电池燃烧锥形量热仪
- 格式:pdf
- 大小:1.66 MB
- 文档页数:7

CONE(锥形量热仪)法在塑料燃烧性能综合评估中的应用研究【摘要】本文介绍了塑料的燃烧性能及其常规测试方法,新型测定聚合物燃烧热性能仪器——锥形量热仪在评定聚合物燃烧性能中的应用,并提出了全面对燃烧性能进行综合评估的新型方法,从而为塑料的正确选型提供了一定的依据【关键词】塑料锥形量热仪层次分析法燃烧性能综合评估聚氯乙烯高抗冲聚苯乙烯1.前言目前,塑料的应用领域已经遍及工农业生产和人民生活的各个领域。
据统计,1999年全球五种主要热塑性塑料的总产量已近1.1亿吨[1],而三大合成材料(塑料,合成纤维,合成橡胶)中塑料占2/3以上的比例。
然而,作为一种高聚物,塑料燃烧迅速并释放出大量的热和有毒烟气,在火灾中暴露出较大的危害性,所以,对塑料的燃烧性能进行全面综合的评估以及正确选型就显得日益重要。
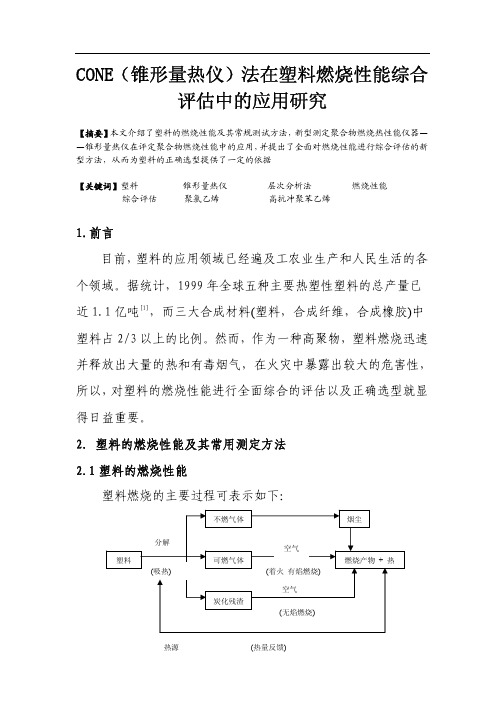
2.塑料的燃烧性能及其常用测定方法2.1塑料的燃烧性能塑料燃烧的主要过程可表示如下:热源(热量反馈)图1 塑料燃烧过程示意图通常塑料在火灾中的燃烧性能主要包括以下几个方面:⑴引燃性引燃性是指材料被引燃的难易程度,是燃烧的初始阶段。
材料在热作用下被引燃时,是热流和时间共同作用的结果。
⑵火焰传播性火焰传播性是指火焰沿材料表面蔓延发展的程度。
其决定因素关键是材料表面有可燃性气体产生,或在材料内部能形成可燃性气体但能逸至材料表面。
火焰传播速度越大,则越易使火灾波及附近的可燃物而使火灾扩大。
⑶释热性由表1[2]中给出的几种塑料的燃烧热值可以看出,塑料燃烧通常能释放出大量的热。
释热性影响着火灾环境温度和火灾传播速度,释热越大的物质,其危险性程度越高,反之越低。
名称 聚苯乙烯 聚乙烯聚氯乙稀 赛璐珞聚酰胺 酚醛树脂燃烧热40.18 45.88 18.05-28.0317.30 30.84 13.47 (KJ/g)表1 几种常见塑料的燃烧热值⑷生烟性烟气的生成不仅大大降低了火场的可见度,影响着人员疏散和救援工作的开展,而且烟气本身的窒息性直接威胁着人身安全。

ZY6243A-10MW热释放速率测试装置(大型锥形量热仪)一、适用范围适用于建筑物和大型复合体(包括机车车辆,车辆,仓库和构成方式)以及对原料、建筑物和实体模型进行广泛的防火性能试验验证。
二、参考标准:根据ISO 9705《Fire tests; full-scale room test for surface products》、ISO5660-1《Reaction to fire tests-Heat release, smoke production and mass loss rate-Part 1: Heat release rate (cone calorimeter method)》设计和制造。
三、性能参数:大型热量计测得的最基本的物理量是热释放率。
当发生火灾时,要测量不知道化学成分的原料的热量,其基本原理是基于这样的事实,即燃烧热量与所需的氧气量成正比的,每消耗1kg氧气生成热量13.1兆焦耳。
该热量的计算方法是通过测量氧气和排出气体的流量来测量热量。
最基本的物理量是热释放率,产烟率,质量损失率和耗氧量,一氧化碳(CO)和二氧化碳(C02)。
热流或可选择性采用的特定气体的浓度。
技术参数:一、大型热量计的结构和功能1)防护罩和管道系统设计防护罩时,主要有两个最重点:防护罩的形式和大小。
与正方形的防护罩相比,1圆形的防护罩能将可能沿着防护罩边缘产生的涡流效应最小化。
因此,采用圆形的防护罩,可调节的高度使试验能够根据测试对象燃烧的烟羽流的特性进行操作。
此外,由于管道和设备的附件提供足够的均匀流长,燃气能彻底分散。
集尘罩的外直径为10cm,这样可以用最大量程为10兆瓦的热量计测量实际尺寸的可燃物品和车辆的热释放速率,火灾荷载,需氧量,产生的一氧化碳和二氧化碳的数量,浓度,温度,流量和燃气的成分。
2)测量因素的构成要测量的因素大概可分为流量/温度、气体浓度和烟密度。
测试仪包含了进程控制和数据测量的程序。



建材锥形量热试验1. 背景介绍建材是指用于建筑工程中的各种材料,如混凝土、砖块、砂浆等。
建材的质量和性能直接影响着建筑物的安全性和耐久性。
锥形量热试验是一种常用于评估建材燃烧性能的方法。
通过对建材在高温下的燃烧特性进行研究,可以为建筑物的设计和材料的选择提供参考依据。
2. 锥形量热试验原理锥形量热试验是利用锥形量热仪对建材样品进行测试,以测定其燃烧性能。
试验中,将建材样品置于锥形量热仪的加热器中,通过控制加热速率,使样品受热并发生燃烧。
同时,通过测量样品的温度和热释放速率等参数,来评估建材的燃烧特性。
3. 锥形量热试验参数在进行锥形量热试验时,常用的参数包括:•最大热释放速率(Peak Heat Release Rate,PHRR):表示样品燃烧时释放的最大热量。
•平均热释放速率(Average Heat Release Rate,AHRR):表示样品燃烧时平均每单位时间释放的热量。
•烟气产生速率(Smoke Production Rate,SPR):表示样品燃烧时产生的烟气的速率。
•烟气毒性(Toxicity):表示样品燃烧时产生的烟气对人体的毒性。
•温度曲线(Temperature Curve):表示样品燃烧时温度的时间变化曲线。
4. 锥形量热试验过程下面是标准的锥形量热试验过程:步骤一:样品制备•准备建材样品,通常为规定尺寸和形状的试块。
•清洁样品表面,确保无油污和杂质。
步骤二:仪器设置•将样品放入锥形量热仪中,并确保样品合适的安装位置。
•设置测试参数,如加热速率、采样频率等。
步骤三:试验开始•启动锥形量热仪,开始测试。
•监测样品温度、热释放速率和烟气产生速率等参数的变化。
步骤四:数据分析•根据实验结果,计算最大热释放速率、平均热释放速率、烟气产生速率等参数。
•分析温度曲线和燃烧过程中的特征。
步骤五:结果评估•根据试验结果评估建材的燃烧性能和烟气产生情况。
•与相应的标准进行对比,判断建材是否符合要求。
聚合物材料燃烧性和阻燃性锥形量热仪测试评价法有机聚合物材料是一种新兴而广泛使用的材料,但由于其内在易燃性,使使用场所的火灾危险性大大增加。
因此,如何正确评价其在实际火情条件下的燃烧与阻燃性能已成为一项迫在眉捷的首要问题。
锥形量热仪( CON E)是美国国家科学技术研究所( N IST)的Babra uskas于1982年提出的。
它是以氧消耗原理为基础的新一代聚合物材料燃烧测定仪,氧消耗原理是指每消耗1 g的氧,材料在燃烧中所释放出的热量是13. 1 kJ(误差为5% 或更好) ,且受燃料类型和是否发生完全燃烧影响很小。
只要能精确地测定出材料在燃烧时消耗的氧量就可以获得准确的热释放速率。
不热辐射强度下的热释放速率( HRR )是CON E给出的最重要的参数之一,同时还能给出其它许多参数。
它们可从不同角度评价聚合物材料的燃烧性和阻燃性。
不同于以往的传统实验室型评价方法(如: 极限氧指数LOI, NBS烟箱等) , CON E的实验结果与大型燃烧实验结果之间存在很好的相关性[2 ]。
以往为了正确评价建筑材料、装饰材料和电线电缆等必须进行大型燃烧实验,浪费了大量的物力和财力。
近年来,由于CON E的出现使评价工作大为改观。
有利的促进了研究和评价工作的进展,并制定了相应的实验标准,如: ASTM E1354- 90 和90A 和ISODIS 5660 /90。
CON E可望在评价聚合物材料燃烧性和阻燃性上代替或部分代替大型燃烧实验,并能进行阻燃机理及烟等方面的研究工作。
1、锥形量热仪可模拟多种火情强度,测定聚合物材料的热释放速率等燃烧参数的CON E由六部分组成: ( 1)截断锥形加热器和有关控制电路; ( 2)通风橱和有关设备; ( 3)天平及试样架; ( 4)氧气和气体分析仪表; ( 5)烟测量系统; ( 6)有关的辅助设备。
该仪器具有较宽的热辐射功率范围( 10 kW /m2~110 kW /m2)。
锥形量热仪FTT和CONE型号对比分析一、CONE锥形量热议锥形量热仪符合的标准:ASTM E 1354 、ASTM E 1740 、ASTM E 1550、ASTM D 5485 、ASTM D 6113、ISO 5660 Parts 1 and 2 、NFPA 271、NFPA 264 、CAN ULC 135 、BS 476 Part 15锥形量热仪技术参数:1、锥形量热仪采用分柜式设计方式,分析柜可移动,既可应用于锥形量热仪测试使用,也可连接大型热释放速率测试系统,符合ISO 5660、ASTM E1354、GB/T 16172 等现行国内外测试标准。
2、集成测试机体和19英寸分析柜,内嵌PC型17英寸触摸屏面板,用于整个控制和测试过程。
3、锥形加热器额定功率5000W,热输出量0~100 kW/m2,可水平或垂直放置4、暴露试样表面的中心部位50X50mm的范围内,于中心处辐照偏差不超过±2%5、样品盒可放置***大100mm x 100mm x 50mm的样品6、样品称量范围 0~2000g;精度:0.1g7、点火系统带有安全切断装置的高压火花发生器,自动定位8、氧气分析顺磁性氧气分析器,浓度范围0~25%,氧分析仪应呈线性响应,在30m in内漂移不得大于士50x10-6OO2,T90时间小于12秒9、无弥散红外线CO和CO2分析器 CO:0~1%;CO2:0~10%10、烟密度分析使用激光系统测量烟雾密度,系统由光电二极管、0.5mW氦氖激光器、主图形检波器和辅助图形检波器组成11、排气系统由风机、集烟罩、风机的进气与排气管道及孔板流量计等所组成。
排风扇流量0~50g/s可调,精度0.1g/s12、环形取样器应装在距集烟罩685 mm处的进气管道内,取样器上应有12个小孔以均化气流组份,小孔与气流方向相反以避免烟灰沉积。
13、排气流量应通过测量风机上方350 mm处的锐缘孔板两侧的压差来确定,锐缘孔板的内径为57mm±1mm。
