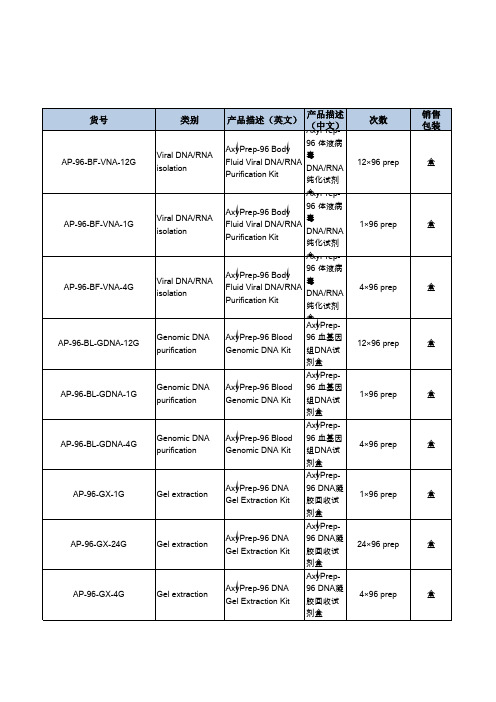
AxyPrep-96 血基因组DNA 试剂盒
- 格式:pdf
- 大小:103.35 KB
- 文档页数:2

3种试剂盒提取烤后烟叶DNA的效果比较陆铮铮;李芳【摘要】[目的]筛选出简单、快速及高效的烤后烟叶DNA提取试剂盒.[方法]选用3种不同的DNA提取试剂盒提取烤后烟叶DNA,通过比较DNA的纯度和浓度、实时荧光定量PCR扩增Ct值以及凝胶电泳图谱分析的方法,综合评价DNA提取效果.[结果]AxyPrep基因组DNA小量试剂盒所提取的DNA,其纯度和浓度较高,用时短,DNA质量好,适用于小量DNA的提取;磁珠法植物基因组DNA试剂盒提取DNA,其浓度和纯度虽然略高于前者,但用时很长,比较适用于批量DNA的提取;GenePure新型植物基因组DNA快速提取试剂盒提取DNA,其纯度高、但浓度低.[结论]烤后烟叶DNA的提取推荐使用AxyPrep基因组DNA小量试剂盒.【期刊名称】《云南农业大学学报》【年(卷),期】2018(033)001【总页数】4页(P172-175)【关键词】烤后烟叶;DNA提取试剂盒;实时荧光定量PCR;凝胶电泳【作者】陆铮铮;李芳【作者单位】遵义师范学院生物与农业科技学院,贵州遵义563002;遵义师范学院生物与农业科技学院,贵州遵义563002【正文语种】中文【中图分类】S572.035.3中国是世界上烟叶出口量较多的国家之一,出口产品覆盖全球70多个国家和地区,在国际市场的地位日趋重要[1]。
贵州是国内的烟叶生产、销售以及出口贸易的重点省之一[2]。
随着科技的快速发展,转基因技术的日渐成熟,烟草作为模式植物被大量研究。
虽然转基因技术在提高作物产量和改善品质等方面有着巨大贡献,但其安全性值令人担忧。
2001年以来,随着国务院对农业转基因作物及其产品实行安全评价制度、生产许可制度、经营许可制度和产品标识制度,海关更是加大力度对出口烟草的检查[3]。
正因如此,对转基因烟叶进行检测和评价是一个重要工作,而在检测的过程中DNA的提取是工作的关键。
提取DNA的方法有很多,目前使用较多的是试剂盒提取法,市面上可供选择的DNA提取试剂盒种类较多,不同试剂盒的提取效果及速度等都存在较大差异[4]。

AxyPrep质粒DNA小量试剂盒本试剂盒采用改进的SDS碱裂解法,结合DNA制备膜选择性地吸附DNA的方法达到快速纯化质粒DNA的目的。
适合于从1-4 ml细菌培养物中提取多至20 μg高纯的质粒DNA,用于测序、体外转录与翻译、限制性内切酶消化、细菌转化等分子生物学实验。
一、试剂盒组成、贮存、稳定性说明书,耗材:DNA制备管,2 ml 离心管,1.5 ml 离心管。
RNase A:50 mg/ml,室温保存。
Buffer S1:细菌悬浮液。
加入RNase A后,4°C贮存。
Buffer S2:细菌裂解液,室温密闭贮存。
(若出现沉淀,应于37°C温浴溶解并冷却至室温后再使用。
)Buffer S3:中和液,室温密闭贮存。
Buffer W1:洗涤液,室温密闭贮存。
Buffer W2 concentrate:去盐液。
使用前,根据瓶上数量加入乙醇,混合均匀,室温密闭贮存。
可用100%乙醇或95%乙醇。
Eluent:2.5 mM Tris-HCl,pH 8.5,室温密闭贮存。
二、注意事项Buffer S2、Buffer S3和Buffer W1含刺激性化合物,操作时要戴乳胶手套和眼镜,避免沾染皮肤、眼睛和衣服,谨防吸入口鼻。
若沾染皮肤、眼睛时,要立即用大量清水或生理盐水冲洗,必要时寻求医疗咨询。
三、实验准备1. 第一次使用前,RNase A全部加入Buffer S1 中,4°C贮存。
2. 准备无核酸和核酸酶污染的Tip头、离心管。
3. 第一次使用前,Buffer W2 concentrate中加入指定体积的无水乙醇。
四、操作步骤第一次使用时,将试剂盒携带的RNase A全部加入到Buffer S1 中,混合均匀,4°C保存。
1. 取1-4 ml 在LB培养基中培养过夜的菌液(若使用丰富培养基,菌液体积应减半或更少),12,000×g离心1 min,弃尽上清。

AxyPrep 动植物基因组DNA小量试剂盒本试剂盒采用独特的裂解和相分离技术,结合DNA制备膜选择性地吸附DNA的方法达到纯化基因组DNA的目的。
每次制备可获得多至20 μg的基因组DNA。
用于PCR、Southern印迹分析、RAPD、RFLD 等分子生物学实验。
一、试剂盒组成、贮存、稳定性说明书,耗材:DNA制备管、小量滤器、2 ml 离心管、1.5 ml 离心管。
RNase A:50 mg/ml,室温保存。
Buffer G-A:裂解液,室温密闭贮存。
Buffer G-B:蛋白去除液,室温密闭贮存。
Buffer DV-A:Buffer DV的制备液,请参照实验准备Buffer DV的配制方法配制,室温密闭贮存。
Buffer DV:相分离液,室温密闭贮存。
Buffer BV:DNA结合液,室温密闭贮存。
Buffer W1:洗涤液,室温密闭贮存。
Buffer W2 concentrate:去盐液。
使用前,按试剂瓶上指定的体积加入无水乙醇,混合均匀,室温密闭贮存。
可用100%乙醇或95%乙醇。
Eluent:2.5 mM Tris-HCl,pH 8.5,室温密闭贮存。
二、注意事项Buffer G-A、Buffer G-B、Buffer BV和Buffer W1含刺激性化合物,操作时要戴乳胶手套和眼镜,避免沾染皮肤、眼睛和衣服,谨防吸入口鼻。
若沾染皮肤、眼睛时,要立即用大量水冲洗,必要时寻求医疗咨询。
三、实验准备1. 第一次使用时,在Buffer W2 concentrate中按试剂瓶上指定的体积加入无水乙醇并混合均匀。
2. 制备Buffer DV:取2 ml Buffer DV-A,125 ml异丙醇,75 ml异丁醇,加入提供的250 ml试剂瓶中,混合均匀。
3. 4℃预冷Buffer DV。
