第7章,工程热力学(哈工大)
- 格式:doc
- 大小:303.50 KB
- 文档页数:11

7-1当水的温度t=80℃,压力分别为0.01、0.05、0.1、0.5及1MPa时,各处于什么状态并求出该状态下的焓值。
解:查表知道t=80℃时饱和压力为0.047359MPa。
因此在0.01、0.05、0.1、0.5及1MPa时状态分别为过热、未饱和、未饱和,未饱和、未饱和。
焓值分别为2649.3kJ/kg,334.9 kJ/kg,335 kJ/kg,335.3 kJ/kg,335.7 kJ/kg。
7-2已知湿蒸汽的压力p=1MPa干度x=0.9。
试分别用水蒸气表和h-s图求出hx,vx,ux,sx。
解:查表得:h``=2777kJ/kg h`=762.6 kJ/kgv``=0.1943m3/kg v`=0.0011274 m3/kgu``= h``-pv``=2582.7 kJ/kg u`=h`-pv`=761.47 kJ/kgs``=6.5847 kJ/(kg.K) s`=2.1382 kJ/(kg.K)hx=xh``+(1-x)h`=2575.6 kJ/kgvx=xv``+(1-x)v`=0.1749 m3/kgux=xu``+(1-x)u`=2400 kJ/kgsx=xs``+(1-x)s`=6.14 kJ/(kg.K)7-3在V=60L的容器中装有湿饱和蒸汽,经测定其温度t=210℃,干饱和蒸汽的含量mv=0.57kg,试求此湿蒸汽的干度、比容及焓值。
解:t=210℃的饱和汽和饱和水的比容分别为:v``=0.10422m3/kg v`=0.0011726 m3/kgh``=2796.4kJ/kg h`=897.8 kJ/kg湿饱和蒸汽的质量:解之得:x=0.53比容:vx=xv``+(1-x)v`=0.0558 m3/kg焓:hx=xh``+(1-x)h`=1904kJ/kg7-4将2kg水盛于容积为0.2m3的抽空了的密闭刚性容器中,然后加热至200℃试求容器中(1)压力;(2)焓;(3)蒸汽的质量和体积。

工程热力学(哈尔滨工程大学)知到章节测试答案智慧树2023年最新绪论单元测试1.工程热力学课程主要研究热能与其它形式能量的相互转化。
()参考答案:对2.热质说无法解释摩擦生热问题。
()参考答案:对第一章测试1.闭口系统是指()的系统。
参考答案:与外界没有物质交换2.孤立系统指的是系统()。
参考答案:A+B+C3.在工质热力状态参数中,可以直接测量的参数是()。
参考答案:压力4.若真空度为0.2个大气压,则该处的的绝对压力为()个大气压。
参考答案:0.85.准静态过程中,系统经历的所有状态都接近于()。
参考答案:平衡态6.在工质热力状态参数中,属于基本状态参数的有()。
参考答案:压力;温度;比体积7.如果容器中气体压力保持不变,那么压力表的读数一定也保持不变。
()参考答案:错8.可逆过程一定是准静态过程,而准静态过程不一定是可逆过程。
()参考答案:对9.热力系统的边界可以是固定的,也可以是移动的;可以是实际存在的,也可以是假想的。
()参考答案:对10.用100℃的热源非常缓慢的给冰、水混合物加热,混合物经历的是准静态过程。
该加热过程是可逆过程。
()参考答案:错第二章测试1.热力学第一定律用于()。
参考答案:任意系统、任意工质、任意过程2.热力学能与推动功之和()。
参考答案:是状态参数3.热力学第一定律的实质是()。
参考答案:能量转换和守恒定律4.工质膨胀功与技术功的关系为。
()参考答案:5.工质膨胀时必须对工质进行加热。
()参考答案:错6.绝热节流过程是等焓过程。
()参考答案:错7.实际气体绝热自由膨胀后热力学能不变。
()参考答案:对8.闭口系与外界不交换流动功,所以不存在焓。
()参考答案:错9.稳定流动能量方程式也适用于有摩擦的热力过程。
()参考答案:对10.热力学能就是热量。
()参考答案:错第三章测试1.通用气体常数R与气体的种类无关,但与气体的性质有关。
()参考答案:错2.理想气体是一种假象的气体,存在2个基本的假设。

绪论(2学时)一、基本知识点基本要求理解和掌握工程热力学的研究对象、主要研究内容和研究方法·理解热能利用的两种主要方式及其特点·了解常用的热能动力转换装置的工作过程1.什么是工程热力学从工程技术观点出发,研究物质的热力学性质,热能转换为机械能的规律和方法,以及有效、合理地利用热能的途径。
电能一一机械能锅炉一一烟气一一水一一水蒸气一一(直接利用) 供热锅炉一一烟气一一水一一水蒸气一一汽轮机一一 (间接利用)发电冰箱一一-(耗能) 制冷2.能源的地位与作用及我国能源面临的主要问题3. 热能及其利用(1).热能:能量的一种形式(2).来源:一次能源:以自然形式存在,可利用的能源。
如风能,水力能,太阳能、地热能、化学能和核能等。
二次能源:由一次能源转换而来的能源,如机械能、机械能等。
(3).利用形式:直接利用:将热能利用来直接加热物体。
如烘干、采暖、熔炼(能源消耗比例大)间接利用:各种热能动力装置,将热能转换成机械能或者再转换成电能,4..热能动力转换装置的工作过程5.热能利用的方向性及能量的两种属性过程的方向性:如:由高温传向低温能量属性:数量属性、,质量属性 (即做功能力)注意:数量守衡、质量不守衡提高热能利用率:能源消耗量与国民生产总值成正比。
6.本课程的研究对象及主要内容研究对象:与热现象有关的能量利用与转换规律的科学。
研究内容:(1).研究能量转换的客观规律,即热力学第一与第二定律。
(2).研究工质的基本热力性质。
(3).研究各种热工设备中的工作过程。
(4).研究与热工设备工作过程直接有关的一些化学和物理化学问题。
7..热力学的研究方法与主要特点(1)宏观方法:唯现象、总结规律,称经典热力学。
优点:简单、明确、可靠、普遍。
缺点:不能解决热现象的本质。
(2)微观方法:从物质的微观结构与微观运动出发,统计的方法总结规律,称统计热力学。
优点:可解决热现象的本质。
缺点:复杂,不直观。

哈工大机械大二课程表【原创实用版】目录1.哈尔滨工业大学简介2.机械大二课程表概述3.课程表详细内容4.课程表分析正文1.哈尔滨工业大学简介哈尔滨工业大学(Harbin Institute of Technology,简称哈工大)位于中国黑龙江省哈尔滨市,是我国著名的工科学府之一。
自 1920 年建校以来,哈工大一直秉持“规格严格,功夫到家”的校训,为国家培养了众多优秀的工程技术人才。
2.机械大二课程表概述本文将介绍哈工大机械大二(即大二年级机械工程专业)的课程表。
课程表包含了学生需要学习的课程及其对应的学时分配,对于学生合理安排学习时间和了解专业发展方向具有重要参考价值。
3.课程表详细内容以下是哈工大机械大二课程表的详细内容:周一:- 机械设计基础(3 学时)- 工程热力学(3 学时)- 计算机程序设计(3 学时)周二:- 机械制造技术基础(3 学时)- 控制理论(3 学时)- 体育(2 学时)周三:- 工程材料(3 学时)- 互换性与测量技术(3 学时)- 物理(3 学时)周四:- 机械工程测试技术(3 学时)- 工程力学(3 学时)- 英语(3 学时)周五:- 机械工程控制基础(3 学时)- 计算机辅助设计(3 学时)- 马克思主义哲学原理(2 学时)周六:- 机械工程专业实验(4 学时)4.课程表分析从课程表中可以看出,哈工大机械大二的课程设置注重理论知识与实践能力的结合,既有机械设计、制造、控制等方面的专业知识,也有计算机程序设计、物理等基础课程。
此外,课程表还安排了体育、英语、马克思主义哲学原理等课程,旨在培养学生的综合素质。

工程热力学名词解释专题注:参考哈工大的工程热力学和西交大的工程热力学第一章——基本概念1、闭口系统:热力系与外界无物质交换的系统。
2、开口系统:热力系与外界有物质交换的系统。
3、绝热系统:热力系与外界无热量交换的系统。
4、孤立系统:热力系与外界有热量交换的系统。
5、热力平衡状态:热力系在没有外界作用的情况下其宏观性质不随时间变化的状态。
6、准静态过程:如果造成系统状态改变的不平衡势差无限小,以致该系统在任意时刻均无限接近于某个平衡态,这样的过程称为准静态过程7、热力循环:热力系从某一状态开始,经历一系列中间状态后,又回复到原来状态。
8、系统储存能:是指热力学能、宏观动能、和重力位能的总和。
9、热力系统:根据所研究问题的需要,把用某种表面包围的特定物质和空间作为具体指定的热力学的研究对象,称之为热力系统。
第二章——热力学第一定律1、热力学第一定律:当热能与其他形式的能量相互转换时,能的总量保持不变。
或者,第一类永动机是不可能制成的。
2、焓:可以理解为由于工质流动而携带的、并取决于热力状态参数的能量,即热力学能与推动功的总和。
3、技术功:技术上可资利用的功,是稳定流动系统中系统动能、位能的增量与轴功三项之和4、稳态稳流:稳定流动时指流道中任何位置上的流体的流速及其他状态参数都不随时间而变化流动。
第三章——热力学第二定律1、可逆过程:系统经过一个过程后,如果使热力系沿原过程的路线反向进行并恢复到原状态,将不会给外界留下任何影响。
2、热力学第二定律:克劳修斯表述:不可能把热从低温物体转移到高温物体而不引起其他变化。
开尔文普朗克表述:不可能从单一热源吸热而使之全部转变为功。
3、可用能与不可用能:可以转变为机械功的那部分热能称为可用能,不能转变为机械功的那部分热能称为不可用能。
4、熵流:热力系和外界交换热量而导致的熵的流动量5、熵产:由热力系内部的热产引起的熵的产生。
6、卡诺定理:工作再两个恒温热源(1T 和2T )之间的循环,不管采用什么工质,如果是可逆的,其热效率均为121T T ,如果不是可逆的,其热效率恒小于121T T 。

哈工大工程热力学试
卷-20套
收集于网络,如有侵权请联系管理员删除
《工程热力学》课程套题库
第1套题……………………………………1~5页 第11套题……………………………………48~52页
第2套题……………………………………6~10页 第12套题……………………………………53~57页
第3套题……………………………………11~15页 第13套题……………………………………58~62页
第4套题……………………………………16~21页 第14套题……………………………………63~68页
第5套题……………………………………22~26页 第15套题……………………………………69~73页
第6套题……………………………………22~26页 第16套题……………………………………
74~78页
第7套题……………………………………27~31页 第17套题……………………………………79~83页
第8套题……………………………………32~36页 第18套题……………………………………84~88页
第9套题……………………………………37~42页 第19套题……………………………………89~94页
第10套题……………………………………43~47页 第20套题……………………………………95~99页
收集于网络,如有侵权请联系管理员删除
收集于网络,如有侵权请联系管理员删除
收集于网络,如有侵权请联系管理员删除
收集于网络,如有侵权请联系管理员删除
收集于网络,如有侵权请联系管理员删除
收集于网络,如有侵权请联系管理员删除
收集于网络,如有侵权请联系管理员删除
收集于网络,如有侵权请联系管理员删除
bar时,饱和水焓:
bar时,饱和水焓:。


第1章基本概念1.1 本章基本要求深刻理解热力系统、外界、热力平衡状态、准静态过程、可逆过程、热力循环的概念,掌握温度、压力、比容的物理意义,掌握状态参数的特点。
1.2 本章难点1.热力系统概念,它与环境的相互作用,三种分类方法及其特点,以及它们之间的相互关系。
2.引入准静态过程和可逆过程的必要性,以及它们在实际应用时的条件。
3.系统的选择取决于研究目的与任务,随边界而定,具有随意性。
选取不当将不便于分析。
选定系统后需要精心确定系统与外界之间的各种相互作用以及系统本身能量的变化,否则很难获得正确的结论。
4.稳定状态与平衡状态的区分:稳定状态时状态参数虽然不随时间改变,但是靠外界影响来的。
平衡状态是系统不受外界影响时,参数不随时间变化的状态。
二者既有所区别,又有联系。
平衡必稳定,稳定未必平衡。
5.注意状态参数的特性及状态参数与过程参数的区别。
1.3 例题例1:绝热刚性容器内的气体通过阀门向气缸充气。
开始时气缸内没有气体,如图 1.1所示。
气缸充气后,气体推动气缸内的活塞向上移动,如图1.2所示。
