Astragaloside IV improves homocysteine-induced acute phase endothelial dysfunction via antioxidation
- 格式:pdf
- 大小:653.45 KB
- 文档页数:6
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Astragaloside IVCatalog No. :HY-N0431CAS No. :84687-43-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C41H68O14Molecular Weight:784.97CAS No. :84687-43-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。

黑龙江医学第43卷2019年第1期HEILONGJIANG MEDICAL JOURNAL Vol.43No.1Jan.2019黄芪甲苷治疗糖尿病及其并发症药理作用研究李杨1,王凡1,贾宁21.天津市南开区中医医院天津3001022.天津市南开医院天津300102摘要黄芪甲苷为中药黄芪的活性成分,具有显著的抗炎、抗氧化、保护神经等作用,其应用于糖尿病治疗中,可控制患者血糖与血脂水平,抑制氧化应激与炎症反应,改善糖尿病肾病、糖尿病视网膜病变等并发症。
故本文则主要对黄芪甲苷治疗糖尿病及其并发症的药理作用进行阐述,其目的在于为临床研究提供相关依据。
关键词黄芪甲苷;糖尿病;并发症;黄芪doi:10.3969/j.issn.1004-5775.2019.01.39学科分类代码:320.67中图分类号:R739.6文献标识码:BPharmacological Study of Astragalus Glucoside on Diabetes Mellitus and its Complication/LI Yang,WANG Fan,JIA Ning//Tianjin Nankai District Traditional Chinese Medicine Hospital,Tianjin,300102,ChinaAbstract:Astragaloside A is an active component of astragalus membranaceus,which has obvious anti-inflammatory,anti-oxi⁃dation and neuroprotective effects.It can control the level of blood glucose and blood lipid,inhibit oxidative stress and inflammatory reaction,improve diabetic nephropathy,diabetic retinopathy and other complications.Therefore,the pharmacological effect of as⁃tragaloside A on diabetes mellitus and its complications is described to provide relevant basis for clinical research.Key words:Astragaloside A;Diabetes mellitus;Complications;Astragalus membranaceus中药黄芪源于豆科草本植物、蒙古黄芪、膜荚黄芪的根,性味微温、甘,具有利尿托毒等功效。

《中国组织工程研究》Chinese Journal of Tissue Engineering Research文章编号:2095-4344(2019)11-01652-051652www.CRTER .org·研究原著·余素姣,女,1980年生,湖北省恩施市人,土家族,2009年武汉大学毕业。
通讯作者:谭慧,恩施土家族苗族自治州中心医院骨科,湖北省恩施市445000文献标识码:A稿件接受:2018-11-15Yu Sujiao,Department of Orthopedics,the Central Hospital of Enshi Autonomous Prefecture,Enshi 445000,Hubei Province,China Corresponding author:Tan Hui,Department of Orthopedics,the Central Hospital of Enshi Autonomous Prefecture,Enshi 445000,Hubei Province,China黄芪甲苷通过炎症小体活化影响软骨细胞炎性因子的表达余素姣,谭慧(恩施土家族苗族自治州中心医院骨科,湖北省恩施市445000)DOI:10.3969/j.issn.2095-4344.1068ORCID:0000-0003-4905-4643(余素姣)文章快速阅读:文题释义:NLRP3炎症小体:NLRP3炎症小体位于巨噬细胞等多种免疫细胞中,主要作用是识别外界的刺激信号,活化的NLRP3炎症小体可以激活半胱天冬氨1,进一步剪切白细胞介素1β,18等促炎介质诱导炎性反应。
黄芪甲苷:提取于豆科草本植物黄芪,具有抗肿瘤、增强免疫及促进生长等药理活性,还发现其可以抑制基质金属蛋白酶活性,改善膝关节炎的临床症状。
摘要背景:有研究表明黄芪甲苷对关节炎具有保护作用,但其对软骨细胞炎症反应及其作用机制尚不明确。


专利名称:用于治疗虚弱、肌肉损伤或少肌症的2-亚烷基-19-去甲-维生素D衍生物
专利类型:发明专利
发明人:安德鲁·G·李
申请号:CN200480027153.X
申请日:20040906
公开号:CN1852718A
公开日:
20061025
专利内容由知识产权出版社提供
摘要:本发明涉及治疗虚弱、肌肉损伤或少肌症的方法,方法包括给药于需要其的患者2-亚烷基-19-去甲-维生素D衍生物。
尤其本发明涉及治疗虚弱、肌肉损伤或少肌症的方法,方法包括给药于需要其的患者治疗有效量的2-亚甲基-19-去甲-20(S)-1α,25-二羟基维生素D3。
申请人:辉瑞产品公司
地址:美国康涅狄格州
国籍:US
代理机构:北京市柳沈律师事务所
更多信息请下载全文后查看。
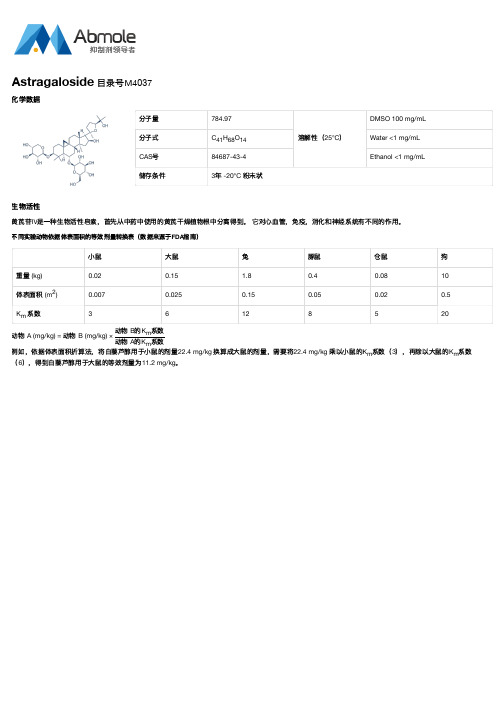
分子量784.97
溶解性(25°C)
DMSO 100 mg/mL
分子式C H O Water <1 mg/mL
CAS号84687-43-4Ethanol <1 mg/mL
储存条件3年 -20°C 粉末状
生物活性
黄芪苷IV是一种生物活性皂素,首先从中药中使用的黄芪干燥植物根中分离得到。
它对心血管,免疫,消化和神经系统有不同的作用。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
小鼠大鼠兔豚鼠仓鼠狗
重量 (kg)0.020.15 1.80.40.0810
体表面积 (m)0.0070.0250.150.050.020.5
K系数36128520
动物 A (mg/kg) = 动物 B (mg/kg) ×
动物 B的K系数
动物 A的K系数
例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K系数(3),再除以大鼠的K系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
Astragaloside 目录号M4037
化学数据
416814
2
m
m
m
m m。
黄芪甲苷对心肌细胞保护作用机制的研究进展张博雅,高晟△摘要:黄芪是一种传统中药材,具有补气升阳、固表止汗、利水消肿、托毒生肌等功效,在中国已经被广泛用于治疗心血管疾病,其中黄芪甲苷(AS-Ⅳ)是其重要的有效成分之一。
黄芪甲苷的主要药理作用包括增强免疫力、抗炎、抗氧化、抗病毒等。
近年开展的AS-Ⅳ相关研究表明,黄芪甲苷通过调节细胞内钙稳态、抗氧化反应、改善心肌能量代谢、抑制细胞凋亡、抗细胞毒性作用等多种机制参与心脏保护作用。
本文就相关研究内容进行综述,以期对临床研究提供参考。
关键词:黄芪;肌细胞,心脏;钙;活性氧;线粒体;黄芪甲苷中图分类号:R777.1+2文献标志码:ADOI :10.11958/20192266The research on mechanism of the protective effect of astragaloside Ⅳon cardiomyocytesZHANG Bo-ya,GAO Sheng △Department of Cardiovascular,Tianjin Nankai Hospital,Tianjin 300100,China△Revisor and Corresponding Author E-mail:gaosheng6747@Abstract:Astragaloside Ⅳ(AS -Ⅳ)is an active ingredient from a kind of traditional Chinese herbal medicineastragalus membranaceus,which has the functions of invigorating and promoting yang,fixing surface and relieving sweat,diffusing edema and detoxifying muscles.Chinese herbal medicine astragalus membranaceus has been widely used in the treatment of cardiovascular diseases in China,and AS -Ⅳis one of its important active ingredients.The main pharmacological