4. 准备65℃水浴。
5. 使用前检查Buffer G-A和Buffer G-B是否有沉淀析出,若出现沉淀,请于65℃水浴加热至沉淀完全溶解后再使用。
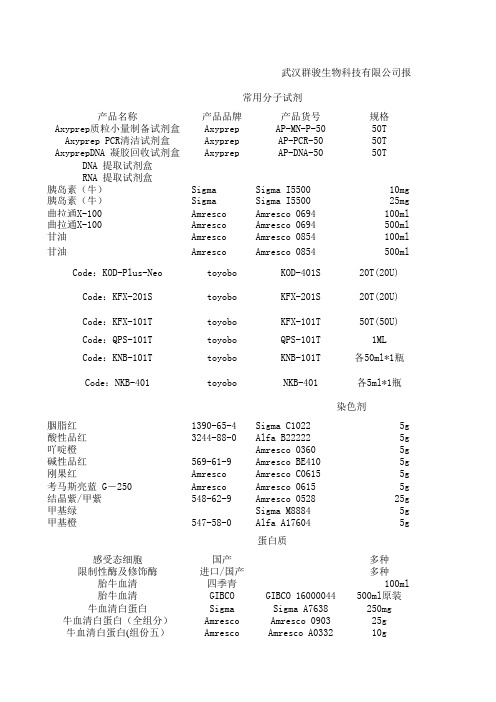
武汉群骏生物科技有限公司报价常用分子试剂产品名称产品品牌产品货号规格Axyprep质粒小量制备试剂盒Axyprep AP-MN-P-5050T Axyprep PCR清洁试剂盒Axyprep AP-PCR-5050T AxyprepDNA 凝胶回收试剂盒Axyprep AP-DNA-5050T DNA 提取试剂盒RNA 提取试剂盒胰岛素(牛)Sigma Sigma I550010mg胰岛素(牛)Sigma Sigma I550025mg曲拉通X-100Amresco Amresco 0694100ml曲拉通X-100Amresco Amresco 0694500ml甘油Amresco Amresco 0854100ml甘油Amresco Amresco 0854500ml Code:KOD-Plus-Neo toyobo KOD-401S20T(20U) Code:KFX-201S toyobo KFX-201S20T(20U)Code:KFX-101T toyobo KFX-101T50T(50U)Code:QPS-101T toyobo QPS-101T1MLCode:KNB-101T toyobo KNB-101T各50ml*1瓶Code:NKB-401toyobo NKB-401各5ml*1瓶染色剂胭脂红1390-65-4Sigma C10225g酸性品红3244-88-0 Alfa B22222 5g吖啶橙Amresco 03605g碱性品红569-61-9Amresco BE4105g刚果红Amresco Amresco C06155g考马斯亮蓝 G-250Amresco Amresco 06155g结晶紫/甲紫548-62-9Amresco 052825g甲基绿Sigma M88845g甲基橙547-58-0Alfa A176045g蛋白质感受态细胞国产多种限制性酶及修饰酶进口/国产多种胎牛血清四季青100ml胎牛血清GIBCO GIBCO 16000044500ml原装牛血清白蛋白Sigma Sigma A7638250mg 牛血清白蛋白(全组分)Amresco Amresco 090325g牛血清白蛋白(组份五)Amresco Amresco A033210g卵清蛋白Sigma Sigma A5253100g缓冲剂培养基琼脂粉Japan Japan500g 琼脂粉Amresco Amresco AJ637500g 牛肉膏国产China500g 酪蛋白(干酪素)Sigma Sigma C5890100g 胆固醇国产china 最高级别25g 脂多糖Sigma Sigma L2880 10mg酶和辅酶乙酰辅酶 A Sigma Sigma A20565mg 乙酰辅酶 A Roche Roche 101018930015mg脱氧核糖核酸酶I Labest Labest100mg 崩溃酶85186-71-6 Sigma D9515500mg 抑肽酶Amresco Amresco E4295mg 溶菌酶Amresco Amresco 06635g 辅酶 A(COA)sigma sigma C428210mg成本价报价备注1501501507015530902895225改善循环后半部分的扩增停滞现象,延伸时间算(30sec./kb)300史上最牛的PCR酶!高成功率,高效450高成功率PCR酶,轻松扩增粗样品,475解决可测定区域,特异性等烦恼。

硕士研究生《昆虫生理生化实验技术》课程代码:3012100018开课单位:植物科技学院责任教师:牛长缨研究生:学号:实验一昆虫血淋巴中蛋白质的分离(聚丙烯酰胺凝胶电泳法)一、实验目的掌握凝胶电泳基本方法,并测定各种昆虫,以及同一种昆虫不同发育阶段的血淋巴的蛋白谱。
二、实验原理昆虫的各种组织,均含有多种蛋白质和各种酶类。
在血淋巴中经分离和纯化后已鉴定的蛋白质有:卵黄原蛋白,脂蛋白,贮存蛋白,滞育蛋白,抗菌蛋白,抗冷蛋白等。
采用聚丙烯酰胺凝胶电泳技术分离蛋白质和酶类,可以得到满意的蛋白谱和酶谱,通常能分离到20-30条带。
聚丙烯酰胺凝胶是由丙烯酰胺单体和交联剂甲叉双丙烯酰胺在催化剂(过硫酸胺)与加速剂(四甲基乙二胺)的作用下,聚合交联而成,具有三维网状结构,能起分子筛的作用,因此常用它作为电泳支持物。
对样品的分离不仅取决于各组分所带电荷的多少,也与分子大小有关。
此外,凝胶电泳还有一种独特的浓缩效应,即在电泳开始阶段,由于不连续pH和凝胶孔径梯度作用,将样品压缩成一条狭窄区带。
从而提高了分离效果。
三、实验材料和仪器供试昆虫:菜青虫试剂及配制:1.Acr:丙烯酰胺Bis:甲叉双丙烯酰胺TEMED:四甲基乙二胺AP:过硫酸胺Tris:三羟甲基氨基甲烷A:1mol/L HCl 48ml;Tris 36.3g;加水至100ml,pH 8.9;贮存于棕色瓶内,4℃保存。
B:Acr 30g;Bis 0.8g;加水至100ml,贮存于棕色瓶内,4℃保存。
C:10%AP 1gAP加9ml水,最好现用现配,贮存于棕色瓶内。
D:1mol/L HCl 48ml;Tris5.98g;加水至100ml,贮存于棕色瓶内,4℃保存.E:Tris6g;甘氨酸28.8g;加水至1000ml,pH8.3(电极缓冲液)2.1%溴酚蓝指示剂,苯硫脲3.染色液:考马斯亮蓝R250 1.25g; 水227ml; 冰乙酸46ml; 甲醇227ml; 共500ml,溶解后过滤。
