)====2NaCl+2HClO+CO2↑错误!4HCl(浓)+MnO2△MnCl2+2H2O+Cl2↑2KMnO4+16HCl(浓)====2KCl+2MnCl2+5Cl2↑+8H2OKCr2O7+14HCl(浓)====2KCl+2CrCl3 +3Cl2↑+2H2OKClO3+6HCl(浓)====KCl+3Cl2↑+3H2OCa(ClO)2+4HCl(浓)====CaCl2+2Cl2↑+2H2O ○13Cl2+H2点燃2HClH2S+Cl2====2HCl+SH2+Cl光照2HClNa2SO3+Cl2+H2O====Na2SO4+2HCl 2FeCl2+Cl2====2FeCl3○14HCl+NH3====NH4Cl (冒白烟)错误!NH4Cl△NH3↑+HCl↑错误!HCl+AgNO3====HNO3+AgCl↓错误!HCl+NaOH====NaCl+H2OHCl+NaHCO3====NaCl+CO2↑+H2O 错误!2HClO光照2HCl↑+O2↑错误!Ca(ClO)2+2HCl====CaCl2+2HClO Ca(ClO)2+CO2+H2O====CaCO3+2HClO○,20NaOH+HClO====NaClO+H2O HClO+Na2CO3====NaClO+2NaHCO3○,212Cl2+2Ca (OH)2====CaCl2+Ca(ClO)2+2H2O5Cl2+I2+6H2O====2HIO3+10HCl错误!3Cl2+8NH3====N2+6NH4Cl氧(O)元素关系图错误!2O 3====3O 2 O 3+2KI+H 2O====2KOH+I 2+O 2错误!3O 放 电2O 3错误!O 3+H 2S====H 2O+O 2+S ↓ 错误!2H 2O 通 电2H 2↑+O 2↑2H 2O+2F 2====4HF+O 2错误!O 2+2H 点 燃2H 2OO 2+2Cu+2H 2SO 4====2CuSO 4+2H 2O O 2+2H 2S====2S ↓+2H 2O错误!2H 2O 2 2 2H 2O+O 2↑5H 2O 2+2KMnO 4+3H 2SO 4====K 2SO 4+2MnSO 4+5O 2↑+8H 2O错误!2H 2O 2MnO 22H 2O+O 2↑H 2O 2+H 2S====S ↓+2H 2OH 2O 2+2KI+H 2SO 4====K 2SO 4+I 2+2H 2O H 2O 2+Na 2SO 3====Na 2SO 4+H 2O H 2O 2+2FeSO 4+H 2SO 4====Fe 2(SO 4)3+2H 2O错误!2H 2O+Na 2O 2====2NaOH+H 2O 2BaO 2+H 2SO 4(稀)低 温H 2O 2+BaSO 4↓错误!O 2+2Na 点 燃Na 2O 2错误!2Na 2O 2+2CO 2====2Na 2CO 3+O 2↑2Na 2O 2+2H 2O====4NaOH+O 2↑错误!Na 2O 2+H 2SO 4(稀)====Na 2SO 4+H 2O 2 Na 2O 2+2H 2O 低 温2NaOH+H 2O 2错误!H 2O 2+SO 2====H 2SO 4 错误!Na 2O 2+SO 2====Na 2SO 4氮(N)元素关系图重要关系:○,4H 2O →O 2:F 2、电解错误!H 2O 2→O 2:KMnO 4、MnO 2 错误!H 2O 2→H 2O :I —、S 2—、SO 42-、Fe 2+重要关系:错误!HNO3(稀)→NO:HBr、HI、H2S、SO2、SO32—、Fe2+、Fe、Cu、Ag○,26HNO3(浓)→NO2:HBr、HI、H2S、SO2、SO32—、Fe2+、S、C、P、Cu、Ag错误!Mg3N2+8HCl====3MgCl2+2NH4Cl错误!2NH4Cl+Ca(OH)2△2NH3↑+CaCl2+2H2ONH4Cl△NH3↑+HCl↑2NH4Cl+CaO△CaCl2+2NH3↑+H2O错误!NH3+HCl====NH4Cl错误!NH3·H2O+HCl====NH4Cl+H2O3NH3·H2O+FeCl3====Fe(OH)3↓+3NH4Cl错误!NH4Cl+H2O====NH3·H2O+H2ONH4Cl+NaOH====NH3·H2O+NaCl错误!NH3+H2O====NH3·H2O错误!NH3·H2O △NH3↑+H2O错误!N2+3H高温高压催化剂2NH3错误!4NH3+3O2(纯)点燃2N2+6H2O2NH3+3CuO△N2+3Cu+3H2O错误!Mg3N2+6H2O====2NH3↑+3Mg(OH)2错误!3Mg+N点燃Mg3N2错误!NaNO2+NH4Cl△N2↑+NaCl+2H2O错误!N2+O放电NO错误!6NO+4NH3△5N2+6H2O错误!6NO2+8NH3△7N2+12H2O错误!NO+NO2+2NaOH====2NaNO2+H2O 错误!2NaNO2+2NaI+2H2SO4====2NO↑+I2 +2H2O+2Na2SO42NaNO2+2HCl====2NaCl+NO↑+NO2(完整word版)高中化学元素关系图↑+H2O错误!2NO+O2====2NO2错误!3NO2+H2O====2HNO3+NONO2+2KI+H2O====I2+NO+2KOH错误!8HNO3(稀)+3Cu====3Cu(NO3)2+2NO↑+4H2O