effects of AS-Ⅳinclude the enhancement of immunity,anti-inflammatory,anti-oxidation and anti-virus.Recent studies on AS-Ⅳdemonstrated that AS-Ⅳcan participate in cardioprotection by regulating intracellular calcium homeostasis,anti-oxidation interaction,improving myocardial energy metabolism,inhibiting apoptosis and anti-cytotoxicity.This article concludes the relevant researches in order to provide reference for clinical study.Key words:astragalus membranaceus;myocytes,cardiac;calcium;reactive oxygen species;mitochondria;astragaloside Ⅳ基金项目:中西结合医学科研课题(2017037)作者单位:天津市南开医院心内一科(邮编300100)作者简介:张博雅(1977),女,硕士,副主任医师,主要从事心脏病的临床和基础研究△审校者及通讯作者E-mail :gaosheng6747@黄芪甲苷(Astragaloside Ⅳ,AS-Ⅳ)是中药材黄芪的重要有效化学成分之一,具有抗氧化、抗癌、抗老化、抗炎症等多种效应[1]。
专利名称:磷酸二酯酶4抑制剂
专利类型:发明专利
发明人:阿肖克·泰西姆,阿伦·霍珀,刘瑞平,埃里克·屈斯特,罗伯特·F.·邓恩,托马斯·E.·雷瑙
申请号:CN200480016778.6
申请日:20040416
公开号:CN1805929A
公开日:
20060719
专利内容由知识产权出版社提供
摘要:式Ⅰ用4-(取代苯基)-2-吡咯烷酮化合物实现选择性PDE4抑制。
这些化合物与诸如咯利普兰的化合物相比显示提高的PDE4抑制,并且相对于其它类PDE的抑制而言表现出选择性。
本发明的化合物是式(I)的化合物,其中R、R和R如本文中定义。
申请人:记忆药物公司,罗氏公司
地址:美国新泽西州
国籍:US
代理机构:永新专利商标代理有限公司
代理人:张晓威
更多信息请下载全文后查看。
Hcy与脑梗死的临床商榷符利锋;Yihyun Kwon;杜俊芳;何佳;赵建国【摘要】针对导致脑卒中的主要危险因素之一同型半胱氨酸,本文旨在对其致病机制、在临床普及中困难重重的原因以及治疗等临床中遇到的问题与各位同道进行商榷.并期待全国的医疗工作者能对这一可控的危险因素提高认识,通过降低这一危险因素,为我国脑卒中发病率的降低做出贡献.%Since homocysteine(Hcy) is increasingly considered as one of the risk (actors for stroke,this paper will discuss wide variety of issues related with homocysteine such as patho - mechanisms,clinical application,problems related to treatment,and other issues frequently impacted in clinic. Furthermore,we expect to contribute to decreasing the incidence of the stroke in the country.【期刊名称】《中西医结合心脑血管病杂志》【年(卷),期】2012(010)004【总页数】2页(P436-437)【关键词】同型半胱氨酸;脑率中;维生素【作者】符利锋;Yihyun Kwon;杜俊芳;何佳;赵建国【作者单位】天津中医药大学 300193;美国健康科学大学;天津中医药大学300193;天津中医药大学 300193;天津中医药大学第一附属医院 300193【正文语种】中文【中图分类】R743;R255.2目前我国脑血管病发病率及死亡率逐年升高,越来越多的研究结果表明,高同型半胱氨酸(Hcy)血症与脑卒中的发生和发展关系十分密切,血浆Hcy水平轻至中度增高是脑卒中的独立危险因素之一[1],而高Hcy导致脑卒中的主要途径是损伤血管[2]。
修正牌斯唯诺的功能主治1. 简介修正牌斯唯诺是一种常用的药物,具有多种功能和主治。
它经过严格的临床试验和研究,被广泛应用于临床实践中。
本文将介绍修正牌斯唯诺的功能主治,帮助读者更好地了解该药物的适应症和作用机制。
2. 功能主治修正牌斯唯诺具有以下功能主治:•缓解疼痛:修正牌斯唯诺作为一种非处方药,常用于缓解轻至中度疼痛,如头痛、牙痛、肌肉酸痛等。
它通过抑制炎症反应和减少疼痛传导来实现镇痛效果。
•退热:修正牌斯唯诺还具有退热作用,可用于降低体温。
它通过影响中枢神经系统中的体温调节中枢,减少体温升高引起的不适。
•抗炎症:修正牌斯唯诺具有一定的抗炎症作用。
它可以抑制炎症细胞产生和释放的炎症介质,减轻组织炎症反应和红肿疼痛。
•抑制血小板聚集:修正牌斯唯诺还具有一定的抑制血小板聚集作用。
它能够阻止血小板在血管壁上聚集,减少血栓的形成,预防心血管疾病的发生。
•缓解过敏症状:修正牌斯唯诺还可以缓解过敏反应引起的症状,如打喷嚏、流鼻涕、鼻塞等。
它通过抑制组织中的过敏介质释放,减轻过敏反应。
•制止咳嗽:修正牌斯唯诺可用于缓解各种类型的咳嗽,包括干咳和有痰咳。
它可以通过抑制咳嗽中枢的兴奋,减少咳嗽次数和程度。
3. 适应症修正牌斯唯诺适用于以下症状和疾病:•头痛和偏头痛。
•牙痛和口腔溃疡。
•肌肉酸痛和关节炎。
•发热和感冒引起的身体不适。
•痛经和月经不调。
•腰背痛和颈椎病。
•过敏性鼻炎和花粉症。
•咳嗽和喉咙痛。
4. 注意事项在使用修正牌斯唯诺之前,需要注意以下事项:•请按照医生或药师的建议使用药物,遵循正确的用药剂量和用药频率。
•长期或过量使用修正牌斯唯诺可能会导致副作用,请遵循医嘱使用,并避免超出推荐剂量。
•如果您有肝肾功能损害或其他严重疾病,请在使用修正牌斯唯诺之前咨询医生的意见。
•对修正牌斯唯诺过敏的患者应避免使用。
•请妥善保存药物,避免儿童接触。
5. 总结修正牌斯唯诺是一种常用的药物,具有多种功能和主治。
它能够缓解疼痛、退热、抗炎症、抑制血小板聚集、缓解过敏症状和制止咳嗽。
H yperhomocysteinemia is an independent risk factor for cardiovascular disease.1)Elevation of homocysteine (H CY)in blood plasma (Ͼ15 mmol/l), resulting from metabolic ab-normalities in H CY remethylation or transsulfuration, is common in subjects with cardiovascular disease. In the last decade, insights from the animal models have established that experimental hyperhomocysteinemia produces several phenotypic effects, including endothelial dysfunction. The most common abnormality of the dysfunction observed dur-ing hyperhomocysteinemia is impaired relaxation of conduit vessels (aorta, carotid arteries, renal, or pulmonary) or im-paired dilatation of micro vessels (mesenteric, cremasteric,coronary, skeletal, or cerebral arterioles) in response to the endothelium-dependent vasodilators in rat, mice, monkey, or guinea pigs, whereas no impairment was seen in response to exogenous nitric oxide (NO) donors.2—8)Therefore, it was suggested that the underlying mechanism by which endo-thelium derived NO, which then diffuses into the adjacent vascular muscle layer and causes its relaxation, may play an important role in mediating these deleterious effects on endothelial function in hyperhomocysteinemia.2,5,8)Further-more, the emerging evidence suggest that hyperhomocys-teinemia leads to increased vascular superoxide production through the upregulation and activation of nicotinamide ade-nine dinucleotide phosphate (NADPH) oxidase, and that re-active oxygen species (ROS) generated