材料概论英文版武汉理工大学
- 格式:doc
- 大小:243.00 KB
- 文档页数:8



记得把精英资料上的两套题背过1。
0 退火的定义和过程:Annealing, in glassblowing and lampworking, is the process of heating, and then slowly cooling glass to relieve internal stresses热处理退火过程中,退火是消除或减少玻璃的热应力至允许值的热处理过程。
Annealing is to heat treat glass near the softening point to relieve stresses.Then to cool more slowly back through the transition region so as to not reintroduce thermal stresses。
Annealing takes time and temperature:1.Thicker glass anneals more slowly, has larger stresses2。
Thinner glass anneals more quickly, has smaller stresses3.Glass with large thermal expansion requires more annealing2.0 材料的力学性质:Mechanical properties of Materials is the behavior of materials deformed by a set of forces。
Strength强度、硬度 Hardness、韧性 Toughness、2.1 硅酸盐水泥的主要的化学成份:(氧化物成份+矿物成份)There are four major minerals in Portland cement clinker(熟料):C3S—3CaO+SiO2 (硅酸三钙)C2S—2CaO+SiO2 (硅酸二钙)C3A—3CaO+Al2O3(铝酸三钙)C4AF—4CaO+Al2O3+Fe2O3 (铁铝酸四钙)Atten. C = CaO, S = SiO2, A = Al2O3, F = Fe2O32.2 物相的定义:a phase is a state of aggregation of matter that has a distinctive structure。


6.2.2 Porosity and DensityHello, everybody, in this Section, we are going to talk about porosity and density.译文:在这一节,我们将讨论气孔率和密度。
When referring to a solid material such as a part made from copper or stainless steel, the word density takes into consideration the microstructures that contains no porosity. By which term we do not mean the voids or vacancies in the atomic structure. In speaking of a solid material we mean the material’s theoretical density or mass density, it is the mass of a material divided by its volume.我们通常说到密度,指的是理论密度,表示结构中不包含气孔。
通过质量除以体积得到。
译文:当提到一种固体材料,尤其是由铜或不锈钢制成的材料时,密度这个词通常会考虑到一个没有气孔率的微观结构,在这个词中,我们指的不是原子结构中的孔洞或空位。
我们通常说到密度,指的是理论密度,表示结构中不包含气孔。
通过质量除以体积得到。
There are two factors influence the density of material. Atomic weight is a major factor in determining the density of the materials. Low-atomic-weight elements have low densities. 影响材料密度的因素有两个。
Material: Solids used by man kind to produce items which constitute the support for his living environmentCharacteristics of materials◆Have certain compositions;◆Can be processed;◆With certain shape and color;◆Can be used and reused or recycled.◆特点:☐具有一定的成分和配比;☐可成型加工;☐保持一定形状和外观;☐具有使用价值并可回收再利用。
材料性能的决定因素◆组成材料的各元素的原子结构,◆原子间的相互作用、相互结合,◆原子或分子在空间的排列分布和运动规律,◆原子集合体的形貌特征。
Classification of materials◆Atomic structures◆Nature of chemical bonds:☐ Metallic bond 金属键☐ Ionic bond 离子键☐ Covalent bond 共价键☐ Secondary bond 次价键☐ Van der Waals bond 范德华力☐ Hydrogen bond 氢键Classification:◆metals and their alloys:- metallic bonding◆Organic polymers: Covalent bonding & secondary bonding◆Ceramics:Ionic bonding & covalent bondingMetals and their alloys:◆ are good conductors of heat and electricity;◆ are opaque to visible light;◆ are hard, rigid;◆ can undergo plastic deformation◆ have a high melting temperature (Tm).Organic polymers:◆made up of long-chain molecules;◆ are electrical and thermal insulators;◆ are light and easily formable;◆ the best-known organic polymers are:☐ poly (vinyl chloride) (聚氯乙烯,PVC);☐ polyethylene (聚乙烯,PE);☐ polystyrene (聚苯乙烯,PS)。