Fe+2HNO3(稀)====Fe(NO3)3+NO↑+4H2O2HNO3(稀)+3H2S====3S↓+2NO↑+4H2O3Na2SO3+2HNO3(稀)====3NaSO4+2NO↑+4H2O2H2O+3SO2+2HNO3(稀)====3H2SO4+2NO6KI+8HNO3(稀)====6KNO3+3I2+2NO↑+4H2O3FeCl2+4HNO3====2FeCl3+NO↑+Fe( NO3)2+2H2O错误!2HNO2====H2O+NO↑+NO2↑错误!5NaNO2+2KMnO4+3H2SO4====5NaN O3+K2SO4+2MnSO4+3H2O错误!2NaNO3△2NaNO2+O2↑错误!2NO2+2NaOH====NaNO3+NaNO2+ H2O○,253NO2+H2O====2HNO3+NO错误!4HNO3(浓)+Cu====Cu(NO3)2+2NO2+2H2OC+4HNO3(浓)△CO2↑+4NO2↑+2H2OS+6HNO3(浓)△H2SO4+6NO2↑+2H2OP+5HNO3(浓)△H3PO4+5NO2↑+H2O4HNO见光或受热2H2O+4NO2↑+O2↑错误!2Cu(NO3)2△2CuO+4NO2↑+O2↑○,28HNO3+NaOH====NaNO3+H2O 错误!NaNO3+H2SO4(浓)微热NaHSO4+HNO3↑错误!2HNO3+Cu(OH)2====Cu(NO3)2+2H2O○312HNO3(浓)+Ag====AgNO3+NO2↑+H2O3Ag+4HNO3(稀)====3AgNO3+NO↑+2H2O○,322AgNO3+Cu====Cu(NO3)2+2Ag磷(P)元素关系图错误!4P+5O2点燃2P2O5○22P+3Cl点燃2PCl3○,3PCl3+Cl2====PCl5错误!PCl3+3H2O====H3PO3+3HCl错误!2P+3Ca △Ca3P2错误!2P+3H2△2PH3错误!Ca3P2+6H2O====3Ca(OH)2+3PH3↑错误!PCl5+4H2O====H3PO4+5HCl错误!P2O5+3H2O====2H3PO4错误!Ca(H2PO4)2+H2SO4====2H3PO4+CaSO4错误!2H3PO4+Ca(OH)2====Ca(H2PO4)2+2H2O 错误!Ca(H2PO4)2+Ca(OH)2====2CaHPO4+2H2O错误!2CaHPO4+H3PO4====Ca(H2PO4)2错误!2CaHPO4+Ca(OH)2====Ca3(PO4)2+2H 2O错误!Ca3(PO4)2+H3PO4====3CaHPO4错误!Ca3(PO4)2+3H2SO4====3CaSO4+2H3PO4错误!2H3PO4+3Ca(OH)2====Ca3(PO4)2+3H2O碳(C)元素、硅(Si)元素关系图错误!错误!C+2H2高温CH4○,2CH高温C+2H2错误!CH4+2O点燃CO2+2H2O 错误!2C+O点燃2COC+CO高温2CO2C+SiO高温Si+2CO↑3C+CaO3000℃CaC2+CO↑C+H2O高温H2↑+CO↑C+ZnO高温Zn+CO↑错误!C+O 点燃CO2C+2CuO△2Cu+CO2↑○6CO2+2Mg点燃2MgO+C ○,72C+SiO高温Si+2CO↑错误!2CO+O2点燃2CO2CO+CuO△Cu+CO2CO+H2O催化剂、△CO2+H2 2CO+Fe2O高温2Fe+3CO2错误!CO2+C高温2CO ○,10CO2+Ca(OH)2====CaCO3↓+H2O 错误!CaCO3煅烧CaO+CO2↑CaCO3+2HCl====CaCl2+CO2↑+H2O 错误!CO2+2NaOH====Na2CO3+H2O错误!Na2CO3+2H+====2Na++CO2↑错误!Na2CO3+Ca (OH)2====CaCO3↓+2NaOH错误!CaCO3+CO2+H2O====Ca(HCO3)2错误!Ca(HCO32△CaCO3↓+CO2↑+H2O Ca(HCO3)2+Ca(OH)2====2CaCO3↓+2H2 O错误!Ca(HCO3)2+2NaOH====CaCO3↓+Na2CO3+2H2O错误!Na2CO3+H2O+CO2====2NaHCO3 Na2CO3+HCl====NaHCO3+NaCl错误!2NaHCO3△Na2CO3+H2O+CO2↑○20Na 2CO 3+SiO 高 温Na 2SiO 3+CO 2↑错误!Si+2NaOH+H 2O====Na 2SiO 3+2H 2↑错误!Si+O 2 △ SiO 2错误!SiO 2+2C 高 温Si+2CO ↑ 错误!Si+2F 2====SiF 4Si+4HF====SiF 4+2H 2↑错误!SiO 2+2NaOH====Na 2SiO 3+H 2O 错误!H 2SiO 3 △ H 2O+SiO 2○,27Na 2SiO 3+6HF====2NaF+SiF 4+3H 2O○28Na 2SiO 3+2HCl+H 2O====2NaCl+H 4Si O 4↓错误!H 4SiO 4====H 2SiO 3+H 2O 错误!SiO 2+4HF====SiF 4↑+2H 2O钠(Na)元素关系图错误!2Na+S△ Na 2S错误!2Na+O点 燃Na 2O 2错误!2Na+2H 2O====2NaOH+H 2↑ 错误!4Na+O 2====2Na 2O 错误!2Na+H 高 温2NaH 错误!2NaCl(熔融)电 解2Na+Cl 2↑ 