by NADPH oxidase contribute to the vascular phenotype.4,9—11)Radix Astragali is the dried root of Astragalus mem-branaceus (F ISCH .) B GE .var. mongholicus (B GE .) H SIAO or As-tragalus membranaceus (F ISCH .) B GE . It has been widely usedin Chinese medicine since ancient times for the improvement of immune disorders and management of cardiovascular dis-eases with an excellent safety.12)Research suggests that this herb was able to inhibit free radicals, decreases lipid peroxi-dation, and increases antioxidant enzymes.13)Many medici-nally active compounds have been isolated from this plant,including polysaccharides, flavones and astragalosides. As-tragalosides is the major active component extracted from the root of Astragalus membranaceus .12)Previous studies from our laboratory demonstrated that crude astragalosides frac-tion can potently protect endothelium-dependent relaxation against the acute injury from HCY through nitric oxide regu-latory pathways, in which antioxidation played a key role.14)Astragaloside IV (AST -IV), 3-o -beta-D -xylopyranosyl-6-o -beta-D -glucopyranosyl-cycloastragenol (Fig. 1), was a small molecular saponin. AST -IV can exert multipotent activities under pathophysiological conditions, such as anti-hyperten-sion,15)positive inotropic action,16)anti-inflammation,17)and anti-infarction.18)Up to now, however, whether AST -IV has an exact protective effect on vessels in hyperhomocysteine-mia and the mechanism are not known. The aim of the pres-ent study is to examine the effect of AST -IV on vasomotor dysfunction induced by HCY in rat aorta and explore the un-derlying mechanism.April 2010641Astragaloside IV Improves Homocysteine-Induced Acute Phase Endothelial Dysfunction via AntioxidationLi-Hong Q IU ,a Xian-Ji X IE ,b and Bi-Qi Z HANG *,aaDepartment of Cardiology, The First Affiliated Hospital, College of Medicine, Zhejiang University; and b Department of Pharmacy, The First Affiliated Hospital, College of Medicine, Zhejiang University; Qingchun road 79#, Hangzhou 310003,P . R. China.Received November 6, 2009; accepted January 22, 2010; published online January 27, 2010Astragaloside IV , the major active component extracted from Astragalus membranaceus , exerts multipotentactivities under pathophysiological conditions. Hyperhomocysteinemia, an independent risk factor for cardiovas-cular disease, induces oxidative stress leading to endothelial dysfunction. We investigated the effect of astragalo-side IV on acute phase endothelial dysfunction induced by homocysteine. In a concentration-dependent manner,endothelial dysfunction was induced by homocysteine. In organ bath experiment using rat aortic rings, treat-ment with astragaloside IV resulted in an improvement of the impaired endothelium-dependent vasorelaxation by homocysteine as reflected by the higher maximal vasorelaxation to acetylcholine. However, the presence of N w -nitro-L -arginine methyl ester hydrochloride could abolish the protective effect of astragaloside IV on homo-cysteine-induced vasomotor dysfunction. In human umbilical vein endothelial cells culture experiment, exposure to astragaloside IV significantly ameliorated the homocysteine-induced inactivation of nitric oxide–nitric oxide synthase signal pathway via reducing oxygen species and increasing the activity of superoxide dismutase. Addi-tionally, pretreatment with superoxide dismutase showed a similar effect to astragaloside IV on attenuation of the homocysteine-induced endothelial dysfunction. These data support the view that astragaloside IV might be ad-vantageous in the treatment of endothelial dysfunction induced by disturbed nitric oxide–nitric oxide synthase pathway due to oxidative stress in hyperhomocysteinemia.Key words astragaloside IV; homocysteine; oxidation; nitric oxide; endothelial functionBiol. Pharm. Bull.33(4) 641—646 (2010)© 2010 Pharmaceutical Society of Japan∗To whom correspondence should be addressed.e-mail: wzzbq@Fig.1.Chemical Structure of Astragaloside IV (C41H 68O14, Molecular Weight: 784)MATERIALS AND METHODSMaterials The AST-IV used in this study was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), with a high purity 99% by H PLC analysis. Phenylephrine (PE), acetylcholine(ACH), H CY, sodium nitroprusside (SNP), Nw -nitro-L-argi-nine methyl ester hydrochloride (L-NAME) and superoxide dismutase (SOD)-polyethylene were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). 