SPE方法开发从此不再复杂-Waters
- 格式:pdf
- 大小:1.56 MB
- 文档页数:16


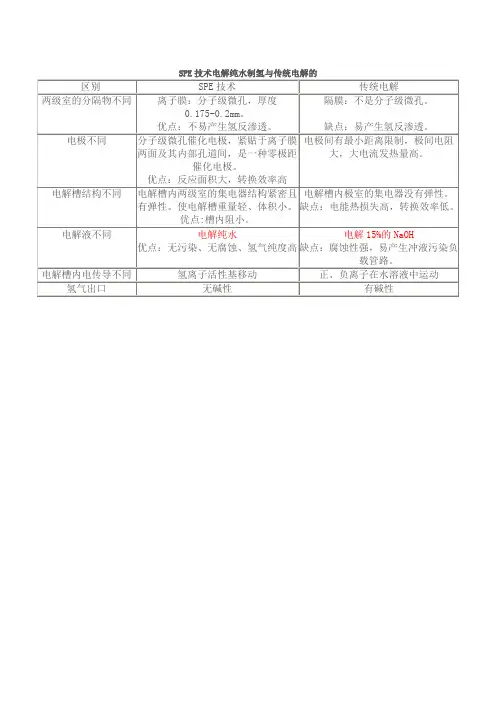
SPE——通过固相萃取进行样品富集和纯化为何使用固相萃取(SPE)技术1. 您需要从样品中去除特定干扰物,以免它们在目标分析物的检测和定量过程中影响实验结果。
在此处所示的示例中,不适当的样品制备方案未能去除干扰物,导致提取物呈现出残留的黄色干扰物,色谱图中目标分析物与多个干扰峰发生了重叠。
2. 您需要提高初始样品中目标分析物的浓度,以便所用的分析技术能够更轻松地对其进行检测和准确定量。
如果目标分析物可被较强地保留,那么可能需要在SPE色谱柱上加载较大的样品量,随后仅以极小体积的洗脱液将此分析物洗脱下来,由此提高样品中分析物的浓度。
3. 您需要去除样品中的干扰物(即使不可见),这些干扰物会在质谱检测中抑制目标分析物的信号。
在此处的示例中,蛋白沉淀法无法去除血浆提取物中的磷脂,从而造成严重的离子抑制。
优化的复合模式SPE方案可获取最纯净的提取物,并可在最大程度上降低离子抑制效应。
What is Solid-Phase Extraction (SPE)?Don't be confused by the term solid-phase extraction [SPE]. A typical SPE device has 50 times more separation power than a simple, single liquid-liquid extraction. SPE is actually column liquid-solid chromatography. Since SPE is liquid chromatography [LC], its practice isgoverned by LC principles. A sample is introduced into a column or a cartridge device containing a bed of appropriate particles, or other form, of a chromatographic packing material [stationary phase]. Solvent [mobile phase] flows through the bed. By choosing an appropriate combination of mobile and stationary phases, sample components may pass directly through the column bed, or they may be selectively retained.Individual compounds in the sample each typically appear to travel at different speeds through the device. Using a weaker solvent causes them to move slowly and/or be strongly retained. A stronger solvent speeds up their passage through the bed and elutes the analyte(s) in a more concentrated volume. Elution from an SPE device is usually done by increasing the strength of the mobile phase in a series of discrete, rather than continuous, steps during which selected analytes or interferences are either fully retained or rapidly eluted-this variation of gradient elution called a step gradient.Most commonly, SPE is practiced using miniature column or cartridge devices. An example is shown here. A mixture of three dyes is loaded onto the cartridge in a weak solvent, causing strong sample retention in a narrow band that appears black at the column inlet. Subsequent gradient steps, each with a successively stronger solvent, are used to elute the dyes individually [yellow, red, then blue].Typical SPE cartridges are low-pressure devices-constructed of solvent-resistant plastic or glass-filled with particles ≥30 µm in diameter. Suitable flow rates may be achieved by gravity or with the assistance of vacuum or low positive pressure. [The latter requires putting a cap on the open inlet of a column or using a sealed device with inlet and outlet fittings.]Importance of Sample PreparationIn the last two decades, dramatic advances in analytical instrumentation and laboratory information management systems shifted the analyst's predominant tasks from assaymeasurements to sample preparation and data processing. As the stringency of requirements for higher sensitivity, selectivity, accuracy, precision, and number of samples to be processed has escalated, the corresponding increases in speed and sophistication of analysis and data collection have outpaced improvements in the many traditional techniques of samplecollection and preparation. By some estimates, 75 to 80% of the work activity and operating cost in a contemporary analytical lab is spent processing and preparing samples forintroduction or injection into an analytical separation and/or measurement device. Clearly, efforts directed and products designed to streamline sample preparation protocols are