脒类化合物的合成工艺研究进展
- 格式:pdf
- 大小:1.02 MB
- 文档页数:4

取代苯甲脒的合成及取代基效应研究
苯甲脒是一种常见的有机化合物,广泛用作杀虫剂。
然而,由于
其对环境和人体健康的潜在风险,寻找替代品已成为重要的研究方向。
近年来,许多研究集中在合成替代苯甲脒化合物并研究其取代基效应。
本文将分步骤阐述这方面的研究最新进展。
第一步:合成替代苯甲脒化合物
为了合成替代苯甲脒化合物,研究人员通常采用2-氨基苯酚或其衍生物作为出发物,通过重氮化或碳酸酯的改性反应,生成目标化合物。
例如,即使在水中也可以使用钠硝酸盐和铁粉将苯胺类物质硝化
成重氮化合物,可以提高产率和选择性。
此外,还有学者基于互变性
和多组分反应策略,构建了一些新的苯甲脒类物质,比如3-(2-氰基
甲酰基)苯酚。
第二步:研究替代基对杀虫活性的影响
不同的取代基对苯甲脒的生物活性有重要影响。
研究人员发现,
在取代苯甲脒时,将部分甲基取代为环氧乙烷,可以增加其杀虫活性。
此外,杂原子的引入也可能提高其活性。
例如,酰胺类取代基可影响
杀虫活性,导致苯甲脒类化合物的不同药效。
其中,氟苯基、硫酰基
和烯丙基等取代基都被证明具有良好的抗虫作用。
第三步:探索扩展应用
除了作为杀虫剂外,取代苯甲脒化合物还有许多潜在应用。
例如,它们可以作为染料、光催化剂和液晶材料的前体,这些应用领域也值
得未来深入研究探索。
总之,合成替代苯甲脒化合物并研究其取代基效应,已经成为当
前有机合成领域的热点之一。
未来,我们可以进一步探索这方面的研究,以寻找更好的苯甲脒类物质替代品,以实现更安全的杀虫和其他
应用。

Pinner脒合成的反应机理及应用进展王阳阳(西北农林科技大学理学院陕西杨凌712100)摘要:脒类化合物在农药、医药以及其他领域上都具有很广泛的用途。
合成脒类化合物的方法主要为:Pinner脒合成法。
本文重点介绍了Pinner脒合成方法的机理和副反应机理,并对其在有机合成中的应用进行了探讨。
关键词:Pinner脒合成;机理;改进;应用The reaction mechanism and application of Pinner amidinesynthesisWang Yangyang(College of science, Northwest A&F University, Yangling, 712100, China)Abstract:The amidine compounds have a very wide range of functions in the pesticide, medicine and other fields. The primary method of synthesis of amidine compounds is Pinner amidine synthesis. This article focuses on the reaction mechanism of Pinner amidine synthesis and the side reactions mechanism Its application in organic synthesis is also discussed.Key words: Pinner amidine synthesis; mechanism; improvement; application1.前言脒类化合物在农药和医药上具有很广泛的用途。
早年发现某些脒盐可以治疗血吸虫病,但毒性较大,一些长链烷氧基取代的苯甲脒盐具有表面活性剂的作用,被称为杀虫脒[1]。
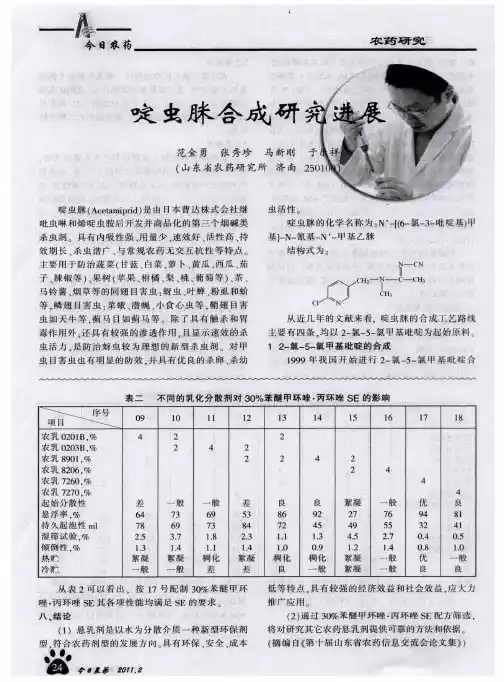

第9卷第2期 南阳理工学院学报 V 〇1.9N 〇. 22 0 1 7 年 3 月J O U R N A L O F N A N Y A N G INSTITUTE O F T E C H N O L O G YMar . 2017微波辅助下的脒类化合物的合成与表征李奎杰,,刘春秀,杨晓宇,王世鹏!工技术大学化学化工学院201620)摘要:本文以芳香胺为起始原料,在微波辅助条件下,发展了一种简便、高效的合成脒类化合物的新方法;通过该方法合成得到了 3类不同母核结构的脒类产物,反应收率最高的达到95%。
所有目标化合物均进行了核磁1HN M R 和LC - M S 表征和确认。
关键词:微波;芳香胺;合成中图分类号:O621文献标识码:A文章编号:1674 -5132(2017)02 -0109 -03〇引言随着医药技术的发展,脒类化合物在农药、医药上的用 。
二脒 合 非洲锥和利原虫症有一定的治 异用[1’2]。
芳香二脒 合物不仅具有抗原,而且表现出杀虫及抗细菌、真菌、病毒和肿瘤的活性[3,4]。
合成脒 合物的方法主要有酰胺合成法[5(、超声合成法[6(、合成法等[7]。
些合成方法往往合成步骤多、后处理复杂、收率低I题。
微波辅助合成方法是近些年来的一种 机合成方法,本文通过微波辅助的方法,发展了一种简 、高效的合成脒类化合物的新方法。
通过该方法 合了 3的脒类产物,最高收率达到95%。
1实验部分1.1仪器和试剂主要仪器:Bruker Advance I I 400 M H z 核磁;安捷伦1200液相;紫外分析仪(Z F —I 型三用紫外分 仪,上海顾村 仪器厂)。