5-(6)-Chloromethyl-2Ј,7Ј-dichloro-dihydrofluorescein diacetate (CM-H2DCF-DA) wasfrom Molecular Probes Inc. (Eugene, U.S.A.). All chemicals were of the highest purity available.Organ Chamber Experiment Animals used in this study were experimentally naive male Sprague–Dawley (SD) rats obtained from Experiment Animal Center of Zhejiang Academy of Medical Sciences, China. The investigation con-forms to the Guide for the Care and Use of Laboratory Ani-mals published by the U.S. National Institutes of H ealth (NIH Publication No. 85-23, revised 1996).Rats were killed by cervical dislocation. Thoracic arterial rings were prepared according to the method described previ-ously by Zhang et al.19)For isometric force recording, aortic rings were mounted between two stainless steel hooks and suspended in the organ bath containing Krebs’ buffer, com-posed of (m M): NaCl, 118; KCl, 4.7; MgSO4·7H2O, 1.2;KH2PO4, 1.2; CaCl2, 2.5; NaH CO3, 25; and glucose, 11; at37°C bubbled with 95% O2ϩ5% CO2(pH 7.4). The tensionof the aortic ring was monitored by a force transducer (Nan-jing Medease Science and Technology Co., Ltd., China), connected to MedLab 5.0v, a computer-based data acquisi-tion system (Nanjing Medease Science and Technology Co., Ltd., China). After equilibrium for 60min at 2.0g resting tension, then rings were challenged with KCl (6.0ϫ10Ϫ2 mol/l) repeatedly until a reproducible maximal contractile re-sponse was obtained. The integrity of the endothelium was assessed in all preparations by determining the ability of ACH (10m mol/l) to induce more than 80% relaxation of rings pre-treated with PE (1m mol/l). Then rings was serially washed and used for the following experiment.Bioassay of Vasoreactivity To assess the effect of HCY on vasorelaxation, rings were incubated with Krebs buffer for 30min and then exposed to H CY (0.1—3mmol/l) for 60min. After the incubation, the endothelium-dependent or independent response curve was evoked by ACH (0.01—10m mol/l) or SNP (0.01—10m mol/l) to PE (1m mol/l)-in-duced contraction.In the subsequent experiments, the effect of AST-IV on va-somotor dysfunction induced by HCY in rat aorta and the un-derlying mechanism were determined. Some rings were pre-treated with different concentrations of AST-IV (5, 10, 50, and 100m g/ml) for 30min; some rings were preincubated with SOD (0.6, 1.2, and 1.8KU/l) alone or coincubated with SOD (1.2KU/l) and AST-IV (100m g/ml) for 30min; and the other rings were exposed to L-NAME (100m mol/l) with or without AST-IV (100m g/ml) for 30min. Continuously, HCY (1mmol/l) were administrated to co-incubate the rings in each group for another 60min. Then, the rings were recon-tracted with 1.0m mol/l PE, and endothelium-dependent re-sponses to ACH were repeated.Cellular Experiments H uman umbilical vein endothe-lial cells (HUVECs) were isolated and cultured as previouslydescribed.20)HUVECs from passages 3—4 were used in this study. For cell culture experiment, HUVECs were incubated with medium, AST-IV (5, 10, 50, and 100m g/ml), SOD (1.2, and 1.8KU/l) alone, and AST-IV (100m g/ml) plus SOD (1.2KU/l) for 30min before treatment with H CY (0.1 or 1mmol/l) for 60min. After above treatment, the supernatant and cultured cells were collected respectively. Cells were counted with microscopy and cellular protein concentrations were determined using a protein assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).Assay of NO Level The amount of NO released by cells was determined using a NO assay kit according to the manu-facturer’s protocol (Nanjing Jiancheng Bioengineering Insti-tute, Nanjing, China). The amount of NO metabolites (nitrate and nitrite), which were more stable than NO, was measured by nitric acid reductase method.21)Assay of NO Synthase (NOS) Activity The collected cells were disrupted with ultrasonic wave. NOS enzymatic activity in the cultured cells was determined from the rate of conversion of L-arginine to L-citrulline using a NOS assay kit according to the manufacturer’s protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).22) Measurement of Intracellular ROS Levels For intra-cellular fluorescence measurement of ROS, some H UVECs were loaded with the probe CM-H2DCF-DA for 30min