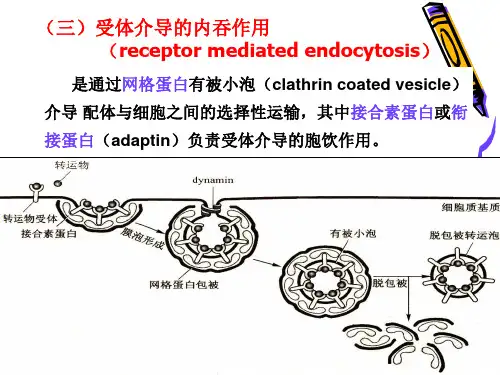
内吞作用
- 格式:docx
- 大小:37.76 KB
- 文档页数:4

9.5细胞的分泌与内吞作用将蛋白质运送给细胞质膜或细胞外是通过细胞的胞吐作用(e x o c yt o s i s),也称细胞的分泌活动。
与分泌过程相反的是细胞的内吞作用。
9.5.1细胞分泌(c e l l s e c r e t i o n)动物细胞和植物细胞将在粗面内质网上合成而又非内质网组成部分的蛋白和脂通过小泡运输的方式经过高尔基体的进一步加工和分选运送到细胞内相应结构、细胞质膜以及细胞外的过程称为细胞的分泌。
■细胞分泌活动的过程细胞分泌活动是非常重要的生命现象,整个过程涉及三种不同的细胞器和细胞结构(图9-50)∶内质网、高尔基体、细胞质膜。
这三部分相当于三道关卡,严格地控制着产品的质量。
这三个部分的职能又是不同的,内质网相当于生产基地,高尔基体相当于产品的精加工和质量检测分配部门,而细胞质膜相当于海关。
图9-50细胞的分泌与内吞作用图中显示了由E R合成的蛋白质经分泌小泡通过高尔基体复合物运向各目的地,包括溶酶体。
分泌泡分泌途径:①核糖体合成的蛋白质与粗面内质网外表面的结合,并在E R腔中糖基化;②从内质网形成的小泡携带新合成并经糖基化的蛋白到达顺面高尔基体;③通过膜融合,蛋白质进入高尔基体,并在高尔基体中进一步加工后通过小泡转运到反面高尔基体,经浓缩并经出芽形成④分泌小泡或⑤溶酶体小泡;分泌小泡移向质膜,或通过组成型(4a)或通过调节型(4b)释放小泡内容物;⑥内吞作用从细胞外摄取蛋白质或其他物质,内吞泡或是与反面高尔基体融合(6a)或是与溶酶体融合(6b)。
■组成型和调节型的分泌蛋白质从内质网经高尔基体到细胞表面的物质运输是通过运输小泡和胞吐作用不断进行的。
这种分泌活动分为两种类型,组成型和调节型(图9-51)。
图9-51组成型和调节型分泌活动●组成型分泌途径(c o n s t i t u t i v e s e c r e t o r y p a t h w a y)这种分泌途径中运输小泡持续不断地从高尔基体运送到细胞质膜,并立即进行膜的融合,将分泌小泡中的蛋白质释放到细胞外,此过程不需要任何信号的触发,它存在于所有类型的细胞中。

核定位信号(NLS):引导蛋白质进入细胞核的一段信号序列,受体为importin 。
核输出信号(NES):引导RNA输出细胞核的一段信号序列,受体为exportin。
着丝粒:处于主缢痕的内部,是主缢痕的染色质部位。
主缢痕:在两条姐妹染色单体相连处,有一个向内凹陷的缢痕,称为主缢痕,光镜下,相对不着色。
次缢痕:在某些染色体上除具有主缢痕外,还有另一个染色较浅的缢痕部位称为次缢痕,其大小和范围是恒定的,常存在于近端着丝粒染色体的短臂上,可作为染色体的鉴别标志。
端粒:是存在于染色体末端的特化部位。
通常由一简单重复的序列组成,进化上高度保守。
人体细胞中序列为GGGTAA。
核基质:是真核细胞间期中除核被膜、染色质和核仁以外的一个精密的网架系统。
又称核骨架。
核仁(nucleolus):见于间期的细胞核内,呈圆球形,一般1~2个,有时多达3~5个。
主要功能是转录rRNA 和组装核糖体单位。
核仁趋边(边集):在生长旺盛的细胞中,核仁常趋向核的边缘,靠近核膜,即发生该现象细胞骨架(cytoskeleton):由蛋白纤维交织而成的立体网架结构,充满整个细胞质的空间,以保持细胞特有的形状并与细胞运动有关。
包括:微管、微丝、中间纤维三种类型。
微管组织中心MTOC :微管聚合从特异性的核心形成位点开始,这些核心形成位点主要是中心体和纤毛的基体,称为微管组织中心,微管在生理状态或实验解聚后重新装配的发生处称为微管中心,其存在位置为间质的中心体。
微管:真核细胞质中的一种中空圆柱状的结构,主要由微管蛋白组成,作为细胞中骨架系统,微管具有维持细胞形态,组成新细胞的功能。
微丝:真核细胞质中含肌动蛋白的细丝,直径约为5-9nm,微丝具有许多重要功能,如细胞形状的维持、细胞运动、细胞收缩等。
中间纤维(IF):中间纤维是一种直径约为10nm的纤维状蛋白,由于其直径介于粗肌丝和细肌丝以及微丝和微管之间,因此命名为中间纤维。
基粒:内膜的内表面附着许多突出于内腔的颗粒,由许多蛋白质亚基构成,分为头部、柄部、基片,又称为A TP酶复合体。


生物化学名词解释遗传密码:mRNA分子中每三个相邻的核苷酸组成一组,在蛋白质翻译合成时代表一个特定的氨基酸,这种核苷酸三联体称为遗传密码翻译:以mRNA为模板,根据碱基排列顺序合成相应的蛋白质的过程限制性内切酶:原核生物中存在着一类能识别外源DNA双螺旋中4-8个碱基对所组成的特异的具有二重螺旋对称性的回文序列,并在此序列的某位点水解DNA链,产生粘性末端或平末端,这类酶称为限制性内切酶中心法则:中心法则是指遗传信息从DNA传递给RNA,再从RNA 传递给蛋白质转录:是指生物体按照碱基互补配对的原则把DNA碱基序列转化为RNA碱基序列,从而将传信息传递到RNA分子上的过程半保留复制:当DNA进行复制时,亲代DNA双链必须解开,两股链分别作为模板,按照碱基互补配对原则指导合成一股新的互补链,最终得到与亲代DNA碱基序列完全一样的两个子代DNA分子,每个子代DNA分子都含有一股亲代DNA和一股新生DNA链,这种复制方式称为半保留复制。
冈崎片段:DNA复制时,不连续合成的亲代单链( 5′→3′)先合成小的DNA片段,再连接称完整的链,这些片段由冈崎等人发现,称为冈崎片。
生物固氮:利用微生物中固氮酶的作用,在常温常压条件下将大气中的氮还原为氨的过程蛋白质的生理价值:指食物蛋白质被动物机体合成组织蛋白质的利用率,即蛋白质的生理价值=氮的保留量/氮的吸收量x100%氧化脱氢基作用:氨基酸在氨基酸氧化酶和谷氨酸脱氢酶的作用下,脱去氨基,生成氨和α-酮酸的酮基,生成相应的α-氨基酸和α-酮酸的过程。
转氨作用:在转氨酶的作用下,把一种氨基酸上的氨基转移到α-酮酸上,形成另一种氨基酸联合脱氨作用:氨基酸的脱氨基既通过转氨作用,又通过氧化脱氨基作用,这种联合脱氨基的方式称为联合脱氨基作用。
脂肪酸β-氧化:脂肪酸在体内氧化时在羧基端的β-碳原子上进行氧化,碳链逐次断裂,每次断下一个二碳单位,即乙酰CoA,该过程称作β-氧化。

adc抗体偶联药物作用原理
ADC(抗体-药物复合物)是一种靶向治疗药物,它将单克隆抗体与细胞毒性药物结合在一起,以便把药物直接传递到癌细胞。
ADC 的作用原理可以从多个角度来解释:
1. 靶向作用,ADC的抗体成分具有特异性,能够识别和结合到癌细胞表面的特定抗原。
一旦抗体与癌细胞表面的抗原结合,ADC 就被内吞进入癌细胞内部。
2. 内吞作用,一旦ADC进入癌细胞内部,它会被内吞体系吞噬到内部囊泡中。
这种内吞过程有助于将ADC释放到癌细胞的细胞质内。
3. 药物释放,一旦ADC进入癌细胞的细胞质,它会被特异性的酶分解,释放出细胞毒性药物。
这种药物释放过程确保了药物的作用仅限于癌细胞内部,减少了对正常细胞的损害。
4. 细胞毒性作用,一旦细胞毒性药物释放到癌细胞内部,它会干扰癌细胞的代谢过程、DNA合成或细胞分裂,最终导致癌细胞的死亡。
总的来说,ADC的作用原理是通过将抗体与细胞毒性药物结合在一起,实现了对癌细胞的精准靶向治疗,最大程度地减少了对正常细胞的损害,从而提高了治疗的有效性和安全性。
这种治疗方法在癌症治疗领域具有广阔的应用前景。

分子生物学专业名词AAllele(等位基因):在染色体上占据给定位点基因的不同形式。
Alu family (Alu 家族):人类基因组中一系列分散的相关序列,每个约300bp 长。
每个成员其两端有Alu 切割位点(名字的由来)。
Amber codon ( 琥珀密码子):核苷酸三联体UAG,引起蛋白质合成终止的三个密码子之一。
Aminoacyl-tRNA ( 氨酰-tRNA):是携带氨基酸的转运RNA,共价连接位在氨基酸的NH2基团和tRNA 终止碱基的3`或者2`-OH基团上。
Aminoacyl-tRNA synthetases ( 氨酰-tRNA 合成酶):催化氨基酸与tRNA 3`或者2`-OH 基团共价连接的酶。
Amplification ( 扩增):指产生一个染色体序列额外拷贝,以染色体内或者染色体外DNA形式簇存在。
Annealing (退火):两条互补单链配对形成双螺旋结构。
Antibody ( 抗体):由B 淋巴细胞产生的蛋白质(免疫球蛋白质),它能识别特殊的外源“抗原”,从而引起免疫应答。
Anticoding strand (反编码链):DNA 双链中作为膜板指导与之互补的RNA合成的链。
Antigen (抗原):进入基体后能引起抗体(免疫球蛋白质)合成的分子。
Antitermination protein ( 抗终止蛋白质):能够使RNA 聚合酶通过一定的终止位点的蛋白质。
Apoptosis ( 细胞凋亡):细胞进行程序性死亡的能力;对刺激应答使通过一系列特定反应摧毁细胞的途径发生。
Attenuation (衰减):控制一些细菌启动子表达中涉及的转录终止调控。
Attenuator (衰减子):衰减发生处的一种内部终止子序列。
Autonomous controlling element ( 自主控制元件):玉米中一种具有转座能力的转座元件。
BBacteriophage (细菌噬菌体):侵染细菌的病毒,通常简称为噬菌体。


Chinese-German Journal of Clinical Oncology June 2009, Vol. 8, No. 6, P360–P365 DOI 10.1007/s10330-009-0023-9In the process of cell metabolism, various kinds of ma-terials including some ions, small molecules, macromo-lecular materials and some granular matters keep in and out of cells. The macromolecular substances and granular materials can not go through the membrane. They com-plete the transfer across the cell membrane in another way, that is, materials cross in and out of cell membrane in the form of vesicle which is fused with membrane and then sciss into the cell, which is currently recognized as the main pathway of intaking biological macromolecules-endocytosis. Endocytosis is broadly divided into two cat-egories, phagocytosis and pinocytosis; the latter is divided into four species according to their different mechanisms: clathrin dependent endocytosis, caveolin dependent en-docytosis, macropinocytosis, and clathrin and caveolin independent endocytosis.The recent study reported that the abnormal expres-sion of endocytosis may be involved in the mechanism of certain diseases, such as diabetes and neurological diseases and also closely related to the malignant transformation of cells. The role of endocytosis was paid increasing atten-tion. Further research in this area will help us understand these diseases, thereby found new treatments. Here we introduce about pathway of cell endocytosis, mechanisms of regulation and endocytotic proteins. Endocytosis pathwayPhagocytosisPhagocytosis refers to endocytosing large granular matters (> 250 nm), and it provides