分析ICP-MS法快速检测农产品中微量有害元素铅镉砷
- 格式:pdf
- 大小:2.92 MB
- 文档页数:1

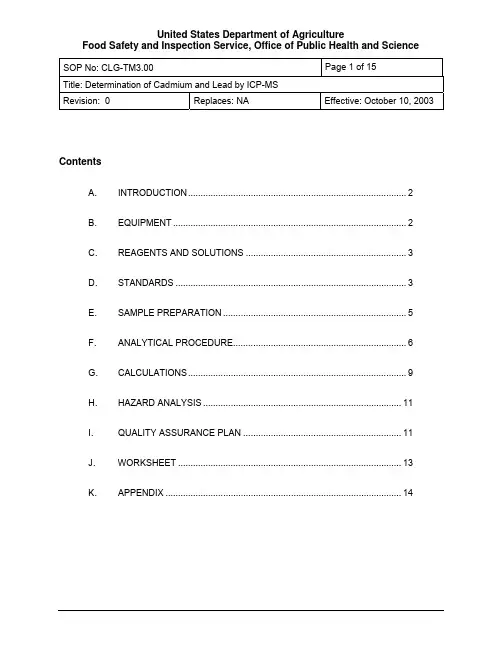
Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003ContentsA. INTRODUCTION (2)B. EQUIPMENT (2)C. REAGENTS AND SOLUTIONS (3)D. STANDARDS (3)PREPARATION (5)E. SAMPLEPROCEDURE (6)F. ANALYTICALG. CALCULATIONS (9)ANALYSIS (11)H. HAZARDPLAN (11)I. QUALITYASSURANCEJ. WORKSHEET (13)K. APPENDIX (14)Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003A. INTRODUCTION1. TheorySample tissue is digested with concentrated nitric acid in a microwave digestionapparatus. The resulting sample digest is diluted, fortified with internal standards, and analyzed using inductively coupled plasma mass spectrometry (ICP-MS).2. ApplicabilityThis method is applicable to the analysis of cadmium (Cd) and lead (Pb) at ppb levels in beef, pork and poultry muscle, liver, and kidney.B. EQUIPMENTNote: Equivalent equipment may be substituted.1. Apparatusa. Analytical balance - sensitive to 0.1 mg.b. Microwave digestion system - Mars5 System, CEM.c. Microwave digestion vessel - high pressure, 100 ml capacity. Model XP-1500,with TFM liners, CEM.d. Vacuum Concentration/Drying apparatus - Microvap accessory set for Mars5system, CEM.e. Stirring rods - Teflon or polypropylene.f. Volumetric flasks - polypropylene or polymethylpentane, 50 and 100 mL.g. Volumetric flasks - glass, 10 - 1000 mL, as needed for preparation of standards,reagents.h. Micropipettors - fixed or variable, covering ranges 10 -1000 µL.i. Bottles - polypropylene, 100 and 250 mL.j. Centrifuge tubes - polypropylene, 15 and 50 mL.k. Argon gas, high purity grade (99.99%).l. Syringe filter - Acrodisc CR 13 mm, with 0.2 um PTFE Membrane, Gelman Laboratory.2. InstrumentationInductively Coupled Plasma Mass Spectrometer - Agilent model 7500a.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003C. REAGENTS AND SOLUTIONSNote: Equivalent reagents/solutions may be substituted.1. Reagentsa. Millipore water - Deionized water polished to ASTM CAP/NCCLS Type 1specifications or better (resistance ≥ 18 megohms).b. Nitric acid - concentrated, ultra-pure, stored in Teflon. Optima by Fisher orDouble Distilled by GFS.c. Sodium Hydroxide - reagent grade.2. Solutionsa. 25% NaOH solution (for evaporation scrubber):Weigh 250 grams of NaOH into a 1 L volumetric flask and bring up to volumewith water.b. 1% and 2% HNO3 solutions:Dilute ultra-pure nitric acid 1:100 and 1:50, respectively, with Millipore water.Prepare and store in polypropylene bottles.D. STANDARDSNote: All elemental standard and internal standard solutions are prepared fromcommercially available reference standard materials. Standard solutions must be ICP-MS grade.1. Sourcea. Elemental standard solutions are available from:i. SPEX CertiPrep 203 Norcross Avenue, Metuchen, NJ 08840.ii. Inorganic Ventures, Inc. 195 Lehigh Ave, Suite 4, Lakewood, NJ · 08701iii. Mass spectrometer tuning solution is available from Agilent Technologies 2. Preparation of Standard SolutionsImportant: Metals may adsorb onto glass surfaces. Store all solutions in polypropylene or other inert containers.a. Internal standard (ISTD), Indium and Terbium, 5000 µg/LAdd 50 mL each of 10,000 µg/L indium and terbium standards to a 100 mLpolypropylene bottle.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003 Note: Other internal standards can be used as long as the element is notcontained in the sample, the mass number is close to that of the analyte, and theionization potential is close to that of the analyte.b. CalibrationStandardsPrepare calibration standards for constructing a multipoint standard curvecovering the range of analyte concentrations anticipated in samples.Prepare intermediate standards by making dilutions of commercially available1000 mg/L standard solutions into 2% HNO3. Suggested concentrations are:i. 10,000µg/L - Pipet 100 µL of 1000 mg/L standard to a 10 mL volumetric flask and dilute to volume.ii. 1000µg/L - Pipet 100 µL of 1000 mg/L standard to a 100 mL volumetric flask and dilute to volume.iii. 100µg/L - Pipet 1000 µL of 10,000 µg/L solution to a 100 mL volumetric flask and dilute to volume.Prepare calibration standards by making appropriate dilutions of intermediatestandards with 2% HNO3 and adding sufficient 5000 µg/L ISTD to result in a finalISTD concentration of 5 µg/L. Prepare these standards using polymericvolumetric flasks.The Table below lists some suggested concentrations for calibration standardsand recommended volumes and concentrations of solutions required forpreparation of 100 mL volumes of each.Calibration STD [Sample Equivalent* in ( )] Amount used x IntermediateStandard concentrationAmountISTDCalibration Blank (0 ppb) (2% HNO3 Only) 100 µL0.05 µg/L (5 ppb) 50 µL x 100 µg/L 100µL0.10 µg/L (10 ppb) 100 µL x 100 µg/L 100µL0.20 µg/L (20 ppb) 200 µL x 100 µg/L 100µL0.50 µg/L (50 ppb) 500 µL x 100 µg/L 100µL1.00 µg/L (100 ppb) 100 µL x 1000 µg/L 100µL2.00 µg/L (200 ppb) 250 µL x 1000 µg/L 100µL5.00 µg/L (500 ppb) 500 µL x 1000 µg/L 100µL10.00 µg/L (1000 ppb) 1000 µL x 1000 µg/L 100µL*Equivalent Analyte concentration in a sample, assuming a sample concentration of 0.01 g/mL (0.5 g/50 mL) in final extract.