设管道阀门以及气缸均可认为是绝热的。
若分别选取开口系统与闭口系统,试说明它们的边界应该如何划定?这些系统与外界交换的功量与热量又如何?解:(1)若以容器内原有的气体作为分析对象,属于闭口系统。
容器放气前,边界如图1.1中的虚线所示。
放气后边界如图1.2中的虚线所示。
气体对活塞作的功W是闭口系统与外界交换的功量。
气体通过活塞与外界交换的热量Q是此闭口系统的传热量。
图1.1 图1.2图1.3 图1.4(2)若以容器放气后残留在容器内的气体作为分析对象,同样也是闭口系统。
这时放气前的边界如图1.3中的虚线所示。
放气后的边界如图1.4的虚线表示。
残留气体对离开容器的那部分放逸气体所作的功,是本闭口系统与外界交换的功,残留气体与放逸气体之间交换的热量是本系统的传热量。
(3)类似地若以放逸气体为分析对象,同样也是闭口系统。

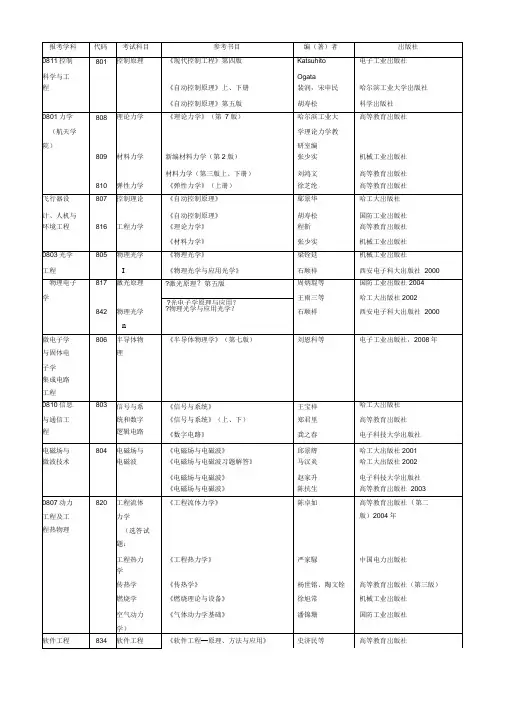
864究方法政治经济学马克思主义中国化《社会统计学》《社会统计软件SPSS 15.0 forwindows简明教程》《政治经济学》卢淑华著尹海洁、刘耳著逢锦聚等北京大学岀版社;社会科学文献岀版社高等教育岀版社2003860《马克思主义中国化研究》(1)(2)庄福龄高等教育岀版社2010 年《马克思主义中国化若干问题研究》李占才、周家伦同济大学出版社2009《毛泽东思想和中国特色社会主义理马克思主义理论体系概论》论研究和建设高等教育岀版社2010 年工程重点教材865中国近代史中国近现代史纲要(2009年修订版)《中国近现代史纲要》教材编写课程组高等教育岀版社2009 年外国语学院相关学868专业综合《新编简明英国文学史》张定铨、吴刚编著上海外语教育岀版社2006年科《美国文学大纲》吴定柏编著上海外语教育岀版社2006年《英国文学选读》王守仁主编高等教育岀版社2005年《美国文学选读》陶洁主编高等教育岀版社2005年《语言学教程》(第三版)胡壮麟主编北京大学岀版社2009年《新编英美概况》(最新修订版)来安方编著河南人民出版社2008年《跨文化交际学》贾玉新著上海外语教育岀版社2006年869翻译《中国翻译简史》马祖毅中国对外翻译岀版公司《西洋翻译简史》谭载喜商务印书馆《英汉对比研究》连淑能高等教育岀版社《中国译学理论史稿》(修订本)陈福康上海外语教育岀版社《实用翻译教程(英汉互译)》(增订冯庆华上海外语教育岀版社本)214法语(二《法语》(1-3册)马晓宏北京外国语学院外)《大学法语简明教程》薛建成外语教学与研究岀版社221俄语(二外)《新大学俄语简明教程》(二外、零起点、成人)蒋财珍主编高等教育岀版社222日语(二《新大学日语标准教程》(基础篇1-2陈俊森总主编高等教育岀版社外)册)(提高篇1-2册)大学日语第二外语课程教学要求课程教学要求高等教育岀版社研订组223德语(二外)《新求精德语强化教程》(初级 1 ,II )第三版教育部直属同济大学留德预同济大学出版社2007 年《新求精德语强化教程》(初级测试题)备部编同济大学出版社2002 年《新求精德语强化教程》(词汇练习册:初级1, II )(修订版)同济大学出版社2008 年246英语(二外)《剑桥国际英语教程》(第三版)入门级,1级,2级,3级[美]理查兹外语教学与研究岀版社;622基础俄语《大学俄语(东方)》(1-8册)北外俄语系外语教学与研究岀版社878 879882883 885流体力学土木工程热力学环境生物化学环境化学细胞生物学《工程流体力学泵与风机》(第一版)可参阅其它各工科院校工程流体力学教材《工程热力学》(第五版)可参阅其他各工科院校工程热力学教材及各种版本的工程热力学习题集《生物化学》第三版(上、下)《环境化学》(第二版)《细胞生物学》伍悦滨、朱蒙生主编化工出版社2006廉乐明王镜岩徐长法戴树桂翟中和谭羽非朱圣庚主编建筑工业岀版社高等教育岀版社高等教育岀版社高等教育岀版社2007 年200220072000交通科学892道路建筑《道路建筑材料》严家汲人民交通岀版社与工程学材料《土木工程材料学》葛勇中国建材工业出版社2007院相关学科863结构设计原理《钢筋混凝土及预应力混凝土桥梁结构设计原理》张树仁人民交通岀版社,2004 894交通工程《交通工程总论》徐吉谦人民交通岀版社学《交通工程学》李江人民交通岀版社2002.7《汽车理论》余志生清华大学岀版社《信号与系统》郑君里高等教育岀版社872结构力学见土木工程学院科目媒体技术与艺术系630艺术理论基础电影的形式与文化[美]罗伯特?考克尔北京大学岀版社12004版次相关学科电影艺术导论黄会林等中国计划岀版社12003版次跨越时空的影像交流一数字电影的媒介形态梁国伟商务印书馆2007版次1个性与共性:中美电影文化比较研究张爱华中国电影岀版社12008版次理解电视[英]大卫?麦克奎尔华夏出版社2003版次1数字电视的媒介形态梁国伟、候薇中国电影岀版社12008版次电视美学大纲胡智锋北京广播学院岀版社版次12003艺术学概论(第三版)彭吉象北京大学岀版社32006版次网络媒体与艺术发展黄鸣奋厦门大学岀版社次12004版美学原理新编杨辛、甘霖北京大学岀版社版次12004853传播理论与传播技传播学总论胡正荣、张磊段鹏、清华大学岀版社2008术传播学教程郭庆光中国人民大学岀版社2004。

工程热力学知到章节测试答案智慧树2023年最新哈尔滨工程大学绪论单元测试1.热力学中常用气态工质就是理想气体。
()参考答案:错2.焦耳首先提出了能量守恒与转换定律。
()参考答案:错3.发电厂循环普遍采用的是蒸汽动力循环。
()参考答案:对第一章测试1.绝热系统与外界没有()交换。
参考答案:热量2.下面所列参数,哪个不是状态参数()。
参考答案:表压力;摄氏温度3.制热系数可以()。
参考答案:大于14.制冷系数可以是()。
参考答案:小于1;大于3;等于1;大于15.不可逆损失来源于()。
参考答案:任何耗散效应;运动摩擦;不等温传热6.绝热闭口系统就是孤立系统。
()参考答案:错7.平衡状态是指在没有外界作用的条件下,热力系统状态不随时间变化的状态。
()参考答案:对8.已知任意两个状态参数,就可以确定简单可压缩系统的状态。
()参考答案:错9.真空度是状态参数。
()参考答案:错10.正向循环的循环净功为正,表现为对外界做功。
()参考答案:对第二章测试1.闭口系统的能量方程不适用于广义功存在的情况。
()参考答案:错2.绝热节流过程是等焓过程。
()参考答案:错3.某工质在过程中热力学能增加15kJ,对外做功15kJ,则此过程中工质与外界交换的热量为30kJ,热量传递方向表现为吸热。
()参考答案:对4.热力循环的净热量等于净功量,所以循环的热效率为1。
()参考答案:错5.下列属于状态参数的有()。
参考答案:推动功6.下列属于状态参数的有()。
参考答案:热力学能7.热力学第一定律是可以通过数学公式进行证明的定律。
()参考答案:错8.简单可压缩系统中,热力学能可以表达为任意两个相对独立状态参数的函数。
()参考答案:对9.闭口系统的能量方程不适用于不可逆过程。
()参考答案:错10.稳定流动开口系统的系统总能不变。
()参考答案:对第三章测试1.工质在相同的初、终状态之间的可逆与不可逆过程,则工质熵的变化是一样的()参考答案:对2.理想气体在绝热容器中作自由膨胀,则气体的温度与压力的表达式是。
《工程热力学》课程简介课程编号:T1023010课程名称:工程热力学课程英文名称:ENGINEERING THERMODYNAMICS总学时:70 讲课学时:64 习题课学时:实验学时:6 上机学时:学分:4.0开课单位:能源科学与工程学院热能动力工程系热工教研室授课对象:能源学院热能动力工程系热能动力工程专业其他相关专业先修课程:高等数学,大学物理教材:《工程热力学》(第三版),沈维道、蒋智敏、童均耕合编,高等教育出版社,2001年6月参考书:《工程热力学》(第三版),严家騄编著、王永青参编,高等教育出版社,2001年1月《工程热力学》(第一版),朱明善、刘颖、林兆庄、彭晓峰,清华大学出版社,1995年11月Required CourseAdvanced Mathematics, College PhysicsTextbookEngineering Thermodynamics (3rd Edition), W. D. Shen, Z. M. Jiang, J.G.Tong, Higher Education Press, Jun., 2001ReferencesEngineering Thermodynamics (3rd Edition), J. L. Yan, Y. Q. Wang, Higher Education Press, Jan., 2001Engineering Thermodynamics (1st Edition), M. S. Zhu, Y. Liu, Z. Z. Lin, X. F. Peng, Tsinghua University Press, Nov., 1995简介工程热力学是研究热能和机械能相互转换规律及热能有效利用的科学。
工程热力学课程是热工类及机械类各专业的主要技术基础课之一。
本课程的教学目的和主要任务是使学生掌握能量转换的基本规律,并能正确运用这些规律进行热工过程和热力循环的分析计算。
CHAPTER4THE SIMPLE COMPRESSIBLE SUBSTANCE4.1IntroductionSince all matter can be compressed if a large enough pressure is applied,a study of the thermodynamic properties of the simple compressible substance is a good starting point for any description of the macroscopic properties of matter in equilibrium.Simple compressible substances are also by far the most important ones for engineering thermodynamics,since most(but not all)power plants and engines employ compression,heating,and expansion of afluid to produce power.A substance may be approximated as a simple compressible substance if effects due to other reversible work modes are negligible.For example,if the surface-to-volume ratio of a large body of water is small enough,then surface tension will not measurably affect the properties of the water except very near the surface.On the other hand,surface tension will have a dramatic influence on the properties of a very small water droplet,and will,for example,cause the pressure inside the droplet to be elevated above the value predicted if surface tension were neglected.Clearly,a very small water droplet can’t be treated accurately as a simple compressible substance,while a large body of water is approximated very well in this way.In this chapter,we examine the properties of simple compressible substances. We will restrict attention to pure substances,which contain only one