ICH Q C R 残留溶剂 培训
- 格式:ppt
- 大小:752.50 KB
- 文档页数:15

ICH常用有机溶剂分类及残留限度国际药品管理机构ICH(International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use)在药品的质量控制方面,制定了一系列规定。
其中,ICH Q3C Guideline for Residual Solvents是针对制药过程中可能存在的残留有机溶剂进行限制和规定的。
该指南中将有机溶剂分为三类:类1、类2和类3。
本文将详细介绍ICH常用的有机溶剂分类及其残留限度。
类1有机溶剂类1有机溶剂是指在制药工业中使用频率最高、风险最低的有机溶剂。
这类有机溶剂在制药过程中的使用应该越少越好,但即使在非常规制造情况下,其残留量也应该受到限制。
以下是ICH对类1有机溶剂的限制:有机溶剂残留限度(mg/每日剂量单位)苯 2甲苯50二氯甲烷601,2-二氯乙烷 41,1,1-三氯乙烷0.61,1-二氯乙烷 61,1,2-三氯乙烷 11,1,2,2-四氯乙烷0.62-氯乙酸乙酯2002-乙醇5000甲醇3000丙酮300乙酸丁酯300乙酸乙酯900甲醛30N,N-二甲基甲酰胺390乙酸乙酰胺600乙醇5000类2有机溶剂类2有机溶剂与类1有机溶剂相比,使用的风险略高一些。
类2有机溶剂的使用应该尽量避免,但如果使用,其残留也应该受到限制。
以下是ICH对类2有机溶剂的限制:有机溶剂残留限度(mg/每日剂量单位)丁酮0.81-丁醇802-丁醇50叔丁醇600溴丁烷 3正丁烷3000甲苯500乙苯420乙醇3000乙酸异丙酯500乙腈20二甲苯150二甲醚3000二氯乙烷 5甲酸甲酯 3甲酸乙酯300甘油正丁醚690甘油乙二醇正丁醚690甘油乙醚/环已醚840马来酸酐30甲酸300三氯乙烯 4类3有机溶剂类3有机溶剂的使用量和风险都比类1和类2更高。
这类有机溶剂在制药过程中必须严格控制,在常规制药过程中尽可能避免使用。

ichq3c溶剂残留指导原则?
答:ICH Q3C是国际药品注册协调委员会(International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use,简称ICH)发布的一系列指导原则之一。
ICH Q3C溶剂残留指导原则旨在提供关于制药产品中有机溶剂残留限量的建议和指导。
这些指导原则主要适用于药品开发和注册过程中的有
机溶剂残留问题,并帮助制药行业确保药品制造过程中使用的有机溶剂不对人体产生不良影响。
ICH Q3C溶剂残留指导原则分为三个部分:
1.ICH Q3C(R6):这个文档提供了关于食品和药品制品中有机溶剂残留限量的概述和指南。
它包含了各种有机溶剂的分类、评估方法和安全性评价。
2.ICH Q3C(R8):这个文档是对Q3C(R6)的更新版本,添加了关于新的有机溶剂的信息,包括氯仿和四氢呋喃等。
3.ICH Q3C(R7):这个文档是对Q3C(R6)的进一步更新版本,修订了关于有机溶剂分类和限量的一些内容。
根据ICH Q3C溶剂残留指导原则,制药企业需要进行有机溶剂残留评估,并确保其产品中的有机溶剂残留量在国际上接受的安全水平范围内。
这有助于确保药品的质量、安全性和有效性。
请注意,具体的有机溶剂残留限量要求可能因地区和国家而异,制药企业在制定和执行相关政策时应考虑当地法规和准则。


ICH杂质残留溶剂的指导原则QCRICH杂质残留溶剂的指导原则QCR(Quality Control Residual Solvents)是国际药品监管机构-国际药品注册、技术和质量控制委员会(ICH)发布的一项指南,用于规范药品制造过程中的溶剂残留物的质量控制。
溶剂残留物是指在药品生产过程中添加的溶剂,但在制剂中未完全蒸发或被移除的溶剂。
导言溶剂在药品生产过程中广泛应用,包括分离、提取、结晶和纯化等步骤。
然而,溶剂中的残留物可能对人体产生毒性或对药品质量产生影响。
因此,在药品开发和生产过程中,需要对残留溶剂进行必要的质量控制。
溶剂分类ICHQCR指导原则将溶剂分为类1、类2A和类2B三类。
类1溶剂是无毒、无致畸、无致癌和生殖毒性的溶剂,如水、乙醇、甘油等。
这些溶剂的使用一般没有限制。
类2A溶剂是有潜在毒性或限制使用的溶剂,但在限定剂量下不会对人体产生危害。
这类溶剂的最大可容许残留量取决于药物用量、剂型和给药途径。
一些常见的类2A溶剂包括氯仿、氯化甲烷、二氯甲烷等。
类2B溶剂是有潜在毒性,并且在制剂中的残留量应尽量减少的溶剂。
这类溶剂的最大可容许残留量取决于药物用量和给药途径。
一些常见的类2B溶剂包括苯、乙酸乙酯、二甲基甲醚等。
质量控制要求ICHQCR指导原则确定了三种质量控制要求来管理溶剂残留物。
1. 可容许残留量(Permitted Daily Exposure, PDE):PDE是指人体每日接触该溶剂残留量的最大可容许量。
它可以通过毒理学评估和毒性数据来确定。
对于类1溶剂,PDE通常设置为无限大。
对于类2A和类2B 溶剂,PDE被用来确定最大可容许残留量。
2.可容许残留量限度:可容许残留量限度是指药品中残留溶剂的最大可容许浓度。
它根据预期剂量、给药途径和药物性质等因素来确定。
通过使用PDE和剂量预设的数学公式,可以计算出每种溶剂的可容许残留量限度。
3.分析方法:为了确保溶剂残留物的质量控制,需要使用适当的分析方法进行检测和分析。

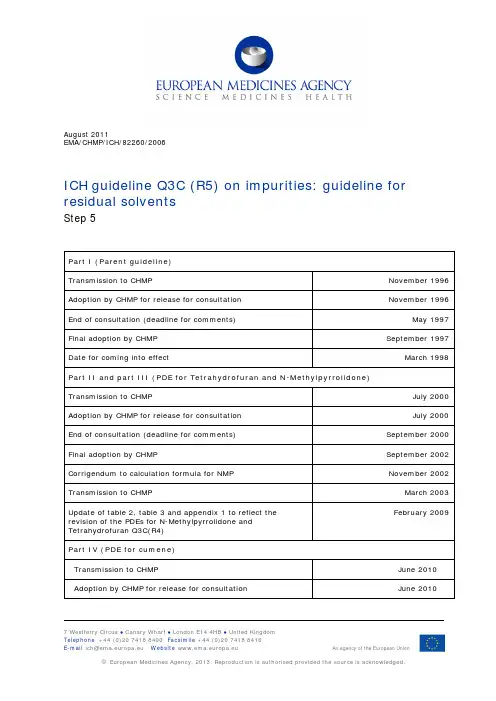
August 2011EMA/CHMP/ICH/82260/2006ICH guideline Q3C (R5) on impurities: guideline for residual solventsStep 5Part I (Parent guideline)Transmission to CHMP November 1996 Adoption by CHMP for release for consultation November 1996 End of consultation (deadline for comments) May 1997 Final adoption by CHMP September 1997 Date for coming into effect March 1998 Part II and part III (PDE for Tetrahydrofuran and N-Methylpyrrolidone)Transmission to CHMP July 2000 Adoption by CHMP for release for consultation July 2000 End of consultation (deadline for comments) September 2000 Final adoption by CHMP September 2002 Corrigendum to calculation formula for NMP November 2002 Transmission to CHMP March 2003February 2009 Update of table 2, table 3 and appendix 1 to reflect therevision of the PDEs for N-Methylpyrrolidone andTetrahydrofuran Q3C(R4)Part IV (PDE for cumene)Transmission to CHMP June 2010 Adoption by CHMP for release for consultation June 20107 Westferry Circus ● Canary Wharf ● London E14 4HB ● United KingdomEnd of consultation (deadline for comments) September 2010 Final adoption by CHMP March 2011 Date for coming into effect August 2011Q3C (R5) on impurities: guideline for residual solventsTable of contentsPart I (4)Impurities: Residual solvents - Parent guideline (4)1. Introduction (4)2. Scope of the guideline (4)3. General principles (5)3.1. Classification of residual solvents by risk assessment (5)3.2. Methods for establishing exposure limits (5)3.3. Options for describing limits of class 2 solvents (6)3.4. Analytical procedures (7)3.5. Reporting levels of residual solvents (7)4. Limits of residual solvents (8)4.1. Solvents to be avoided (8)4.2. Solvents to be limited (8)4.3. Solvents with low toxic potential (9)4.4. Solvents for which no adequate toxicological data was found (10)Glossary (11)Appendix 1: List of solvents included in the guideline (12)Appendix 2: Additional background (16)Appendix 3: Methods for establishing exposure limits (17)PART II: (20)PDE for Tetrahydrofuran (20)PART III: (22)PDE for N-Methylpyrrolidone (NMP) (22)PART IV (24)PDE for cumene (24)Part IImpurities: Residual solvents - Parent guideline1. IntroductionThe objective of this guideline is to recommend acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. The guideline recommends use of less toxic solvents and describes levels considered to be toxicologically acceptable for some residual solvents. Residual solvents in pharmaceuticals are defined here as organic volatile chemicals that are used or produced in the manufacture of drug substances or excipients, or in the preparation of drug products. The solvents are not completely removed by practical manufacturing techniques. Appropriate selection of the solvent for the synthesis of drug substance may enhance the yield, or determine characteristics such as crystal form, purity, and solubility. Therefore, the solvent may sometimes be a critical parameter in the synthetic process. This guideline does not address solvents deliberately used as excipients nor does it address solvates. However, the content of solvents in such products should be evaluated and justified.Since there is no therapeutic benefit from residual solvents, all residual solvents should be removed to the extent possible to meet product specifications, good manufacturing practices, or other quality-based requirements. Drug products should contain no higher levels of residual solvents than can be supported by safety data. Some solvents that are known to cause unacceptable toxicities (Class 1, Table 1) should be avoided in the production of drug substances, excipients, or drug products unless their use can be strongly justified in a risk-benefit assessment. Some solvents associated with less severe toxicity (Class 2, Table 2) should be limited in order to protect patients from potential adverse effects. Ideally, less toxic solvents (Class 3, Table 3) should be used where practical. The complete list of solvents included in this guideline is given in Appendix 1.The lists are not exhaustive and other solvents can be used and later added to the lists. Recommended limits of Class 1 and 2 solvents or classification of solvents may change as new safety data becomes available. Supporting safety data in a marketing application for a new drug product containing a new solvent may be based on concepts in this guideline or the concept of qualification of impurities as expressed in the guideline for drug substance (Q3A, Impurities in New Drug Substances) or drug product (Q3B, Impurities in New Drug Products), or all three guidelines.2. Scope of the guidelineResidual solvents in drug substances, excipients, and in drug products are within the scope of this guideline. Therefore, testing should be performed for residual solvents when production or purification processes are known to result in the presence of such solvents. It is only necessary to test for solvents that are used or produced in the manufacture or purification of drug substances, excipients, or drug product. Although manufacturers may choose to test the drug product, a cumulative method may be used to calculate the residual solvent levels in the drug product from the levels in the ingredients used to produce the drug product. If the calculation results in a level equal to or below that recommended in this guideline, no testing of the drug product for