主要 :苯胺(分析纯,上海国药集团);原甲酸三甲酯(分析纯,药集团); 基苯胺(分析纯,阿 );基 (分析纯,阿);邻苯二(纯,安耐 );2-胺基苄胺(纯,安耐);(分析纯,上海国药集团);无水 手册步骤处理。
1.)化合物)a -2e的合成脒的合成路线如图1所示,以芳香胺(化合物 1a -e )始原料,在甲酸做的条件下,与原酯经过缩合脱水,得到目标产物(化合物 2a - e )。

丙烷脒中间体的合成工艺研究的开题报告题目:丙烷脒中间体的合成工艺研究一、研究背景及意义丙烷脒是一种重要的有机合成中间体,在生物医药、染料、涂料、塑料等领域有着广泛的应用。
其中,丙烷脒的合成工艺研究成为了当前有机化学领域的热点之一。
丙烷脒的合成有多种方法,但目前针对某些用途所需丙烷脒的化学纯度和产率需求,对其生产工艺提出了更高的要求。
因此,探究一种高效、环保、经济、简便的丙烷脒制备工艺具有重要的现实意义。
二、研究内容和目标本课题旨在通过合理的工艺设计和反应条件优化,建立一种高产、高纯度、高效、环保、经济、简便的丙烷脒合成工艺,并验证其可行性。
具体研究内容包括:1. 确定丙烷脒的最佳制备路线及反应机理;2. 筛选和优化反应条件以提高丙烷脒的收率和纯度;3. 通过不同方法分离纯化丙烷脒,比较分析各种技术对产物纯度和收率的影响,寻找最优方案;4. 对所得的丙烷脒进行结构表征和性质测试。
三、研究方法和计划本课题将采用实验室合成结合理论计算的方法,通过反应机理分析、反应条件优化实验、产物纯化实验、结构表征实验等多种手段,逐步建立完整、可行的丙烷脒合成工艺。
具体计划如下:1. 文献研究及实验前准备(2个月);2. 确定丙烷脒制备路线和反应机理,设计反应条件和实验方案(2个月);3. 进行反应条件优化实验,选取最佳反应条件(3个月);4. 进行产物纯化实验,比较分析各种技术对产物纯度和收率的影响,寻找最优方案(3个月);5. 对所得的丙烷脒进行结构表征和性质测试(2个月);6. 整理实验数据,撰写研究论文(2个月)。
四、预期结果本研究预期可以建立一种高产、高纯度、高效、环保、经济、简便的丙烷脒合成工艺,具体表现为:1. 实现丙烷脒的高产率和高纯度制备;2. 对技术路线和反应机理的探究和机制解析;3. 对所得丙烷脒的结构表征和性质测试结果。
五、参考文献1. Bal R. et al. Preparation of N-methylpropanamine catalyzed by acidic ionic liquids. Journal of Molecular Liquids, 2016, 220: 353-358.2. Guo K. et al. Facile synthesis of aliphatic amine derivatives by nickel-catalyzed intermolecular reductive amination of aldehydes with ammonia or amine gas. Journal of the American Chemical Society, 2017, 139(48): 17261-17264.3. Wang W. et al. Synthesis and biological evaluation of new 1,2-oxazole-based compounds on angiogenesis. Journal of Heterocyclic Chemistry, 2017, 54(4): 2254-2259.。

基于脒的嘧啶杂环化合物的合成研究
本文主要介绍基于脒的嘧啶杂环化合物的合成研究。
脒是一种重要的有机化合物,具有广泛的应用价值。
嘧啶杂环化合物是一类具有重要生物活性的化合物,具有抗肿瘤、抗病毒、抗菌等多种药理作用。
因此,合成嘧啶杂环化合物具有重要的意义。
本文首先介绍了脒的化学性质和合成方法,然后详细阐述了基于脒的嘧啶杂环化合物的合成方法。
其中,涉及了多种反应,如催化氢化、单质氨化、环化等反应。
本文还介绍了嘧啶杂环化合物的生物活性和应用前景,以及合成方法的改进和优化研究。
最后,本文总结了基于脒的嘧啶杂环化合物的合成方法研究的现状和发展趋势,并展望了其在药物研发和合成化学领域的重要作用。
- 1 -。


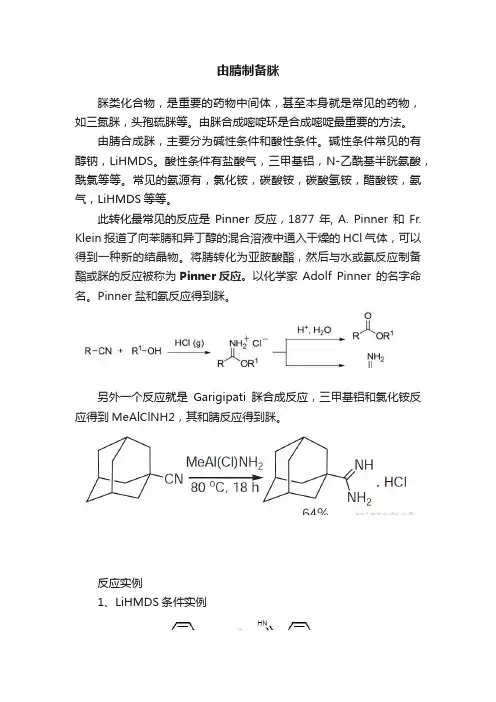
由腈制备脒脒类化合物,是重要的药物中间体,甚至本身就是常见的药物,如三氮脒,头孢硫脒等。
由脒合成嘧啶环是合成嘧啶最重要的方法。
由腈合成脒,主要分为碱性条件和酸性条件。
碱性条件常见的有醇钠,LiHMDS。
酸性条件有盐酸气,三甲基铝,N-乙酰基半胱氨酸,酰氯等等。
常见的氨源有,氯化铵,碳酸铵,碳酸氢铵,醋酸铵,氨气,LiHMDS等等。
此转化最常见的反应是Pinner反应,1877年, A. Pinner和Fr. Klein报道了向苯腈和异丁醇的混合溶液中通入干燥的HCl气体,可以得到一种新的结晶物。
将腈转化为亚胺酸酯,然后与水或氨反应制备酯或脒的反应被称为Pinner反应。
以化学家Adolf Pinner 的名字命名。
Pinner盐和氨反应得到脒。