错误!2Na+Cl 点 燃2NaCl○,8 +H 2O+H 2↑错误!NaH+H 2O====NaOH+H 2↑(完整word 版)高中化学元素关系图错误!Na 2O+H 2O====2NaOH○,112Na 2O+O 2 △ 2Na 2O 2○,122Na 2O 2+2H 2O====4NaOH+O 2↑错误!2Na 2O 2+2CO 2====2Na 2CO 3+O 2↑ 错误!2NaOH+CO 2====Na 2CO 3+H 2O 错误!Na 2CO 3+Ca(OH)2====2NaOH+CaCO 3↓错误!Na 2CO 3+2HCl====2NaCl+H 2O+CO 2↑○17NaOH+CO 2====NaHCO 3错误!2NaCl+2H 2O电 解2NaOH+H 2↑+Cl 2↑○19NaOH+HCl====NaCl+H 2O错误!NaCl+NH 3+CO 2=H 2O====NH 4Cl+Na HCO 3错误!NaHCO 3+HCl====NaCl+H 2O+CO 2↑错误!NaHCO 3+NaOH====Na 2CO 3+H 2O错误!Na 2CO 3+CO 2+H 2O====2NaHCO 3铝(Al)元素关系图错误!2Al 2O 3(熔融)电解、冰晶石4Al+3O 2↑○,24Al+3O 2====2Al 2O 3重要关系:错误!Al 3+→Al (OH)3:OH -、NH 3·H 2O 、CO 32-、HCO 3—、S 2-、HS —、ClO —、AlO 2-○11 AlO 2— →Al(OH )3:H +、CO 2、Al 3+、Fe 3+、HCO 3—、NH 4+、HSO 3—、H 2PO 4—、Cl 2、NO 2(完整word版)高中化学元素关系图2Al+Fe2O3高温2Fe+2Al2O34Al+3MnO高温3Mn+2Al2O32Al+WO3高温W+Al2O3错误!2Al+6H+====2Al3++3H2↑2Al+3Hg2+====2Al3++3Hg错误!Al2O3+6H+====2Al3++3H2O错误!Al(OH)3+3H+====Al3++3H2O错误!Al3++3OH-====Al(OH)3↓Al3++3NH3·H2O====Al(OH)3↓+3NH4+2Al3++3CO32—+3H2O====2Al(OH)3↓+3CO2↑Al3++3HCO3—====Al(OH)3↓+3CO2↑2Al3++3S2—+6H2O====2Al(OH)3↓+3H2S↑Al3++3HS-3H2O====Al(OH)3↓+3H2S↑Al3++3ClO-+3H2O====Al(OH)3↓+3HCl OAl3++3AlO2—+6H2O====4Al(OH)3↓○7AlO2-+4H+====Al3++2H2O错误!Al3++4OH-====AlO2—+2H2O错误!2Al+2OH—+2H2O====2AlO2-+3H2↑错误!Al(OH)3+OH-====AlO2—+2H2O 错误!AlO2-+H++H2O====Al(OH)3↓2AlO2-+CO2(少量)+3H2O====2Al(OH)3↓+CO32-AlO2-+CO2(足量)+2H2O====Al(OH)3↓+HCO3-3AlO2-+Fe3++6H2O====3Al(OH)3↓+Fe (OH)3↓AlO2-+HCO3-+H2O====Al(OH)3↓+CO3 2—AlO2-+NH4++2H2O====Al(OH)3↓+NH3·H2OAlO2—+HSO3—+H2O====Al(OH)3↓+SO32—2AlO2-+Cl2+3H2O====2Al(OH)3↓+Cl-+ ClO—2AlO2-\+3NO2+3H2O====2Al (OH)3↓+NO+2NO3-AlO2—H2PO4-+H2O====Al(OH)(完整word版)高中化学元素关系图3↓+HPO42-错误!2Al(OH)3△Al2O3+3H2O错误!Al2O3+2OH-====2AlO2—+H2O 铁(Fe)元素关系图重要关系:错误!Fe→Fe2+:Fe3+、Cu2+、H+、I2○,3Fe2+→Fe3+:Cl2、Br2、O2、H2O2、HNO3、MnO4—错误!Fe3+→Fe2+:Zn、Fe、Cu、I—、H2S、SO2错误!Fe→Fe3+:Cl2、Br2、HNO3、H2SO4(浓)△错误!Fe3+→Fe(OH)3:OH—、S2—、HS-、CO32—、HCO3-、AlO2—、ClO-(完整word版)高中化学元素关系图错误!Fe2++Zn====Fe+Zn2+错误!Fe+Cu2+====Cu+Fe2+Fe+2Fe3+====3Fe2+Fe+2H+====Fe2++H2Fe+I2====Fe2++2I—○32Fe2++Cl2====2Fe3++2Cl—2Fe2++Br2====2Fe3++2Br—4Fe2++O2+4H+====4Fe3++2H2O2Fe2++H2O2+2H+====2Fe3++2H2O5Fe2++MnO4-+8H+====5Fe3++Mn2++ 4H2O3Fe2++NO3-+4H+====3Fe3++NO↑+2H 2O错误!2Fe3++Zn====2Fe2++Zn2+2Fe3++Cu====2Fe2++Cu2+2Fe3++2I-====2Fe2++I22Fe3++H2S====2Fe2++S↓+2H+2Fe3++SO2+2H2O====SO42-+2Fe2++4 