residual solvents need be considered. If, however, the calculated level is above the recommended level, the drug product should be tested to ascertain whether the formulation process has reduced therelevant solvent level to within the acceptable amount. Drug product should also be tested if a solvent is used during its manufacture.This guideline does not apply to potential new drug substances, excipients, or drug products used during the clinical research stages of development, nor does it apply to existing marketed drug products.The guideline applies to all dosage forms and routes of administration. Higher levels of residual solvents may be acceptable in certain cases such as short term (30 days or less) or topical application. Justification for these levels should be made on a case by case basis.See Appendix 2 for additional background information related to residual solvents.3. General principles3.1. Classification of residual solvents by risk assessmentThe term "tolerable daily intake" (TDI) is used by the International Program on Chemical Safety (IPCS) to describe exposure limits of toxic chemicals and "acceptable daily intake" (ADI) is used by the World Health Organization (WHO) and other national and international health authorities and institutes. The new term "permitted daily exposure" (PDE) is defined in the present guideline as a pharmaceutically acceptable intake of residual solvents to avoid confusion of differing values for ADI's of the same substance.Residual solvents assessed in this guideline are listed in Appendix 1 by common names and structures. They were evaluated for their possible risk to human health and placed into one of three classes as follows:Class 1 solvents: Solvents to be avoidedKnown human carcinogens, strongly suspected human carcinogens, and environmental hazards. Class 2 solvents: Solvents to be limitedNon-genotoxic animal carcinogens or possible causative agents of other irreversible toxicity such as neurotoxicity or teratogenicity.Solvents suspected of other significant but reversible toxicities.Class 3 solvents: Solvents with low toxic potentialSolvents with low toxic potential to man; no health-based exposure limit is needed. Class 3 solvents have PDEs of 50 mg or more per day.3.2. Methods for establishing exposure limitsThe method used to establish permitted daily exposures for residual solvents is presented in Appendix 3. Summaries of the toxicity data that were used to establish limits are published in Pharmeuropa, Vol. 9, No. 1, Supplement, April 1997.3.3. Options for describing limits of class 2 solventsTwo options are available when setting limits for Class 2 solvents.Option 1: The concentration limits in ppm stated in Table 2 can be used. They were calculated using equation (1) below by assuming a product mass of 10 g administered daily. Concentration (ppm) = 1000 x PDE(1)Here, PDE is given in terms of mg/day and dose is given in g/day.These limits are considered acceptable for all substances, excipients, or products. Therefore this option may be applied if the daily dose is not known or fixed. If all excipients and drug substances in a formulation meet the limits given in Option 1, then these components may be used in any proportion. No further calculation is necessary provided the daily dose does not exceed 10 g. Products that are administered in doses greater than 10 g per day should be considered under Option 2.Option 2: It is not considered necessary for each component of the drug product to comply with the limits given in Option 1. The PDE in terms of mg/day as stated in Table 2 can be used with the known maximum daily dose and equation (1) above to determine the concentration of residual solvent allowed in drug product. Such limits are considered acceptable provided that it has been demonstrated that the residual solvent has been reduced to the practical minimum. The limits should be realistic in relation to analytical precision, manufacturing capability, reasonable variation in the manufacturing process, and the limits should reflect contemporary manufacturing standards. Option 2 may be applied by adding the amounts of a residual solvent present in each of the components of the drug product. The sum of the amounts of solvent per day should be less than that given by the PDE.Consider an example of the use of Option 1 and Option 2 applied to acetonitrile in a drug product. The permitted daily exposure to acetonitrile is 4.1 mg per day; thus, the Option 1 limit is 410 ppm. The maximum administered daily mass of a drug product is 5.0 g, and the drug product contains two excipients. The composition of the drug product and the calculated maximum content of residual acetonitrile are given in the following table.Acetonitrile content Daily exposure Component Amount informulationDrug substance 0.3 g 800 ppm 0.24 mgExcipient 1 0.9 g 400 ppm 0.36 mgExcipient 2 3.8 g 800 ppm 3.04 mgDrug Product 5.0 g 728 ppm 3.64 mgExcipient 1 meets the Option 1 limit, but the drug substance, excipient 2, and drug product do not meet the Option 1 limit. Nevertheless, the product meets the Option 2 limit of 4.1 mg per day and thus conforms to the recommendations in this guideline.Consider another example using acetonitrile as residual solvent. The maximum administered daily mass of a drug product is 5.0 g, and the drug product contains two excipients. The composition of the drug product and the calculated maximum content of residual acetonitrile is given in the following table.Acetonitrile content Daily exposure Component Amount informulationDrug substance 0.3 g 800 ppm 0.24 mgExcipient 1 0.9 g 2000 ppm 1.80 mgExcipient 2 3.8 g 800 ppm 3.04 mgDrug Product 5.0 g 1016 ppm 5.08 mgIn this example, the product meets neither the Option 1 nor the Option 2 limit according to this summation. The manufacturer could test the drug product to determine if the formulation process reduced the level of acetonitrile. If the level of acetonitrile was not reduced during formulation to the allowed limit, then the manufacturer of the drug product should take other steps to reduce the amount of acetonitrile in the drug product. If all of these steps fail to reduce the level of residual solvent, in exceptional cases the manufacturer could provide a summary of efforts made to reduce the solvent level to meet the guideline value, and provide a risk-benefit analysis to support allowing the product to be utilised with residual solvent at a higher level.3.4. Analytical proceduresResidual solvents are typically determined using chromatographic techniques such as gas chromatography. Any harmonised procedures for determining levels of residual solvents as described in the pharmacopoeias should be used, if feasible. Otherwise, manufacturers would be free to select the most appropriate validated analytical procedure for a particular application. If only Class 3 solvents are present, a non-specific method such as loss on drying may be used. Validation of methods for residual solvents should conform to ICH guidelines Text on Validation of Analytical Procedures and Extension of the ICH Text on Validation of Analytical Procedures.3.5. Reporting levels of residual solventsManufacturers of pharmaceutical products need certain information about the content of residual solvents in excipients or drug substances in order to meet the criteria of this guideline. The following statements are given as acceptable examples of the information that could be provided from a supplier of excipients or drug substances to a pharmaceutical manufacturer. The supplier might choose one of the following as appropriate:Only Class 3 solvents are likely to be present. Loss on drying is less than 0.5%.Only Class 2 solvents X, Y, ... are likely to be