另外一个反应就是Garigipati脒合成反应,三甲基铝和氯化铵反应得到MeAlClNH2,其和腈反应得到脒。
反应实例1、LiHMDS条件实例to a 50 mL dry reaction flask charged with 1 M LiHMDS in THF (22 mmol), p- chlorobenzonitrile (2.76 g, 20.0mmol) in 2 mL of THF is added, and the reaction mixture is kept stirring at RT for 4 h, at which point 5-6 N HCI (in/PrOH, 15 mL) is added. The crude reaction mixture is kept at 0oC overnight.The precipitated product is filtered, washed with diethyl ether to yield 3.5 g (93percent) of the compound of formula 2b as a white solid, m.p. 238 oC (litm.p. 243-245oC) (E. Ragona, D. L. Nelson, M. Mares-Guis, J. Amer. Chem. Soc. EPO 1975, 97, 6844-6848). - IR (KBr): nu(tilde) = 3239 crrf1, 3054, 1678, 1460, 1401 , 1036, 715. - 1H NMR (250 MHz, [D6]DMSO): δ = 7.60-7.77 (m, 2 H), 7.85-7.97 (m, 2 H), 8.4 (br. s, 3 H, NH). - 13C NMR (62.9 MHz, [D6]DMSO), δ = 126.79(Cquat), 129.36 (+), 130.57 (+), 139.1 (Cquat), 165.1 (NCN).【Patent; MERCK PATENT GMBH; WO2006/94604; (2006); (A1) English】2、甲醇钠条件下反应To a solution of sodium methoxide (5.55 mmol) in methanol (50 mL) was added 2- furonitrile (5.0 g, 53.2 mmol, leq.). The mixture was stirred at room temperature for 3 hours. To the resulting solution was slowly added ammonium chloride (3.14 g, 58.7 mmol, l.leq.) and the mixture was stirred at room temperature for 68 hours. The resulting suspension was filtered and the solvent removed under reduced pressure. The solid obtained was washed with ethyl ether (3x25 mL) to give 7.5 g (96percent yield) of 2- furancarboxamidine (HCl). δ (200 MHz, DMSO-d6):6.88-6.86 (m, IH); 7.89 (d, J=3.8 Hz, IH); 8.19 (s, IH);9.22 (s, 3H).【Patent; NEUROCRINE BIOSCIENCES, INC.; LABORATORIOS ALMIRALL, S. A.; WO2008/70661; (2008); (A1)English】3、盐酸气条件下反应100 gm(0.207 mol) Ethyl3 - { [(2- { [(4-cyanophenyl)amino]methyl } - i-methyl-i Hbenzimidazol-5 -yl)carbonylj(pyridin-2-yl)amino} propanoate was added to 500 ml ethanolic hydrochloride solution having HCl content between 34 to 36 at temperature not more than 35°C. Upon domplete addition reaction mixture was stirred for 10-15 mm. and temperature of reaction was raised to 38-42°C. Completion of reaction was monitored by HPLC. Reaction mixture was then cooled to 25-30oC and diluted with 1500 ml ethanol. reaction mixture was then cooled