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003c. Quality Control StandardsPrepare Quality Control standards from commercially available 1000 mg/Lstandard solutions obtained from a different source than that used to prepareCalibration Standards. Two types of quality control standard must be prepared:Standard:i. QCPrepare a combined Pb/Cd standard having concentrations near themidpoint of the calibration curve, but different from those used in anycalibration standard. Prepare in same manner calibration standards areprepared, diluting with 2% HNO3 and adding sufficient 5000 µg/L ISTD toresult in a final ISTD concentration of 5 µg/L.Standard:ii. FortificationPrepare a combined Pb/Cd standard suitable for preparation of positivecontrols by making quantitative dilutions of the 1000 mg/L standard into2% HNO3. Prepare solutions at a concentration sufficient to permit afortification volume between 25 - 250 µL to be used. A 500 µg/Lfortification standard, for example, would be applicable over a 25-250 ppbrange, based on a nominal sample weight or 0.5 g.d. Mass Spectrometer Tuning SolutionThis is a commercially available 10 ng/mL solution of Lithium, Yttrium, Cerium,Thallium, and Cobalt in 2% HNO3. (Agilent Cat # 5184-3566).3. Storage and Stability.a. All standards may be stored at room temperature.b. Commercially available standard solutions may be used until their expirationdate.c. Solutions made from these may be used up to the earliest expiration date of anystandard used to prepare them.PREPARATIONE. SAMPLENote: Since trace amounts of lead and cadmium are ubiquitous in the environment andmay be present in dust particles, efforts should be made to avoid external contamination.All areas involved in sample preparation and analysis should be kept as dust-free aspossible to minimize the chance of sample or apparatus contamination.Samples must be thoroughly blended to assure uniformity prior to removal of a testportion.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003PROCEDUREF. ANALYTICALDigestion1. Microwavea. Weigh homogenized sample to nearest .01 g into a clean1 microwave vesselliner. Use approximately 0.5 g for muscle tissues, 0.5 - 1 g for liver and kidney2.Note: Prepare negative and positive controls at this time (See section I.5,“Sample Set”). Fortify positive control(s) using 25 - 250 µL of an appropriateFortification Standard.1Vessel liners must be cleaned after each use to reduce the possibility of cross-contamination. Refer to Section K.2 for recommended cleaning procedure.2Caution! Mixing sample types or sample weights may produce unacceptablylarge variations in pressures developed during digestion, possibly resulting indamage to vessels if unvented caps are used. In order to maintain relativelyconstant digestion conditions in all vessels, analyst should attempt to digest likequantities of similar samples in each batch.b. Add 5mL of concentrated HNO3.c. Assemble the vessel according to the manufacturer’s instructions.d. Place assembled vessels into the microwave according to the manufacturer’sinstructions.e. Set microwave oven program as follows:Watts*Power: 1200Ramp Time: 10 minutesFinal Temperature: 180 °CTemperature Hold Time: 10 minutesCool down time: 10 minutes*If there are less than eight vessels in the microwave the wattage can belowered to 600.f. Initiate oven program and digest samples.g. Allow vessels to cool, then transfer to a fume hood and allow coming to roomtemperature.h. Slowly open the vent fittings and vent to atmospheric pressure, then disassemblevessels.Evaporation2. Microwavea. Place vessel liners into the evaporation carousel and assemble according to theTitle: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003manufacturer’s instructions.b. Place the evaporation assembly into the microwave.c. Set microwave oven program as follows:Power: 600WattsRamp Time: 5 minutesFinal Temperature: 120 °CTemperature Hold Time: 3.5 minutes*Cool down time: 10 minutes*Typical value required when 8 vessels are used. Hold times required toachieve a final volume of 1 mL for any given number of vessels must bedetermined experimentally.d. Initiate oven program and evaporate samplese. Once vessels have cooled to room temperature, remove the evaporationassembly from the oven and dismantle.Preparation3. Extracta. If the solution volume remaining in the vessel liner is less than 1 mL, addconcentrated HNO3 to bring volume to approximately 1 mL. Note: residual acidvolumes of up to 2.5 mL are acceptable, but should be avoided if possible. Pourextract solution into a 50 mL plastic tube containing approximately 10 mLMillipore water.b. Quantitatively transfer residual digest by rinsing the liner 3 - 4 times with Milliporewater, adding each rinse to the extract in the tube. Keep total rinse volume < 35mL.