光伏太阳能电池板组件火灾危险性探究摘要:现阶段,太阳能电池板应用十分普遍,但是在应用过程中也面临着一定的火灾隐患.文章中采取实验的方式对太阳能电池板火灾产生原因以及特征展开了探究。
实验过程中以锥形量热仪为基本平台。
主要表现为电池板正面朝上反面朝上的两种工况,前者是模拟电池板的自燃,后者则是模拟电弧故障火灾。
太阳能电池板基于热辐射状态下被电火花引燃,并且持续性燃烧,然后熄灭。
通过分析实验了解到了太阳能电池板的基本燃烧规律以及存在的危险性。
关键词:光伏太阳能电池板组件;火灾危险性;实验要点太阳能属于一项新型能源,本身有着清洁性、充足性等一系列特征,因此得到了广泛青睐。
国外相继开展了光伏并网发电计划,我国也展开了西部太阳能发电工程。
当前阶段,国务院颁布了有关于推进光伏产业健康发展的基本文件,目的是加快太阳能产业进程。
不过从太阳能电池板实际开展情况来看,周围工作环境十分复杂,面临着老化和积热等一系列现象,同时出现火灾隐患的概率是特别高的。
通过分析光伏火灾案例可以看出,引起火灾的原因表现为多方面,比如电弧故障、因为生产不符合标准要求、安装行为出现错误、导致组件连接不到位、在接盒处和电控箱处引起电弧现象,从而造成了火灾。
然后是自燃现象,这种现象的产生原因是因为采取的组件不合格、太早老化、热斑效应等因素的影响,导致电池板在高温状态下燃烧。
在本篇文章中主要开展了相关的实验探究,明确了基本的实验要点以及论述了光伏板燃烧的情况和实验结果。
1、实验探究太阳能光伏电池组件亦称太阳能电池组件、光伏组件,是由一系列的太阳能电池片按照不同的列阵组成,单体太阳电池不能直接做电源使用。
太阳能电池着火有着一定的特殊性特征。
太阳能电池着火是一种带电隐患,光伏建筑将电源切断以后并没有彻底对组件的末端电压有效消除。
经过相关测试表明,太阳能板能从消防车警笛、火光中吸收光能进行发电,诱使消防人员触电。
其次,屋顶通道既能供通风井释放火苗和过热气体,又有助于消防员确定着火点及其他危险源头,是消防救援中的黄金通道。
An experimental study on burning behaviors of18650lithium ion batteries using a cone calorimeterYangyang Fu,Song Lu,Kaiyuan Li,Changchen Liu,Xudong Cheng,Heping Zhang* State Key Laboratory of Fire Science,University of Science and Technology of China,Hefei,Anhui230026,Chinah i g h l i g h t sFire risks and behaviors of charged lithium ion batteries were investigated.The thermal runaway of charged lithium ion batteries was experimentally studied.The effects of state of charge on burning behaviors of LIBs were evaluated.The heat release rates of LIBs were experimentally measured.The internal generated oxygen accounts for up to13%of total heat release rate.a r t i c l e i n f oArticle history:Received26May2014Received in revised form15August2014Accepted7September2014 Available online16September2014Keywords:Lithium ion batteryHeat release rateThermal runawayThermal hazardExplosion a b s t r a c tNumerous of lithium ion batteryfires and explosions enhance the need of precise risk assessments on batteries.In the current study,18650lithium ion batteries at different states of charge are tested using a cone calorimeter to study the burning behaviors under an incident heatflux of50kW mÀ2.Several parameters are measured,including mass loss rate,time to ignition,time to explosion,heat release rate (HRR),the surface temperature and concentration of toxic gases.Although small quantities of oxygen are released from the lithium ion battery during burning,it is estimated that the energy,consuming oxygen released from the lithium ion battery,accounts for less than13%of total energy released by a fully charged lithium ion battery.The experimental results show that the peak HRR and concentration of toxic gases rise with the increasing the states of charge,whereas the time to ignition and time to explosion decrease.The test results of the fully charged lithium ion batteries at three different incident heatfluxes show that the peak HRR increases from6.2to9.1kW and the maximum surface temperature increases from662to934 C as the incident heatflux increases from30to60kW mÀ2.©2014Elsevier B.V.All rights reserved.1.IntroductionThe lithium ion batteries(LIBs)have been widely used in elec-tronic products,vehicles and aerospace applications owing to their excellent features of high power capacity,stable voltage,long life cycle and low self-discharge[1,2].However,the LIBsfires and ex-plosions have occurred occasionally in the transportation because their thermal stability is sensitive to temperature,overcharging, extrusion and collision.For instance,the Asiana Airlines'B747 freighter crashed into the sea on July29,2011,killing two pilots,due to a cargofire caused by LIBs[3].Therefore,to ensure the safety transportation of LIBs,it is worthwhile to study the burning be-haviors of LIBs.Previous researches have reported that the LIBs underwent thermal runaway reactions which lead to the combustion of or-ganics electrolyte and rupture under heating conditions[4,5].Jhu et al.[6]conducted a set of experiments in an adiabatic calorimeter and found that the charged LIBs were more hazardous than un-charged ones and the maximum surface temperature of the charged LIB could reach903 C.Roth et al.[7]showed that the thermal runaway reactions of LIBs were affected by the state of charge(SOC)using an accelerating rate calorimeter.Increasing SOC reduced the onset temperature of thermal runaway reactions and increased acceleration rate.Ribi e re et al.