AxyPrep DNA Gel Extraction KitFor the rapid purification of DNA fragments from agarose gelsKit contents, storage and stabilityCat. No. AP-GX-4 AP-GX-50 AP-GX-250Kit size 4 preps 50 preps 250 prepsMiniprep column 4 50 2502 ml microfuge tube 4 50 2501.5 ml microfuge tube 4 50 250Buffer DE-A 6 ml 66 ml 2x165 mlBuffer DE-B 3 ml 33 ml 165 mlBuffer W1 2.8 ml 28 ml 135 mlBuffer W2 concentrate 2.4 ml24 ml2x72 mlEluent 1 ml 5 ml 25 mlProtocol manual 1 1 1All buffers in this kit are stable for a period of at least 12 months from the date of receipt whenstored under ambient conditions. Please avoid exposure to direct sunlight or extremes intemperature.Buffer DE-A: Gel solubilization buffer. Store at room temperature.Buffer DE-B: Binding buffer. Store at room temperature.Buffer W1: Wash buffer. Store at room temperature.Buffer W2 concentrate: Desalting buffer. Before use, add the amount of ethanol specified.Store at room temperature. Either 100% or 95% denatured ethanol can be used.Eluent: 2.5 mM Tris-Cl, pH 8.5. Store at room temperature.IntroductionThe AxyPrep DNA Gel Extraction Kit employs optimized reagents in combination with a convenient Miniprep column to purify DNA fragments from either TAE or TBE agarose gels (regular and low-melt).Each Miniprep column will bind up to 8 μg of DNA. DNA fragments in a size range of 70 bp to 10 kbcan be efficiently recovered. Depending upon the length of the DNA fragments, the recovery rate is approximately 60-85%. Buffer DE-A contains a reagent for solubilizing agarose gels, in combinationwith a second reagent that protects DNA fragments against degradation during heating. DNA fragments purified by this method are full-length with high biological activity. These fragments are suitable for all routine molecular biology applications, such as ligation, PCR, sequencing, etc.CautionBuffers DE-A, DE-B and W1 contain chemical irritants. When working with the buffers, always wear suitable protective clothing such as safety glasses, laboratory coat and gloves. Be careful to avoid contact with eyes and skin. In the case of such contact, wash immediately with water. If necessary, seek medical assistance.Equipment and consumables required•Heated water bath or temperature block•AxyVac Vacuum manifold with luer-type fittings (#AP-VM)•Vacuum source and regulator (-25-30 inches Hg required)capable of 12,000xg• Microcentrifuge•100% or 95% (denatured) ethanol• 100%isopropanolPreparation before experiment1) Before using the kit, add amount of ethanol specified on the bottle label to the Buffer W2 concentrate.Either 100% or 95% (denatured) ethanol can be used. Mix well and store at room temperature.2) Adjust water bath or temperature block to 75°C.3) Pre-warming