ALKENES and POTASSIUM MANGANATE(VII) Test for a double
- 格式:ppt
- 大小:106.50 KB
- 文档页数:2
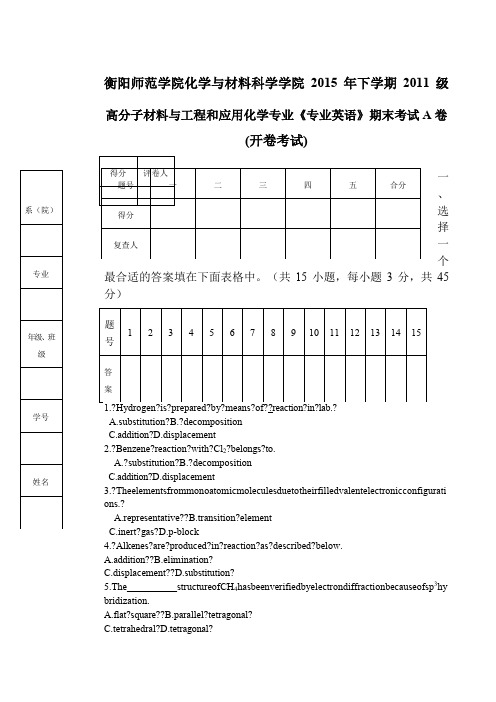
衡阳师范学院化学与材料科学学院2015年下学期2011级高分子材料与工程和应用化学专业《专业英语》期末考试A 卷(开卷考试)一、选择一个最合适的答案填在下面表格中。
(共15小题,每小题3分,共45分)6.?The?octet?rule?simply?states?that?an?atom?tends?to?gain?or?lose?electrons?until?th ere?are??electrons?in?its?valent?shell.A.12?B.8??C.6??D.127.?When?water?combines?with?pure?CuSO4,?a?CuSO4 5H2O?forms.A.hydrogenate??B.hydrate??C.hydrocarbon??D.hydride?8.?InamoleculeofCH4,thehydrogenatomsarespatiallyorientedtowardthecentersofaregul ar.A.pyramidB.tetrahedronC.squareD.rectangle9.?Thesubstancesontheleftsideofachemicalequationareknownas.A.reactant(s)B.reactor(s)C.reductant(s)D.reaction(s)?10.?The?seawater?is?saline?solution.?To?desalinate?seawater?is?the?process?tosalt? component.A.?add??B.dilute?C.concentrate?D.remove11.?There?are(is)paired?electrons?in?hydrogen.A.1?B.2??C.3?D.4?12.?The?valent?electronic?configuration?for?fluorine?is.?A.1s2?B.2s2?C.2s22p5?D.2s22p4?13.?Whichofthefollowingbelongstothefamilyofnitrites?A.HNO3B.KNO2C.Mg3N2D.NH314.Whichofthefollowingreactionwilloccurwhenweblendanacidwithaalcohol?A.eliminationB.replacementC.esterificationD.neutralization15.?Which?of?the?following?belongs?to?transition?metal??A.?S?B.Na?C.He?D.V二、词汇题(将中文词组翻译成英文;对英文和缩写Array给出中文释义;共20分,每题2分)1.tetrahedron2.chiralmolecule5.benzyl6.过氧化物7.阴离子8.滴定(n.)9.滤液10.天平他科学家能够重复你的实验和证实你的实验结果。


关于新批准阿尔茨海默病药物甘露特钠简介8648资料一、甘露特钠的研究过程简介1、上世纪八十年代,中国海洋大学管华诗院士团队为了对褐藻胶进行系统深入研究将其嵌段分离提取,分别得到聚甘露糖醛酸(M段)和聚古罗糖醛酸(G段)两大片段寡糖。
2、1997年管华诗院士团队从褐藻寡糖当中筛选发现了抗阿尔茨海默症的寡糖片段,取名““971”,此正式立项对971开展系统的成药性研究。
3、2001年,管华诗院士团队在国内申请了第一个化合物发明专利“褐藻胶寡糖作为制备预防因东莨菪碱所致痴呆药物的应用”4、2006年,经国家食品药品监督管理局批准,“971”顺利获得药物临床试验批件。
5、2009年,美国Sinova公司以8100万美元(合同额)获得了“971”全球实施许可选择权。
之后,上海绿谷制药有限公司又以高价买回“971”的国内独占许可实施,并与中国科学院上海药物所签订《技术开发合同》,共同推进“971”的临床研究。
6、2010年,管华诗院士领衔完成的项目“海洋特征寡糖的制备技术(糖库构建)与应用开发”,获得2009年度国家技术发明一等奖,成为新中国为数不多的技术一等奖之一。
7、2011年,启动实施二期临床。
8、2013年,中国海洋大学与上海绿谷、上海药物所签订《备忘录》,明确了各方在971化合物的临床试验和新药注册过程中的权利义务。
9、2014年,启动实施三期临床研究。
10、2019年11月2日,糖类多靶治疗阿尔兹海默病的创新药物“971”获批上市!药品取名甘露特钠胶囊,注册商标:九期一®。
二、甘露特钠基本资料1、甘露特钠(GV-971)就是海藻酸钠的降解产物.化学名:甘露糖醛酸钠寡糖(1997年管华诗院士团队从褐藻寡糖当中筛选发现了抗阿尔茨海默症的寡糖片段。
此寡糖片段为M段,即代号为“1”的聚甘露糖醛酸,故取名为“971”。
自此正式立项对971开展系统的成药性研究。
)2、以海洋褐藻提取物为原料,制备获得的低分子酸性寡糖化合物,3、是我国自主研发并拥有自主知识产权的创新药,获得国家重大新药创制科技重大专项支持。

阿卡波糖片与二甲双胍治疗2型糖尿病的临床效果及药物不良反应分析李剑珠,宋宏,沈燕萍莆田学院附属医院药剂科,福建莆田351100[摘要]目的探究阿卡波糖片与二甲双胍治疗2型糖尿病(diabetes mellitus type 2, T2DM)的临床效果及药物不良反应分析。
方法选取2020年10月—2022年10月于莆田市荔城区莆田学院附属医院进行治疗的86例T2DM患者作为研究对象,采用信封法随机分为二甲双胍组与阿卡波糖片组,每组43例,分别予以二甲双胍与阿卡波糖片治疗。
比较两组血糖水平、不良反应发生情况以及生活质量。
结果治疗后,阿卡波糖片组空腹血糖、餐后2 h血糖、糖化血红蛋白水平均低于二甲双胍组,差异有统计学意义(P<0.05)。
两组不良反应发生率比较,差异无统计学意义(P>0.05)。
治疗后,阿卡波糖片组世界卫生组织生活质量测定简表中的躯体、社会、环境、心理、综合维度评分均高于二甲双胍组,差异有统计学意义(P<0.05)。
结论针对T2DM患者,阿卡波糖片与二甲双胍疗效确切,安全性相当,但阿卡波糖片相对能更好地控制患者血糖,患者的生活质量更高。
[关键词] 2型糖尿病;阿卡波糖片;二甲双胍;临床效果;安全性;生活质量[中图分类号] R587 [文献标识码] A [文章编号] 1672-4062(2023)11(b)-0077-04Clinical Effects and Adverse Drug Reactions of Acarbose Tablets and Met⁃formin in the Treatment of Type 2 Diabetes MellitusLI Jianzhu, SONG Hong, SHEN YanpingDepartment of Pharmacy, the Affiliated Hospital of Putian University, Putian, Fujian Province, 351100 China[Abstract] Objective To explore the clinical effects and adverse drug reactions of Acarbose tablets and Metformin in the treatment of type 2 diabetes mellitus (T2DM). Methods A total of 86 T2DM patients treated in the Affiliated Hos⁃pital of Putian University, Licheng District, Putian City from October 2020 to October 2022 were selected as the study objects. They were randomly divided into Metformin group and Acarbose tablet group by envelope method, with 43 cases in each group receiving Metformin andAcarbose tablet treatment, respectively. Blood glucose levels, adverse re⁃actions and quality of life were compared between the two groups. Results After treatment, fasting blood glucose, 2 hours postprandial blood glucose and glycated hemoglobin levels in Acarbose tablet group were lower than those in Metfor⁃min group, and the differences were statistically significant (P<0.05). There was no statistically significant difference in the incidence of adverse reactions between the two groups (P>0.05). After treatment, the scores of physical, social, environmental, psychological and comprehensive dimensions of Acarbose tablet group were higher than those of Met⁃formin group, and the differences were statistically significant (P<0.05). Conclusion For T2DM patients, Acarbose tab⁃lets have the same efficacy and safety as Metformin, but Acarbose tablets can better control patients' blood glucose and improve patients' quality of life.[Key words] Type 2 diabetes; Acarbose tablets; Metformin; Clinical effect; Security; Quality of life糖尿病是一种因胰岛素分泌不足而导致的糖类代谢紊乱疾病,患者一旦发病,则需要终身服药控制血糖。

2'-岩藻糖基乳糖的国外标准包括:
1.欧洲药典:欧洲药典委员会发布的《欧洲药典》是欧洲药品质量的法定标
准。
该标准中包含了2'-岩藻糖基乳糖的详细质量要求和测试方法。
2.美国药典:美国药典是美国的官方药品标准,其中也包含了2'-岩藻糖基乳
糖的质量要求和测试方法。
3.日本药典:日本药典是日本的官方药品标准,其中也包含了对2'-岩藻糖基
乳糖的质量要求和测试方法。
这些标准通常会包括对2'-岩藻糖基乳糖的物理性质、化学组成、微生物学特性、安全性、有效性等方面的详细要求和测试方法。
需要注意的是,不同国家和地区的标准可能会有所不同,因此在实际应用中需要根据具体情况选择适用的标准。