µL of 5000 µg/L ISTD solution to the extract.50c. Addd. Bring extract volume to 50 mL with Millipore water.e. Cap tube and invert several times to mix.Note: The percentage of dissolved solids in the 50 mL extract, which is higherthan that recommended by instrument manufacturer, can be reduced byincreasing the dilution volume. Analyst must balance detrimental effects of highdissolved solids content (matrix effects, instrument contamination) againstdetrimental effects resulting from environmental contamination and lower analyteconcentrations when considering this. If additional dilutions are made, care mustbe taken to maintain ISTD concentration at 5 ng/mL and adjust standard curveconcentrations accordingly, if necessary.Analysis4. ICP-MSTitle: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003a. Tuningi. Prior to sample analysis check the instrument’s tuning parameters byanalyzing the tuning solution as specified by the manufacturer. Check thesensitivity, % RSD, % oxide, % doubly charged, peak shape, andresolution.ii. If these parameters are outside the manufacturer’s specifications, retune the instrument.Parametersb. ICP-MSSet up instrument to monitor isotopes of cadmium and lead, indium and terbium(or other selected internal standards), and molybdenum (interferes with cadmiumquantitation if present).Table 1. ICP-MS Isotopes Monitored for Pb and Cd AnalysisMonitortoMetal IsotopesCadmium 106, 108, 110, 111*, 112, 113, 114, 116Lead 204, 206, 207, 208*Molybdenum 92, 94, 95, 96, 97, 98, 100115Indium 113,Terbium 159* Isotope used for quantitationc. Instrumentcalibrationi. Analyze a calibration blank followed by at least 4 calibration standards(D.2.b) covering the range of interest. Using linear regression analysis,plot relative response (response relative to ISTD response) vs.concentration in µg/L and determine slope (m), intercept (b), andcorrelation coefficient (r) of the calibration curve. Correlation coefficientmust be ≥ 0.995, or calibration must be repeated.ii. Analyze the calibration blank and a QC standard (D.2.c.i)immediatelyafter the calibration curve. The value of the blank should be close to thatinitially recorded, and the concentration calculated for the QC standardmust be within ± 10% of its accepted value. If these conditions are notmet, the calibration sequence must be repeated until results areacceptable.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003Analysisd. SampleOnce instrument meets calibration requirements, analyze all controls and testsamples, taking care to meet conditions listed below.i. Calibration blanks and QC standards must be included in the sampleanalysis sequence after at least every 12 consecutive samples analyzed,and also at the end of the sample sequence to verify instrumentperformance over the course of the run.ii. If response of any sample exceeds highest standard in the calibrationcurve, make an appropriate dilution with a 5 µg/L solution of ISTD in 2%HNO3 and re-analyze.G. CALCULATIONSNote: Instrument software can be programmed to perform all necessary calculations.1. Using values for m, b determined for the calibration curve (F.4.c), determine analyteconcentration (C E, in ng/mL) in any extract having a relative response R using:C E (ng/mL) = C E, µg/L = (R-b)/mNote: If sample is found to contain molybdenum, instrument software must be set tocompensate for contribution of molybdenum oxide to the 111 ion used for quantitation of cadmium in the sample.2. Calculate analyte concentrations in controls and samples (C S) using:C S (ppb) = C E x V x DWWhere:C E = Analyte Concentration in final extract, in ng/mLV = Final extract volume in millilitersD = Dilution factor (Diluted volume/aliquot volume), if secondary dilution made.W = Sample Weight in grams.3. Calculate Relative % Difference (RPD) for duplicate results using:RPD = |C1 - C2| x 100(C1 + C2)/2Where:C1 = first duplicate’s concentration.C2 = second duplicate’s concentration.