and a paste composed of Portland cement and water. The aggregates generally used are sand and gravel or crushed stone. These aggregates have no cementing value of their own; their function in concrete is to serve as a filler. The cement-water paste changes from a semifluid substance into a solid binder as a result of chemical reactions between the water and the various compounds in the cement. The final quality of the concrete depends upon the effectiveness of the hardened paste binding the aggregate particles together and in filling the voids between the particles.How many types of raw materials involving in concrete production? And what are their functions, respectively?Analyze the major factors affecting concrete quality.strain plots are shown in Figure 1. One of the lines shows the character of fiber. Strain, △L /LS t r e s s , (N ·c m -2)Fiber(1) (2)(3)标准答案(B卷)一、Fill in the blanks (1’×10 = 10’)1.The advantage of microwave heating is that it is extremely rapid .2.The mechanical properties of ceramics are good in compression , The greatest weakness is brittleness .3.Ferrous Alloys normally contains major phases, called austenite, cementite, graphite, martensitic.4.The components as well as the interface between them can usually be physically identified.5.Surface treatment of reinforcing materials is done to improve the adhesion of fillers and fibers tomatrix resin by modifying the surface of the solid.6.The properties of materials are defined by the nature of their chemical bonds, their atomic ordering andtheir microstructure.二、True or false (indicate with “√” or “×”, 1’×10 = 10’)1.(×) All amorphous materials are glasses.2.(×) Aluminum alloys offer superior specific strength in high temperatures.3.(√) The properties of crystals are different in various crystallographic directions.4.(√) Titanium alloys have lower density and high strength.5.(√) Most of life and industrial glass are made from silica and silicate glasses.6.(√) Magnesium is the lightest of all structural metals.7.(√) Glasses is made from elements, simple chemical compounds, complex organic molecules, salt mixturesand alloys.8.(√) Glass when newly formed, with a perfect surface, is very strong about five times as strong as steel.9.(×) MMC、PMC、CMC are all composite, and matrix are metal, ceramic and organic polymer.10.(√) 玻璃化温度和熔点是表征聚合物热性能的重要指标。

Material: Solids used by man kind to produce items which constitute the support for his living environmentCharacteristics of materials◆Have certain compositions;◆Can be processed;◆With certain shape and color;◆Can be used and reused or recycled.◆特点:☐具有一定的成分和配比;☐可成型加工;☐保持一定形状和外观;☐具有使用价值并可回收再利用。
材料性能的决定因素◆组成材料的各元素的原子结构,◆原子间的相互作用、相互结合,◆原子或分子在空间的排列分布和运动规律,◆原子集合体的形貌特征。
Classification of materials◆Atomic structures◆Nature of chemical bonds:☐ Metallic bond 金属键☐ Ionic bond 离子键☐ Covalent bond 共价键☐ Secondary bond 次价键☐ Van der Waals bond 范德华力☐ Hydrogen bond 氢键Classification:◆metals and their alloys:- metallic bonding◆Organic polymers: Covalent bonding & secondary bonding◆Ceramics:Ionic bonding & covalent bondingMetals and their alloys:◆ are good conductors of heat and electricity;◆ are opaque to visible light;◆ are hard, rigid;◆ can undergo plastic deformation◆ have a high melting temperature (Tm).Organic polymers:◆made up of long-chain molecules;◆ are electrical and thermal insulators;◆ are light and easily formable;◆ the best-known organic polymers are:☐ poly (vinyl chloride) (聚氯乙烯,PVC);☐ polyethylene (聚乙烯,PE);☐ polystyrene (聚苯乙烯,PS)。

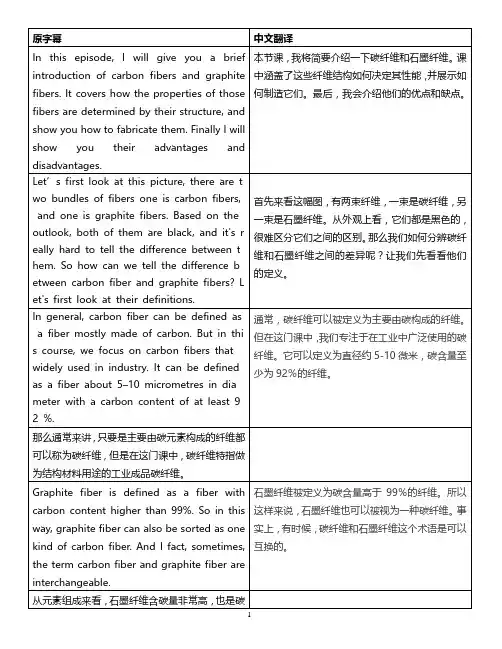
原字幕 中文翻译In this episode, I will give you a brief introduction of carbon fibers and graphite fibers. It covers how the properties of those fibers are determined by their structure, and show you how to fabricate them. Finally I will show you their advantages and disadvantages.本节课,我将简要介绍一下碳纤维和石墨纤维。
课中涵盖了这些纤维结构如何决定其性能,并展示如何制造它们。
最后,我会介绍他们的优点和缺点。
Let’s first look at this picture, there are t wo bundles of fibers one is carbon fibers, and one is graphite fibers. Based on the outlook, both of them are black, and it's r eally hard to tell the difference between t hem. So how can we tell the difference b etween carbon fiber and graphite fibers? L et's first look at their definitions.首先来看这幅图,有两束纤维,一束是碳纤维,另一束是石墨纤维。
