水的粘度对照表
- 格式:docx
- 大小:36.61 KB
- 文档页数:3

水的黏度表(0~40℃)水的物理性质F3Viscosity decreases with pressure (at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them。
As the pressure increases, the volume decreases and the volume of thesevoids reduces,so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534]can be explained by the increased pressure (up to about 150 MPa)causing deformation,so reducing the strength of the hydrogen—bonded network,which is also partially responsible for the viscosity。
This reduction in cohesivity more than compensates for the reduced void volume。
It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558]in water;hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities),the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity,then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength。

水的粘度与温度的关系对照表
水的粘度是它分子间的相互作用力以及分子间距离的影响。
随着温度的升高,水分子之间的作用力减弱,分子间的距离变大,这会导致水的粘度减小。
下表的数据显示了粘度和温度之间的相关性:| 水的温度 | 水的粘度 |
| :---: | :---: |
| 0℃ | 1.794mPa|s |
| 10℃ | 1.002mPa|s |
| 15℃ | 0.890mPa|s |
| 20℃ | 0.794mPa|s |
| 25℃ | 0.711mPa|s |
从上表中可以看出,随着温度的升高,水的粘度逐渐降低,这是由于水分子在高温下逐渐分散,相互之间的作用力变弱,水分子之间的距离变大,因此水的粘度减小了。
粘度在普通条件下水的粘度是 1.794mPa|s,当水的温度升到10℃时,水的粘度降低到1.002mPa|s,水的粘度还会继续降低,到15℃和20℃,它的粘度减少到0.890mPa|s和0.794mPa|s;再到25℃时,水的粘度还会再次减少到0.711mPa|s。
因此,我们清楚地可以看到,随着温度的升高,水的粘度也会随之降低,这是由于水的分子在高温下,会减弱它们之间的作用力和距离,从而导致水的粘度也会变得更低。

水的粘度计算表WTD standardization office【WTD 5AB- WTDK 08- WTD 2C】水的黏度表(0~40℃)水的物理性质350360 109370 264F3??? Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence ofthe between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。

水的黏度随温度的对照表如下:10℃水:1.308*10-3μ/Pa*s或者
1.308*10-6ν/m2s-1;20℃水:1.005*10-3μ/Pa*s或者1.007*10-6ν/m2s-1
水的粘度约为2.98×10-3Pa·s。
在压强为101.325kPa、温度为20℃的条件下,水的动力粘度为1.01×10^(-3) Pa·s。
粘度在数值上等于面积为1㎡相距1m的两平板,以1m/s的速度作相对运动时,因之间存在的流体互相作用所产生的内摩擦力。
不同流体的粘度数值不同。
同种流体的粘度显著地与温度有关,而与压强几乎无关。
气体的粘度随温度升高而增大,液体则减小。
水的密度异乎寻常。
通常,在冷却过程中,液体密度会越来越大。
然而,水在4摄氏度时达到最大密度。
在这个温度以下,水的密度反而会下降。
所以,冰的密度比水小。
因此,冰会漂浮在水面,而水的冷冻是自上而下的。
水的表面张力非常高。
除水银外,它在所有液体中具有最高的表面张力。
因此,水蜘蛛能够站立在水上。
此外,水的沸点也高得异乎寻常。
还有,相较于其它液体,水能够溶解许多化学物质这点也很奇特。

