细胞外基质2
- 格式:ppt
- 大小:2.10 MB
- 文档页数:35

11.4 细胞外基质同学好!大家很容易理解,在多细胞生物体内,每一个细胞作为细胞社会中的一个成员,彼此相互作用,需要一个和谐稳定的生活环境。
我们前几讲一起学习了细胞表面、细胞连接和黏附分子,依赖这些重要的结构和分子,细胞与细胞,细胞与环境之间发生着各种各样的细胞社会联系。
那么由近及远,细胞表面邻近的周边环境怎么样?都由哪些成分构成呢?这就是我们今天要讨论的主题:细胞外基质(extracellular matrix, ECM)。
它是由细胞分泌产生的多种蛋白质和多糖大分子构成的精密有序的结构网络,填充在细胞间隙并介导细胞社会联系,构筑起细胞赖以生存的外部微环境。
一、细胞外基质的主要成分细胞外基质主要是由一些蛋白质和多糖构成。
包括:(一)胶原蛋白(collagen)胶原是细胞外最重要的水不溶性纤维蛋白,是构成细胞外基质的骨架。
1、结构单位:胶原蛋白的基本结构单位是原胶原,原胶原肽链的一级结构具有(Gly-X-Y)n重复序列,其中X常为脯氨酸,Y常为羟脯氨酸或羟赖氨酸,羟赖氨酸可发生糖基化修饰,糖在胶原中约占10%。
2、结构模式:原胶原是由三条α肽链盘绕而成的三股螺旋纤维结构,长约300nm,直径仅为1.5nm左右,原胶原进一步交联成原纤维,继而可形成直径达数微米的胶原纤维,形同绳索,其中原胶原的三股螺旋是维系胶原稳定和坚韧的重要结构基础。
3、主要类型:目前已发现胶原为类型有10多种,最主要有是I、II、III、IV 型胶原。
其中前三种为纤维状,广泛分布于各种组织的细胞外基质中。
IV型胶原则不形成螺旋纤维,而以分子头对头相接的方式形成三聚体,再相互交联成网络层结构,是各种基膜的重要构造。
胶原与多种疾病的发生和病理过程有关。
例如:过去远洋航海船员因维生素C缺乏引起的坏血病,就与胶原异常有关。
现已证明,当机体缺乏维生素C时,原胶原中的脯氨酸、赖氨酸不能有效羟基化,难以形成稳定的螺旋结构,导致组织中胶原减少,严重时引起血管脆弱,表现渗血出血倾向,称为坏血病。




第十一章细胞外基质及其与细胞的相互作用细胞外基质(ECM):是由细胞分泌到细胞外空间,由蛋白和多糖构成的精密有序的网络结构。
不仅对组织细胞起支持、保护、营养作用,而且还与细胞的增殖、分化、代谢、识别、黏着、迁移等基本生命活动密切相关。
糖胺聚糖(AGA):是细胞外基质的主要成分,是由重复的二糖单位构成的直链多糖,过去称为黏多糖,其二糖单位之一是氨基己糖(N-乙酰氨基葡萄糖或N-乙酰氨基半乳糖),故又称氨基聚糖,另一个糖残基多为糖醛酸(葡萄糖醛酸或艾杜糖醛酸)糖胺聚糖可分为六种:透明质酸HA、硫酸软骨素CS、硫酸皮肤素DS、硫酸乙酰肝素HS、肝素、硫酸角质素KS蛋白聚糖(PG):是由糖胺聚糖(除透明质酸外)与核心蛋白共价结合形成的高分子量复合物,是一种含糖量极高的糖蛋白。
黏多糖累积病:由于基因突变引起先天性缺乏降解糖胺聚糖的酶(如糖苷酶或硫酸酯酶)可导致糖胺聚糖或蛋白聚糖及其降解中间产物在体内一定部位堆积,造成黏多糖累积病如Hunter综合征。
胶原(collagen):是细胞外基质中的骨架结构,动物体内高度特化的纤维蛋白家族,是人体内含量最丰富的蛋白质,遍布于体内各种器官和组织,在结缔组织中特别丰富,可由成纤维细胞、软骨细胞、成骨细胞以及某些上皮细胞合成并分泌到细胞外。
原胶原:典型的胶原分子呈纤维状,是由3条α多肽链盘绕而成的3股螺旋结构,称原胶原。
胶原合成与组装始于内质网,在高尔基体修饰,最后在细胞外组装成胶原纤维①前α链:在糙面内质网附着核糖体上合成,不仅含有内质网信号肽,而且在其N端和C端各含有一段不含Gly-X-Y序列的前肽。
②前胶原:胶原合成过程中带有前肽的3股螺旋胶原分子称为前胶原,其两端的前肽部分保持非螺旋卷曲。
③原胶原分子:在细胞外,前胶原在前胶原N-蛋白酶和前胶原C-蛋白酶的作用下,分别水解去除两端的前肽,在两端各保留一段非螺旋的端肽区形成原胶原分子。
④胶原原纤维:原胶原分子在细胞外基质中相互呈阶梯式有序排列并发生侧向交联,自组装成胶原原纤维。

细胞外基质(Extracellular Matrix,ECM)是由成纤维细胞、间质细胞、上皮细胞等体内各类组织和细胞合成和分泌的一类散布和聚集在细胞表面和细胞间质的大分子物质所组成的复杂网络结构,故称细胞外基质(间质),是细胞和组织赖以生存、活动和调剂的外环境。
要紧作用:一方面为细胞和组织提供支持、联结、固定、保水、缓冲等物理性的爱惜作用,另一方面又是细胞与外环境进行物质互换、信息传递和聚集的中介。
它可通过各类信号传递系统,调剂细胞生长、增殖、迁移、分化、粘附、代谢、损伤修复、组织重构等各类生理功能。
被称为是人体细胞和组织内稳态的要紧调剂者(The Central Regulator of Cell and Tissue Homeostasis)。
细胞外基质的成份十分复杂,除各型胶原之外,还有各类粘连蛋白(FN)、层连蛋白(LN)、氨基聚糖(GAG)、蛋白聚糖(PG)、弹性蛋白(Elastin)、内动素(Cytotatin)、血栓结合素(Thrombospondin)、整合素(Integrin)、玻连蛋白(Vitronetin VN)、连结蛋白(Connexins)、钙粘素(Cadherins)、选择素(Selectin)、粘附素(细胞粘合素)、细胞粘合素(Cytotatin)等几十个类别。
每一种类别又有几种至十几种亚型。
细胞不同产生和分泌的细胞外基质成份亦不同;组织不同所含的细胞外基质的成份和比例亦不同;即便同一种细胞,同一种组织,在不同的生理、病理和反映条件下,细胞外基质的成份、结构和构型亦不同;结构和构型不同,细胞外基质的功能和作用亦不同。
随着基因和蛋白质组生物学的研究进展,新的细胞外基质分子还在不断诞生,其类型、构型、构像还有更多发觉,其功能亦在不断的扩展,组成了一个十分复杂的细胞外基质的网络家族和体系。
细胞外基质尽管来源、成份、分型和功能不同,各司其责,但在结构和功能上,它们又排列有序、疏密相间、彼此联结、彼此协同,在细胞间质、组织间隙和器官内,形成各类复杂的相对固定的形式和分层网状结构,形成许多不同的功能结构区域,如在血管,能够形成内膜表面的粘附爱惜层、内膜基层、基底膜层、内弹力层、外弹力层、血管中层和外层系膜结缔组织等等。


细胞外基质的生物学功能和应用细胞外基质(extracellular matrix,ECM)是由细胞合成并分泌到周围环境中的一组复杂的蛋白质、多糖和小分子组成的结构。
细胞外基质的主要功能包括提供对细胞的支持、促进信号传递、调节细胞分化和增殖、参与细胞外基质重塑等。
在本文中,我们将详细介绍细胞外基质的生物学功能以及其在生物技术和医学应用中的价值。
细胞外基质的生物学功能1.提供对细胞的支持和结构细胞外基质是细胞及组织之间的接口,它将细胞连接成组织和器官。
过去,人们通常将细胞外基质视为静态的结构,但现在已经发现,它是一种动态的结构,具有调节细胞行为的重要作用。
细胞外基质中最重要的成分是胶原蛋白,它是一种纤维性蛋白质,质地坚韧。
除了机械支持之外,胶原蛋白还可以通过与细胞外基质中其他成分的相互作用,调节胞外基质的物理性质和化学性质,从而影响细胞的生理过程。
此外,细胞外基质中还有积累在地面物质(ground substance)中的大量的葡萄糖胺聚糖(glycosaminoglycans)。
这些多糖具有负电张和胶冻状态的特性,可以在形成透明质酸之前,提供一种有效的滑润层。
这可以保护细胞免受机械性损伤,并协助它们在经历的现实环境变化时维持其形态和生理功能。
2.促进信号传递除了为细胞提供支持之外,细胞外基质还具有信号传递的作用。
细胞外基质上存在着许多导向细胞行为和增殖的细胞外生信号分子,包括肽、糖和脂质等。
这些信号分子作用于细胞表面的受体,从而触发一系列的细胞信号级联反应,包括细胞增殖、分化和转移等。
此外,细胞外基质本身也可以促进信号传递。
例如,细胞外基质中的蛋白质可以与调节蛋白相互作用,从而改变它们的空间位置和功能,影响细胞的行为。
3.调节细胞分化和增殖细胞外基质可以通过许多不同的方式调节细胞的行为,其中增殖和分化是最为重要的两个方面。
细胞外基质可以激活或抑制某些信号通路,进而影响细胞的生长和偏向性。
另外,在某些时候,细胞外基质可以促进细胞分化,例如在骨骼生长相和软骨分化中。