type of molecule.Mixtures will be considered in a later chapter.4.2Phases of a Simple Compressible SubstanceA simple compressible substance may exist in different phases:solid,liquid,or gas.Some substances have multiple solid phases,some even have multiple liquid phases(helium),but all have only one gas phase.An experimental apparatus is shown in Fig.4.1which can be used to measure the properties and phases of a simple compressible substance as a function of temperature and pressure.A cylindrical solid sample is placed in a vertical cylinder of the same diameter,which isfitted with a piston.The ambient pressure is P0,and the piston weight provides a constant downward force F=68CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE69PistonFigure4.1:Constant-pressure heating experiment.M p g.The pressure in the cylinder isP=F/A=M p g/A+P0.(4.1)The sample height is small enough that the pressure may be taken to be uniform within the sample.The cylinder and piston are well insulated,so there is no heat loss to the environment.A small amount of heat Q is added by briefly passing current through a resistor mounted in the cylinder wall,after which the system is allowed to re-establish equilibrium.Once the system has come back to equilibrium,both the temperature and the volume may have changed.The new temperature is measured with a thermometer,and the new volume by the piston height.If Q is sufficiently small,the expansion will occur slowly enough that friction between the piston and cylinder is negligible.In this case,even if the piston oscillates for a while due to the perturbation,once the oscillations have died out and the piston has settled down at a new height,the work done by the substance on the piston will be equal to the work done against atmospheric pressure,plus the change in the gravitational potential energy of the piston:1W=(M p g+P0A)∆y=(M p g+P0)(∆V/A)=(M p g/A+P0)∆V=P∆V.(4.2) An energy balance on the substance yields the change in its internal energy:∆U=Q−P∆V.(4.3)1If friction were not negligible,some kinetic energy of the piston would be converted to internal energy in the piston or cylinder due to friction,and therefore the work would be >P∆V.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE70The quantities Q,P,and∆V are all measured,so we can calculate∆U.Since P is a constant in this experiment,this equation may be rearranged in the form∆(U+P V)=Q.(4.4)The combination U+P V occurs often in analysis of problems at constant pressure.Since U,P,and V are material properties,so is U+P V.Rather than always write U+P V,we give this property its own name and symbol:the enthalpy!definition H is defined byH=U+P V.(4.5)Like U and V,H is an extensive property.In terms of the enthalpy,Eq.(4.4)becomes∆H=Q.(4.6)For heat addition at constant pressure,the heat added equals the change in enthalpy of the substance.In contrast,recall from the First Law that if heat is added at constant volume(W=0),then∆U=Q.Returning to our experiment,the process in now repeated many times,and the resulting property values are recorded at every step:heat Q is added,time is allowed to elapse to re-establish equilibium,the new T and V are measured, H is incremented by Q.After n heat addition steps,the volume and temperature have values V n and T n,and enthalpy H n of the substance relative to its starting value H0isH n−H0=nQ.(4.7)Since the extensive properties(V and H)depend on how much of the substance was placed in the container,it is preferable to convert them to specific quantities (v=V/M,h=H/M).In Fig.4.2,the measured temperature and change in specific enthalpy are shown plotted vs.the measured specific volume(connecting the individual measurements with solid lines).When heat isfirst added to the solid,its temperature increases and it ex-pands slightly(region a-b in Fig.4.2).At point b,the temperature stops in-creasing,although the volume still increases.A look inside the cylinder reveals the presence of some liquid–the solid is melting.At point c,all of the solid has melted,and precisely at this point the temper-ature begins to rise again.But when point d is reached,it stops again and the volume begins to increase significantly.Bubbles are observed to begin formingCHAPTER 4.THE SIMPLE COMPRESSIBLE SUBSTANCE 71T V abc de h - h v a b c d e0Figure 4.2:Measured temperature (a)and specific enthalpy change (b)vs.mea-sured specific volume for constant-pressure heating.in the liquid at point d –the liquid is boiling.Moving across from point d to e,the amount of vapor in the cylinder increases,and the amount of liquid decreases.At point e,no liquid remains,and both temperature and volume increase upon further heat addition.Figure 4.2shows that when two phases are present (solid/liquid or liq-uid/vapor),h −h 0continues to increase with v even though T is constant,since energy input is required to convert solid to liquid,or liquid to vapor.Note that the only way to measure h is by means of Eq.