the host a direct way digesting exogenous substances, which is one of the most important immune protecting mechanisms [1]. In mamma-lian, phagocytosis can only be completed by specific cells, such as macrophages and neutrophils which are called phagocytic cells. Phagocytic cells recognize and combine with IgFc and complement packing pathogens through IgFc receptor and complement receptor respectively. When the cell surface receptors combined with the cor-responding ligands, downstream signal transduction was activated, which caused actin polymerization under plas-ma membrane of intaking site, actin contraction makes phagocytic cell membrane form pseudo-foot fusing into vesicle to pack pathogens. In cytoplasm, dynamin assem-bled into ring at the neck of vesicle, and hydrolysed the binding GTP, dynamin contraction forced vesicle to sciss from membrane at the neck and form phagosome which integrated with the lysosome, the acid hydrolysis enzyme of lysosomal digested pathogens. In addition to phagocy-tosing pathogens, macrophages of phagocytic cells canStudy progress of cell endocytosis*Li Chen1, Hui Li1, Ren Zhao2, jianwei Zhu31Department of Pathology, Nantong University Medical College, Nantong 226001, China2Department of General Surgery, Ruijin Hospital, Shanghai Jiaotong University, Shanghai 230002, China 3Department of General Surgery, Affiliated Hospital, Nantong University, Nantong 226001, ChinaReceived: 19 February 2009 / Revised: 13 March 2009 / Accepted: 10 April 2009Abstract Endocytosis is a process through which extracellular materials are transported into cell through membrane defor-mation. This process is not a simple step-by-step process in which a series of proteins function according to the chronological order, but rather a complex process comprising many members which are regulated precisely. The role of endocytosis is broadly divided into two categories, phagocytosis and pinocytosis, the latter is divided into four species in accordance with the size of endocytosis substances: clathrin dependent endocytosis, the diameter of clathrin-coated vesicle is 100–150 nm;caveolin dependent endocytosis, the diameter of caveolin protein-coated vesicle is 50–100 nm; macropinocytosis, the diam-eter of macropinocytosis is generally 0.5–2 μm, sometimes up to 5 μm; clathrin and caveolin independent endocytosis. Many proteins including endophilin A1, A2, A3, and endocytotic proteins B, B1a, and B1b as well as dynamin, actin and Rab protein families are involved in endocytosis and play an important role in different stages. The abnormal endocytosis may be involved in the development of certain diseases.Key words endocytosis; clathrin; caveolin; endophilin; signalling pathwayCorrespondence to: Jianwei Zhu. Email: usazhujianwei@* Supported by grants from the National Natural Sciences Foundationof China (No. 30771126 and 30772106).361 Chinese-German J Clin Oncol, June 2009, Vol. 8, No. 6swallow foreign bodies through ligand-receptor binding pattern. Identify and kill tumor cells, identify and remove degenerative plasma protein, lipids, and other macromol-ecules. Remove aging and damaged cells and cell debris. PinocytosisPinocytosis refers to the intake process that endocyto-ses extracellular liquid and the dissolving materials. Clathrin dependent endocytosisClathrin plays an important role in the regulation of composition of plasma membrane proteins, and research on clathrin can help us understand how cells interact with the surrounding environment, signal transduction of mitogenic, nutrition intake of the cell, establishment of extracellular environment cell identity including the interaction with the immune system, keep a balance in the stability of the environment of cell.(1) Clathrin and adaptor protein (AP)Clathrin is the skeleton protein outside the vesicle, clathrin-coated vesicle is 100–150 nm in diameter and exists in all eukaryotic cells and it mediates the way of transportation from the plasma membrane to intracellu-lar of proteins, lipids, nutrients, antibodies and growth factors and also the vector through which proteins and lipids transport from trans Golgi net work (TGN) to endosome. Clathrin is spider-like and polymerized by three chains at the top, which is known as triskelion [1]. Adapter lies inside the clathrin-coated vesicle, which mediates membrane binding, localization, sorting signals, identification and inositol phosphate. It not only serves to combine cargo and clathrin, but also connect with polyphosphatidylinositol headgroup. It is now found four kinds of adapters (AP1–4), all of which comprise a pair of 100–130 kD subunit. These subunits are able to identify 6-phosphate mannose receptor, transferrin, low-density lipoprotein and epidermal growth factor receptor, prote-ase and de-sialic acid receptor.