石墨烯平面结构 GRAPHENE MATERIALS IN THE FLATLAND ppt
- 格式:pdf
- 大小:8.89 MB
- 文档页数:31

石墨烯的制备及其在铅酸电池中的应用石沫;杨新新;周明明;吴亮;柯娃;李厚训;戴贵平【摘要】添加炭材料能够明显地提高铅酸电池的性能。
石墨烯是具有独特平面二维结构的炭材料,具有很多优异的性能,如良好的导电性和很高的比表面积。
本文综述了石墨烯的制备方法,并对目前石墨烯在铅酸电池中的应用情况进行了研究和总结。
%Carbon materials can significantly improve the performance of lead-acid batteries. Graphene is a kind of carbon materials with unique two-dimensional structure, which has a lot of excellent performance, such as good electrical conductivity and high speciifc surface area. This paper reviews the preparation methods of graphene, and its application in lead-acid batteries.【期刊名称】《蓄电池》【年(卷),期】2015(000)003【总页数】4页(P142-145)【关键词】炭材料;石墨烯;导电性;铅酸电池【作者】石沫;杨新新;周明明;吴亮;柯娃;李厚训;戴贵平【作者单位】超威电源有限公司研究院,浙江湖州313100;超威电源有限公司研究院,浙江湖州313100;超威电源有限公司研究院,浙江湖州313100;超威电源有限公司研究院,浙江湖州313100;超威电源有限公司研究院,浙江湖州313100;超威电源有限公司研究院,浙江湖州313100;超威电源有限公司研究院,浙江湖州313100【正文语种】中文【中图分类】TM912.1石墨烯是碳原子紧密堆积的二维蜂窝状晶格结构的碳质材料,碳原子排列呈平面六边形结构,在二维平面上每个碳原子以 sp2杂化轨道相连接[1]。

Comsol软件在二维材料教学中的应用摘要:石墨烯是一种典型的二维材料,具有优良的光学和电学性能,光-物质响应能力强并且易于进行光电调控,在小型化、多功能化的光电子学器件研究中具有广阔应用前景。
为了进行石墨烯的理论与实验教学,首先需进行准确的光电特性建模。
目前,始终缺乏针对石墨烯精准、直观的光电仿真方法,导致教学内容晦涩难懂。
有限元分析软件Comsol Multiphysics具有多物理场综合仿真能力,可自主编译并且剖分精确,可为石墨烯的理论教学提供直观、易于理解的仿真手段。
本文通过研究石墨烯的光电特性,确定了准确的建模参数,之后利用Comsol进行了建模仿真,通过与公开实验数据对比验证了模型的正确性。
该建模方法可用于进行多种二维材料的教学演示。
关键词:Comsol软件,石墨烯,二维材料,仿真建模一、石墨烯特性石墨烯(graphene)是由单层碳原子以六角形式排列的蜂巢状晶格平面结构,2004年,英国科学家Andre Geim和Konstantin Novoselov利用机械剥离法成功制备出单层石墨烯,掀起了对二维材料的研究热潮[1]。
石墨烯每个碳原子都有六个电子,其中2个为内壳层电子,4个为外壳层价电子。
形成石墨烯晶格时,碳原子外壳层4个价电子中的3个电子按sp2杂化轨道分别与邻边三个碳原子构成平面共价键,用“σ”键表示,相比于钻石的sp3杂化轨道共价键,石墨烯具有更为坚固的轨道键,这决定了其卓越的机械性能。
共价键外的一个电子被称为‘π’电子,由于石墨烯的平面结构,其可以自由移动且具有超高迁移率,这一特性使石墨烯展示出了诸多奇异光电子学性质。
不同于其他半导体材料,石墨烯具有零带隙特性,如图1。
其特殊的能量-色散关系决定了石墨烯的超高电导率。
科学家们在理论上证明了石墨烯载流子迁移率可达到100000,实验中诸多研究者获得了超过15000的载流子迁移率。
这一数值超过硅材料的10倍,是目前已知载流子迁移率最高的物质,因此石墨烯也被称为“半金属”。
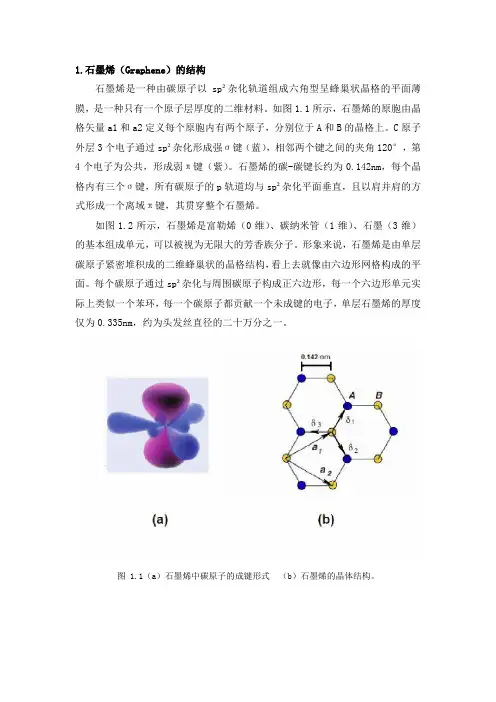
1.石墨烯(Graphene)的结构石墨烯是一种由碳原子以sp²杂化轨道组成六角型呈蜂巢状晶格的平面薄膜,是一种只有一个原子层厚度的二维材料。
如图1.1所示,石墨烯的原胞由晶格矢量a1和a2定义每个原胞内有两个原子,分别位于A和B的晶格上。
C原子外层3个电子通过sp²杂化形成强σ键(蓝),相邻两个键之间的夹角120°,第4个电子为公共,形成弱π键(紫)。
石墨烯的碳-碳键长约为0.142nm,每个晶格内有三个σ键,所有碳原子的p轨道均与sp²杂化平面垂直,且以肩并肩的方式形成一个离域π键,其贯穿整个石墨烯。
如图1.2所示,石墨烯是富勒烯(0维)、碳纳米管(1维)、石墨(3维)的基本组成单元,可以被视为无限大的芳香族分子。
形象来说,石墨烯是由单层碳原子紧密堆积成的二维蜂巢状的晶格结构,看上去就像由六边形网格构成的平面。
每个碳原子通过sp²杂化与周围碳原子构成正六边形,每一个六边形单元实际上类似一个苯环,每一个碳原子都贡献一个未成键的电子,单层石墨烯的厚度仅为0.335nm,约为头发丝直径的二十万分之一。
图 1.1(a)石墨烯中碳原子的成键形式(b)石墨烯的晶体结构。
图1.2石墨烯原子结构图及它形成富勒烯、碳纳米管和石墨示意图石墨烯按照层数划分,大致可分为单层、双层和少数层石墨烯。
前两类具有相似的电子谱,均为零带隙结构半导体(价带和导带相较于一点的半金属),具有空穴和电子两种形式的载流子。
双层石墨烯又可分为对称双层和不对称双层石墨烯,前者的价带和导带微接触,并没有改变其零带隙结构;而对于后者,其两片石墨烯之间会产生明显的带隙,但是通过设计双栅结构,能使其晶体管呈示出明显的关态。
单层石墨烯(Graphene):指由一层以苯环结构(即六角形蜂巢结构)周期性紧密堆积的碳原子构成的一种二维碳材料。
双层石墨烯(Bilayer or double-layer graphene):指由两层以苯环结构(即六角形蜂巢结构)周期性紧密堆积的碳原子以不同堆垛方式(包括AB堆垛,AA堆垛,AA‘堆垛等)堆垛构成的一种二维碳材料。
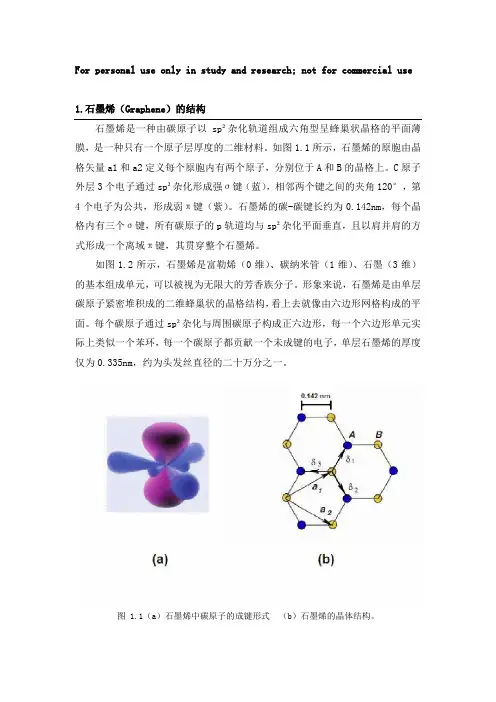
For personal use only in study and research; not for commercial use1.石墨烯(Graphene)的结构石墨烯是一种由碳原子以sp²杂化轨道组成六角型呈蜂巢状晶格的平面薄膜,是一种只有一个原子层厚度的二维材料。
如图1.1所示,石墨烯的原胞由晶格矢量a1和a2定义每个原胞内有两个原子,分别位于A和B的晶格上。
C原子外层3个电子通过sp²杂化形成强σ键(蓝),相邻两个键之间的夹角120°,第4个电子为公共,形成弱π键(紫)。
石墨烯的碳-碳键长约为0.142nm,每个晶格内有三个σ键,所有碳原子的p轨道均与sp²杂化平面垂直,且以肩并肩的方式形成一个离域π键,其贯穿整个石墨烯。
如图1.2所示,石墨烯是富勒烯(0维)、碳纳米管(1维)、石墨(3维)的基本组成单元,可以被视为无限大的芳香族分子。
形象来说,石墨烯是由单层碳原子紧密堆积成的二维蜂巢状的晶格结构,看上去就像由六边形网格构成的平面。
每个碳原子通过sp²杂化与周围碳原子构成正六边形,每一个六边形单元实际上类似一个苯环,每一个碳原子都贡献一个未成键的电子,单层石墨烯的厚度仅为0.335nm,约为头发丝直径的二十万分之一。
图 1.1(a)石墨烯中碳原子的成键形式(b)石墨烯的晶体结构。
图1.2石墨烯原子结构图及它形成富勒烯、碳纳米管和石墨示意图石墨烯按照层数划分,大致可分为单层、双层和少数层石墨烯。
前两类具有相似的电子谱,均为零带隙结构半导体(价带和导带相较于一点的半金属),具有空穴和电子两种形式的载流子。
双层石墨烯又可分为对称双层和不对称双层石墨烯,前者的价带和导带微接触,并没有改变其零带隙结构;而对于后者,其两片石墨烯之间会产生明显的带隙,但是通过设计双栅结构,能使其晶体管呈示出明显的关态。
单层石墨烯(Graphene):指由一层以苯环结构(即六角形蜂巢结构)周期性紧密堆积的碳原子构成的一种二维碳材料。

石墨平台微结构的纳米级红外光谱表征史云胜;刘秉琦;杨兴【摘要】具有原子级光滑平面的石墨平台微结构是实现特殊功能微机电器件、微系统的重要基础.石墨微结构的化学信息表征对微机电器件、微系统的制备及性能有着重要的意义.先使用原子力显微镜获得形貌信息,再使用纳米级红外光谱对微结构的不同区域进行表征,获得了多个特征位置的红外光谱.通过对红外光谱的分析发现相对于其他位置,石墨平台表面具有非常有序的碳六元环结构,并且吸附的水分子最少.而石墨平台微结构的边缘由于悬键及微加工等原因是吸附水分子最多的位置,石墨基底由于微加工的破坏已经不具有碳六元环结构.这些信息为了解微结构的化学状态提供了帮助,明确所处环境对石墨平台微结构不同位置的影响,能够指导微机电器件的制备与应用.%The graphite mesa microstructure with the atomically flat surface is an important basis for the realization of the special function of MEMS devices and micro systems. Chemical information characterization of graphite microstructure has important significance on the manufacture and properties of MEMS devices and micro systems. Atomic force microscopy was used to obtain the morphology information, and then the different regions of the microstructure were characterized by the nanoscale infrared spectroscopy. Through the analysis of the infrared spectra, it is found that the surface of the graphite mesa has a very ordered carbon hexatomic ring structure with respect to the other places, and adsorbs least water molecules. The edge of the graphite mesa microstructure adsorbed most water molecules because of the dangling bond and microfabrication. The graphite substrate has no carbonhexatomic structure due to the destruction of the microfabrication. This information helps to understand the chemical state of the microstructure. Above information can make it clear that the influence of the environment on the different regions of graphite mesa microstructure and can also guide the manufacture and application of MEMS devices.【期刊名称】《红外技术》【年(卷),期】2016(038)011【总页数】6页(P914-919)【关键词】微机电器件;红外光谱;纳米级;石墨微结构【作者】史云胜;刘秉琦;杨兴【作者单位】军械工程学院电子与光学工程系,石家庄 050003;清华大学精密仪器系,北京 100084;军械工程学院电子与光学工程系,石家庄 050003;清华大学精密仪器系,北京 100084;清华大学精密测试技术及仪器国家重点实验室,北京 100084【正文语种】中文【中图分类】O657.3石墨具有很多极端的物理性质,如超高面内刚度、良好导电率和极高热导率[1-3]。