(4.7),which actually only allows the change in h from the initial state to be determined.There is no experiment we could do to measure the value in the initial state h 0.2Since h =u +P v ,if h 0can’t be determined,then u 0can’t be either.This isn’t a problem,however,since only differences of energy (or enthalpy)have any phys-ical significance.We can start the experiment in some convenient,reproducible state,and simply assign any value we like to h 0(for example,h 0=0).We call the initial state with arbitrarily-chosen h 0the reference state or datum state .The results shown in Fig.4.2are for a single pressure,P =M p g/A +P 0.By changing the mass of the piston,we can repeat the experiment for different pressures,determining T (v )and h (v )−h 0curves for a range of pressures.Typical T (v )curves are shown in Fig.4.3(a)with the points on different curves where the slope is discontinuous connected by dotted lines.The curve corresponding to Fig.4.2(a)is labeled P 1on this plot.In Fig.4.3(b),the dashes2Wecould measure it by starting in some other state,but it wouldn’t be the initial state;the measured value would be relative to the enthalpy in this new initial state,which would still be undetermined.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE72 TVPP1P2P3P4TVliquid +gassolid + gassolidliquidgasliquid+ solidFigure4.3:(a)T−v curves for constant-pressure heating;(b)phase diagram.lines are drawn solid,and each region is labeled by which phase or phases are present in the cylinder.Diagrams like this are called phase diagrams.The experimental observations are as follows.As the pressure is increased, the temperature at which liquid appears(the melting point)and the tempera-ture at which vapor appears(the boiling point)both increase.Also,the spe-cific volume of the liquid increases to a larger value before boiling begins,and the specific volume of the vapor once the last liquid has evaporated decreases. Therefore,the change in specific volume upon boiling decreases as the pressure increases.Beyond a particular pressure P3,the T(v)curve changes character.As P3is approached,the change in specific volume upon boiling goes to zero–the liquid and vapor approach the same density,and in fact become identical in all respects at P3.For P>P3,there is no longer a meaningful distinction between liquid and vapor,and there is no longer any conventional boiling behavior observed. In this pressure regime,as heat is added the high-densityfluid simply expands continuously and homogeneously to a low-densityfluid,without ever breaking up into separate liquid and vapor regions within the cylinder.Pressure P3is known as the critical pressure P c.Below P c,the transfor-mation from liquid to vapor upon heating occurs by means of thefluid in the cylinder splitting into two separate regions(high-density liquid and low-density vapor);as more heat is added,the liquid portion shrinks,and the vapor portion grows.Above P c,the transformation from liquid to vapor occurs continuously, with thefluid remaining uniform throughout the cylinder at all times.As P approaches P c from below,the limiting value approached by the boilingCHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE73Table4.1:Critical temperature,pressure,and density for a few substances.T c(K)P c(MPa)ρc(kg/m3)Helium-4 5.200.227569.64Hydrogen32.94 1.2831.36Nitrogen126.2 3.4314.03Oxygen154.6 5.04436.15Methane190.6 4.60160.43Carbon Dioxide304.27.38464.00Water647.322.1317.0temperature is known as the critical temperature T c.The T(v)curve for P=P c has an inflection point at T=T c:∂T ∂vP=0and∂2T∂v2P=0at T=T c,P=P c.(4.8)The specific volume of thefluid at the point where P=P c and T=T c is known as the critical specific volume v c;the reciprocal of v c is the critical densityρc.The quantities T c,P c,and v c(orρc)define the critical point.The critical point quantities for a few substances are listed in Table4.2.If the pressure is now lowered below P1,another change in the character of the T(v)curve is observed.On the P0curve,there is only one segment where T is constant,not two.On theflat segment,a solid/vapor mixture is found in the cylinder,rather than a solid/liquid or liquid/vapor mixture.Evidently,at sufficiently low pressure no liquid phase forms–instead,the solid transforms directly to vapor.This process is known as sublimation.4.3P−v−T SurfacesSince any two independent properties serve to define the thermodynamic state for a simple compressible substance,we may regard any other property as a function of these two.For example,at every(T,v)point in Fig.4.3we know the pressure,so we can construct P(T,v).The function P(T,v)defines a surface over the T−v plane.A typical P−v−T surface is shown in Fig.4.4.Every equilibrium state of the substance corresponds to some point on the P−v−T surface.In the pure solid region and in the pure liquid region below T c,the slope of the surface is very steep,since compressing a solid or liquid even a little requires a huge increase in pressure. In the gas or vapor region,the surface is gently sloped,since gases are easilyCHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE74Figure4.4:A P−v−T surface for a substance which expands upon melting.compressed.Of course,above the critical point the liquid and gas regions merge smoothly.In Fig.4.4the term“gas”is used above the critical point,and“vapor”below it.This convention dates back to the early19th century,when it was thought that“gases”like oxygen were different than“vapors”like steam.Va-pors could be condensed to liquid,but gases(it was believed)could not be. When it was demonstrated in1877that oxygen and nitrogen could be liquified at sufficiently low temperature,it became clear that there was no