essential to future progress in analytical science.Goals of Sample PreparationSuccessful sample preparation for most analytical techniques [HPLC, GC, spectrophotometry, RIA, etc.] has a threefold objective: namely, to provide the sample component of interest▪in solution▪free from interfering matrix elements▪at a concentration appropriate for detection or measurement.To accomplish these goals, a sample, or a representative portion thereof [not always easy to obtain], is prepared via traditional methods of dissolution, homogenization, extraction[liquid- or solid-phase], filtration, concentration, evaporation, separation, chemicalderivatization, standardization [internal or external], etc.Usually such methods are used in combinations of multiple steps, which form a sample prep protocol. The fewer steps and methods used in any given protocol, the simpler, moreconvenient, cost effective, and less time consuming it is. Simpler protocols lend themselves more readily to automation and also lead to increased accuracy, reliability, reproducibility, and safety.Innovation in Sample Preparation MethodsThere are many ways to combine standard tools and techniques to accomplish the goals of sample prep. However, it is best to seek innovative means to streamline sample prepprotocols:▪to combine the functions of several steps, if possible, into one operation;▪to eliminate needless sample transfers and manipulations;▪to reduce the scale as much as practicable [gaining economies of time, labor, and cost];▪to use new tools in creative ways.Benefits of Solid-Phase Extraction [SPE] CartridgesWhen compared to other sample preparation processes, solid-phase extraction using SPE cartridges offers:Lower Cost • lower solvent consumption• lower reagent consumption• less apparatusGreater Recoveries • min imal sample transferFaster Protocol • fewer stepsGreater Safety • less exposure to toxic agentsGreater Accuracy • no cross contaminationNo Emulsion Problems • less sample handling• fewer stepsNo Transporting of Samples to Lab • direct field samplingReduced Harm to Labile Samples • minimal evaporationMinimal Glass Breakage • less glassware used, less to washAchieving Sample Preparation Objectives with Solid-Phase Extraction [SPE]▪To remove sample constituents that elute after the analytes of interest or are strongly adsorbed:▪use solid-phase extraction with sorbent surface chemistry that is the same as that in the analytical HPLC column.▪tailor the gradient steps to elute analytes selectively.▪To remove sample constituents that coelute with an analyte of interest:▪use solid-phase extraction with sorbent surface chemistry and/or separation mode different from that in the analytical column.▪tailor the gradient steps to elute analytes selectively.▪To enrich sample components present in low concentration:▪tailor the gradient steps to elute analytes selectively.▪use "large" sample volumes in adsorption-promoting solvent.▪use "small" collection volume in desorption-promoting solvent.▪use sorbent chemistry tailored to the analyte, independent of that in analytical column.▪carefully choose chemistry of solid-phase extraction column so further sample prep will be unnecessary.▪To desalt samples:▪first, adsorb analytes on reversed-phase sorbent while salt breaks through unretained.▪then, after using water to wash away residual salt, desorb analytes using water-miscible organic solvent.▪To exchange solvents:▪adsorb the sample completely onto a strongly retentive sorbent and flush away the original solvent with a weaker eluent.