石墨烯材料简介在构成纳米材料的众多元素中,碳元素值得我们格外重视。
作为自然界中性质最为奇特的元素,碳(C)在原子周期表中的序号为六,属于第Ⅳ族。
碳原子一般是四价的,最外层有4个电子,可与四个原子成键。
但是其基态只有两个单电子,所以成键时总是要进行杂化。
由于较低的原子序数,碳原子对外层电子的结合力强,表现出较高的键能,容易形成共价键,故自然界中碳元素形成的化合物形式丰富多彩。
关于碳与碳原子之间或碳与其它原子间以共价键相结合,有杂化轨道和分子轨道的理论。
在形成共价键过程中,由于原子间的相互影响,同一个原子中参与成键的几个能量相近的原子轨道可以重新组合,重新分配能量和空间方向,组成数目相等的,成键能力更强的新的原子轨道,称为杂化轨道。
在有机化合物中,碳原子的杂化形式有三种:sp3、sp2和sp杂化轨道。
以甲烷分子(CH4)为例,碳原子在基态时的电子构型为1S22S22Px12Py12Pz0按理只有2px和2py可以形成共价键,键角为90°。
但实际在甲烷分子中,是四个完全等同的键,键角均为109°28′。
这是因为在成键过程中,碳的2s轨道有一个电子激发到2Pz轨道,3个p轨道与一个s轨道重新组合杂化,形成4个完全相同的sp3杂化轨道。
每个轨道是由s/4与3P/4轨道杂化组成。
这四个sp3轨道的方向都指向正四面体的四个顶点,轨道间的夹角是109°28´。
得益于碳原子丰富多样的键合方式和强大的键合能力,氧、氢、氮等各种元素被有机的组合在一起,形成碳的化合物,最终构成了令人惊叹的生命体。
碳元素广泛存在于自然界,其独特的物性和多样的形态随着人类文明的进步而逐渐被发现。
由于碳原子之间不同的杂化方式,能形成结构和性质迥异的多种同素异型体,其中最为人知的存在形式是金刚石和石墨。
当每个碳原子与四个近邻碳原子以共价键结合(sp3杂化)时,形成各向同性的金刚石。
此时,四个价电子平均分布在四个轨道中,形成稳定的σ键,而且没有孤电子对的排斥,非常稳定。

石墨烯的研究进展刘乐浩,李铁虎,赵廷凯,王大为(西北工业大学材料科学与工程学院,西安710072)摘要石墨烯是碳的又一同素异形体,具有独特的二维结构和优异的力学、电学、光学、热学等性能,成为富勒烯和碳纳米管之后的又一研究热点。
全面综述了近几年来石墨烯的制备方法,洋细讨论了微机械剥离法、化学剥离法、化学合成法、外延生长法、电弧法、化学气相沉积法的优缺点,并针对制备方法存在的产量低、结构不稳定、高污染等问题,提出了一些大规模可控制备高质量石墨烯的建议。
还结合石墨烯的结构和特性,概括了石墨烯在复合材料、微电子、光学、能源、生物医学等领域的应用进展,并展望了其主要研究方向和发展趋势。
关键词石墨烯制备方法应用中图分类号:〇613. 71 文献标识码:Research Progress on GrapheneLIU Lehao,LI Tiehu,ZHAO Tingkai,WANG Dawei (School of Materials Science and Engineering,Northwestern Polytechnical University,Xi,an 710072)Abstract As an allotrope of carbon,graphene has become a research hotspot due to its unique two-dimensional structure and excellent mechanical,electrical,optical and thermal properties. Synthesis of graphene via different approaches ,such as micro mechanical stripping, chemical stripping, chemical synthesis, epitaxial growth, arc dis- charge,and chemical vapor deposition, are discussed in detail, and strategies for producing homogeneous graphene with improved yield and structural stability while limiting its pollution are proposed. Also application progress of gre- phene in polymer composites,micro electronics, optics, energy and biomedicine are summarized, and the main research direction and development trend are imagined.Key words graphene,preparation methods,applicationo引言富勒烯[1]和碳纳米管[2]已经成为碳材料研究的热点,而在2004年,Geim等[3]又发现了碳的又一同素异形体——石墨烯(Graphene)。

石墨烯石墨烯声明:百科词条人人可编辑,词条创建和修改均免费,绝不存在官方及代理商付费代编,请勿上当受骗。
详情>> 石墨烯(二维碳材料)编辑本词条由“科普中国”百科科学词条编写与应用工作项目审核。
石墨烯(Graphene)是一种由碳原子以sp2杂化方式形成的蜂窝状平面薄膜,是一种只有一个原子层厚度的准二维材料,所以又叫做单原子层石墨。
英国曼彻斯特大学物理学家安德烈·盖姆和康斯坦丁·诺沃肖洛夫,用微机械剥离法成功从石墨中分离出石墨烯,因此共同获得2010年诺贝尔物理学奖。
石墨烯常见的粉体生产的方法为机械剥离法、氧化还原法、SiC外延生长法,薄膜生产方法为化学气相沉积法(CVD)。
[1] 由于其十分良好的强度、柔韧、导电、导热、光学特性,在物理学、材料学、电子信息、计算机、航空航天等领域都得到了长足的发展。
作为目前发现的最薄、强度最大、导电导热性能最强的一种新型纳米材料,石墨烯被称为“黑金”,是“新材料之王”,科学家甚至预言石墨烯将“彻底改变21世纪”。
极有可能掀起一场席卷全球的颠覆性新技术新产业革命。
中文名石墨烯外文名Graphene 发现时间2004年主要制备方法机械剥离法、气相沉积法、氧化还原法、SiC外延法主要分类单层、双层、少层、多层(厚层)基本特性强度柔韧性、导热导电、光学性质应用领域物理、材料、电子信息、计算机等目录1 研究历史2 理化性质? 物理性质? 化学性质3 制备方法? 粉体生产方法? 薄膜生产方法4 主要分类? 单层石墨烯? 双层石墨烯? 少层石墨烯? 多层石墨烯5 主要应用? 基础研究? 晶体管? 柔性显示屏? 新能源电池? 航空航天? 感光元件? 复合材料6 发展前景? 中国? 美国? 欧洲? 韩国? 西班牙? 日本研究历史编辑实际上石墨烯本来就存在于自然界,只是难以剥离出单层结构。
石墨烯一层层叠起来就是石墨,厚1毫米的石墨大约包含300万层石墨烯。

毕业论文题目:_________ 石墨烯复合材料的制备______及其性能研究讲展学院:化学化工学院____________________专业:___________ 化学工程与工艺__________________毕业年限:________ 2015年 ______________________学生姓名:_________________________学号:____________________________指导教师:__________________________石墨烯复合材料的制备及其性能研究进展摘要:石墨烯以其优异的性能和独特的二维结构成为材料领域研究热点。
本文综述了石墨烯的制备方法并分析比较了各种方法的优缺点,简单介绍了石墨烯的力学、光学、电学及热学性能。
基于石墨烯的复合材料是石墨烯应用领域中的重要研究方向,本文详细介绍了石墨烯聚合物复合材料和石墨烯基无机纳米复合材料的制备及应用,以及石墨烯复合材料的展望。
关键词:石墨烯;制备;性能;复合材料Research Progress on Preparation and properties ofgraphene composite materialsAbstract: Graphene has become a hot researchfield of material for its excellent performanee and unique two-dimensional structure. This paper summarizes the method for preparing graphene and compared the advantagesand disadvantagesof various methods, in troduces the mecha ni cs, graphe ne optical, electrical and thermal properties. Composite materials based on graphe ne is an importa nt research direct ion in the field of application of graphene, this paper introduces the preparation and application of graphene polymer composites and graphene based inorganic nano composite material, and the prospect of graphe ne composite materials.Key words: graphe ne; preparati on; properties; composite materials1•刖言石墨烯自2004年被发现以来,就引起了材料科学家的广泛关注,在世界范围内掀起了石墨烯材料的制备和应用研究的热潮。

姓名:李雄杰学号:20071050198专业:物理学石墨烯的结构性能及应用(云南大学物理科学技术学院物理系云南昆明650091)摘要:石墨烯是2004年才发现的一种有奇异性能的新型材料,它是由碳原子组成的二维六角点阵结构,具有单一原子层或几个原子层厚。
石墨烯因其具有独特的电子能带结构和具相对论电子学特性,是迄今为止人类发现的最理想的二维电子系统,且具有丰富而新奇的物理特性。
本文详细介绍了石墨烯的结构,特殊性能及相关应用。
关键词:石墨烯;结构性能;相关应用一、引言石墨烯是2004年以来发现的新型电子材料【1】石墨烯是sp2杂化碳原子形成的厚度仅为单层原子的排列成蜂窝状六角平面晶体。
在单层石墨烯中,碳碳键长为0.142nm,厚度只有0.334nm。
石墨烯是构成下列碳同素异型体的基本单元:例如:石墨,碳纳米管和富勒烯。
石墨烯被认为是平面多环芳香烃原子晶体。
石墨烯在电子和光电器件领域有着重要和广阔的应用前景【2】正因为如此,石墨烯的两位发现者获得了2010年的诺贝尔物理学奖。
图1石墨烯结构图石墨烯是一种没有能隙的半导体,具有比硅高100倍的载流子迁移率(2×105cm2/v),在室温下具有微米级自由程和大的相干长度,因此石墨烯是纳米电路的理想材料石墨烯具有良好的导热性[3000W/(m·K)]、高强度(110GPa)和超大的比表面积(2630mZ/g)。
这些优异的性能使得石墨烯在纳米电子器件、气体传感器、能量存储及复合材料等领域有光明的应用前景【3-4】二.石墨烯的特殊性能石墨烯是一种半金属或者零带隙二维材料,在靠近布里渊区6个角处的低能区,其E-k色散关系是线性的【5】,因而电子或空穴的有效质量为零,这里的电子或空穴是相对论粒子,可以用自旋为1/2粒子的狄拉克方程来描述。
石墨烯的电子迁移率实验测量值超过15000cm2/(V·s)(载流子浓度n≈1013cm-2),在10~100K范围内,迁移率几乎与温度无关,说明石墨烯中的主要散射机制是缺陷散作者简介:李雄杰(1987-)、男,湖南人,云南大学物理学专业在读本科生,主要研究碳纳米材料及应用。