fundamental distinction between gases and vapors;the only difference is that substances such as oxygen have critical temperatures well below room temperature,while the critical temperature of water is above room temperature.Thus,liquid water is commonplace,but liquid oxygen,nitrogen,hydrogen,or helium are not.How-ever,processes to produce these as liquids are now straightforward,and liquid oxygen,nitrogen,and helium have very significant technological applications.3 The slope of the P−v−T surface is discontinuous on the boundary between the single-phase and the two-phase regions.Note that since T remains constant3For example,liquid hydrogen and oxygen as used as propellants in the Space Shuttle Main Engine;liquid nitrogen is widely used to cool electronic equipment and photodetectors;liquid helium is used to cool large superconducting magnets to temperatures a few degrees above absolute zero.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE75Table4.2:Triple-point temperatures and pressures.T t(K)P t(kPa)Helium-4 2.18 5.1Hydrogen13.87.0Nitrogen63.1512.5Oxygen54.340.14Methane90.6811.7Carbon Dioxide216.54517.3Water273.160.61in the two-phase regions during constant-pressure heating,any horizontal slice through the surface in a two-phase region must produce a line perpendicular to the T axis;in other words,the slope of the surface in the T direction is zero in the two-phase regions.The solid-vapor two-phase region intersects the solid-liquid and liquid-vapor regions in a single line parallel to the volume axis.This line is known as the triple line,since along this line all three phases may coexist in equilibrium.The pressure and temperature are the same everywhere along the triple line.There-fore,for a given substance,there is only one pressure P t and one temperature T t at which solid,liquid,and vapor may coexist in equilibrium.The combination (T t,P t)is known as the triple point.Of course it is only a point in the P−T plane;when the volume axis is considered,it is a line.This is in contrast to the critical point,which is really a point in(P,v,T)space.The triple points for several substances are listed in Table4.3.Note that of the ones listed,only carbon dioxide has P t>1atm(1atm=101.325kPa). Therefore,at1atm pressure,solid carbon dioxide(“dry ice”)sublimates,while solid water melts.Th P−v−T surface shown in Fig.4.4is appropriate for a substance which expands upon melting,which we assumed implicitly in our discussion above. However,a few substances–including water–contract when they melt.For these substances,the P−v−T surface looks like that shown in Fig.4.5.If a substance has multiple solid phases,the P−v−T surface can become very complex.A portion of the actual surface for water is shown in Fig.4.6, showing the different phases of ice.Two-dimensional phase diagrams may be obtained by projecting the P−v−T surface onto the P−T,T−v,or P−v planes.We have already looked at the T−v projection in constructing the P−v−T surface[Fig.4.3(b)].The P−TCHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE76Figure4.5:A P−v−T surface for a substance which contracts upon melting.Figure4.6:A portion of the P−v−T surface for water.CHAPTER 4.THE SIMPLE COMPRESSIBLE SUBSTANCE77PTPT Figure 4.7:P −T phase diagrams for (a)a substance which expands on melting;(b)a substance which contracts on melting.projection is shown in Fig.4.7for a substance which expands on melting (a)and for one which contracts on melting (b).The critical point (c)and the triple point (t)are both shown.The lines separating the single-phase regions on a P −T plot are known as coexistence lines ,since on these lines two phases may coexist.The liquid-vapor coexistence line terminates at the critical point.In contrast,the solid-liquid coexistence line never terminates,no matter how high the pressure.This is because solid and liquid are fundamentally different –in a solid,atoms are arranged in a highly regular,periodic way,while in a liquid they are arranged randomly.There is no way for these states with very different symmetry to transform into one another continuously,and so it is not possible for a critical point to exist on the solid-liquid coexistence line.Each of the coexistence lines in a P −T phase diagram can be described by some function P (T ),so clearly P and T are not independent when two phases are simultaneously present.On the liquid-vapor and solid-vapor coexistence lines,the term vapor pressure is used to denote P (T ),since this is the pressure of the vapor in equilibrium with the solid or liquid.An equivalent term is saturation pressure P sat (T ).If pressure is specified,the saturation temperature T sat (P )is defined to be the temperature on the coexistence curve where the pressure is P .The saturation temperature is just another name for the boiling temperature.For example,for water,T sat (1atm)is 373.15K (100◦C),and P sat (373.15K)=1atm.4.4Determining Properties in the Mixed-Phase RegionsUnder conditions where two phases coexist in equilibrium,some care must be taken to correctly determine the properties and amount of each phase from aCHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE78Tv Pf v gFigure4.8:In the liquid/vapor two-phase region,the liquid has specific volume v f and the vapor has specific volume v g.phase diagram.The two phases have very different