Carbohydrate Polymers 111(2014)149–182Contents lists available at ScienceDirectCarbohydratePolymersj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /c a r b p olReviewReview on flammability of biofibres and biocompositesMfiso E.Mngomezulu a ,c ,1,Maya J.John a ,b ,∗,Valencia Jacobs a ,b ,2,Adriaan S.Luyt c ,3aCSIR Materials Science and Manufacturing,Polymers and Composites Competence Area,P.O.Box 1124,Port Elizabeth 6000,South Africa bDepartment of Textile Science,Faculty of Science,Nelson Mandela Metropolitan University,P.O.Box 1600,Port Elizabeth 6000,South Africa cDepartment of Chemistry,Faculty of Natural and Agricultural Sciences,University of the Free State (Qwaqwa Campus),Private Bag X13,Phuthaditjhaba 9866,South Africaa r t i c l ei n f oArticle history:Received 6November 2013Received in revised form 7March 2014Accepted 20March 2014Available online 3April 2014Keywords:Flammability Flame retardants Biopolymers Natural fibre Compositea b s t r a c tThe subject on flammability properties of natural fibre-reinforced biopolymer composites has not been broadly researched.This is not only evidenced by the minimal use of biopolymer composites and/or blends in different engineering areas where fire risk and hazard to both human and structures is of critical concern,but also the limited amount of published scientific work on the subject.Therefore,it is necessary to expand knowledge on the flammability properties of biopolymers and add value in widening the range of their application.This paper reviews the literature on the recent developments on flammability studies of bio-fibres,biopolymers and natural fibre-reinforced biocomposites.It also covers the different types of flame retardants (FRs)used and their mechanisms,and discusses the principles and methodology of various flammability testing ©2014Elsevier Ltd.All rights reserved.Contents 1.Introduction (150)2.Flame retardants ....................................................................................................................................1512.1.Mode of action of flame retardants..........................................................................................................1512.1.1.Physical action .....................................................................................................................1512.1.2.Chemical action ....................................................................................................................1512.2.Types of flame retardant agents .............................................................................................................1522.2.1.Phosphorus based flame retardants ...............................................................................................1522.2.2.Halogen based flame retardants ...................................................................................................1532.2.3.Silicon based flame retardants.....................................................................................................155Abbreviations:APP,ammonium polyphosphate;ATH,aluminum tri-hydrate;BAl,boehemite aluminum;BDP,bisphenyl A bis(diphenyl phosphate);DNA,deoxyribonu-cleic acid;DTG,derivative thermogravimetric analysis;EG,expandable graphite;EVA,ethylene vinyl acetate;FRs,flame retardants;FRAs,flame retardant agents/additives;HPCA,hyperbranched polyamine charring agent;HBCD,hexabromocyclododecane;HRR,heat release rate;HRC,heat release capacity (Ác );IFR,intumescent flame retardant;LOI,limited oxygen index;LDPE,low density polyethylene;MA,melamine;MCC,Microscale combustion calorimetry;MH or MDH,magnesium hydroxide or magnesium dihydroxide;MPD,methacryloyloxyethylorthophosphorotetraethyl diamidate;MA-g -PP,maleic acid grafted polypropylene;MMP,melamine phosphate;MMB,melamine borate;MMT,montmorillonite;MLR,mass loss rate;MWNTs,multi walled nanotubes;NAs,normal additives;NFs,natural fibres;NFRBC,natural fibre reinforced biopoly-mer composites;OSU,Ohio State University;PMMA,polymethyl methacrylate;PCFC,pyrolysis combustion flow calorimetry;PA6,polyamide 6;PU,polyurethane;PC,polycarbonate;PP,polypropylene;PE,polyethylene;PS,polystyrene;POSS,polyhedral oligomeric silsesquioxane;PCL,polycaprolactone;PLA,polylactic acid;PHBV,poly(3-hydroxybutyrate-co-3-hydroxyvalerate);PBT,poly(butylene terephthalate);PBAT,poly(butylene adipate-co-terephthalate);PTT,poly(trimethylene terephthalate);PPTA,poly(p-phenylenediamineterephthalamide);PBDE,polybromodiphenyl ether;PHRR,peak heat release rate;PEBAX,polyether blockamide;PC,polycarbonate;RAs,reactive additives;RTM,resin transfer moulding;SPR,smoke production rate;SPDPM,spirocyclic pentaerythritol bisphosphorate disphosphoryl melamine;SEA,soot extinction area;SEM,Scanning electron microscopy;SWNTs,single walled nanotubes;TBBPA,tetrabromobisphenol A;TBPA,tetrabromophthalic anhydride;TTI,time to ignition;THR,total heat release;TPOSS,trisilanolphenylpolyhedral oligomeric silsesquioxane;UL 94,underwriter laboratories 94;UV,ultraviolet;ZB,zinc borate.∗Corresponding author at:Corresponding author.Tel.:+270415083292.E-mail addresses:MMngomezulu1@csir.co.za (M.E.Mngomezulu),MJohn@csir.co.za (M.J.John),VJacobs@csir.co.za (V.Jacobs),LuytAS@qwa.ufs.ac.za (A.S.Luyt).1Tel.:+270415083241.2Tel.:+270415083229.3Tel.:+270587185314./10.1016/j.carbpol.2014.03.0710144-8617/©2014Elsevier Ltd.All rights reserved.150M.E.Mngomezulu et al./Carbohydrate Polymers111(2014)149–1822.2.4.Nano metric particles (155)2.2.5.Mineralflame retardant (159)3.Flammability testing techniques (161)3.1.Cone calorimetry (161)3.2.Pyrolysis combustionflow calorimetry(PCFC) (162)3.3.Limiting oxygen index(LOI) (163)3.4.UnderwritersLaboratories94(UL94) (164)3.5.Ohio State University heat release apparatus(OSU) (164)4.Flammability of biofibres and biocomposites (166)4.1.Biofibres(naturalfibres) (166)4.2.Biopolymers (171)4.3.Biofibre reinforced biopolymer