静脉注射纯度级别人血浆丙种球蛋白(IgG)纯化流程
- 格式:rtf
- 大小:400.72 KB
- 文档页数:9

丙球蛋白制作过程丙球蛋白是一种重要的免疫球蛋白,它在人体中具有重要的免疫调节功能。
丙球蛋白的制备过程主要包括采集血浆、分离、纯化和灭菌等步骤。
下面将详细介绍丙球蛋白的制作过程。
丙球蛋白的制备需要采集血浆。
血浆是血液中没有凝固的部分,它含有丰富的蛋白质和其他营养物质。
采集血浆的过程一般通过静脉采血实现,采血后将血液置于离心机中离心分离,得到血浆。
接下来,需要对血浆进行分离。
分离的目的是将血浆中的各种成分分离出来,以得到丙球蛋白。
分离的方法有多种,其中最常用的是冷沉淀法。
该方法通过在低温下将血浆进行冷冻,然后将冷冻的血浆进行离心,将沉淀物与上清分离开来。
分离后的上清中含有丙球蛋白,但其中还夹杂着其他蛋白质和杂质。
因此,需要对上清进行纯化。
纯化的目的是去除杂质,提高丙球蛋白的纯度。
纯化的方法有多种,常用的是离子交换层析法和凝胶过滤法。
离子交换层析法是利用离子交换树脂的特性,将血浆中的蛋白质分离开来。
这种方法可以根据蛋白质的电荷大小来进行分离,从而得到纯净的丙球蛋白。
凝胶过滤法则是利用凝胶的孔隙大小来分离蛋白质,将丙球蛋白与其他蛋白质分离开来。
经过纯化后,得到的丙球蛋白需要进行灭菌处理,以确保其无菌。
灭菌的方法有多种,常用的是高温灭菌法和紫外线灭菌法。
高温灭菌法是将丙球蛋白置于高温环境中,使其中的微生物被杀灭。
紫外线灭菌法则是利用紫外线的杀菌作用,对丙球蛋白进行灭菌处理。
经过灭菌处理的丙球蛋白就可以进行包装和贮存了。
包装的目的是保护丙球蛋白不受外界环境的污染,贮存的目的是延长丙球蛋白的保质期。
常见的包装方式有玻璃瓶和注射器等,贮存一般在低温下进行,以确保丙球蛋白的质量。
丙球蛋白的制备过程包括采集血浆、分离、纯化、灭菌和贮存等步骤。
这个过程需要严格的操作和控制,确保丙球蛋白的质量和安全性。
丙球蛋白的制备是一项复杂的工艺,但通过科学的方法和技术,可以获得高纯度和高活性的丙球蛋白,为临床应用提供重要的免疫治疗药物。

血清免疫球蛋白G的分离纯化及鉴定实验八血清免疫球蛋白G的分离纯化及鉴定为了初步掌握蛋白质的分离纯化技术,选择了从家畜血清中分离纯化免疫球蛋白G (Immunoglobulin G,简称IgG)的实验。
血清中的蛋白质有数十种之多,IgG是球蛋白的一种。
分离纯化蛋白质的方法是利用不同蛋白质的某些物理、化学性质(如在一定条件下带电的情况、分子量、溶解度等)的不同而建立起来的,其中有盐析、离子交换、凝胶过滤、亲和层析、制备电泳和超速离心等。
在分离纯化时,要根据情况选用几种方法互相配合才能达到分离纯化一种蛋白质的目的。
本实验采用硫酸铵盐析、DEAE-纤维素离子交换及凝胶过滤等方法,提取家畜血清中IgG。
其原理及操作如下:㈠硫酸铵盐析1.试剂饱和硫酸铵溶液pH7.0:称取(NH4)2SO4760g,加蒸馏水至1000ml,加热至5℃,使绝大部分硫酸铵溶解,置室温过夜,取上清液,用氢氧化铵调pH至7.0。
(内含0.15mol/L NaCl,简称PBS):取0.2mol/L Na2HPO4磷酸缓冲溶液(0.01mol/L,pH7.0)溶液30.5ml,0.2mol/L NaH2PO4溶液19.4ml,加NaCl8.5g,加蒸馏水至1000ml。
磷酸缓冲溶液(0.0175mol/L,pH6.7)(不含NaCl,简称PB):取0.2mol/L Na2HPO4溶液43.5ml,0.2mol/L NaH2PO4溶液56.6ml混合,用蒸馏水稀释至1000ml。
2.操作①取家畜血清5ml,加磷酸缓冲溶液(0.1mol/L,pH7.0)5ml,混匀,滴加(边摇边搅拌)饱和硫酸铵4ml(此时溶液硫酸铵饱和度约为20%)。
静置20min,以3000r/min 离心15min,沉淀为纤维蛋白(弃去),上清液中含清蛋白、球蛋白。
②取上清液,再加饱和硫酸铵溶液6ml(方法同前),此时溶液硫酸铵饱和度为50%,静置20min,以3000r/min 离心15min,上清液中含清蛋白,沉淀为球蛋白。
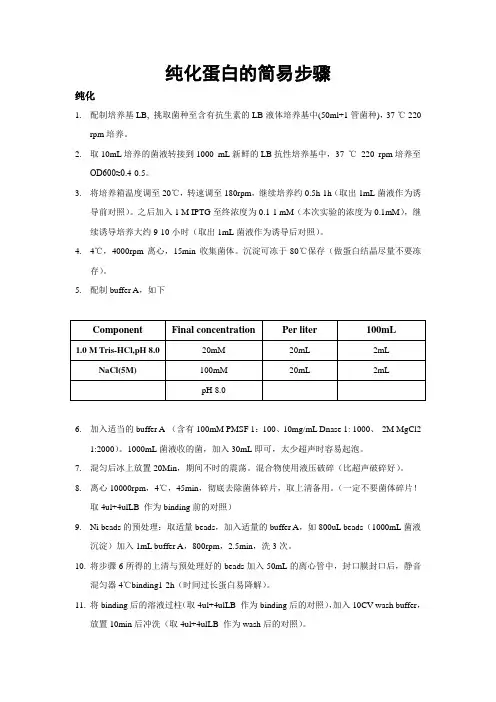
纯化蛋白的简易步骤纯化1.配制培养基LB, 挑取菌种至含有抗生素的LB液体培养基中(50ml+1管菌种),37 ℃ 220rpm培养。
2.取10mL培养的菌液转接到1000 mL新鲜的LB抗性培养基中,37 ℃220 rpm培养至OD600≈0.4-0.5。
3.将培养箱温度调至20℃,转速调至180rpm,继续培养约0.5h-1h(取出1mL菌液作为诱导前对照)。
之后加入1 M IPTG至终浓度为0.1-1 mM(本次实验的浓度为0.1mM),继续诱导培养大约9-10小时(取出1mL菌液作为诱导后对照)。
4.4℃,4000rpm离心,15min收集菌体。
沉淀可冻于-80℃保存(做蛋白结晶尽量不要冻存)。
5.配制buffer A,如下6.加入适当的buffer A (含有100mM PMSF 1:100、10mg/mL Dnase 1: 1000、2M MgCl21:2000)。
1000mL菌液收的菌,加入30mL即可,太少超声时容易起泡。
7.混匀后冰上放置20Min,期间不时的震荡。
混合物使用液压破碎(比超声破碎好)。
8.离心10000rpm,4℃,45min,彻底去除菌体碎片,取上清备用。
(一定不要菌体碎片!取4ul+4ulLB 作为binding前的对照)9.Ni beads的预处理:取适量beads,加入适量的buffer A,如800uL beads(1000mL菌液沉淀)加入1mL buffer A,800rpm,2.5min,洗3次。
10.将步骤6所得的上清与预处理好的beads加入50mL的离心管中,封口膜封口后,静音混匀器4℃binding1-2h(时间过长蛋白易降解)。
11.将binding后的溶液过柱(取4ul+4ulLB 作为binding后的对照),加入10CV wash buffer,放置10min后冲洗(取4ul+4ulLB 作为wash后的对照)。
Wash buffer: buffer A+20mM imidazole12.洗好的beads加入适量(5CV)的elution buffer,放置10min后洗脱。

人血丙种球蛋白制造及检定规程本品系由乙型肝炎疫苗免疫健康人的血浆或血清经低温乙醇法提取的免疫球蛋白制剂。
含两种球蛋白90%以上。
用于常见病毒性感染的被动免疫,主要用于预防麻疹和传染性肝炎。
1 制造1.1制造要求与《人血白蛋白(低温乙醇法)制造及检定规程》中1.1项规定相同。
1.2制造工艺1.2.1采用低温乙醇法。
可含适宜稳定剂(如含甘氨酸,应为0.3或0.4mol/L)。
1.2.2分批每批制品最少应由1000名以上健康献血员的血浆(清)混合制成。
同一制造工艺同一空中溶解稀释的制品作为一批,不同滤器除菌过滤或不同机柜冻干的制品应分为亚批。
1.2.3半成品检定液体制剂于除菌过滤后应做理化检查(残余乙醇含量≤0.03%)及热原质试验,并按亚批抽样做无菌试验。
直接分装时应留样做上述实验。
1.2.4 冻干半成品经检定合格后及时分装,并按《人血白蛋白(低温乙醇法)制造及检定规程》1。
2。
5项规定进行冻干,但制品温度不得超过35℃。
1.3剂型与规格1.3.1剂型分为液体及冻干两种。
1.3.2规格液体制剂蛋白质浓度为10%。
液体和冻干制剂每瓶(支)蛋白质装量均为150mg、300mg。
1.4制品重滤和再制与《人血白蛋白(低温乙醇法)制造及检定规程》中1.4项相同。
2 成品检定2.1抽样每批成品应抽样做全面检定,不同机柜冻干的制品应分别抽样做无菌试验及水分测定。
2.2物理检查2.2.1外观冻干制剂应为白色或灰白色的疏松体,无融化迹象。
液体制剂和冻干制剂溶解后溶液应为接近无色,可带乳光,或淡黄色澄明液体,不应含有异物、混浊或摇不散的沉淀。
2.2.2冻干制剂溶解时间冻干制剂加入20~25℃标示量的灭菌注射用水后,应在15分钟内完全溶解。
2.2.3热稳定性试验液体制剂置57±0.5℃水浴保温4小时后,应无凝胶化或絮状物。
2.3化学检定按《生物制品化学检定规程》进行。
2.3.1水分冻干制剂的水分含量应≤3%(g/g)。


丙型肝炎病毒抗体(胶体金法)标准操作规程1.检验目的用于体外定性检测人血清或血浆样本中的丙型肝炎病毒(HCV)抗体。
2.检验程序的原理和方法采用胶体金免疫层析技术,应用间接法原理定性检测人血清(浆)HCV抗体。
在玻璃纤维素膜上预包被金标鼠抗人IgG抗体(anti-IgGAb),在硝酸纤维素膜上检测线和对照线处分别包被重组丙肝混合抗原(Core、NS3、NS4、NS5,源自大肠杆菌)和人IgG 抗体(丙种球蛋白)。
检测阳性样本时,血清样本中HCV-Ab与胶体金标记鼠抗人IgG 抗体结合形成复合物,由于层析作用复合物沿纸条向前移动,经过检测线时与预包被的抗原结合形成“Au-anti-IgGAb-HCVAb-HCVAg”夹心物而凝聚显色,游离金标鼠抗人IgG抗体则在对照线处与人IgG抗体结合而富集显色。
阴性标本则仅在对照线处显色,15分钟内观察结果即可。
3.性能特征3.1用国家参考品测定,产品性能应符合以下要求。
3.1.1阴性参考品符合率:对20份阴性参考品进行检测,要求在15分钟内全部显示阴性结果。
3.1.2阳性参考品符合率:对20份阳性参考品进行检测,要求在15分钟内全部显示阳性结果。
3.1.3最低检出量:灵敏度1号1:8进行检测,要求在15分钟内显示阳性结果。