at37°C. CM-H2DCF-DA fluorescence was monitored using a fluorescence microscope (Nikon TE2000, Japan) (excitation 495nm, emission 520nm). The fluorescent intensities were recorded and analyzed by a charge-coupled device Camera (CoolSNAP HQ; Nippon Roper, Chiba, Japan) with an image analysis system (MetaMorph; Nippon Roper).Assay of SOD Activity SOD activity in the cultured cells was determined by inhibition of nitroblue tetrazolium reduction due to superoxide anion generation by xanthine–xanthine oxidase system using a SOD activity assay kit ac-cording to the manufacturer’s protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).23)Statistical Analysis The ACH-induced maximal relax-ation (Emax) in aortic rings was calculated as a percentage of the contraction in response to PE (1m mol/l). The half maxi-mum effective concentration (EC50) was designated as the concentration of ACH that induced 50% of maximum relax-ation from the contraction elicited by PE (1m mol/l). These values were determined by nonlinear regression of the log concentration–response curves. Sensitivity was expressed aspD2(Ϫlog EC50). All values are expressed as meanϮS.E.M. Statistical analysis was performed with one-way ANOV A followed by Newman–Keuls test. Differences were accepted as statistically significant at p values Ͻ0.05 (Graph Pad Prism).RESULTSOrgan Chamber Experiment In each group, a sus-tained vascular contraction with a peak tension of about 2.65Ϯ0.31g was induced by PE (1m mol/l). In any group, no difference in relaxation responses to ACH (10m mol/l) in aor-tic rings was observed at the initial experiment.Compared with the control group (Emax: 98.37Ϯ0.65%,pD2: 6.88Ϯ0.05), H CY (0.1—3mmol/l) treatment inhibited642V ol. 33, No. 4endothelium-dependent relaxation evoked by ACH , and the E max fell to 84.90Ϯ1.01%, 76.99Ϯ1.56%, 65.20Ϯ1.50%, and 51.18Ϯ1.89%, with a reduction of pD 2(6.74Ϯ0.06, 6.62Ϯ0.05, 6.53Ϯ0.05, and 6.50Ϯ0.05). There was no significant difference of pD 2was found between 1mmol/l and 3mmol/l HCY group, although E max exerted a further reduction (Fig.2). Moreover, H CY (0.1—3mmol/l) failed to deteriorate the endothelium-independent relaxation to SNP (data not shown). Therefore, 1mmol/l HCY treatment-induced inhibi-tion of endothelium-dependent relaxation to ACH was used in the subsequent experiment as a control.Treatment with AST -IV (50 or 100m g/ml) was able to improve the inhibition of endothelium-dependent relaxation induced by H CY (1mmol/l). E max and pD 2were shown as (74.67Ϯ2.10% or 81.73Ϯ1.87% versus 65.20Ϯ1.50%,p Ͻ0.05) and (6.78Ϯ0.06 or 6.87Ϯ0.06 versus 6.53Ϯ0.05,p Ͻ0.05), respectively. Additionally, due to the presence of L -NAME (100m mol/l), the inhibitor of nitric oxide synthase (NOS), AST -IV (100m g/ml) failed to show any ameliorating effect on endothelium-dependent vasomotor response caused by HCY (1mmol/l) (Fig. 3).To confirm the oxidative effect of H CY on endothelium-dependent relaxation, SOD was used. With the increase of the concentration of SOD in organ bath (0.6, 1.2, and 1.8KU/l), a more significant effect was observed (E max :70.59Ϯ1.72%, 82.18Ϯ1.26%, and 93.51Ϯ1.30% versus 65.20Ϯ1.50%; pD 2: 6.68Ϯ0.06, 6.91Ϯ0.06, and 9.94Ϯ0.06versus 6.53Ϯ0.05) and the inhibitory effect of HCY was al-most completely abolished. In an analogous manner, com-bined treatment of the rings with AST -IV (100m g/ml) and SOD (1.2KU/l) totally reversed the H CY -induced impair-ment of endothelium-dependent relaxation (E max : 95.10Ϯ2.04%; pD 2: 6.91Ϯ0.06), which was very similar to the ef-fect of 1.8KU/l SOD group (Fig. 4).Cellular Experiments Incubation of H UVECs with H CY (0.1 or 1mmol/l) for 60min resulted in the reduction both of NO content and NOS activity. Treatment with AST -IV (50 and 100m g/ml) was able to attenuate the inhibition both of NO content and NOS activity. Furthermore, SOD (1.8KU/l) exerted more significant ameliorating effects onthese parameters than AST -IV (100m g/ml). Meanwhile,combined treatment with SOD (1.2KU/l) and AST -IV (100m g/ml) induced a similar effect with SOD (1.8KU/l)(Figs. 5, 6).Compared to the control group, treatment with H CY (1mmol/l) for 60min significantly increased the ROS level and decreased the SOD activity in H UVECs. AST -IV (10,50, and 100m g/ml) markedly prevented this accumulation of ROS and reduction of SOD