Eluent to 65°C will generally improve elution efficiency.Protocols:DNA Gel Extraction Vacuum ProtocolAny vacuum manifold with complementary fittings, such as the AxyVac Vacuum Manifold can be used with the Miniprep columns. A negative pressure of -25-30 inches Hg is be required. We recommend the use of a vacuum regulator to adjust the negative pressure.Note: -25-30 inches Hg is equivalent to -850-1,000 mbar and -12-15 psi.1. Excise the agarose gel slice containing the DNA fragment of interest with a clean, sharp scalpelunder ultraviolet illumination. Briefly place the excised gel slice on absorbent toweling to remove residual buffer. Transfer the gel slice to a piece or plastic wrap or a weighing boat. Mince the gel into small pieces and weigh. In this application, the weight of gel is regarded as equivalent to the volume. For example, 100 mg of gel is equivalent to a 100 μl volume. Transfer the gel slice into a1.5 ml microfuge tube.Note: Alternatively, the gel slice can be placed into the 1.5 ml microfuge tube and then crushed with a pipette tip or other suitable device. Spin the tube for 30 sec at 12,000xg to consolidate the gel at the bottom of the tube.Use the graduations to estimate the volume of the agarose gel.2. Add a 3x sample volume of Buffer DE-A.Note: The color of Buffer DE-A is red. This color is used to add contrast in the next step, so that any pieces of unsolubilized agarose can be visualized.3. Resuspend the gel in Buffer DE-A by vortexing. Heat at 75°C until the gel is completely dissolved(typically, 6-8 minutes). Heat at 40°C if low-melt agarose gel is used. Intermittently vortexing (every 2-3 minutes) will accelerate gel solubilization.IMPORTANT: Gel must be completely dissolved or the DNA fragment recovery will be reduced.IMPORTANT: Do not heat the gel for longer than 10 minutes.4. Add 0.5x Buffer DE-A volume of Buffer DE-B, mix. If the DNA fragment is less than 400 bp,supplement further with a 1x sample volume of isopropanol.Example: For a 1% gel slice equivalent to 100 μl, add the following:• 300 μl Buffer DE-A• 150 μl Buffer DE-BIf the DNA fragment is <400 bp, you would also add:• 100 μl of isopropanol.Note: The color of the mixture will turn yellow after the addition of Buffer DE-B. Please make sure the contents area uniform yellow color before proceeding.5. Attach the vacuum manifold to a vacuum source. Position a Miniprep column securely into one ofthe complementary fittings. Transfer the binding mix from Step 4 to the Miniprep column(s). Switch on the vacuum source and adjust the negative