present. All are below the Option 1 limit. (Here the supplier would name the Class 2 solvents represented by X, Y, ...)Only Class 2 solvents X, Y, ... and Class 3 solvents are likely to be present. Residual Class 2 solvents are below the Option 1 limit and residual Class 3 solvents are below 0.5%.If Class 1 solvents are likely to be present, they should be identified and quantified."Likely to be present" refers to the solvent used in the final manufacturing step and to solvents that are used in earlier manufacturing steps and not removed consistently by a validated process.If solvents of Class 2 or Class 3 are present at greater than their Option 1 limits or 0.5%, respectively, they should be identified and quantified.4. Limits of residual solvents4.1. Solvents to be avoidedSolvents in Class 1 should not be employed in the manufacture of drug substances, excipients, and drug products because of their unacceptable toxicity or their deleterious environmental effect. However, if their use is unavoidable in order to produce a drug product with a significant therapeutic advance, then their levels should be restricted as shown in Table 1, unless otherwise justified. 1,1,1-Trichloroethane is included in Table 1 because it is an environmental hazard. The stated limit of 1500 ppm is based on a review of the safety data.TABLE 1. Class 1 solvents in pharmaceutical products (solvents that should be avoided).Solvent Concentration limitConcern(ppm)Benzene 2 CarcinogenCarbon tetrachloride 4 Toxic and environmental hazard1,2-Dichloroethane 5 Toxic1,1-Dichloroethene 8 Toxic1,1,1-Trichloroethane 1500 Environmental hazard4.2. Solvents to be limitedSolvents in Table 2 should be limited in pharmaceutical products because of their inherent toxicity. PDEs are given to the nearest 0.1 mg/day, and concentrations are given to the nearest 10 ppm. The stated values do not reflect the necessary analytical precision of determination. Precision should be determined as part of the validation of the method.TABLE 2. Class 2 solvents in pharmaceutical products.Solvent PDE (mg/day) Concentration limit(ppm)Acetonitrile 4.1 410Chlorobenzene 3.6 360Chloroform 0.6 60Cyclohexane 38.8 38801,2-Dichloroethene 18.7 1870Dichloromethane 6.0 6001,2-Dimethoxyethane 1.0 100N,N-Dimethylacetamide 10.9 1090N,N-Dimethylformamide 8.8 8801,4-Dioxane 3.8 3802-Ethoxyethanol 1.6 160Ethyleneglycol 6.2 620Formamide 2.2 220Hexane 2.9 290Methanol 30.0 30002-Methoxyethanol 0.5 50Methylbutyl ketone 0.5 50Methylcyclohexane 11.8 1180N-Methylpyrrolidone1 5.3 530Nitromethane 0.5 50Pyridine 2.0 200Sulfolane 1.6 160Tetrahydrofuran27.2 720Tetralin 1.0 100Toluene 8.9 8901,1,2-Trichloroethene 0.8 80Xylene* 21.7 2170*usually 60% m-xylene, 14% p-xylene, 9% o-xylene with 17% ethyl benzene4.3. Solvents with low toxic potentialSolvents in Class 3 (shown in Table 3) may be regarded as less toxic and of lower risk to human health. Class 3 includes no solvent known as a human health hazard at levels normally accepted in pharmaceuticals. However, there are no long-term toxicity or carcinogenicity studies for many of the solvents in Class 3. Available data indicate that they are less toxic in acute or short-term studies and negative in genotoxicity studies. It is considered that amounts of these residual solvents of 50 mg per day or less (corresponding to 5000 ppm or 0.5% under Option 1) would be acceptable without justification. Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and good manufacturing practice.Table 3: Class 3 solvents which should be limited by GMP or other quality-based requirements. Acetic acid HeptaneAcetone Isobutyl acetateAnisole Isopropyl acetate1 The information included for N-Methylpyrrolidone reflects that included in the Revision of PDE Information for NMP which reached Step 4 in September 2002 (two mistyping corrections made in October 2002), and was incorporated into the core guideline in November 2005. See Part III (pages 20-21).2 The information included for Tetrahydrofuran reflects that included in the Revision of PDE Information for THF which reached Step 4 in September 2002, and was incorporated into the core guideline in November 2005. See Part II (pages 18-19).1-Butanol Methyl acetate2-Butanol 3-Methyl-1-butanolButyl acetate Methylethyl ketonetert-Butylmethyl ether Methylisobutyl ketoneCumene 2-Methyl-1-propanolDimethyl sulfoxide PentaneEthanol 1-PentanolEthyl acetate 1-PropanolEthyl ether 2-PropanolEthyl formate Propyl acetateFormic acid4.4. Solvents for which no adequate toxicological data was foundThe following solvents (Table 4) may also be of interest to manufacturers of excipients, drug substances, or drug products. However, no adequate toxicological data on which to base a PDE was found. Manufacturers should supply justification for residual levels of these solvents in pharmaceutical products.Table 4 Solvents for which no adequate toxicological data was found.1,1-Diethoxypropane Methylisopropyl ketone1,1-Dimethoxymethane Methyltetrahydrofuran2,2-Dimethoxypropane Petroleum etherIsooctane Trichloroacetic acidIsopropyl ether Trifluoroacetic acidGlossaryGenotoxic Carcinogens:Carcinogens which produce cancer by affecting genes or chromosomes.LOEL:Abbreviation for lowest-observed effect level.Lowest-Observed Effect Level:The lowest dose of substance in a study or group of studies that produces biologically significant increases in frequency or severity of any effects in the exposed humans or animals.Modifying Factor:A factor determined by professional judgment of a toxicologist and applied to bioassay data to relate that data safely to humans.Neurotoxicity:The ability of a substance to cause adverse effects on the nervous system.NOEL:Abbreviation for no-observed-effect level.No-Observed-Effect Level:The highest dose of substance at which there are no biologically significant increases in frequency or severity of any effects in the exposed humans or animals.PDE:Abbreviation for permitted daily exposure.Permitted Daily Exposure:The maximum acceptable intake per day of residual solvent in pharmaceutical products. Reversible Toxicity:The occurrence of harmful effects that are caused by a substance and which disappear after exposure to the substance ends.Strongly Suspected Human Carcinogen:A substance for which there is no epidemiological evidence of carcinogenesis but there are positive genotoxicity data and clear evidence of carcinogenesis in rodents.Teratogenicity:The occurrence of structural malformations in a developing fetus when a substance is administered during pregnancy.Appendix 1: List of solvents included in the guideline Solvent Other Names Structure Class Acetic acid Ethanoic acid CH3COOH Class 3Acetone 2-PropanoneCH3COCH3 Class 3Propan-2-oneAcetonitrile CH3CN Class 2Anisole Methoxybenzene OCHClass 33Benzene Benzol Class 11-Butanol n-Butyl alcoholCH3(CH2)3OH Class 3Butan-1-olCH3CH2CH(OH)CH3 Class 3 2-Butanol sec-Butyl alcoholButan-2-olButyl acetate Acetic acid butyl ester CH3COO(CH2)3CH3 Class 3tert-Butylmethyl ether 2-Methoxy-2-methyl- propane (CH3)3COCH3 Class 3Carbon tetrachloride Tetrachloromethane CCl4 Class 1Chlorobenzene Cl Class 2Chloroform Trichloromethane CHCl3 Class 2Cumene IsopropylbenzeneCH(CH3)2Class 3(1-Methyl)ethylbenzeneCyclohexane Hexamethylene Class 2CH2ClCH2Cl Class 1 1,2-Dichloroethane sym-DichloroethaneEthylene dichlorideEthylene chloride1,1-Dichloroethene 1,1-DichloroethyleneH2C=CCl2 Class 1Vinylidene chloride1,2-Dichloroethene 1,2-DichloroethyleneClHC=CHCl Class 2Acetylene dichlorideDichloromethane Methylene chloride CH2Cl2 Class 2H3COCH2CH2OCH3 Class 2 1,2-Dimethoxyethane Ethyleneglycol dimethyl etherMonoglymeDimethyl CellosolveN,N-Dimethylacetamide DMA CH3CON(CH3)2 Class 2 N,N-Dimethylformamide DMF HCON(CH3)2 Class 