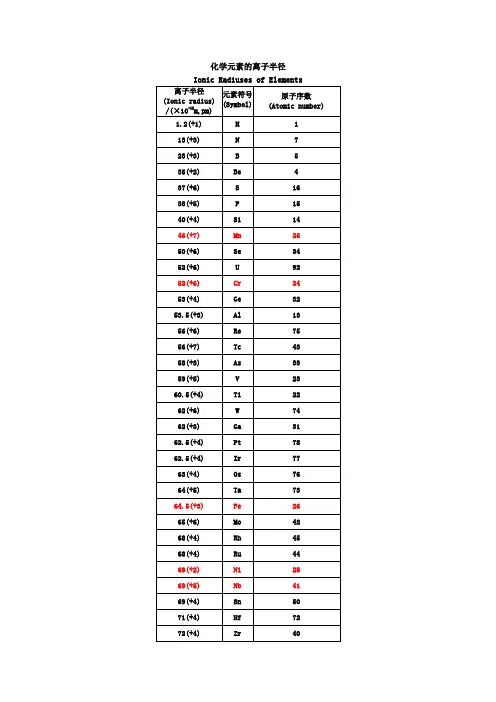
离子半径表xls
- 格式:xls
- 大小:157.00 KB
- 文档页数:22

∙0.001 纳米(nm) = 1 皮米 pm(皮米) ∙0.000 001 微米(µm) = 1 皮米∙0.000 000 001 毫米(mm) = 1 皮米∙ 1 000 皮米 = 1 纳米(nm)∙ 1 000 000 皮米 = 1 微米(µm)∙ 1 000 000 000 皮米 = 1 毫米(mm)∙ 1 000 000 000 000 皮米 = 1 米(m)离子半径数据(除注明外均为六配位,非六配位时以上标标注,如+34。
ls =低自旋,hs=高自旋。
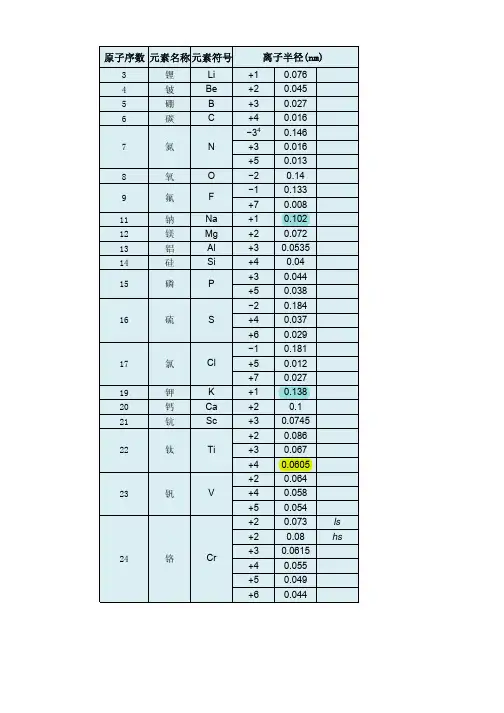
[2])原子序数元素名称元素符号离子半径pm3 锂Li +1764 铍Be +2455 硼 B +3276 碳 C +4167 氮N −34 146 +316 +5138 氧O −21409 氟 F −1133 +7811 钠Na +110212 镁Mg +27213 铝Al +353.514 硅Si +44015 磷P +344 +53816 硫S −2184+437+62917 氯Cl−1181+512+72719 钾K +113820 钙Ca +210021 钪Sc +374.522 钛Ti+286+367+460.523 钒V+264+458+55424 铬Cr+273 ls+280 hs+361.5+455+549+64425 锰Mn+2 67+3 58 ls+3 64.5 hs+4 53+5433+6425.5+7 4626 铁Fe +2 61 ls- 1 -+2 78 hs +3 55 ls +3 64.5hs +4 58.5+642527 钴Co +265 ls +274.5hs +354.5ls +361 hs +45328 镍Ni +269+356 ls +360 hs +448 ls29 铜Cu +177+273+354 ls30 锌Zn +27431 镓Ga +36232 锗Ge +273 +45333 砷As +358 +54634 硒Se −2198 +450 +64235 溴Br −1196+34sq59+5431+73937 铷Rb +115238 锶Sr +211839 钇Y +39040 锆Zr +47241 铌Nb+372+468+56442 钼Mo+369+465+561+65943 锝Tc+464.5+560+75644 钌Ru+3 68+4 62+5 56.5+7438+843645 铑Rh+366.5+460+55546 钯Pd+1259+2 86+3 76+4 61.547 银Ag+1115+294+37548 镉Cd +29549 铟In +38050 锡Sn +2112- 2 -+46951 锑Sb +376 +56052 碲Te −2221 +497 +65653 碘I −1220 +595 +75354 氙Xe +84855 铯Cs +116756 钡Ba +213557 镧La +3103.258 铈Ce +3102 +48759 镨Pr +399 +48560 钕Nd +28129 +3 98.361 钷Pm +39762 钐Sm +24122 +3 95.863 铕Eu +2117 +394.764 钆Gd +393.865 铽Tb +392.3 +47666 镝Dy +2107 +391.267 钬Ho +390.168 铒Er +38969 铥Tm+2103+38870 镱Yb+2102+386.871 镥Lu +386.172 铪Hf +47173 钽Ta+372+468+56474 钨W+466+562+66075 铼Re+463+558+655+75376 锇Os+4 63+5 57.5+6 54.5+7 52.5+843977 铱Ir+368+462.5+55778 铂Pt+286+462.5+55779 金Au+1137+385+55780 汞Hg +1119- 3 -+210281 铊Tl +1150 +388.582 铅Pb +2119 +477.583 铋Bi +3103 +57684 钋Po +494 +66785 砹At +76287 钫Fr +118088 镭Ra +2814889 锕Ac +311290 钍Th +49491 镤Pa +3104 +490 +57892 铀U +3102.5+489+578+67393 镎Np+2110+3101+487+575+672+77194 钚Pu+3100+486+574+67195 镅Am+28126+3 97.5+4 8596 锔Cm+397+48597 锫Bk+396+48398 锎Cf+395+482.1[编辑] 参见原子半径[编辑] 参考文献1.^Pauling, L.(1960). The Nature of the Chemical Bond(3rd Edn.). Ithaca,NY: Cornell University Press.2.^ 2.0 2.1Shannon, R.D.. Revised effective ionic radii and systematic studiesof interatomic distances in halides and chalcogenides. Acta. Cryst. A.1976, 32: 751–767. doi:10.1107/S0567739476001551.3.^ 通常来讲,Ag+(129 pm)的离子半径事实上比Na+(116 pm)的大。