从外观上看,它们都是黑色的,很难区分它们之间的区别。
那么我们如何分辨碳纤维和石墨纤维之间的差异呢?让我们先看看他们的定义。
In general, carbon fiber can be defined as a fiber mostly made of carbon. But in thi s course, we focus on carbon fibers that widely used in industry. It can be defined as a fiber about 5–10 micrometres in dia meter with a carbon content of at least 92 %.通常,碳纤维可以被定义为主要由碳构成的纤维。

GlassIt is well known that glasses play an important role as one of building materials ordinary living products. Advanced and specialty glasses also play important roles in several industries. In the last several years, these materialshave continued to find new applications in the areas of telecommunications, electronics, and biomedical uses. Glass compositions and processing techniques continue to evolve to suit the increasing number of applications. Some of the glass compositions have distinctive properties that make them the most preferred materials for certain applications, such as optical fibers, electronic displays, biocompatible implants, dental posterior materials, and high-performance composites.众所周知,玻璃作为建筑材料普通生活产品之一起着重要的作用。
先进和特种玻璃也在许多行业也起着重要作用。
在过去几年中,这些材料继续在电讯,电子,生物医学领域发现新的应用。
为适应日益剧增的应用,玻璃的组分和加工技术不断发展。
Chapter 1 An Overview第一章概述1.1 Introduction1.1介绍Materials are the matter of the universe. These substances have properties that make them useful in structures, machines, devices, products, and systems. The term properties describe behavior of materials when subjected to some external force or condition. For example, the tensile strength of a metal is a measure of the material's resistance to a pulling force. The Family of Materials consists of four main groups of materials: Metals (e.g., steel), Polymers (e.g., plastics), Ceramics (e.g., porcelain), and Composites (e.g., glass-reinforced plastics). The materials in each group have similar properties and/or structures, as will be described later.材料是宇宙的物质。
这些物质的特性使其有用的结构、机器设备、产品和系统。
这个术语属性描述材料的行为时,受到一些外部力量或状态。
例如,抗拉强度的金属是测量的材料抵抗了拉力。
这个家庭的材料由四个主要群体的材料:金属(如钢)、高分子材料(例如:塑料)、陶瓷(如瓷),复合材料(例如,增强塑料)。
武汉理工英文文献如下:The School of Foreign Languages and Literature at Wuhan University of Technology is one of the oldest schools within the university, with a history that can be traced back to the Ziqiang Institute. The school is responsible for teaching and research in the field of foreign languages and literatures, which includes English language and literature.The curriculum at the School of Foreign Languages and Literature typically covers a wide range of topics in linguistics, literature, translation studies, and cultural studies. Students are likely to study both the theoretical foundations of language and literature as well as practical skills such as language proficiency, translation, and interpretation.In addition to undergraduate programs, the school may also offer graduate programs leading to master's and doctoral degrees. These programs usually involve more specialized research and may include topics such as comparative literature, world literature, and advanced language education methodologies.For those interested in accessing English literature or other scholarly works related to the field, the school may have a library or resource center that contains a collection of books, journals, and digital materials. These resources can be invaluable for students and researchers looking to conduct research or deepen their understanding of the subject matter.If you are seeking specific information on courses, faculty, or research opportunities related to English literature at Wuhan University of Technology, it would be advisable to visit the university's official website or contact the School of Foreign Languages and Literature directly for the most up-to-date and detailed information.。