▪elute the analyte with the desired solvent.▪To fractionate classes of compounds:▪use a step-gradient sequence to divide a sample-on the basis of hydrophobicity, polarity, or charge-into fractions containing groups of analytes that share common properties.▪To derivatize analytes using solid-phase reagents:▪adsorb a derivatization reagent on the surface of the sorbent; then, collect the sample (usually a gas) under conditions that favor complete adsorption of the analyte; wait for the reaction to occur and then selectively elute the derivative.SPE is ChromatographyKeep in mind that solid-phase extraction has the same fundamental basis as HPLC. Any knowledge of the chromatographic behavior of the analytes of interest, and of other matrix components, can help in choosing the proper sorbent and eluents. If, for example, you know that certain chromatographic conditions provide excellent separation of your analyte from interferences, then you may choose a similar SPE sorbent and solvent combination. Similarly, if you are trying to remove an interference that coelutes in HPLC, then you know a priori that similar SPE conditions will not be successful.General Elution ProtocolsThere are two general strategies for isolating and cleaning up sample components of interest:▪adsorb matrix interferences while components of interest pass through the cartridge unretained.▪adsorb components of interest while matrix interferences pass through the cartridge unretained.The first strategy is usually chosen when the desired sample component is present in high concentration. When components of interest are present at low levels, or multiplecomponents of widely differing polarities need to be isolated, then the second strategy is generally employed. Trace enrichment of compounds present at extremely low levels and concentration of dilute samples are also achieved by the second strategy.Steps of a Solid-Phase Extraction ProcedureThe following section describes the steps involved in a complete solid-phase extraction procedure. In many applications, one or more of the steps, listed below and subsequently described by general examples, can be omitted, thereby simplifying the procedure. The procedures illustrated here use samples containing dyes so that separations may be easily visualized. Keep in mind that most samples contain colorless components that require some type of detector or test to locate them in the collected fractions. Use the following information as a guideline in the development of your own procedure or when modifying procedures published in the literature.1.Pretreatment of the sample2.Conditioning of the cartridge3.Loading the sample4.Elution of the fractionsPrincipal Separation Modes in Solid-Phase Extraction [SPE]Normal-Phase ChromatographyThis mode is classically used to separate neutral organic compounds whose chemical nature ranges from hydrophobic to moderately polar.To perform normal-phase chromatography with SPE cartridges, use a step gradient of nonpolar solvents with a polar packing material.1.Condition the cartridge with six to ten hold-up volumes of non-polar solvent, usually thesame solvent in which the sample is dissolved.2.Load the sample solution onto the cartridge bed.3.Elute unwanted components with a non-polar solvent.4.Elute the first component of interest with a more polar solvent.5.Elute remaining components of interest with progressively more polar [stronger]solvents.6.When