to 0-5°C and was purged with ammonia gas till to achieve neutral pH. To the reaction mixture was added 75 gm ammonium carbonate and reaction mixture was warmed to 28-32°C and stirred for 10-12 hr. completion of reaction was monitored by HPLC. Reaction mixture was filtered and the residue was washed with ethanol 500 ml. combined filtrate was concentrated under vacuum to obtain residue. To the obtained residue was added 500 ml ethanol and 1000 ml ethyl acetate. Mixture was refluxed for 30 mm. and then cooled to 25-30°C. precipitated product was stirred for 60 mm. at 25-30°C and then filtered and dried at 40-45°C to obtain crude Ethyl3-{[(2-{[(4- {carbamimidoyl } phenyl)amino]methyl} -1-methyl-i H-benzimidazol-5-yl)carbonyl](pyridin-2-yl)amino }propanoate.Yield: l00g (96.61percent); Purity by HPLC: 95.3 percent.【Patent; MEGAFINE PHARMA (P) LTD.; MATHAD, Vijayavitthal Thippannachar; SOLANKI, Pavankumar Vrajlal; UPPELLI, Sekhar Babu; SARODE, Ganesh Gitaram; WO2015/128875; (2015); (A2) English】【Synthesis2003, 1603–1609】【Tetrahedron2008, 64, 11594–11602】4、N-乙酰基半胱氨酸条件In four flask equipped with a mechanical stirrer, thermometer, reflux condenser. take For bromoxynil 36.4g (0.2mol) was poured into a beaker, add 100ml of absolute ethanol, stirring to dissolve completely; then weighed 32.4g N- acetylcysteine (0.2mol), into another beaker was then added 150ml of absolute ethanol, stirring the mixture to complete dissolution, then a solution of ethanol will bromoxynil and N- acetylcysteine ethanol solution was poured into four flask, stirred and heated to 45 The reaction 20h . Weigh 8.6g ammonium carbamate (0.11 mol), was slowlyadded portionwise to a 4-neck flask, 20h, after complete dissolution the reaction, the reactionAfter completion of standing 48h, suction filtered, the filter cake was washed with ethanol, and drying, a white solid product was obtained 38g of bromobenzene formamidine (Compound ), yield 95.4percent, HPLC purity was 99.6percent.