H+错误!2Fe3++3Zn====2Fe+3Zn2+○6Fe+NO3—+4H+====Fe3++NO↑+2H2OFe+6H2SO4(浓)△Fe2(SO4)3+3SO2↑+6H2O错误!FeO+2H+====Fe2++H2O○,8Fe(OH)2+2H+====Fe2++2H2O错误!Fe2++2OH-====Fe(OH)2↓错误!Fe3O4+8H+====Fe2++2Fe3++4H2O Fe3O4+8HI====3FeI2+I2+4H2O错误!4Fe(OH)2+O2+2H2O====4Fe(OH)3○123Fe3O4+28HNO3====9Fe (NO3)3+NO↑+14H2O错误!Fe3++3NH3·H2O====Fe(OH)3↓+3NH4+○,14Fe(OH)3+3H+====Fe3++3H2O错误!Fe2O3+6H+====2Fe3++3H2O○,16Fe3++3SCN-====Fe(SCN)3错误!Fe(OH)2隔绝空气、△FeO+H2O○182Fe(OH)3△FeO+H2O错误!Fe2O3+3CO高温2Fe+3CO23Fe3O4+8Al高温9Fe+4Al2O32FeO+Si高温2Fe+SiO2○,206FeO+O2△2Fe3O4○213Fe+2O2点燃Fe3O43Fe+4H2O(g)高温Fe3O4+4H2○,222Fe(过量)+O2高温2FeO (炼钢炉中的反应)错误!Fe+S△FeS错误!FeS2△FeS+S错误!4FeS2+11O高温2Fe2O3+8SO2错误!2Fe2++5ClO-+5H2O====2Fe(OH)3↓+4HClO+Cl-6Fe2++3ClO-+3H2O====2Fe(OH)3↓+4Fe3++3Cl-4Fe2++4Na2O2+6H2O====4Fe(OH)3↓+8Na++O26Fe2++3Na2O2+6H2O====4Fe(OH)3↓+2Fe3++6Na+铜(Cu)元素关系图重要关系:错误!CuO→Cu:H2、CO、NH3、C、CH3CH2OH错误!Cu→Cu2+:O2、HNO3、H2SO4(浓)、H2O2、Fe3+错误!Cu(OH)2→C u2O:CH3CHO、HCHO、H—COOH错误!Cu2(OH)2CO3+4H+====2Cu2++CO2↑+3H2O错误!2Cu+S △Cu2S错误!2Cu+O2+CO2+H2O====Cu2(OH)2CO3错误!CuO+H2△Cu+H2O3CuO+2NH3△N2+3Cu+3H2OCuO+CO△Cu+CO2CuO+CH3CH2OH−→−∆Cu+CH3CHO+H2 O错误!2Cu+O2△2CuO4Cu+2NO2△4CuO+N2错误!Cu2++Zn====Zn2++Cu○,72Cu+O2+2H2SO4(稀)△2CuSO4+2H2O3Cu+8HNO3(稀)====3Cu (NO3)2+2NO↑+4H2OCu+2H2SO4△CuSO4+SO2↑+2H2O Cu+H2O2+H2SO4(稀)====CuSO4+2H2O2FeCl3+Cu====CuCl2+2FeCl2○8Cu2(OH)2CO3△2CuO+H2O+CO2↑错误!4CuO 高温2Cu2O+O2↑错误!Cu(OH)2△CuO+H2O错误!CuO+2H+====Cu2++H2O 错误!Cu2+H2S====CuS↓+2H+错误!3CuS+8HNO3(稀)====3Cu(NO3)2+3S↓+2NO↑+4H2O错误!Cu(OH)2+2H+====Cu2++2H2O错误!Cu2++2OH—====Cu(OH)2↓错误!2Cu (OH)2+R—CHO+NaOH−→−∆R—COO Na+Cu2O↓+3H2O2Cu(OH)2+H-COOH+2NaOH−→−∆Na2CO3 +Cu2O↓+4H2O4Cu(OH)2+H—CHO+2NaOH−→−∆Na2CO3+2Cu2O↓+6H2O错误!Cu(OH)2+4NH3====[Cu(NH3)4](OH)2错误!Cu2O+2H+====Cu+Cu2++H2O高中化学17≡≡种常见气体的实验室制法Cl2:4HCl+MnO2△Cl2+MnCl2+2H2O HCl:2NaCl+H2SO4△Na2SO4+2HClHBr:NaBr+H3PO4△NaH2PO4+HBr HI:KI+H3PO4△KH2PO4+HIO2:2KMnO4△K2MnO4+MnO2+O2 SO2:Na2SO3+H2SO4====Na2SO4+H2O +SO2H2S:FeS+H2SO4====FeSO4+H2SNH3:2NH4Cl+Ca(OH)2△2NH3+CaCl2+2H2ON2:NH4NO2△N2+2H2ONO:3Cu+8HNO3====3Cu(NO3)2+2NO+4H2ONO2:Cu+4HNO3====Cu(NO3)2+2NO2+2H2OCO2:CaCO3+2HCl====CaCl2+CO2+H2O CO:HCOOH△、浓硫酸CO+H2OCH4:CH2COONa+NaOH△、碱石灰Na2CO3+CH4CH2=CH2:C2H5OH浓硫酸、170℃CH2=CH2+H2O CH≡CH:CaC2+2H2O−→−Ca(OH)2+CH≡CHH2:Zn+H2SO4====ZnSO4+H2。