[8]demonstrated thefire-induced hazards of Li-ion polymer batteries using a Tewarson calorimeter.It was found that the HRR and toxic gases productions depended significantly on the SOC.Golubkov et al.[9]concluded that the maximum surface temperature of LIBs with the format 18650was850 C as measured using a custom-designed test stand. The above researches have concentrated on the LIB burning*Corresponding author.Tel.:þ8655163600572(office).E-mail address:zhanghp@(H.Zhang).Contents lists available at ScienceDirect Journal of Power Sourcesjournal ho mep age:/locate/jpowsour/10.1016/j.jpowsour.2014.09.0390378-7753/©2014Elsevier B.V.All rights reserved.Journal of Power Sources273(2015)216e222behaviors while heated using different calorimeters.However,cone calorimeter,as an important apparatus in hazardous material as-sessments,has not yet been applied to evaluate the burning be-haviors of LIBs.Of the manyfire reaction properties measured by the cone calorimeter,it is generally recognized that the HRR is the most important factor in controllingfire hazards,which corre-sponds directly to the intensity offire.The HRR has been studied by Ribi e re using the Tewarson calorimeter.Their estimate of the maximum energy,liberated by the Joule effect which cannot indeedbe related to O2consumption,is about10%of the overall energy of a fully charged Li-ion polymer battery.The present work is to esti-mate the HRR of format18650LIB using the cone calorimeter.The HRR is estimated by oxygen depletion and mass loss methodolo-gies,providing experimental data to infer the amount of released energy using up oxygen liberated from the LIB.Then this work investigates the burning behaviors of LIBs.The experiments focus on two aspects:(1)the effect of the SOC on the burning behaviors of LIBs,(2)the burning behaviors of the fully charged LIBs at different incident heatfluxes.The collected data by the cone calorimeter can be either used directly by researchers or used as input data for mathematical models to analyze the thermal and chemical threats [10].2.Brief introduction to format18650lithium ion batteryAn18650lithium ion battery mainly consists of a positive electrode(cathode),a negative electrode(anode),a separator and electrolyte.The cathode is a lithium cobalt oxide and the anode is graphite.Lithium ions are extracted from the anode andflow into the cathode during discharge.The ions reverse direction during charge[11].As shown in Fig.1,the LIB has a diameter of18mm and a length of65mm.The separator is a very thin sheet of micro-perforated plastic allowing ions to pass through,which is located between the cathode and anode separating the positive and negative elec-trodes.The electrolyte is a solution consisting of organic solvent and inorganic salt,which provides the media for lithium ions transport.The LIB is constructed by winding long strips of elec-trodes into a“jelly roll”configuration.Electrode stacks or rolls can be inserted into hard cases that are sealed with gaskets.The enclosure of a hard case cell is aluminum.A burst disk is used as safety vent[12].3.Experimental setup3.1.SamplesThe LIBs used in the current study are manufactured by Sanyo. Table1shows the information of LIBs.The nominal capacity of the LIB is2.6Ah.The LIB is charged to the expected SOC and then put in the cone calorimeter to research the burning behaviors.3.2.ApparatusThe cone calorimeter experiments were carried out according to the procedures in the ISO5660-1standard[13].The samples were put horizontally in a sample holder staying on a load cell.Ceramic fiber blanket was used underneath the samples for thermal insu-lation.The sample holder was covered by a wire grid to prevent the LIB explosion splashing sparks from attaching the cone heater during the test.Two K-type thermocouples were wound around the sample using steel wires,which attaching the sample surface at different locations in order to obtain the surface temperature.The cone calo-rimeter was calibrated according to the procedure in the ISO5660-1 standard before experiments.Protection screen was put down to prevent explosion splashing sparks from widely spreading.Fig.2 presents the experimental