you recover all of your components, discard the used cartridge in a safe andappropriate manner.This procedure is illustrated in the figure below for a sample containing a mixture of three neutral, relatively non-polar organic dyes [yellow, red, and blue] that appears black when initially loaded onto the cartridge bed.Illustration of a General Elution Protocol for Normal-Phase Chromatography on SPECartridges(Silica, Florisil, Alumina, Diol, CN, NH2)Reversed-Phase ChromatographyBecause of the multiplicity of aqueous samples spanning a breadth of applications from environmental water to fruits and vegetables, from beverages to biological fluids, reversed-phase chromatography has become the predominant mode of SPE.To perform reversed-phase chromatography with SPE cartridges, use a gradient of strongly to weakly polar solvents [from weak to strong solvent elution strength] with a non-polar packing material.1.Solvate the silica-bonded phase or polymer packing with six to ten hold-up volumes ofmethanol or acetonitrile. Flush the cartridge with six to ten hold-up volumes of water or buffer. Do not allow the cartridge to dry out [unless using HLB].2.Load the sample dissolved in a strongly polar [weak] solvent [typically water].3.Elute unwanted components with a strongly polar solvent.4.Elute weakly retained components of interest with a less polar solvent.5.Elute more tightly bound components with progressively more non-polar [stronger]solvents.6.When you recover all the components of interest, discard the used cartridge in a safe andappropriate manner.This procedure is illustrated in the figure below for a sample of an aqueous grape drink containing two polar food dyes [red and blue], as well as sugar and artificial flavor [but no real grape juice!]. As prepared, this drink appears light purple in a glass, since the dye concentration is dilute. When a portion is loaded onto a prepared SPE cartridge, the strongly retained dyes become concentrated near the inlet in a dark purple band.Illustration of a General Elution Protocol for Reversed-Phase Chromatography on SPECartridges(C18, tC18, C8, CN, Diol, HLB, Porapak RDX, NH2)Ion-Exchange ChromatographyCompounds that are ionic or ionizable are often best isolated using some form ofion-exchange chromatography. This separation mode is orthogonal to the more widely used normal-phase and reversed-phase modes and provides a powerful, selective second dimension to sample preparation protocols.Illustration of the Two Major Types of Phases—Anion and Cation Exchange—and How They Selectively Attract and Retain Molecules of Opposite ChargeTo perform ion-exchange chromatography with SPE cartridges, use a gradient of pH or ionic strength with an ion exchange packing material.1.Condition the cartridge with six to ten hold-up volumes of deionized water or weak buffer.2.Load the sample dissolved in a solution of deionized water or buffer.3.Elute unwanted, weakly bound components with a weak buffer.4.Elute the first component of interest with a stronger buffer (change the pH or ionicstrength).5.Elute other components with progressively stronger buffers.6.When you recover all of your components, discard the used cartridge in an appropriatemanner.This procedure is illustrated in the figure below for a sample of an aqueous mixture of two ionic dyes with different pK a values. When loaded onto the cartridge, both are strongly retained, and the combination of blue and yellow components appears as a green band near