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 20034. Calculate recoveries of fortified controls and check samples using% Rec = (C F - C B) x W x 100V FS x C FSWhereC F, C B = Analyte concentrations determined for the fortified sample and the blanktissue from which it was prepared, in ppb (ng/g).W = Weight of fortified control, in grams.V FS = Volume of fortification standard added, in mL.C FS = Concentration of fortification standard, in ng/mL.5. Acceptability Requirements:Before results can be reported, the following quality control acceptability requirementsmust be met:Each Sample batch must meet the following criteria:a. The instrument calibration meets acceptance criteria specified in section F.4.c.b. The recovery calculated for the set’s positive control meets acceptance criteriaspecified in Section I.1.c. If a positive control duplicate is run, the calculated RPD is ≤ 20%.d. All calibration blanks injected show responses similar to those seen in previousblanks, and all QC standards calculate to be within ± 10% of their acceptedvalue.In addition, each test sample must meet the following criteria:a. The sample internal standard areas must be within ± 20% of the averageinstrument calibration internal standard areas.b. For a positive identification the analyte’s isotope ratios must be correct.This may be determined by using Agilent software to visually compare relativeion abundances for all monitored isotopes against a plot of expected values. Theanalyte may be considered to be positively identified if ions from all monitoredisotopes are present and at least 3 isotope ions are within approximately ± 15%of expected value after any necessary corrections for interferences have beenapplied.Alternatively, identity may be confirmed by comparing response ratios of allmonitored isotope ions (relative to the quantitation ion) in the sample againstthose of a reference standard. Analyte will be positively identified if all monitoredions are present and at least two of the calculated ion ratios are within 80 -120%of those calculated for a reference standard.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003 H. HAZARDANALYSIS1. Required Protective Equipment - Safety glasses, lab coat, protective gloves.2. HazardsItem/Procedure Hazard Recommended Safe ProceduresNitric Acid Strong oxidizer. May be fatalif swallowed or inhaled.Extremely corrosive. Contactwith skin or eyes may causesevere burns and permanentdamage. Perform operations using concentrated acid in fume hood. Use protective eyewear, gloves and clothing. Store in approved acid safety cabinet away from basic or other reactive materials.Microwave Digester Possible explosion hazard Follow manufacturerrecommendations Pb, Cd Standards Poisonous if ingested. Do not pipet by mouth3. DisposalProceduresItem/Procedure Hazard Recommended Safe Procedures Nitric Acid See above Store in cabinet away frombases or other reactivematerials. Follow applicablestate and local regulations whendisposing of acid solutions.Pb, Cd Standards Follow applicable state and localregulations when disposing ofsolutions or their residues.I. QUALITY ASSURANCE PLAN1. Performance Standard (FSIS requirements)Analyte Analytical Range Acceptable RecoveryPb, Cd ≥ 25 ppb (Pb)≥ 10 ppb (Cd) < 25 ppb: 70 – 110% ≥ 25 ppb: 80 – 110%Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 20032. Readiness to Performa. Familiarizationi. Phase I: Standards - Analyze a ≥ 6 level standard curve in duplicate forPb and Cd over range 0 – 5 µg/L (0r higher) on 3 different days.Suggested concentrations are:µg/L (calibration blank)(a) 0µg/L(b) 0.2µg/L(c) 0.5µg/L(d) 1.0µg/L(e) 2.0(f) 5.0µg/Lii. Phase II: Fortified samples - For muscle, liver, and kidney tissues: Fortify blank tissue in duplicate with Cd and Pb at 4 levels (including 0)representing low, medium, and high values in the range of interest for thattissue. Recommended ranges are muscle: up to100 ppb; liver andkidney: up to 250 ppb. Analyses must be conducted over a minimum ofthree separate days.NOTE: Phase I and Phase II may be performed concurrently.iii. Phase III: Check samples for analyst accreditation.(a) A minimum of six check samples, having concentrations unknownto the analyst. Set must include one blank tissue, with theremainder containing Pb at levels between 25 - 250 ppb and Cd atany levels between 10 - 250 ppb.(b) Approval from Quality Assurance Manager (QAM) is required tocommence official analysis.criteria.b. AcceptabilityRefer to section I.1 above.3. Intralaboratory Check Samplescontents.a. System,minimumi. Frequency: One per week per analyst, when samples are analyzed.ii. Records are to be maintained by the analyst and reviewed by thesupervisor and QAM.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003criteria.b. AcceptabilityIf unacceptable values are obtained, then:i. Stop all official analyses by that analyst.ii. Take corrective action.4. Sample Acceptability and Stabilitya. Sample size: 450 g minimum.b. Sample condition: Cold on receipt.storage:c. SampleIndefinite.i. Time:Frozen.ii. Condition:Set5. SampleA sample set consists of:a. One tissue blank (Negative control).Note: Truly blank tissues are not available. Use previously analyzed tissueshaving low analyte levels for this purpose.b. One or more fortified blanks (Positive controls), prepared using the same matrixused for the tissue blank.Note: Use fortification levels greater than the amount naturally present in theblank to minimize the blank’s contribution to the uncertainty of the calculatedrecovery.c. As many samples as can be digested simultaneously with the positive andnegative controls in the microwave apparatus.6. MethodSensitivitya. Lowest detectable level (LDL): Not Determined.