灵敏度2号1:64进行检测,要求在15分钟内显示阳性结果。
3.1.4精密性:用精密性参考品平行检测10次,均在15分钟内显示出清晰可辨的检测线,且显色深度无显著差别。
3.1.5稳定性:试剂盒置37℃20天,阴性参考品符合率、阳性参考品符合率、最低检出量和精密性均应符合要求。
3.2乙肝、人类免疫缺陷病毒(HIV)、甲肝、庚肝、戊肝、类风湿因子(RF)、红斑狼疮等各种类型的标本不会对测试产生干扰。
3.3胆红素(342.0μmol/L)、胆固醇(20.7mmol/L)、血红蛋白(5.0g/L)、甘油三酯(28.2mmol/L)),不影响检测结果。

蛋白质分离纯化的一般程序可分为以下几个步骤:(一)材料的预处理及细胞破碎分离提纯某一种蛋白质时,首先要把蛋白质从组织或细胞中释放出来并保持原来的天然状态,不丧失活性。
所以要采用适当的方法将组织和细胞破碎。
常用的破碎组织细胞的方法有: 1. 机械破碎法这种方法是利用机械力的剪切作用,使细胞破碎。
常用设备有,高速组织捣碎机、匀浆器、研钵等。
2. 渗透破碎法这种方法是在低渗条件使细胞溶胀而破碎。
3. 反复冻融法生物组织经冻结后,细胞内液结冰膨胀而使细胞胀破。
这种方法简单方便,但要注意那些对温度变化敏感的蛋白质不宜采用此法。
4. 超声波法使用超声波震荡器使细胞膜上所受张力不均而使细胞破碎。
5. 酶法如用溶菌酶破坏微生物细胞等。
(二) 蛋白质的抽提通常选择适当的缓冲液溶剂把蛋白质提取出来。
抽提所用缓冲液的pH、离子强度、组成成分等条件的选择应根据欲制备的蛋白质的性质而定。
如膜蛋白的抽提,抽提缓冲液中一般要加入表面活性剂(十二烷基磺酸钠、tritonX-100等),使膜结构破坏,利于蛋白质与膜分离。
在抽提过程中,应注意温度,避免剧烈搅拌等,以防止蛋白质的变性。
(三)蛋白质粗制品的获得选用适当的方法将所要的蛋白质与其它杂蛋白分离开来。
比较方便的有效方法是根据蛋白质溶解度的差异进行的分离。
常用的有下列几种方法:1. 等电点沉淀法不同蛋白质的等电点不同,可用等电点沉淀法使它们相互分离。
2. 盐析法不同蛋白质盐析所需要的盐饱和度不同,所以可通过调节盐浓度将目的蛋白沉淀析出。
被盐析沉淀下来的蛋白质仍保持其天然性质,并能再度溶解而不变性。
3. 有机溶剂沉淀法中性有机溶剂如乙醇、丙酮,它们的介电常数比水低。
能使大多数球状蛋白质在水溶液中的溶解度降低,进而从溶液中沉淀出来,因此可用来沉淀蛋白质。
此外,有机溶剂会破坏蛋白质表面的水化层,促使蛋白质分子变得不稳定而析出。
由于有机溶剂会使蛋白质变性,使用该法时,要注意在低温下操作,选择合适的有机溶剂浓度。

实训血浆中IgG的分离纯化[任务描述]IgG是免疫球蛋白(简称IgG)的主要成分之一,分子量约为15万~16万。
IgG是动物和人体血浆的重要成分之一。
血浆蛋白质的成分多达70余种,要从血浆中分离出IgG,首先要进行尽可能除去其他蛋白质成分的粗分离程序,使IgG 在样品中比例大为增高,然后再纯化而获得IgG。
盐析法是粗分离蛋白质的重要方法之一,是利用各种蛋白质所带电荷不同、相对分子质量不同,致使在高浓度的盐溶液中溶解度就不同,因此一个含有几种蛋白质的混合液,就可用不同浓度的中性盐来使其中各种蛋白质先后分别沉析下来,达到分离纯化的目的,这种方法称为分级盐析。
其中最常用的盐析剂是硫酸铵,本实训任务利用硫酸铵饱和溶液沉淀并分离目的蛋白质。
[任务实施]一、准备工作1.建立工作小组,制定工作计划,确定具体任务,任务分工到个人,并记录到工作表。
2.收集硫酸铵盐析血浆中的IgG工作中的必须信息,掌握相关知识及操作要点,与指导教师共同确定出一种最佳的工作方案。
3.完成任务单中实际操作前的各项准备工作。
(1)材料准备新鲜动物血清(2)试剂饱和硫酸铵溶液:取化学纯(NH4)2SO4800g,加蒸馏水1000ml,不断搅拌下加热至50~60℃,并保持数分钟,趁热过滤,滤液在室温中过夜,有结晶析出,即达到100%饱和度,使用时用浓NH4OH调至PH7.0。
0.2mol/L pH7.2的磷酸盐缓冲液(PBS)其配制方法如下:①配A液:0.2mol/L 磷酸氢二钠溶液(称取磷酸氢二钠5.37g加去离子水定容至100mL)②配B液:0.2mol/L磷酸二氢钠溶液(称取磷酸二氢钠3.12g加去离子水定容至100mL)③取A液约72mL,取B液约28mL,然后将这两种溶液边混合边用高精度pH试纸检测,调配成约100mL浓度为0.2mol/L pH7.2的磷酸盐缓冲液备用。
(3)器具离心机、烧杯(500mL和250mL)、高精度pH试纸、大容量瓶、移液管、玻璃棒等。


丙种球蛋白的电泳制备相关概念:【别名】免疫血清球蛋白,普通免疫球蛋白,人血丙种球蛋白,丙种球蛋白,静脉注射用人免疫球蛋白。
丙种球蛋白药理作用:注射丙种球蛋白是一种被动免疫疗法。
它是把免疫球蛋白内含有的大量抗体输给受者,使之从低或无免疫状态很快达到暂时免疫保护状态。
由于抗体与抗原相互作用起到直接中和毒素与杀死细菌和病毒。
因此免疫球蛋白制品对预防细菌、病毒性感染有一定的作用。
适应症:丙种球蛋白是血液中一种蛋白质,它通常来源于许多人献的血液。
含有健康人群血清所具有的各种抗体,因而有增强机体抵抗力以预防感染的作用。
主要用于免疫缺陷病以及传染性肝炎、麻疹、水痘、腮腺炎、带状疱疹等病毒感染和细菌感染的防治,也可用于哮喘、过敏性鼻炎、湿疹等内源性过敏性疾病。
电泳基本原理:电泳——带电颗粒在电场的作用下,向着与其电性相反的电极移动,称为电泳电泳技术——指利用电泳现象对混合物进行分离分析的技术。
其他电泳技术:1、纸电泳2、乙酸纤维素薄膜电泳3、凝胶电泳4、等电聚焦电泳5、等速电泳6、双向凝胶电泳(二维电泳)盘状电泳的原理:聚丙烯酰胺凝胶盘状电泳是根据被分离物质所带的电荷多少及其分子的大小,形状的不同,在电场的作用下,产生不同的速度而分离的方法。
它是利用丙烯酰胺和双丙烯酰胺在催化剂的作用下聚合成大分子的凝胶物。
它同时兼有分子筛和电泳效应。
当样品通过凝胶进行电泳时,便可以根据其样品中各分子的电荷和分子量的不同而泳动出不同的区带。
由于聚丙烯酰胺凝胶化学性质较琼脂稳定,很少带有侧基,在电泳过程中,吸附作用与电渗作用均小,所以该技术的分辨率均高于其它琼脂电泳技术盘状电泳法的三个效应:1、样品浓缩效应2、电荷效应3、分子筛效应在这三重效应的共同作用下使血清蛋白中不同的蛋白质分离开来。
影响电泳速度的因素:电场强度、溶液的pH值、溶液的离子强度、电渗作用、粒子的迁移率、吸附作用。
制备丙种球蛋白的方法丙种球蛋白的两种分离制备的方法①层析法(如:Sephadex G-50)定义:是一种利用混合物中诸组分在两相间的分配原理以获得分离的方法。

人血丙种球蛋白制造及检定规程本品系由乙型肝炎疫苗免疫健康人的血浆或血清经低温乙醇法提取的免疫球蛋白制剂。
含两种球蛋白90%以上。
用于常见病毒性感染的被动免疫,主要用于预防麻疹和传染性肝炎。
1 制造1.1制造要求与《人血白蛋白(低温乙醇法)制造及检定规程》中1.1项规定相同。
1.2制造工艺1.2.1采用低温乙醇法。
可含适宜稳定剂(如含甘氨酸,应为0.3或0.4mol/L)。
1.2.2分批每批制品最少应由1000名以上健康献血员的血浆(清)混合制成。
同一制造工艺同一空中溶解稀释的制品作为一批,不同滤器除菌过滤或不同机柜冻干的制品应分为亚批。
1.2.3半成品检定液体制剂于除菌过滤后应做理化检查(残余乙醇含量≤0.03%)及热原质试验,并按亚批抽样做无菌试验。
直接分装时应留样做上述实验。
1.2.4 冻干半成品经检定合格后及时分装,并按《人血白蛋白(低温乙醇法)制造及检定规程》1。
2。
5项规定进行冻干,但制品温度不得超过35℃。
1.3剂型与规格1.3.1剂型分为液体及冻干两种。
1.3.2规格液体制剂蛋白质浓度为10%。
液体和冻干制剂每瓶(支)蛋白质装量均为150mg、300mg。
1.4制品重滤和再制与《人血白蛋白(低温乙醇法)制造及检定规程》中1.4项相同。
2 成品检定2.1抽样每批成品应抽样做全面检定,不同机柜冻干的制品应分别抽样做无菌试验及水分测定。
2.2物理检查2.2.1外观冻干制剂应为白色或灰白色的疏松体,无融化迹象。
液体制剂和冻干制剂溶解后溶液应为接近无色,可带乳光,或淡黄色澄明液体,不应含有异物、混浊或摇不散的沉淀。
2.2.2冻干制剂溶解时间冻干制剂加入20~25℃标示量的灭菌注射用水后,应在15分钟内完全溶解。
2.2.3热稳定性试验液体制剂置57±0.5℃水浴保温4小时后,应无凝胶化或絮状物。
2.3化学检定按《生物制品化学检定规程》进行。
2.3.1水分冻干制剂的水分含量应≤3%(g/g)。
直接抗人球蛋白IgG检查标准操作程序1. 试验原理和目的将抗球蛋白血清加入已经致敏的红细胞盐水悬液中,红细胞表面的抗体球蛋白与抗球蛋白血清中的抗体发生特异性反应,通过桥梁作用使红细胞发生凝聚。
通过直接抗人球蛋白试验可检查红细胞是否被不完全抗体所致敏,如新生儿溶血病(胎儿红细胞被母亲血型抗体致敏)、溶血性输血反应(输入的不相合红细胞被受者不完全抗体致敏)、自身免疫性溶血性贫血(患者红细胞被自身抗体致敏)以及药物诱导产生的自身抗体(由甲基多巴类药物、青霉素等所致)。
2.标本要求2.1 病人准备标本采集前患者处于安静状态,避免剧烈运动、情绪激动。
2.2 标本要求2.2.1 标本种类 EDTA抗凝管2.2.2 标本容器 01开头的紫色短管(EDTA 1:9抗凝)2.2.3 标本存放采完标本立即送检,标本禁忌放入4℃~8℃冰箱中。