the inlet.Illustration of General Elution Protocol for Ion-Exchange Chromatography on SPE Cartridges (NH2, Accell™ Plus QMA, Accell Plus CM, SCX, SAX, WCX, WAX)Cation and anion exchangers are further categorized as either weak or strong exchangers, depending upon the type of ionic group on their surface. Strong cation exchangers possess an acidic surface moiety such as a sulfonic acid that is always ionized [negatively charged] over the whole pH range. Weak cation exchangers possess an acidic surface moiety such as a carboxylic acid that is negatively charged at high pH but neutral at low pH. Similarly, strong anion exchangers typically bear quaternary ammonium groups that are always positively charged, while weak anion exchangers possess primary, secondary, or tertiary amine groups that may be positively charged at low pH but neutral at high pH.Use the following table as a guideline to choose the appropriate SPE ion-exchange cartridgetype for your particular analyte.Mixed-mode ion exchange chromatography combines the use of reversed-phase andion-exchange modes into a single protocol on a single SPE cartridge. It can be used to isolate and separate neutral, acidic, and basic compounds from a single complex matrix. An ideal mixed-mode SPE sorbent substrate remains water-wettable while exhibiting strong reversed-phase retention of hydrophobic compounds. On its surface are ion-exchange functionalities of one of the four general types just described above. Intermediate washes with organic solvent mixtures of appropriate elution strength may be used to isolate neutral compounds [including ionizable analytes in their neutral state]. Selective elution of ionically bound analytes may be attained by manipulating the charge of either the analyte [when bound to strong ion exchangers] or of the sorbent [for analytes bound to weak ion exchangers].下表汇总了各种SPE模式,为方法开发工作的开展提供了一个良好的起点:固相萃取选择色谱模式及吸附剂分析物中-低极性低-高极性/中性带电荷、可电离分离机制基于疏水性的分离基于极性的分离基于电荷的分离样品基质水溶液非极性有机溶剂水溶液/低离子强度SPE吸附剂的活化/平衡1. 用极性有机溶剂得到的溶剂化物2. 水非极性有机溶剂低离子强度缓冲液初步冲洗步骤水溶液/缓冲液非极性有机溶剂低离子强度缓冲液洗脱步骤增加极性有机溶剂的含量增加混合有机溶剂的洗脱强度更强的缓冲液——通过调节离子强度或pH值而中和电荷AX[阴离子交换]CX[阳离子交换]吸附剂官能团C18, tC18, C8, tC2,CN, NH2, HLB, RDX,Rxn RPSilica, Alumina,Florisil, Diol, CN,NH2Accell Plus QMA,NH2, SAX, MAX,WAXAccell Plus CM,SCX, MCX, WCX,Rxn CX吸附剂表面极性低至中等高至中等高高典型溶剂的极性范围高至中等低至中等高高典型的上样溶剂水、低强度缓冲液正己烷、氯仿、二氯甲烷水、低强度缓冲液水、低强度缓冲液典型的洗脱溶剂MeOH/水、CH3CN/水乙酸乙酯、丙酮、CH3CN缓冲液、高离子强度的盐类,提高pH缓冲液、高离子强度的盐类,降低pH样品洗脱顺序最大极性样品组分最先洗脱出来最弱极性样品组分最先洗脱出来最弱电离样品组分最先洗脱出来最弱电离样品组分最先洗脱出来为洗脱化合物而做的流动相溶剂改变减弱溶剂极性增强溶剂极性增加离子强度或提高pH值增加离子强度或降低pH值This has been a brief introduction to sample enrichment and purification using solid-phase extraction [SPE]. The best way to start using SPE is to first learn what others have done with analytes and/or matrices similar to those of interest to you. You will find > 7,700 references to the use of SPE in the Resource Library on . Fill in the blank with a partial compound or matrix name in the following search phrase:“Sep-Pak” OR “Oasis” AND ______*NOTE: Rather than risk a spelling error, use an asterisk [*] with a root name for best results. Using this same search string, even more references [> 60,000] may be found on GOOGLE Scholar.Further reading:J.C. Arsenault and P.D. McDonald, Beginners Guide to Liquid Chromatography, Waters [2007]; Order P/N 715001531on P.D. McDonald and E.S.P. Bouvier, A Sample Preparation Primer and Guide to Solid-Phase Extraction Methods Development, Waters [2001] Search for WA20300 on Waters, Purity by SPE [2008]; Search for 720001692en on U.D. Neue, P.D. McDonald, Topics in Solid-Phase Extraction. Part 1. Ion Suppression inLC/MS Analysis: A Review. Strategies for its elimination by well-designed, multidimensional solid-phase extraction [SPE] protocols and methods for its quantitative assessment [2005]; Search for 720001273en on 。