setup in the cone calorimeter.As shown in Fig.2,the surface of the sample was radiated by the cone heater and heated up.The combustion productions were collected by an exhaust hood and transported away through a ventilation system.CO and CO2gas analyzers were used to measure the generation of carbon monoxide and carbon dioxide.An O2analyzer was used to measure the oxygen depletion.The fanflow rate was set as24L sÀ1.A camera was positioned to observe the burning process of the LIB.The ambient temperature was around30 C with natural ventilation.The experiments ended whenflame was extinguished.Each experiment was repeated at least three times and the average values were taken.During the test,the following parameters were determined: mass loss rate,the time to ignition and the time to explosion,the yields of CO and CO2,the surface temperature and HRR.The sample was weighted using a load cell.The time to ignition is defined as the duration time which is needed by the sample from being exposed to incident radiation till a visible and sustainableflame isfirstly established.The time to explosion is time from experiment start to explosion.The observation of time to ignition and explosion were recorded.The temperature readings were recorded using the Agi-lent34970A data acquisition system.The HRR and the yields of CO and CO2were recorded automatically by the cone calorimeter.4.Results and discussion4.1.Burning processThe experimental process shows the LIB reacts strongly while being heated.Fig.3presents the typical burning process of afully Fig.1.Structure of the lithium ion battery.Table1Information of commercial18650lithium-ion batteries.Sample Producer Type Capacity(Ah)SOC(%)A Sanyo UR18650FM 2.6100B Sanyo UR18650FM 2.670C Sanyo UR18650FM 2.665D Sanyo UR18650FM 2.650E Sanyo UR18650FM 2.6Fig.2.Experimental setup in the cone calorimeter.Y.Fu et al./Journal of Power Sources273(2015)216e222217charged LIB during the experiment.The sample undergoes 3noticeable stages during the experiment.At the stage 1,the sample mainly experiences a smoldering stage with no flame presented while in phase 2a sustainable flame is observed above the sample.Stage 2would last for several tens of seconds before the burning process goes into stage 3in which a severe explosion takes place.When the sample is exposed to heat flux,the surface temperature of the sample gradually increases.The temperature curve versus time is plotted in Fig.4,where the surface temperature is the average value obtained from the two thermocouples attached at the exposed sample surface.The increasing surface temperature makes the package plastic to melt and the generation of gas in the LIB results in the swelling of the LIB.Soon after,it is seen that the burst disk cracks and small amount of gas releases.At the same time,the surface temperature increases signi ficantly at 125 C because of solid electrolyte interface (SEI)layer breaking down exothermically.Consequently the intercalated lithium directly re-acts with the organic electrolyte and generates more heat [14,15].Due to the new SEI layer preventing the electrolyte from continu-ously reacting with intercalated lithium,there is a minor temper-ature decrease at approximately 255 C.The new SEI layer is formed by intercalated lithium reacting with the electrolyte [15].Then the surface temperature further rises because the new SEI layer decomposes exothermically with the increasing temperature.When the temperature is over 264 C,the surface temperature increases sharply from 264 C to the maximum temperature of 747 C within 10s,which is a result of the thermal runaway re-actions.It is also observed that a violent explosion occurs with the cap assemblies of the LIB ejected and a large amount of gas released,and then the fragments and evolved gases are ignited.When the explosion ends,the temperature decreases slowly,as shown in Fig.4.The temperature increasing rate against the surface tempera-ture is plotted in Fig.5.In the experiment,the cone heater heats up the sample surface and raises the surface temperature from 30to 125 C.Then the sample starts to react exothermically and reaches the first peak of increasing rate at 148 C.However,a sudden decline is caused by the endothermic separator fusing [7],as shown in Fig.5.It is noted that the temperature increasing rate starts to arise distinctly at the temperature around 264 C which is regarded as the onset temperature of thermal runaway reactions,at which a suf ficient concentration of flammable vapor is produced.By reference to Roth et al.