高中化学必修一元素转换关系图1、钠及其化合物的转化关系①Na 2O +2HCl=2NaCl +H 2O ②2Na +2HCl=2NaCl +H 2↑Na 2O +2H +=2Na ++H 2O 2Na +2H +=2Na ++H 2↑ ③2Na 2O 2+4HCl=4NaCl +O 2↑+2H 2O ④4Na +O 2=2Na 2O2Na 2O 2+4H +=4Na ++O 2↑+2H 2O ※⑥2Na +2H 2O=2NaOH +H 2↑ ※⑤2Na +O 2△Na 2O 2 2Na +2H 2O=2Na ++2OH -+H 2↑⑦Na 2O +H 2O=2NaOH ※⑧2Na 2O 2+2H 2O=4NaOH +O 2↑Na 2O +H 2O=2Na ++2OH - 2Na 2O 2+2H 2O=4Na ++4OH -+O 2↑ ⑨Na 2O +CO 2=Na 2CO 3 ※⑩2Na 2O 2+2CO 2=2Na 2CO 3+O 2⑾2NaOH +CO 2=Na 2CO 3+H 2O ⑿Na 2CO 3+Ca(OH)2=CaCO 3↓+2NaOH2OH -+CO 2=CO 32-+H 2O CO 32-+Ca 2+=CaCO 3↓⒀NaOH +CO 2=NaHCO 3 ※⒁Na 2CO 3+H 2O +CO 2=2NaHCO 3OH -+CO 2=HCO 3- CO 32-+H 2O +CO 2=2HCO 3-※⒂2NaHCO 3△Na 2CO 3+H 2O +CO 2↑ 或 ※NaHCO 3+NaOH=Na 2CO 3+H 2OHCO 3-+OH -=CO 32-+H 2O⒃Na 2CO 3+2HCl=2NaCl +H 2O +CO 2↑ ⒄NaHCO 3+HCl=NaCl +H 2O +CO 2↑CO 32-+2H +=H 2O +CO 2↑ HCO 3-+H +=H 2O +CO 2↑补充:①石灰水中加入少量NaHCO 3 : Ca(OH)2+NaHCO 3=CaCO 3↓+NaOH +H 2OCa 2++OH -+HCO 3-=CaCO 3↓+H 2O②石灰水中加入过量NaHCO 3 : Ca(OH)2+2NaHCO 3=CaCO 3↓+Na 2CO 3+2H 2OCa 2++2OH -+2HCO 3-=CaCO 3↓+CO 32-+2H 2O 2、铝及其化合物的转化关系NaCl Na NaOH Na 2CO 3 NaHCO 3 NaCl Na 2O Na 2O 2 ① ② ③ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩ ⑾ ⑿ ⒀ ⒁ ⒂⒃ ⒄ ④ Al Al 2O 3 A l (O H )3 KAl(SO 4)2 AlCl 3① ② ③ ④ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩ ⑾⑿①2Al +6HCl=2AlCl 3+3H 2↑ ②4Al +3O 2△2Al 2O 32Al +6H +=2Al 3++3H 2↑※③2Al +2NaOH +2H 2O=2NaAlO 2+3H 2↑ ④Al 2O 3+6HCl=2AlCl 3+3H 2O2Al +2OH -+2H 2O=2AlO 2-+3H 2↑ Al 2O 3+6H +=2Al 3++3H 2O ※⑤Al 2O 3+2NaOH=2NaAlO 2+H 2O ⑥AlCl 3+3NH 3·H 2O=Al(OH)3↓+3NH 4ClAl 2O 3+2OH -=2AlO 2-+H 2O Al 3++3NH 3·H 2O = Al(OH)3↓+3NH 4+⑦Al(OH)3+3HCl=AlCl 3+3H 2O ⑧2Al(OH)3△Al 2O 3+3H 2OAl(OH)3+3H +=Al 3++3H 2O☆⑨NaAlO 2+HCl +H 2O =Al(OH)3↓+NaCl 或NaAlO 2+2H 2O +CO 2=Al(OH)3↓+NaHCO 3AlO 2-+H ++H 2O=Al(OH)3↓ AlO 2-+2H 2O +CO 2=Al(OH)3↓+HCO 3-※⑩Al(OH)3+NaOH = NaAlO 2+2H 2O ☆⑾Al 3++3H 2O Al(OH)3胶体+3H +Al(OH)3+OH -= AlO 2-+2H 2O 明矾净水⑿AlCl 3+4NaOH = NaAlO 2+3NaCl +2H 2O Al 3++4OH -= AlO 2-+2H 2O 3、铁及其化合物的转化关系①Fe 3O 4+4CO△3Fe +4CO 2 ※② 3Fe +4H 2O(g)高温Fe 3O 4+4H 2 ③ Fe +2HCl=FeCl 2+H 2↑ 或3Fe +2O 2点燃Fe 3O 4Fe +2H +=Fe 2++H 2↑ ④2Fe +3Cl 2点燃2FeCl 3※⑤2FeCl 2+Cl 2 = 2FeCl 3 ※⑥Fe +2FeCl 3 = 3FeCl 22Fe 2++Cl 2 = 2Fe 3++2Cl - Fe +2Fe 3+= 3Fe 2+⑦FeCl 2+2NaOH = Fe(OH)2↓+2NaCl ⑧Fe(OH)2+2HCl = FeCl 2+2H 2OFe 2++2OH -= Fe(OH)2↓ Fe(OH)2+2H += Fe 2++2H 2O※⑨4Fe(OH)2+O 2+2H 2O = 4Fe(OH)3 ⑩FeCl 3+3NaOH = Fe(OH)3↓+3NaCl白色沉淀迅速变成灰绿色,最后变成红褐色 Fe 3++3OH -= Fe(OH)3↓⑾Fe(OH)3+3HCl = FeCl 3+3H 2O Fe 3++3H 2O Fe(OH)3胶体+3H +(净水) Fe(OH)3+3H += Fe 3++3H 2O ⑿2Fe(OH)3△Fe 2O 3+3H 2O⒀Fe 2O 3+6HCl = 2FeCl 3+3H 2O ※⒁FeCl 3+3KSCN = Fe(SCN)3+3KClFe 2O 3+6H += 2Fe 3++3H 2O Fe 3++3SCN -= Fe(SCN)3Fe 3O 4 Fe FeCl 2 Fe(OH)2 Fe(SCN)3 FeCl 3 Fe(OH)3 Fe O 3 ①② ③ ④⑤ ⑥ ⑦ ⑧ ⑨⑩ ⑾⑿ ⒁ ⒀①Si +O 2△SiO 2②SiO 2+2C 高温 Si +2CO ↑※③SiO 2+4HF = SiF 4↑+2H 2O (刻蚀玻璃)④Si +4HF = SiF 4↑+2H 2↑ ⑤SiO 2+CaO高温CaSiO 3※⑥SiO 2+2NaOH = Na 2SiO 3+H 2O SiO 2+CaCO 3高温CaSiO 3+CO 2↑SiO 2+2OH -= SiO 32-+H 2O ※⑦Na 2SiO 3+2HCl = H 2SiO 3↓+2NaCl SiO 2+Na 2CO 3高温Na 2SiO 3+CO 2↑ SiO 32-+2H += H 2SiO 3↓※⑦Na 2SiO 3+H 2O +CO 2=H 2SiO 3↓+Na 2CO 3或Na 2SiO 3+2H 2O +2CO 2=H 2SiO 3↓+2NaHCO 3SiO 32-+H 2O +CO 2=H 2SiO 3↓+CO 32-或SiO 32-+2H 2O +2CO 2=H 2SiO 3↓+2HCO 3-⑧H 2SiO 3+2NaOH = Na 2SiO 3+2H 2O ⑨H 2SiO 3 △H 2O +SiO 2H 2SiO 3+2OH -= SiO 32-+2H 2O 5、氯及其化合物的转化关系①2Fe +3Cl 2 点燃2FeCl 3 ②Cu +Cl 2 点燃CuCl 2 ③2FeCl 3+Cu = 2FeCl 2+CuCl 22Fe 3++Cu = 2Fe 2++Cu 2+④H 2+Cl 2 2HCl※⑤MnO 2+4HCl(浓) △MnCl 2+Cl 2↑+2H 2OMnO 2+4H ++2Cl - △ Mn 2++Cl 2↑+2H 2O ※⑥Cl 2+H 2O = HCl +HClO ※⑦2HClO 2HCl +O 2↑Cl 2+H 2O = H ++Cl -+HClO 2HClO 2H ++2Cl -+O 2↑※⑧Cl 2+2NaOH = NaCl +NaClO +H 2O ※⑨2Cl 2+2Ca(OH)2 = CaCl 2+Ca(ClO)2+2H 2OCl 2+2OH -= Cl -+ClO -+H 2O 工业制漂白粉※⑩Ca(ClO)2+H 2O +CO 2 = CaCO 3↓+2HClO 或Ca(ClO)2+2HCl = CaCl 2+2HClOCa 2++2ClO -+H 2O +CO 2= CaCO 3↓+2HClO 或ClO -+H += HClO 漂白粉的漂白原理向漂白粉溶液中通入过量的CO 2:Ca(ClO)2+2H 2O +2CO 2 = Ca(HCO 3)2+2HClOClO -+H 2O +CO 2 = HCO 3-+HClONa 2SiO 3 SiF 4 Si SiO 2 H 2SiO 3 CaSiO 3 ①②③ ④ ⑤⑥ ⑦ ⑧⑨ CuCl 2 HClO HCl Cl 2 FeCl 3NaClO Ca(ClO)2① ② ③ ④⑤ ⑥ ⑦ ⑧ ⑨ ⑩ 点燃或光照 光照光照①S +O 2 点燃SO 2②2H 2S +SO 2=3S +2H 2O※③SO 2+O 2 催化剂加热 2SO 3 ④SO 3+H 2O = H 2SO 4 ⑤SO 2+CaO△CaSO 3 或 SO 2+Ca(OH)2 = CaSO 3↓+H 2O⑥SO 3+CaO = CaSO 4 SO 2+Ca 2++2OH -=CaSO 3↓+H 2O SO 3+Ca(OH)2 = CaSO 4+H 2O ⑦2CaSO 3+O 2△2CaSO 4※⑧SO 2+Cl 2+2H 2O = H 2SO 4+2HCl ※⑨Cu +2H 2SO 4(浓) △CuSO 4+SO 2↑+2H 2OSO 2+Cl 2+2H 2O = 4H ++SO 42-+2Cl -Cu +2H 2SO 4(浓) △Cu 2++SO 42-+SO 2↑+2H 2O※⑩C +2H 2SO 4(浓)△CO 2↑+2SO 2↑+2H 2O7、氮及其化合物的转化关系 ※①N 2+O 22NO※②2NO +O 2 = 2NO 2※③3NO 2+H 2O = 2HNO 3+NO 3NO 2+H 2O =2H ++2NO 3-+NO以上三个反应为“雷雨发庄稼”原理 扩展反应有:4NO 2+O 2+2H 2O = 4HNO 3 4NO +3O 2+2H 2O = 4HNO 3 ※④Cu +4HNO 3(浓) = Cu(NO 3)2+2NO 2↑+2H 2O 上面两个反应主要用于气体溶于水时的计算Cu +4H ++2NO 3-=Cu 2++2NO 2↑+2H 2O 或 4HNO 3 4NO 2↑+ O 2↑+ 2H 2OC +4HNO 3(浓)△CO 2↑+2NO 2↑+2H 2O 浓硝酸见光易变黄的原因※⑤3Cu +8HNO 3(稀) = 3Cu(NO 3)2+2NO ↑+4H 2O ⑥N 2+3H 2催化剂 高温高压2NH 33Cu +8H ++2NO 3-=3Cu 2++2NO ↑+4H 2O 合成氨反应是人工固氮的主要途径 ⑦NH 3+HCl = NH 4Cl ⑧NH 4Cl△NH 3↑+HCl ↑NH 3+H += NH 4+(水溶液中) 补充:NH 4HCO 3 △NH 3↑+H 2O+CO 2↑氨气与酸均能反应生成铵盐,且与挥发性酸 铵盐受热都易分解,但并不是所有的铵盐 (如浓HCl 、浓HNO 3)相遇时空气中有白烟 都分解出氨气,如NH 4NO 3、(NH 4)2SO 4※⑧NH 4Cl +NaOH△NaCl +NH 3↑+H 2O NH 4++OH-△NH 3↑+H 2O所有的铵盐都能与碱作用放出氨气,可利用此反应鉴别铵离子。