(2) MechanismThe process from recruitment to disassemble is very short. It comprises as follows:1) Recruitment of adapter and clathrin. Recruitment of AP2 complex to high activity, saturated, easy enzymolysis site, activate formation of clathrin-coated vesicle in the plasma membrane under the effect of sorting signal and docking protein [2].2) Invagination, scission and budding of clathrin-coat-ed vesicle. The planar clathrin protein can be transformed into curved one without nucleic acid and cytoplasm in vitro; In vivo, clathrin-coated vesicle curves to some de-gree. Invagination may be due to structure changes or rearrangement of clathrin assembling in the crystal lat-tice or between clathrin. The budding of clathrin-coated vesicle is involved with GTPase dynamin, actin tubulizes or forms ring-shape in vitro, it is the trigger that vesicle dissociated from the membrane.3) Decapsulation of clathrin-coated vesicle. This is a wasting process that needs hsc 70, auxilin and ATP. The large chain of clathrin has two sites which interact with the adapter and also with hsc 70. The interaction between clathrin and hsc 70 destroyed the interaction between clathrin and adapter. Over-expression of hsc 70 mutant blocked the cycle of transferrin receptor so that the “as-sembly – dismantle” balance moved to the assembly di-rection. Hsc 70 dissociated clathrin from the vesicle in vitro, but could not dissociate adapter. Auxilin plays an important role in decapsulation. Auxilin can not only re-cruit hsc 70 to clathrin-coated vesicle, but also stimulate activity of hsc 70 ATP enzyme.Caveolin dependent endocytosisCaveolae/caveolins mediate endocytosis of many sub-stances and is the main form of clathrin-independent en-docytosis.(1) As early as 1950, Japanese scholar Yamada used transmission electron microscopy to observe some small caveolae which was about 50–100 nm in diameter for the first time. These vesicles appeared alone or string-like, in the form of invagination of the cytoplasmic membrane, it had typical lipid bilayer structure [2]. Caveolae is currently considered to be the signal transduction center, and many signal transduction receptors, protein kinase and binding proteins are highly enriched in caveolae region. In the regulation of caveolae endocytosis, activation of tyrosine kinase-dependent signal is an important step. After the treatment of phosphatase inhibitor, the endocytosis of cell by caveolae had increased notably.(2) Caveolin is the main surface marker of a caveolae, a kind of membrane integrin family modified in the inner surface of caveolae, 21–25 kD. It consists of N-terminal region, transmembrane region and C-terminal region, N-terminal and C-terminal cytoplasmic moves inward into the cytoplasm and its peptide chains like hairpin struc-ture. Caveolin plays a critical role in the assembly process of caveolae and cholesterol and is the signaling molecule of the scaffold proteins and negative regulatory proteins and belongs to a highly conserved integrity membrane protein family [3]. Rajjayabun [4] confirmed that caveolin-1 was the key factor of caveolae endocytosis. If caveolin gene was knockout, caveolae can not be formed. So far, four kinds of caveolin isomers have been found in mammals: caveolin-1α, 1β, 2 and 3, which are products of differ-ent genes, most cells expressed caveolin-1 and caveolin-2, the two form a stable heterologous oligomers complexes, particularly rich in terminal differentiated cells, such as fat cells, endothelial cells and fibroblasts, and caveolin-3 mainly exists in various muscle cells (such as myocardial cells, rhabdomyosarcoma cells and skeletal muscle cells) and is closely related to the synthesis of muscle cells [5]. Neither caveolin protein nor caveolae structure appeared362 www. springerlink. com/content/1613-9089in peripheral blood cells and nerve cells.(3) The biological feature of caveolin. Caveolae as a sig-naling molecule plays a role as a platform in signal trans-duction. Caveolin-1 is in the center of signaling pathways at all these platforms. Caveolin, as a signaling molecule’s scaffold protein and negative regulatory protein, inhibits kinase activities of signaling molecules in the normal sig-nal transduction pathways. Caveolin-2’s skeleton region has no inhibitory activities, and other signaling molecules inhibitory regions may exit in it. Caveolin-3 is involved in energy metabolisms in muscle cells. Caveolin-1 has strong affinity to cholesterol, thus the cholesterol levels of caveolae is far higher than other biofilm. The study showed that the absence of caveolae eventually resulted in formation of foam cells, suggesting that caveolae and caveolin-1 can maintain the cholesterol balance by re-moving the excessive lipoprotein cholesterol out of cells [6].The correlation between caveolin and tumors have become one of the hot spots in tumor biology, among which, the relationship between caveolin-1 and tumor occurrence and metastasis has been studied a lot. Gene coding for caveolin-1 (CAV) locates in the suspicious tu-mor suppressive site (D7S522; 7q31.1). This site depletes or fractures in a variety of tumors (such as liver cancer, ovarian cancer, breast cancer, uterine fibroids, gastric ad-enocarcinoma, etc. [7]). The normal NIH 3T3 cell which was introduced by antisense caveolin-1 was transplanted into nude mice to observe the formation of tumor, sug-gesting caveolin-1 has tumor suppressive function. In many tumors and activated oncogene transfected cells, the expression of mRNA and its protein of caveolin-1 de-creased or lost [8]. The experiments in vivo demonstrated mutation or deletion of caveolin-1 could lead to the ex-cessive proliferation of breast epithelial cells, and then increase the occurrence of breast cancers [9]. Besides, the expression of caveolin uprehulated in diabetes and hyper-cholesterolemia and plays a significant role in Alzheim-er’s disease [10], degenerative muscle disease [11], heart and lung diseases.MacropinocytosisLarge and irregular original endocytosis vesicles are formed by folding membrane on the verge of extending cell under stimulation by certain factors, and they are known as macropinosome whose size varies with diam-eter generally 0. 