A new technique to expand human mesenchymal stem cellsusing basement membrane extracellular matrixTakehiro Matsubara,a,c,f Shinichi Tsutsumi,b Haiou Pan,a Hisatada Hiraoka,c Ryo Oda,d Masahiro Nishimura,e Hiroshi Kawaguchi,c Kouzou Nakamura,c and Yukio Kato a,f,*aJapan Science and Technology Corporation (JST),Chiyoda-ku,Tokyo 102-8666,JapanbDepartment of Orthopedic Surgery,School of Medicine,Gunma University,Maebashi 371-8511,Japan cDepartment of Orthopedic Surgery,Graduate School of Medicine,University of Tokyo,Tokyo 113-8655,JapandDepartment of Operative Dentistry,Graduate School of Biomedical Science,Hiroshima University,Hiroshima 734-8553,Japan eDepartment of Prosthetic Dentistry,Graduate School of Biomedical Science,Hiroshima University,Hiroshima 734-8553,JapanfDepartment of Dental and Medical Biochemistry,Graduate School of Biomedical Science,Hiroshima University,Hiroshima 734-8553,JapanReceived 12November 2003Abstractshort proliferative life span and readily lose the differentiation potential in culture.span of the stem cells markedly increased using tissue culture dishes coated with a which was produced by PYS-2cells or primary endothelial cells.Furthermore,the stem cells expanded on the extracellular matrix,but not those on plastic tissue culture dishes,retained the osteogenic,chondrogenic,and adipogenic potential throughout many mitotic divisions.The extracellular matrix had greater effects on the proliferation of MSC and the maintenance of the multi-lineage differentiation potential than basic fibroblast growth factor.Mesenchymal stem cells expanded on the extracellular matrix should be useful for regeneration of large tissue defects and repeated cell therapies,which require a large number of stem or progenitor cells.Ó2003Elsevier Inc.All rights reserved.Keywords:Mesenchymal stem cell;Basement membrane;Extracellular matrix;Regeneration;DifferentiationMesenchymal stem cells (MSC)can be induced to differentiate into a variety of tissues including bone,cartilage,tendon,fat,heart,muscle,and brain,in vitro and in vivo [1,2].Autologous MSC have advantages over ES cells:there is no teratocarcinoma formation,no immune rejection,and there are no ethical problems.However,compared with ES cells,which have an un-limited proliferative life span (period before the cells reach growth arrest in culture)and consistently high telomerase activity,MSC have very poor replicative capacity and short proliferative longevity [3,4].Thus,an important challenge in regenerative medicine is to im-prove the replicative capacity of MSC,thereby to obtain a number of MSC sufficient to repair large defects.Forced expression of telomerase in MSC markedlyincreases their proliferative life span and MSC with a high telomerase activity showed osteogenic potential [5].However,it is unknown whether these cells can maintain the chondrogenic and adipogenic potential or whether these cells have a risk of transformation.We report here that the growth rate and the proliferative life span of MSC markedly increased using tissue culture dishes coated with a basement membrane-like extracellular matrix (“bmECM”).Furthermore,MSC that expanded 106-fold on bmECM retained its osteogenic,chondro-genic,and adipogenic potential.Materials and methodsPreparation of bmECM-coated dishes .PYS-2cells,which produce laminin,type IV collagen,and heparan sulfate proteoglycans [6–8],were supplied by Dr.Atsumi (Riken,Wako,Japan),and bovine corneal endothelial cells were isolated and maintained as described [9].