材料概论(双语)知到章节测试答案智慧树2023年最新东华理工大学第一章测试1.结构材料和功能材料是依据材料组成来划分的。
参考答案:错2.Metals in general have high electrical conductivity, high thermal conductivity,and high density.参考答案:对3.金属材料中不含非金属元素。
参考答案:错4.铜是黑色金属。
参考答案:错5.锌是有色金属。
参考答案:对6.以下哪种是黑色金属?参考答案:铁第二章测试1.哪种原料为陶瓷体提供玻璃相?参考答案:长石2.哪种原料为陶瓷体提升白度?参考答案:石英3.陶瓷瓶的制作一般采用注浆成型。
参考答案:对4.干燥的方法主要排除坯体中的哪类水?参考答案:自由水5.Glass is a non-crystalline product of fusion which has been cooled to a rigidcondition.参考答案:对6.水泥生产用回转窑的高端是进料口,低端是燃料进口。
参考答案:对7.下列哪个不是硅酸盐水泥的化学组成?参考答案:氧化钠8.下列哪些是硅酸盐水泥的矿物组成?参考答案:硅酸三钙;铝酸三钙;硅酸二钙第三章测试1.高碳钢的碳含量在百分之零点六到百分之一点四之间。
是一类硬度最大、强度最高、延性最差的碳钢。
参考答案:对2.哪种元素是不锈钢不锈的原因?参考答案:铬3.黄铜的主要合金元素是什么?参考答案:锌4.青铜的主要合金元素是什么?参考答案:锡5.黄铜的强度比青铜要高。
参考答案:错6.相的状态可以是固体、液体,或者是气体。
可以是一类纯物质或包含几种组成的溶液。
参考答案:对7.相图是用以描述一个材料系统在不同温度、压力和组成下存在什么相的图形表示。
参考答案:对8.二元匀晶合金体系的两种成分在固液两态下能完全互溶。
参考答案:对9.共析钢的碳含量是多少?参考答案:0.8%10.Substitutional solid solution是指间隙固溶体。
As a key university under the direct administration of the Education Ministry, Wuhan University of Technology (WUT) has been one of the "State 211 Project" institutions. Under the auspices of this program, WUT has achieved great advances as an institution of higher education, particularly in science and technology related fields.WUT is located in Wuhan, the biggest metropolitan city along the Yangtze River, in central China. Renowned as "the Thoroughfare Leading to Nine Provinces", Wuhan serves as the biggest inland junction of water, road and air transportation and offers an abundance of natural resources both regionally and within the city itself. The city is also famous as one of the foremost center for science, education and culture in Central China. As one of the most important universities in Wuhan, WUT occupies an area of 267 hectares with the total construction area of over 1.52 million square meters. It has numerous campuses spread through the Wuhan metropolitan area.WUT has formed a system of diverse disciplines, including engineering, science, literature, management, economics, law, philosophy, history, education, medicine and many more. There are 79 undergraduate programs, 153 postgraduate programs (including MBA, MPA and MFA), 62 doctoral programs and 13 post-doctoral research stations. WUT has also established 3 national key laboratories and industrial experiment bases as well as 24 provincial/ministerial key laboratories and engineering research centers.WUT has attached great importance to the development of its faculty. Currently, there are approximately 6,000 full time employees, among whom there are 3,000 faculty members, including 582 professors, 1,173 associate professors, 6 academicians both of the Chinese Academy of Sciences and the Chinese Academy of Engineering, 2 scholars of the "One-thousand-talent Plan", 2 chief scientists of the "973 Program" and the "Important Sci-tech Research Plan", 5 Cheung Kong scholars, 13 Chutian Scholars, 75 young experts with prominent national and provincial contributions, 4 experts of the "National Excellent Youth Foundation", 9 experts of the "New CenturyOne-hundred-million-talent Project" and 212 experts with special governmental allowances. In total, there are 36,100 undergraduate, 16,200 postgraduate and doctorate students and 450 overseas students.In recent years, WUT has undertaken massive projects in collaboration with the National Natural Science Foundation, National 973 Program, National 863 Program, National Sci-tech Supporting Plan as well as ministerial