5–2 μm. Macropinocytosis plays a major role in macrophages and dendritic cells, also in many tu-mor cells [11].Macropinosome has no clathrin or caveolins coating; it is closely related to actin at the early stages of formation, which provides an effective way for non-selective endo-cytosis of extracellular nutrients and liquid phase macro-molecules. Macropinocytosis and phagocytosis, regulated by a variety of proteins, such as actin, Scar protein, Ap21 complexes and RabB. Different membrane wrinkle results in different rates of developing macropinocytosis.The formation of macropinosomes can be significantly inhibited by cytochalasin D and colchicine, suggesting that microtubules and microfilaments play an important role in this process. The mechanism may be the stimulus by a variety of factors that activate corresponding recep-tor tyrosine kinase and then self-phosphorylate quickly. Phosphorylated residues recruit PI3 kinase and activate it, the activated PI3 kinase activates Rac1 which may cause microfilament reconstruction through two ways. Firstly, the activated Rac1 activates PAK1 which regulates phos-phorylation of myosin light chain. The interaction be-tween myosin and actin is regulated by the phosphory-lated myosin light chain and the interaction promotes reconstruction of microfilaments, development of the cell membrane ruffles and formation of macropinosomes; Secondly, the activated Rac1 binds with the amino-ter-minal of its target protein IRSp53, SH3 region at the car-boxyl end of IRSp53 combines with WAVE to form three molecular complexes which could activate WAVE [12]. The latter further activates actin related protein Arp2/3 complexes which stimulate microfilament nucleation, promote microfilament reconstruction, development of cell membrane ruffles and formation of macropinosomes [13]. In-depth regulation mechanism needs further study. MicrodomainMicrodomain has similar lipid composition with caveolae but not function through caveolin. It functions through dynamin and Rho A-dependent mechanism, or through clathrin-independent, dynamin-2-independent, Cdc4 mediated pathway to endocytose glycosylated phos-phatidylinositol-hexanol anchored proteins [13]. The path-way plays a role in endocytosis of IL-2. EndophilinThe biological features of endophilinThe main function of endophilin is related to the en-docytosis of neurotransmitter. Endophilin has a common special structure, whose C-terminal has a unique SH3 do-main with the unique binding ability [14], which can in-teract with a number of special proteins such as dynamin, thus affecting the functions of other proteins. While N-terminal participates in membrane invagination of vesi-cles. They were named endophilin A1, A2, A3 in 2002. Huntingtin belongs to binding protein of endophilin A1, the complex composed of it and the huntingtin binding protein 40 (HAP40) inhibited early microtubule-depen-dent endosome movement, increased connection be-tween early endosome and actin [15]. Then a new family was discovered, which was named endophilin B.363 Chinese-German J Clin Oncol, June 2009, Vol. 8, No. 6Endophilin and its role in signal transduction Endophilin can interact with various proteins through which involved in different signal transduction pathway Endophilins which interact with cell membrane re-ceptors are mainly distributed in the cytoplasm and cell membrane, it can interact with cytoplasmic membrane receptor and conduct signals, such as beta 1-adrenergic receptor. Endophilin is also involved in phospholipid sig-nal transduction. Many endophilins contain the binding sites of inositol 4,5-diphosphate (PIP2). The degradation of PIP2 is necessary for the completion of the endocytosis. Mark PIP2 connected domain by immunohistochemical fluorescent protein and track endocytosis movement of living cells, and PIP2 was found present in the cell mem-brane surface in the form of many small plaques, these plaques entered gradually into the cell, which confirmed that the shift was the endocytosis. The disappearance of PIP2 matched the recruitment of phosphoinositide phosphatase enzyme, and we can propose that the latter can degraded the former. When PIP2 degradation was blocked, abnormal invagination can be observed, shear-ing machine of endocytosis gathered around the plaque on the membrane and failed to complete endocytosis. Therefore, PIP2 degradation is the necessary step of ves-icle scission [16].Recycling of the neurotransmitter Neurotransmitter recycle is essentially a process of cell endocytosis, clathrin interact with AP2 or AP180 (adapter protein) and membrane lipid to form clathrin-coated ves-icles. The process of its formation is just that endophilin A1 drove the rupture of membrane vesicles from the do-nor membrane at the neck of vesicles, LPAAT at N-termi-nal of endophilin A1 can catalyze lysophosphatidic acid (LPA) and arachidonic ene-CoA to form lysophosphatidic acid (PA), LPA is a three-dimensional structure like in-verted cone-type and PA is cone-type, the former struc-ture is beneficial for positive membrane deformation, the later is conducive to negative membrane deformation. It is the transformation of lysophosphatidic acid that drive rupture between vesicle and the donor vesicle membrane. After endophilin mutation, it will severely affect recycle mechanism of synaptic particle [17].Function besides endocytosisEndophilin A2 is actually involved in absorbing nu-trients and growth factors and endocytosis of pathogens and receptors, participating in a variety of membrane transport mechanism . While endophilin B1 locates in the membrane of the cell and its main function is relat-ed to cell membrane dynamics. Apoptosis receptor Fas/ CD95 can not correctly located in the membrane