[16],the SEI layer decomposes at 90e 130 C,intercalated lithium reacts with the electrolyte at 90e 290 C (electrolyte decomposes at 200e 300 C),and positively active materials decompose and react with the solvent at 150e 500 C.The endothermic reactions start about 170 C,fol-lowed by exothermic reactions in the temperature range of 170e 330 C according to the ARC tests [17].The results of the cone calorimeter experiments are basically consistent with those ob-tained by other calorimetric methodologies.4.2.Time to ignition and time to explosionThe time to ignition and time to explosion of LIBs are listed in Table 2.It can be seen that the time to ignition signi ficantly changes with the SOCs under the same incident heat flux.The higher the SOC is,the shorter time to ignition is.However,the LIBs with 0and 50%SOC are shorter than that of the LIB with 65%SOC.The timetoFig.3.Burning process of the fully charged LIB obtained in theexperiment.Fig.4.Temperature pro file with time of the fully charged LIB under an incident heat flux of 50kW m À2.Fig.5.The temperature rate versus the temperature of the fully charged LIB under an incident heat flux of 50kW m À2.Y.Fu et al./Journal of Power Sources 273(2015)216e 222218ignition of the fully charged LIB is the shortest of the LIBs atdifferent SOCs,indicating that the fully charged LIB ignites more easily and shows high fire risk.It also can be observed that the ignition time is inverse function of the incident heat flux.The higher the incident heat flux is,the shorter is the ignition time.In theory,the temperature and concentration of combustible gas are directly relevant to ignition.Fig.6presents the surface tempera-tures measured in the experiments.From Fig.6,the surface tem-perature increases as the incident heat flux increases.For high incident heat fluxes,the surface temperature increases speedy,the thermal decomposition of the LIB is also rapid and produces a large amount of gas.As a result,the time to ignition is short.On the other hand,for low incident heat fluxes,the surface temperature in-creases slowly,the emission of gas is slow so that the time to ignition is relatively long.The time to explosion decreases with the increasing SOCs.Table 2presents the fully charged LIB explodes at 81s while the LIB with 65%SOC explodes at 200s.However,the LIBs with the 50and 0%SOC do not explode during the experiments.4.3.Heat release rateAs the HRR is the most critical factor which governs thermal hazards in a given scenario and determines whether a neighbor LIB will be ignited in a battery pack [18],it needs to be seriously considered when assessing the fire risks of battery pack.There are a series of methodologies developed to evaluate the HRR,such as methodologies of oxygen depletion,mass loss [19,20]and tem-perature rise [21].The oxygen depletion based on Thornton's principle [22]is the most frequently used methodology.For a largenumber of organic solids or liquids or gaseous,Thornton found that the amount of energy released by a complete burning material was proportional to the amount of O 2consumed by the combustion reactions.The advantage of this methodology is the direct use of oxygen mass without the knowledge of the material chemical composition or the combustion chemistry.The HRR can be deter-mined using Equation (1)._q¼E_m 0O 2À_m O 2 (1)where _q is the heat release rate (kW),_m 0O 2and _m O 2are the massflow rates of oxygen from the incoming air and in the exhaust duct,respectively (kg s À1).E ¼13.1±5%MJ kg À1is the energy released per mass unit of O 2consumed for a given fuel [23].In this work,the HRR is measured by oxygen depletion method and calculated by mass loss method.Fig.7shows the HRRs estimated using the two methods.The mass loss rate is recorded by the load cell and the heat of com-bustion of a fully charged LIB is found in the literature [12].The HRR is calculated using the Equation (2)[18],_q¼c D H c _m fuel (2)where _mfuel