*Corresponding author.Fax:+81-82-257-5629.E-mail address:ykato@hiroshima-u.ac.jp (Y.Kato).0006-291X/$-see front matter Ó2003Elsevier Inc.All rights reserved.doi:10.1016/j.bbrc.2003.11.143Biochemical and Biophysical Research Communications 313(2004)503–508BBRC/locate/ybbrcBmECM-coated dishes were prepared according to the method of Dr. Gospodarowicz[10].The cells were seeded at2Â104cells/cm2on60-mm tissue culture dishes(Corning,Corning,NY)and maintained in 4ml of Dulbecco’s modified Eagle’s medium(DMEM)-Ham’s F12 medium(1:1)(Sigma,St.Louis,MO)in the presence of10%fetal bo-vine serum(Hyclone,Logan,Utah)and antibiotics(100U/ml penicillin G and100l g/ml streptomycin)(medium-A).Medium was changed every other day.Once the cultures became confluent,the media were renewed by4ml of medium-A supplemented with5%dextran (200,000Da,Wako,Osaka,Japan)and the cultures were further in-cubated for7days.Treatment of the cultures with20mM NH3resulted in cell lysis,exposing the extracellular matrix adhering to the substrata of tissue culture dishes.The substratum was washedfive times with PBS.Previous studies have shown that bmECM is composed of lami-nin,heparan sulfate,entactin,and type IV collagen[11,12].Preparation of laminin-coated dishes,type IV collagen-coated dishes, and ECM gel-coated dishes.Three milliliters of10mM NaHCO3 containing30l g/ml type IV collagen or30l g/ml laminin(Koken, Tokyo,Japan)was incubated in60-mm plastic tissue culture dishes at 4°C for12h.Two hundred micrograms per milliliter ECM gel solution (Sigma)was made by diluting with DMEM-high glucose.Three mil-liliters of the ECM gel solution was incubated in60-mm tissue culture dishes at4°C for12h.These concentrations of laminin,type IV col-lagen,and ECM gel were optimal for proliferation of MSC(data not shown).MSC culture.Human MSC were obtained from the ilium or the alveolar bone according to a protocol approved by ethical authorities at Hiroshima University.Cells in marrow aspirates(1ml/100-mm dish) were seeded on plastic tissue culture dishes.Passages were performed when cells were approaching confluence.Unless otherwise specified, MSC obtained from the primary cultures were seeded at1Â103cells/ cm2on60-mm of laminin-,type IV collagen-or ECM gel-coated dishes,on bmECM-coated dishes or on plastic tissue culture dishes, and cells were fed with DMEM-low glucose supplemented with10% fetal bovine serum and antibiotics(medium-B)every3days.Sub-sequent passages were performed on the appropriate substrata.In these studies,we seeded MSC at a low density(1Â103cells/cm2)to avoid frequent passages and the risk of contamination considering clinical application,although MSC showed a higher growth rate and a longer proliferative life span at a high seeding cell density(5Â103cells/ cm2)(data not shown).Differentiation.Chondrogenic,osteogenic or adipogenic conversion of MSC was determined according to the procedures reported by Pittenger et al.[1],with some modifications.For chondrogenic differ-entiation,cells were seeded at2.5Â105cells per15ml plastic centrifuge tube and maintained in0.5ml of serum-free a-MEM supplemented with3500mg/ml glucose, 6.25l g/ml insulin, 6.25l g/ml transferrin, 6.25ng/ml selenite,5.33l g/ml linolate,1.25mg/ml bovine serum al-bumin,10ng/ml transforming growth factor-b3,100nM dexametha-sone,and50l g/ml ascorbic acid-2-phosphate.The cultures were fed with0.5ml of the medium until3days after seeding.Thereafter,the cultures were fed with1ml of the medium every other day.Cells were cultured under the chondrogenic status for28days.For osteogenic differentiation,cells were seeded at4Â104cells per16-mm dish and maintained for21–28days in DMEM-low glucose supplemented with 10l g/ml insulin,10mM b-glycerophosphate,100nM dexamethasone, and50l g/ml ascorbic acid-2-phosphate.For adipogenic differentia-tion,cells were seeded at2Â105cells per35-mm dish and grown to confluence in medium-B.Thereafter,adipogenic differentiation was induced by subjecting confluent monolayers to3–4rounds of adipo-genic treatments.Each round had two steps;incubation with adipo-genic medium(DMEM-high glucose,10%fetal bovine serum,0.2mM indomethacin,1l M dexamethasone,0.5mM methyl-isobutylxanthine, and10l g/ml insulin)for72–96h