activity. H owever, AST -IV showed a lower potency than SOD (Figs. 7, 8).April 2010643Fig.2.Cumulative Concentration–Response Curves to Acetylcholine in Phenylephrine-Precontracted Endothelium-Intact Aorta Rings Incubated with Homocysteine (HCY) for 60minData are expressed as means ϮS.E.M. n ϭ10. ∗∗p Ͻ0.01 vs.control group.Fig.3.Effect of Astragaloside IV (AST -IV) and/or N w -Nitro-L -arginine Methyl Ester H ydrochloride (L -NAME) on Cumulative Concentration–Re-sponse Curves to Acetylcholine in Endothelium-Intact Aorta Rings Incu-bated with Homocysteine (HCY) for 60minData are expressed as means ϮS.E.M. n ϭ10. ∗∗p Ͻ0.01 vs.1mmol/l HCY group.Fig.4.Effect of Superoxide Dismutase (SOD) with or without Astragalo-side IV (AST -IV) on Cumulative Concentration–Response Curves to Acetyl-choline in Endothelium-Intact Aorta Rings Incubated with H omocysteine (HCY) for 60minData are expressed as means ϮS.E.M. n ϭ10. ∗p Ͻ0.05, ∗∗p Ͻ0.01 vs.1mmol/l HCY group.DISCUSSIONThe present study indicated that AST -IV improved the endothelium dysfunction induced by H CY . Furthermore, a favorable mechanism on activation of NO pathway through the antioxidant defense has been postulated.H yperhomocysteinemia is considered an established risk factor for atherosclerosis. Increased HCY levels are found in 40% of patients with coronary, cerebral or peripheral artery diseases, and only in 15% of healthy individuals.24)Accumu-lating evidence suggests a significant role of altered cellular redox reactions in the vascular phenotype of hyperhomocys-teinemia.25)Redox effects are particularly important in medi-ating the adverse effects of hyperhomocysteinemia on the endothelium, leading to loss of endothelium-derived nitric oxide and vasomotor dysfunction.Consistent with the previous report, the endothelial speci-ficity of HCY was clearly demonstrated in that a significant effect on endothelium-dependent relaxation to ACH was seen both in our present and previous studies.14,26)The result of this study that HCY did not alter the relaxation responses to SNP and the other observation that SNP-induced relaxation in endothelium-denuded tissues was also unaffected by HCY 27)would tend to support the hypothesis that the HCY -induced vasomotor dysfunction occurs within endothelial rather than vascular smooth muscle cells.A reduction in both bioavailability and production of endothelium-derived NO is the hallmark of endothelial dys-function. NO produced through the calcium-dependent acti-vation of endothelial nitric oxide synthase in endothelial cells diffuses into the adjacent vascular muscle layer, and causes its relaxation by activity of cGMP pathway.28)In agree-ment with previous reports,21,29)our data suggested that both NOS activity and NO formation were inhibited by H CY in H UVECs. Thus, we have shown H CY impaired endothelial dysfunction mediated by NOS-NO pathway.Redox reactions resulting in increased vascular levels of ROS may play an important role in mediating endothelial dysfunction, particularly the loss of endothelium-derived NO, during hyperhomocysteinemia. The present study indi-cated that HCY significantly increased intracellular ROS lev-644Vol. 33, No. 4Fig.5.Effects of Astragaloside IV (AST -IV) and/or Superoxide Dismu-tase (SOD) on Content of Nitric Oxide (NO) Released from Human Umbili-cal Vein Endothelial Cells (HUVECs) Treated with Homocysteine (HCY)HUVECs were pretreated with AST -IV (5—100m g/ml) and/or SOD (1.2, 1.8KU/l)for 30min and then stimulated with 1mmol/l HCY for 60min. The content of NO in the supernatant was measured by nitric acid reductase method. The values are ex-pressed as a percentage of the control group from five individual experiments (means ϮS.E.M.). ∗p Ͻ0.05, ∗∗p Ͻ0.01, vs.control group; ϩp Ͻ0.05, ϩϩp Ͻ0.01, vs.1mmol/l HCY group; #p Ͻ0.05 ##p Ͻ0.01, vs.100m g/ml AST-IV group.Fig.6.Effects of Astragaloside IV (AST -IV) and/or Superoxide Dismu-tase (SOD) on the Activity of Nitric Oxide Synthase (NOS) in Human Um-bilical Vein Endothelial Cells (H UVECs) Treated with H omocysteine (HCY)HUVECs were pretreated with AST -IV (5—100m g/ml) and/or SOD (1.2, 1.8KU/l)for 30min and then stimulated with 1mmol/l HCY for 60min. NOS enzymatic activity in the cultured cells was determined from the rate of conversion of L -arginine to L -cit-rulline. The values are