已检标本封盖后室温放置,保存48小时后由医院按感染标本统一处理。
2.2.3标本拒收样品标识不清或标识错误、采血管不正确、严重溶血或脂血、与申请单内容不符等标本。
3.试剂3.1 试剂成分:直接抗人球蛋白试剂盒(上海血液生物医药有限公司):抗IgG,C3d(多特异性)、抗IgG、抗C3d;理盐水。
3.2 用不完全抗D致敏的5%Rh(D)阳性红细胞盐水悬液供阳性对照用:取3人O型红细胞,混合,经盐水洗涤后取压积红细胞,加等量抗D血清,置于37℃水浴致敏1h,盐水洗涤3次,用盐水配成5%红细胞悬液。
3.3 正常人5%D阳性红细胞悬液(未致敏),供阴性对照用,除不用抗D血清致敏外,按上述方法配制。
3.4 试剂贮藏要求:2~8℃冰箱.4. 仪器4.1 日本OLYMPUS CX31普通光学显微镜。
4.2 SSW型微电脑电热恒温水槽(上海博迅实业有限公司医疗设备厂)5.操作步骤5.1 取受检者红细胞,以生理盐水洗涤3次(3000rpm 1min),末次洗涤后,将上层盐水倒尽,再以滤纸将管口附着的盐水吸去,配成5%红细胞盐水悬液。
血浆IgG的分离,纯化及鉴定实验报告一、实验目的掌握盐析法的原理及盐析法分离制备血浆IgG的基本过程。
二、实验原理1.血浆IgG是免疫球蛋白(简称Ig)的主要成分之一,相对分子质量为15~16万。
要从身姿中分离出血浆IgG,首先要尽可能除去其他蛋白质成分的粗分离程序,使血浆IgG在样品中比例大为增高,然后再纯化而获得血浆IgG。
2.盐析法是粗分离蛋白质的重要方法,许多蛋白质在纯水或低盐溶液中溶液度低,若稍加一些无机盐则溶解度增加,这种现象为盐溶。
而当盐浓度继续继续增加到某一浓度时,蛋白质又变得不溶而自动析出,这种现象称为盐析。
这现象的原理大致是由于蛋白质带有羟基解离的负电荷或氨基解离的正电荷,其极性基团使分子间相互排斥,同时与水分子形成水膜,这些因素保证了蛋白质不溶于水,当加入少量盐后,破坏水膜结构,增多了蛋白质的极性基团,蛋白质的溶解度增大,出现盐溶的现象,另一方面加入的盐离子中和蛋白质表面电荷,蛋白质分子相互蛋白质混合溶液中的各个成分分步盐析出来,达到分离目的蛋白质。
3.用硫酸铵分级盐析蛋白质时,盐析出某种蛋白质成分所需的硫酸铵浓度一般饱和度来表示。
实际工作中将饱和硫酸铵溶液的饱和度为100%或1。
盐析某种蛋白质成分所需的硫酸铵数量折算成100%或1的百分之几。
即称为该蛋白质盐析的饱和度。
4.饱和度可以用饱和硫酸铵溶液法计算:在蛋白质要求达到50%以下时采用:V=V0*(C2-C1)/(1-C2)V0--蛋白质溶液的原始体积C1--所要达到硫酸铵饱和度C2--原来溶液的硫酸铵饱和度三、试剂与仪器饱和硫酸铵溶液,0.01mol/l,PH7.0的磷酸盐缓冲溶液,0.0175mol/L,PH6.7的磷酸盐缓冲溶液四、实验步骤1.取1支离心管,向其中加入5ml血浆和5ml0.01mol/l,PH7.0的磷酸盐缓冲溶液,混合。
向其中加入2.5ml饱和硫酸铵溶液,使其饱和度为20%(加入过滴加边搅拌),加完后,应在4度放置15min,使之充分盐析,然后以3000r/min离心10min,弃去沉淀(沉淀为纤维蛋白)2.量取10ml清液体积,置于另一离心管,用滴管加入6ml饱和硫酸铵溶液,使其饱和度达到50%,加完后,4度放置15min,以3000r/min离心10min,弃去溶液,留下沉淀。
(19)中华人民共和国国家知识产权局(12)发明专利申请(10)申请公布号 (43)申请公布日 (21)申请号 201810576441.4(22)申请日 2018.06.06(71)申请人 华兰生物工程重庆有限公司地址 408000 重庆市涪陵区鹤凤大道66号申请人 华兰生物工程股份有限公司(72)发明人 刘余江 滕世超 杨柳 周康森 张宝献 李长明 (74)专利代理机构 重庆中之信知识产权代理事务所(普通合伙) 50213代理人 张景根(51)Int.Cl.C07K 16/00(2006.01)C07K 1/36(2006.01)C07K 1/34(2006.01)C07K 1/18(2006.01)(54)发明名称一种静注人免疫球蛋白的提纯方法(57)摘要本发明公开了一种静注人免疫球蛋白的提纯方法,对二次沉淀组分进行提纯,其特征在于:所述静注人免疫球蛋白的提纯方法包括如下步骤:S1溶解:用注射用水溶解二次沉淀组分,并在2.0~8.0℃下搅拌2~4小时,得溶解液;S2过滤:过滤溶解液,先用0.45μm滤膜过滤,再用0.2μm滤膜过滤,得过滤液;S3调整过滤液:调整过滤液的pH为5.60~6.00,蛋白浓度为10~13g/L,电导率为0.2~1.90ms/cm,得层析前溶液;S4层析:利用强阴离子交换凝胶进行层析,层析前,先对凝胶进行冲洗平衡:平衡至凝胶的pH与层析前溶液的pH值差值为-0.10~0.10,按照0.5~1.5cm/min线性流速,按照层析载量不高于600g/L凝胶层析上样,并收集层析后溶液。
本发明的方法简单、可控,大大降低了静注人免疫球蛋白中IgA、IgM的含量。
权利要求书1页 说明书11页CN 108623677 A 2018.10.09C N 108623677A1.一种静注人免疫球蛋白的提纯方法,对经低温乙醇法得到的二次沉淀组分进行提纯,其特征在于:所述静注人免疫球蛋白的提纯方法包括如下步骤:S1溶解:用相当于二次沉淀组分体积5~10倍的注射用水溶解二次沉淀组分,并在2.0~8.0℃下搅拌2~4小时,得溶解液;S2过滤:过滤溶解液,先用0.45μm孔径过滤,再用0.2μm孔径过滤,得过滤液;S3调整过滤液:调整过滤液的pH为5.60~6.00,蛋白浓度为10~13g/L,电导率为0.20~1.90ms/cm,得层析前溶液;S4层析:利用强阴离子交换凝胶进行层析,层析前,先对凝胶进行冲洗平衡:平衡至凝胶的pH与层析前溶液的pH值差值为-0.10~0.10;依据层析前溶液的蛋白浓度,按照层析载量不高于600g/L凝胶层析上样,控制流速为0.5~1.5cm/min线性流速,并收集层析后溶液,层析结束后,用洗脱液将吸附于凝胶上的杂质冲洗下来,得杂质溶液。
静注人免疫球蛋白制作工艺流程下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by the editor. I hope that after you download them, they can help yousolve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you!In addition, our shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts,other materials and so on, want to know different data formats and writing methods, please pay attention!静注人免疫球蛋白(IVIG)是一种通过提取健康人群血浆中的免疫球蛋白进行高效纯化制备的生物制品,具有广泛的临床应用价值。
人血清IgG分离纯化方法探讨2 IgG的纯化方法根据IgG的不同特性,IgG的纯化方法主要有以下几种:2.1 饱和硫酸铵盐析法盐析法是粗分离蛋白质的重要方法之一.因为蛋白质分子吸附某种盐离子后,其带电表层使蛋白质分子彼此排斥,而蛋白质分子与水分子间的相互作用却加强,因而溶解度提高.但当大量中性盐加入,使水的活度降低,进而导致蛋白质分子表面电荷逐渐被中和,水化膜逐渐被破坏,最终引起蛋白质分子间相互聚集并从溶液中析出.硫酸铵是盐析最常用的无机盐,其主要特点是溶解度大,随温度变化小;对蛋白质有保护作用,高浓度时可抑制微生物和蛋白酶的活性;溶解于水时不产生热量,价格低廉,但在碱性环境中不能用硫酸铵作为沉淀剂进行盐析反应.在血清中加入硫酸铵,当饱和度为28 ~33 时,优球蛋白析出;33 ~55 时,拟球蛋白析出;饱和度大于50%后,白蛋白析出,这是硫酸铵分析沉淀血清蛋白的一般规律[3].2.2 有机溶剂沉淀法有机溶剂沉淀法是利用有机溶剂能破坏溶质分子周围形成的水化层,使溶质分子脱水而相互聚集析出,也就是降低了溶质的溶解度;而且有机溶剂的介电常数比水小,随着有机溶剂的加入,整个溶液的介电常数降低,带电溶质分子之间的库仑引力逐渐增强,于是发生相互吸引而聚集.一般来说,溶质分子量越大,越容易被有机溶剂沉淀,发生沉淀所需要的有机溶剂浓度越低[7].在这主要介绍辛酸法.2.3 聚乙二醇(PEG)置换法水溶性非离子型聚合物沉淀法常用的聚合物为聚乙二醇(PEG)及右旋糖酐.水溶性聚合物沉淀蛋白质的机制还不清楚,大致有如下解释:聚合物与蛋白质形成共沉物;聚合物与蛋白质之间发生水的重分配;聚合物与蛋白质形成复合物.此法受许多因素影响,主要是pH、离子强度、蛋白质浓度和PEG的分子量等.PEG在浓度为3 ~4时可沉淀去除了蛋黄脂磷蛋白及脂质,6 ~7可沉淀IgM,8 ~12 沉淀IgG,12 ~15 沉淀其他球蛋白,25 则沉淀白蛋白.在10mL人血清中加入等量0。