*b. Lowest reliable quantitation (LRQ): Not Determined.*c. Minimum proficiency level (MPL): Pb: 25 ppb; Cd: 10 ppb.* Background levels measured in tissues were too high or variable to allowaccurate determination.J. WORKSHEETNot Included.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003K. APPENDIX1. ReferencesAgilent 7500 ICP-MS Hardware Manual, G1833-90004, January 2001.CEM XP-1500 Plus Vessel Accessory Sets and Autovent Option Instruction for Use,600493, Rev. 5, 8/01.CEM Vacuum Concentration/Drying Accessory Set Instructions for Assembly and Use, 600484, Rev. 1, 6/99.EPA Method 6020, Inductively Coupled Plasma-Mass Spectrometry, Revision 0,September 1994.2. Cleaning Vessel LinersThe following procedure was shown to be adequate for removal of residual adsorbed Cd and Pb from Teflon liners used in this method. Other procedures are available and may be used if shown to be effective.a. Add approximately 20 ml of 2 % HNO3 to each digestion vessel liner.b. Assemble vessels as specified by manufacturer.c. Place in microwave.d. Digest at 600W, Ramp to 125 °C over 10 minutes, then hold at temperature for10 minutes.e. Cool vessels to room temperature, then disassemble.f. Rinse vessel liners and caps with Millipore water several times to remove alltraces of acid.g. Place in a clean environment to dry.Title: Determination of Cadmium and Lead by ICP-MSRevision: 0Replaces: NA Effective: October 10, 2003APPROVALSApproval Records are on file.Approved by Date ApprovedEric Flynn 9/10/2003Gina McLeroy 9/10/2003Bill Koscinski 9/02/2003Jess Rajan 9/08/2003Charles Pixley 9/08/2003Phyllis Sparling 9/03/2003。

ICP-MS测试卷烟纸中铅、砷、汞、镉、铬、镍的方法研究(牡丹江恒丰纸业技术中心李党国157013)摘要采用微波消解前处理样品,建立电感耦合等离子体质谱法同时测定卷烟纸中的Cr、Ni、As、Cd、Hg和Pb6种重金属含量。
该方法采用内标补偿基体效应,采用碰撞池(CCT)模式消除Ni的质谱干扰。
该方法相对标准偏差均小于10%、回收率为89.9%—102.7%、检出限为1.00—62.2ng/L,该方法操作简单、分析速度快、灵敏度高、准确度好, 能快速、准确测定彩色卷烟纸中重金属。
关键词电感耦合等离子体-质谱法卷烟纸重金属微波消解碰撞池1 引言铅、铬、镉、镍等金属易在人体内蓄积,对人体毒害很大,1990年Hoffman将As、Cd、Cr、Pb、Ni和Hg等列为烟草44种有害成分【1】。
其中砷及其化合物都具有毒性,世界卫生组织(WHO)已将砷排在优先研究的有毒金属的第一位,国际癌症研究所(IARC)、美国环境卫生科学研究院(NIEHS)等诸多权威机构已将砷公认为人类已确定的致癌物,我国烟草行对卷烟配套用纸中砷含量也规定了严格的限量。
香烟在抽吸过程中纸中含有的这些重金属元素,可经呼吸道、消化道进入人体。
随着卷烟减害降焦工作的不断深入,卷烟配套用纸中有害元素残留逐渐受到高度重视,因此,准确测定卷烟纸中重金属含量对于卷烟烟气的安全性评价及降低卷烟危害具有一定意义。
在国标中,Pb、Cr、Cd、Ni采用石墨炉原子吸收光谱法(GFAAS),As、Hg采用氢化物原子吸收法(HG-AAS)或氢化物原子荧光(HGAFS),但是这些方法分别具有操作繁琐、检测周期长、试剂用量大、灵敏度低、重现性差等缺点。
因此建立一种简便、快速、准确、检出限低的测试方法具有十分重要的意义。
电感耦合等离子体质谱分析技术(ICP-MS)是近十几年来发展最快的无机微量元素分析应用的先进技术之一。
由于其具有较高的灵敏度和精密度,且易于进行多元素同时分析且检出限低,干扰少、精度高、线性范围宽、简便、快捷,已经广泛用于测定海产品中的微量元素,化妆品中的有害元素,水、土壤中的矿物元素,生物样品痕量分析等。

ICP-MS快速检测超纯水中43种元素新方法
余少丹
【期刊名称】《广州化工》
【年(卷),期】2024(52)1
【摘要】电感耦合等离子质谱法(ICP-MS)检测超纯水中43项超痕量金属杂质。
将传统多仪器简化成一台ICP-MS,多次数实验减至一次,1天检验周期压缩至2 h。
保证检验准确性及精密度的同时,达到降本增效的目的。
实验结果证明:新检测方法实验校正曲线线性相关系数>0.999;检验回收率99.1%~104.8%;实验数据相对标准偏差<5%。
该方法操作简便,分析速度快,结果准确可靠,可满足超纯水的43项元素快速检测需求。
【总页数】4页(P122-125)
【作者】余少丹
【作者单位】西陇科学股份有限公司
【正文语种】中文
【中图分类】TQ421.22
【相关文献】
1.分析ICP-MS法快速检测农产品中微量有害元素铅镉砷
2.湿法快速消解-ICP-MS 法同时测定化妆品中的37种元素
3.ICP-MS法同时快速测定八角中多种重金属元素
4.氟化氢铵快速分解-电感耦合等离子体质谱(ICP-MS)法测定多金属矿中痕量稀土元素
5.直接稀释ICP-MS法快速测定尿液中14种元素的方法建立
因版权原因,仅展示原文概要,查看原文内容请购买。


安全监测ICP-MS法测定18种动物药中重金属及有害元素的残留量及初步风险分析*左甜甜,李耀磊,金红宇**,马双成**(中国食品药品检定研究院,北京100050)摘要 目的:建立动物药中重金属及有害元素残留量的测定方法,为相关品种重金属及有害元素的风险评估、标准完善和质量监督提供依据。
方法:采用电感耦合等离子体质谱(ICP-MS)法测定动物药中铅、镉、砷、汞的含量。
等离子气流量:15.0 L·min-1;蠕动泵0.20 r·s-1;雾化室温度:2 ℃;辅助气流量:0.8 L·min-1;He气流量:5 mL·min-1;载气流量:0.8 L·min-1;射频功率:1 550 W;数据采样模式:跳峰采集模式;采样深度:10 mm;重复次数:3次;扫描次数:100次。
结果:4种重金属及有害元素的线性关系良好(r>0.990),回收率为80%~120%,重复性良好。
按照现行2015年版中国药典水蛭等动物药重金属及有害元素限量标准,18种58批动物药的总体合格率为74.1%,其中铅、镉、砷、汞元素均存在超标的情况,其中合格率分别为91.4%、89.7%、86.2%和96.6%。
结论:本文所建立的ICP-MS法适用于动物药中重金属及有害元素残留量的分析和评定。
个别动物药品种重金属及有害元素污染情况较为严重。
建议重视动物药的质量,从风险评估的角度,完善动物药重金属及有害元素的限量标准。
关键词:电感耦合等离子体质谱(ICP-MS);动物药;重金属;有害元素;铅;镉;砷;汞;风险评估中图分类号:R 917 文献标识码:A 文章编号:0254-1793(2017)02-0237-06doi:10.16155/j.0254-1793.2017.02.08Determination of residues of heavy metals and harmful elements in 18 types of animal medicines by ICP-MS and preliminary risk analysis* ZUO Tian-tian, LI Yao-lei, JIN Hong-yu**, MA Shuang-cheng**,(National Institutes for Food and Drug Control, Beijing 100050, China)Abstract Objective:To establish a method for the determination of heavy metals and harmful elements in animal medicines, so as to provide a reference for risk evaluation, standard improvement and quality supervision for the related medicines.Methods: The contents of lead, cadmium, arsenic and mercury in animal drugs were * 国家十二五“重大新药创制”课题“中药质量安全检测和风险控制技术平台”(2014ZX09304307-002) ** 通信作者 金红宇 Tel:(010)67095994;E-mail:jhyu@ 马双成 