水的粘度对照表水的粘度是指水流动时所表现出的阻力大小,也就是水的黏度。
水的黏度值与温度有关,在不同温度下的水黏度值也是不同的。
以下是水的粘度对照表,列出了不同温度下水的粘度值。
一、水在0℃以下的黏度:水在0℃以下始终处于冰冻状态,此时无法测量其粘度值。
二、水在0℃~5℃的黏度:水在0℃~5℃范围内属于冷水,虽然温度较低但黏度并不高,粘度值约为0.00153 Pa·s。
三、水在5℃~10℃的黏度:水在5℃~10℃的温度下,黏度值开始升高,约为0.00131 Pa·s。
四、水在10℃~15℃的黏度:水在10℃~15℃的温度下,黏度值约为0.00114 Pa·s。
五、水在15℃~20℃的黏度:水在15℃~20℃的温度下,黏度值逐渐升高,约为0.001 Pa·s。
六、水在20℃~25℃的黏度:水在20℃~25℃的温度下,黏度值约为0.00089 Pa·s。
七、水在25℃~30℃的黏度:水在25℃~30℃的温度下,黏度值约为0.00081 Pa·s。
八、水在30℃~35℃的黏度:水在30℃~35℃的温度下,黏度值逐渐升高,约为0.00075 Pa·s。
九、水在35℃~40℃的黏度:水在35℃~40℃的温度下,黏度值约为0.0007 Pa·s。
十、水在40℃以上的黏度:水在40℃以上的温度下,黏度值不断升高,约为0.00064 Pa·s。
总结来说,水的黏度随着温度的升高而升高,但这种变化趋势并不是线性的,大致呈现出曲线形态。
在一定温度范围内,水的黏度值并不会随着温度的升高而在同样的比例增加,因为水的黏度受到温度、压力、浓度等多方面的影响,因此需要对不同条件下的水进行具体的测试才能得到精确的黏度值。
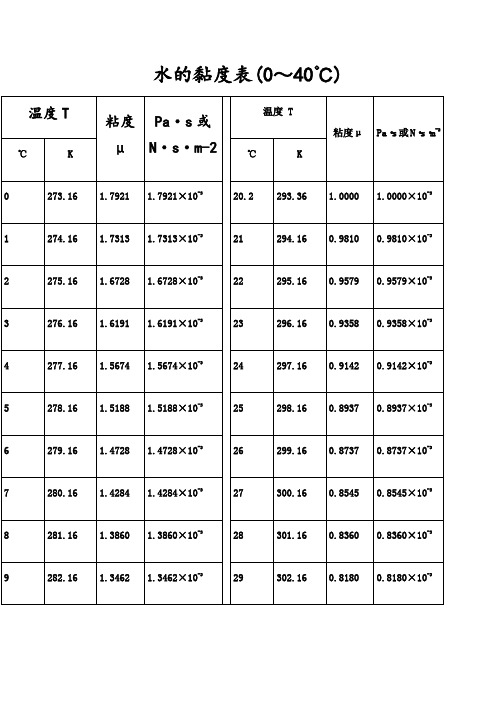
水的黏度表(0~40℃)水的物理性质F3??? Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume.It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表 Revised as of 23 November 2020水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of thesevoids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的黏度表(0~40℃)水的物理性质370 264F3Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist betweenthem. As the pressure increases, the volume decreases and the volume of thesevoids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increasedpressure (up to about 150 MPa) causing deformation, so reducing the strength ofthe hydrogen-bonded network, which is also partially responsible for the viscosity.This reduction in cohesivity more than compensates for the reduced void volume. Itis thus a direct consequence of the balance between hydrogen bonding effects andthe van der Waals dispersion forces [558] in water; hydrogen bonding prevailing atlower temperatures and pressures. At higher pressures (and densities), the balancebetween hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure (at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersionforces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的动力粘度与温度对照表表:水的动力粘度与温度对照① 0℃时:动力粘度为:1.7989×10-3 PXa.s② 15℃时:动力粘度为:1.0037×10-3 PXa.s③ 30℃时:动力粘度为:0.588×10-3 PXa.s④ 50℃时:动力粘度为:0.334×10-3 PXa.s⑤ 70℃时:动力粘度为:0.1956×10-3 PXa.s⑥ 90℃时:动力粘度为:0.1144×10-3 PXa.s水是自然界最常用的液体物质,在生物的各个过程中起着至关重要的作用。
动力粘度是温度对水流动粘度的表现,对于农业水利、机械工程、液压传动及温度传递设备等工业部门都有很重要的意义。
以下是水的动力粘度与温度间的对照表:以0℃为基准,其动力粘度为1.7989×10-3 PXa.s;水的温度升高到15℃时,其动力粘度会变成1.0037×10-3 PXa.s;当水的温度升高到30℃时,动力粘度变成0.588×10-3 PXa.s;随着水的温度继续上升到50℃时,其动力粘度变成0.334×10-3 PXa.s;继续往上加温至70℃时,动力粘度会变成0.1956×10-3 PXa.s;最后在90℃时,动力粘度变成0.1144×10-3 PXa.s。
根据此表可知,随着温度的升高,水的动力粘度呈现出逐渐减弱的趋势,其中升高每10℃温度所引起的动力粘度变化差值可以逐步趋于0.66X×10-3 PXa.s。
因此,水的动力粘度的变化受温度的大幅影响,只有准确地测量了水的温度,才能够准确测量出水的动力粘度。
而温度这一因素也是一些水利建设及机械设备运行过程中必不可少程度的一种因素。
因此,为了更好地推进水利建设、机械设备发展,以及各类水流体运行过程,研究者们必须更精确准确地测量及分析水的温度及动力粘度间的关系。
水的粘度对照表
水的粘度是指水流动时所表现出的阻力大小,也就是水的黏度。
水的黏度值与温度有关,在不同温度下的水黏度值也是不同的。
以下是水的粘度对照表,列出了不同温度下水的粘度值。
一、水在0℃以下的黏度:
水在0℃以下始终处于冰冻状态,此时无法测量其粘度值。
二、水在0℃~5℃的黏度:
水在0℃~5℃范围内属于冷水,虽然温度较低但黏度并不高,粘度值约为0.00153 Pa·s。
三、水在5℃~10℃的黏度:
水在5℃~10℃的温度下,黏度值开始升高,约为0.00131 Pa·s。
四、水在10℃~15℃的黏度:
水在10℃~15℃的温度下,黏度值约为0.00114 Pa·s。
五、水在15℃~20℃的黏度:
水在15℃~20℃的温度下,黏度值逐渐升高,约为0.001 Pa·s。
六、水在20℃~25℃的黏度:
水在20℃~25℃的温度下,黏度值约为0.00089 Pa·s。
七、水在25℃~30℃的黏度:
水在25℃~30℃的温度下,黏度值约为0.00081 Pa·s。
八、水在30℃~35℃的黏度:
水在30℃~35℃的温度下,黏度值逐渐升高,约为0.00075 Pa·s。
九、水在35℃~40℃的黏度:
水在35℃~40℃的温度下,黏度值约为0.0007 Pa·s。
十、水在40℃以上的黏度:
水在40℃以上的温度下,黏度值不断升高,约为0.00064 Pa·s。
总结来说,水的黏度随着温度的升高而升高,但这种变化趋势并不是线性的,大致呈现出曲线形态。
在一定温度范围内,水的黏度值并不
会随着温度的升高而在同样的比例增加,因为水的黏度受到温度、压力、浓度等多方面的影响,因此需要对不同条件下的水进行具体的测试才能得到精确的黏度值。