A chromatographic method forthe production of a humanimmunoglobulin G solution forintravenous useK. Tanaka, E. Sawatani, Divisão de Produção e Desenvolvimento Industrial,E.M. Shigueoka, Fundação Pró-Sangue Hemocentro de São Paulo, São Paulo, SP, BrasilT.C.X.B. Campos,H.C. Nakao, G.A. Dias,R.K. Fujita and F. ArashiroAbstractCorrespondence Immunoglobulin G (IgG) of excellent quality for intravenous use was Key wordsK. Tanaka obtained from the cryosupernatant of human plasma by a chromato-•Immunoglobulin G Divisão de Produção e productiongraphic method based on a mixture of ion-exchange, DEAE-SepharoseDesenvolvimento Industrial •Hemoderivate productionFF and arginine Sepharose 4B affinity chromatography and a finalFundação Pró-Sangue Hemocentro •Intravenous gammapurification step by Sephacryl S-300 HR gel filtration. The yield of 10de São Pauloglobulin productionexperimental batches produced was 3.5 g IgG per liter of plasma. AAv. Enéas C. Aguiar, 155, 1º andar•Industrial chromatography05403-000 São Paulo, SP solvent/detergent combination of 1% Tri (n-butyl) phosphate and 1%•Downstream processTriton X-100 was used to inactivate lipid-coated viruses. Analysis of the final product (5% liquid IgG) based on the mean for 10 batches Publication supported by FAPESP.showed 94% monomers, 5.5% dimers and 0.5% polymers and aggregates.Anticomplementary activity was 0.3 CH50/mg IgG and prekallikreinactivator levels were less than 5 IU/ml. Stability at 37o C for 30 days in theliquid state was satisfactory. IgG was stored in flasks (2.5Received April 28, 1998 Accepted August 18, 1998 g/flask) at 4 to 8o C. All the characteristics of the product wereconsistent with the requirements of the 1997 Pharmacopée Européenne.Introduction or purification by ion-exchange and gel filtrationchromatography (4,5). The IgG iso-Commercially available liquid or lyo-lated from human plasma in the 1940’s and philizedimmunoglobulins G (IgG) are pro-1950’s by the method of fractionation with duced from pooledhuman plasma from a cold ethanol of Cohn and Oncley (2,6,7) was large number of donors,usually more than suitable for intramuscular use and could not one thousand, so that a widevariety of anti-be administered intravenously because of bodies will be present in the product (1). undesirable effects due to the modifications Several production processes are employed, in the IgG molecule, such as aggregates re-most of them based on the method of Cohn sulting from the fractionation process with (2), i.e., fractionation with cold ethanol, with ethanol and other agents which increased its polyethyleneglycol (PEG) precipitation (3) anticomplement activity (8,9).Thus, the major goal of IgG producers was to develop methods that would guarantee intravenous tolerance, eliminating or preventing the aggregation of molecules without affecting the activity of the antibodies present in the IgG preparation. The first process for the production of intravenous(iv) IgG was based on treatment of IgG with a quantity of pepsin (10) or plasmin (11) that caused enzymatic cleavage of IgG molecules. This process, corresponding to the preparation of first-generation iv IgG, is today considered to be obsolete (9).Specific structural changes of IgG were introduced in the enzymatic methods to re-duce anticomplementary activity. The prod-ucts thus obtained usually have reduced in vivo survival times and their continuous use may cause an antigen response depending on the enzyme used (12). The second-gen-eration iv IgG consisted of chemically modi-fied preparations with more or less impaired Fc-related effector functions (9). From 1975 to 1989, the chemical modification of the protein was probably the most successful approach to the preparation of iv IgG (12). The reagents used for this modification range from ß-propiolactone (13) to reducing/alkylating agents (12,14), reducing/amidating agents (15) and reducing/sulfonating agents (16).Today, all of these preparations are being replaced with third-generation products con-taining intact IgG molecules which retain effector functions. The latest generation in-cludes preparations that are free of comple-ment-activating aggregates thanks to the use of small or trace amounts of pepsin, pH 