石墨烯(Graphene)作为一种平面无机纳米材料,在物理、化学、科技、数码方面的发展都是极具前景的。
它的出现为科学界带来极大的奉献,机械强度高,导热和导电功能极具优势,原材料来源即石墨也相当丰富,是制造聚合复合物的最正确无机纳米技术。
由于石墨烯的运用很广泛,导致在工业界的发展存在很严重的一个问题就是其制作过程规模浩大,所以应该将其合理地分散到相应的聚合物内部,到达均匀分布的效果,同时平衡聚合物之间的作用力。
石墨烯的内部结构是以碳原子以sp2 杂化而成的,是一种单原子结构的平面晶体,其以碳原子为核心的蜂窝状结构。
一个碳原子相应的只与非σ键以外的三个碳原子按照相应的顺序连接,而其他的π则相应的与其他的的碳原子的π电子有机地组成构成离域大π键,在这个离域范围内,电子的移动不受限制,因为此特性使得石墨烯导电性能优异。
另一方面,这样的蜂窝状结构也是其他碳材料的基础构成元素。
如图1-1 所示,单原子层的最外层石墨烯覆盖组成零维的富勒烯,任何形状的石墨烯均可以变化形成壁垒状的管状[1]。
因为在力学规律上,受限于二维晶体的波动性,所以任何状态的石墨烯都不是平整存在的,而是稍有褶皱,不管是沉积在最底层的还是不收区域限制的。
,如图1-2 所示,蒙特卡洛模拟〔KMC〕做出了相应的验证[3]。
上面所提的褶皱范围在横向和纵向上都存在差异,这种微观褶皱的存在会在一定程度上引起静电,所以单层的会很容易聚集起来。
同时,褶皱的程度也会相应的影响其光电性能[3-6]图1-1. 石墨烯:其他石墨结构碳材料的基本构造单元,可包裹形成零维富勒烯,卷曲形成一维碳纳米管,也可堆叠形成三维的石墨[7]。
Figure 1-1. Graphene: the building material for other graphitic carbon materials. It can be wrapped up into 0D buckyballs, rolled into 1D nanotubes or stacked into 3D graphite[7].图1-2. 单层石墨烯的典型构象[1]。
高中物理石墨烯知识点2004年,Science杂志首次报道了曼彻斯特大学的AndreGeim和KonstantinNovoselov成功分离出稳定的石墨烯,并论述了石墨烯材料的基本性质他们关于石墨烯的研究被授予2010年诺贝尔物理奖,轰动世界,推动了石墨烯材料的研究,促进了石墨烯在物理化学材料生物医学和环境方面的研究。
石墨烯(Graphene)是一种由碳原子以sp2杂化方式形成的蜂窝状平面薄膜,是当前发现的唯一一种二维自由态原子晶体,是除金刚石以外其他碳晶体的基本结构单元,具有许多极佳的电子及机械性能,是当前使用的材料中最薄强度最大导电和导热性能最好的一种纳米材料。
近年来,科学界对石墨烯的研究逐渐从石墨烯的制备研究转变到对石墨烯的应用研究,并对石墨烯在光电医学计算机晶体管等领域都进行了大量的研究,取得了较好的成果,其主要结构及特性如下。
(一)结构特征石墨烯是一种单原子层的碳二维纳米材料,其点阵结构是由碳六元环组成的二维蜂窝状,是构成其他石墨材料的基本单元,石墨烯主要分为单层石墨烯双层石墨烯少层石墨烯多层或厚层石墨烯4个类别石墨烯中每个碳原子之间都以很强的共价σ键相互结合,且相互成为120°的角度,其C—C键长大约为0.142nm,构成了特有的六角晶格,这种特殊的成键方式和结构使石墨烯成为史上最牢固的材料之一。
(二)导电特性石墨烯原子之间具有极强的相互作用力,在室温条件下,即使碳原子之间发生相互碰撞和挤压,石墨烯中的电子在轨道中移动时也不会发生散射,其电子受到的干扰也非常小,其电子迁移率可达到2x10^5cm^2/V·s,约为硅中电子迁移率的140倍,砷化镓的20倍,温度稳定性也较高,电导率可达10^8Ω/m,电阻约为31Ω/sq,比铜或银更低,是室温下导电最好的材料因此,石墨烯具有较好的导电性能。
(三)导热特性石墨烯的导热效应在高温时由光子传导,在低温时由其中的弹道传输所决定其热导率室温下是5000W·m^-1·K^-1,是硅的36倍,砷化镓的20倍,是铜在室温下的十倍多,因此,石墨烯的导热性是目前已知材料中最高的,这使石墨烯在许多微热电器件等领域有非常广阔的应用前景。
Progress in Polymer Science 35 (2010) 1350–1375Contents lists available at ScienceDirectProgress in PolymerSciencej o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /p p o l y s ciRecent advances in graphene based polymer compositesTapas Kuilla a ,Sambhu Bhadra b ,Dahu Yao a ,Nam Hoon Kim c ,Saswata Bose d ,Joong Hee Lee a ,d ,∗a BIN Fusion Research Team,Department of Polymer &Nano Engineering,Chonbuk National University,Jeonju,Jeonbuk 561-756,Republic of Koreab School of Polymers and High Performance Materials,The University of Southern Mississippi,118College Drive #10076,Hattiesburg,MS 39406-0001,USAc Department of Hydrogen and Fuel Cell Engineering,Chonbuk National University,Jeonju,Jeonbuk 561-756,Republic of Korea dDepartment of BIN Fusion Technology,Chonbuk National University,Jeonju,Jeonbuk 561-756,Republic of Koreaa r t i c l e i n f o Article history:Received 8March 2010Received in revised form 13July 2010Accepted 21July 2010Available online 27 July 2010Keywords:GrapheneGraphene modification Polymer composites Mechanical properties Electrical conductivitya b s t r a c tThis paper reviews recent advances in the modification of graphene and the fabrication of graphene-based polymer nanocomposites.Recently,graphene has attracted both academic and industrial interest because it can produce a dramatic improvement in properties at very low filler content.The modification of graphene/graphene oxide and the utilization of these materials in the fabrication of nanocomposites with different polymer matrixes have been explored.Different organic polymers have been used to fabricate graphene filled polymer nanocomposites by a range of methods.In the case of modified graphene-based polymer nanocomposites,the percolation threshold can be achieved at a very lower filler loading.Herein,the structure,preparation and properties of polymer/graphene nanocomposites are discussed in general along with detailed examples drawn from the scientific literature.© 2010 Elsevier Ltd. All rights reserved.Contents 1.Introduction ........................................................................................................................13512.Graphene............................................................................................................................13522.1.Discovery of graphene (1352)Abbreviations:AFM,atomic force microscopy;CNT,carbon nanotubes;CNF,carbon nanofiber;CCl 4,carbon tetrachloride;CTEs,coefficient of thermalexpansions;CVD,chemical vapor deposition;S-CCG,chemically converted graphene;CHCl 3,chloroform;CMG,chemically modified graphene;Cryo-TEM,cryogenic transmission electron microscopy;DCM,dichloro methane;DMSO,dimethyl sulfoxide;DSC,differential scanning calorimetry;DMA,dynamic mechanical analysis;DMTA,dynamic mechanical thermal analysis;EELS,electron energy loss spectroscopy;EMI,electromagnetic interference; ,elec-trical conductivity;ESEM,environmental scanning electron microscopy;xGnP,exfoliated graphite nanoplatelet;FLG,few layer graphene;GNS,graphene nanosheets;EVA,ethylene vinyl acetate;EG,expanded graphite;FG,foliated graphite;FT-IR,fourier transform infra red;GPa,gigapascal;T g ,glass transition temperature;GO,graphite oxide;HMPA,hexamethylphosphoramide;HDPE,high density polyethylene;IR,infra red;GNP IL ,ionic-liquid-functionalized graphene;KMG,KOH treated GO;LDH,layered double hydroxide;LM,light microscopy;LLDPE,linier low density polyethylene;e //,loss factor;MPa,mega-pascal;MMA,methyl methacrylate;NMP,N-methylpyrrolidone;DMF,N,N -dimethylformamide;DMAc,N,N -dimethylacetamide;ODA,octadecylamine;PETI,phenylethynyl-terminated polyimide;PANI,polyaniline;PPS,poly(phenylene sulfide);PNIPAAm,poly(N-isopropylacrylamide);PSS,poly(sodium 4-styrenesulfonate);PVA,poly(vinyl alcohol);PS,polystyrene;PMMA,poly(methyl methacrylate);PI,polyimide;PET,poly(ethylene terepthalate);PE-g-MA,polyethylene grafted maleic anhydride;PP,polypropylene;PVC,poly(vinyl chloride);PA6,polyamide;SIS,poly(styrene-b -isoprene-b -styrene);PS,polystyrene;PU,polyurethane;PVDF,poly(vinylidene fluoride);PEDOT,poly(3,4-ethyldioxythiophene);PB −,pyrenebutyrate;PBA,pyrene butyric acid;SEM,scanning electron microscopy;SiO 2,silicaSiCsilicon carbide;SWNTs,single wall carbon nanotubes;SDS,sodium dodecyl sulfate;SDBS,sodium dodecyl benzene sulfonate;E /or G /,storage modulus;SSSP,solid-state shear pulverization;SPANI,sulfonated polyaniline;THF,tetrahydrofuran;TMEDA,tetramethylethylenediamine;TCNQ,7,7,8,8-tetracyanoquinodimethane;TGA,thermogravimetric analysis;TPU,thermoplastic polyurethane;XRD,X-ray diffraction.∗Corresponding author at:BIN Fusion Research Team,Department of Polymer &Nano Engineering,Chonbuk National University,Jeonju,Jeonbuk 561-756,Republic of Korea.Tel.:+82632702342;fax:+82632702341.E-mail address:jhl@chonbuk.ac.kr (J.H.Lee).0079-6700/$–see front matter © 2010 Elsevier Ltd. All rights reserved.doi:10.1016/j.progpolymsci.2010.07.005T.Kuila et al./Progress in Polymer Science35 (2010) 1350–137513512.2.Synthesis and structural features of graphene (1352)2.3.Surface modification of graphene (1352)2.3.1.Chemical modification of graphene (1353)2.3.2.Electrochemical modification of graphene (1356)2.3.3.–interaction (1357)3.Polymer/graphene nanocomposites (1358)3.1.Preparation methods of polymer/graphene nanocomposites (1358)3.1.1.In situ intercalative polymerization (1358)3.1.2.Solution intercalation (1359)3.1.3.Melt intercalation (1359)3.2.Graphenefilled different polymer composites (1360)3.2.1.Epoxy/graphene nanocomposites (1360)3.2.2.Polystyrene/graphene nanocomposites (1360)3.2.3.Polyaniline/graphene nanocomposites (1362)3.2.4.Nafion/graphene nanocomposites (1364)3.2.5.PVA/graphene nanocomposites (1364)3.2.6.PU/graphene nanocomposites (1365)3.2.7.PVDF/graphene nanocomposites (1366)3.2.8.Poly(3,4-ethyldioxythiophene)/graphene nanocomposites (1368)3.2.9.Polyethylene terephthalate/graphene nanocomposites (1368)3.2.10.Polycarbonate/graphene nanocomposites (1369)4.Conclusions (1369)Acknowledgements (1370)References (1371)1.IntroductionThefield of nanoscience has blossomed over the last twenty years,and the importance for nanotechnology will increase as miniaturization becomes more important in areas,such as computing,sensors,biomedical and many other applications.Advancements in these disciplines depend largely on the ability to synthesize nanoparti-cles of various materials,sizes and shapes,as well as to assemble them efficiently into complex architectures [1].Currently,nanomaterials have an enormous range of applications owing to their structural features.However, material scientists are examining materials with improved physicochemical properties that are dimensionally more suitable in thefield of nanoscience and technology.In this regard,the discovery of graphene and graphene-based polymer nanocomposites is an important addition in the area of nanoscience,playing a key role in modern science and technology[2].The discovery of polymer nanocomposites by the Toyota research group[3]has opened a new dimension in thefield of materials science.In particular,the use of inorganic nanomaterials asfillers in the preparation of polymer/inorganic composites has attracted increasing interest owing to their unique properties and numerous potential applications in the automotive,aerospace,con-struction and electronic industries[4–11].Thus far,the majority of research has focused on polymer nanocom-posites based on layered materials of a natural origin,such as a montmorillonite type of layered silicate compounds or synthetic clay(layered double hydroxide)[5–17]. However,the electrical and thermal conductivity of clay minerals are quite poor[18–20].In order to overcome these shortcomings,carbon-based nanofillers,such as carbon black,EG,CNT,and CNF have been introduced to the preparation of polymer nanocomposites[21–47].Among these,CNTs have proven to be very effective as conductivefillers[22,33–39].The only drawback of CNTs as a nanofiller is their higher production cost[48]. Therefore,the mass production of CNT based functional composite materials is very difficult.As Nicholas A.Kotov wrote in his review in Nature[49]“When carbonfibers just won’t do,but nanotubes are too expensive,where can a cost-conscious materials scientist go tofind a practical conductive composite?The answer could lie with graphene sheets”.Graphene is considered a two-dimensional carbon nanofiller with a one-atom-thick planar sheet of sp2bonded carbon atoms that are densely packed in a honeycomb crystal lattice.It is regarded as the“thinnest material in the universe”with tremendous application potential[50,51].Graphene is predicted to have remarkable properties,such as high thermal con-ductivity,superior