【Patent; Shandong Luoxin Pharmaceutical Co., Ltd.; Zhao, Jinlong; Yu, Defeng; Zhang, Guijie; (15 pag.);CN105669651; (2016); (A) Chinese】Preparation of ethyl 3-(2-((4-carbamimidoylphenylamino)methyl)-l-methyl-N- (pyridin-2-yl)-IH-benzo[d]imidazole-5-carboxamido) propanoate of Formula (ll)using N-acetyl cysteine 10 g (0.020 mol) of ethyl 3-(2-((4-cyanophenylamino)methyl)- l-methyl-N- (pyridin-2-yl)-IH- benzo[d]- imidazole-5-carboxamido) propanoate of Formula (IV) was dissolved in 600 ml EtOH.NH3 (15-18percentw/w) and stirred at 25°C. Added 3.38 g (0.020 mol) of N-acetylcysteine to the reaction mass and stirred for 24 hours at 70-75°C under 2.0-2.3 kg of pressure. The ethanol was distilled under vacuum and residue was purified by column. Yield: 5.5 g Efficiency: 53percent【Patent; CIPLA LIMITED; KING, Lawrence; RAO, Dharmaraj Ramachandra; MALHOTRA, Geena; PULLELA, Venkata Srinivas; ACHARYA, Vinod Parameshwaran; SINARE, Sudam Nanabhau; (43 pag.); WO2016/27077;(2016); (A1) English】A solution of Boc-Aze-NH-CH2-((5-cyano)-2-pyrimidinyl)(0.83 g, 2.6 mmol; see step (iv) above), N-acetylcysteine (0.43 g, 2.6 mmol) and ammonium acetate (0.60 g, 7.8 mmol) in 10 mL of methanol was heated at 60° C. under nitrogen for 2 days. The solvent was evaporated and the crude material was purified by preparative RPLC using a gradient of CH3CN: 0.1M NH4OAc (5:95 to 100:0).The fractions of interest were freeze dried to give 1.0 g (93percent) of the desired material. 1H NMR (300 MHz, D2O, signals obscured by the HDO signal) δ9. 17 (s, 2H), 4.1-3.9 (m, 2H), 2.60 (m, 1H), 2.29 (m,1H), 1.93 (s, 3H), 1.44 (s, 9H) 【Patent; Inghardt, Tord; Johansson, Anders; Svensson, Arne; US2004/19033; (2004); (A1) English】5、三甲基铝条件下反应14.5 mL (29 mmol) of trimethyl aluminum (2.0 M toluene solution) was dropwise added to a 20 mL of toluene containing 1.55 g (28.9 mmol) of ammonium chloride at room temperature. After stirring for 1.5 hours, 2 g (28.9mmol) of isobutironitrile was added thereto and the resulting mixture was heated to 85°C for 9 hours. After completion of a reaction, the reaction solution was poured into 200 mL of chloroform containing 500 g of silicagel and filtered. The residue was washed with 200 mL of methanol and distillation was conducted to give 2.3 g (26.7 mmol) of the title compound in a yield of 92percent. Mass(EI) 87(M++.)