4.0, PEG and bulk adsorption with ion-exchange gel (9). This development was encouraged by the fact that the chromatographic method provides a safe and effective iv IgG product meeting the 1982 requirements of the World Health Organization Committee (17). The choice of a third-generation preparation which exhibits all the functions of the IgG molecule will be determined on the basis of safety in terms of viral transmission and absence of contaminants. Furthermore, the preparation should contain a normal distri-bution of IgG subclass molecules and have a half-life after infusion (9) which is in the physiological range of 21 to 36 days. Material and MethodsProduction equipmentPharmacia liquid chromatography equip-ment was employed using a Bio Process controller (Uppsala, Sweden). The following columns were used: step 1, desalting, gel filtration on Sephadex G-25, 1 column, mo-del BPSS 400/600 (60 cm in height by 40 cm in diameter). Step 2, anion-exchange DEAE-Sepharose FF, 1 column, model PS-370/15 (15 cm in height and 37 cm in diameter). Step 3, affinity gel Arginine Sepharose 4B (40%) + anion-exchange DEAE-Sepharose FF (60%), 1 column, model PS-370/15 (15 cm in height by 37 cm in diameter) and cation-exchange CM-Sepharose FF, 1 column, model Index 200/500 (15 cm in height and 20 cm in diameter). Step 4, cation-exchange CM-Sepharose FF, 1 column, model Index 200/500. Step 5, gel filtration on Sephacryl S-300, 1 column, model BPG 200/ 950 (95 cm in height and 20 cm in diameter).Other instruments used were a tangential flow ultrafiltration Pellicon cassette system and a filtration system with a stainless steel sanitary filter holder, 293 mm in diameter (Millipore, Bedford, MA, USA), and a con-tinuous flow centrifuge model AG BKA-6 (Westphalia Separator, Oeld, Germany). Details about the buffer solutions are given in Table 1.Preparation of immunoglobulin G Approximately 1200 human plasma bagsstored at -30o C were thawed at a temperature of 2 to 4o C in order to form a 200-l pool. Thawed plasma was centrifuged at 2o C toBraz J Med Biol Res 31(11) 1998obtain a cryoprecipitate to be used for the production of factor VIII. The supernatant of the cryoprecipitate was cleared by filtration through a 30-S depth filter (Zeta Plus, Cuno, Meriden, CT, USA) and desalted with coarse Sephadex G-25 filtration gel on a BPSS 400/ 600 column using 5 mM sodium acetate as elution solution. The pH was adjusted to 5.2 with 1 M CH 3CO 2H and the protein solution (410 l) was allowed to stand overnight in a cold chamber at 4oC for euglobulin precipi tation. On the following day the preparation was centrifuged at 4oC to remove the pre cipitated euglobulins and the supernatant was cleared by filtration. The pH was readjusted to 5.2 with 1 M CH 3CO 2H and conductivity was adjusted to 1.4 mS/cm with NaCl. The sample was applied to a PS-370/ 15 column containing DEAE-Sepharose FF in order to separate gamma-globulin (unadsorbed) from other plasma proteins such as albumin, etc.The gamma-globulin fraction was submitted to chromatography for the preparation of IgG as described below. The albumin fraction was further purified by a chromatographic method (18). The pH of the gamma-globulin fraction (480 l) was adjusted to pH6.0 with 1 M NaOH and conductivity was adjusted to 1.4 mS/cm. The fraction was then applied to two columns, PS-370/15 and Index 200/500, coupled in series. The first column was packed with a mixture of two gels, 40% arginine Sepharose 4B and 60% DEAE-Sepharose FF, and the second with 8 l of CM-Sepharose FF gel.Arginine + DEAE-Sepharose and CM-Sepharose FF Equilibration 20 mM sodium acetate, pH 6.0, and conductivity 1.4 mS/cm Elution of arginine + DEAE-Sepharose FF 0.5 M sodium acetate, pH 7.0, and conductivity >20 mS/cm Elution of CM-Sepharose FF 1.0 M NaCl, pH 7.0 Cleaning and storage 0.5 M NaOH and 10 mM NaOHCM-Sepharose FF (after virusinactivation) Equilibration 10 mMglycine, pH 7.0 Elution 0.1 M glycine + 0.15 M NaCl, pH 9.0 Cleaning and storage 0.5 M NaOH and 10 mM NaOHSephacryl S-300 HR 1st Equilibration 