2(CH3)2SO Class 3 Dimethyl sulfoxide MethylsulfinylmethaneMethyl sulfoxideDMSO1,4-Dioxane p-DioxaneO O Class 2[1,4]DioxaneEthanol Ethyl alcohol CH3CH2OH Class 3 2-Ethoxyethanol Cellosolve CH3CH2OCH2CH2OH Class 2 Ethyl acetate Acetic acid ethyl ester CH3COOCH2CH3 Class 3HOCH2CH2OH Class 2 Ethyleneglycol 1,2-Dihydroxyethane1,2-EthanediolCH3CH2OCH2CH3 Class 3 Ethyl ether Diethyl etherEthoxyethane1,1’-OxybisethaneEthyl formate Formic acid ethyl ester HCOOCH2CH3 Class 3 Formamide Methanamide HCONH2 Class 2 Formic acid HCOOH Class 3 Heptane n-Heptane CH3(CH2)5CH3 Class 3Hexane n-Hexane CH3(CH2)4CH3 Class 2Isobutyl acetate Acetic acid isobutyl ester CH3COOCH2CH(CH3)2 Class 3 Isopropyl acetate Acetic acid isopropyl ester CH3COOCH(CH3)2 Class 3 Methanol Methyl alcohol CH3OH Class 2 2-Methoxyethanol Methyl Cellosolve CH3OCH2CH2OH Class 2 Methyl acetate Acetic acid methyl ester CH3COOCH3 Class 33-Methyl-1-butanol Isoamyl alcoholIsopentyl alcohol3-Methylbutan-1-ol(CH3)2CHCH2CH2OH Class 3Methylbutyl ketone 2-HexanoneHexan-2-oneCH3(CH2)3COCH3 Class 2Methylcyclohexane Cyclohexylmethane CH3Class 2 Methylethyl ketone 2-ButanoneMEKButan-2-oneCH3CH2COCH3 Class 3Methylisobutyl ketone 4-Methylpentan-2-one4-Methyl-2-pentanoneMIBKCH3COCH2CH(CH3)2 Class 32-Methyl-1-propanol Isobutyl alcohol2-Methylpropan-1-ol(CH3)2CHCH2OH Class 3 N-Methylpyrrolidone 1-Methylpyrrolidin-2-one1-Methyl-2-pyrrolidinone NCH3OClass 2Nitromethane CH3NO2 Class 2 Pentane n-Pentane CH3(CH2)3CH3 Class 3 1-Pentanol Amyl alcohol CH3(CH2)3CH2OH Class 3Pentan-1-olPentyl alcohol1-Propanol Propan-1-olPropyl alcoholCH3CH2CH2OH Class 32-Propanol Propan-2-olIsopropyl alcohol(CH3)2CHOH Class 3 Propyl acetate Acetic acid propyl ester CH3COOCH2CH2CH3 Class 3PyridineNClass 2Sulfolane Tetrahydrothiophene 1,1-dioxideSO OClass 2Tetrahydrofuran1Tetramethylene oxideOxacyclopentane OClass 2Tetralin 1,2,3,4-Tetrahydro-naphthalene Class 2Toluene Methylbenzene CH3Class 2 1,1,1-Trichloroethane Methylchloroform CH3CCl3 Class 1 1,1,2-Trichloroethene Trichloroethene HClC=CCl2 Class 2Xylene* DimethybenzeneXylolCH3CH3Class 2*usually 60% m-xylene, 14% p-xylene, 9% o-xylene with 17% ethyl benzene1 The information included for Tetrahydrofuran reflects that included in the Revision of PDE Information for THF which reached Step 4 in September 2002, and was incorporated into the core guideline in November 2005. See Part II (pages 18-19).Appendix 2: Additional backgroundA2.1 Environmental Regulation of Organic Volatile SolventsSeveral of the residual solvents frequently used in the production of pharmaceuticals are listed as toxic chemicals in Environmental Health Criteria (EHC) monographs and the Integrated Risk Information System (IRIS). The objectives of such groups as the International Programme on Chemical Safety (IPCS), the United States Environmental Protection Agency (USEPA), and the United States Food and Drug Administration (USFDA) include the determination of acceptable exposure levels. The goal is protection of human health and maintenance of environmental integrity against the possible deleterious effects of chemicals resulting from long-term environmental exposure. The methods involved in the estimation of maximum safe exposure limits are usually based on long-term studies. When long-term study data are unavailable, shorter term study data can be used with modification of the approach such as use of larger safety factors. The approach described therein relates primarily to long-term or life-time exposure of the general population in the ambient environment, i.e. ambient air, food, drinking water and other media.A2.2 Residual Solvents in PharmaceuticalsExposure limits in this guideline are established by referring to methodologies and toxicity data described in EHC and IRIS monographs. However, some specific assumptions about residual solvents to be used in the synthesis and formulation of pharmaceutical products should be taken into account in establishing exposure limits. They are:1) Patients (not the general population) use pharmaceuticals to treat their diseases or forprophylaxis to prevent infection or disease.2) The assumption of life-time patient exposure is not necessary for most pharmaceuticalproducts but may be appropriate as a working hypothesis to reduce risk to human health.3) Residual solvents are unavoidable components in pharmaceutical production and will oftenbe a part of drug products.4) Residual solvents should not exceed recommended levels except in exceptionalcircumstances.5) Data from toxicological studies that are used to determine acceptable levels for residualsolvents should have been generated using appropriate protocols such as those describedfor example by OECD, EPA, and the FDA Red Book.Appendix 3: Methods for establishing exposure limitsThe Gaylor-Kodell method of risk assessment (Gaylor, D. W. and Kodell, R. L.: Linear Interpolation algorithm for low dose assessment of toxic substance. J Environ. Pathology, 4, 305, 1980) is appropriate for Class 1 carcinogenic solvents. Only in cases where reliable carcinogenicity data are available should extrapolation by the use of mathematical models be applied to setting exposure limits. Exposure limits for Class 1 solvents could be determined with the use of a large safety factor (i.e., 10,000to 100,000) with respect to the no-observed-effect level (NOEL). Detection and quantitation of these solvents should be by state-of-the-art analytical techniques.Acceptable exposure levels in this guideline for Class 2 solvents were established by calculation of PDE values according to the procedures for setting exposure limits in pharmaceuticals (Pharmacopeial Forum, Nov-Dec 1989), and the method adopted by IPCS for Assessing Human Health Risk of Chemicals (Environmental Health Criteria 170, WHO, 1994). These methods are similar to those used by the USEPA (IRIS) and the USFDA (Red Book) and others. The method is outlined here to give a better understanding of the origin of the PDE values. It is not necessary to perform these calculations in order to use the PDE values tabulated in Section 4 of this document. PDE is derived from the no-observed-effect level (NOEL), or the lowest-observed effect level (LOEL) in the most relevant animal study as follows:PDE =NOEL x Weight Adjustment(1)F1 x F2 x F3 x F4 x F5The PDE is derived preferably from a NOEL. If no NOEL is obtained, the LOEL may be used. Modifying factors proposed here, for relating the data to humans, are the same kind of "uncertainty factors" used in Environmental Health Criteria (Environmental Health Criteria 170, World Health Organization, Geneva, 1994), and "modifying factors" or "safety factors" in Pharmacopeial Forum. The assumption of 100% systemic exposure is used in all calculations regardless of route of administration.The modifying factors are as follows:F1 = A factor to account for extrapolation between speciesF1 = 5 for extrapolation from rats to humansF1 = 12 for extrapolation from mice to humansF1 = 2 for extrapolation from dogs to humansF1 = 2.5 for extrapolation from rabbits to humansF1 = 3 for extrapolation from monkeys to humansF1 = 10 for extrapolation from other animals to humans。

ICH_Q3c_杂质:残余溶剂的指导原则(中文版)纯净版杂质:残留溶剂的指导原则杂质:残留溶剂的指导原则1.介绍本指导原则旨在介绍药物中残留溶剂在保证人体安全条件下的可接受量,指导原则建议使用低毒的溶剂,提出了一些残留溶剂毒理学上的可接受水平。
药物中的残留溶剂在此定义为在原料药或赋形剂的生产中,以及在制剂制备过程中产生或使用的有机挥发性化合物,它们在工艺中不能完全除尽。
在合成原料药中选择适当的溶剂可提高产量或决定药物的性质,如结晶型。
纯度和溶解度。
因此.有时溶剂是合成中非常关键的因素。
本指导原则所指的溶剂不是谨慎地用作赋形剂的溶剂,也不是溶剂化物,然而在这些制剂中的溶剂含量也应进行测定,并作出合理的判断。
出于残留溶剂没有疗效,故所有残留溶剂均应尽可能.去,以符合产品规范、GMP 或其他基本的质量要求。
制剂所含残留溶剂的水平不能高于安全值,已知一些溶剂可导致不接受的毒性(第一类,表 1),除非被证明特别合理,在原药、赋形剂及制剂生产中应避免使用。
一些溶剂毒性不太大(第二类,表2)应限制使用,以防止病人潜在的不良反应。
使用低毒溶剂(第三类,表 3)较为理想。
附录 1 中列出了指导原则中的全部溶剂。
第 1 页共 18 页杂质:残留溶剂的指导原则表中所列溶剂并非详尽无遗,其他可能使用的溶剂有待日后补充列人。