【Patent:LG LIFE SCIENCES, LTD.; WO2006/104356; (2006); (A1) English】Adamantane amidine hydrochloride (5). Cold Me3Al 1 (25 mL, 50 mmol) in PhMe was added gradually with stirring to a suspension of NH4Cl 2 (2.9 g, 54 mmol) in dry PhMe (20 mL) at 5 C under N2. The mixture was warmed to r.t. and stirred for 2 h until CH4 evolution stopped, the cyano adamantane 4 (4.83 g, 30 mmol) was added in 10 mL PhMe and the mixture was heated to 80 C under Ar for 18 h (TLC). The mixture was poured into a slurry of SiO2 (15 g) and CHCl3 (50 mL) and after 5 min silica was filtered and washed with MeOH and the combined solvents were concentrated to 15 mL. NH4Cl was filtered off and MeOH/HCl (10 mL, conc 2 g, 54 mmol) followed by Et2O (400 mL)was added. After 10 h of stirring, filtration provided 5.8 g of crude 5. Recrystallization (4:1 iPrOH: Me2CO) afforded 4.1 g of 5 (64%), mp 257–259 C.【Moss RA, Tet Lett., 1995, 36, 8761】14.5 mL (29 mmol) of trimethyl aluminum (2.0 M toluene solution) was dropwise added to a 20 mL of toluene containing 1.55 g (28.9 mmol) of ammonium chloride at room temperature. After stirring for 1.5 hours, 2 g (28.9mmol) of isobutironitrile was added thereto and the resulting mixture was heated to 85°C for 9 hours. After completion of a reaction, the reaction solution waspoured into 200 mL of chloroform containing 500 g of silicagel and filtered. The residue was washed with 200 mL of methanol and distillation was conducted to give 2.3 g (26.7 mmol) of the title compound in a yield of 92percent. Mass(EI) 87(M++.) 【Patent:LG LIFE SCIENCES, LTD.; WO2006/104356; (2006); (A1) English】6、乙酰氯条件To a solution of 3-(3-bromo-4-fluorophenyl)propanenitrile (5.0 g, 21.05 mmol) in toluene (20 mL) and methanol (6.32 mL, 156 mmol) at 0°C was added acetyl chloride (7.51 mL, 105 mmol) dropwise over 5 min. The reaction mixture was allowed to warm to room temperature and stirred for 2 h. The mixture was cooled to 0°C by an ice bath, to which ammonia (30.1 mL, 210 mmol) was added dropwise. The reaction mixture was stirred at room temperature overnight. The mixture was filtered, and the filtrate was concentrated to give a crude product. Recrystallization from toluene/methanol (1: 1) then afforded the title compound as a white solid (5.2 g, 93 percent yield). [M+H+] =245【Patent; GLAXO GROUP LIMITED; JIN, Yun; WAN, Zehong; ZHANG, Qing; WO2012/76435; (2012); (A1) English】。