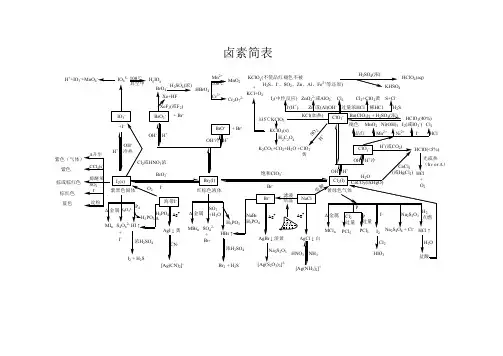

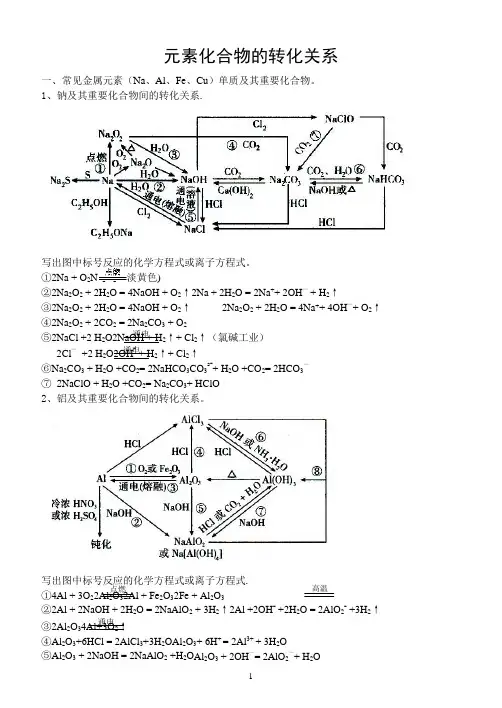
元素化合物的转化关系一、常见金属元素(Na 、Al 、Fe 、Cu )单质及其重要化合物。
1、钠及其重要化合物间的转化关系.写出图中标号反应的化学方程式或离子方程式。
①2Na + O 2Na 2O 2(淡黄色)②2Na 2O 2 + 2H 2O = 4NaOH + O 2↑2Na + 2H 2O = 2Na ++ 2OH —+ H 2↑③2Na 2O 2 + 2H 2O = 4NaOH + O 2↑ 2Na 2O 2 + 2H 2O = 4Na ++ 4OH —+ O 2↑ ④2Na 2O 2 + 2CO 2 = 2Na 2CO 3 + O 2⑤2NaCl +2 H 2O2NaOH + H 2↑+ Cl 2↑(氯碱工业)2Cl — +2 H 2O2OH - + H 2↑+ Cl 2↑⑥Na 2CO 3 + H 2O +CO 2= 2NaHCO 3CO 32-+ H 2O +CO 2= 2HCO 3—⑦ 2NaClO + H 2O +CO 2= Na 2CO 3+ HClO 2、铝及其重要化合物间的转化关系。
写出图中标号反应的化学方程式或离子方程式.①4Al + 3O 22Al 2O 32Al + Fe 2O 32Fe + Al 2O 3②2Al + 2NaOH + 2H 2O = 2NaAlO 2 + 3H 2↑2Al +2OH - +2H 2O = 2AlO 2- +3H 2↑③2Al 2O 34Al+3O 2↑ ④Al 2O 3+6HCl = 2AlCl 3+3H 2OAl 2O 3+ 6H + = 2Al 3+ + 3H 2O⑤Al 2O 3 + 2NaOH = 2NaAlO 2 +H 2O Al 2O 3 + 2OH —= 2AlO 2—+ H 2O通电 通电 通电 点燃高温⑥AlCl 3 +3NH 3·H 2O = Al (OH )3↓+ 3NH 4Cl Al 3+ + 3 NH 3·H 2O = Al(OH )3↓+ 3NH 4+ AlCl 3 +3Na OH = Al (OH)3↓+ 3NaCl Al 3+ + 3OH — = Al(OH )3↓⑦Al (OH)3 + NaOH =NaAlO 2 +2 H 2O Al (OH)3 + OH -= AlO 2— +2H 2O ⑧Al 3+ +3 AlO 2— +6H 2O =4Al(OH)3↓ 3、铁及其重要化合物间的转化关系。
元素化合物的转化关系之五兆芳芳创作一、罕有金属元素(Na 、Al 、Fe 、Cu )单质及其重要化合物.1、钠及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式.①2Na + O 2Na 2O 2(淡黄色)②2Na 2O 2 + 2H 2O = 4NaOH + O 2↑2Na + 2H 2O = 2Na ++ 2OH - + H 2↑③2Na 2O 2 + 2H 2O = 4NaOH + O 2↑ 2Na 2O 2 + 2H 2O = 4Na ++ 4OH -+ O 2↑④2Na 2O 2 + 2CO 2 = 2Na 2CO 3 + O 2⑤2NaCl +2 H 2O2NaOH + H 2↑+ Cl 2↑(氯碱产业) 2Cl - +2 H 2O2OH - + H 2↑+ Cl 2↑⑥Na 2CO 3 + H 2O +CO 2= 2NaHCO 3CO 32-+ H 2O +CO 2= 2HCO 3- ⑦ 2NaClO + H 2O +CO 2= Na 2CO 3+ HClO2、铝及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式. ①4Al + 3O 22Al 2O 32Al + Fe 2O 32Fe + Al 2O 3②2Al + 2NaOH + 2H 2O = 2NaAlO 2 + 3H 2↑2Al +2OH - +2H 2O = 2AlO 2- +3H 2↑③2Al 2O 34Al+3O 2↑ 通电 通电通电点燃高温④Al 2O 3+6HCl = 2AlCl 3+3H 2OAl 2O 3+ 6H + = 2Al 3+ + 3H 2O ⑤Al 2O 3 + 2NaOH = 2NaAlO 2 +H 2O Al 2O 3 + 2OH -= 2AlO 2-+ H 2O ⑥AlCl 3 +3NH 3·H 2O = Al(OH)3↓+ 3NH 4Cl Al 3+ + 3 NH 3·H 2O = Al(OH)3↓+ 