properties.For example, the specific volume of a liquid is much less than that of a gas.How can we determine both values from a phase diagram?The key is to realize that in a two-phase region,the properties of each phase present are those at the“edges”of the region.For example,consider boiling a liquid at constant pressure.Just before the temperature where gas bubbles first appear,the cylinder is stillfilled with liquid.Call the specific volume at this point v f.4As more heat is added at constant pressure,the only thing that happens is that some liquid becomes vapor–the properties(per unit mass) of the remaining liquid don’t change.Although there is less liquid,the liquid remaining still has specific volume v f.What is the specific volume of the vapor which has been created?It too is constant during the constant-pressure boiling process,and thus must equal the value of v obtained once the cylinder contains only vapor.Call this value v g.Suppose now that the system is somewhere in the two-phase region on the isobar5labeled P in Fig.4.8and the measured total volume V results in a value for v=V/M as shown in thisfigure.This v does not actually correspond to the specific volume of either the liquid or the vapor in the container.These are v f and v g,respectively.Instead,v is an average of v f and v g,weighted by the mass of each in the container.We use the term“saturated”to denote the states on either side of the vapor dome(Fig.4.9).Thus,“saturated liquid”has specific volume v f,and“saturated4Although it is not entirely logical,it is conventional to use the subscript“f”to denote properties of the liquid and“g”to denote properties of the vapor in an equilibrium liquid/vapor mixture.5An isobar is a line of constant pressure.The P in a circle simply labels the pressure of this isobar.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE79 TFigure4.9:Definition of saturated,superheated,and subcooled states.vapor”has specific volume v g.When liquid and vapor are present together in equilibrium,the liquid is always saturated liquid,and the vapor is saturated vapor.Vapor at a temperature above T sat(P)is called superheated vapor,and liquid at a temperature below T sat(P)is called subcooled liquid.Subcooled liquid is also called compressed liquid,since it is at a higher pressure than P sat(T).Let the mass of the vapor in the container be Mx,where0≤x≤1.Then the mass of the liquid must be M(1−x),since the total mass is M.The total volume is thenV=M(1−x)v f+Mxv g,(4.9)orv=VM=(1−x)v f+xv g.(4.10)If we know v,we can then solve for x:x=v−v fv g−v f.(4.11)The vapor mass fraction x is an intensive thermodynamic property of a liquid/vapor mixture.The common name in engineering thermodynamics for x is the quality.This name was given to x by engineers developing steam engines and power plants:the presence of liquid droplets in the steam damages engine parts such as turbine blades,hence from the engineer’s perspective higher “quality”steam had less liquid content.Of course,for other applications the relative merits of liquid and vapor might be reversed.In this book,we will usually refrain from making a value judgement about liquid vs.vapor,and simply call x the vapor mass fraction.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE80Equation(4.11)is known as the lever rule.It may be interpreted in terms Fig.4.8as follows.To determine the mass fraction of a phase in a saturated vapor/liquid mixture,locate the system point in the two-phase region on a T−v or P−v plot.Now take the length of the horizontal line segment from v to the saturation line corresponding to the other phase,and divide this length by the total width of the2-phase region(v g−v f).Note that this rule works for any 2-phase region,for example for liquid/solid mixtures.Example4.1A bottle contains10kg of carbon dioxide at260K.If the volume of the bottle is100liters,does the bottle contain liquid,solid,gas,or a mixture? How much of each?What is the pressure?Solution:Since260K is greater than the triple-point temperature for CO2, no solid will be present.Calculate v=V/M=(100liters)/(10kg):v=0.01 m3/kg.From a phase diagram for CO2at260K,wefind that v f=0.001001 m3/kg,and v g=0.01552m3/kg.Since v is between these two values,the bottle contains a mixture of liquid and gaseous CO2.The vapor mass fraction isx=0.01−0.0010010.01552−0.001001=0.62.Since it is in the mixed phase region,the pressure is the saturation pressure at 260K,which is2.421MPa.4.5Software for Property EvaluationTo solve problems involving real substances(such as the last example),some source of property data is required.Traditionally in thermodynamics courses, properties were looked up in tables,or estimated from detailed phase diagrams.A typical diagram for oxygen is shown in Fig.4.10.Here the pressure is plotted against the enthalpy,and many curves representing particular values of other properties are shown.Since P and h are two valid,independent properties,the thermodynamic state is represented by a point on this plot.The point may befixed by interpolation if any two properties are known for which curves are plotted on the chart.Once the state point is found,any other properties can be read off(with some care and practice).Most thermodynamics textbooks now come with at least some rudimentary software to evaluate properties.In many cases,the programs are simply elec-tronic versions of tables,which print to the screen the property values.In other cases,more elaborate software is provided which allows you to do a complete thermodynamic analysis.But even these packages are specialized applications, which you use for thermodynamics but nothing else.