第一、二类溶剂的建议限度或溶剂的分类会随着。
新的安全性资料的获得而调整。
含有新溶剂的新药制剂、其上市申请的安全性资料应符合本指导原则或原料药指导原则(Q3A 新原料药中的杂质)或新药制剂(Q3B 新药制剂中的杂质)中所述的杂质控制原则,或者符合上述三者。
2. 指导原则的范围指导原则范围包括原料药、赋形剂或制剂中所含残留溶剂.因此,当生产或纯化过程中会出现这些溶剂时。
应进行残留溶剂的检验。
也只有在上述情况下,才有必要作溶剂的检查。
虽然生产商可以选择性地测定制剂,但也可以从制剂中各成分的残留溶液水平来累积计算制剂中的残留溶剂。



手把手教您计算制剂产品中残留溶剂的限度值做好国产药,除了要有相关文件作为引导,一些必备的技术知识也是非常重要的。
前期小编介绍了如何确保中间产品的均匀性,今天再结合USP<467>、ICH Q3C介绍一下如何计算制剂产品残留溶剂的限度及控制。
残留溶剂(Residual Solvents)是指在原料药、辅料、以及在制剂制备过程中使用到的但又不能够通过实际生产技术使之完全去除的具有挥发性有机化合物。
残留溶剂不仅不会产生治疗作用,有时反而会对人体产生一些危害,因此残留溶剂必须要尽可能的去除干净,至少是要达到基本的质量标准。
溶剂一般分为I、II、III类,具体溶剂的风险评估归类可参见USP<467>中的附件1。
因为Class I是强烈不建议在生产过程中使用的,这里就以Class II限度计算方式为例进行介绍。
有两种方式可用于计算Class II的残留限度值:Option 1(每日剂量≤ 10 g的制剂):其中,PDE:mg/天;剂量:g/天。
Option 1限度计算方式适合于所有的原料药、辅料和制剂产品,尤其适合于日剂量不明确或不固定的制剂产品。
如果制剂产品中所有的原料药和辅料限度均满足于Option 1计算出的限度值,那么它们在处方中可以任意比例存在。
制剂产品中的每一种成分没有必要都符合option1计算出的限度值。
某些PDE值可参考USP<467>中的tablet 2,再结合已知的最大日剂量计算制剂产品中允许残留的浓度。
(为了最大化保证用药安全,小编建议,对于日剂量不明确或不固定的制剂产品,在计算时日剂量以10g计算最为严格。
)而对于日剂量超过10g/day的产品,需采用下面的option 2计算。
Option 2(每日剂量>10g的制剂):将制剂处方中每种成分中的残留溶剂相叠加,总的限度值应低于PDE值。
以处方中的乙腈(acetonitrile)为例,采用Option 2计算制剂产品允许残留的浓度值(乙腈):处方中含有乙腈的成分为原料药、辅料1和辅料2,通过制剂产品折算后的每日服用量分别为0.3g、0.9g和3.8g,总日剂量5.0g。

杂质:残留溶剂的指导原则1.介绍本指导原则旨在介绍药物中残留溶剂在保证人体安全条件下的可接受量,指导原则建议使用低毒的溶剂,提出了一些残留溶剂毒理学上的可接受水平。
药物中的残留溶剂在此定义为在原料药或赋形剂的生产中,以及在制剂制备过程中产生或使用的有机挥发性化合物,它们在工艺中不能完全除尽。
在合成原料药中选择适当的溶剂可提高产量或决定药物的性质,如结晶型。
纯度和溶解度。
因此.有时溶剂是合成中非常关键的因素。
本指导原则所指的溶剂不是谨慎地用作赋形剂的溶剂,也不是溶剂化物,然而在这些制剂中的溶剂含量也应进行测定,并作出合理的判断。
出于残留溶剂没有疗效,故所有残留溶剂均应尽可能.去,以符合产品规范、GMP或其他基本的质量要求。
制剂所含残留溶剂的水平不能高于安全值,已知一些溶剂可导致不接受的毒性(第一类,表1),除非被证明特别合理,在原药、赋形剂及制剂生产中应避免使用。
一些溶剂毒性不太大(第二类,表2)应限制使用,以防止病人潜在的不良反应。
使用低毒溶剂(第三类,表3)较为理想。
附录1中列出了指导原则中的全部溶剂。
表中所列溶剂并非详尽无遗,其他可能使用的溶剂有待日后补充列人。
第一、二类溶剂的建议限度或溶剂的分类会随着。
新的安全性资料的获得而调整。
含有新溶剂的新药制剂、其上市申请的安全性资料应符合本指导原则或原料药指导原则(Q3A新原料药中的杂质)或新药制剂(Q3B新药制剂中的杂质)中所述的杂质控制原则,或者符合上述三者。
2. 指导原则的范围指导原则范围包括原料药、赋形剂或制剂中所含残留溶剂.因此,当生产或纯化过程中会出现这些溶剂时。
应进行残留溶剂的检验。
也只有在上述情况下,才有必要作溶剂的检查。
虽然生产商可以选择性地测定制剂,但也可以从制剂中各成分的残留溶液水平来累积计算制剂中的残留溶剂。
如果计算结果等于或低于本原则的建议水平,该制剂可考虑不检查残留溶剂,但如果计算结果高于建议水平则应进行检测,以确定制剂制备过程中是否降低了有关溶剂的量以达到可接受水平。
人用药品注册技术要求国际协调会ICH协调指导原则杂质:残留溶剂的指导原则Q3C(R6)现行第四阶段版本2016年10月20日本指导原则由相应的ICH专家工作组制定,并根据ICH进程已提交给管理当局征询意见。
在ICH进程的第四阶段,最后的草案被推荐给欧盟、日本、美国、加拿大和瑞士的管理机构采纳。
Q3C(R5)文件历史母指导原则:杂质:残留溶剂的指导原则对母指导原则所含THF的PDE信息的修订修订母指导原则所含NMP的PDE信息母指导原则:杂质:残留溶剂的指导原则对母指导原则所含异丙基苯的PDE信息的修订修订母指导原则所含甲基异丁基酮的PDE信息,并纳入三乙胺的PDE杂质:残留溶剂的指导原则ICH协调指导原则目录第一部分:1. 引言 (1)2. 指导原则的适用范围 (1)3. 通则 (2)3.1 基于风险评估的残留溶剂的分类 (2)3.2 建立暴露限度的方法 (2)3.3 2类溶剂限度的表示方法 (2)3.4 分析方法 (4)3.5 残留溶剂的报告水平 (4)4. 残留溶剂的限度 (4)4.1 应避免的溶剂 (4)4.2 应限制的溶剂 (5)4.3 低潜在毒性的溶剂 (6)4.4 没有足够毒理学数据的溶剂 (7)词汇表 (8)附录1:指导原则中包括的溶剂列表 (9)附录2:其他背景 (13)A2.1 环境领域对有机挥发性溶剂的监管 (13)A2.2 药物中的残留溶剂 (13)附录3:建立暴露限度的方法 (14)第二部分:四氢呋喃的PDE (17)第三部分:N-甲基吡咯烷酮(NMP)的PDE (19)第四部分:异丙基苯的PDE (21)第五部分:三乙胺的PDE和甲基异丁基酮的PDE (24)第一部分:杂质:残留溶剂的指导原则在1997年7月17日的ICH指导委员会会议上进入ICH进程第四阶段,并建议ICH的三方监管机构采纳该指导原则1. 引言本指导原则旨在建议为保证患者安全而应规定的药物中残留溶剂的可接受量。
一类溶剂:已知可致癌且对人和环境有害的溶剂,尽可能避免使用(事实上很多大公司如罗氏等已将这些甚至包括某些二类溶剂打入“黑名单”),如果实在无法避免,残留量必须控制在规定限度:苯(2ppm)四氯化碳(4ppm)1,2-二氯乙烷(5ppm)(我注意到隆莱还有使用)1,1-二氯乙烯(8ppm)1,1,1-三氯乙烷(1500ppm)二类溶剂:有动物致癌性的溶剂,按每日允许接触量计算的规定限度如下:乙腈(410ppm)氯苯(360ppm)氯仿(60ppm)(罗氏禁用)环己烷(3880ppm)1,2-二氯乙烯(1870ppm)二氯甲烷(600ppm)1,2-二甲氧乙烷(100ppm)N,N-二甲基乙酰胺(1090ppm)DMF(880ppm)二氧六环(380ppm)2-乙氧基乙醇(160ppm)乙二醇(620ppm)甲酰胺(220ppm)正己烷(290ppm)甲醇(3000ppm)乙二醇甲醚(50ppm)甲丁酮(50ppm)甲基环己烷(1180ppm)N-甲基吡咯烷酮(4840ppm)硝基甲烷(50ppm)吡啶(200ppm)环丁砜(160ppm)1,2,3,4-四氢化萘(100ppm)甲苯(890ppm)1,1,2-三氯乙烯(80ppm)二甲苯(2170ppm)三类溶剂:低毒溶剂,限度为≤0.5%,即5000ppm,如我们常用的乙醇、EA、TBME、丙酮、THF、庚烷、异丙醇等除上述三类溶剂外,还有一些溶剂如我们用过的石油醚、甲基四氢呋喃等尚无毒理资料,必须证明其残留量的合理性。
尤其石油醚,建议今后避免使用,尤其在后道反应最好避免,由于其成分复杂,无法测定准确残留量。
总之,在工艺开发阶段应优先选择三类溶剂,控制二类溶剂,尽量避免一类溶剂的使用。
European Medicines Agency7 Westferry Circus, Canary Wharf, London, E14 4HB, UKTel. (44-20) 74 18 85 75 Fax (44-20) 75 23 70 40E-mail: mail@emea.eu.int http://www.emea.eu.intMarch 1998 CPMP/ICH/283/95ICH Topic Q 3 C (R3)Impurities: Residual SolventsStep 5NOTE FOR GUIDANCE ONIMPURITIES: RESIDUAL SOLVENTS(CPMP/ICH/283/95)TRANSMISSION TO CPMP November 1996 TRANSMISSION TO INTERESTED PARTIES November 1996 COMMENTS REQUESTED BEFORE May 1997 FINAL APPROVAL BY CPMP September 1997 DATE FOR COMING INTO OPERATION March 1998ICH Harmonised Tripartite GuidelineTable of Contents1. INTRODUCTION (3)2. SCOPE OF THE GUIDELINE (3)3. GENERAL PRINCIPLES (4)3.1 Classification of Residual Solvents by Risk Assessment (4)3.2 Methods for Establishing Exposure Limits (4)3.3 Options for Describing Limits of Class 2 Solvents (4)3.4 Analytical Procedures (6)3.5 Reporting levels of residual solvents (6)4. LIMITS OF RESIDUAL SOLVENTS (7)4.1 Solvents to Be Avoided (7)4.2 Solvents to Be Limited (7)4.3 Solvents with Low Toxic Potential (8)4.4 Solvents for which No Adequate Toxicological Data was Found (9)GLOSSARY (10)APPENDIX 1. LIST OF SOLVENTS INCLUDED IN THE GUIDELINE (11)APPENDIX 2. ADDITIONAL BACKGROUND (16)A2.1 Environmental Regulation of Organic Volatile Solvents (16)A2.2 Residual Solvents in Pharmaceuticals (16)APPENDIX 3. METHODS FOR ESTABLISHING EXPOSURE LIMITS (16)PART II: (21)IMPURITIES : RESIDUAL SOLVENTS (MAINTENANCE) (21)PART III: (23)IMPURITIES : RESIDUAL SOLVENTS (MAINTENANCE) (23)PART I:1. INTRODUCTIONThe objective of this guideline is to recommend acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. The guideline recommends use of less toxic solvents and describes levels considered to be toxicologically acceptable for some residual solvents.Residual solvents in pharmaceuticals are defined here as organic volatile chemicals that are used or produced in the manufacture of drug substances or excipients, or in the preparation of drug products. The solvents are not completely removed by practical manufacturing techniques. Appropriate selection of the solvent for the synthesis of drug substance may enhance the yield, or determine characteristics such as crystal form, purity, and solubility. Therefore, the solvent may sometimes be a critical parameter in the synthetic process. This guideline does not address solvents deliberately used as excipients nor does it address solvates. However, the content of solvents in such products should be evaluated and justified.Since there is no therapeutic benefit from residual solvents, all residual solvents should be removed to the extent possible to meet product specifications, good manufacturing practices, or other quality-based requirements. Drug products should contain no higher levels of residual solvents than can be supported by safety data. Some solvents that are known to cause unacceptable toxicities (Class 1, Table 1) should be avoided in the production of drug substances, excipients, or drug products unless their use can be strongly justified in a risk-benefit assessment. Some solvents associated with less severe toxicity (Class 2, Table 2) should be limited in order to protect patients from potential adverse effects. Ideally, less toxic solvents (Class 3, Table 3) should be used where practical. The complete list of solvents included in this guideline is given in Appendix 1The lists are not exhaustive and other solvents can be used and later added to the lists. Recommended limits of Class 1 and 2 solvents or classification of solvents may change as new safety data becomes available. Supporting safety data in a marketing application for a new drug product containing a new solvent may be based on concepts in this guideline or the concept of qualification of impurities as expressed in the guideline for drug substance (Q3A, Impurities in New Drug Substances) or drug product (Q3B, Impurities in New Drug Products), or all three guidelines.2. SCOPE OF THE GUIDELINEResidual solvents in drug substances, excipients, and in drug products are within the scope of this guideline. Therefore, testing should be performed for residual solvents when production or purification processes are known to result