Pinner脒合成的反应机理及应用进展王阳阳(西北农林科技大学理学院陕西杨凌712100)摘要:脒类化合物在农药、医药以及其他领域上都具有很广泛的用途。
合成脒类化合物的方法主要为:Pinner脒合成法。
本文重点介绍了Pinner脒合成方法的机理和副反应机理,并对其在有机合成中的应用进行了探讨。
关键词:Pinner脒合成;机理;改进;应用The reaction mechanism and application of Pinner amidinesynthesisWang Yangyang(College of science, Northwest A&F University, Yangling, 712100, China)Abstract:The amidine compounds have a very wide range of functions in the pesticide, medicine and other fields. The primary method of synthesis of amidine compounds is Pinner amidine synthesis. This article focuses on the reaction mechanism of Pinner amidine synthesis and the side reactions mechanism Its application in organic synthesis is also discussed.Key words: Pinner amidine synthesis; mechanism; improvement; application1.前言脒类化合物在农药和医药上具有很广泛的用途。
早年发现某些脒盐可以治疗血吸虫病,但毒性较大,一些长链烷氧基取代的苯甲脒盐具有表面活性剂的作用,被称为杀虫脒[1]。

脒的合成及应用教案脒是一种有机化合物,化学式为R-CN,其中R代表一种有机基。
它是由一个氰基和一个有机基联结而成的,由于氰基中的碳原子与氮原子之间的三键,使得脒具有较高的稳定性和惰性。
一、脒的合成方法:1. 酰氯与氨的反应:通过酰氯与氨反应可以得到脒。
反应过程中,氨与酰氯发生取代反应,生成脒和HCl。
2. 酰异氰酸酯与胺的反应:酰异氰酸酯与胺反应也可以得到脒。
反应过程中,酰异氰酸酯发生取代反应,生成脒和酯。
3. 先对羟基化合物的氨解反应,再与硝酸银反应:先将羟基化合物进行氨解反应,生成相应的胺,然后与硝酸银反应,生成脒和相应的硝酸盐。
二、脒的应用:1. 蒸汽动力机械:脒是一种常用的溶剂,它可以在高温高压的情况下作为蒸汽动力机械领域中的传热介质使用。
脒在高温下具有较低的蒸发损失和较高的热传导性能,因此在核电站、航空航天等领域中广泛应用。
2. 腐蚀抑制剂:脒具有较强的腐蚀抑制能力,可以用作金属腐蚀抑制剂。
在水处理、石油化工等领域中,加入适量的脒可以降低金属材料的腐蚀速率,保护设备和管道的安全运行。
3. 医药领域:脒化合物在医药领域中具有广泛的应用。
例如,一些脒类化合物可以作为抗生素药物,抑制细菌的生长。
此外,还有一些脒类化合物被用作局部麻醉药物和抗癌药物等。
4. 化学合成:脒可以作为化学合成中的原料或中间体使用。
例如,脒可以与卤代烃反应,生成相应的胺化合物。
此外,脒还可以与酸、酮等反应,生成相应的酰胺化合物。
5. 有机合成催化剂:脒也可以作为有机合成中的催化剂使用。
例如,在研发新型的有机合成反应时,脒可以作为催化剂参与反应过程,加速反应速率并提高产率。
总之,脒具有较高的稳定性和惰性,可以在高温高压等严苛条件下使用。
它的合成方法较为简单,应用领域广泛,包括蒸汽动力机械、腐蚀抑制剂、医药领域、化学合成以及有机合成催化剂等。
随着科学技术的不断发展,脒的合成方法和应用领域还有很大的发展潜力。
一种简便的合成脒的新方法
方乐平;吴华悦
【期刊名称】《浙江师范大学学报(自然科学版)》
【年(卷),期】2003(026)003
【摘要】脒类化合物是重要的有机合成中间体,鉴于目前脒类化合物合成方法中的诸多不足,对Sm/TMSCl/H2O(微量)体系促进的腈与叠氮化合物分子间的还原偶联反应进行了研究.结果表明,在室温条件下,在Sm/TMSCl/H2O(微量)体系中,芳香族叠氮化合物与芳腈或脂肪腈反应,可以很容易地得到相应的脒,条件温和,产率较高,且环境友好.
【总页数】3页(P262-264)
【作者】方乐平;吴华悦
【作者单位】温州师范学院,化学与材料科学系,浙江,温州,325027;温州师范学院,化学与材料科学系,浙江,温州,325027
【正文语种】中文
【中图分类】O627.4
【相关文献】
1.一种简便合成2-丁炔-1,4-二醇衍生物的新方法 [J], 程金生;江焕峰;张群健;欧阳小月
2.偶氮二异丁脒盐酸盐的合成新方法 [J], 胡志勇;程原;李蕾;张巧玲
3.一种合成3H-1,2-苯并二磺酚-3-硫酮的简便新方法 [J], 蒋栋; 金红卫; 杨振平; 王海滨; 高建荣
4.合成二苯基卡巴腙的一种简便有效的新方法 [J], 时蕾;潘峰;贾学顺;王玉炉
5.一种简便、有效的合成6-OTs-β-CD的新方法 [J], 刘育;张毅民;孙世新;陈荣悌
因版权原因,仅展示原文概要,查看原文内容请购买。