and incubation with maintenance medium(DMEM-high glucose,10%fetal bovine serum,and10l g/ml insulin)for72–96h.Cells were cultured under the adipogenic status for 28days.Glycosaminoglycan content,alkaline phosphatase activity,calcium level,glycerol-3-phosphate dehydrogenase activity,and DNA content. The glycosaminoglycan(GAG)content was determined using a sul-fated GAG assay kit(Biocolor,Newtownabbey,UK)[13].The alka-line phosphatase(ALP)activity was determined by the method of Bessey[14].The calcium level was determined by the method of Git-elman[15].The glycerol-3-phosphate dehydrogenase activity was de-termined using an assay kit(Hokudo,Sapporo,Japan)[16].The DNA content was determined using afluorescent DNA quantification kit (Bio-Rad,Chicago,IL).RT-PCR.Total RNA was extracted using Isogen(Nippon Gene, Tokyo,Japan).Thefirst-strand cDNA was synthesized from1l g of total RNA using the SUPERSCRIPT II RNase HÀreverse trans-criptase(Life Technologies,Rockville,MD).Using the cDNAs as a template,PCR was carried out under the following conditions:dena-turation at94°C for30s and primer extension at65°C for1.5min in 27cycles.Pairs of nucleotides,50-TGGTGGAGCAGCAAGAGCAA-30and50-TGCCCAGTTCAGGTCTCTTA-30for type II collagen,50-CCCAACACCAAGACACAGTT-30and50C-ATCACCTTTGATG CCTGGCT-30for type X collagen,and50-GTCAAGGCCGAGAAT GGGAA-30and50-GCTTCACCACCTTCTTGATG-30for GAPDH, 50-CATTTTGGGAATGGCCTGTG-30and50-ATTGTCTCCTCCG CTGCTGC-30for bone sialoprotein,50-CTAGGCATCACCTGTGC CATACC-30and50-CAGTG ACCAGTTCATCAGATTCATC-30for osteopontin,50-CCACCGAGACACCATGAGAG-30and50-CCATA GGGCTGGGAGGTCAG-30for osteocalcin,and50-CATTCTGGC CCACCAACTT-30and50-CCTTGCA TCCTTCACAAGCA-30for PPAR-c2were used as primers for RT-PCR.Obtained PCR products were separated on1%agarose gels and stained with ethidium bromide.Statistical analysis.Student’s t test was used.ResultsThe extracellular matrix produced by PYS-2cells or endothelial cells adhered to the substratum of plastic tissue culture dishes and could be easily cut with a needle and turned over like a sheet of paper(Fig.1A).When cells in marrow aspirates were seeded on plastic culture dishes,adherent cells—MSC—proliferated in the pres-ence of10%fetal bovine serum at a high growth rate in primary cultures[3],but their growth rate rapidly de-creased in secondary and tertiary cultures(Figs.1B–D). In cultures on plastic tissue culture dishes,non-adherent cells were removed completely by thefirst passage. However,when cells in marrow aspirates were seeded directly on bmECM,both MSC and many other cells adhered to the substratum and these adherent cells were not removed by changing the medium.Accordingly,we harvested MSC when the cells were approaching con-fluence in primary cultures on plastic tissue culture dishes,and seeded the isolated MSC on bmECM or plastic tissue culture dishes without the matrix (“plastic”)to examine the effects of bmECM on the proliferation of MSC.The growth rate of human MSC isolated from the ilium(Fig.1B)or the alveolar bone(Fig.1C)on bmECM was much higher than that on plastic,and thus the cumulative cell number in the cultures on bmECM was105-fold greater than that on plastic on day50. After MSC obtained from primary cultures were seeded504T.Matsubara et al./Biochemical and Biophysical Research Communications313(2004)503–508on bmECM or plastic,the proliferative life span of MSC on bmECM(50.3Æ1.5days)was also significantly (p<0:0001)longer than that of MSC on plastic (29.2Æ4.4days)(Figs.1B and C).The effect of bmECM produced by PYS-2was similar to that of endothelial cell bmECM(Fig.1).MSC seeded at a low density and grown on plastic lost their spindle-like appearance,be-comingflat with an increase in the passage number (Fig.1E).Theflat appearance is characteristic of