in the presence of such solvents. It is only necessary to test for solvents that are used or produced in the manufacture or purification of drug substances, excipients, or drug product. Although manufacturers may choose to test the drug product, a cumulative method may be used to calculate the residual solvent levels in the drug product from the levels in the ingredients used to produce the drug product. If the calculation results in a level equal to or below that recommended in this guideline, no testing of the drug product for residual solvents need be considered. If, however, the calculated level is above the recommended level, the drug product should be tested to ascertain whether the formulation process has reduced the relevant solvent level to within the acceptable amount. Drug product should also be tested if a solvent is used during its manufacture.This guideline does not apply to potential new drug substances, excipients, or drug products used during the clinical research stages of development, nor does it apply to existing marketeddrug products. The guideline applies to all dosage forms and routes of administration. Higher levels ofresidual solvents may be acceptable in certain cases such as short term (30 days or less) or topical application. Justification for these levels should be made on a case by case basis.See Appendix 2 for additional background information related to residual solvents.3. GENERAL PRINCIPLES3.1 Classification of Residual Solvents by Risk AssessmentThe term "tolerable daily intake" (TDI) is used by the International Program on Chemical Safety (IPCS) to describe exposure limits of toxic chemicals and "acceptable daily intake" (ADI) is used by the World Health Organization (WHO) and other national and internationalhealth authorities and institutes. The new term "permitted daily exposure" (PDE) is defined in the present guideline as a pharmaceutically acceptable intake of residual solvents to avoidconfusion of differingvalues for ADI's of the same substance.Residual solvents assessed in this guideline are listed in Appendix 1 by common names and structures. They were evaluated for their possible risk to human health and placed into one ofthree classes as follows:Class 1 solvents: Solvents to be avoided Known human carcinogens, strongly suspected human carcinogens, and environmentalhazards.Class 2 solvents: Solvents to be limited Non-genotoxic animal carcinogens or possible causative agents of other irreversible toxicitysuch as neurotoxicity or teratogenicity. Solvents suspected of other significant but reversible toxicities.Class 3 solvents: Solvents with low toxic potential Solvents with low toxic potential to man; no health-based exposure limit is needed. Class 3solvents have PDEs of 50 mg or more per day.3.2 Methods for Establishing Exposure LimitsThe method used to establish permitted daily exposures for residual solvents is presented inAppendix 3. Summaries of the toxicity data that were used to establish limits are published in Pharmeuropa, Vol. 9, No. 1, Supplement, April 1997.3.3 Options for Describing Limits of Class 2 SolventsTwo options are available when setting limits for Class 2 solvents.Option 1: The concentration limits in ppm stated in Table 2 can be used. They were calculated using equation (1) below by assuming a product mass of 10 g administered daily.dose PDE x 1000(ppm)ion Concentrat :(1) =Here, PDE is given in terms of mg/day and dose is given in g/day.These limits are considered acceptable for all substances, excipients, or products. Therefore this option may be applied if the daily dose is not known or fixed. If all excipients and drugsubstances in a formulation meet the limits given in Option 1, then these components may be used in any proportion. No further calculation is necessary provided the daily dose does notexceed 10 g. Products that are administered in doses greater than 10 g per day should be considered under Option 2. Option 2: It is not considered necessary for each component of the drug product to complywith the limits given in Option 1. The PDE in terms of mg/day as stated in Table 2 can be used with the known maximum daily dose and equation (1) above to determine theconcentration of residual solvent allowed in drug product. Such limits are considered acceptable provided that it has been demonstrated that the residual solvent has been reducedto the practical minimum. The limits should be realistic in relation to analytical precision, manufacturing capability, reasonable variation in the manufacturing process, and the limitsshould reflect contemporary manufacturing standards.Option 2 may be applied by adding the amounts of a residual solvent present in each of the components of the drug product. The sum of the amounts of solvent per day should be lessthan that given by the PDE.Consider an example of the use of Option 1 and Option 2 applied to acetonitrile in a drug product. The permitted daily exposure to acetonitrile is 4.1 mg per day; thus, the Option 1limit is 410 ppm. The maximum administered daily mass of a drug product is 5.0 g, and the drug product contains two excipients. The composition of the drug product and the calculated maximum content of residual acetonitrile are given in the following table.Component Amount in formulation Acetonitrile contentDaily exposure Active substance0.3 g 800 ppm 0.24 mg Excipient 10.9 g 400 ppm 0.36 mg Excipient 23.8 g 800 ppm 3.04 mg Medicinal product 5.0 g 728 ppm 3.64 mgExcipient 1 meets the Option 1 limit, but the drug substance, excipient 2, and drug product do not meet the Option 1 limit. Nevertheless, the product meets the Option 2 limit of 4.1 mg per day and thus conforms to the recommendations in this guideline.Consider another example using acetonitrile as residual solvent. The maximumadministered daily mass of a drug product is 5.0 g, and the drug product contains two excipients. Thecomposition of the drug product and the calculated maximum content of residual acetonitrile is given in the following table.Component Amount in formulation AcetonitrilecontentDaily exposure Active substance0.3 g 800 ppm 0.24 mg Excipient 10.9 g 2000 ppm 1.80 mg Excipient 23.8 g 800 ppm 3.04 mg Medicinal product 5.0 g 1016 ppm 5.08 mgIn this example, the product meets neither the Option 1 nor the Option 2 limit according to this summation. The manufacturer could test the drug product to determine if the formulationprocess reduced the level of acetonitrile. If the level of acetonitrile was not reduced during formulation to the allowed limit, then the manufacturer of the drug product should take othersteps to reduce the amount of acetonitrile in the drug product. If all of these steps fail to reduce the level of residual solvent, in exceptional cases the manufacturer could provide asummary of efforts made to reduce the solvent level to meet the guideline value, and provide a riskbenefit analysis to support allowing the product to be utilised with residual solvent atahigher level.3.4 Analytical ProceduresResidual solvents are typically determined using chromatographic techniques such as gas chromatography. Any harmonised procedures for determining levels of residual solvents as described in the pharmacopoeias should be used, if feasible. Otherwise, manufacturers wouldbe free to select the most appropriate validated analytical procedure for a particular application. If only Class 3 solvents are present, a nonspecific method such as loss on dryingmay be used.Validation of methods for residual solvents should conform to ICH guidelines Text on Validation of Analytical Procedures and Extension of the ICH Text on Validation ofAnalytical Procedures .3.5 Reporting levels of residual solventsManufacturers of pharmaceutical products need certain information about the content of residual solvents in excipients or drug substances in order to meet the criteria of thisguideline. The following statements are given as acceptable examples of the information that could be provided from a supplier of excipients or drug substances to a pharmaceuticalmanufacturer. The supplier might choose one of the following as appropriate:• Only Class 3 solvents are likely to be present. Loss on drying is less than 0.5%.