Tel:(010)67095272;E-mail: masc@第一作者 Tel:(010)67095994;E-mail: zuotiantian2011@determined by inductively coupled plasma mass spectrometry (ICP-MS).The determination conditions were as follows:plasma gas flow:15.0 L·min-1;peristaltic pump:0.20 r·s-1; spray chamber temperature: 2 ℃; auxiliary gas flow rate: 0.8 L·min-1; He gas flow rate: 5 mL·min-1; sample carrier gas flow rate:0.8 L·min-1;radio frequency power:1 550 W;data sampling mode:peak-jump acquisition mode;sampling depth:10 mm;repeat times:3 times;and scanning times:100 times.Results:The linear relationship of the four heavy metal elements was good (r>0.990),the recovery rate ranged from 80% to120%, and the repeatability was also good.The established method was applicable for determining the amount of heavy metals and harmful elements of animal medicines.According to limits of heavy metals and harmful elements for animal medicines such as leech in Chinese Pharmacopoeia of 2015 edition, the qualified rate of heavy metals and harmful elements of these animal medicines was 74.1%.The qualified rates of Pb,Cd,As and Hg were 91.4%,89.7%,86.2% and 96.6%,respectively.Conclusion:The ICP-MS method established in this paper is suitable for the analysis and evaluation of heavy metals and harmful elements in animal drugs.The problem of heavy metals and harmful elements in animal medicines cannot be ignored.It is suggested to pay more attention to the quality of animal medicines and improve the standards of heavy metals and harmful elements of animal medicines from the view of risk assessment.Keywords: inductively coupled plasma mass spectrometry (ICP-MS); animal medicines; heavy metals; harmful elements; lead; cadmium; arsenic; mercury; risk assessment近年,随着环境污染日益严重,未经处理的工业“三废”的排放,农药、化肥等的不规范使用,成方制剂生产加工及贮藏运输过程中产生的污染,均造成中药重金属污染急剧加重。

ICP-MS法测定中药中铜、砷、镉、汞、铅含量的不确定度评定王欣美;王柯;季申【摘要】目的对电感耦合等离子体质谱法(ICP-MS)测定中药材中铜、砷、镉、汞、铅含量的不确定度进行分析,以期找出影响不确定度的因素,为评价检验结果提供科学依据.方法用电感耦合等离子体质谱法测定中药中铜、砷、镉、汞、铅的含量,通过对测定方法流程进行分析,确定不确定度来源为样品称重、样品消解、稀释定容、仪器分析,并根据<测量不确定度评定与表示>(JJF1059-1999)中有关规定量化不确定度分量,计算合成不确定度.结果用ICP-MS法测定铜、砷、镉、汞、铅的扩展不确定度分别为铜0.08 mg·kg-1、砷0.06 mg·kg-1、镉0.012 mg·kg-1、汞0.016 mg·kg-1、铅0.14 mg·kg-1.样品溶液的制备过程、测定样品溶液中各元素的浓度是影响中药中重金属定量分析的测量不确定度的主要因素.结论在实际工作中,应选择最佳的消解方法,按规范做好标准溶液的配制、样品溶液的制备、仪器测量等几个步骤,以保证测量结果准确、可靠.测量不确定度的评定为评价分析结果的准确程度和方法的可靠性研究提供了参考.【期刊名称】《药学研究》【年(卷),期】2012(031)003【总页数】5页(P136-140)【关键词】电感耦合等离子体质谱;不确定度;影响因素【作者】王欣美;王柯;季申【作者单位】上海市食品药品检验所,上海,201210;上海市食品药品检验所,上海,201210;上海市食品药品检验所,上海,201210【正文语种】中文【中图分类】R927.1在化学测试过程中,由于测量用仪器和工具的限制,测量方法的不完善、分析操作和测量环境的变化、测量人员本身的技术水平或经验的影响,测量往往带有误差,不能得到真值。
为评价测定结果的质量,需要进行不确定度的评定。
不确定度越小,结果与真值接近,其测定的质量越高,使用价值越大[1,2]。

饲料中重金属元素检测研究进展饲料中重金属元素检测是饲料安全的重要保障措施之一。
重金属元素的存在会对动物的生长和健康产生不利影响,因此必须采用有效的检测方法,及时监测饲料中重金属元素的含量。
本文综述了目前饲料中常见的重金属元素——铅、镉、汞、砷的检测方法,以及各种检测方法的优缺点和应用范围。
一、铅的检测方法铅是饲料中常见的重金属元素之一,对动物的神经、心脏和消化系统都有不利影响。
铅的检测方法主要有原子吸收光谱法(AAS)、电感耦合等离子体质谱法(ICP-MS)和荧光光度法。
AAS法是铅检测方法中最常用的一种方法,其优点是检测准确度高、灵敏度高、操作简单,但它的缺点是只能检测其中铅元素的含量。
ICP-MS法是一种新型的检测方法,其检测范围更广,能侦测更小的铅含量,以及其他重金属元素。
但ICP-MS法在操作过程中比较复杂,需要对仪器的调试、检测参数等进行较为严格的管理,且仪器的造价较高,因此其使用范围受到一定的限制。
荧光光度法也是铅的检测方法之一,主要适用于草料和谷类饲料的测试,具有简单易操作、精度高、成本低等优点,但该方法不能检测其他重金属元素,且检测范围较窄。
镉是一种极易积累在生物体内的重金属元素,其长期摄入会导致慢性中毒,危害人和动物的健康。
镉的检测方法主要有AAS法、ICP-MS法、原子荧光光谱法(AFS)等。
AAS法对镉的检测是最常用的方法之一,检测准确度高、灵敏度高、成本低廉,是广泛使用的定量分析方法之一。
ICP-MS法在镉检测中的应用也越来越广泛,它能够同时检测多种重金属元素,且具有更高的检测灵敏度和精度。
AFS法则是一种适用于镉含量较低的饲料样品的检测方法。
此方法基于镉离子与氢气生成亚砷酸化合物来检测出镉的含量,具有高灵敏度、高精度和低检测下限的优点。
AAS法对汞的检测精度较高,但其特异性较差,不能区分汞的各种化合物。
ICP-MS法对汞的检测能力较强,能够同时检测多种重金属元素,但需要高成本的设备和技术支持。

微波消解-ICP-OES测定大米中铝、铅、镉的含量建立微波消解-ICP-OES测定大米中有害元素铝、铅、镉的方法。
方法用微波消解法消解样品,测定其中铝、铅、镉含量。
结果Al、Pb、Cd的线性范围为0.O1μg/mL~3.O0μg/mL,相关系数r为0.9996~0.9999,加标回收92.0%~106.5%,RSD为1.83%~4.47%,检出限分别为1.6、8.2、1.5μg/L。
结论该方法具有检出限低、精密度和回收率好、测量范围宽、并可进行多种元素的同时测定等特点,简便易行。
大米是生活中的必备食品,含有多种有益健康的微量元素,其质量好坏直接影响着人们的身体健康,但是随着环境污染的不断加重,大米被有害元素污染的事件也时有发生,为了保护人们的健康,国家制定了相应的污染物限量标准[1]。
目前食品中铝、铅、镉的测定,实验室多采用石墨炉原子吸收法或氢化物原子荧光法测定[2],但这些方法都只能对元素逐项进行分析,并且湿式消解用酸量大、注意事项多、耗时长,且易消化不全或待测元素损失、污染等缺点[3]。
微波消解操作简便,能同时处理多个样品,所用试剂和样品量少,污染或损失的程度大大减少。
电感耦合等离子体发射光谱仪(ICP-OES)可以对样品进行多元素的同时测定,技术发展成熟。
为了充分利用两种方法的优点,本文研究并建立了微波消解一ICP-OES同时测定大米中有害元素铝、镉、铅含量方法,取得了满意的结果。
1 材料和方法1.1 实验原理消化后的样品导入炬管高温等离子体中,被测元素受激发释放出特征辐射,根据特征辐射信号波长及强弱进行定性与定量分析。
1.2 仪器与试剂1.2.1 仪器美国PE公司Optime7000DV ICP-OES;美国CEM公司CEM MARS 240/50密闭微波消解仪;玻璃器皿用10%HNO3溶液(V/V)浸泡过夜,用纯水冲洗干净后备用。
1.2.2 试剂氩气(99.999%);硝酸(优级纯);铝、铅、镉标准储备液(中国计量科学研究院提供)。