composites (175)5.Summary (178)Acknowledgements (179)References (179)1.IntroductionIn recent years,the research on biofibre reinforced biopolymer composites has advanced.This development is motivated by factors such as shortage of and high fossil energy cost,and the current shift towards environmentally tolerant or“green”composite materials. The shift towards environmentally friendly biocomposite materi-als is due to environmental legislation,the REACH Act(Registration, Evaluation,Authorization and Restriction of Chemical substances), comparable properties to syntheticfibre counterparts,green attri-bution and low cost.Most of the components in biocomposites are based on agricultural products as a source of raw materials.Thus, their use provides solution for waste disposal,reduction in agricul-tural residues and hence environmental pollution resulting from the burning of these.Additionally,it offers an economical solution for farming and rural areas in developing countries(Anandjiwala et al.,2013;Chapple&Anandjiwala,2010;Faruk,Bledzki,Fink,& Sain,2012;Horrocks,2011;Jang,Jeong,Oh,Youn,&Song,2012; John&Thomas,2008;Kandola,2012;Sahari&Sapuan,2011; Satyanarayana,Arizaga,&Wypych,2009).Biofibre reinforced biopolymer composite materials largely have appealing properties.They are renewable,recyclable(par-tially or completely),relatively cheap,biodegradable and thus environmentally friendly.However,there are some inherent dis-advantages such as their hydrophilic nature and poorflammability properties(i.e.poorfire resistance).The attractive properties clearly outweigh the undesirable ones and the latter have remedial measures.For example,remedies may be chemical and/or phys-ical modifications such as the incorporation offlame retardant additives(FRAs)to improveflammability of biocomposites(John &Thomas,2008).Previous research observed limitations in the use of biofibre reinforced biopolymer composites,especially in areas that posefire hazard and risk.This is because naturalfibre reinforced biopoly-mer composites are largely used in the packaging and automotive industries wherefire safety regulatory requirements are not as stringent as those in the aerospace industry.Therefore,to broaden the range of applications of these biocomposites into other sec-tors of advanced engineering(i.e.aerospace,marine,electronics equipment and construction),both theirflammability characteris-tics andfire retardance strategies need more research(Bourbigot& Fontaine,2010;Chapple&Anandjiwala,2010;Kandola,2012).There are different strategies that can be demonstrated forfire retardancy of biocomposites.Fire retardancy is the phenomenon in which materials such as plastics and/or textiles are rendered less likely ignitable or,if they are ignitable,should burn with less efficiency(Price,Anthony,&Carty,2001).It may be achieved by use of several approaches.These may be chemical modification of existing polymers,addition of surface treatment to the polymers,use of inherentlyfire resistant polymers or high performance poly-mers,and direct incorporation offlame retardants(FRs)and/or micro or nanoparticles in materials.The direct incorporation of flame retardants is achieved through use of various additives.These flame retardance strategies may range from the use of phospho-rus additives(e.g.intumescent systems),halogen additives(e.g. organobromine),silicon additives(e.g.silica),nanometric particles (e.g.nanoclays)and minerals based additives(e.g.metal hydrox-ide).The broader information onflame retardant additives(FRAs) in natural polymers,wood and lignocellulosic materials has been reviewed by Kozlowski and Wladyka-Przybylak(2001).Thus,the primary duty offlame retardant systems is to prevent,minimize, suppress or stop the combustion of a material(Laoutid,Bonnaud, Alexandre,Lopez-Cuesta,&Dubois,2009;Morgan&Gilman,2013; Price et al.,2001;Wichman,2003).Flame retardant systems can either act chemically or physically in the solid,liquid or gas phase.These mechanisms are dependent on the nature of theflame retardant system.The chemical mode of action may be manifested by reaction in the gaseous and condensed phases,whereas the physical mode occurs by a cooling effect,for-mation of a protective layer or by fuel dilution.FRs may be classified into three classes.They are normal additives(NAs),reactive addi-tives(RAs)and a combination of FRs(Laoutid et al.,2009;Price et al.,2001;Wichman,2003).Theflammability offire retarded materials may be tested through differentfire testing techniques.The most widely used laboratoryflammability testing techniques have been reported in literature(Laoutid et al.,2009;Price et al.,2001;Wichman,2003).A number of small,medium and full scaleflammability tests are used in both academic and industrial laboratories.They are employed for either screening the materials during production or testing the manufactured products.These techniques are cone calorime-try,pyrolysis combustionflow calorimetry(PCFC),limiting oxygen index(LOI),and underwriters’laboratories94(UL94)and Ohio State University(OSU)heat release rate tests.These techniques involve the measurement of variousflammability parameters by appropriate tests depending on the targeted application of a poly-meric material.Theflammability of polymers can be characterized by parameters such as ignitability(ignition temperature,delay time,critical heatflux),burning rates(heat release rate,solid degra-dation rate),spread rates(flame,pyrolysis,and