• Only Class 2 solvents X, Y, ... are likely to be present. All are below the Option 1 limit. (Here the supplier would name the Class 2 solvents represented by X, Y, ...)• Only Class 2 solvents X, Y, ... and Class 3 solvents are likely to be present. Residual Class 2 solvents are below the Option 1 limit and residual Class 3 solvents are below0.5%.If Class 1 solvents are likely to be present, they should be identified and quantified."Likely to be present" refers to the solvent used in the final manufacturing step and tosolvents that are used in earlier manufacturing steps and not removed consistently by avalidated process.If solvents of Class 2 or Class 3 are present at greater than their Option 1 limits or 0.5%, respectively, they should be identified and quantified.4. LIMITS OF RESIDUAL SOLVENTS4.1 Solvents to Be AvoidedSolvents in Class 1 should not be employed in the manufacture of drug substances, excipients,and drug products because of their unacceptable toxicity or their deleterious environmentaleffect. However, if their use is unavoidable in order to produce a drug product with asignificant therapeutic advance, then their levels should be restricted as shown in Table 1,unless otherwise justified. 1,1,1- Trichloroethane is included in Table 1 because it is an environmental hazard. The stated limit of 1500 ppm is based on a review of the safety data.ConcernLimitSolvent Concentration(ppm)Benzene 2 Carcinogen Carbon tetrachloride 4 Toxic and environmentalhazard1,2-Dichloroethane 5 Toxic1,1-Dichloroethene 8 Toxic1,1,1-Trichloroethane 1500 Environmentalhazard4.2 Solvents to Be LimitedSolvents in Table 2 should be limited in pharmaceutical products because of their inherenttoxicity. PDEs are given to the nearest 0.1 mg/day, and concentrations are given to the nearest10 ppm. The stated values do not reflect the necessary analytical precision of determination.Precision should be determined as part of the validation of the method.TABLE 2. Class 2 solvents in pharmaceutical products.Solvent PDE (mg/day) Concentration Limit(ppm)Acetonitrile 4.1 410 Chlorobenzene 3.6 360 Chloroform 0.6 60 Cyclohexane 38.8 3880 1,2-Dichloroethene 18.7 1870 Dichloromethane 6.0 600 1,2-Dimethoxyethane 1.0100 N,N-Dimethylacetamide 10.91090 N,N-Dimethylformamide 8.8 8801,4-Dioxane 3.8 380 2-Ethoxyethanol 1.6 160 Ethylene glycol 6.2 620Formamide 2.2 220 Hexane 2.9 290 Methanol 30.0 3000 2-Methoxyethanol 0.5 50 Methylbutylketone 0.5 50 Methylcyclohexane 11.8 1180 N-Methylpyrrolidone 48.4 4840Nitromethane 0.5 50Pyridine 2.0 200 Sulfolane 1.6 160 Tetralin 1.0 100 Toluene 8.9 8901,1,2-Trichloroethene 0.8 80 Xylene* 21.7 2170 * usually 60% m-xylene, 14% p-xylene, 9% o-xylene with 17% ethyl benzene.4.3 Solvents with Low Toxic PotentialSolvents in Class 3 (shown in Table 3) may be regarded as less toxic and of lower risk to human health. Class 3 includes no solvent known as a human health hazard at levels normallyaccepted in pharmaceuticals. However, there are no long-term toxicity or carcinogenicity studies for many of the solvents in Class 3. Available data indicate that they are less toxic inacute or short-term studies and negative in genotoxicity studies. It is considered that amounts of these residual solvents of 50 mg per day or less (corresponding to 5000 ppm or 0.5% underOption 1) would be acceptable without justification. Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and good manufacturingpractice.Table 3. Class 3 solvents which should be limited by GMP or other qualitybased requirements.Acetic acid Heptaneacetate Acetone IsobutylAnisole Isopropylacetateacetate1-Butanol Methyl2-Butanol 3-Methyl-1-butanol Butyl acetate Methylethyl ketonetert-Butylmethyl ether Methylisobutyl ketoneCumene 2-Methyl-1-propanol Dimethylsulfoxide PentaneEthanol 1-PentanolEthyl acetate 1-PropanolEthyl ether 2-PropanolEthyl formate Propyl acetateFormic acid Tetrahydrofuran4.4 Solvents for which No Adequate Toxicological Data was FoundThe following solvents (Table 4) may also be of interest to manufacturers of excipients, drug substances, or drug products. However, no adequate toxicological data on which to base aPDE was found. Manufacturers should supply justification for residual levels of these solvents in pharmaceutical products.Table 4. Solvents for which no adequate toxicological data was found.1,1-Diethoxypropane Methylisopropylketone1,1-Dimethoxymethane Methyltetrahydrofuranether2,2-Dimethoxypropane PetroleumIsooctane Trichloroaceticacid Isopropyl ether Trifluoroacetic acidGLOSSARYGenotoxic Carcinogens:Carcinogens which produce cancer by affecting genes or chromosomes.LOEL:Abbreviation for lowest-observed effect level.Lowest-Observed Effect Level:The lowest dose of substance in a study or group of studies that produces biologically significant increases in frequency or severity of any effects in the exposed humans or animals.Modifying Factor:A factor determined by professional judgment of a toxicologist and applied to bioassay data to relate that data safely to humans.Neurotoxicity:The ability of a substance to cause adverse effects on the nervous system.NOEL:Abbreviation for no-observed-effect level.No-Observed-Effect Level:The highest dose of substance at which there are no biologically significant increases in frequency or severity of any effects in the exposed humans or animals.PDE:Abbreviation for permitted daily exposure.Permitted Daily Exposure:The maximum acceptable intake per day of residual solvent in pharmaceutical products.Reversible Toxicity:The occurrence of harmful effects that are caused by a substance and which disappear after exposure to the substance ends.Strongly Suspected Human Carcinogen:A substance for which there is no epidemiological evidence of carcinogenesis but there are positive genotoxicity data and clear evidence of carcinogenesis in rodents.Teratogenicity:The occurrence of structural malformations in a developing fetus when a substance is administered during pregnancy.APPENDIX 1. LIST OF SOLVENTS INCLUDED IN THE GUIDELINESolvent OtherNames Structure Class Acetic acid Ethanoic acid CH3COOH Class3Acetone 2-Propanone CH3COCH3 Class 3Propan-2-oneAcetonitrile CH3CN Class2Anisole Methoxybenzene OCH3Class3Benzene Benzol Class11-Butanol n-Butyl alcohol CH3(CH2)3OH Class3Butan-1-ol2-Butanol sec-Butyl alcohol CH3CH2CH(OH)CH3 Class 3Butan-2-olButyl acetate Acetic acid butyl ester CH3COO(CH2)3CH3 Class3tert-Butylmethyl ether 2-Methoxy-2-methyl-propane (CH3)3COCH3 Class3Carbon tetrachloride Tetrachloromethane CCl4 Class1Chlorobenzene ClClass2Chloroform Trichloromethane CHCl3 Class 2Cumene IsopropylbenzeneCH(CH 3)2Class 3(1-Methyl)ethylbenzeneCyclohexane Hexamethylene Class 21,2-Dichloroethane sym -Dichloroethane CH 2ClCH 2Cl Class 1Ethylene dichloride Ethylene chloride 1,1-Dichloroethene 1,1-Dichloroethylene H 2C=CCl 2 Class 1 Vinylidene chloride 1,2-Dichloroethene 1,2-Dichloroethylene ClHC =CHCl Class 2Acetylene dichloride Dichloromethane Methylene chlorideCH 2Cl 2Class 21,2-Dimethoxyethane Ethyleneglycol dimethyl ether H 3COCH 2CH 2OCH 3 Class 2 Monoglyme Dimethyl Cellosolve N,N-Dimethylacetamide DMACH 3CON(CH 3)2 Class 2 N,N-Dimethylformamide DMFHCON(CH 3)2 Class 2Dimethyl sulfoxide Methylsulfinylmethane (CH 3)2SO Class 3 Methyl sulfoxide DMSO1,4-Dioxane