SPE定义SPE,固相萃取( Solid Phase Extraction,简称SPE)是1 种用途广泛而且越来越受欢迎的样品前处理技术。
SPE 是利用固体吸附剂将液体样品中的目标化合物吸附, 与样品的基体和干扰化合物分离, 然后再用洗脱液洗脱或加热解吸附, 达到分离和富集目标化合物的目的。
与传统的液液萃取法相比较可以提高分析物的回收率,更有效的将分析物与干扰组分分离,减少样品预处理过程,操作简单、省时、省力。
广泛的应用在医药、食品、环境、商检、化工等领域。
因其具有安全、回收率高, 重现性好、操作简便、快速、应用范围广、易实现自动化操作[等特点, 从而显示出良好的发展前景, 在相关领域的应用越来越多[。
从1978 年美国Waters 公司首先将一次性SPE 商品柱投放到市场以来,据不完全统计, SPE 商品柱一直以每年10%的增长速度。
SPE装置SPE 装置一般由柱管、烧结垫, 固定相3 部分组成,其中固定相是SPE 柱中最重要的部分。
最常见的固定相是键合的硅胶材料, 也有很多非硅胶基的固定相被广泛应用。
工作原理固相萃取是一个包括液相和固相的物理萃取过程,主要适用于水中组分的处理。
吸附剂是固相,而液相是萃取过程中的水样或解析过程的有机溶剂。
当水样通过装有合适吸附剂的SPE 柱时,其中某些痕量待测物质被保留在固定相当中,然后再用少量的选择性溶剂浏兑,因此, SpE 是同时进行萃取和浓缩的有效方法。
由于其工作原理、固定相、溶剂的选择等方面与高效液相色谱有许多相似之处,SPE也可以近似的看成是一个简单的柱色谱过程。
固相萃取(SPE) 技术基于液-固相色谱理论,采用选择性吸附、选择性洗脱的方式对样品进行富集、分离、纯化,是一种包括液相和固相的物理萃取过程;也可以将其近似的看作一种简单的色谱过程。
SPE是利用选择性吸附与选择性洗脱的液相色谱法分离原理。
较常用的方法是使液体样品通过一吸附剂,保留其中被测物质,再选用适当强度溶剂冲去杂质,然后用少量良溶剂洗脱被测物质,从而达到快速分离净化与浓缩的目的。



SPE-GC/MS法检测鱼塘水中微量毒物李晓民;杨崇俊;曲筱静;崔文;张璟【摘要】目的:建立固相萃取-气质联用法检测(SPE‐GC/MS/SIM )分析方法,检测鱼塘水中微量敌敌畏、氯氰菊酯、硫丹和五氯酚含量。
方法取1000ml 鱼塘水进行固相萃取,洗脱后浓缩进行GC/MS/SIM 定性定量测定。
结果固相萃取对4种毒物浓缩提取后,各项定量参数符合分析要求。
鱼塘水中敌敌畏农药的最低检测限为0.1ng/ml ,氯氰菊酯的最低检出限为5ng/ml ,硫丹和五氯酚的最低检出限为1ng/mm。
结论SPE‐GC/M S法简单、灵活、经济、快速、无溶剂,适用于鱼塘水中微量毒物分析。
%Objective To establish an analysis method of detection of trace dichlorvos (DDVP) ,cypermethrin , endosulfan and pentachlorophenol (PCP) in fishpond water by gas chromatography‐mass spectrometry in selected ion monitoring (GC/MS/SIM ) with solid phase extraction (SPE) .Methods 1000ml fishpond water was treated with SPE .After being eluted and concentrated ,the water sample was treated with qualitative and quantitative anal‐ysis by GC/MS/SIM .Results After concentration and extraction of four toxicants with SPE ,the quantitative pa‐rameters for the fishpond sample conformed to the requirements of analysis .The detection limit for DDVP in fish‐pond water was 0 .1 ng/ml ,5 ng/ml for cypermethrin ,1 ng/ml for endosulfan and PCP .Conclusion The SPE‐GC/MS was simple ,flexible ,economic ,fast ,solvent‐free ,which was suitable for traces analysis of poison in fishpond water .【期刊名称】《济宁医学院学报》【年(卷),期】2014(000)006【总页数】3页(P425-426,429)【关键词】法医毒物分析;固相萃取;气质联用【作者】李晓民;杨崇俊;曲筱静;崔文;张璟【作者单位】菏泽市公安局刑事科学技术研究所,山东菏泽 274099;济宁市公安局刑事侦查支队;济宁市公安局刑事侦查支队;济宁医学院司法鉴定中心,山东济宁272067;济宁医学院司法鉴定中心,山东济宁 272067【正文语种】中文【中图分类】DF795.4近年来鱼塘投毒案件骤增,鱼塘水中毒物是投毒案的最直接证据。
一、固相萃取基本原理与操作1、固相萃取吸附剂与目标化合物之间的作用机理固相萃取主要通过目标物与吸附剂之间的以下作用力来保留/吸附的1)疏水作用力:如C18、C8、Silica、苯基柱等2)离子交换作用:SAX, SCX,COOH、NH2等3)物理吸附:Florsil、Alumina等2、p H值对固相萃取的影响pH值可以改变目标物/吸附剂的离子化或质子化程度。
对于强阳/阴离子交换柱来讲,因为吸附剂本身是完全离子化的状态,目标物必须完全离子化才可以保证其被吸附剂完全吸附保留。
而目标物的离子化程度则与pH值有关。
如对于弱碱性化合物来讲,其pH值必须小于其pKa 值两个单位才可以保证目标物完全离子化,而对于弱酸性化合物,其p H值必须大于其pKa值两个单位才能保证其完全离子化。
对于弱阴/阳离子交换柱来讲,必须要保证吸附剂完全离子化才保证目标物的完全吸附,而溶液的pH值必须满足一定的条件才能保证其完全离子化。
3、固相萃取操作步骤及注意事项针对填料保留机理的不同(填料保留目标化合物或保留杂质),操作稍有不同。
1)填料保留目标化合物固相萃取操作一般有四步(见图1):Ø 活化---- 除去小柱内的杂质并创造一定的溶剂环境。
(注意整个过程不要使小柱干涸)Ø 上样---- 将样品用一定的溶剂溶解,转移入柱并使组分保留在柱上。
(注意流速不要过快,以1ml/min为宜,最大不超过5ml/min)Ø 淋洗---- 最大程度除去干扰物。
(建议此过程结束后把小柱完全抽干)Ø 洗脱---- 用小体积的溶剂将被测物质洗脱下来并收集。
(注意流速不要过快,以1ml/min为宜)此主题相关图片如下11.jpg:2)填料保留杂质固相萃取操作一般有三步(见图2):Ø 活化--除去柱子内的杂质并创造一定的溶剂环境。
(注意整个过程不要使小柱干涸)Ø 上样--将样品转移入柱,此时大部分目标化合物会随样品基液流出,杂质被保留在柱上,故此步骤要开始收集(注意流速不要过快)Ø 洗脱---用小体积的溶剂将组分淋洗下来并收集,合并收集液。