smoulder),product distribution(emissions of toxic products)and smoke production (Carvel,Steinhaus,Rein,&Torero,2011;Laoutid et al.,2009;Price et al.,2001).Theflammability properties of naturalfibrereinforced biopoly-mer composites have not been studied extensively.The aim of this paper is to review the current research and developments related toflammability of biofibre reinforced biopolymer composites for the period2000–2013.This review will explore aspects such as theM.E.Mngomezulu et al./Carbohydrate Polymers111(2014)149–182151different types offlame retardants,laboratoryflammability testing techniques and recent studies onflammability of biopolymers and biocomposites.2.Flame retardantsFRs impartflame retardancy character to materials such as coatings,thermoplastics,thermosets,rubbers and textiles.These FRs may prevent,minimize,suppress or stop the combustion pro-cess of materials.They act to break the self sustaining polymer combustion cycle shown in Fig.1,and consequently reduce the burning rate or extinguish theflame in several ways(Guillaume, Marquis,&Saragoza,2012;Grexa&Lübke,2001;Kandola,2001; Kandola&Horrocks,2001;Ke et al.,2010;Kozlowski&Wladyka-Przybylak,2001;Laoutid et al.,2009;Morgan&Gilman,2013;Price et al.,2001;Wichman,2003).The possible ways to reduce the burning rate or extinguish theflames are:(i)the modification of the pyrolysis process in order to lower the quantity of evolvedflammable volatiles,with normally an increase in the formation of char(lessflammable)serv-ing as barrier between the polymer andflame(stage‘a’,Fig.1); (ii)the isolation of theflame from the oxygen/air supply(stage ‘b’);(iii)introduction into the polymer formulations those com-pounds that will release efficientflame inhibitors(e.g.chlorine and bromine)(stage‘c’);and(iv)the lowering of thermal feedback to the polymer to prevent further pyrolysis(stage‘d’)(Price et al., 2001).Toflame retard polymer materials or to protect them fromfire, there are three main approaches to be considered.These are the engineering approach,use of inherently lowflammable polymers and the use offlame retardant additives(FRAs)(Morgan&Gilman, 2013).The engineering approach is cost effective and relatively easy to implement.It requires the use of afire protection shield.However, the method has some limitations such as tearing and/or ripping off(offire proof fabric),loss of adhesion(in metalfire protection), and scratching away and falling off due to impact or ageing(of intumescent paint).Consequently,the underlying material may be left exposed tofire damage.The inherentlyflame retarded polymers can be made in various forms and are easy to implement in different applications.Their use,though,can be limited by high cost and difficulty to recycle(i.e.fibre reinforced polymer composites).As a result,lowflammability polymers are less used except for applications demanding their use (e.g.aerospace and military sectors).The use of FRAs is a well known approach,cost effective and relatively easy to incorporate into polymers.The challenges with this approach,however,include potential for leaching into envi-ronment,difficulty with recycling and a compromise in reaching a balance in properties of a polymer.Regardless of these problems, FRAs are still used.FRs are classified into three categories.They are normal additives(NAs)flame retardants,reactive additives(RAs)flame retardants and combinations of FRs.NAs are incorporated during polymerization or during melt mixing processing and react with the polymer only at higher temperatures at the start of afire.They are commonflame retardant additives and their interaction is physical with the substrate.NAs usually include mineralfillers,hybrids or organic compounds that can include macromolecules.RAs,on the other hand,are usually introduced into polymers during polymer-ization or in a post reaction process.During polymerization,RAs are introduced as monomers or precursor polymers whereas in a post reaction process their introduction is by chemical grafting.These flame retardants chemically bond to the polymer -binations of NAs and RAs can produce an additive(sum),synergistic (higher)or antagonistic(lower)effect.A synergistic effect typically occurs when they are used together with specificflame retardants (Kozlowski&Wladyka-Przybylak,2001;Morgan&Gilman,2013; Price et al.,2001;Troitzsch,1998).2.1.Mode of action offlame retardantsFlame retardant systems can act either chemically or physi-cally in the solid,liquid or gas phase.Such actions do not occur singly but should be considered as complex processes in which var-ious individual stages occur simultaneously,with one dominating. They are dependent on the nature offlame retardant system in place(Bourbigot&Duquesne,2007;Laoutid et al.,2009;Morgan& Gilman,2013;Price et al.,2001;Troitzsch,1998;Wichman,2003). Various modes offlame retardants are discussed in subsequent sections.2.1.1.Physical actionThe physical mode occurs by(i)cooling effect,(ii)fuel dilu-tion or(iii)via formation of a protective layer(coating)(Chapple& Anandjiwala,2010;Jang,Jeong,Oh,Youn,&Song,2012;Kandola, 2012;Laoutid et al.,2009;Price et al.,2001;Troitzsch,1998; Wichman,2003).(i)Cooling effect:Some FRAs(e.g.hydrated trialumina and mag-nesium hydroxide)decompose by an endothermic process and trigger temperature decrease in the system.Cooling of the medium to below the polymer combustion temperatures is effected.Such endothermic reaction is known to act as a heat sink.