without endophilin, resulting in inhibition of the mechanism of apoptosis [18]. Endocytosis related proteinsDynaminDynamin is a 100 kD GTPase, and its GTPase region at N-terminal can bind and hydrolyse GTP, PH (pleck-strin homology) region can bind with membrane and me-diate polymerization between dynamins, PRD (praline arginine rich) region at C-terminal mediate the interac-tion between other proteins. In addition, dynamin has a small GED (GTPase effector) region which is necessary in hydrolysis of GTP [18], and also plays an important role in some clathrin independent endocytosis. Dynamin is necessary in pinching and formation of vesicles. After its binding with ligand amphiphysin-1, dynamin-1 plays a key role in dynamin dependent endocytosis under regu-lation of cycle-dependent kinase 5 (Cdk5). Dynamin-1-dependent endocytosis occurs quickly, usually only a few seconds, while dynamin-2 mediated endocytosis is slow, usually ten minutes.Dynamin uses mechanochemical activity – specifi-cally a twisting action – to pinch off endocytic vesicles [19]. Dynamin was, early on, localized to the collar around the neck of forming endocytic vesicles. This suggested that dynamin may use the energy of GTP hydrolysis to directly pinch a membranous neck. Indeed, dynamin could tubulate lipids and break apart the tubules in vitro, although later it seemed that the breaking apart was hap-pening as the samples dried on EM grids. Meanwhile, Schmid had come up with a “regulatory GTPase” mode: that dynamin was active not as it hydrolyzed GTP but in its GTPbound form, which recruited other proteins to do the pinching. The Yale group has more evidence for the earlier “pinchase” model. They used light microscopy rather than EM to follow tubulation and fission directed by dynamin in vitro. Longitudinal tension was needed with constriction to achieve fission. In mammalian, the dynamin collars are relatively short, so cortical actin is the most likely source of tension that would help the dynamin to wrench an endocytic vesicle free [20–23]. Dynamin has intrinsic activity of GTP and is also the cerebellum Dyrk1A kinase substrate, with its own ag-gregation feature of the endometrium can help new pro-cesses from the cell membrane vesicles isolated. Dynamin is involved in tubulation by PH epsin and ENTH (epsin NH2-terminal homology) [21].Wild-type dynamin mutant block the tube, which can be restricted to speculate dynamin of these membrane protein of the ability to control grid protein coated vesi-cle endocytosis and other media invagination degree. ActinActin is the main component of microfilament. Microfilament is involved in cell shape and polarity of the maintenance of endocytosis, intracellular transport,364 www. springerlink. com/content/1613-9089cell shrinkage and movement, cell division, and many other functions. Actin has two kinds: G-actin and F-ac-tin. The importance of F-actin in endocytosis was clearly illustrated by the effect of addition of the actin-mono-mersequestering drug latrunculin A. Within 5 minutes of its addition, actin patches were no longer visible and endocytosis was completely abrogated. This was the point at which the yeast WASP orthologue Las17p and the type I myosins (Myo3p and Myo5p) began to nucleate actin filaments through activation of the Arp2/3 complex [24]. This group of proteins has been called the “actin net-work growth machinery”. Some researchers combined epifluorescence with total internal reflection microscopy (TIRF) to follow the internalisation of individual coated pits in living cells expressing a fluorescent tagged form of clathrin light chain (DsRed clathrin). They showed that transient recruitment of actin coincides with the inward movement of vesicles, they also observed recruitment of the Arp2/3 complex to clathrin-coated pits. PH-sensitive probes have been developed that allow internalisation of cargo into individual clathrin-coated vesicles to be visual-ized, allowing us to follow the time course of invagination and identify the point of scission. The recruitment of actin during invagination is thought to provide the force that drives the invagination of the coated pit [25]. It is currently identified that the vesicle scission module contains the two yeast amphiphysin proteins Rvs161p and Rvs167p. These proteins contain BAR (Bin-Amphiphysin-Rvs) domains that bind to and tabulate, actin and associated proteins recruit amphiphysins and thus mark the site at which membrane tubulation should occur. Tubulation is then suggested to facilitate the scission process. Following scission, the vesicle is uncoated and moves away from the membrane until it fuses with an endosome. There are two possible roles for actin at this final stage: vesicles could move along actin cables; alternatively actin could be nu-cleated at the vesicle surface to facilitate their movement within the cell. Then the actin depolymerized from the endocytosis site. The most representative depolymeriz-ing factor is cofilin. Cofilin is necessary for endocytosis in mamamals, but its mechanism is still unknown [26]. In the process of invagination, Sac6p/microfilament binding protein/fimbrin is also required, invagination induced by actin failed without Sac6p [27].PCH (Pombe Cdc15 homology) / F-BARPCH (Pombe Cdc15 homology) / F-BAR is another family of proteins which plays an important role in en-docytosis. The BAR region of these proteins combine with phosphoinositide. FBP17 is a member of PCH fam-ily, containing PCH domain and extended FC domain. The EFC domains show weak homology to the Bin-am-phiphysin-Rvs (BAR) domain. The EFC domains bound strongly to phosphatidylserine and phosphatidylinositol 4,5-bisphosphate and deformed the plasma membrane and liposomes into narrow tubules. Most PCH proteins possess an SH3 domain that is known to bind to dynamin and that recruited and activated neural Wiskott-Aldrich syndrome protein (N-WASP) at the plasma membrane. FBP17 contributed to the formation of the protein com-plex, including N-WASP and dynamin-2, in the early stage of endocytosis. Furthermore, knockdown of endog-enous FBP17 impaired endocytosis, suggesting FBP17 is necessary for dynamin-dependent endocytosis [28].Rab proteinsRab protein which is a small GTP enzyme is an im-portant regulator of endocytosis. Rab5 is involved in tubulization and its role in endocytosis has been clear. Rab5 protein has three isomers, Rab5A, Rab5B and Rab5C. Rab5 protein exists primarily on the plasma mem-brane, clathrin-coated vesicle and early endosome, tak-ing charge of vesicle fusion and recycling. Rab5 protein functions with ongoing GTP/GDP cycle. As molecular switch of vesicle transport, the activated state of combin-ing with GTP is “open”, the unactivated state of combin-ing with GTP is called “close”. Rab5 regulates transport of endocytotic materials between membrane and early en-dosome, and help vesicle movement along microtubules. HAP40 is an effective regulatory factor of Rab5, and Rab5 increase connections of endosome with actin un-der existence of HAP40. RAB5A protein was related to endocytosis of prostacyclin [29]. A-synuclein is the root of Parkinson’s disease, Lewy body Dementia and Alzheim-er’s disease and other central nervous system diseases. Moreover, Young found the mutant can decrease endo-cytosis of A-synuclein according to RAB5A GTP mutant enzyme research while Lewy body-like decreased in cy-toplasm, the cytotoxicity also decreased. Over-expression of RAB5A protein in immune system deficiency, which can slower macrophage phagocytosis of pathogens and decrease the rate of degradation of pathogens and IFN-C-mediated phagocytosis weakened.ConclusionWe have found many molecules involved in endocy-tosis in the past few decades, and had a preliminary un-derstanding about its process and metabolism. We now know that the process of endocytosis is not a simple step-by-step process in which a series of proteins function ac-cording to the chronological order, but rather a complex process comprising many members which are regulated precisely. Although the mechanism of regulation of en-docytosis is still unknown, it can be predicted that as more and more new research techniques applied in this area, we will be able to understand the mechanism of cell endocytosis more comprehensively. Whether tumor cells365Chinese-German J Clin Oncol, June 2009, Vol. 8, No. 6endocytose more nutrients than normal cells and wheth-er tumor cells increase sizes through endocytosing more growth factors? Can we inhibit tumor growth through inhibiting cell endocytosis or can we cure tumor by in-ducing specific drug endocytosis of tumor cells? Whether the endocytosis of 6-phosphate mannose receptor (MPR) which plays a very important role in cell death signal transduction is restrained in tumor cells? The mechanism of cell endocytosis can be further elucidated by solving all of the above problems.ReferencesGiodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosiselicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci USA, 2009, 106: 3324–3329.Kirchhausen T. Clathrin. Annu Rev Biochem, 2000, 69: 699–727.Kiss AL, Turi A, Müller N, et al . Caveolae and caveolin isoforms in ratperitoneal macrophages. Micron, 2002, 33: 75–93.Rajjayabun PH, Garg S, Durkan GC, et al . Caveolin-1 expressionis associated with high-grade bladder cancer. Urology, 2001, 58: 811–814.Frank PG, Woodman SE, Park DS, et al . Caveolin, caveolae andendothelial cell function. Arterioscler Thromb Vasc Biol, 2003, 23: 1161–1168.Krajewska WM, Masłowska I. Caveolins: structure and function in sig -nal transduction. Cell Mol Biol Lett, 2004, 9: 195–220.Pascariu M, Bendayan M, Ghitescu L. Correlated endothelial caveolinoverexpression and increased transcytosis in experimental diabetes. J Histochem Cytochem, 2004, 52: 65–76.Fra AM, Pasqualetto E, Mancini M, et al . Genomic organization andtranscriptional analysis of the human genes coding for caveolin-1 and caveolin-2. Gene, 2000, 243: 75–83.Cameron PL, Liu C, Smart DK, et al . Caveolin-1 expression is main-tained in rat and human astroglioma cell lines. Glia, 2002, 37: 275–290.Williams TM, Cheung MW, Park DS, et al. Loss of caveolin-1 gene ex-pression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell, 2003, 14: 1027–1042.Gaudreault SB, Dea D, Poirier J. Increased caveolin-1 expression inAlzheimer′s disease brain. Neurobiol Aging, 2004, 25: 753–759.Müller JS, Piko H, Schoser BG, et al . Novel splice site mutation in thecaveolin-3 gene leading to autosomal recessive limb girdle muscular dystrophy. Neuromuscul Disord, 2006, 16: 432–436.Lim JP, Wang JT, Kerr MC, et al . A role for SNX5 in the regulation ofmacropinocytosis. BMC Cell Biol, 2008, 9: 58.1.2.3.4.5.6.7.8.9.10.11.12.13.Miki H, Yamaguchi H, Suetsugu S, et al . IRSp53 is an essential in -termediate between Rac and WAVE in the regulation of membrane ruffling. Nature, 2000, 408: 732–735.Sun P, Yamamoto H, Suetsugu S, et al . Small GTPase Rah/Rab34 isassociated with membrane ruffles and macropinosomes and promotes macropinosome formation. J Biol Chem, 2003, 278: 4063–4071.