mechanical properties and excellent electronic transport properties[52–56].These intrinsic properties of graphene have generated enormous interest for its possible implementation in a myriad of devices [57].These include future generations of high speed and radio frequency logic devices,thermally and electrically conducting reinforced nanocomposites,ultra-thin carbon films,electronic circuits,sensors,and transparent and flexible electrodes for displays and solar cells[57–71]. Graphene,as a nanofiller,may be preferred over other conventional nanofillers(Na-MMT,LDH,CNT,CNF,EG,etc.) owing to high surface area,aspect ratio,tensile strength (TS),thermal conductivity and electrical conductivity, EMI shielding ability,flexibility,transparency,and low CTE[52–56,72].Table1gives a comparative chart on the mechanical,thermal and electrical properties of graphene with CNT,steel,plastic,rubber andfiber.The tensile strength of graphene is similar or slightly higher than CNT, but much higher than steel,Kevlar,HDPE and natural rub-ber.The thermal conductivity of graphene is higher than1352T.Kuila et al./Progress in Polymer Science35 (2010) 1350–1375Table1Properties of graphene,CNT,nano sized steel,and polymers.Materials Tensile strength Thermal conductivity(W/mk)at room temperature Electrical conductivity(S/m)ReferencesGraphene130±10GPa(4.84±0.44)×103to(5.30±0.48)×1037200[73–84] CNT60–150GPa35003000–4000[35,85–88] Nano sized steel1769MPa5–6 1.35×106[89,90] Plastic(HDPE)18–20MPa0.46–0.52Insulator[91–93] Rubber(natural rubber)20–300.13–0.142Insulator[94,95] Fiber(Kevlar)3620MPa0.04Insulator[96,97]all these materials.The electrical conductivity of graphene is also higher than these materials except for steel[73–97].The superior properties of graphene compared to polymers are also reflected in polymer/graphene nanocom-posites.Polymer/graphene nanocomposites show superior mechanical,thermal,gas barrier,electrical andflame retardant properties compared to the neat polymer [2,44–47,98–101].It was also reported that the improve-ment in mechanical and electrical properties of graphene based polymer nanocomposites are much better in com-parison to that of clay or other carbonfiller-based polymer nanocomposites[2,98–101].Although CNTs show compa-rable mechanical properties compared to graphene,still graphene is a better nanofiller than CNT in certain aspects, such as thermal and electrical conductivity(Table1) [73–97].However,the improvement in the physicochem-ical properties of the nanocomposites depends on the distribution of graphene layers in the polymer matrix as well as interfacial bonding between the graphene layers and polymer matrix.Interfacial bonding between graphene and the host polymer dictates thefinal properties of the graphene reinforced polymer nanocomposite.Pristine graphene is not compatible with organic polymers and does not form homogeneous composites.In contrast,graphene oxide sheets are heavily oxygenated graphene(bearing hydroxyl,epoxide,diols,ketones and carboxyls functional groups)that can alter the van der Waals interactions sig-nificantly and be more compatible with organic polymers [68,102–106].There are also some additional carbonyl and carboxyl groups located at the edge of the sheets,which makes graphene oxide sheets strongly hydrophilic,allow-ing them to readily swell and disperse in water[107,108]. For this reason,GO has attracted considerable attention as a nanofiller for polymer nanocomposites.However,GO sheets can only be dispersed in aqueous media,which are incompatible with most organic polymers.In addition, unlike graphene,graphene oxide is electrically insulating, which makes it unsuitable for the synthesis of conducting nanocomposites.The surface modification of graphene is an essential step for obtaining a molecular level dispersion of individual graphene in a polymer matrix.The electri-cal conductivity of the resulting nanocomposites can be increased by the chemical reduction of GO,presumably by restoring the graphitic network of sp2bonds[2].2.Graphene2.1.Discovery of grapheneGraphene is the basic structural unit of some car-bon allotropes,including graphite,carbon nanotubes and fullerenes(Fig.1).It is believed to be composed of ben-zene rings stripped of their hydrogen atoms.The rolling up of graphene along a given direction can produce a car-bon nanotube.A zero-dimensional fullerene can also be obtained by wrapping-up graphene[109,110].In1940,it was established theoretically that graphene is the building block of graphite[111].In2004,Geim and co-workers at Manchester University successfully identified single lay-ers of graphene and other2-D crystals[112]in a simple tabletop experiment,which were previously considered to be thermodynamically unstable and could not exist under ambient conditions[113,114].The promising mechani-cal,electrical,optical,thermal and magnetic properties of graphene have“led to the creation of a new and exciting ‘laboratory’for the study of fundamental science.”2.2.Synthesis and structural features of grapheneGraphene can be prepared using four different meth-ods[84].Thefirst is chemical vapor deposition(CVD)and epitaxial growth,such as the decomposition of ethylene on nickel surfaces[115,116].The second is the microme-chanical exfoliation of graphite,which