(ii)Fuel dilution:During decomposition offlame retardants(e.g.aluminum hydroxide),the formation of gases such as H2O,CO2, and NH3lead to dilution of the mixture of combustible gases.Consequently,this limits both the concentration of reagents and the possibility of materials to ignite.(iii)Formation of a protective layer(coating):Some FRAs(e.g.phos-phorus and boron compounds)form a protective solid or gaseous layer between the gaseous and solid combustible phases.This limits the transfer of combustible volatile gases, excludes oxygen necessary for combustion and thus reducing the amount of decomposition gases.2.1.2.Chemical actionThe chemical mode of action may be manifested by reaction in the(i)gaseous and(ii)condensed phase(Chapple&Anandjiwala, 2010;Jang et al.,2012;Kandola,2012;Laoutid et al.,2009;Price et al.,2001;Troitzsch,1998;Wichman,2003).(i)Gaseous phase:By incorporation of FRAs that favour the releaseof specific radicals(e.g.halogenflame retardants,Cl•and Br•) in the gas phase,the free radical mechanism of the combustion process can be stopped.These radicals can react with highly reactive species such as H•and OH•to form less reactive or inert molecules.The exothermic reactions are then stopped;the system cools down and the supply offlammable gases is subsequently reduced.(ii)Condensed phase:Two types of chemical reaction initiated by FRAs are possible:(a)flame retardants can speed up the rupture of polymer chains and the polymer will drip,thus moving away from theflame action zone;(b)FRs can cause the formation ofa carbonized or vitreous layer at the surface of the polymer.This occurs by chemical transformation of degraded polymer chains.The formed char and/or vitreous layer acts as a physical insulating barrier between the gas and condensed phases.152M.E.Mngomezulu et al./Carbohydrate Polymers 111(2014)149–182Fig.1.Demonstration of the self-sustaining polymer combustion cycle;a–d represent potential modes of flame retardants.Adapted from Price et al.(2001).2.2.Types of flame retardant agentsFRAs are based on various chemical compounds.This subsection discusses chemical compounds based on phosphorus,halogen,sil-icon,nanometric particles and mineral additives.The phosphorus based additives include organic phosphorus,inorganic phospho-rus,red phosphorus and intumescent flame retardant systems.The silicon based additives consist of silica and silicones,the nanomet-ric particles based ones may be carbon nanotubes,nanoclays and nanoscale particulate additives,and the minerals based flame retar-dant additives are hydrocarbonates,metal hydroxides and borates.2.2.1.Phosphorus based flame retardantsPhosphorus based FRs include phosphorus into their structure.Their structure can vary from inorganic to organic forms,and with oxidation states of 0,+3,or +5.Phosphorus based FRs consist of phosphates,phosphonates,phosphinates,phosphine oxide and red phosphorus.These FRAs are used as NAs or RAs incorporated into the polymer chain during synthesis.They are effective with oxy-gen or nitrogen containing polymers (cellulose,polyesters,and polyamides).Phosphorated FRs are unique in that they can be con-densed phase or vapour phase FRs depending on their chemical structure and interaction with the polymer under fire conditions (Faruk et al.,2012;Jang et al.,2012;Laoutid et al.,2009).In the condensed phase ,their thermal decomposition leads to the production of phosphoric acid that readily condenses to give phosphorylated structures and gives off water.Released water dilutes the oxidizing gas phase (physical action:fuel dilution).Addi-tionally,phosphoric acid and pyrophosphoric acid can facilitate a dehydration reaction resulting in the formation of carbon to carbon double bonds and charring.This can then lead to the generation of crosslinked or carbonized structures at high temperatures (Faruk et al.,2012;Jang et al.,2012;Laoutid et al.,2009;Morgan &Gilman,2013;Troitzsch,1998).At high temperatures both ortho and pyrophosphoric acid are turned into metaphosphoric acid (OPOOH)and their corresponding polymers (PO 3H)n .Phosphate anions (pyro and polyphosphates)then partake in char formation (with carbonized residue).This car-bonized layer isolates and protects the polymer from the flames,limits the volatilization of fuel,prevents formation of new free radicals,limits the diffusion of oxygen thus reducing combustion,and insulates the polymer underneath from the heat (Faruk et al.,2012;Jang et al.,2012;Laoutid et al.,2009;Morgan &Gilman,2013;Troitzsch,1998).Phosphorus based flame retardants can also volatilize into vapour phase forming active radicals (PO 2•,PO •and HPO •)and act-ing as scavengers of H •and OH •radicals.Volatile phosphorated compounds are among the effective inhibitors of combustion com-pared to bromine and chlorine radicals.Since phosphorus based flame retardants are significantly effective in oxygen and nitrogen containing polymers,it is thus important to have these atoms in the polymer chain.In case the used polymer lacks these atoms in its chain and cannot contribute to charring,a highly charring coadditive {e.g.polyol (pentaerythritol)}has to be introduced in combination with the phosphorated flame retardant.Polymers such as polyamides and polyurethane can also be used as char-ring agents in intumescent flame retardant systems (Faruk et al.,2012;Jang et al.,2012;Laoutid et al.,2009;Morgan &Gilman,2013;Troitzsch,1998).anic phosphorus.Many