se-nescent cells.However,most MSC grown on bmECM maintained the spindle-like appearance until the5th passage culture on day45(Fig.1F),suggesting that bmECM suppressed cell senescence.Laminin and type IV collagen are the major com-ponents of bmECM,but MSC on laminin-or type IV collagen-coated dishes showed lower growth rates than on bmECM(Fig.1F).The ECM gel isolated from Engelbreth–Holm–Swarm murine sarcoma also showed less growth stimulation than bmECM(Fig.1F),sug-gesting a loss of active substances during isolation of the extracellular matrix components or the necessity of an intact structure for growth stimulation.The chondrogenic potential of MSC was examined as a function of the passage number.MSC obtained from the2nd and the5th passage cultures on day15and day 51(Fig.1B)were maintained in pellet cultures for28 days(Fig.2A).The amount of cartilage proteoglycan stained with toluidine blue was greater in the pellets obtained from the5th passage cultures grown on bmECM than in the pellets from the2nd and the5th passage cultures grown on plastic.The expressions of type II collagen and type X collagen mRNAs were higher in pellets from the5th passage cultures on bmECM than in pellets from the5th passage cultures on plastic(Fig.2B).GAG content and ALP activity in the pellets decreased with the increase in the passage num-ber,irrespective of the presence or absence of bmECM. However,at each passage number,the GAG content (Fig.2C)and ALP activity(Fig.2D)were higher with MSC from cultures on bmECM than with MSC from cultures on plastic.Next,MSC from the2nd and the5th passage cultures on bmECM or plastic were incubated under the osteo-genic status on plastic tissue culture dishes.DuringT.Matsubara et al./Biochemical and Biophysical Research Communications313(2004)503–508505osteogenesis,we did not use bmECM to discriminate the effect of the extracellular matrix on proliferation from its direct effect on differentiation.MSC from cultures on bmECM became stained with alizarin red more in-tensely than MSC from cultures on plastic on day 21(Fig.2E).The expressions of bone sialoprotein,osteo-pontin,and osteocalcin mRNAs on day 28were also higher in cultures of MSC from cultures on bmECM than in cultures of MSC from cultures on plastic at the 5th passage (Fig.2F).ALP activity and calcium level on day 28decreased with the increase in the passage num-ber.However,MSC from cultures on bmECM showed a higher ALP activity (Fig.2G)and a higher calcium level (Fig.2H)than MSC from cultures on plastic at the 2nd and the 5th passages.To examine the adipogenic potential,MSC from the 2nd and the 5th passage cultures on bmECM or plastic were incubated under the adipogenic status on plastic tissue culture dishes for 28days.MSC from cultures on bmECM showed higher adipogenic differentiation,which was indicated by more intense staining with oil-red O (Fig.2I),higher PPAR-c 2mRNA expression (Fig.2J),and higher glycerol-3-phosphate dehydroge-nase activity (Fig.2K).Next,human MSC were grown on bmECM or plastic with 10%human serum,since human serum may be safer than fetal bovine serum for clinical use.Under these conditions,MSC proliferated more rapidly and showed a longer proliferative life span on bmECM than on plastic (Fig.3A).Furthermore,this effect of bmECM was greater than that of basic fibroblast growth factor (FGF).The MSC grown on bmECM for 66days de-veloped into a cartilage-like tissue (Fig.3B),even though these MSC on bmECM had lost proliferation capability.In contrast,scarcely any cartilage-like tissue was formed with MSC obtained from 66-day-old cul-tures on plastic (Fig.3C).DiscussionThe extracellular matrix (ECM)plays a vital role in organ morphogenesis,maintenance,and reconstruction following injury,and actions of ECM can be attributed to its effect on proliferation of stem cells,since stem cells reside on the basement membrane in the epithelium and some other tissues [17].In muscle,satellite cells (stem cells)—which can be induced to differentiate intomuscle,Fig.2.Retention of chondrogenic,osteogenic,and adipogenic potential on bmECM.The MSC obtained from the 2nd and the 5th passage cultures on plastic or bmECM produced by PYS-2cells (Fig.1B)were transferred into the chondrogenic status in pellet cultures for 28days (A–D)and stained with toluidine blue (A).