pressure to -25-30 inches Hg. Continue to apply vacuum until no liquid remains in the Miniprep column.6. Pipette 500 μl of Buffer W1 into the Miniprep column(s). Draw all liquid through the column(s).7. Pipette 700 μl of Buffer W2 along the wall of the Miniprep column(s) to wash off all residual BufferW1. Draw all liquid through the column(s).Note: Make sure that ethanol has been added into Buffer W2 concentrate.Note: Be sure to add Buffer W2 along the tube wall to wash off all residual salt.8. Repeat this wash step with a second 700 μl aliquot of Buffer W2.Note: Two washes with Buffer W2 are used to ensure the complete removal of salt, eliminating potential problems in subsequent enzymatic reactions, such as ligation and sequencing reaction.9. Transfer the Miniprep column into a 2 ml microfuge tube (provided) and centrifuge at 12,000xg for1 minute to purge residual Buffer W2 from the binding membrane.10. Transfer the Miniprep column into a clean 1.5 ml microfuge tube (provided). To elute the DNA,add 25-30 μl of Eluent or deionized water to the center of the membrane. Let it stand for 1 minute at room temperature. Centrifuge at 12,000xg for 1 minute.Note: Pre-warming the Eluent to 65°C will generally improve elution efficiency.Note: Deionized water can also be used to elute the DNA fragments.DNA Gel Extraction Spin Protocol1. Excise the agarose gel slice containing the DNA fragment of interest with a clean, sharp scalpel under ultraviolet illumination. Briefly place the excised gel slice on absorbent toweling to remove residual buffer. Transfer the gel slice to a piece or plastic wrap or a weighing boat. Mince the gel into small pieces and weigh. In this application, the weight of gel is regarded as equivalent to the volume. For example, 100 mg of gel is equivalent to a 100 μl volume. Transfer the gel slice into a 1.5 ml microfuge tube.Note: Alternatively, the gel slice can be placed into the 1.5 ml microfuge tube and then crushed with a pipette tip or other suitable device. Spin the tube for 30 sec at 12,000xg to consolidate the gel at the bottom of the tube.Use the graduations to estimate the volume of the agarose gel.2. Add a 3x sample volume of Buffer DE-A.Note: The color of Buffer DE-A is red. This color is used to add contrast in the next step, so that any pieces of unsolubilized agarose can be visualized.3. Resuspend the gel in Buffer DE-A by vortexing. Heat at 75°C until the gel is completely dissolved (typically, 6-8 minutes). Heat at 40°C if low-melt agarose gel is used. Intermittent vortexing (every 2-3 