organic phosphorus derivatives show flame retardancy properties.But,those of commercial impor-tance are limited by the processing temperature and the nature of the polymer to be modifianic phosphorus based FRs can act as NAs or as RAs monomers or co monomers/oligomers.Their main groups are phosphate esters,phosphonates and phosphinates.Due to their high volatility and relatively low fire retardant efficiency,the use of alkyl substituted triaryl phosphate (i.e.triphenyl phos-phate,TPP,cresyl diphenyl phosphate,isopropylphenyl diphenyl phosphate,tertbutylphenyl diphenyl phosphate or tricresyl phos-phate)is limited in plastics engineering.Oligomeric phosphates with lower volatility and higher thermal stability than triaryl phos-phate can be used for plastics engineering.These may be resorcinol bis(diphenyl phosphate)(RDP)and bisphenol A bis(diphenyl phosphate (BDP).The combination of volatile and nonvolatile phosphates can also lead to a synergistic effect.This may be a positive combination of the condensed phase and gas phase of phosphates.The use of reactive phosphorus flame retardants isM.E.Mngomezulu et al./Carbohydrate Polymers111(2014)149–182153also a solution for avoiding volatilization during thermal decompo-sition and migration towards the surface of a polymer.They can be incorporated directly within the polymer chain structure and can be used either as monomers for copolymerization with one or two co-monomers to get phosphorated polymers or as oligomers that react with polymers to form branched or grafted phosphorated polymers(Faruk et al.,2012;Jang et al.,2012;Laoutid et al.,2009; Morgan&Gilman,2013).2.2.1.2.Inorganic phosphorus.A typical example of an inorganic phosphorus salt is a combinationof polyphosphoric acid and ammonia called ammonium polyphosphate(APP).It is either a branched or unbranched polymeric compound with variable chain length(n).For short and linear chain APPs(where n is less than100, crystalline form I),they are more water sensitive and less thermally stable,whereas APPs with longer chain(n is greater than1000, crystalline form II)exhibit very low water solubility(<0.1g/100ml) (Jang et al.,2012;Laoutid et al.,2009).The APPs are stable and nonvolatile compounds.Those with long chains start decomposing at temperatures above300◦C giv-ing polyphosphoric acid and ammonia,whereas the short chain ones decompose at150◦C.It is thus important to adapt a crys-talline form of APP to the decomposition temperature of a polymer. When an APP is incorporated into a polymer that contains oxygen and/or nitrogen atoms,polymer charring occurs.Thermal degrada-tion of APP creates free acidic hydroxyl groups that condense by thermal dehydration yielding a crosslinked structure of ultraphos-phate and polyphosphoric acid with a highly crosslinked structure. Polyphosphoric acid reacts with oxygen and/or nitrogen containing polymers and catalyses their dehydration reaction and char for-mation.However,the effectiveness of an APP is dependent on the loading concentration.Low concentrations of APP are not efficient in aliphatic polyamides,but at high concentrations it becomes effi-cient.In non self-charring polymeric materials,the APP can modify the degradation mechanism of the polymer(Bourbigot&Fontaine, 2010;Ke et al.,2010;Zhu et al.,2011).2.2.1.3.Red phosphorus.This is the most concentrated source of phosphorus forflame retardancy and is used in small quantities (i.e.<10%).It is effective in oxygen and nitrogen containing poly-mers(i.e.polyesters,polyamides and polyurethanes).For oxygen containing polymers only,the mode of action involves specific scav-enging of oxygen containing radicals leading to the generation of gaseous fuel species.For oxygen and nitrogen containing polymers, red phosphorus turns into phosphoric acid or phosphoric anhy-dride,which gives polyphosphoric acid upon heating.This happens through thermal oxidation and the formed polyphosphoric acid catalyses the dehydration reaction of the polymer chain ends and triggers char formation(Laoutid et al.,2009;Laoutid,Ferry,Lopez-Cuesta,&Crespy,2006).Additionally,red phosphorus is also effective in non oxygenated polymers(e.g.polyethylene).Consequently,red phosphorus depolymerizes into white phosphorus(P4).This white phosphorus can volatilize at high temperatures and act in the gaseous phase or it can diffuse from the bulk of the polymer to the burning sur-face where it oxidizes to phosphoric acid derivatives.These can come into close contact with theflame and form phosphoric acid. This acid can act as a char forming agent and therefore physically limiting oxygen access and fuel volatilization(Laoutid et al.,2009).Red phosphorus is active in both the gas and condensed phase in polyethylene.In the gas phase,the produced PO•radicals quench the free radical process.In the condensed phase,red phosphorus lowers the heat of oxidation and also traps the free radicals.This results in improved thermal stability leading to a decrease in fuel production during burning of a material(Laoutid et al.,2009).The disadvantage of red phosphorus is that it releases toxic phosphine(PH3)through reaction with moisture due to its poor thermal stability.However,phosphine formation can be avoided by prior encapsulation of red phosphorus to improve its effective-ness as aflame retardant.Alternatively,phosphine formed at high temperatures can be trapped by taking advantage of its capacity to react with metallic