最新产5万吨乙二醇工艺流程
- 格式:doc
- 大小:405.50 KB
- 文档页数:21

乙二醇生产工艺流程研究乙二醇生产工艺流程研究进程本文由南通润丰石油化工整理乙二醇是非常重要的化工生产原料,可用于合成多种聚酯类化合物。
综述了解目前合成乙二醇工艺中石化流程、煤化工流程和生物质资源流程,并对比了各种生产工艺流程的优缺点,对其工业发展前景作了展望。
乙二醇俗称甘醇,英文名称ethyleneglycol,简称EG;分子式C2H6O2,其结构简式HO-CH2-CH2-OH,是生产聚酯涤纶,聚酯树脂、合成纤维、增塑剂、表面活性剂等有机产品的主要原料。
乙二醇是最简单的二元醇,具有醇的通性,可与无机酸或有机酸发生酯化反应,与多元酸发生聚合反应生成聚酯。
乙二醇也可发生脱水反应,其分子内脱水生成醛,分子间脱水生成醚,和强氧化剂如KMnO4(高锰酸钾)、O3(臭氧)、K2CrO7(重铬酸钾)等还可以发生氧化反应。
乙二醇分子内有两个羟基可与羰基发生缩醛反应生成缩醛,水解后又生成羰基和乙二醇,这个性质常在有机合成中使用,是很好的保护羰基的试剂。
由于乙二醇的熔点非常低,在零下四十摄氏度下才开始凝固,因此还可作为抗冻剂用于发动机等机器部件。
正是基于乙二醇在工业上广泛的用途,吸引了人们的注意力。
1石化路线以石油化工产品乙烯(CH2=CH2)为原料,在贵金属Ag的催化作用下,CH2=CH2先氧化制成环氧乙烷(化学式:C2H4O,简称EO),然后通过EO再直接与水反应合成乙二醇,这是石化路线中较为成熟的生产工艺。
2煤化工路线随着科技的不断进步,再加上世界石油危机的影响,人们普遍认识到了石油是不可再生的,开始逐渐探索研究其它生产乙二醇的方法。
我国拥有十分丰富煤炭资源,因此,从原料选择的经济合理性以及我国的能源结构组成出发,采用以煤为原料的煤化工路线制备乙二醇还是较适合我国国情。
以煤为原料制取乙二醇,目前主要是以煤先制取合成气(主要是CO 和H2),然后再制取乙二醇。
现阶段,该合成乙二醇的工艺路线一般分为直接合成法和间接合成法[6]。

[煤制甲醇]环氧乙烷水合制乙二醇环氧乙烷水合制乙二醇乙二醇是合成聚酯树脂的主要原料,大家熟知的涤纶纤维就是由乙二醇与对苯二甲酸合成的。
乙二醇还可用作防冻液,w(乙二醇)=55%的水溶液的冰点为—36℃,可用作中国北方冬天汽车必需的冷却液。
此外,乙二醇还可用作溶剂和用于化妆品、毛皮加工、烟叶润湿和纺织工业染整等。
据预测,2000年乙二醇的世界产量将达到10Mt/a.中国1995年的产量为53×104t/a,到2000年将达72×104t/a。
1。
乙二醇生产方法综述现在,乙二醇有多种工业生产方法,但环氧乙烷水合制乙二醇法仍占主导地位.(1)环氧乙烷法可用酸作催化剂,但用得较多的是加压水合:反应中生成约10%的二乙二醇醚(二甘醇)和三乙二醇醚(三甘醇),它们是有用的化工产品,故反应所得的有用产品总产率按环氧乙烷计接近100%,生成的二乙二醇醚用作纤维素、树胶、涂料、喷漆的溶剂或稀释剂.三乙二醇醚主要用来生产刹车液。
它们的售价比乙二醇还高,因此可改善生产装置的经济效益。
环氧乙烷法因环氧乙烷售价高,生产总成本也比较高。
(2)乙烯乙酰氧基化法乙烯乙酰氧基化法又称奥克西兰(Oxirane)法,它可由乙烯为原料生产乙二醇。
工艺分二步进行,第一步乙烯与醋酸反应生成乙二醇-醋酸酯和乙二醇二醋酸酯:反应条件:反应温度160℃,反应压力2。
8MPa,催化剂TeO2/HBr[w(HBr)=48%的水溶液],还可用醋酸锰加碘化钾作催化剂,乙烯转化率60%,选择性95%~97%,产品分布:乙二醇二醋酸酯70%,乙二醇一醋酸酯25%,乙二醇5%。
第二步是醋酸酯水解生成乙二醇和醋酸:反应条件为:反应温度107~130℃,压力0.117MPa,选择性95%。
该法的总反应式为:2CH2=CH2+2H2O+O2→2HOCH2-CH2OH以乙烯计的摩尔产率为94%,高于以环氧乙烷法生产乙二醇的产率。
该法虽然以廉价的乙烯作原料,但投资和能耗比环氧乙烷法高,经济上是否比环氧乙烷法好尚有争论,再加上醋酸对设备的腐蚀是一个头痛问题,催化剂的再生和回收问题也没有很好解决,致使已开工生产的0.36Mt/a生产装置被迫停产关闭。