广东化工2020年第2期·154·第47卷总第412期Simultaneous Determination of Copper,Lead,Manganese,Cadmium and Chromium in Coconut Water by Microwave Digestion ICP-MSWang Dacheng(No.9Geological Part of CAPF,Haikou571127,China)Abstract:A method of the determination of Cu,Mn,Pb,Cr,Cd in coconut water by ICP-MS after microwave digestion was established.Five elements of Cu, Mn,Pb,Cr and Cd in coconut water were determined by ICP-MS.63Cu,208Pb,55Mn,52Cr,111Cd were selected as analysis isotopes of Cu,Pb,Mn,Cr and Cd respectively.187Re was selected as internal standard correction Pb,Cd,and74Ge as internal standard correction Cu,Mn,Cr.The detection limits of Cu,Pb,Mn,Cd and Cr were4.11,0.219,2.21,0.023and0.119ng/mL respectively,RSD was3.6%,3.2%,2.6%,4.6%and4.5%respectively,and the recovery of reagent samples was86%-105%.The method met the analysis requirements of copper,lead,manganese,cadmium and chromium in coconut water.Keywords:coconut water;microwave digestion;ICP-MS;heavy metal elements椰子(cocos nucifera L.)属棕榈科(Palmaceae)椰子属(Cocos),属热带地区主要多年生木本作物以及食品能源作物,同时又是一种即食水果[1-3]。

ICP-MS测定沙葱中5种重金属元素于颖【摘要】建立电感耦合等离子体质谱法(ICP-MS)同时测定沙葱样品中铅(Pb)、铬(Cr)、镉(Cd)和镍(Ni)、铜(Cu)含量的方法.样品加HNO3和H2O2后采用微波消解进行预处理,探索了微波消解样品的实验条件,并对ICP-MS工作参数进行优化和选择.结果表明:该方法测定沙葱中5种元素的线性关系良好(r=0.9997~0.9999),方法检出限为0.0015μg/L~0.0101μg/L,重复性实验的相对标准偏差为1.3%~3.4%,回收率为97.85%~100.09%.%The method of simultaneous determination of 5 heavy metals (Ni, Pb, Cd, Cr, Cu)in Allium mon-golicum regel by inductively coupled plasma mass spectroscopy (ICP-MS) was studied. The sample of Allium mongolicum regel after added into HNO3 and H2O2 was pre-processed by means of microwave dissolving tech-nique. The experimental conditions of the microwave digestion sample were researched and the experimental pa-rameters of ICP-MS were discussed and optimized. The results showed that the determined method of the simul-taneous determination of 5 heavy metals in Allium mongolicum regel had good linear relationship (r=0.9997-0.9999), the detection limit of the method was 0.0015 μg/L-0.0101 μg /L, the relative standard derivation of the developed method was 1.3 % -3.4 %, its mean recovery was 97.85 % -100.09 %, the measured value of the standard matter in agreement with the standard value.【期刊名称】《食品研究与开发》【年(卷),期】2017(038)016【总页数】3页(P136-138)【关键词】电感耦合等离子体质谱法;微波消解;沙葱;重金属元素【作者】于颖【作者单位】辽东学院化学工程学院,辽宁丹东 118003【正文语种】中文沙葱,又名蒙古葱(Allium mongolicum regel)属百合科,多年生草本植物。
质量控制Quality Control中国果菜China Fruit & Vegetable第 40 卷, 第 12 期2020 年 12 月ICP-MS 同时测定蔬菜中的多种元素姜大勇,徐立清* ,刘佩,解恒杰,林晓收稿日期院2020-01-19第一作者简介:姜大勇(1982—),男,工程师,本科,主要从事食品质量检测工作*通信作者简介:徐立清(1987—),男,工程师,本科,主要从事食品质量检测工作(山东省食品药品检验研究院,山东济南250100)摘要:目前随着人民生活水平的提高,消费者对无公害蔬菜的需求也越来越高,因此蔬菜中多种元素的测定具有重 要意义。
采用电感耦合等离子体质谱法(ICP-MS )同时测定不同蔬菜中铬、申、钒、锰、铜、锶、铯、银、钡、汞,与以往测定方法相比,具有前处理简单,测定速度快,灵敏度高,多种元素同时测定等优势。
该方法的线性相关系数在0.999 2 以上,检出限为0.001〜0.050 mg/kg ,加标回收率在91.08%-96.37%之间,精密度RSDW7.58%。
关键词:蔬菜;ICP-MS ;多元素中图分类号:O657.63文献标志码:A 文章编号:1008-1038(2020)12-0032-04DOI :10.19590/ki.1008-1038.2020.12.007Simultaneous Determination of Various Elements in Vegetables by ICP-MSJIANG Da-yong, XU Li-qing *, LIU Pei, XIE Heng-jie, LIN Xiao (Shandong Institute for Food and Drug Control, Jinan 250100, China)Abstract: At present, with the improvement of people's living standards, consumers'demand for pollution-freevegetables is also increasing, so the determination of various elements in vegetables is of great significance. ICP-MSwas used to determine chromium, arsenic, vanadium, manganese, copper, strontium, cesium, silver, barium and mercury in different vegetables at the same time. Compared with the previous methods, it has the advantages of simple pretreatment, fast determination speed, high sensitivity, simultaneous determination and so on. The linearcorrelation coefficient was above 0.999 2, the detection limit was 0.001-0.050 mg/kg, the recovery rate was 91.08% -96.37%, the precision RSDw 7.58%.Key words: Vegetables; ICP-MS; multielement蔬菜是维生素C 、硫胺素、吡哆醇、烟酸、矿物质、叶 作用。
科技创新ICP-MS法测定中药材中的重金属及有害元素冯华业,邱富源(无限极(中国)有限公司,广东 江门 529156)摘要:随着人们的保健意识越来越强烈,对有毒有害元素进行严格控制已成为国际国内法律法规的重要要求。
而目前中药材的质量标准和检测技术相对落后。
常用的检测方法是特异性和灵敏度较差的比色法,易受干扰导致不能对有毒有害元素进行定量分析。
它们中的大多数使用原子吸收分光光度法,其缺点是样品制备复杂、测量速度慢、灵敏度低和严重的测量干扰。
关键词:中药材;ICP-MS;重金属;有害元素;测定现在,我国经济在高速的发展,人民生活水平也随之提升,仅饮食已不能满足人民的需求,人们往往会选择日常生活中加入中药材食疗,因此中药材检验工作是必要的,它变得越来越重要。
的确,确保社会的和谐与稳定以及人民的健康已成为政府的责任和义务。
由于中药材制药或熬制后是直接服用的,因此其中所含的有害成分会对人体造成非常直接的伤害,因此世界各地的国家都建立了严格的限制措施,以控制有害成分的摄入。
因此,必须对中药材中可能存在的有害元素进行测定,并且需要高水平的分析技能才能完成这些元素的测定。
中医药作为中华传统文化的瑰宝,历史悠久,是千百年来抗击疾病的人们积累的宝贵财富,为中国的繁荣做出了巨大的贡献。
中药材作为一种天然药物,它具有丰富的资源和独特的治疗作用,并在世界范围内日益受到人们的喜爱。
由于药材的原料受到地理和环境条件,生产和加工技术等许多因素的影响,因此很可能造成重金属污染,而重金属含量直接影响到药材的质量和功效。
中药材会影响使用者的健康,因此在使用中药材的情况下,准确检测中药材中的重金属含量和有害元素尤为重要。
铅、汞、砷、镉等重金属对人体非常有害,会使人类蛋白质变性并失去酶活性,从而导致各种病理变化。
当前,主要是通过原子吸收分光光谱法(AAS)、电感耦合等离子体质谱法(ICP-MS)和原子荧光光谱(AFS)的方法来测量中药材中的重金属元素。
ICP-MS法测定石菖蒲中的微量元素含量李璐【摘要】用ICP-MS测定石菖蒲中的微量元素含量,为深入研究石菖蒲药理作用提供实验依据.将石菖蒲样品粉碎,用硝酸和高氯酸的混酸及过氧化氢硝解,用电感耦合等离子体发射质谱仪测定样品中的微量元素含量.结果显示,石菖蒲样品中含有丰富的铁、锰、铜、锌等微量元素,含量分别为(mg/Kg):Fe-676,Mn-1275,Cu-13.3,Zn-36.7.含砷为1.1 mg/Kg,铬、镍、铅含量小于0.5 mg/Kg,镉、汞含量小于0.05 mg/Kg.常量元素钙、磷、镁含量比较高,含量分别为(mg/Kg):Ca-6101,P-3706,Mg-130.【期刊名称】《楚雄师范学院学报》【年(卷),期】2017(032)006【总页数】4页(P157-160)【关键词】石菖蒲;微量元素;ICP-MS【作者】李璐【作者单位】楚雄师范学院,云南楚雄 675000【正文语种】中文【中图分类】Q949.717.2石菖蒲为天南星科多年生草本植物石菖蒲(Acorus tatarinowii Schott)的干燥根茎,始载于《神农本草经》,具有多种药用价值,被列为上品[1]。
石菖蒲性微温、味辛、苦,具有化痰开窍、化湿行气、祛风利痹、消肿止痛的作用,用于治疗健忘、耳鸣、耳聋、脘腹胀痛、跌打损伤等[2]。