0.1 M sodium acetate + 50 mM NaCl, pH 6.0 2nd Equilibration 0.15 M NaCl, pH 6.0 Elution with2nd equilibration buffer Cleaning and storage 0.5 M NaOH and 10 mM NaOHTable 1 - Buffer solutions used. Sephadex G-25 C Equilibration 5 mM sodium acetate, pH 7.0, and conductivity 0.37 mS/cmElutionwith equilibration buffer Cleaning and storage 0.5 M NaOH and 10 mM NaOHDEAE-Sepharose FFEquilibration 20 mM sodium acetate, pH 5.2, and conductivity 1.4 mS/cm1st Elution with equilibration buffer2nd Elution25 mM sodium acetate, pH 4.5, and conductivity 1.75 mS/cmThe proteins not of interest for the presentstudy, IgM, IgA, transferrin, etc, wereadsorbed to the first column. IgG wasadsorbed to the second column and eluted andconcentrated to 5% (w/v) using the-Thawing 4o CCryoprecipitate-CentrifugationCryosupernatantDesalting on Sephadex G-25 CSephadex fraction -Euglobulin precipitation at pH 5.2 -Kept overnight at 4o C -Centrifugation -Filtration SupernatantAlbumin fraction -Purification Unadsorbed + Gamma-globulin 60% DEAE-Sepharose FF 40% Arginine-Sepharose 4 BUnadsorbed protein fractionCM-Sepharose FFIgG -Concentration to 5% (w/ v) -S/D Virus inactivationCM-Sepharose FFIgG without S/D-Enzyme treatment (0.1 mg pepsin/g of IgG, pH 4) -Temperature 37o C/12 h -pH adjustment to 6.0 with 1.0 M NaOHSephacryl S-300IgG without enzyme or polymers-Concentration to 7% (w/v) -Formulation-Sterilization by filtrationFigure 1 - Flow diagram for the production of liquid intravenous IgG.Pellicon Cassette System 30,000 NMWL. The pH of the IgG solution (16 l) was adjusted to 5.5 and the material was submitted to viral inactivation with a solvent/detergent combination (19), i.e., 1% Tri (n-butyl) phosphate and 1% Triton X-100 at 35o C for 10 h. After viral inactivation, the solvent/deter-gent combination was removed by ion-ex-change chromatography on CM-Sepharose FF in an Index 200/500 column containing 8 l of gel. The IgG solution was concentrated to 7%, pepsin was added (0.1 mg/g protein), pH 4.0, and the solution was heated at 37o C for 10 h. The preparation was cooled to 20o C and then applied to a BPG 200/950 column containing 20 l of Sephacryl S-300 HR fil tration gel to remove the aggregated IgG molecules and pepsin. The eluted IgG solution was concentrated to 6.5% and then formulated as follows: the pH was adjusted to5.0 with 1 N HCl, the conductivity to 9 to 10 mS/cm with solid NaCl, and 7.5% (w/v) maltose and 0.1 M glycine were added as stabilizers (12,20). The material was steril-ized by filtration through a 0.22-µm mem-brane (Millipore) and the final product (14 l), containing 5% protein (w/v), was bottled in 50-ml type I (neutral) flasks, with 2.5 g IgG per flask and was stored at 4 to 8o C (see Figure 1).Analytical methodsThe characteristics of normal liquid 5% IgG for intravenous use were evaluated by the methods described in the Pharmacopée Européenne (1) and in Regulation No. 2.419 of 12/17/1996 of the Brazilian Ministry of Health.The immunochemical tests were carried out by cellulose acetate electrophoresis, by the micro-Ouchterlony method and by im-munoelectrophoresis using anti-total human protein and anti-animal protein antisera of domestic species such as horses, cows and sheep. Protein concentration was determined by the biuret method, pH was measured by Braz J Med Biol Res 31(11) 1998diluting to 1% in a 0.9% sodium chloride solution, and anti-A and anti-B hemaggluti-nins were determined by the indirect Coombs method. The presence of other plasma proteins such as IgA and IgM and of the IgG subclasses IgG1, IgG2, IgG3 and IgG4 was determined by radial immunodiffusion on Bindarid plates (The Binding Site Inc., San Diego, CA, USA).Anticomplementary activity was deter-mined by the method of Mayer using guinea pig complement and sheep red cells. The prekallikrein activator was determined using the S2302 chromogenic substrate of Chro-mogenix (Mölndal, Sweden).The biological safety of the product was evaluated by tests for the detection of anti-HIV, anti-HTLV-1 and 2, anti-HCV, and HB antigens at the