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE81Figure4.10:A pressure-enthalpy plot for oxygen.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE82A new software package is provided as a supplement to this book which im-plements thermodynamic property functions in Microsoft Excel(a spreadsheet program).The thermodynamic property functions are provided by an“add-in”module called TPX(“T hermodynamic P roperties for E X cel”).Details of how to load it into Excel and use it are given in Appendix A.Calculating properties is easy with TPX.For example,if you want to know the specific enthalpy of oxygen at1MPa and500K,you simply type into a cell:=h("o2","PT",1,500)The parameters are:the substance name(case is unimportant);a string stating the properties which will be used tofix the state(here P and T);the value of thefirst parameter(P);the value of the second parameter(T).The value of the function returned in the cell is655.83kJ/kg.You can select any system of units you like;all inputs and outputs will then be in those units.(This example assumes the user selected units of Kelvin for temperature,MPa for pressure,kJ for energy,and kg for mass.)The real power of using a spreadsheet becomes apparent in more complex analyses.For example,the temperature and pressure may not be specified inputs,but are themselves the result of calculations in other cells.In this case, simply replace the numerical value in the function parameters by the appropriate cell address(e.g.B4).The same functions implemented by TPX are also available in a WWW property calculator.The calculator is convenient for simple calculations,if you have access to the Web but not to Excel.Example4.2One kg of water is placed in a closed container and heated at constant volume.The initial temperature is300K.If the desiredfinal state is the critical point,determine the necessary container volume,initial pressure, initial vapor mass fraction,and energy transfer as heat.Solution:Two properties are needed to specify the initial state.The tem-perature is given,so one more is required.Since the process occurs at constant volume,the initial volume V1must equal thefinal volume V2.Thefinal state is the critical point,soV2=(1kg)×v c.The critical specific volume of water may be calculated using TPX:v c=vcrit("h2o")=0.003155m3/kg.Therefore,state1isfixed by T1=300K and v1=v ing TPX,any otherCHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE83 desired property for state1may be computed:P1=3528.2Pa P("H2O","TV",300,vcrit("H2O"))X1=5.49×10−5x("H2O","TV",300,vcrit("H2O"))u1=112.727kJ/kg u("H2O","TV",300,vcrit("H2O"))h1=112.738kJ/kg h("H2O","TV",300,vcrit("H2O"))Note that the initial state is a mixed liquid/vapor state,and so P1=P sat(300 K).The vapor fraction is very small,since v c is only slightly greater than the specific volume of the saturated liquid.The required heat transfer Q is determined from the First Law:∆U=Q+W.Since the volume is constant,W=0,soQ=∆U=M(u2−u1).Using TPX,u2=u("h2o","tv",tcrit("h2o"),vcrit("h2o"))=2029.6kJ/kg.so Q=(1kg)(2029.6kJ/kg-112.7kJ/kg)=1916.9kJ.4.6More Properties:Partial Derivatives of Equations of StateConsider an equation of state like P(T,v).Clearly,a partial derivative of this function,for example(∂P/∂T)v,is some new function of(T,v)and may rightly be regarded as a thermodynamic property of the system.Some useful derivative properties are defined here.4.6.1Thermal Expansion CoefficientMost substances expand when heated.The property which tells us how much a substance expands when heated at constant pressure is the thermal expansion coefficientβ,defined byβ=1v∂v∂TP(4.12)To calculateβ,the equation of state v(T,P)would be differentiated with respect to T.CHAPTER4.THE SIMPLE COMPRESSIBLE SUBSTANCE84Note the1/v in the definition–βis defined as the fractional change in volume per degree of temperature increase.Also,note that this definition is only really meaningful if a single phase is present,since otherwise T can’t be increased holding P constant.Example4.3What is the thermal expansion coefficient of liquid water at300 K and1atm?Solution:βmay be calculated approximately using TPX,evaluating the partial derivative by afinite-difference approximation.Taking a small increment of,say,0.1K,use TPX(with units set to K and atm)to evaluate(∂v/∂T)P:∂v ∂TP≈(v("h2o","tp",300.1,1)-v("h2o","tp",300,1)/0.1)=2.75×10−7m3/kg-K.(4.13)Sincev=v("h2o","tp",300,1)=0.001003378m3/kg,β≈2.74×10−4K−1.4.6.2Isothermal CompressibilityAll matter decreases slightly in volume if the pressure is increased at constant temperature.The property how the volume varies with pressure at constant temperature is the isothermal compressibilityκ,defined byκ=−1v∂v∂PT(4.14)As withβ,if the equation of state v(T,P)is known,it can be differentiated to findκ(T,P).And as with the thermal expansion coefficient,κis only meaningful if the substance is in a single phase.4.6.3Specific HeatsSuppose a unit mass of a substance absorbs an amount of heat¯d Q;how much does the temperature increase?It depends in part on how the heating is done. From the First Law,du=¯d Q+¯d W=¯d Q−P dv.(4.15)。