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal coldsensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem, 2009, 284: 1570–1582.Gad H, Ringstad N, Löw P, et al . Fission and uncoating of synapticclathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron, 2000, 27: 301–312.Petrelli A, Gilestro GF, Lanzardo S, et al . The endophilin-CIN85-Cb1complex mediates ligand-dependent downregulation of c-Met. Na-ture, 2002, 416: 187–190.Williams R. PIP2 in endocytosis. J Cell Biol, 2007, 177: 185.Guichet A, Wucherpfennig T, Dudu V, et al . Essential role of endophilinA in synaptic vesicle budding at the Drosophila neuromuscular junc-tion. EMBO J, 2002, 21: 1661–1672.Takei K, McPherson PS, Schmid SL, et al . Tubular membrane invagi-nations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature, 1995, 374: 186–190.Zhang X, Wang F, Chen X, et al . Post-endocytic fates of delta-opi-oid receptor are regulated by GRK2-mediated receptor phosphory-lation and distinct beta-arrestin isoforms. J Neurochem, 2008, 106: 781–792.William A. Twisting endocytosis. J Cell Biol, 2006, 173: 456.Damke H, Binns DD, Ueda H, et al . Dynamin GTPase domain mu-tants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell, 2001, 12: 2578–2589.Tsujita K, Suetsugu S, Sasaki N, et al . Coordination between the ac-tin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol, 2006, 172: 269–279.Sun Y, Martin AC, Drubin DG. Endocytic internalization in buddingyeast requires coordinated actin nucleation and myosin motor activity. Dev Cell, 2006, 11: 33–46.Okreglak V, Drubin DG. Cofilin recruitment and function during actin-mediated endocytosis dictated by actin nucleotide state. J Cell Biol, 2007, 178: 1251–1264.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin-and actin-mediated endocytosis machinery. Cell, 2005, 123: 305–320.O’Keeffe MB, Reid HM, Kinsella BT. Agonist-dependent internaliza-tion and trafficking of the human prostacyclin receptor: a direct role for Rab5a GTPase. Biochim Biophys Acta, 2008, 1783: 1914–1928.14.15.16.17.18.19.20.21.22.23.24.25.26.27.28.29.。
细胞生物学名词解释1. intermediate filament中间纤维:中间纤维是细胞的第三种骨架成分,由于这种纤维的平均直径介于微管和微丝之间,故称为中间纤维。
微管和微丝都是由球形蛋白装配起来的,而中间纤维则是由长的、杆状的蛋白装配的。
中间纤维是最稳定的细胞骨架成分,主要起支撑作用。
中间纤维在细胞中围绕着细胞核分布,成束成网,并扩展到细胞质膜,与质膜相连结。
2. MPF成熟促进因子:成熟促进因子是能够促使染色体凝集,使细胞由G2期进入M期的因子。
在结构上,它是一种复合物,由周期蛋白依赖性蛋白激酶和G2期周期蛋白组成,其中,周期蛋白对蛋白激酶起激活作用,周期蛋白依赖性蛋白激酶是催化亚基,它能够将磷酸基团从ATP转移到特定底物的丝氨酸和苏氨酸残基上。
3. confocal共聚焦:从一个点光源发射的探测光通过透镜聚焦到被观测物体上,如果物体恰在焦点上,那么反射光通过原透镜应当汇聚回到光源,这就是所谓的共聚焦,简称共焦。
4. integrin整合蛋白:也称内在蛋白、跨膜蛋白,部分或全部镶嵌在细胞膜中或内外两侧,以非极性氨基酸与脂双分子层的非极性疏水区相互作用而结合在质膜上。
5. clathrin-coated vesicle (COP)笼形衣被小泡:笼形蛋白形成的衣被中还有衔接蛋白,介于笼形蛋白与配体受体复合物之间,起连接作用。
目前至少发现4种不同类型的衔接蛋白,可分别结合不同类型的受体,形成不同性质的转运小泡。
6. transformation转化:细菌转化是一种常见的分子生物学技术,通过热激、冰浴、复苏等步骤后,把外源DNA转入细菌感受态细胞,使外源基因在宿主细菌胞内扩增、表达等。
7. transfection转染:起初指外源基因通过病毒或噬菌体感染细胞或个体的过程。
现在常泛指外源DNA (包括裸DNA)进入细胞或个体导致遗传改变的过程。
8. polyclonal antibody多克隆抗体:多种抗原表位刺激机体免疫系统后,机体产生的针对不同抗原表位的混合抗体。
细胞膜的内吞和溶酶体降解机制细胞膜内吞和溶酶体降解机制是细胞内重要的分解和清除废物的过程。
通过这一过程,细胞能够消化、回收和排除各种物质,维持细胞内环境的稳定。
本文将介绍细胞膜的内吞和溶酶体降解机制的基本概念、过程和调控。
一、细胞膜的内吞过程细胞膜的内吞是指细胞通过膜的融合和膜囊的形成将溶质、液滴或细胞外部分包裹进来的过程。
细胞膜内吞的主要过程包括三个步骤:引导、融合和内吞囊的形成。
1. 引导:在内吞开始之前,特定的分子会与特异性的适配分子结合,发生一系列的信号传导和蛋白质互作。
这些分子通常被称为配体,它们能够与细胞膜上的受体结合,从而将其引导到内吞的位置。
2. 融合:一旦配体和受体结合后,细胞膜上会形成富集了这些配体和受体的特定区域。
在这个区域内,细胞膜会与细胞质内的细胞器膜融合,形成一个膜泡。
3. 内吞囊的形成:膜泡形成后,细胞膜会进一步固化形成内吞囊,将配体和受体以及周围的液滴或溶质包裹在内。
内吞囊的形成依赖于细胞骨架和分子马达的参与,这些蛋白质可以将膜泡定向到特定的位置。
二、溶酶体的降解过程溶酶体是一种特殊的细胞质器,其主要功能是分解和消化细胞内外的废物和降解产物。
巨噬细胞和多核白细胞是溶酶体最为典型的例子,它们主要通过吞噬和吞吐作用将外来物质或被细胞膜内吞形成的内吞囊引入溶酶体进行降解。
1. 吞噬:巨噬细胞和多核白细胞通过细胞膜的伸缩和膜的融合将外来物质吞噬进细胞内部形成吞噬体。
吞噬体与溶酶体逐渐融合形成吞噬溶酶体。
2. 吞吐:细胞膜内吞形成的内吞囊也可以通过与溶酶体膜的融合形成吞吐泡。
吞吐泡会将内吞囊内的废物和降解产物引入溶酶体进行降解。
3. 降解:一旦吞噬体或吞吐泡与溶酶体融合,溶酶体内的酶会被激活并开始降解和分解引入的废物。
这些酶能够分解蛋白质、核酸、脂质和多糖等生物大分子,使其转化为小分子物质,供细胞再利用或排出体外。
三、内吞和溶酶体降解的调控机制内吞和溶酶体降解的过程需要复杂的调控机制来保证其准确性和高效性。
Amphipathic molecule/兼性分子0109 Anchoring junction/锚定连接150 Adhesion belt/黏合带501 adhesion plaque/粘合斑154 Active transport/主动运输123 Autophagy/自体吞噬502 Autophgosome/自噬体202 Autolysis/自溶作用205 Acrosome/顶体207 Apoptosis/细胞凋亡410 Biological membrane/生物膜108 Ca2+ pump/钙泵0118 caryoty pe/核型310 Cell membrane/细胞膜0101 Cell surface/细胞表面0102 Cell coat/细胞外被0103 Cell junction/细胞连接148 Cell recognition/细胞识别141 Cell cycle/细胞周期401 Cell cycle engine/细胞周期引擎503 Cell cycle checkpoint/细胞周期检控点504 Cell differentiation/细胞分化0414 cell determination/细胞决定413 Cell communication/细胞通讯140 cellular respiration/细胞呼吸228 Cellular aging/细胞衰老0433 Cellular death/细胞死亡0434 Centromere/着丝粒0505 Centromere domain/着丝粒结构域506 Chromatin/染色质507 Chromosome/染色体0508 Chromatids/染色单体307 Centriole cycle/中心粒周期406 Communication junction/通讯连接152 Carrier protein/载体蛋白0114 Channel protein/通道蛋白0115 Channel diffusion/通道扩散127 Clathrin/网格蛋白0133 Coated pit/有被小窝510 Coated vesicle/有被小泡511 Cytoskeleton/细胞骨架231 Contact inhibition/接触抑制145 Cristae/(线粒体)嵴222 central body;centrosome/中心体241 Desmosomes/桥粒151 Dedifferentiation/去分化415 Euchromatin/常染色质0305 Endosome/内体512 Endomembrane system/内膜系统0201 Endoplasmic reticulum;ER/内质网513 Exportin;Export protein/输出蛋白0514 Endocytosis/胞吞/内吞/入胞作用0129 Exocytosis/胞吐作用515Elementary particle/基粒223embryonic induction/胚胎诱导417Fluid mosaic model/液态镶嵌模型0112Facilitated diffusion/易化扩散0126free diffusion/自由扩散125free radical/自由基435Free ribosomes/游离核糖体319Fixed ribosome/附着核糖体320Golgi apparatus/complex/高尔基复合体0219G protein/G蛋白155Gap junction/间隙连接153Glycosy lation/(蛋白质)糖基化216Gene differential expression/基因差次表达0416Heterophagy/异体吞噬516Heterochromatin/异染色质0306housekeeping gene/持家基因0420hemidesmosomes/半桥粒159human leucocyte antigen ,HLA/人白细胞抗原147Intermediate filaments;IF/中间纤维239Integral protein/膜内在蛋白0104Intermediate junction/中间连接ly sosome/溶酶体518mosaic protein/镶嵌蛋白519ionic pump/离子泵116ionic channel/离子闸门通道122informational molecule/信号分子karyotype;cary