Serology Laboratory of Fundação Pró-Sangue Hemocentro de São Paulo. Sterility was evaluated by the Steritest membrane method (Sterility Testing System, Millipore). Pyrogenicity and toxicity were tested at Medlab, São Paulo, Brazil.The distribution of monomers, dimers and polymers was evaluated by HPLC, and anti-polio I, II and III, anti-measles and anti-herpes activities were determined at Instituto Adolfo Lutz, São Paulo, Brazil. Anti-rubeola, anti-CMV and anti-streptolysin O activities were determined at the Immunology Laboratory of IAMSPE, São Paulo, Brazil. Stability was evaluated by incubating the preparation at 57o C for 4 h and observing the presence of jelling.Results and DiscussionThe size distribution of the product, evaluated by HPLC, indicated 94% monomers, 5.5% dimers and 0.5% polymers, corresponding to standard values. The profile of the IgG molecule did not show any alterations when the IgG preparation was incubated at 37o C for one month; the anticomplementary activ ity was 0.3 CH50/mg IgG, in agreement with the specifications that determine a value of less than 1 CH50/mg IgG, and the functional activity of Fc was unchanged, presenting an index of 125 to 128% of Fc, meeting the recommendationof Pharmacopée Européenne, whichrequires >60%. On the basis of these evaluations,we concluded that the product contained intactmolecules. The subclasses determined were IgG1= 65.6%, IgG2 = 28.7%, IgG3 = 4.2%, and IgG4= 1.4%, with no significant variations comparedto normal plasma. For prekallikrein activator(PKA) determination we used a reference PKAfrom the FDA and the S2302 chromogenicsubstrate of Chromogenix. Values of less than 5IU/ml 5% (w/v) IgG were obtained, well belowthe specification limit of 35 IU/ml of PKA. Theremaining data which characterize the productare presented in Tables 2 and 3, and celluloseacetateTable 2 - Characteristics of liquid intravenous IgG.N = 10. ND, Not detected. *Pharmacopée Européenne(1).Table 1 - Buffer solutions used.Sephadex G-25 CEquilibration 5 mM sodium acetate, pH 7.0, and conductivity 0.37mS/cmElution with equilibration bufferCleaning and storage 0.5 M NaOH and 10 mM NaOHDEAE-Sepharose FFEquilibration 20 mM sodium acetate, pH 5.2, and conductivity 1.4mS/cm1st Elution with equilibration buffer2nd Elution 25 mM sodium acetate, pH 4.5, and conductivity 1.75mS/cm3rd Elution 0.15 M sodium acetate, pH 4.0, and conductivity 8.0mS/cmCleaning and storage 0.5 M NaOH and 10 mM NaOHAnalysis Results Specifications*Protein concentration 5.0 ±0.2% >90 to <110%pH determination 4.5-5.0 4.0-7.4PKA determination <5.0 IU/ml <35 IU/mlPurity (electrophoresis) >99% >95%IgA ND -IgM ND -Anti-A hemagglutinin ≤1:8 <1:64Anti-B hemagglutinin ≤1:8 <1:64 Anticomplementaryactivity<0.3 CH50/mg IgG <1 CH50/mg IgGAluminum <20 µg/ml -Fc function 125-128% >60%electrophoresis and immunoelectrophoresis data are shown in Figures 2 and 3, respectively.We describe the preparation of intravenous 5% immunoglobulins in the liquid state from a pool of human plasma from 1200 donors by the chromatographic method. The analysesperformed for quality control showed that the IgG met international specifications. In thestability test involving heat-Table 3 - Antibodies detected in liquid intravenous IgG.N = 10.Antibody Method Titer and/or uniting to 57o C for 4 h there was no jelling, and in the quarantine test involving incubation in an oven at 37o C for 4 weeks, again there were no alterations in the IgG molecule.Viral inactivation was performed with a solvent/detergent system (19), i.e., 1% Tri (n-butyl) phosphate and 1% Triton X-100, pH 5.5, at 35o C for 10 h, which was then removed by the ion-exchange gel, CM-Sepharose FF. The protein aggregates gener ated during the process were removed by gel filtration chromatography through Sephacryl S-300 HR. Thus, the product obtained consisted mainly of the monomer, presenting only trace amounts of polymers, consistent with low anticomplementary activity, i.e.,less than 1 CH50/mg IgG.Anti-polio I Neutralization test 1:256 Anti-polio II Neutralization