expressed as a percentage of the fluorescence intensity of the control group from five individual experiments (means ϮS.E.M.). ∗∗p Ͻ0.01 vs.control group; ϩϩp Ͻ0.01 vs.1mmol/l HCY group; #p Ͻ0.05 vs.100m g/ml AST-IV group.Fig.7.Effects of Astragaloside IV (AST -IV) and/or Superoxide Dismu-tase (SOD) on H omocysteine (H CY)-Induced Reactive Oxygen Species (ROS) ProductionH uman umbilical vein endothelial cells (H UVECs) were pretreated with AST -IV (5—100m g/ml) and/or SOD (1.2, 1.8KU/l) for 30min and then stimulated with HCY for 60min. The probe 5-(6)-chloromethyl-2Ј,7Ј-dichloro-dihydrofluorescein diacetate (CM-H 2DCF-DA) was used to detect the ROS production. The values are expressed as a percentage of the fluorescence intensity of the control group from five individual ex-periments (means ϮS.E.M.). ∗∗p Ͻ0.01, vs.control group; ϩp Ͻ0.05, ϩϩp Ͻ0.01, vs.1mmol/l HCY group; ##p Ͻ0.01, vs.100m g/ml AST-IV group.Fig.8.Effects of Astragaloside IV (AST -IV) on the Activity of Super-oxide Dismutase (SOD) in H uman Umbilical Vein Endothelial Cells (HUVECs) Treated with Homocysteine (HCY)HUVECs were pretreated with AST -IV (5—100m g/ml) for 30min and then stimu-lated with 1mmol/l HCY for 60min. SOD activity was determined by inhibition of ni-troblue tetrazolium reduction due to superoxide anion generation by xanthine–xanthine oxidase system. The values are expressed as units per milligram protein (means ϮS.E.M.). ∗p Ͻ0.05, ∗∗p Ͻ0.01 vs.control group; ϩp Ͻ0.05, ϩϩp Ͻ0.01 vs.1mmol/l HCY group.els and decreased the SOD activity in HUVECs, which was consistent with the reported by Lin et al.30)Treatment with SOD, an antioxidant enzyme, reversed the H CY-impaired endothelium-dependent vasomotor responses. Other re-searchers have suggested that SOD showed similar protective effects on injury aorta, pulmonary artery, or skeletal muscle arterioles.27)During these years, natural products with antioxidant activity have drawn the most attention. Astragalus mem-branaceus is widely used for prevention of ROS-mediated in-jury in pathological situation through its antioxidant proper-ties.13,31—33)In an in vitro study, it was demonstrated by elec-tron paramagnetic resonance imaging technique that Astra-galus membranaceus can potently inhibit ROS produced by the dimethyl sulphoxide system and scavenge over 90% of ROS.34)Recently, the emerging experiments reported that a herbal formulation, comprising Astragalus membranaceus, improved the pancreatic beta cell function via the antioxida-tive effect.35,36)Furthermore, total saponins extracted from Astragalus membranaceus had the effects on enhancing free radical removal and decreasing lipid peroxidation, thus pre-venting the calcium overload in isoproterenol-treated car-diomyocytes.37)According to our previous study, it has been shown that total saponins isolated from Astragalus mem-branaceus ameliorated endothelium-dependent relaxation in-jured by HCY via scavenging free radical species.14) Astragalus membranaceus contains a series of cycloartane triterpene glycosides denoted astragalosides I—VII (sapo-nins), which are based on the aglycone cycloastragenol and contain from one to three sugars attached at the 3-, 6-, and 25-positions.12)AST-IV is regarded as the main criterion of for quality control of Astragalus membranaceus in the Phar-macopoeia of the People’s Republic of China.38)There is emerging evidence that AST-IV has the biological property of eliminating ROS, due to which it protects cardiomyocytes from oxidative stress-mediated injury under hypoxic condi-tions, delays the aging effect in rats treated by hydrocorti-sone, and protects coxsackievirus B3-induced murine my-ocarditis.33,39,40)In the present study, we have shown that AST-IV is effective in protecting endothelium-dependent va-somotor function from injury by HCY, and this ameliorating effect of AST-IV was attenuated by L-NAME, a NOS in-hibitor. In order to clarify whether this effect of AST-IV was exerted through NO pathway, we tested the influence of AST-IV on NO pathway in endothelial cells. The result showed that AST-IV was able to improve content of NO and activity of NOS. However, whether the beneficial effects of AST-IV on vascular endothelial dysfunction injured by HCY are re-lated with scavenging ROS remain largely unknown. It is worth noting that AST-IV