(B)The mRNA levels of type II collagen (type II)and type X collagen (type X)were analyzed by RT-PCR.GAG content (C)and ALP activity (D)were determined on day 28.The MSC from the 2nd and the 5th passage cultures on plastic or bmECM were transferred into the osteogenic status (E–H).(E)The cell layers were stained with alizarin red on day 21.(F)The mRNA levels of bone sialoprotein (BSP),osteopontin (OP),and osteocalcin (OC)were analyzed by RT-PCR on day 28.The ALP activity (G)and calcium level (H)of the cell-matrix layers were determined on day 28.The MSC obtained from the 2nd and the 5th passage cultures on plastic or bmECM were cultured under the adipogenic status for 28days (I–K).(I)The cell layers were stained with oil-red O.(J)The mRNA level of PPAR-c 2was analyzed by RT-PCR.(K)Glycerol-3-phosphate dehydrogenase activity was determined.(C,D,G,H,and K)“)”and “+”represent MSC from culture on plastic and bmECM,respectively.Values are averages þ=ÀSD for four cultures.*p <0:05,**p <0:01,and ***p <0:001vs plastic.506T.Matsubara et al./Biochemical and Biophysical Research Communications 313(2004)503–508bone,cartilage,and fat —are also in close contact with the basement membrane;satellite cells became myo-blasts after detachment from the basal membrane [18].These observations suggest that the basement membrane and/or some other ECMs play a crucial role in the proliferation of stem cells and the maintenance of their undifferentiated state.In this study,we showed that bmECM markedly increased both the growth rate and the proliferative life span of MSC.Furthermore,MSC that had expanded 106-fold on bmECM maintained its multi-lineage differentiation potential.The mechanism by which bmECM stimulates MSC proliferation and maintains their differentiation potential is not known,but even MSC transfected with telomerase gradually decreased its osteogenic potential with the increase in the passage number [5],although their replicativecapacity was maintained.In contrast,MSC on bmECM maintained the differentiation potential even after they lost their replicative capacity.Thus,the extracellular matrix and telomerase may have complementary effects on the proliferation of MSC and their differentiation potential.In any case,the remarkable effects of bmECM in MSC cultures demonstrated here meet the expecta-tions of doctors eager to expand MSC extensively in vitro from a small volume of marrow aspirates before transplantation.Henceforth,bmECM will be prepared using human ES or human cell lines,and in the near future bmECM-coated dishes will probably have a great use in MSC studies and regeneration medicine.References[1]M.F.Pittenger, A.M.Mackay,S.C.Beck,R.K.Jaiswal,R.Douglas,J.D.Mosca,M.A.Moorman,D.W.Simonetti,S.Craig,D.R.Marshak,Multilineage potential of adult human mesenchy-mal stem cells,Science 284(1999)143–147.[2]R.J.Deans,A.B.Moseley,Mesenchymal stem cells:biology and potential clinical uses,Exp.Hematol.28(2000)875–884.[3]S.Tsutsumi,A.Shimazu,K.Miyazaki,H.Pan,C.Koike,E.Yoshida,K.Takagishi,Y.Kato,Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF,mun.288(2001)413–419.[4]A.Muraglia,R.Cancedda,R.Quarto,Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model,J.Cell Sci.113(2000)1161–1166.