salts(i.e.AgNO3,HgCl2,MoS2,HgO,PbO2,CuO, FeCl3·H2O)(Laoutid et al.,2009).2.2.1.4.Intumescentflame retardant system.Intumescentflame retardant systems were initially developed to protect fabrics,wood and coatings for metallic structures fromfire.Intumescent mate-rials are classed into thick or thinfilm intumescent coatings.The thickfilms are usually based on epoxy resins,contain agents that intumesce when exposed to heat and are available as solvent free systems.Thinfilms are available as solvent or water based sys-tems,and are applied by spray or brush roller in thinfilm coats. An intumescent system is based on the formation of an expanded carbonized layer on the surface of a polymer during thermal degra-dation.This layer acts as an insulating barrier by reducing heat transfer between the heat source and the polymer surface,by limiting the fuel transfer from the polymer towards theflame,and limiting the oxygen diffusion into a material.The formulation of an intumescent system consists of three components:an acid source,a carbonizing agent and a blowing agent.Table1tabulates examples of each component category(Bourbigot&Duquesne,2007).The intumescent FRs are widely used due to their advantages of low smoke and low toxicity(Jimenez,Duquesne,&Bourbigot,2006;Ke et al.,2010;Laoutid et al.,2009;Morgan&Gilman,2013).An acid source promotes dehydration of the carbonizing agent and results in the formation of a carbonaceous layer.It has to be liberated at a temperature below the decomposition temper-ature of a carbonizing agent and its dehydration should happen around the decomposition temperature of a polymer.A carboniz-ing agent is generally a carbohydrate that can be dehydrated by an acid to form a char.Its effectiveness relates to the number of car-bon atoms and the reactive hydroxyl sites containing carbon source agent molecules.The quantity of char produced is dependent on the number of carbon atoms present.Reactive hydroxyl(OH)sites determine the rate of the dehydration reaction and thus the rate of formation of the carbonized structure.A blowing agent decomposes and releases gas leading to expansion of the polymer and forma-tion of swollen multicellular layer.The gas must be released during thermal decomposition of a carbonizing agent in order to trigger the expansion of the carbonized layer(Bourbigot&Duquesne,2007; Jimenez et al.,2006;Ke et al.,2010;Laoutid et al.,2009;Morgan& Gilman,2013).2.2.2.Halogen basedflame retardantsHalogenated FRs are molecules that include elements from group VII of the periodic table(F,Cl,Br and I).Their effectiveness increases in the order F<Cl<Br<I.The type of halogen dictates the effectiveness of the halogenatedflame retardant.However,fluorine (F)and iodine(I)are not used because they do not interfere with the polymer combustion process.Fluorinated compounds are more thermally stable than most polymers and do not release halogen radicals at the same temperature range or below the decomposition of the polymers.Iodine compounds are less thermally stable than most commercial polymers and therefore release halogen species during polymer processing.Bromine and chlorine can readily be released and partake in the combustion process because of their low bonding energy with carbon atoms(Chen&Wang,2010; Laoutid et al.,2009;Morgan&Gilman,2013;Troitzsch,1998).2.2.2.1.Halogenatedflame retardant additives.Halogenated FRs differ in chemical structure from aliphatic to aromatic carbon。
AL Chemistry Part 7 (Alkanes and Alkenes) [91I1a]1.C H 3CHCH 2(a) Using E , give reagents and a mechanism to explain what is meant by each of the following:A reaction obeying Markovnikoff ’s rule.A polymerisation.(4 marks)[91II7a]2. State with explanation, what you would observe in each of the following experiments, and writeequations for the reactions. (i) A mixture of pentane and bromine in tetrachloromethane is exposed to sunlight. (ii) Propene is bubbled into aqueous alkaline potassium manganate(VII).(5 marks)[93II9b]3. Outline the mechanism for the reaction between but-1-ene and HBr to give bromobutane. Explain why2-bromobutane is the major product, rather than 1-bromobutane.(3 marks)[94I3aii]4.Dehydration of C gives 3 products, E , F and G all with the formula C 4H 8. On treatment with ozone followed by hydrolysis, E gives methanal among other products, but F and G do not give methanal. Give structures for E , F and G and an equation for the ozonolysis reaction involving E .CH 3CH 2CHCH 3OH(4 marks)5. Ethene and chloroethene can undergo polymerization to give polyethene (PE) and polyvinyl chloride(PVC) respectively. PVC is more rigid and durable than PE, but incineration of PVC causes a more serious pollution problem.(a) Describe the bonding and shape of the ethene molecule in terms of the type and spatialarrangement of the orbitals involved.(b) Use equations to show a mechanism of the polymerization of ethene.(c) Show how ethene can be converted to chloroethene.(d) Explain why PVC is more rigid than PE.(e) Explain why the incineration of PVC causes a more serious pollution problem than theincineration of PE.(10 marks)[95I3c]6. (a) Give the structure of the major product formed from the following reaction :CH3(b) Outline a mechanism for the above reaction. (Movement of electron pairs should be indicated bycurly arrows.)