minutes) will accelerate gel solubilization.IMPORTANT: Gel must be completely dissolved or the DNA fragment recovery will be reduced.IMPORTANT: Do not heat the gel for longer than 10 minutes.4. Add 0.5x Buffer DE-A volume of Buffer DE-B, mix. If the DNA fragment is less than 400 bp, supplement further with a 1x sample volume of isopropanol.Example: For a 1% gel slice equivalent to 100 μl, add the following:• 300 μl Buffer DE-A•150 μl Buffer DE-BIf the DNA fragment is <400 bp, you would also add:• 100 μl of isopropanol.Note: The color of the mixture will turn yellow after the addition of Buffer DE-B. Please make sure the contents area uniform yellow color before proceeding.5. Place a Miniprep column into a 2 ml microfuge tube (provided). Transfer the solubilized agarose from Step 4 into the column. Centrifuge at 12,000xg for 1 minute.6. Discard the filtrate from the 2 ml microfuge tube. Return the Miniprep column to the 2 ml microfuge tube and add 500 μl of Buffer W1. Centrifuge at 12,000xg for 30 seconds.7. Discard the filtrate from the 2 ml microfuge tube. Return the Miniprep column to the 2 ml microfuge tube and add 700 μl of Buffer W2. Centrifuge at 12,000xg for 30 seconds.Note: Make sure that 95-100% ethanol has been added into Buffer W2 concentrate. Make a notation on the bottle label for future reference.8. Discard the filtrate from the 2 ml microfuge tube. Place the Miniprep column back into the 2 ml microfuge tube. Add a second 700 μl aliquot of Buffer W2 and centrifuge at 12,000xg for 1 minute.Note: Two washes with Buffer W2 are used to ensure the complete removal of salt, eliminating potential problems in subsequent enzymatic reactions, such as ligation and sequencing reaction.9. Discard the filtrate from the 2 ml microfuge tube. Place the Miniprep column back into the 2 mlmicrofuge tube. Centrifuge at 12,000xg for 1 minute.10. Transfer the Miniprep column into a clean 1.5 ml microfuge tube (provided). To elute the DNA,add 25-30 μl of Eluent or deionized water to the center of the membrane. Let it stand for 1 minute at room temperature. Centrifuge at 12,000xg for 1 minute.Note: Pre-warming the Eluent at 65°C will generally improve elution efficiency.Note: Deionized water can also be used to elute the DNA fragments.OverviewExcise gelAdd Buffer DE-A, heat at 75°C to melt gel Add 0.5×Buffer DE-A volume of Buffer DE-BAdd 500 μl of Buffer W1Add 700 μl of Buffer W2Repeat wash with Buffer W2Elute with 25-30 μl of Eluent or deionized water Gel containing DNASolubilizationBindingWashingElutionTroubleshooting1. Low or no