is the mass loss rate (kg s À1),D H c ¼6.2MJ kg À1is the heat of combustion,and c denotes the combustion ef ficiency (c ¼0.78)[18].From Fig.7,the HRR curves can be also divided into 3stages.At the stages 1and 2,the HRR values are relatively low,while the ones at the stage 3are extremely high representing the LIB explosion happening.A comparison between the two methods indicates that the HRR curves are substantially identical.As shown in Fig.7,the HRR curves of the two methods almost overlap as zero at the stage 1due to the absence of flame.However,the HRR values estimated by oxygen depletion method are lower than that calculated values using Equation (2)at the stages 2and 3.This can be attributed to that the oxygen depletion method underestimates the actual oxy-gen consumed as the chemical reactions inside the LIB can produce a spot of oxygen during the burning.The calculated HRR values using Equation (2)increase slightly beyond 40s.However,at approximately 77s,the calculated HRR curve presents a steep decrease,which is caused by the pressure generated by the explosion.The load cell is pressed suddenly andTable 2Time to ignition and time to explosion of LIBs.Incident heat flux (kW m À2)SOC (%)Time to ignition (s)Time toexplosion (s)5010040815070831565065131200505049Not explode 50072Not explode 30100157182601001755Fig.6.Temperature pro files of the fully charged LIBs under incident heat fluxes of 30,50and 60kW m À2.Fig.7.HRR pro files of the fully charged LIB under an incident heat flux of 50kW m À2.Y.Fu et al./Journal of Power Sources 273(2015)216e 222219results in a sharp increase of mass reading.Shortly afterward,the calculated HRR value increases again and reaches the peak HRR.At the stage 2,the positive HRR values obtained by oxygen depletion method correspond to the packaged plastic melting and gas burning while the negative HRR values at about 44s and 75s are caused by the oxygen released from the decompositions of cathode and anode,as observed in Fig.6.Kumari et al.[24]claims that generation of O 2is due to the decomposition of cathode.MacNeil et al.[25,26]reports that above 200 C the delithiated Li 0.5CoO 2decompose and release O 2as:Li 0:5CoO 2/12LiCoO 2þ16Co 3O 4þ16O 2(3)Co 3O 4can decompose with the presence of solvent at 400 Cand release O 2and CoO.The CoO can further decompose at 473 C and release O 2.Co 3O 4/3CoO þ1O 2(4)CoO !Co þ12O 2(5)In the meantime,the negative electrode also releases O 2.ðCH 2OCO 2Li Þ2/Li 2CO 3þC 2H 4þCO 2þ12O 2(6)At the stage 3,the HRR values obtained by oxygen depletion method correspond to the thermal runaway reactions and explo-sion.The energy is fleetly released and the peak HRR swiftly rea-ches,which might be explained by the presence of acidic C e H bonds of imidazolium cations easily converted into NHC-carbenes under heat conditions [27].It should be noted that although small quantities of oxygen are released from the energetic LIB during the burning,the internally generated oxygen has insigni ficant effect on the combustion pro-cess and the HRR.The comparison between the measured and calculated HRR values shows the biggest discrepancy is approxi-mately 13%,as shown in Fig.7.Therefore,it is estimated that the energy,consuming the O 2released by the internal redox reactions,accounts for less than 13%of the overall energy released by a fully charged LIB.This con firms the feasibility of using cone calorimeter for the HRR determination.4.3.1.Effect of the state of charge on heat release rateThe SOC of the LIB is one of the most critical factors to the chemical reactions and it should be less than 50%to minimize degradation and aging during the transportation [28].Fig.8shows the HRR pro files of LIBs with the SOCs from 0to 100%under an incident heat flux of 50kW m À2.The HRR pro files of LIBs with 50and 0%SOC exhibit similarly while the others are closely resemble.As shown in Fig.8,the SOC has an effect on both the time to the peak HRR and the magnitude of the peak HRR.The peak HRR values reach 6.8,6.5,5.8,1.5and 1.1kW for 100,70,65,50and 0%SOC,respectively.It can be concluded that the fully charged LIB is more risky compared to the others as it produces the highest HRR,whereas the LIB with 0%SOC is the safest case with the lowest HRR and