(3 marks)[96II9b]7. Give the structure of the major product in the following reaction and outline the mechanism of thereaction. ( Movement of electron pairs should be indicated by curly arrows. )CH2(3 marks)[97I4cii]8. Outline a free radical mechanism for the conversion of ethene to poly(ethene). Your answer shouldinclude appropriate arrows to show how the new bonds are made.(2 marks)9.Alcohol E has the structure CH 3CH(OH)C 2H 5 .On treatment with dilute H 2SO 4(aq), E gives mainly two isomeric compounds, F and G , both of which have the formula C 4H 8. On treatment with bromine, both F and G give a product H with formula C 4H 8Br 2. (a) Draw structures for F , G and H .(b) What is the isomeric relationship between F and G ?(c) Outline the mechanism for the formation of H from either F or G .(5 marks)[98II5c]10. Give the structure of the major organic product, G , in (i) below.Outline a mechanism for the formation of the major product in the following reaction.C CCH3H3CH 3HBr(3 marks)[99I5a]11. Under certain conditions, methane reacts with chlorine to give chloromethane as the major product. (a) State the conditions for the reaction.(b) Outline the mechanism and name the mechanistic steps of the reaction.(c) Is the reaction of methane with chlorine an appropriate method for the preparation ofdichloromethane? Explain.(5 marks)[00I5a]12.Consider the reaction:BrHBr(a) Name the type of the reaction. (b) Outline a mechanism of the reaction.(c) Draw the structures of all possible stereoisomers of the product.(d) Would he product rotate a beam of plane polarized light? Explain your answer.(5 marks)13. Product J , from the transformation below, is a commonly-used household detergent.CH 3(CH 2)11H 2SO 4(1) conc.(2) NaOH(aq)(a) Draw the structure of J .(b) State one advantage of using J as a detergent.(c) State one environmental problem associated with the use of J .(3 marks)[01I5a]14. Consider the reaction:(D is deuterium, an isotope of hydrogen.) (a) Draw the structure of the major product. (b) Outline a mechanism of the reaction.(c) Is the product optically active? Explain your answer.(5 marks)[01IA6b]15. After some lessons in organic chemistry, a student remarked, ‘Alkanes are more stable than alkenes,therefore alkanes do not react with chlorine but alkenes do. Do you agree with the student ? Explain.(3 marks)[01II5c]16. Consider the information below concerning the production of low density polyethenefrom ethene.(a) Outline a mechanism for the polymerization and name each mechanistic step. (b) Explain why benzoyl peroxide is used.(c) Why is high pressure needed for polymerization? (d) Is the product a single compound? Explain.(7 marks)C CCH3CH 3CH 2HHCH 2CH 2*CH 2CH 2n。
高中化学高锰酸钾化学式与作用素材新人教版必修1高锰酸钾(potassium permanganate)亦名“灰锰氧”、“PP粉”,是一种常见的强氧化剂,常温下为紫黑色片状晶体,易见光分解:2KMnO₄(s)—hv→K2MnO₄(s)+MnO₂(s)+O₂(g),故需避光存于阴凉处,严禁与易燃物及金属粉末同放。
高锰酸钾以二氧化锰为原料制取,有广泛的应用,在工业上用作消毒剂、漂白剂等,在实验室,高锰酸钾因其强氧化性和溶液颜色鲜艳而被用于物质的鉴定,酸性高锰酸钾溶液是氧化还原滴定的重要试剂。
在医学上,高锰酸钾可用于消毒、洗胃。
性质参数高锰酸钾主要参数见下高锰酸钾在水中的溶解度化学性质化学式:KMnO4,高锰酸钾常温下即可与甘油等有机物反应甚至燃烧(但有时与甘油混合后反应极为缓慢,甚至感受不到温度的升高,其原因尚不明确);在酸性环境下氧化性更强,能氧化负价态的氯、溴、碘、硫等离子及二氧化硫等。
与皮肤接触可腐蚀皮肤产生棕色染色,数日不褪;粉末散布于空气中有强烈刺激性,可使人连打喷嚏。
尿液、二氧化硫等可使其褪色。
与较活泼金属粉末混合后有强烈燃烧性,危险。
该物质在加热时分解:2KMnO₄(s)—△→K2MnO₄(s)+MnO₂(s)+O₂(g)·高锰酸钾在酸性溶液中还原产物为二价锰离子·高锰酸钾在中性溶液中还原产物一般为二氧化锰。
·高锰酸钾在碱性环境下还原产物为墨绿色的锰酸钾(K2MnO₄)维生素C的水溶液能使高锰酸钾溶液褪色,并且维生素C溶液越浓,水溶液用量就越少。
根据这一特性,就能够用高锰酸钾测定蔬菜或水果中的维生素含量。
高锰酸钾造成的污渍可用还原性的草酸、维生素C等去除。
[编辑本段]制备高锰酸钾常见的制备方法有以下两种:矿石中取得的二氧化锰和氢氧化钾在空气中或混合硝酸钾(提供氧气)加热,产生锰酸钾,再于碱性溶液中与氧化剂进行电解氧化得到高锰酸钾。
2MnO₂+ 4KOH + O₂——→ 2K2MnO₄+ 2H2O2K2MnO₄+ Cl₂——→2KMnO₄+ 2KCl也可以用MnSO4在酸性环境中和二氧化铅(PbO₂)或铋酸钠(NaBiO₃)等强氧化剂反应产生。
代谢“废物”乳酸在肿瘤微环境中的免疫抑制作用苑思羽1侯俊杰2张片红1(1.浙江大学医学院附属第二医院营养科,杭州 310009;2.吉林省人民医院肿瘤综合治疗科,长春 130021)中图分类号R730.3 文献标志码 A 文章编号1000-484X(2024)04-0832-08[摘要]近年来,肿瘤微环境(TME)备受科学家们的关注,它是由肿瘤细胞、肿瘤相关成纤维细胞(CAFs)、免疫细胞、血管、细胞外基质、周围支持组织及其所在的代谢环境等共同组成的复杂体系。
免疫逃逸和代谢改变(葡萄糖有氧代谢转至无氧代谢产生乳酸)是此体系的两个基本特征。
虽然过去一直认为乳酸是TME中的代谢废物,但现在人们普遍认为乳酸的增加和TME的酸化在肿瘤发生发展中发挥关键作用,包括免疫逃逸、组织侵袭/肿瘤转移、血管生成和肿瘤耐药等。
因此,研究TME 中乳酸代谢、免疫抑制、血管生成、肿瘤耐药等关键过程的调控机制,可为靶向TME的新治疗策略提供理论基础和实践依据。
[关键词]乳酸代谢;免疫抑制;肿瘤微环境Immunosuppressive effect of metabolic "waste" lactic acid in tumor microenvironmentYUAN Siyu1, HOU Junjie2, ZHANG Pianhong1. 1. Department of Clinical Nutrition, the Second Affiliated Hospital,Zhejiang University School of Medicine,Hangzhou 310009,China;2. Department of Tumor Comprehensive Therapy, Jilin Provincial People's Hospital, Changchun 130021, China[Abstract]In recent years, the tumor microenvironment (TME) has garnered significant attention from scientists. It is a com‑plex system composed of tumor cells, cancer-associated fibroblasts (CAFs), immune cells, blood vessels, extracellular matrix, sur‑rounding supportive tissues and their metabolic environment. Two fundamental characteristics of this system are immune escape and metabolic changes (the shift from aerobic to anaerobic metabolism of glucose, leading to lactate production). Although lactate has tra‑ditionally been considered a metabolic "waste" product in the TME, it is now widely recognized that the increase in lactate and the acidification of the tumor microenvironment play key roles in tumor development and progression, including immune escape, tissue in‑vasion/tumor metastasis,angiogenesis and tumor drug resistance. Therefore,studying the regulatory mechanisms of lactate metabo‑lism, immune suppression, angiogenesis, and tumor drug resistance in the TME can provide a theoretical basis and practical evidence for new therapeutic strategies targeting the TME.[Key words]Lactic acid metabolism;Immunosuppressive;Tumor microenvironment1 乳酸的分子结构与生理功能乳酸为三碳分子的酸性物质,属于羧酸,其分子式为C3H6O3。