is also known as the‘scotch tape’or‘peel-off’method[112,117].The third method is epitaxial growth on electrically insulating sur-faces,such as SiC,and the fourth is the solution-based reduction of graphene oxide[84,118].Graphene is a single layer of carbon atoms packed densely in a honeycomb crystal lattice.The carbon-carbon bond(sp2)length in graphene is approximately0.142nm [119–121].Depending on the research group,the graphene layer thickness ranges from0.35to1nm relative to the SiO2substrate[122].Novoselov et al.measured platelet thicknesses of1–1.6nm[112].2.3.Surface modification of graphenePristine graphene materials are unsuitable for interca-lation by large species,such as polymer chains,because graphene as a bulk material has a pronounced tendency to agglomerate in a polymer matrix[106,123].It is likely that oxidation followed by chemical functionalization will facilitate the dispersion and stabilize graphene to prevent agglomeration[123,124].The functional groups attached to graphene can be small molecules[105,125–127]or polymer chains[128–130].The chemical functionalization of graphene is a particularly attractive target because it can improve the solubility and processability as well as enhance the interactions with organic polymers[131,132]. Considerable work has been carried out on the amination, esterification[104,132],isocyanate modification[133],andT.Kuila et al./Progress in Polymer Science 35 (2010) 1350–13751353Fig.1.Graphene (top left)is a honeycomb lattice of carbon atoms.Graphite (top right)can be viewed as a stack of graphene layers.Carbon nanotubes are rolled-up cylinders of graphene (bottom left).Fullerenes (C60)are molecules consisting of wrapped graphene through the introduction of pentagons on the hexagonal lattice (Reproduced with permission from Neto et al.[110]).polymer wrapping [128–130]as routes for the functional-ization of graphene.The electrochemical modification of graphene using ionic liquids has also been reported [48].The general approaches for preparing organo-modified graphene are as follows.2.3.1.Chemical modification of grapheneThis method is based on the modified Hummers method [134].Initially,graphite oxides are generally prepared from naturally occurring graphite.After oxidation,several chemical methods to obtain soluble graphene have been examined,including the reduction of graphite oxide (GO)in a stabilization medium [135],covalent modification by the amidation of the carboxylic groups [131,132],non-covalent functionalization of reduced graphene oxide [128–130],nucleophilic substitution to epoxy groups [105],and dia-zonium salt coupling [136].2.3.1.1.Reduction of graphite oxide (GO)in a stabilization medium.Park et al.[135]reported a simple route for the preparation of a homogeneous aqueous suspension of chemically modified graphene with good electrical conduc-tivity.In this method,the precursor graphene oxide was first dispersed in water followed by the addition of an aque-ous KOH solution (Fig.2).According to Park et al.,KOH,a strong base,can confer a large negative charge through reactions with the reactive hydroxyl,epoxy and carboxylic acid groups on the graphene oxide sheets,resulting in extensive coating of the sheets with negative charges and K +ions.The addition of hydrazine monohydrate to KOH-treated graphene oxide produces a homogeneous suspension of hKMG,which can remain stable for at least 4months.Recently,Li et al.prepared stable aqueous colloids of graphene sheets through the electrostatic stabilization of graphite [84].This discovery enabled the development of a facile approach to the large scale production of aque-ous graphene dispersions without the need for polymeric or surfactant stabilizers.2.3.1.2.Covalent modification of graphene.For the prepa-ration of organomodified graphene oxide/graphene,most studies followed a covalent modification technique.Differ-ent organic amines,alkyl lithium reagents,isocyanates,and diisocyanate compounds have been used for this purpose1354T.Kuila et al./Progress in Polymer Science35 (2010) 1350–1375Fig.2.A simple route for the preparation of a homogeneous aqueous suspension of graphene sheets from graphite stack:(a)graphite stack is oxidized to separate the individual layers of graphene oxide (GO),(b)GO is dispersed in water and treated with aqueous KOH solution to obtain KOH modified oxidized graphene (KMG)and finally (c)KMG is reduced using hydrazine to produce hydrazine reduced KOH modified graphene (hKMG)in the form of stable aqueous dispersion of individual graphene sheets.[131–133,137].These surface modifying agents reduce the hydrophilic character of graphene oxide sheets by forming amide and carbamate ester bonds with the carboxyl and hydroxyl groups,respectively [133].Niyogi et al.