not only scavenged OHϪgenerated from the Fenton reaction in an in vitro reaction system,41)but also entered cells and upregulation of SOD content and activ-ity.40)This study provided detailed information that AST-IV inhibited the production of ROS and improved the SOD ac-tivity in endothelial cells incubated by HCY. Moreover, SOD, a scavenger of superoxide anions showed a similar but higher maximum effect than AST-IV.In summary, it was demonstrated for the first time that AST-IV has the ability to regulate NO pathway impaired by HCY through antioxidant defense. In light of these findings, it could lead to the development of a new therapy for the vas-cular lesion in the pathological condition characterized by elevation of HCY in blood plasma.Acknowledgements This study supported by grants from Province Administration of Traditional Chinese Medi-cine, Zhejiang, P.R. China (Project Number: 2009CA055). REFERENCES1)Fowler B., Semin. V asc. Med., 5, 77—86 (2005).2)Eberhardt R. T., Forgione M. A., Cap A., Leopold J. A., Rudd M. A.,Trolliet M., H eydrick S., Stark R., Klings E. S., Moldovan N. I., Y aghoubi M., Goldschmidt-Clermont P. J., Farber H. W., Cohen R., Loscalzo J., J. Clin. Invest., 106, 483—491 (2000).3)Dayal S., Bottiglieri T., Arning E., Maeda N., Malinow M. R., SigmunC. D., Heistad D. D., Faraci F. M., Lentz S. R., Circ. Res., 88, 1203—1209 (2001).4)Bagi Z., Ungvari Z., Koller A., Arterioscler. Thromb. V as. Biol., 22,28—33 (2002).5)Dayal S., Arning E., Bottiglieri T., Boger R. H., Sigmund C. D., FaraciF. M., Lentz S. R., Stroke, 35, 1957—1962 (2004).6)Jacobsen D. W., Catanescu O., Dibello P. M., Barbato J. C., Clin.Chem. Lab. Med., 43, 1076—1083 (2005).7)Symons J. D., Rutledge J. C., Simonsen U., Pattathu R. A., Am. J.Physiol., 290, H181—H191 (2006).8)Tasatargil A., Sadan G., Karasu E., Pulm. Pharmacol. Ther., 20, 265—272 (2007).9)Ungvari Z., Csiszar A., Edwards J. G., Kaminski P. M., Wolin M. S.,Kaley G., Koller A., Arterioscler. Thromb. V asc. Biol., 23, 418—424 (2003).10)Shukla N., Koupparis A., Jones R. A., Angelini G. D., Persad R., Je-remy J. Y., Eur. J. Pharmacol., 531, 201—208 (2006).11)Suematsu N., Ojaimi C., Kinugawa S., Wang Z., Xu X., Koller A.,Recchia F. A., Hintze T. H., Circulation, 115, 255—262 (2007).12)No authors listed, Altern. Med. Rev., 8, 72—77 (2003).13)Ko J. K., Lam F. Y., Cheung A. P., World J. Gastroenterol., 11, 5787—5794 (2005).14)Zhang B. Q., Hu S. J., Qiu L. H., Zhu J. H., Xie X. J., Sun J., Zhu Z.H., Xia Q., Bian K., V ascul. Pharmacol., 46, 278—285 (2007).15)Zhang W. D., Zhang C., Wang X. H., Gao P. J., Zhu D. L., Chen H.,Liu R. H., Li H. L., Planta Med., 72, 621—666 (2006).16)Li Z. P., Cao Q., Acta Pharmacol. Sin., 23, 898—904 (2002).17)Zhang W. J., Hufnagl P., Binder B. R., Wojta J., Thromb. Haemost., 90,904—914 (2003).18)Luo Y., Qin Z., Hong Z., Zhang X., Ding D., Fu J. H., Zhang W. D.,Chen J., Neurosci. Lett., 363, 218—223 (2004).19)Zhang B. Q., Hu S. J., Qiu L. H., Shan Q. X., Sun J., Xia Q., Bian K.,Biol. Pharm. Bull., 28, 1450—1454 (2005).20)Lin R., Liu J., Peng N., Y ang G., Gan W., Wang W., Biol. Pharm. Bull.,28, 1630—1634 (2005).21)Jin L., Caldwell R. B., Li-Masters T., Caldwell R. W., J. Physiol. Phar-macol., 58, 191—206 (2007).22)Ferro A., Queen L. R., Priest R. M., Xu B., Ritter J. M., Poston L.,Ward J. P. T., Br. J. Pharmacol., 126, 1872—1880 (1999).23)Liu H. T., Li W. M., Xu G., Li X. Y., Bai X. F., Wei P., Yu C., Du Y. G.,Pharmacol. Res., 59, 167—175 (2009).24)Welch G. N., Upchurch G. J. R., Loscalzo J., Ann. N.Y. Acad. Sci., 811,48—58 (1997).25)Dayal S., Lentz S. R., Antioxid. Redox Signal., 9, 1899—1909 (2007).26)Fu Y. F., Xiong Y., Fu S. H., J. Cardiovasc. Pharmacol., 42, 566—572(2003).27)Lang D., Kredan M. B., Moat S. J., Hussain S. A., Powell C. A., Bel-lamy M. F., Powers H. J., Lewis M. J., Arterioscler. Thromb. V asc.Biol., 20, 422—427 (2000).28)Linder L., Kiowski W., Buhler F. R., Luscher T. F., Circulation, 81,1762—1767 (1990).29)Jiang X., Y ang F., Tan H., Liao D., Bryan R. M. Jr., Randhawa J. K.,Rumbaut R. E., Durante W., Schafer A. I., Y ang X., Wang H., Arte-rioscler. Thromb. V asc. Biol., 25, 2515—2521 (2005).30)Lin R., Liu J., Gan W., Ding C., Basic Clin. Pharmacol. Toxicol., 101,197—202 (2007).31)Hei Z. Q., Huang H. Q., Zhang J. J., Cheng B. X., Li X. Y., World J.April 2010645。