and key provincial research projects. By grasping this myriad of opportunities to collaborate with industry and government, WUT has generated an annualincrease in its research fund, which reached RMB 420 million in 2008. In that same year, WUT also obtained 57 scientific and technological achievement prizes at the national, ministerial and provincial levels, 205 granted patents together with 1,223 Sci-tech papers indexed by SCI, EI and ISTP. Over the years, a series of advanced research achievements have been attained in a diverse array of applied and research oriented fields including fiber optic sensors, high-speed ship engineering, electric vehicles, new materials, harbor machinery and advanced manufacturing. The WUT Sci-tech Park, located in Wuhan-China Optics Valley, has an area of 53.3 hectares, contributing an income of RMB 1.08 billion in 2008.On the basis of cultivating high-quality and adaptable talent, great motivation and a strong atmosphere of innovation, WUT has demonstrated its dedication to the improvement of students' overall qualities and innovation abilities. Moreover, a set of national experimental teaching and demonstration centers (Material Science and Engineering Experiment Teaching Center, Mechanical and Electronic Engineering Experiment Training Center, National Chemical Instruction Base for Fundamental Courses of Engineering, National College Students' Culture and All-round Development Education Base, 3 Innovative Experiment Bases for National Talent Cultivation Mode) have become more and more mature, continually producing cutting edge educational achievements. Furthermore, WUT is also a model university for the National College English Teaching Reform. In 2007, WUT was graded as having excellent achievement in undergraduate education by the Higher Education Evaluation Committee of the Ministry of Education. Up until now, there have been 16 national excellence courses, 63 provincial and ministerial excellence courses, 9 national excellence teaching achievements and 68 provincial and ministerial teaching achievements. The post-graduation employment rate has been kept stable at over 94%.WUT has and continues to greatly emphasize internationalization. The university has established programs with over 100 universities and research institutes in the US, the UK, Japan, Australia, Germany, Holland, Russia, France and Italy. WUT has also invited distinguished scholars from all over the world to give lectures on campus and granted 200 of them guest and honorary professorships. Each year, as many as 1,000 foreign visiting scholars spend time on WUT's campuses, 600 students go abroad for further education and almost 400 teachers spend time overseas for visiting and cooperative research fellowships.WUT has also developed strong ties with industrial and governmental sectors. To this end, the university has successfully established 3 Boards ofDirectors supported by enterprises in the building materials and construction engineering, transportation and automotive industries. These boards include representatives of 65 key businesses in the building materials sector, 50 in transportation industry and 66 in automotive industry in China. There are also now 52 Alumni Associations collaborating with the General WUT Alumni Office and 95 local-governmental joint bases for production, study and research throughout China's 32 provincial administration areas. WUT holds regular meetings of the board of industries and the board of alumni.With the principle of "Educating People First and Academic Advancement Prioritized", WUT has always been striving to keep her motto--"Sound in Morality, Broad in Learning and Pursuing Excellence". The university also endeavors to extend itself in four fundamental directions: promoting individuality, harmony, internationalization and innovation. WUT presently aims to transform itself into a first-class university in China and establish some of its featured programs as being among the best in the world. In attaining these goals, WUT will be an open, multi-disciplinary, and individualized research-oriented university by 2020.。