3NH 4+AlCl 3 +3Na OH = Al(OH)3↓+ 3NaCl Al 3+ + 3OH - = Al(OH)3↓ ⑦Al(OH)3 + NaOH =NaAlO 2 +2 H 2O Al(OH)3 + OH -= AlO 2- +2H 2O⑧Al 3+ +3 AlO 2- +6H 2O =4Al(OH)3↓3、铁及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式. ①3 Fe+4H 2O Fe 3O 4 + 4H 2↑3 Fe+2O 2 Fe 3O 4②Fe + 2HCl = FeCl 2 + H 2↑Fe + 2H + = Fe 2+ + H 2↑③2FeCl 3 + Fe = 3FeCl 22Fe 3+ + Fe = 3Fe 2+2FeCl 3 + Cu = 2FeCl 2+ CuCl 22Fe 3+ + Cu = 2Fe 2++Cu 2+④2FeCl 2 + Cl 2 = 2FeCl 32Fe 2+ + Cl 2 = 2Fe 3+ + 2Cl-或O 2高温点燃△⑤4Fe(OH)2 + O2 + 2H 2O = 4Fe(OH)3⑥ 2Fe(OH)3Fe 2O3 + 3H 2O ⑦Fe 2O 3 + 3CO 2Fe + 3CO 2⑧FeCl 3 +3NH 3·H 2O =Fe (OH)3↓+ 3NH 4ClFe 3+ + 3 NH 3·H 2O = Fe (OH)3↓+ 3NH 4+4、铜及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式.①2Cu + S Cu 2S②Cu+4HNO 3 (浓)= Cu(NO 3)2+ 2H 2O + 2NO 2↑Cu+4H ++2NO 3-= Cu 2++ 2H 2O + 2NO 2↑3Cu+8HNO 3 (稀)= 3Cu(NO 3)2+ 4H 2O + 2NO ↑ 3Cu+8H ++2NO 3-= Cu 2++ 2H 2O + 4NO ↑③Fe + Cu(NO 3)2 = Fe(NO 3)2+ Cu Fe + Cu 2+= Fe 2+ + Cu ④CuCl 2 Cu + Cl 2↑二、罕有非金属元素(H 、C 、N 、O 、Si 、S 、Cl )单质及其重要化合物.1、氯及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式. ①Cu + Cl 2 CuCl 2②2Fe + 3Cl 2 3FeCl 3③2NaOH+ Cl 2= NaClO + NaCl + H 2O 2OH -+ Cl 2= ClO - + Cl - + H 2O通电△点燃点燃④MnO 2+4HCl(浓)MnCl 2+Cl 2↑+ 2H 2OMnO 2+4H ++2Cl -Mn 2++Cl 2↑+2H 2O⑤2NaCl 2Na+ Cl 2↑⑥Cl 2 + H 2O HCl + HClOCl 2 + H 2O H ++ Cl - + HClO ⑦Ca(ClO)2 + CO 2 + H 2O =CaCO 3 + 2HClO2、硫及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式. ①Fe + S FeS ②2SO 2+O 22SO 3③Cu+2H 2SO 4 (浓) CuSO 4 +2H 2O+SO 2↑C+2H 2SO 4(浓) 2H 2O+CO 2↑+2SO 2↑④H 2SO 4+Ba(OH)2= Ba SO 4↓+2H 2O 2H ++SO 42-+Ba 2++2OH -= Ba SO 4↓+2H 2O⑤SO 2 +2NaOH = Na 2SO 3 + H 2O SO 2 +2OH -= SO 32-+H 2O ⑥SO 2 +Cl 2+ 2H 2O = H 2SO 4 + 2HCl SO 2 +Cl 2+ 2H 2O = 4H ++SO 42-+ 2Cl -3、氮及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式. ①2NH 4Cl+Ca(OH)2△ CaCl 2+2H 2O+2NH 3↑②N 2 + 3H 2 催化剂 高温高压2NH 3③4NH 3 +5O 2 4NO + 6H 2O ④3NO 2 + H 2O = 2HNO 3 + NO 通电 催化剂 加热⑤3Cu+8HNO3(稀)= 3Cu(NO3)2+ 4H2O + 2NO↑3Cu+8H++2NO3-= Cu2++ 2H2O + 4NO↑⑥Cu+4HNO3 (浓)= Cu(NO3)2+ 2H2O + 2NO2↑Cu+4H++2NO3-= Cu2++ 2H2O + 2NO2↑⑦8NH3 +6NO2= 7N2 + 12H2O4、碳硅及其重要化合物间的转化关系.写出图中标号反响的化学方程式或离子方程式.① 2Mg + CO②SiO2↑③SiO2 + 2NaOH = Na2SiO3 + H2O④Na2Si O3+ 2HCl = 2NaCl + H2Si O3↓Na2Si O3+ H2O +CO2= H2Si O3↓+Na2CO3⑤2NaHCO2CO3 + H2O +CO2↑NaHCO3+ NaOH = Na2CO3 + H2O HCO3-+ OH-= CO32-+ H2O⑥Ca(HCO3)2 + 2NaOH=CaCO3↓+Na2CO3+ 2H2O。