test 1:128 Of the several stabilizing agents tested,Anti-polio III Neutralization test 1:64 glucose, sucrose and maltose, the last one at Anti-measles Hemagglutination inhibition 1:64a 7.5% concentration, and 0.1 M glycine, pH Anti-rubeola ELISA 756 IU/ml5.0 (12,20), were found to be the most ap Anti-herpes Immunofluorescence 1:128 Anti-cytomegalovirus ELISA 898 IU/ml propriate. The IgG solution was limpid andAnti-streptolysin O Nephelometry 716 IU/mltransparent with no detectable molecular al-terations even when stored for more than 2years at 5 to 8o C.On the basis of in vitro and in vivo laboratorytests, we conclude that the product fullysatisfies all the requirements of thePharmacopée Européenne (1), as well as thenorms of the Brazilian Ministry of Health(Regulation No. 2419 of December 17, 1996).We are awaiting the results of clinical testscurrently underway to liberate the productfor use.Acknowledgments009 009Figure 2 - Cellulose acetate electrophoresis. a, Plasma pool (25 µl of 10 g/l); b, immunoglo-The authors wish to thank Dr. Hiroyoshi bulin G (25 µl of 30 g/l). Protein was detected with Ponceau S and the data are reported asIto from the Japanese Red Cross, Chitose, percent of total densitometer units.Hokkaido, Dr. Komei Ohashi fromKaketsuken, Kumamoto, and Dr. KentaroNakamura from Green Cross, Osaka, forFigure 3 - Immunoelectrophoretheir helpful suggestions and the opportunitysis. a , Plasma pool (2 µl of 10 g/ l); b , immunoglobulin G (2 µl ofof training at theirPlasmaFractionation a30 g/l). Protein was detected Plants inJapan, and Dr. Erica Kitahara, withlight green stain.bPharmacia Biotech, forsupplying the reagents.Braz J Med Biol Res 31(11) 199814. Pappenhagen A, Lundblad J & Schroeder D (1975). Pharmaceutical compositionscomprising intravenously injectable modified serum globulin, its production and use. US Patent No. 3,903,262.15. Schmidtberger R (1978). Amidated immune globulins and process for preparing them. US Patent No. 4,118,379.16. Masuho Y, Tomibes S, Matsuzawa K & Ohtdu A (1977). Development of an intravenous gamma-globulin with Fc activities.I. Preparation and characterization of S-sulfonated human gamma-globulin. VoxSanguinis , 32: 175-181.17. WHO Expert Committee on Biological Standardization (1982). Report of an informal meeting on intravenous immunoglobulins (human), Geneva.18. Tanaka K, Sawatani E, Nakao HC, Dias GA & Arashiro F (1996). An alternative column chromatographic process for the productionof human albumin. Brazilian Journal of Medical and Biological Research , 29: 185-191.19. Horowitz B, Wieb ME, Lippin A & Stryker H (1985). Inactivation of viruses in labile blood derivatives. Transfusion , 25: 516522. 20. McCue JP, Hein RH & Tendold R (1986). Three generations of immunoglobulin G preparations for clinical use. Reviews of InfectiousDiseases , 8 (Suppl 4): 374-381.Table 1 - Buffer solutions used. Sephadex G-25 C Equilibration 5 mM sodium acetate, pH 7.0, and conductivity 0.37 mS/cmElutionwith equilibration buffer Cleaning and storage 0.5 M NaOH and 10 mM NaOHDEAE-Sepharose FFEquilibration 20 mM sodium acetate, pH 5.2, and conductivity 1.4 mS/cm1st Elution with equilibration buffer2nd Elution 25 mM sodium acetate, pH 4.5, and conductivity 1.75 mS/cm3rd Elution0.15 M sodium acetate, pH 4.0, and conductivity 8.0 mS/cmCleaning and storage 0.5 M NaOH and 10 mM NaOH AnalysisResults Specifications* Protein concentration 5.0 ± 0.2% >90 to <110% pH determination 4.5-5.0 4.0-7.4 PKA determination <5.0 IU/ml <35 IU/ml Purity (electrophoresis) >99% >95% IgA ND - IgMND - Anti-A hemagglutinin ≤1:8 <1:64 Anti-B hemagglutinin ≤1:8<1:64Anticomplementary activity <0.3 CH 50/mg IgG <1 CH 50/mg IgG Aluminum <20 µg/ml - Fc function125-128%>60%Molecular distributionMonomers 94.0% mono + dimers = 90%Dimers5.5%A PRIVATE BRAZILIAN BIOTECHNOLOGY COMPANYLEADER IN THE BRAZILIAN INSULINMARKETDO YOU KNOW OUR COMPANY?If you don't know us or, if you already know us, did you realize we do all that? Call us! It will be a pleasure toserve you. BIOBRÁS S.A. Fax: 55-31-335-79-88。