recoveryGel not completely solubilizedIncomplete solubilization of the agarose gel will allow the DNA fragments to be masked from the AxyPrep membrane surface, preventing interaction and binding. Depending upon the amount of incompletely solubilized gel remaining in the sample, only partial binding of the fragments may occur, resulting in premature elution and fragments loss during the ensuing wash steps. Usually, this is attributable to processing too much agarose (too large and/or too high percentage). Be sure to use the correct amount of Buffer DE-A. Carefully inspect the sample during heating to be sure that no solid agarose remains. Use frequent vortexing during heating to enhance solubilization.Poor fragment bindingTo ensure complete solubilization of the agarose, increase the amount of Buffer DE-A to 4×the sample volume. Trim the gel as close to the DNA fragments as possible to minimize the amount of agarose processed. Be sure to supplement the solubilized agarose containing DNA fragments <400 bp with 1× sample volume of isopropanol (100%).Premature elution of bound DNA fragmentsAs described above, premature elution of the DNA fragments can be attributable to the presence of excessive agarose. In addition, omission of the ethanol from the Buffer W2 or misformulation with 70% ethanol (instead of 95-100%) will also cause the DNA fragments to detach during the desalting step.Poor elution efficiencyDo not allow the Miniprep column to remain under vacuum for an excessive period of time after the last Buffer W2 wash step. To improve elution efficiency, heat the eluent to 65°C.2. DNA fragments do not perform well in enzymatic reactionsResidual saltBe sure to perform 2× Buffer W2 washes.Residual ethanolSpin the column for 1 additional minute (2 minutes total) after the last Buffer W2 wash step.Residual agaroseTo ensure complete removal of the agarose be sure that the agarose slice is fully solubilized by Buffer DE-A. Try to trim the gel as close to the DNA fragments as possible to minimize the amount of agarose processed.For technical inquiries about AxyPrep Kits, please contact Axygen Biosciences at support.axyprepkits@。
AxyPrep DNA凝胶回收试剂盒本试剂盒适合从各种琼脂糖凝胶中提取多至8 μg DNA(70bp-10Kb),回收率为60-85%。
琼脂糖凝胶在温和的缓冲液(DE-A溶液)中溶解,其中的保护剂能防止线状DNA在高温下降解,然后在DE-B溶液的作用下使DNA选择性结合到膜上。
纯化的DNA纯度高,并保持片断完整性和高生物活性,可直接用于连接、体外转录、PCR扩增、测序、微注射等分子生物学实验。
一、试剂盒组成、贮存、稳定性说明书,耗材:DNA制备管、2 ml离心管、1.5 ml离心管。
Buffer DE-A:凝胶熔化剂,含DNA保护剂,防止DNA在高温下降解。
室温密闭贮存。
Buffer DE-B:结合液(促使大于70 bp的DNA片段选择性结合到DNA制备膜上)。
室温密闭贮存。
若出现沉淀,应于70°C温育溶解并冷却至室温后再使用。
Buffer W1:洗涤液,室温密闭贮存。