Material:Solids used by man kind to produce items which constitute the support for hisliving environmentCharacteristics of materials◆Have certain compositions;◆Can be processed;◆With certain shape and color;◆Can be used and reused or recycled.◆特点:☐具有一定的成分和配比;☐可成型加工;☐保持一定形状和外观;☐具有使用价值并可回收再利用。
材料性能的决定因素◆组成材料的各元素的原子结构,◆原子间的相互作用、相互结合,◆原子或分子在空间的排列分布和运动规律,◆原子集合体的形貌特征。
Classification of materials◆Atomic structures◆Nature of chemical bonds:☐Metallic bond 金属键☐Ionic bond 离子键☐Covalent bond 共价键☐Secondary bond 次价键☐Van der Waals bond 范德华力☐Hydrogen bond 氢键Classification:◆metals and their alloys:- metallic bonding◆Organic polymers: Covalent bonding & secondary bonding◆Ceramics:Ionic bonding & covalent bondingMetals and their alloys:◆are good conductors of heat and electricity;◆are opaque to visible light;◆are hard, rigid;◆can undergo plastic deformation◆have a high melting temperature (Tm).Organic polymers:◆made up of long-chain molecules;◆are electrical and thermal insulators;◆are light and easily formable;◆the best-known organic polymers are:☐poly (vinyl chloride) (聚氯乙烯,PVC);☐polyethylene (聚乙烯,PE);☐polystyrene (聚苯乙烯,PS)。
Composite materials: Are constituted by two or more different materials with specific properties.➢Glass fiber reinforced resins:lightweight composites with highmechanical strength➢Concrete:an agglomeration of cement, sandand gravelMechanical properties:☐The behavior of materials deformed by a set of forces.☐弹性Elasticity☐塑性Plasticity☐强度Strength☐硬度Hardness☐韧性Toughness☐疲劳特性Fatigue behavior☐耐磨性Abrasion resistancePhysical properties:◆The behavior of materials subjected to the action of temperature, electric ormagnetic fields, or light.☐电性能Electric properties☐磁性能Magnetic properties☐热性能Thermal properties☐光性能Optical propertiesChemical properties:◆The behavior of material in a reactive environment.◆抗腐蚀能力Corrosion resistance☐Atmospherically,☐Chemically (salts, acid, alkali)Materials Engineering:◆Concerned with☐Manufacturing,☐Transformation☐Shaping☐Processing.The three most common crystal structures of metals are☐Body-centered cubic structure,☐Face-centered cubic structure,☐Hexagonal close-packed structureProduction of steel:◆Pig iron contains too much carbon and too many impurities to be used directly inmost applications.◆It must be converted to steel in one of several types of converters. Theseconverters may differ in appearance, but they do the same thing:☐burn off the carbon in the iron.☐Impurities are removed as gases and in the slag.☐Phosphorus and sulfur are reduced to less than 0.05 percent of the steel.☐Manganese content is reduced to an amount from 0.2 to 2.0 percent;☐Silicon is reduced to an amount from 0.01 to 3.5 percent.Three primary ores are☐magnetite,☐hematite,☐taconite铸铁概念:cast iron, essentially an alloy of iron, carbon and silicon, is composed of iron and from 2 to 6.67 percent carbon, plus manganese, sulfur, and phosphorus, and shaped by being cast in a mold.The types and properties of cast iron ;white cast iron( hard , brittle ) , gray cast iron (brittle ,withstand large compressive loads but small tensile loads ), alloy cast iron , nodular or ductile cast iron (good