乙二醇是一种广泛应用于化工和纺织行业的重要有机化合物。
它具有良好的溶解性、低毒性和稳定性,可以用作溶剂、抗冻剂、塑化剂等。
在这篇文章中,我将详细介绍年产5万吨乙二醇的工艺流程设计。
1.原料准备2.反应装置设计乙二醇的生产主要通过乙烯的氧化反应实现。
反应装置通常由反应器、加热器、冷却器、分离器等组成。
反应器中的乙烯和空气在催化剂存在下进行氧化反应生成乙二醇。
加热器用于提升反应温度,使得反应可以进行。
冷却器则用于降低反应液的温度,防止过高的温度对催化剂产生不良影响。
分离器主要用于将反应生成的乙二醇与其他副产物进行分离。
3.催化剂选择在乙二醇的生产过程中,催化剂的选择对反应效率和产物质量有很大影响。
常用的催化剂包括金属铜、铁、钴等催化剂。
这些催化剂具有良好的活性和选择性,可以有效促进乙烯和空气的氧化反应。
4.控制参数在乙二醇的生产过程中,控制参数的选择对反应效率和产物质量起着决定性的作用。
温度、压力和物料流速是常用的控制参数。
适当的反应温度和压力可以促进反应的进行,同时不会造成催化剂的热解或催化剂的失活。
物料流速的控制可以调节反应速率,使得反应达到最佳状态。
5.产品分离和纯化在乙二醇的生产过程中,由于反应中会生成一些副产物,因此需要进行产品分离和纯化。
常用的分离方法包括蒸馏、结晶等。
通过适当的蒸馏条件,可以将乙二醇与其他挥发性副产物进行分离,得到纯度较高的乙二醇产品。
结晶则可以通过控制温度和压力,使得溶解度较低的乙二醇晶体从反应溶液中析出。
6.副产物处理乙二醇的生产过程中会产生一些副产物,包括残留的催化剂、水分和其他杂质。
这些副产物需要进行处理,以保证产品的纯度和质量。
常用的处理方法包括过滤、蒸发等。
通过适当的过滤条件,可以去除残留的催化剂颗粒;蒸发则可以去除溶液中的水分和其他杂质。
7.产品质量检测乙二醇的生产过程中,对产品的质量进行检测是必要的。
常用的检测方法包括物理性质检测和化学成分检测。
物理性质检测可以通过测定产品的密度、粘度和凝固点等指标进行。

摘要乙二醇在国民经济中有着极其重要的地位,广泛用于生产聚酯纤维、薄膜、容器瓶类等聚酯系列产品和汽车防冻剂,但国内乙二醇的产量一直无法满足国内市场的强劲需求。
因此,本设计以乙二醇精制为中心和重点,经过严密的计算和论证,得到了肯定的结果。
关键词:乙二醇;环氧乙烷;水合法。
目录前言 (1)1文献综述....................................1.1? 乙二醇工业的发展[1][2] .................前?言乙二醇在国民经济中有着极其重要的地位,是大宗有机化工产品。
广泛用于生产聚酯纤维、薄膜、容器瓶类等聚酯系列产品和汽车防冻剂,还可用于除冰剂、表面涂料、表面活性剂、增塑剂、不饱和聚酯树脂以及合成乙二醇醚、乙二醛、乙二酸等化工产品的原料,虽然乙二醇产品用途极广,但国内乙二醇的产量一直无法满足国内市场的强劲需求,乙二醇自给率不足50%,如图1有相当大的部分需要进口,易受国际市场供求关系的影响。
因此,发展和技术改造乙二醇工艺设计对我国经济发展有着重要的意义。
随着我国市场经济的发展,以前那种单纯*增大原料和能源的消耗来提高产量的做法已逐渐被淘汰,继续这种做法的企业已经濒临破产倒闭;现在只有依*科技的力量,通过技术的改造来降低能源的消耗,同时使各种生产数据得到优化的配置,才是摆脱困境最有效的方法。
乙二醇工艺设计中,乙二醇的精制是整个工艺流程的核心部分,关系着乙二醇产品的质量和产量。
因此,本设计以乙二醇精制为中心和重点,经过严密的计算和论证,得到了肯定的结果。
该技术具有世界共同发展趋向的节能性,是生产乙二醇工艺的重大突破。
图1 我国近些年乙二醇的供需情况年份产量万吨/年进口量万吨/年需求量万吨/年自给率%2000901051954620018016024033200290214304302003962513472820049433943322200511040051021200615640656228200717448065427200821452273629第1章?文献综述1.1乙二醇工业的发展[1][2]乙二醇是最简单和最重要的脂肪族二元醇,它在有机化工生产中是一种重要的基本原料,尤其广泛用于聚酯纤维、聚酯塑料的生产。