[132]reported an amide coupling reac-tion between the carboxyl acid groups of graphene oxides and octadecylamine (ODA).Thionyl chloride was used to activate the COOH groups in the presence of N,N -dimethylformamide (DMF)under reflux conditions.ODA-modified GO had a solubility of 0.5mg ml −1in THF,and was also soluble in CCl 4and 1,2-dichloroethane.Soluble graphene layers have also been prepared by react-ing graphite fluoride with alkyl lithium reagents [131].Vacuum-dried graphite fluoride and tetramethylethylene-diamine (TMEDA)were dissolved in hexane at 0◦C in an ice bath.An alkyl-lithium reagent was then added drop wise and stirred for 3days to produce functionalized graphene (Fig.3).IR spectral studies confirmed the covalent attach-ment of alkyl chains to the graphene layers [131].Xu et al.modified the surface of graphene via the cova-lent attachment of a porphyrin ring on the GO surfaces [138].Thionyl chloride was used to activate the carboxylic acid group in the presence of porphyrin using DMF as a sol-vent.Fig.4shows a schematic diagram for the modification of graphene via the covalent attachment of a porphyrin ring on to the GO surfaces.Organic isocyanates were added to a GO dispersion in anhydrous DMF and allowed to react with GO for a spe-cific period of time.The reaction was then quenched by adding methylene chloride,and the modified graphene was washed and dried [133].The isocyanate compound was attached to the hydroxyl and carboxyl groups of GO via the formation of carbamate and amide function-alities,respectively.Fig.5shows a schematic diagram of the formation of carbamate and amide functionali-ties.Isocyanate-modified graphene oxide readily forms a stable colloidal dispersion in all polar aprotic solvents,such as DMF,N-methylpyrrolidone (NMP),dimethyl sul-foxide (DMSO)and hexamethylphosphoramide (HMPA).The modification of GO with diisocyanate compounds was recently reported [137].The preparatory method was sim-ilar to isocyanate modification.However,there was an additional step.The diisocyanate treated graphene oxide was reduced by hydrazine monohydrate at 80◦C for 48h.The building blocks of the GO sheets were crosslinked to form lamellar porous structures via a reaction of diiso-cyanate with the carboxyl and hydroxyl groups on both sides of the sheets.2.3.1.3.Non-covalent functionalization of graphene.The reduction of a stable aqueous dispersion of exfoliated graphite oxide nanoplatelets with hydrazine hydrateT.Kuila et al./Progress in Polymer Science35 (2010) 1350–13751355Fig.3.Production of functionalized graphene layers from graphitefluoride and alkyl lithium reagent.Annealing of functionalized graphene produces 3-dimensional graphite.(Reproduced from Worsley et al.by permission of Elsevier Science Ltd.,UK[131]).resulted in the formation of aggregates[130].The precip-itated materials could not be re-dispersed,even after a prolonged ultrasonic treatment in water in the presence of surfactants,such as SDS and TRITON X-100.In order to overcome this problem,the reduction was carried out in the presence of polymer/polymeric anions,resulting in a stable polymer-grafted dispersion of graphene[130].Stable aqueous dispersions of graphitic nanoplatelets were prepared by coating reduced graphite oxide nanoplatelets with an amphiphilic polymer,poly(sodium 4-styrenesulfonate)(PSS)[130].In2008,an aqueous dispersion of graphene was prepared from expanded graphite using7,7,8,8-tetracyanoquinodimethane(TCNQ)anion as a stabilizer.This could provide a facile route for producing high quality water soluble and organic solvent soluble graphene sheets for a range of applications [139].It was suggested that TCNQ anions adsorb on the graphene sheets,which stabilizes them in water.The TCNQ-stabilized graphene sheets could be re-dispersed in water,DMF and dimethyl sulfoxide(DMSO).Xu et al.[140] reported the preparation of stable aqueous dispersions of graphene sheets using a water soluble pyrene derivative, 1-pyrenebutyrate(PB−),as a stabilizer because the pyrene moiety has been reported to have strong affinity to the basal plane of graphite via-stacking[141,142].For this, an alkaline solution of pyrene butyric acid(PBA)wasFig.4.Schematic showing the modification of graphene via the covalent attachment of a porphyrin ring on to the GO surfaces.The–NH2groups of prophyrene ring are covalently bonded to the carboxyl group of graphene by the amide linkage.Thionyl chloride was used to activate the carboxylic acid group in the presence of porphyrin in DMF.(Reproduced from from Xu et al.by permission of Wiley-VCH,Germany[138]).1356T.Kuila et al./Progress in Polymer Science35 (2010) 1350–1375Fig.5.Schematic showing the modification of GO using organic isocyanate in DMF medium.Scheme shows the reactions of isocyanate group with different functional groups of GO.Isocyanates react with the hydroxyl (left oval)and carboxyl groups (right oval)of graphene oxide sheets to form carbamate and amide functionalities,respectively (Reproduced from Stankovich et al.by permission of Elsevier Science Ltd.,UK [133]).reacted with a GO dispersion followed by reduction with hydrazine monohydrate at 80◦C for 24h.Both the modi-fied and reduced GO dispersed well in water and showed an electrical conductivity of 2×102S m −1.Homogeneous aqueous dispersions of reduced exfoliated graphite oxide in the presence of sulfonated polyaniline (SPANI)have been reported [128].The successful reduction of GO was confirmed by electrical conductivity measurements.The conductivity of the composite film was 30S m −1,which is similar to that of the reduced GO sheets reported in the literature [140].2.3.1.4.Nucleophilic substitution.Aliphatic amine-modified GO was prepared by a reaction of amine with an aqueous dispersion of GO [105].Nucleophilic substitution occurs on the epoxy groups of GO [143].For the small chain primary amine,C n H 2n +1NH 2(n =2,4,8,12),grafting was completed at room temperature.On the other hand,for long chain aliphatic amines (octadecylamine),the reaction mixture was heated under reflux for 24h.However,the later GO derivatives were dispersed more easily in organic solvents.X-ray diffraction (XRD)showed that the interlayer distance of the amine intercalated GO derivatives depends on the amine chain lengths and their orientation relative to the layers.According to Bourlinos et al.,the tilted orientation is more likely to be due to the hydrophilic nature of the layers [105].Bourlinos et al.