castability ,toughness, good wear resistance ,low meltingpoint ,and hardenability ), malleable cast iron (strength ,toughness, ductility ,and machinability)Stainless Steel :●Ferritic●Martensitic●Austenitic●PH Alloysadvantages of using Al:one-third of the weight of steel ;good thermal and electrical conductivity ;high strength-to-weight ratio ;can be given a hard surface by anodizing and hard coating ;most alloys are weldable ;will not rust ;high reflectivity ;can be die cast ;easily machined ;good formability; nonmagnetic ;nontoxic and one –third of the stiffness of steel.Magnesium:1.The lightest of all structural metals2.Specific gravity:1.753. Magnesium weighs 1.5 times less than an equal volume of aluminum and 4 times less than zinc.4.High strength, stiffness, dimensional stability,5.High strength-to-weight ratio. Magnesium alloys have☐relatively high thermal and electrical conductivities;☐good energy absorption characteristics;☐Nonmagnetic propertiesThe four types of stresses:ductility, tensile strength, proportional limit, elastic limit, modulus of elasticity, resilience, yield point, yield strength, ultimate strength, and breaking strengthFour basic types of stresses : tensile, compressive, shear ,torsion (拉力,压力,剪切力,扭转力)Metals and nonmetals◆Ability to donate electrons and form a positive ion;◆Crystalline structure – grain structure;◆High thermal and electrical conductivity;◆Ability to be deformed plastically, and◆Metallic luster or reflectivityCrystalline unit structures◆Body-centered cubic (BCC)◆Face-centered cubic (FCC)◆Hexagonal close-packed (HCP)◆Cubic◆Body-centered tetragonal◆RhombohedralThere are several factors that may speed the corrosion process. Among these factors are ☐an increase in temperature,☐the presence of certain gases,☐environmental factors such as acid rain,☐metal fatigue,☐cold working/forming,☐and other similar factorsCeramic compounds:Can be defined as inorganic compounds made by heating clay or other mineral matter to a high temperature at which they partially melt and bond together Ceramics Classification☐traditional ceramics: derived and processed from clay or nonclay minerals including refractories(耐火材料), white wares, cement(水泥), porcelain(瓷器), and structural clay ceramics.☐advanced ceramics: high purity, better mechanical, electrical, magnetic, and optical propertiesNature of Ceramics◆Crystalline solids composed of metallic and nonmetallic materials◆Ceramics are crystal structures made of metallic ions and Inorganic materials◆Bonding is either partially or completely ionic.◆Variables include☐The magnitude of the electrical charge on the ions;☐The relative size of the ionsProperties of Ceramics◆Corrosion resistance, chemical inertness, thermal shock resistance wear-resistance◆Electrical properties: solid electrolytes in experimental batteries and fuel cells◆Other uses: include automotive sensors, packaging for integrated circuits,electronic/optical devices, fiber optics, microchips, and magnetic headsPorosity and Density◆Mass density, which uses the mass of a material divided by its volume refers to thistheoretical density◆Atomic weight is a major factor in determining the density of a material◆Close-packed metals are more dense than open-structured material s◆The optimum density of spherical particles can be reached by varying the sizedistribution of particles to permit smaller particles (50 nm or less) to locate in the interstices (空隙) of the larger ones.Ceramic Processing◆Traditional Processing☐structural clay products and the whitewares.☐Formation → Drying →Firing◆Advanced Ceramic Processing1.制备工艺:不再以熔炉为主要烧结工具, 如真空烧结、气氛烧结、热压烧结、气压烧结等。