Buffer W2 concentrate:去盐液,使用前,根据瓶上数量加入乙醇,混合均匀,室温密闭贮存。
可用100%无水乙醇或95%乙醇。
Eluent:2.5 mM Tris-HCl,pH 8.5,室温密闭贮存。
二、注意事项1. 在步骤1中,将凝胶切成细小的碎块可大大缩短凝胶熔化时间(线型DNA长时间暴露在高温条件下易于水解),从而提高回收率。
勿将含DNA的凝胶长时间地暴露在紫外灯下,减少紫外线对DNA 造成的损伤。
2. 在步骤2中凝胶必须完全熔化,否则将严重影响DNA回收率。
3. 将Eluent或水加热至65°C,有利于提高洗脱效率。
4. DNA分子呈酸性,建议在2.5 mM Tris-HCl,pH 8.5洗脱液中保存。
三、实验准备1. 第一次使用前,Buffer W2 concentrate中加入指定体积的无水乙醇。
2. 准备无核酸和核酸酶污染的Tip头、离心管。
3. 准备75°C水浴。
四、操作步骤1. 在紫外灯下切下含有目的DNA的琼脂糖凝胶,用纸巾吸尽凝胶表面液体并切碎。
AxyPrep-96血基因组DNA试剂盒
本试剂盒采用特殊的细胞裂解和蛋白去除液(包括蛋白酶K裂解)从抗凝全血中得到基因组DNA。
适用于从冻全血、血浆、血清、骨髓、其他体液、淋巴细胞、培养细胞、病毒和线粒体中提取DNA。
一、试剂盒组成、贮存、稳定性
说明书,耗材:96孔深孔板(1.6 ml),96圆孔板,96孔DNA制备板,96圆孔硅胶片,BF-400膜。
Proteinase K:冻干的蛋白酶K可室温贮存6个月,长时间保存请置于4℃;溶解后,在2-8℃可贮存2个月,长时间保存请勿置于室温中。
Buffer PK:蛋白酶K溶解液,室温密闭贮存。
Buffer BL:细胞裂解液,室温密闭贮存。
Buffer W1B concentrate:洗涤液。
使用前,根据瓶上数量加入乙醇,混合均匀,室温密闭贮存。
可用100%乙醇或95%乙醇。
Buffer W2 concentrate:去盐液。
使用前,根据瓶上数量加入乙醇,混合均匀,室温密闭贮存。
可用100%乙醇或95%乙醇。
10×Buffer W2(12×96试剂盒):用于配制Buffer W2。
Eluent B:7.5 mM Tris-HCl,pH 8.5,0.3 mM EDTA,室温密闭贮存。
二、注意事项
Buffer BL和Buffer W1B含刺激性化合物,操作时要戴乳胶手套和眼镜,避免沾染皮肤、眼睛和衣服,谨防吸入口鼻。
若沾染皮肤、眼睛时,要立即用大量清水和生理盐水冲洗,必要时寻求医疗咨询。
三、实验准备
1. 第一次使用时,在Buffer W2 concentrate和Buffer W1B concentrate中按试剂瓶上指定
的体积加入无水乙醇。
2. Buffer W2(12×96试剂盒)配制:在提供的500 ml空瓶中加入15 ml 10×Buffer W2,
135 ml去离子水和350 ml乙醇,可用100%无水乙醇或95%乙醇。
3. 根据瓶上标签将蛋白酶K溶解于Buffer PK中,请勿旋涡振荡。
4. 准备70℃温浴。
5. 使用前检查Buffer BL是否有沉淀析出,若出现沉淀,请于70℃温浴加热至沉淀完全溶解
后再使用。
四、操作步骤
1. 向96圆孔板的每孔中加入20 μl蛋白酶K。
2. 加200 μl抗凝全血到96圆孔板中。
* 若全血样品体积少于200 μl,用PBS补充到200 μl。
* 若样品为鸟类血,样品用量须低于10 μl。
* 若需得到RNA-free的基因组DNA,在步骤3加入Buffer BL前加入DNase-free的
RNase A(20 mg/ ml)。
3. 加200 μl Buffer BL,注意不要打湿每孔的边缘,用96圆孔硅胶片密封各孔。
4. 用力混合30 s。
5. 3000 rpm简短离心使96圆孔硅胶片上的溶液到96圆孔板内。
启动离心机,当速度达
到3000 rpm后即停止。
6. 在培养箱或烘箱中70℃温浴至少10 min。
7. 3000 rpm简短离心使96圆孔硅胶片上的溶液到96圆孔板内。
启动离心机,当速度达
到3000 rpm后即停止。
8. 取下96圆孔硅胶片,每孔加入200 μl乙醇(96-100%)。
9. 用96圆孔硅胶片密封各孔,用力混匀混合15 s。
3000 rpm简短离心使96圆孔硅胶片
上的溶液到圆孔板内。
启动离心机,当速度达到3000 rpm后即停止。
10. 将96孔DNA板放到一洁净的96孔1.6 ml深孔板。
取下圆孔板上的96圆孔硅胶片,
将每孔中的溶液转移至96孔DNA板中,6000 rpm离心4 min。
11. 丢弃96孔1.6 ml深孔板的滤液,将96孔DNA板放回到96孔1.6 ml深孔板上,每孔
加入500 μl Buffer W1B,用一新的BF-400膜密封96孔DNA板,6000 rpm离心4 min。
* 确认在Buffer W1B concentrate中已按试剂瓶上的指定体积加入无水乙醇。
12. 丢弃96孔1.6 ml深孔板的滤液,将96孔DNA板放回到96孔1.6 ml深孔板上,每孔
加入850 μl Buffer W2,用另一新的BF-400膜密封96孔DNA板,6000 rpm离心4 min。
* 确认在Buffer W2 concentrate中已按试剂瓶上的指定体积加入无水乙醇。
13.弃滤液,将96孔DNA板放回到96孔1.6 ml深孔板上,每孔加入400 μl Buffer W2,
用另一新的BF-400膜密封96孔DNA板,6000 rpm离心4 min。
14.将96孔DNA板放在一洁净的96孔1.6 ml深孔板上,用BF-400膜密封96孔DNA板,
6000 rpm离心15 min。
15.将96孔DNA板放在另一洁净的96孔1.6 ml深孔板上,每孔加入100-200 μl Eluent B
或去离子水,室温静置2 min,用另一新的BF-400膜密封96孔DNA板,6000 rpm离
心4 min洗脱得到DNA。