[105]also reported amino acid modified graphene.In this case,an alkaline solution of amino acids was used to modify GO.Nucleophilic attack of the –NH 2end group on the epoxide groups of GO occurs,suggesting that the amino acid molecules adopt a flat orientation in the interlayer zone of GO.2.3.1.5.Diazonium salt coupling.Graphene (S-CCG)sheets wrapped chemically with sodium dodecyl benzene sul-fonate (SDBS)were obtained by the reduction of graphene oxide with hydrazine and functionalized by a treatment with aryl diazonium salts [136].Thefunctionalized graphene could be dispersed readily in N,N -dimethylformamide (DMF),N,N -dimethylacetamide (DMAc)and 1-methyl-2-pyrrolidinone (NMP)up to 1mg/mL with minimal sedimentation.Cryogenic trans-mission electron microscopy (Cryo-TEM)revealed the existence of individual sheets and a few multiple sheet structures of SDBS-coated graphene Thermogravimetric analysis showed that the thermal stability of functional-ized CCG was significantly higher than that of neat GO but lower than that of S-CCG.The degree of functionalization calculated from these weight losses was estimated to be ∼1functional group in 55carbon atoms.The chemical modifi-cation of epitaxial graphene by the covalent attachment of aryl groups to the basal carbon atoms was reported [125].Epitaxial graphene grown on SiC wafers was modified chemically with aryl groups via the formation of cova-lent bonds to the conjugated carbon atoms [118,125,144].The grafting of aryl groups was confirmed by FT-IR spec-troscopy.Nitrophenyl-functionalized epitaxial graphene exhibited some new bands at 1565and 1378cm −1,which correspond to the symmetric and asymmetric stretching modes of NO 2.2.3.2.Electrochemical modification of grapheneGraphite was treated electrochemically to produce a colloidal suspension of chemically modified graphene (CMG)[48].Fig.6shows a schematic diagram of the exper-imental set up.A commercial graphite electrode was used as the cathode,which was immersed in a phase-separated mixture of water and imidazolium-based ionic liquids.A constant potential of 10–20V was applied across the electrodes.After 30min electrochemical reaction,ionic liquid functionalized graphene (GNP IL )sheets originating from the graphite anode precipitated.Ultrasonication of dried GNP IL in DMF resulted in a homogeneous dispersion (1mg ml −1).The average length and width of the GNP IL were 700and 500nm,respectively,as evidenced from the TEM images.AFM image analysis revealed the thickness of GNP IL to be ∼1.1nm.。
纳米科技前沿Page1of 18题目:纳米材料——石墨烯摘要随着纳米材料的快速发展,纳米材料有着众多优秀的理化性质,同时,还包括在应用领域优秀的应用性能,本文从纳米材料的基本性质出发,叙述纳米材料的特有性质,继而本文叙述了对于标志这纳米材料发展的有着重要意义的三种材料——富勒烯,碳纳米管,石墨烯。
而本文的核心是关于目前最具前景的纳米材料——石墨烯。
石墨烯是一种碳纳米二维材料,原子以sp2杂化轨道方式构成,平面像六角的蜂巢结构,质料非常牢固坚硬,在室温状况,传递电子的速度比已知导体都快,而全材料仅一个碳原子厚度,是全世界已知材料最薄的材料。
本文从石墨烯的发展历史出发,叙述石墨烯的优异理化性质,最后叙述石墨烯的不同制备方法以及该方法的优劣之处。
关键词:石墨烯理化性质制备方法AbstractWith the rapid development of nanomaterials, nanomaterials have many excellent physical and chemical properties, as well as excellent application properties in the field of application. Starting from the basic properties of nanomaterials, this paper describes the unique properties of nanomaterials, and then describes three kinds of materials which are of great significance to mark the development of nanomaterials: fullerenes, carbon nanotubes, carbon nanotubes, Graphene. The core of this paper is about the most promising nano material graphene.Graphene is a kind of carbon nano two-dimensional material. The atoms are composed of SP2 hybrid orbitals. The plane is like a hexagonal honeycomb structure. The material is very firm and hard. At room temperature, the speed of electron transfer is faster than that of known conductors. The whole material is only one carbon atom thick, which is the thinnest known material in the world. Starting from the development history of graphene, this paper describes the excellent physical and chemical properties of graphene, and finally describes the different preparation methods of graphene and the advantages and disadvantages of this method.Key words: physical and chemical properties of graphene, preparation methods.目录1纳米材料概述 (4)1.1纳米材料 (4)1.2纳米材料的基本特性 (4)1.2.1 表面效应 (4)1.2.2 小尺寸效应 (4)1.2.3 磁学性质 (6)1.2.4 量子尺寸效应 (6)1.2.5 宏观量子隧道效应 (6)1.2.6 纳米材料奇特的物理性能 (7)1.3纳米材料的发展 (7)1.3.1 富勒烯 (7)1.3.2 碳纳米管 (9)1.3.3 石墨烯 (10)2石墨烯 (13)2.1石墨烯概述 (13)2.2石墨烯的性质 (13)2.2.1 结构性质 (13)2.2.2 电子性质 (14)2.2.3 其他性值 (16)2.3石墨烯的制备 (16)2.3.1 机械剥离法 (17)2.3.2 碳化硅表面外延生长法 (17)2.3.3 化学气相沉积法 (18)2.3.4 氧化石墨还原法 (18)3参考论文............................................................................................ 错误!未定义书签。