[5]S.Shi,S.Gronthos,S.Chen,A.Reddi,C.M.Counter,P.G.Robey,C.Y.Wang,Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression,Nat.Biotechnol.20(2002)587–591.[6]A.Oohira,T.N.Wight,J.McPherson,P.Bornstein,Biochemical and ultrastructural studies of proteoheparan sulfates synthesized by PYS-2,a basement membrane-producing cell line,J.Cell Biol.92(1982)357–367.[7]B.Tyree,E.A.Horigan,D.L.Klippenstein,J.R.Hassell,Heter-ogeneity of heparan sulfate proteoglycans synthesized by PYS-2cells,Arch.Biochem.Biophys.231(1984)328–335.[8]J.R.Couchman,A.Woods,M.Hook,J.E.Christner,Character-ization of a dermatan sulfate proteoglycan synthesized by murine parietal yolk sac (PYS-2)cells,J.Biol.Chem.260(1985)13755–13762.[9]D.Gospodawowicz,A.L.Mesher,C.R.Birdwell,Stimulation of corneal endothelial cell proliferation in vitro by fibroblast and epidermal growth factors,Exp.Eye Res.137(1977)15–23.[10]D.Gospodarowicz,R.Gonzalez,D.K.Fujii,Are factors origi-nating from serum,plasma,or cultured cells involved in the growth-promoting effect of the extracellular matrix produced by cultured bovine corneal endothelial cells?J.Cell.Physiol.114(1983)191–202.[11]A.Martinez-Hernandez,A.E.Chung,The ultrastructural local-ization of two basement membrane components:entactin and laminin in rat tissues,J.Histochem.Cytochem.32(1984)289–298.[12]E.Hahn,G.Wick,D.Pencev,R.Timpl,Distribution of basementmembrane proteins in normal and fibrotic human liver:collagen type IV,laminin,and fibronectin,Gut 21(1980)63–71.Fig.3.Effects of bmECM on proliferation of MSC and their chon-drogenic potential in cultures with human serum.(A)Human MSC,in the presence of 10%human serum,showed faster proliferation and a longer proliferative life span on bmECM than on plastic.Cells in marrow aspirates (1ml/100-mm dish)from the ilium were seeded and maintained on plastic tissue culture dishes,as described in the legend of Fig.1.The first passage was performed on plastic at a seeding density of 5000cells/cm 2.In this study,MSC obtained from the 1st passage cultures on plastic were used for experimentation.The isolated cells were seeded on bmECM (endothelial)or on plastic as described in Materials and methods.FGF at 1ng/ml was added to some cultures on plastic.*;**Differs significantly from the cell number in cultures on plastic dishes with FGF (“FGF”)or without FGF (“plastic”)on days 66–87(*p <0:05,**p <0:01).The MSC from 66-day-old cultures on bmECM (B)or plastic (C)were transferred into the chondrogenic status in pellet cultures for 28days and stained with toluidine blue.T.Matsubara et al./Biochemical and Biophysical Research Communications 313(2004)503–508507[13]R.W.Farndale,C.A.Sayersm,A.J.Barrett,A direct spectropho-tometric microassay for sulfated glycosaminoglycans in cartilage cultures,Connect.Tissue Res.9(1982)247–248.[14]O.A.Bessey,O.H.Lowry,O.H.Brock,A method for the rapiddetermination of alkaline phosphatase withfive cubic millimeters of serum,J.Biol.Chem.164(1946)321–329.[15]H.J.Gitelman,An improved automated procedure for thedetemination of calcium in biological specimens,Anal.Biochem.18(1967)521–531.[16]W.Guo,J.K.Choi,J.L.Kirkland,B.E.Corkey,J.A.Hamilton,Esterification of free fatty acids in adipocytes:a comparison between octanoate and oleate,Biochem.J.349(2000)463–471.[17]P.Kaur,A.Li,Adhesive properties of human basal epidermalcells:an analysis of keratinocyte stem cells,transit amplifying cells,and postmitotic differentiating cells,J.Invest.Dermatol.114 (2000)413–420.[18]D.R.Campion,The muscle satellite cell:a review,Int.Rev.Cytol.87(1984)225–251.508T.Matsubara et al./Biochemical and Biophysical Research Communications313(2004)503–508。

细胞外基质的结构和功能细胞外基质是细胞外的一种重要结构,它由一系列互相作用的分子组成,包括纤维蛋白、胶原蛋白、基质分子和其他结构蛋白。
细胞外基质在支持细胞形态、细胞活动、刺激细胞分化和维持细胞功能方面都具有重要作用。