1、例题例1:有一容积为23m 的气罐(内有空气,参数为1bar ,20℃)与表压力为17bar 的20℃的压缩空气管道连接,缓慢充气达到平衡(定温)。
求:1.此时罐中空气的质量 2.充气过程中气罐散出的热量 3.不可逆充气引起的熵产(大气压1bar ,20℃)解:充气前1p =1bar 1T =20℃ 质量1m ,充气后2p =0p =17bar 2T =1T =20℃ 质量2m ①2m =22RgT V P =12RgT VP ②热力学第一定律:Q=E ∆+⎰-)(12)(12τm m d e de +tot WE ∆=u ∆=2u -1u =22u m -11u m ;⎰-)(12)(12τm m d e de =00dm u ⎰-τ=in m u 0=)(120m m u --;tot W =in m -00V p =)(1200m m P V --;得:Q=22u m -11u m )(120m m u --)(1200m m P V --=22u m -11u m )(120m m h -- 由缓慢充气知为定温过程,1u =2u =0V C 1T ; 0h =0P C 0T ;Q=)(12m m -0V C 1T -)(12m m -0P C 0T =)(12m m -0V C (1T -0γ0T )=(2p -1p )V)1(01001--γγT T T③S ∆=g f S S ++⎰-)(21)(21τm m d S d S =2m 2S -1m 1S ; f S =T Q ; ⎰-)(21)(21τm m d S d S =in S )(12m m -;g S =(2m 2S -1m 1S )-in S )(12m m --0T Q =2m (2S -in S )+1m (in S -1S )-0T Q ;S ∆=2S -1S =P C 12lnT T -g R 12ln p p; g S =2m (P C in T T 2ln-g R in p p2ln )+1m (P C 1ln T T in -g R 1ln p p in )-0T Q ; g L S T E 0= 例2:1mol 理想气体2o ,在(T ,V )状态下,1S ,1Ω,绝热自由膨胀后体积增加到2V ,此时2S ,2Ω。
第7章 水 蒸 汽7.1 本章基本要求理解水蒸汽的产生过程,掌握水蒸汽状态参数的计算,学会查水蒸汽图表和正确使用水蒸汽h -s 图。
掌握水蒸汽热力过程、功量、热量和状态参数的计算方法。
自学水蒸汽基本热力过程(§7-4)。
7.2 本章难点1.水蒸汽是实际气体,前面章节中适用于理想气体的计算公式,对于水蒸汽不能适用,水蒸汽状态参数的计算,只能使用水蒸汽图表和水蒸汽h-s 图。
2.理想气体的内能、焓只是温度的函数,而实际气体的内能、焓则和温度及压力都有关。
3.查水蒸汽h -s 图,要注意各热力学状态参数的单位。
7.3 例题例1:容积为0.63m 的密闭容器内盛有压力为3.6bar 的干饱和蒸汽,问蒸汽的质量为多少,若对蒸汽进行冷却,当压力降低到2bar 时,问蒸汽的干度为多少,冷却过程中由蒸汽向外传出的热量为多少解:查以压力为序的饱和蒸汽表得:1p =3.6bar 时,"1v =0.51056kg m /3 "1h =2733.8kJ /kg蒸汽质量 m=V/"1v =1.1752kg查饱和蒸汽表得:2p =2bar 时,'2v =0.0010608kg m /3 "2v =0.88592kg m /3 '2h =504.7kJ /kg ''2h =2706.9kJ /kg 在冷却过程中,工质的容积、质量不变,故冷却前干饱和蒸汽的比容等于冷却后湿蒸汽的比容即: "1v =2x v或"1v =''22'22)1(v x v x +- 由于"1v ≈''22v x =≈"2"12v v x 0.5763 取蒸汽为闭系,由闭系能量方程 w u q +∆=由于是定容放热过程,故0=w所以 1212u u u q -=∆=而u =h -pv 故)()("11"1222v p h v p h q x x ---=其中:2x h =''22'22)1(h x h x +-=1773.8kJ /kg 则 3.878-=q kJ /kgQ=mq=1.1752⨯(-878.3) =-1032.2kJ例2:1p =50bar C t 01400=的蒸汽进入汽轮机绝热膨胀至2p =0.04bar 。
设环境温度C t 0020=求:(1)若过程是可逆的,1kg 蒸汽所做的膨胀功及技术功各为多少。
(2)若汽轮机的相对内效率为0.88时,其作功能力损失为多少 解:用h -s 图确定初、终参数初态参数:1p =50bar C t 01400=时,1h =3197kJ /kg 1v =0.058kg m /31s =6.65kJ /kgK则1111v p h u -==2907 kJ /kg6.65kJ /kgK终态参数:若不考虑损失,蒸汽做可逆绝热膨胀,即沿定熵线膨胀至2p =0.04bar ,此过程在h-s 图上用一垂直线表示,查得2h =2020 kJ /kg 2v =0.058kg m /3 2s =1s =6.65kJ /kgK2222v p h u -==1914 kJ /kg膨胀功及技术功:21u u w -==2907-1914=993 kJ /kg21h h w t -==3197-2020=1177 kJ /kg2)由于损失存在,故该汽轮机实际完成功量为t ri t w w η='=0.88⨯1177=1036 kJ /kg此不可逆过程在h-s 图上用虚线表示,膨胀过程的终点状态可以这样推算,按题意'21'h h w t -=,则'12't w h h -==3197-1036=2161 kJ /kg这样利用两个参数'2p =0.04bar 和'2h =2161 kJ /kg ,即可确定实际过程终点的状态,并在h-s 图上查得'2s =7.12kJ /kgK ,故不可逆过程熵产为22's s s g -=∆=7.12-6.65=0.47kJ /kgK作功能力损失)(00g f s s T s T w ∆+∆=∆=∆因绝热过程0=∆f s则kg kJ s T w g /7.13747.0)20273(0=⨯+=∆=∆例3:0.1kg 水盛于一绝热的刚性容器中,工质的压力的0.3Mpa ,干度为0.763。
一搅拌轮置于容器中,由外面马达带动旋转,直到水全变为饱和蒸汽。
求:(1)完成此过程所需的功;(2)水蒸汽最终的压力和温度。
解:取容器内的工质为系统,搅拌轮搅拌工质的功变为热,是不可逆过程。
(1)系统绝热,q =0。
系统的边界为刚性,因而没有容积变化功,可是由于搅拌轮搅拌,外界对系统作了功。
由热力学第一定律)(21u u m U W -=∆=即外界消耗的功变了热,工质吸收热内能增加,因而只要计算初终态的内能变化就可求得耗功量。
对于初态,已知p 1=0.3Mpa ,x =0.763,于是/kgm 4643.06058.0763.0001074.0)763.01()1(3111=⨯+-=''+'-=v x v x v1(1(1-=-=h 11=-=h u 而终态是v 2和水与饱和v 2=2v ''=0.4624t 2=143.62态的内能u 2W (2t 2=143.62℃。
例4:1kg 水贮存于有负载的活塞-气缸装置中,压力为3Mpa ,温度为240℃。
定压下对工质慢慢加热直至其温度达320℃,求:(1)举起负载活塞作多少功?(2)外界需加入多少热量?解:取装置中的工质水为系统,由饱和水与饱和蒸气表查得对应于3Mpa 的饱和温度为233.84℃。
现初态温度高于此值,可知初态为刚高于饱和温度的过热蒸气,故工质在整个加热过程均为过热蒸气。
(1)在缓慢加热的条件下,可认为热源与工质温差很小而视过程为可逆过程故⎰-==2112)(v vp pdv w比容值可由“未饱和水与过热蒸汽表”查出,代入上式得kJ/kg 4.5010)06818.0085.0(10366=⨯-⨯=-w(2)对可逆定压加热过程12h h h q -=∆=由过热蒸气表查得焓值后代入上式得kJ/kg 8.2193.28241.3044=-=q若根据水蒸气的h -s 图读取v 和h 的数据亦可得到相近的结果。
图中数据连续,且可显示过程,但所读数据不如表中数据准确。
7.4 思考及练习题1.物质的临界状态究竟是怎样一种状态?2.水的定压产生过程可分为哪几个阶段,经历哪几种状态,写出它们的名称。
3.对水蒸汽的热力过程和理想气体热力过程 ,在分析方法上有何异同之处。
456.的焓值呢?7.压力为并将该过程表示在h 8.1kg 状态为p 1压下被加热到300变化。
如果工质为同之处。
9现将压力为0.1MPa0.1MPa,温度为20℃的自来水相混合,如果供应4吨开水,则需要蒸气、水各为多少?混合过程的熵产是多少?10.图7.2表示一台锅炉的示意图,每小时生产1MPa,240℃的过热蒸气15吨。
已知给水温度为20℃锅炉效率为0.75,煤的值为29400kJ/kg,进入过热嚣的湿蒸汽干度为0.95,试求:(1)耗煤量;(2)温蒸气在过热器中的吸热量。
11.一刚性容器被绝热隔板分成A和B两部分。
已知A和B中的状态分别为:m A=1kg,x A=1,p A=0.5MPa;m B=1kg,x B=1,p B=0.5MPa抽去隔板后,容器内的最终压力为0.7MPa,若环境温度为300K,求:(1)刚性容器的容积及蒸汽的最终状态;(2)过程中交换的热量;(3)过程中总的熵产。
12.一容器内装有1kg的湿蒸汽,其压力p s=10bar,如果容器内的饱和水与饱和蒸汽各自恰好占去容器容积的一半。
求容器内湿蒸汽的焓。
13.在容积V=6m3的容器中,有t s=150℃的饱和水与饱和蒸气共存,其中饱和水占总容积的1/3。
求蒸汽的干度x。
14.在一内径为60cm、高为3m的圆柱形密闭容器中,充以压力p1=80at 的饱和水与饱和水蒸汽混合物,其中饱和水的液面高h1=15cm。
若使容器内湿饱和蒸汽的压力由于冷却而降至p2=40at,求此时饱和水液面的高度h2以及冷却过程中所放出的热量Q。
15.在压力p=8bar下,对1kg的饱和液体加入711.76kJ的热量,结果其内能增加为628kJ。
试求:(1)加热后混合物的干度x=?(2)内汽化潜热和外汽化潜热各为多少?某物质的汽化潜热为1130kJ/kg。
16.现有两个容积相等、材质相同的容器,它们的容积V=1m3,其中一个充满了压力为10bar饱和水,另一个装有压力为10bar的饱和干蒸汽。
如果由于某种意外的原因发生爆炸,问哪一个容器爆炸所引起的危害大些?提示:由于爆炸是在瞬间发生的,饱和水饱和蒸汽进行绝热膨胀其,压力由10bar降至1bar,所以s1=s2,其危害性可粗略按其绝热膨胀所作功的大小来衡量。
由第一定律可得:W=-mΔu。
计算结果饱和水容器爆炸的危害约为饱和汽容器产生危害的30倍。
17.把压力为1bar的干饱和蒸气10kg,在定压下变为t w=50℃的热水,用其所放出的热量来加热空气。
问在定压加热的情况下,这些热量可将多少公斤的空气由t1=50℃加热至t2=100℃。
18.在压力p=30bar,温度t=500℃的过热蒸汽中的加入t w=25℃的水,使其混合后温度t m=400℃,问对1kg蒸汽而言,需要加入多少公斤的水,设过热蒸汽的平均比热c pm=0.5399kcal/kg·℃。
19.有一废热锅炉每小时可把200kg温度为t1=10℃的水,变为t2=100℃的干饱和蒸气。
进入锅炉的烟气温度为t g1=600℃,排烟温度为t g2=200℃,若锅炉的效率为60%,求每小时通过的烟气量。
已知烟气的比热为c p=1.047kJ/kg℃,水的汽化潜热T=2260.87kJ/kg·℃。
20.一加热器每小时换热量9010kJ/h,现送入压力p=1.078bar的蒸气(h=2658.6kJ/kg),蒸气在加热器内放热后变为t2=83℃的凝结水排入大气,问此换热器每小时所需蒸汽量。
21.把压力p=2.94bar的干饱和蒸汽,通过1英寸的管道(内径为2.54cm)送往用户。
无论管内的蒸汽流量是多大,每1m2的管子内表面每小时向管外保温层散出的热量为2093.4kJ/m2·h。
问蒸气在管内流动时每流过10m有多少蒸汽由于散热而冷凝?如要使蒸气的冷凝量限制在管内蒸汽总流量的10%,求每小时需要通过的蒸汽量。