without explosion.This is probably because that the HRR is affected by the stored electrical energy which is stored as potential chemical energy [29].The more energy a LIB has stored,the more energetic the exothermic reactions of the electrodes with the electrolytes will be,the more heat and gas generated will be,the easier the explosion occurs.Thus,it can be concluded that the burning hazards of the LIB rises with the increasing SOCs and less than 50%SOC is reasonable to be suggested in the transportation.Integrating the HRR curves gives the total energy released during the burning which ends up as approximately 100,101,104,120and 109kJ for 100,70,65,50and 0%SOC,respectively.It is noted that the fully charged LIB releases the least energy but has the highest HRR value.This might be attributed to two aspects:(1)the explosion suddenness restricts the oxygen consumption,lead-ing to incomplete combustion [8],(2)the internal released oxygen is involved in the combustion in relatively short burning process.4.3.2.Effect of incident heat flux on heat release rateFig.9shows the HRR pro files of LIBs with 100%SOC under incident heat fluxes of 30,50and 60kW m À2.It is found that the LIBs burn much more vigorously under high incident heat fluxes of 50and 60kW m À2than that under 30kW m À2.The increasing incident heat fluxes have a strong in fluence on the intensities of thermal degradation and thermal runaway reactions.It can be seen in Fig.9that the peak HRR values increase with the increasing incident heat fluxes.According to the experimental results,the peak HRR increase from 6.2to 9.1kW as the incident heat flux increase from 30to 60kW m À2.Fig.8.HRR pro files of LIBs at different states of charge under an incident heat flux of 50kW m À2.Fig.9.HRR pro files of the fully charged LIBs under incident heat fluxes of 30,50and 60kW m À2.Y.Fu et al./Journal of Power Sources 273(2015)216e 2222204.4.Gas analysisAnother key factor related to fire is the toxic gases released.Figs.10and 11show the CO 2and CO productions pro files for different experimental conditions.From Figs.10and 11,CO and CO 2productions increase with the increasing SOCs.O 2is generated from the decomposition of the overcharged delithiated LiCoO 2as in Equation (3).The air inside the LIB contains CO 2and the redox reactions in the LIB also generate CO 2as in Equations (7)e (9).The oxidation reactions of electrolyte and O 2in the LIB release CO 2[30]:C 3H 6O 3þ3O 2/3CO 2þ3H 2O(7)The other reactions of water and HF (decomposition product of LiPF6)in the electrolyte are:LiOCO 2CH 3þHF /LiF þCH 3OH þCO 2(8)ðCH 2OCO 2Li Þ2þH 2O /Li 2CO 3þ2CH 2OH þCO 2(9)The carbon monoxide is likely to be generated from the reduc-tion of CO 2with intercalated Li at the anode.2CO 2þ2Li þþ2e À/Li 2CO 3þCO(10)Another source of the carbon monoxide may be the incomplete combustion.As a result,higher SOCs will lead to larger amount of O 2.The increasing concentration of oxygen will lead to more CO 2and CO productions according to Equations (7)and (10)consequently.At the ignition point both the CO and CO 2emitted from the LIB increase because the CO and CO 2increase when the flame appears.The CO and CO 2generation rates present a signi ficant increase at which the LIB goes into thermal runaway reactions till explosion and then decrease rapidly.5.ConclusionsThis study has examined the burning behaviors of LIBs using the cone calorimeter with key parameters including the HRR,mass loss rate,the time to ignition and concentration of toxic gases investi-gated.The HRR was analyzed using oxygen depletion and mass loss methodologies.From the comparison of the two methods,it is concluded that the energy,consuming oxygen released from theLIB,accounts for less than 13%of the overall energy released by a fully charged LIB.The LIBs with 100,70,65,50and 0%SOC were evaluated under incident heat flux of 50kW m À2while the LIBs with 100%SOC were tested under different incident heat fluxes of 30,50and 60kW m À2.The results show that the SOC has signi ficant effects on the burning behaviors as the stored electrical energy plays an important role in burning process.According to result,the fully charged LIB presents the highest surface temperature of 797 C,the maximum peak