p-DioxaneOO Class 2[1,4]Dioxane Ethanol Ethyl alcoholCH 3CH 2OH Class 32-Ethoxyethanol Cellosolve CH3CH2OCH2CH2OH Class 23Ethyl acetate Acetic acid ethyl ester CH3COOCH2CH3 Class2 Ethyleneglycol 1,2-Dihydroxyethane HOCH2CH2OH Class1,2-Ethanediol3Ethyl ether Diethyl ether CH3CH2OCH2CH3 ClassEthoxyethane1,1’-Oxybisethane3Ethyl formate Formic acid ethyl ester HCOOCH2CH3 Class2 Formamide Methanamide HCONH2 Class Formic acid H C O O H Class 33 Heptane n-Heptane CH3(CH2)5CH3 Class2 Hexane n-Hexane CH3(CH2)4CH3 Class Isobutyl acetate Acetic acid isobutyl ester CH3COOCH2CH(CH3)2 Class 33Isopropyl acetate Acetic acid isopropyl ester CH3COOCH(CH3)2 Class2alcohol CH3OH Class Methanol MethylCellosolve CH3OCH2CH2OH Class22-Methoxyethanol Methyl3 Methyl acetate Acetic acid methyl ester CH3COOCH3 Classalcohol (CH3)2CHCH2CH2OH Class 33-Methyl-1-butanol IsoamylalcoholIsopentyl3-Methylbutan-1-ol2 Methylbutyl ketone 2-Hexanone CH3(CH2)3COCH3 ClassHexan-2-oneMethylcyclohexane Cyclohexylmethane CH3Class2Methylethyl ketone 2-Butanone CH3CH2COCH3 Class3 MEKButan-2-oneMethylisobutyl ketone 4-Methylpentan-2-one CH3COCH2CH(CH3)2 Class 34-Methyl-2-pentanoneMIBK2-Methyl-1-propanol Isobutyl alcohol (CH3)2CHCH2OH Class3 2-Methylpropan-1-olN-Methylpyrrolidone 1-Methylpyrrolidin-2-one N OCH3 Class21-Methyl-2-pyrrolidinoneNitromethane CH3NO2 Class2 Pentane n-Pentane CH3(CH2)3CH3 Class31-Pentanol Amylalcohol CH3(CH2)3CH2OH Class3Pentan-1-olPentylalcohol1-Propanol Propan-1-ol CH3CH2CH2OH Class3Propylalcohol2-Propanol Propan-2-ol (CH3)2CHOH Class3IsopropylalcoholPropyl acetate Acetic acid propyl ester CH3COOCH2CH2CH3 Class 3Pyridine NClass2Sulfonane Tetrahydrothiophene1,1-dioxideSO OClass2Tetrahydrofuran Tetramethyleneoxide O Class3 OxacyclopentaneTetralin 1,2,3,4-Tetrahydro-naphthalene Class2Toluene Methylbenzene CH3Class21,1,1-Trichloroethane Methylchlororoform CH3CCl3 Class1 1,1,2-Trichloroethene Trichloroethene HClC=CCl2 Class2Xylene* Dimethybenzene CH3CH3Class2Xylol* usually 60 % m-xylene, 14 % p-xylene, 9 % o-xylene with 17 % ethyl benzeneAPPENDIX 2. ADDITIONAL BACKGROUNDA2.1 Environmental Regulation of Organic Volatile SolventsSeveral of the residual solvents frequently used in the production of pharmaceuticalsare listed as toxic chemicals in Environmental Health Criteria (EHC) monographs andthe Integrated Risk Information System (IRIS). The objectives of such groups as theInternational Programme on Chemical Safety (IPCS), the United States EnvironmentalProtection Agency (USEPA), and the United States Food and Drug Administration(USFDA) include the determination of acceptable exposure levels. The goal is protectionof human health and maintenance of environmental integrity against the possible deleterious effects of chemicals resulting from long-term environmental exposure. The methods involved in the estimation of maximum safe exposure limits are usually based on long-term studies. When long-term study data are unavailable, shorter term study data can be used with modification of the approach such as use of larger safety factors. The approach described therein relates primarily to long-term or life-time exposure of the general population in the ambient environment, i.e. ambient air, food, drinking water and other media.A2.2 Residual Solvents in PharmaceuticalsExposure limits in this guideline are established by referring to methodologies and toxicity data described in EHC and IRIS monographs. However, some specific assumptions about residual solvents to be used in the synthesis and formulation of pharmaceutical products should be taken into account in establishing exposure limits. They are:1) Patients (not the general population) use pharmaceuticals to treat their diseases or forprophylaxis to prevent infection or disease.2) The assumption of life-time patient exposure is not necessary for mostpharmaceuticalproducts but may be appropriate as a working hypothesis to reduce risk to human health3) Residual solvents are unavoidable components in pharmaceutical production and willoften be a part of drug products.4) Residual solvents should not exceed recommended levels except in exceptionalcircumstances.5) Data from toxicological studies that are used to determine acceptable levels forresidual solvents should have been generated using appropriate protocols such as those described for example by OECD, EPA, and the FDA Red Book.APPENDIX 3. METHODS FOR ESTABLISHING EXPOSURE LIMITSThe Gaylor-Kodell method of risk assessment (Gaylor, D. W. and Kodell, R. L.: Linear Interpolation algorithm for low dose assessment of toxic substance. J Environ. Pathology, 4, 305, 1980) is appropriate for Class 1 carcinogenic solvents. Only in cases where reliable carcinogenicity data are available should extrapolation by the use of mathematical models be applied to setting exposure limits. Exposure limits for Class 1 solvents could be determined with the use of a large safety factor (i.e., 10,000 to 100,000) with respect to the no-observed-effect level (NOEL). Detection and quantitation of these solvents should be by state-of-the-art analytical techniques.Acceptable exposure levels in this guideline for Class 2 solvents were established by calculation of PDE values according to the procedures for setting exposure limits in pharmaceuticals (Pharmacopeial Forum, Nov-Dec 1989), and the method adopted by IPCS for Assessing Human Health Risk of Chemicals (Environmental Health Criteria 170, WHO, 1994). These methods are similar to those used by the USEPA (IRIS) and the USFDA (Red Book) and others. The method is outlined here to give a better understanding of the origin of the PDE values. It is not necessary to perform these calculations in order to use the PDE values tabulated in Section 4 of this document.PDE is derived from the no-observed-effect level (NOEL), or the lowest-observed effect level (LOEL) in the most relevant animal study as follows:PDE =NOEL x Weight Adjustment F1x F2x F3x F4x F5The PDE is derived preferably from a NOEL. If no NOEL is obtained, the LOEL may be used. Modifying factors proposed here, for relating the data to humans, are the same kind of "uncertainty factors" used in Environmental Health Criteria (Environmental Health Criteria 170, World Health Organization, Geneva, 1994), and "modifying factors" or "safety factors" in Pharmacopeial Forum. The assumption of 100% systemic exposure is used in all calculations regardless of route of administration.The modifying factors are as follows:F1 = A factor to account for extrapolation between speciesF1 = 5 for extrapolation from rats to humansF1 = 12 for extrapolation from mice to humansF1 = 2 for extrapolation from dogs to humansF1 = 2.5 for extrapolation from rabbits to humansF1 = 3 for extrapolation from monkeys to humansF1 = 10 for extrapolation from other animals to humansF1 takes into account the comparative surface area:body weight ratios for the species concerned and for man. Surface area (S) is calculated as:S = kM0.67in which M = body mass, and the constant k has been taken to be 10. The body weights used in the equation are those shown below in Table A3.1.F2 = A factor of 10 to account for variability between individuals.A factor of 10 is generally given for all organic solvents, and 10 is used consistently in this guideline.F3 = A variable factor to account for toxicity studies of short-term exposureF3 = 1 for studies that last at least one half lifetime (1 year for rodents or rabbits; 7 years for cats, dogs and monkeys).F3 = 1 for reproductive studies in which the whole period of organogenesis is covered.F3 = 2 for a 6-month study in rodents, or a 3.5-year study in non-rodents.F3 = 5 for a 3-month study in rodents, or a 2-year study in non-rodents.F3 = 10 for studies of a shorter duration.。