牛小飞等人对小鼠进行的试验证明,石菖蒲可提高小鼠机体的免疫能力,因为石菖蒲可以通过促进脾脏和胸腺发育而提高机体免疫力[3]。
石菖蒲挥发油有数十种成分,产地不同、采收时间不同,成分也有差异,但主要成分相似,主要是α-细辛醚和β-细辛醚,占挥发油总量的80%左右[4、5、6]。
除了药用价值外,石菖蒲提取物对番茄灰霉病菌具有抑制作用,以石菖蒲为材料可研发中药杀菌剂[7]。
尽管石菖蒲具有很多药用价值,但有报导认为长期使用石菖蒲可能有一定的致癌作用,仅研究主要有机成分是不够的,所以必须全面、深入的对石菖蒲进行研究,保障更好的指导临床用药的安全[8]。
ICP-MS法测定大黄蛰虫胶囊(丸)中5种有害元素
孙鹏飞;李龙囡;丁晴;金坚
【期刊名称】《海峡药学》
【年(卷),期】2017(29)5
【摘要】目的对大黄蛰虫胶囊(丸)中铅、镉、砷、汞、铜5种有害元素含量进行检测与评价.方法采用石墨炉及微波消解仪分别对样品进行消解,并用ICP-MS法进行检测分析.结果所用的方法线性、精密度、准确度良好;石墨消解法与药典标准方法微波消解法具有很高相关性.结论 6批样品中有5批有害元素超标,建议将重金属及其有害元素检测纳入大黄蛰虫胶囊(丸)的质量评价当中.
【总页数】3页(P41-43)
【作者】孙鹏飞;李龙囡;丁晴;金坚
【作者单位】江南大学无锡214000;无锡市药品检验所无锡214000;无锡市药品检验所无锡214000;江南大学无锡214000
【正文语种】中文
【中图分类】R914.1
【相关文献】
1.百令胶囊、大黄蛰虫丸联合基础治疗对早期糖尿病性肾病蛋白尿的疗效观察 [J], 吴健;张振忠;刘宁州;张军军
2.百令胶囊联合大黄蛰虫丸对慢性肾衰竭患者TGF-β1和Col-Ⅳ的影响 [J], 熊有明;蒋背乐;唐镜;邓晓蔚;陈源
3.ICP-MS法测定人参再造丸中5种有害元素 [J], 张博; 田兰; 智雪枝; 陈睿; 王彦;
闫超
4.丹鹿胶囊联合大黄蛰虫丸治疗乳腺增生的临床效果观察 [J], 张鑫山;盛勇;卜晓莉;郭亚荣
5.微波消解ICP-MS法测定清肺十八味丸中5种有害元素含量 [J], 赵猛;李玲
因版权原因,仅展示原文概要,查看原文内容请购买。
电感耦合等离子体发射光谱仪(ICP-AES)检测铅、镉的光谱干扰分析及解决方案摘要:电感耦合等离子体发射光谱仪(ICP-AES)广泛应用于铅和总镉等重金属含量的测定,实际检测中由于基质和仪器的局限性而对待测物质产生干扰,使得测试结果失真,本文针对常见的干扰情况进行分析及给出相应的解决措施。
关键词:电感耦合等离子体发射光谱仪(ICP-AES);铅;镉;光谱干扰。
1引言电感耦合等离子体发射光谱仪(ICP-AES)因其检测速度快,检出限低、线性范围广、电离和化学干扰少、准确度和精密度高等分析性能[1],而广泛应用于重金属检测。
实际检测实践中,我们发现由于含待测元素的样品中存在其它原子或分子在选定分析波长处的谱线,使待测元素谱线和其它谱线重叠,无法分辨,易造成光谱干扰,从而影响测定结果的准确性。
2铅的光谱干扰及解决方案ICP-AES测试金属试样中的铅含量,因样品中同时有较大含量的铁元素,造成铅元素明显的光谱干扰。
图4-1和图4-2分别为铁块经消解后,使用ICP-AES测试铅含量时,Pb220.353和Pb283.305的峰图。
图4-3和图4-4为1 mg/L的铅标准溶液,Pb220.353和Pb283.305的峰图。
对比图4-1和图4-3,图4-2和图4-4,可见,使用ICP-AES测试铅的含量时,高浓度的铁元素的存在对Pb220.353和Pb283.305的光谱干扰都很明显。
图4-1 ICP-AES测试铁材质金属消解液的峰图(波长Pb220.353)图4-2 ICP-AES测试铁材质金属消解液的峰图(波长Pb283.306)图4-3 ICP-AES测试1 mg/L的铅标准溶液的峰图(波长Pb220.353)图4-4 ICP-AES测试1 mg/L的铅标准溶液的峰图(波长Pb283.305)针对这种多个特征光谱都不同程度被干扰的情况,需要采用特异性更强的仪器进行确认测试才能得出准确的测试结果。
针对上述被干扰样品,采用电感耦合等离子体发射质谱仪(ICP-MS)对样品液进行确认测试。
超声提取-ICP-MS法测定土壤中有效态铅和镉农云军;谢继丹;黄名湖;郭鹏然;苏流坤;马名扬【摘要】本实验以DTPA为提取剂,建立了超声提取-电感耦合等离子体质谱(ICP-MS)测定土壤中有效态铅和镉的方法.实验考察了超声提取时间、溶液稀释倍数、测定条件等因素,并对基体干扰的消除进行了对比研究.在优化的ICP-MS条件下,有效态铅和镉在1~200 μ g/L浓度范围内线性关系良好,方法检出限分别为0.019 mg/kg、0.001 4 mg/kg,相对标准偏差小于6%(n=6).用国家土壤标准物质进行方法精密度和准确度验证,相对误差小于5%(n=6),该测定结果与国家标准方法(GB/T 23739-2009)一致,经t检验表明两者无显著性差异.该方法简便快捷、检出限低,具有良好的准确度和精密度,可满足大批量农业土壤污染调查样品中有效态铅和镉的分析要求.【期刊名称】《质谱学报》【年(卷),期】2016(037)001【总页数】7页(P68-74)【关键词】土壤;超声提取;电感耦合等离子体质谱(ICP-MS);有效态;铅;镉【作者】农云军;谢继丹;黄名湖;郭鹏然;苏流坤;马名扬【作者单位】中国广州分析测试中心,广东省化学危害应急检测技术重点实验室,广东广州510070;中国广州分析测试中心,广东省化学危害应急检测技术重点实验室,广东广州510070;中国广州分析测试中心,广东省化学危害应急检测技术重点实验室,广东广州510070;中国广州分析测试中心,广东省化学危害应急检测技术重点实验室,广东广州510070;中国广州分析测试中心,广东省化学危害应急检测技术重点实验室,广东广州510070;中国广州分析测试中心,广东省化学危害应急检测技术重点实验室,广东广州510070【正文语种】中文【中图分类】O657.63doi:10.7538/zpxb.2016.37.01.0068土壤有效态是指在土壤中容易被植物实际吸收利用的元素存在形态[1]。
※农业科学
农业与技术
2018, Vol.38, No.22 39
分析ICP-MS法快速检测农产品中微量有害元素铅镉砷
章 路 刘 岩
(绿城农科检测技术有限公司,浙江 杭州 310000)
摘 要:
文章主要对ICP-MS法详细研究,以农产品中微量有害元素铅、镉、砷为研究对象,详细规划试验操作步骤,保
证实验准确性,目的是准确掌握农产品中微量有害元素铅、镉、砷。
关键词:
ICP-MS法;微量有害元素;质谱仪
中图分类号:TS207 文献标识码:A DOI:
10.11974/nyyjs.20181132033
人们生活品质提升对农产品质量的要求越来越高。
农产品生产过程中,为了获得更高的生产质量,会选择
各种肥料进行灌溉,但是这会造成农产品出现有害元素
铅、镉、砷。这些元素主要是污染所致,对人们的身体
健康产生直接影响。农产品生产与销售期间,必须关注
重金属污染控制情况,保障农产品的安全。利用ICP-MS
法快速检测农产品重金属元素,可保证检测的精准性。
1 ICP-MS法试验材料
对于ICP-MS法快速检测农产品中的微量有害元素
铅、镉、砷,需要准备如下设备与试剂:质谱仪。型
号为X Series Ⅱ,属于电感耦合等立体体制;微波处
理设备。具体仪器为微波消解仪(Berghof speedwave
four),试验需要的所有器皿都必须使用硝酸(20%)比
例浸泡与清洗;超纯水。使用条件为电阻率必须控制在
18.2MΩ·cm
-1
,硝酸、盐酸、过氧化氢。其中硝酸必须保证优级纯,并且需要利用聚四氟乙烯酸纯化仪蒸馏纯化,次数为2次,盐酸也需要保证优级纯,同硝酸相同条件纯化2次。过氧化氢必须为优级纯;混合标准储备液。混合标准储备液具体包含砷、镉、铅元素,浓度都为500mg/L,标准储备液必须在国家标准物质研究中心购买;测定样品。测定样品主要是送检样品为主。从Tgermo公司购买质朴调谐液(Y、Li、Co、TI),浓度标准为0.1mg/L。2 ICP-MS试验方法制备试验样品。农产品中的水果、蔬菜等都是试验样品,具体取其中1.0~2.0g,将样品放置于消解罐中,按照顺序加入硝酸(5mL)/硝酸(4mL);过氧化氢(1mL)。对水分较少的粮食以及肉类试验样品提取,称取重量一般控制在0.4~0.1g,同样将其放置于消解罐中,按照顺序加入硝酸(10/9mL);盐酸(3mL),根据实际情况确定是否加入双氧水,密封存储,放置于微波消解仪中,对提取物质进行消解。消解参数主要功率为1200w,升温时间分别设定为5min、3min、3min,控制温度为120℃、160℃、180℃,保持时间分别为2min、10min、10min。配置标准系列溶液。吸收混合标准储备液,选择HNO3(1%),按顺序将其稀释,分别配置0.0、1.0、5.0、10.0、50.0、100.0(μg/L)标准溶液。优化仪器的具体工作环境。需要应用到质谱仪,因此必须准备0.1ng/mL的质谱调谐液与试验内的标准溶液(1.0mg/L)。ICP-MS试验的仪器测定期间,工作环境的协调能够进一步提升其检测的灵敏度,并且保持氧化物与双电荷水平变化。具体工作条件要求如下。正向功率项目中,要求功率为1300w;冷却器流量项目要求为13.1L/min;雾化气流量项目要求为0.94L/min;镍截取锥孔径项目要求为0.75mm;采样深度项目要求为150;采样时间项目要求为22s。对仪器进行开机检测。质谱调谐液微调仪器包含很多参数,保证这些参数准确性,控制最佳灵敏度[1]。样品在编辑与测定期间,明确方法、计算方程以及各种影响元素,保证所有条件达到检测要求,按顺序将空白试剂、标准样品等分别引入到微调仪器中。输入试验要求参数,绘制标准试验曲线。铅、镉、砷含量计算。计算公式为。计算公式中,样品铅、镉、砷的具体含量用X表示,单位为mg/kg。消化液中砷、铅、镉含量为C,单位为μg/L,样品空白消化液含量为C0,单位为μg/L,公式中的V主
要表示样品消化液总体积,单位为mL。样品质量为M,
单位为g。
3
试验讨论
试验操作与计算期间需要科学选择同位素,这也是
试验操作的主要影响因素,期间还包含双电荷与多原子。
因为样品试验的微量元素质量数分别为As75、Pb208、
Cd111。因此需要以干扰校正方程形式抗干扰。非质谱干
扰现象主要是因为样品本身基质影响出现,需要利用内
标校正的方式克服
[2]
。当然具体落实方案设计如下,分
析样品中是否隐藏以上元素,对元素质量进行研究分析,
必须保证沸点临近,保证电离能相同。
4
结论
通过微波消解样品的方式,对ICP-MS法快速检测
农产品中微量有害元素铅、镉、砷进行讨论得出如下结
论:试验过程中必须优化测定仪器以及保证环境适当,
对准确度与精密度严格测试,校准偏差≤10%。该方法
可以实现多元素检测研究,灵敏性良好,检测结果准确
无误。
参考文献
[1]霍燕,刘淑华,柴庆伟.ICP-MS
法快速检测农产品中微量有
害元素铅、镉、砷
[J].新疆农业科技,2013(4):36-38.
[2]
王勇,陈民辉,曹玲.电感耦合等离子质谱法(ICP-MS)法
测定茵栀黄注射液中有害元素铅、砷、镉、汞、铜的含量
[J].
中国野生植物资源
,2012,31(3):26-28.