HRR value of 6.8kW,the shortest time to ignition of 40s,the shortest time to explosion of 81s and the highest CO and CO 2productions of respective 38kg kg À1and 348kg kg À1.As a result,the fully charged LIBs are more dangerous than the others.With respect to the effect of incident heat flux,exposing the LIBs to high incident heat fluxes would be very dangerous,as the accumulation of heat at the surface might cause severe thermal decomposition and thermal runaway reactions.The temperature control of the LIB is crucial to prevent the occurrence of thermal runaway reactions.The surface temperature and the peak HRR in-crease as the incident heat flux increases while the time to ignition and time to explosion decrease.The highest temperature reaches 934 C at 60kW m À2which is far more than the ignition temper-ature of normal combustible materials.The burning LIB could ignite adjacent batteries in the battery pack and cause the entire batteries to ignite or rupture.The current results also indicate that applying the cone calorimeter to evaluate the thermal hazards of LIB is a reasonable technique in LIB safety assessments.Future work will focus on the thermal behaviors of multiple LIBs in a module trig-gered by heating conditions.AcknowledgementsThis study was supported by the National Natural Science Foundation of China (No.71403254)and the China Postdoctoral Science Foundation (No.2014M551823).References[1]T.M.Bandhauer,S.Garimella,T.F.Fuller,J.Electrochem.Soc.158(2011)R1e R25.[2]H.J.Noh,S.Youn,C.S.Yoon,Y.K.Sun,J.Power Sources 233(2013)121e 130.[3]AirportWatch,Air Freight News,2011./?p ¼3521.Fig.10.CO production pro files of LIBs at different states of charge under an incident heat flux of 50kW m À2.Fig.11.CO 2production pro files of LIBs at different states of charge under an incident heat flux of 50kW m À2Y.Fu et al./Journal of Power Sources 273(2015)216e 222221[4] C.Arbizzani,G.Gabrielli,M.Mastragostino,J.Power Sources196(2011)4801e4805.[5] C.Y.Jhu,Y.W.Wang,C.Y.Wen,C.C.Chiang,J.Therm.Anal.Calorim.106(2011)159e163.[6] C.Y.Jhu,Y.W.Wang,C.M.Shu,J.C.Chang,H.C.Wu,J.Hazard.Mater.192(2011)99e107.[7] E.P.Roth,D.H.Doughty,J.Power Sources128(2004)308e318.[8]P.Ribi e re,S.Grugeon,M.Morcrette,S.Boyanov,ruelle,Energy Environ.Sci.5(2012)5271.[9] A.W.Golubkov,D.Fuchs,J.Wagner,H.Wiltsche,C.Stangl,G.Fauler,G.Voitic,A.Thaler,V.Hacker,RSC Adv.4(2014)3633.[10]K.C.Tsai,J.Hazard.Mater.172(2009)763e772.[11]Q.Wang,P.Ping,X.Zhao,G.Chu,J.Sun,C.Chen,J.Power Sources208(2012)210e224.[12]M.Celina,K.Michael,W.Kevin,T.L.Richard,Fire Protection Research Foun-dation,2011.[13]ISO5660-1,Reaction to Fire Tests e Heat Release Smoke Production and MassLoss Rate Part1Heat Release Rate,2002.[14]T.Ohsaki,T.Kishi,T.Kuboki,N.Takami,N.Shimura,Y.Sato,M.Sekino,A.Satoh,J.Power Sources146(2005)97e100.[15]Q.Wang,J.Sun,X.Yao,C.Chen,J.Electrochem.Soc.153(2006)A329.[16] E.P.Roth, D.H.Doughty,J.Franklin,J.Power Sources134(2004)222e234.[17]J.S.Gnanaraj,E.Zinigrad,L.Asraf,H.E.Gottlieb,M.Sprecher,D.Aurbach,M.Schmidt,J.Power Sources119e121(2003)794e798.[18]G.G.Eshetu,S.Grugeon,ruelle,S.Boyanov, A.Lecocq,J.P.Bertrand,G.Marlair,Phys.Chem.Chem.Phys.:PCCP15(2013)9145e9155.[19]ISO5660,Fire Tests e reaction to Fire e part1:Rate of Heat Release fromBuilding Products(Cone Calorimeter),ISO,Geneva,1993.[20]ASTM1354e905,Standard Test Method for Heat and Visible Smoke ReleaseRates for Materials and Products Using an Oxygen Consumption Calorimeter, ASTM,Philadelphia,1995.[21]ASTM E-906,Standard Test Method for Heat and Visible Smoke Release Ratesfor Materials and Products,ASTM,Philadelphia,1983.[22]W.M.Thornton,Philos.Mag.Ser.633(1917)196e203.[23] C.Huggett,Fire Mater.4(2)(1980)61e65.[24]K.Kumai,H.Miyashiro,Y.Kobayashi,K.Takei,R.Ishikawa,J.Power Sources81(1999)715.[25] D.D.MacNeil,J.R.Dahn,J.Electrochem.Soc.149(2002)A912e A919.[26] D.D.MacNeil,J.R.Dahn,J.Electrochem.Soc.148(2001)A1205e A1210.[27]H.Yao,J.Zhang,Y.Zhang,H.Sun,Q.Shen,Organometallics29(2010)5841e5846.[28] D.Michael,J.Power Sources96(2001)260e265.[29]K.White,Q.Horn,S.Singh,R.Spray,N.Budiansky,in:Proceedings,28th In-ternational Battery Seminar and Exhibit,uderdale,FL,2011,pp.14e17.[30]K.Kazuma,M.Hajime,J.Power Sources81e82(1999)715e719.Y.Fu et al./Journal of Power Sources273(2015)216e222 222。