otype/核型310karyotype analysis/核型分析0521kinetochore动粒308Luxury gene/奢侈基因0418lipid rafts model/脂筏模型0522leader sequence/peptide/导肽0523liposome/脂质体0524ligand gated channel/配体闸门通道120ligand /配体135microfilaments;MF/微丝0236microtubule;MT/微管0232mitosis/有丝分裂402meiosis/减数分裂411mitotic apparatus/有丝分裂器409membrane receptor/膜受体139mitochondrion/线粒体221mtDNA/线粒体DNA 0227microsome/微粒体211microtubule-associated protein;MAP/微管相关蛋白质0233MTOC/微管组织中心235membrane flow/膜流0220membrane transport protein/膜转运蛋白113membrane structure/膜相结构157myosin/肌球蛋白238nucleoid/类核体210nuclear envelope/membrane/核膜525nuclear pore complex/核孔复合体0302nuclear lamina/核纤层0303nucleoskeleton/核骨架0526nucleosome/核小体0304nucleolus/核仁0527Nuclear localization signal;NLS/核定位序列0528Na+K+pump/钠钾泵0117nucleolar organizing region,NOR/核仁组织区0311NP/核质指数;核质比301oxidative phosphorylation/氧化磷酸化230peripheral protein/膜周边蛋白质0105phase transition/相变110passive transport/被动运输124peroxisome/过氧化物酶体529primary messenger/第一信使143primary lysosome/初级溶酶体208primary constriction/主缢痕530phagocytosis/吞噬作用131pinocytosis/胞饮作用130polyribosome/多聚核糖体0217proteoglycan /蛋白聚糖107polarization/极化138ribosome/核糖体531respiratory chain/呼吸链226restriction point/R点;限制点0403residualbody/残余小体203Russell’s body/罗氏小体212receptor mediated endocytosis/受体介导的胞吞作用0132Resting Potential,RP/静息电位137signal transduction/信号传导532signaling molecules/信号分子142second messenger/第二信使143signal recognition particle;SRP/信号识别颗粒0215signal peptide/信号肽0214secondary lysosome/次级溶酶体209structural protein/结构蛋白质0533solenoid/螺线管534supersolenoid/超螺线管535simple diffusion/简单扩散125synaptonemal complex;SC/联会复合体0412spindle/纺锤体0240semiautonomous organelle/半自主性细胞器224sarcoplasmic reticulum/肌质网218tetrad/四分体0538Telomeres/端粒309transmembrane protein/跨膜蛋白0106tight junction/紧密连接149transport by vesicle foration /膜泡运输0128transcytosis/穿胞吞吐作用136telolysosome/终末溶酶体0203transmembrane signal transduetion/膜受体介导的跨膜信号转导536tricarboxylic acid cycle/三羧酸循环229unit membrane/单位膜0111uniport/单运输0537voltage-gated-channel/电压闸门通道1216119质子泵6134出胞作用6144细胞膜抗原6146 AB0血型抗原6156细胞体积守恒定律6158非膜相结构6160受体的激活6204粒溶作用6206异噬作用6213脱粒6225 F1因子6234微管蛋白流6237肌动蛋白微丝6242中心粒小轮6312染色体病6313翻译6314遗传密码6315密码子6317同义密码子6318核糖体循环6321密码子兼并性6322同功受体6404 G0期细胞6405终端分化细胞6406中心粒周期6407细胞分裂周期基因6419奢侈蛋白6421管家蛋白6422干细胞6423畸胎瘤6424全能性6425多能细胞pleuripotent stem cell;PSC6426单能细胞6427免疫细胞6428裸细胞6429双重标志细胞6430嵌合体6431癌基因6432抑癌基因6436细胞坏死性死亡6437阻遏物repressor6438癌细胞0开头的四位编号是可能的重点,所以提到最前面,保留原三位编号顺序,1至4的三位编号为单元分布,5开头的是补充编号,6开头的四位编号是无英译非重点0101细胞膜又称细胞质膜是位于细胞最外层,围绕整个细胞质的一层薄膜,主要由脂类和蛋白质构成。
癌症和癌症治疗中的内吞作用前言细胞内吞是一个复杂的过程,来自细胞外环境的细胞表面蛋白、脂质和液体被包装、分类并内化进细胞。
多种内吞途径与癌症的发展、进展和调节有关,癌症细胞中的每一种内吞途径都可能失调,不同癌症类型中的这些变化呈现高度复杂性和异质性。
此外,细胞内吞也是药物进入到细胞中的一种机制,有多种内吞途径决定药物分子的命运,从溶酶体中的降解到再循环回质膜。
过去十年的研究表明,内吞作用已成为肿瘤细胞营养摄取、免疫监测和肿瘤免疫逃避、肿瘤转移和治疗药物递送的关键调节因子。
随着抗体疗法、抗体偶联药物(ADC)和肿瘤放射性配体靶向的出现,调节内吞作用以改变药物递送药理学特性的能力已成为一个令人兴奋的新研究领域。
内吞的信号途径一般来说,正常的内吞作用可分为三个阶段:(1)芽的形成,(2)膜的弯曲和囊泡的成熟,(3)膜的断裂并释放到细胞质中。
多种内吞途径有重叠的方面,因此内吞的一般过程是高度灵活和复杂的。
网格蛋白介导的内吞作用网格蛋白介导的内吞作用(CME)在概念上是一个简单的过程,包括几个连续和部分重叠的步骤。
CME可由质膜上的某些受体结构性启动,或者需要配体和/或抗体结合后启动。
当细胞质中的内吞衣壳蛋白开始聚集在质膜的内小叶上时,CME就开始了。
衣壳蛋白通过从细胞质中招募并与额外的蛋白质适配器相互作用而继续组装和生长。
关键的衔接蛋白使膜弯曲,从而将内化受体/配体集中到一个“网格蛋白包被坑”(CCP)中。
由于CCP内陷增大,CCP颈缩窄时,通过一个断裂过程与质膜分离。
肌动蛋白聚合有助于将CCP向内拉入细胞质,直到断裂完成,CCP释放并成为一个网格蛋白包被的囊泡(CCV)。
最后,CCV外壳被分解,CCV与内涵体融合以运输到特定的亚细胞位置,或者可以被回收回细胞表面。
网格蛋白是CME的关键成分,由重链和轻链组成。
三个网格蛋白重链和轻链形成一个三聚体,它与其他三聚体相互作用,并在新兴的CCP周围形成一个多边形晶格。
内吞小泡名词解释内吞小泡呢,这可是细胞生物学里一个超有趣的概念哦。
内吞小泡简单来说就是细胞在进行内吞作用的时候形成的一种小泡结构。
细胞啊,它可不是一个封闭的、与世隔绝的小世界。
它需要与外界进行物质的交换,内吞作用就是它获取外界物质的一种重要方式。
当细胞要摄取某些大分子物质或者颗粒物质的时候呢,细胞膜就会发生凹陷。
哇,你能想象吗?就像一个柔软的袋子凹进去一块一样。
然后呢,这凹陷的部分就会逐渐把要摄取的物质包裹起来。
这个包裹起来的结构就慢慢形成了一个小泡,这个小泡就是内吞小泡啦。
内吞小泡就像是一个小小的运输囊,把从细胞外摄取到的东西包裹在里面,防止它们在运输过程中到处乱跑呢。
内吞小泡里面包裹的物质种类可多啦。
比如说一些营养物质,像蛋白质之类的大分子营养物质,细胞可不会放过把它们吸收进来的机会。
还有可能是一些信号分子,细胞通过内吞小泡把这些信号分子摄取进来,然后在细胞内部进行信号的传递和处理,就像是在细胞内部悄悄地传递着外界的信息呢。
内吞小泡形成之后,它会在细胞内部进行移动哦。
它可不是静止不动的。
它会带着自己的“货物”朝着细胞内部的特定区域移动,这个过程就像是一个小小的快递员在细胞这个大公司里按照特定的路线送货一样。
而且啊,内吞小泡在细胞内部还可能会和其他的细胞器或者结构发生相互作用呢。
比如说,它可能会和溶酶体融合。
溶酶体可是细胞里的一个“消化车间”,当内吞小泡和溶酶体融合之后,内吞小泡里面包裹的物质就会被溶酶体里的各种水解酶进行分解。
这就像是把包裹里的东西拿出来放到专门的处理车间进行处理一样。
分解后的小分子物质就可以被细胞重新利用啦,比如被用来合成细胞自身需要的物质或者提供能量等。
内吞小泡在细胞的生命活动中扮演着不可或缺的角色呢。
如果没有内吞小泡,细胞就很难有效地摄取外界的物质,那细胞的正常生长、发育以及功能的维持都会受到极大的影响。
所以说啊,这个小小的内吞小泡虽然看起来不起眼,但它对细胞来说就像一个小宝藏一样重要哦。
内吞作用名词解释
内吞作用是指一个组织或企业通过收购、合并或整合其他公司或资源来扩大自身规模、增强竞争力和市场份额的行为和效果。
在商业领域,内吞作用通常指的是一个公司通过吞并其他公司或业务部门,将其纳入自己的组织结构和运营体系中。
这种收购和整合的目的可以是获取更多的市场份额、扩大产品线、提高效率、降低成本、实现协同效应等。
内吞作用可以带来多种好处,例如:
1.增强市场竞争力:通过收购或合并其他公司,可以扩大
市场份额、提高市场竞争力。
2.扩大产品线或服务范围:通过吞并其他公司,可以获取
其产品或服务,并扩大自身的产品线或服务范围。
3.实现协同效应:通过整合不同公司或业务部门的资源和
能力,可以实现协同效应,提高整体运营效率和业绩。
4.获取资源和技术:通过收购其他公司,可以获得其资源
、技术、知识产权等,加速自身的发展和创新能力。
然而,内吞作用也存在一些潜在的风险和挑战,如整合困难、文化冲突、管理问题等。
因此,在进行内吞作用时,需要进行充分的尽职调查和战略规划,以确保顺利实现预期的效益和目标。
2、吞饮作用
网格蛋白示意图
运输作用
网格蛋白在人体中起运输的作用,生物分子激素、神经递质、膜蛋白等物质都可通过网格蛋白进行运输。
在内吞过程中,质膜上受体与配体特异结合部位的胞质面(将形成有被小泡的外衣)有一些蛋白附着:网格蛋白是其中最主要的一种蛋白。
它是一种纤维蛋白,与另一种较小的多肽形成了有被小泡外衣的结构单位,即三腿蛋白复合物。
三腿蛋白复合物包括三个网格蛋白和三个较小的多肽。
由许多三腿蛋白复合物聚合构成五边形或六边形的网格样结构,覆于有被小泡或有被小窝的胞质面。
由网格蛋白装配成的外衣提供了牵动质膜的机械力,导致有被小窝的下凹,也有助于捕获膜上的特异受体及与之结合的被转运分子;调节素是有被小泡中组成外衣的另一类重要的蛋白,它是多亚基的复合物,能识别特异的跨膜蛋白受体,并将其连接至三腿蛋白复合物上,起选择性介导作用。
跨膜受体蛋白胞质面肽链尾部,常在一个由四个氨基酸残基构成的区域内高度转折,形成一个内吞信号,由调节素识别它。
所以调节素可介导不同类型受体,使细胞能捕获不同类型的物质。
保证细胞正常分裂
2012年9月,美国加州大学旧金山分校生物工程与治疗科学系教授弗朗西斯·布罗茨基和她的研究小组发现,如果没有网格蛋白,细胞分裂会变得极不规律,而这正是癌症等人类疾病的一个特征之一。
研究人员通过RNA干扰技术,向原有基因中注入一小段基因片段,以阻止网格蛋白的生成,删除了细胞中的网格蛋白。
结果发现,在没有网格细胞的情况下,细胞分裂过程中中心体内的中心粒不是成对出现,而是毫无规律且越来越多。
在经过进一步的筛选和识别后,布罗茨基的小组发现真正起作用的是一种名为CHC17的网格蛋白。
如果删除
CHC17或用化学方法使其钝化,就会导致细胞外观异常。
通常当一个细胞分裂时,会产生结构蛋白形成纺锤体,并以此来分裂成两个具有同样DNA的新细胞。
在删除网格蛋白后,这一过程的对称性和稳定性就遭到了破坏。
这说明网格蛋白对细胞分裂至关重要。
该发现找到了网格蛋白的一项“隐藏功能”,同时也为人们对癌症的认识和治疗提供了一个新角度。
有被小泡
配体与膜上受体结合后,网格蛋白聚集在膜下一侧,逐渐形成50-100nm的质膜凹陷,称网格有被小窝,一种小分子GTP结合蛋白在深陷有被小窝的颈部装备成环,dynamin蛋白水解与其结合的GTP引起颈部缢缩,最终脱离质膜形成网格蛋白有被小泡。