在本文中,我们将介绍细胞外基质的结构和功能,以及它的关键作用。
一、解剖学细胞外基质由胶原纤维、弹性纤维、基质分子和其他结构蛋白组成。
胶原纤维是最主要的分子,它是一种硬、纤维状蛋白,可支持许多类型的细胞。
弹性纤维包含一种富含弹性的蛋白质,支持一些需要伸展或缩回的组织,如大动脉。
基质分子通常是链多糖,它们和其他分子一起构成细胞外基质的基础。
其他结构蛋白包括黏附分子,它们能够使细胞在基质上停留,支持细胞和基质之间的相互作用。
二、功能1. 细胞形态的维护细胞外基质能够提供结构上的支撑和细胞定位,这对于维持细胞的形态和空间组织结构非常重要。
细胞所处的环境和周围物质的物理属性和化学组成都可以影响细胞形态。
2. 细胞活动细胞外基质直接影响细胞运动、黏附和迁移。
这些活动关键地依赖于细胞表面的黏附分子,在与基质分子和胶原纤维之间形成联系。
在危及健康的过程中,某些蛋白质性簇可以释放到细胞外,促进细胞黏附和迁移,并启动某些类型的炎症反应。
3. 刺激细胞分化细胞外基质对细胞分化和发展方面也有着深远的影响。
它们可以影响干细胞和基质干细胞的行为,导致它们不断分裂和成熟,最终,要么不再分裂,要么分化成特定细胞类型。
4. 维持细胞功能细胞外基质还可以通过持续、高依赖性的生化互动和信号传递,包括通过细胞表面和细胞内受体调控特定信号通路,以维持细胞功能。
三、关键作用尽管细胞外基质对于细胞形态的维持、细胞的活动、细胞分化和发展以及细胞功能的维持都有重要作用,但是它可能最为重要的也许是其在细胞外病理过程中的重要作用。
例如,固态肿瘤生长与菌丝细胞之间的交互作用,常常会受到基质分子的影响。
良性肿瘤和恶性肿瘤发展的情形也很大程度上受到细胞外基质的影响。
细胞外基质
细胞外基质(extracellular matrixc,ECM),是由动物细胞合成并分泌到胞外、分布在细胞表面或细胞之间的大分子, 主要是一些多糖和蛋白, 或蛋白聚糖。
这些物质构成复杂的网架结构,支持并连接组织结构、调节组织的发生和细胞的生理活动。
细胞外基质是动物组织的一部分, 不属于任何细胞。
它决定结缔组织的特性,对于一些动物组织的细胞具有重要作用。
定义1:存在于组织中,由细胞合成并分泌至胞外的成分,包括纤维性成分(胶原蛋白、弹性蛋白和网织蛋白)、连接蛋白(纤维粘连蛋白、层粘连蛋白)和空间充填分子(主要为糖胺聚糖)等,其对细胞增殖和分化发挥重要调控作用。
定义2:与细胞表面基底膜缔合的存在于细胞外空间的筛网状物质,由蛋白质和多糖等大分子构成。
可促进细胞增殖,并为细胞提供支持结构。
定义3:由细胞分泌到细胞外间充质中的蛋白质和多糖类大分子物质。
构成复杂的网架,连接组织结构、调节组织的发育和细胞生理活动。
定义4:由细胞产生并分泌到细胞外周质中的物质,主要包括纤维成分(如胶原和弹性蛋白)、连接蛋白(如纤连蛋白等)和填充分子(通常是糖胺聚糖)等。
细胞外基质的特性常决定组织的特性。
细胞的细胞外基质细胞是构成生物体的基本单位,除了细胞本身的细胞质,还存在着细胞外基质。
细胞外基质是一种复杂的结构,由许多不同成分组成。
本文将介绍细胞外基质的定义、成分及其在生物体中的重要功能。
一、细胞外基质的定义细胞外基质是指细胞外的一种胶状物质,包围在细胞周围,并与细胞直接接触。
它起到细胞支撑、细胞间通信和细胞附着等重要作用。
细胞外基质的主要成分为纤维蛋白和胶原蛋白。
二、细胞外基质的成分1. 纤维蛋白:纤维蛋白是细胞外基质中的主要成分之一,主要由胶原聚合而成。
它具有良好的弹性和抗拉性,能够提供细胞支撑和保护作用。
纤维蛋白还能够帮助细胞附着并参与组织修复过程。
2. 胶原蛋白:胶原蛋白是一种结构丰富的蛋白质,其分子具有长链结构。
胶原蛋白是细胞外基质中最丰富的一种蛋白质,它具有细胞附着的能力,可以帮助细胞粘附在周围的基质上。
3. 糖类和多糖类物质:细胞外基质中还含有多种糖类和多糖类物质,如葡萄糖、核酸和糖蛋白等。
糖类和多糖类物质具有保湿和润滑的作用,能够维持细胞外基质的稳定性。
同时,它们还能够参与细胞信号传导和细胞间通讯。
三、细胞外基质的功能1. 细胞支撑:细胞外基质能够提供细胞的结构支持,帮助细胞保持形状的稳定性。
纤维蛋白和胶原蛋白的存在使得细胞外基质具备了一定的刚性和弹性,有效支撑和保护细胞。
2. 细胞间通讯:细胞外基质中的糖类和多糖类物质能够参与细胞间的信号传导和通讯。
这些物质能够调节细胞的生长、分化和迁移等过程,维持组织和器官的正常功能。
3. 细胞附着:细胞外基质中的纤维蛋白和胶原蛋白能够帮助细胞附着在周围的基质上。
细胞附着是细胞生存和繁殖的基础,它使得细胞能够保持在正确的位置,并参与组织修复和器官再生的过程。
4. 组织修复:细胞外基质对于组织修复起到重要的作用。
当组织受到损伤时,细胞外基质能够为损伤区域提供支持和结构,促进细胞的迁移和增殖,帮助组织恢复正常功能。
细胞外基质是细胞活动中不可或缺的一部分,它通过提供支持、参与信号传导和调节细胞间通讯等方式,保持了生物体的结构稳定和正常功能。
细胞外被及外基质4.3.1细胞外被细胞表面包括细胞被(cell coat)和细胞质膜1.概念:细胞质膜通常是由覆盖在细胞表面的保护层保护着,这种保护层即是细胞被。
由于这层结构的主要成份是糖,所以又称为糖萼(glycocalyx)。
2.成分:糖蛋白和蛋白聚糖。
这些糖蛋白和蛋白聚糖都是在细胞内合成的,然后分泌出来并附着到细胞质膜上。
3.作用(1)保护:如消化道、呼吸道、生殖腺等上皮细胞的外被有助于润滑、防止机械损伤,同时又可保护上皮组织不受消化酶的作用和细菌的侵袭。
植物和细菌的细胞壁不仅可以保护细胞质膜和细胞器,同时还赋予细胞以特定的形状。
革兰氏阳性菌的细胞壁是一种蛋白聚糖,图4-15细胞的外被青霉素通过抑制它的合成从而抑制细菌的生长。
(2)参与细胞与环境的相互作用,参与细胞与环境的物质交换,细胞增殖的接触抑制、细胞识别等。
4.3.2细胞外基质植物细胞的表面结构是细胞壁,而动物细胞的表面结构是细胞外基质1.外基质概念:分布于细胞外空间(如细胞之间或细胞表面),由细胞分泌的蛋白和多糖构成的网络结构。
2.外基质成分(1)结构蛋白——胶原蛋白,弹性蛋白。
它们赋予细胞外基质一定的强度和韧性,并赋予组织抗张力。
(2)连接蛋白——层粘连蛋白,纤连蛋白。
它们促使细胞同基质结合。
(3)多糖——胺聚糖和蛋白聚糖。
它们能够形成水性的胶状物,增加组织耐压性。
3.外基质功能(1)结构蛋白赋予组织弹性和柔韧性,使组织具有抗张力(2)多糖吸水向外膨胀,使组织具有抗压力(3)连接蛋白将细胞外基质通过跨膜蛋白与细胞连接起来概括为:细胞间粘着;保护作用;维持细胞外环境(调节细胞周围的物质浓度);过滤作用等等。
在形态发生中作用重大,包括:细胞迁移、增殖、形态变化、分化、保护、组建等4.结构:蛋白作为基质的骨架和连接细胞,多糖填充其间。
细胞外基质细胞外基质(extracellular matrix,ECM)是由大分子构成的错综复杂的网络。
为细胞的生存及活动提供适宜的场所,并通过信号转导系统影响细胞的形状、代谢、功能、迁移、增殖和分化。
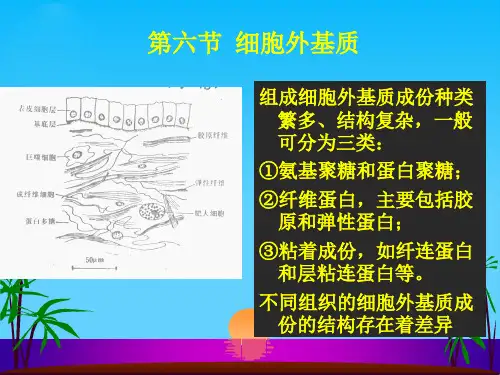
细胞外基质的成分构成细胞外基质的大分子种类繁多,可大致归纳为四大类:胶原、非胶原糖蛋白、氨基聚糖与蛋白聚糖、以及弹性蛋白(图10-1,2)。
上皮组织、肌组织及脑与脊髓中的ECM含量较少,而结缔组织中ECM含量较高。
细胞外基质的组分及组装形式由所产生的细胞决定,并与组织的特殊功能需要相适应。
例如,角膜的细胞外基质为透明柔软的片层,肌腱的则坚韧如绳索。
细胞外基质不仅静态的发挥支持、连接、保水、保护等物理作用,而且动态的对细胞产生全方位影响。
图10-1 细胞外基质的成分图10-2 上皮组织的细胞外基质一、胶原(collagen)胶原是动物体内含量最丰富的蛋白质,约占人体蛋白质总量的30%以上。
它遍布于体内各种器官和组织,是细胞外基质中的框架结构,可由成纤维细胞(图10-3)、软骨细胞、成骨细胞及某些上皮细胞合成并分泌到细胞外。
图10-3 成纤维细胞周围的胶原纤维目前已发现的胶原至少有19种(表10-1),由不同的结构基因编码,具有不同的化学结构及免疫学特性。
Ⅰ、Ⅱ、Ⅲ、Ⅴ及Ⅺ型胶原为有横纹的纤维形胶原。
各型胶原都是由三条相同或不同的肽链形成三股螺旋,含有三种结构:螺旋区,非螺旋区及球形结构域。
其中Ⅰ型胶原的结构最为典型。
表10-1 胶原的类型图10-4 胶原的结构(左模式图,右电镜照片)Ⅰ型胶原的原纤维平行排列成较粗大的束,成为光镜下可见的胶原纤维,抗张强度超过钢筋。
其三股螺旋由二条α1(Ⅰ)链及一条α2(Ⅰ)链构成。
每条α链约含1050个氨基酸残基,由重复的Gly-X-Y序列构成。
X常为Pro(脯氨酸),Y常为羟脯氨酸或羟赖氨酸残基。
重复的Gly-X-Y序列使α链卷曲为左手螺旋,每圈含3个氨基酸残基。