乙二醇是一种重要的有机化学品,被广泛应用于涂料、塑料、纺织品、化妆品等行业中。
为了满足市场需求,设计一套年产5万吨乙二醇的工艺流程。
乙二醇的制备可以通过乙烯的加氧甲醇化反应得到。
在甲醇的存在下,将乙烯与空气或氧气反应生成乙二醇。
该反应的反应条件及催化剂的选择对乙二醇的产率和选择性有一定的影响。
乙烯的加氧反应需要催化剂的存在,常用的催化剂包括铬酸盐、钒酸盐和铬酸-钼酸盐等。
催化剂选择应综合考虑催化剂的活性、稳定性、价格等因素。
甲醇的存在可以促进乙烯分子的氧化,同时抑制副反应的发生。
甲醇可以作为乙二醇的溶剂和载体,提供反应的热量。
在实际工业生产中,甲醇需要反复使用,进行回收再利用,以提高经济性。
工艺流程设计如下:1.原料准备:准备高纯度的乙烯、甲醇以及所需的氧气或空气。
2.反应器:选用适当的反应器,反应器材料应能耐受高温、高压和有害物质的腐蚀。
同时应考虑反应器的传热性能和搅拌性能。
3.氧化反应:将乙烯、甲醇和氧气或空气按一定的摩尔配比加入反应器中,调节适宜的反应温度和压力。
根据催化剂的选择,可能需要进行预处理步骤,如还原剂的添加。
4.分离和净化:经过反应后,产物和反应物混合物需要经过分离和净化过程。
通过汽提、蒸馏、萃取等方法,将乙二醇与其他杂质分离开来。
同时要进行催化剂的去除和再生。
5.尾气处理:处理反应过程中生成的废气,采用适当的吸附、洗涤等方法,将有害气体去除,以达到环境保护的要求。
6.乙二醇产品回收:将分离得到的乙二醇进行进一步处理,包括干燥、贮存等步骤。
同时回收甲醇进行再利用,减少原料的消耗和生产成本。
7.催化剂再生与回收:对使用过的催化剂进行再生和回收处理,以实现催化剂的可持续使用和经济性。
以上是一套年产5万吨乙二醇的工艺流程设计。
在实际工业生产中,还需要考虑能源消耗、化学物品的储存和运输、工艺控制等方面的问题。
工艺流程的设计应综合考虑经济性、环境保护和安全性等因素,以实现高效、可持续的生产。

乙二醇生产工艺综述摘要:通过对石油化工路线和C1路线生产乙二醇的比较,分析了两条路线的各种工艺的优缺点。
针对我国石油紧缺、煤炭资源丰富的现状,重点介绍了合成气制乙二醇工艺的发展现状。
1.前言:乙二醇是一种重要的有机化工原料,主要用来生产聚酯纤维(pet)、塑料、橡胶、聚酯漆、胶粘剂、非离子表面活性剂、乙醇胺以及炸药,也大量用作溶剂、润滑剂、增塑剂和防冻剂等。
目前,乙二醇的工业生产方法主要是由乙烯在银催化剂上气相氧化制备环氧乙烷,然后进行液相非催化水合反应得到乙二醇产品。
工艺路线完全依赖于不可再生的石油资源。
随着近年来国民经济的快速发展,中国的石油消费量一直在上升。
面对世界石油资源日益短缺的形势,开发新的乙二醇生产工艺已成为研究热点。
2.石油化工路线合成乙二醇在石化路线中有环氧乙烷(eo)直接催化水合法和碳酸乙烯酯(ec)法路线,ec路线又分ec直接水合生产eg、ec法和甲醇反应联产eg、碳酸二甲酯(dmc)法。
上述方法的基础首先是乙烯氧化生成环氧乙烷,因而环氧乙烷的生产效率直接关系到石化路线生产乙二醇的成本。
1938年,UCC公司首先建立了一座工业装置,用银催化剂对乙烯进行气相氧化生产环氧乙烷(EO),然后环氧乙烷与水蒸气反应合成乙二醇,从而开启了大规模工业化生产乙二醇的时代。
目前乙二醇的生产基本上是以乙烯为原料,通过eo非催化液相水合法进行,而银则是乙烯氧化制环氧乙烷唯一有效的催化剂。
通过对环氧乙烷生产成本的分析表明,原料乙烯的消耗占生产成本的70%,所以工业上eo、eg生产技术的进展很大程度上取决于eo催化剂的选择性的进一步提高,以实现有效的节约乙烯,提高经济效益。
总的来说,虽然对石油化工法合成乙二醇的催化剂和水合效率进行了大量研究,但乙烯氧化制环氧乙烷的选择性较低;环氧乙烷水合副产物多(主要为二乙二醇、三乙二醇),分离精制工艺复杂,能耗大;原料乙烯是石油产品,伴随原油价格逐渐上涨,产品的经济性会逐渐降低等问题。
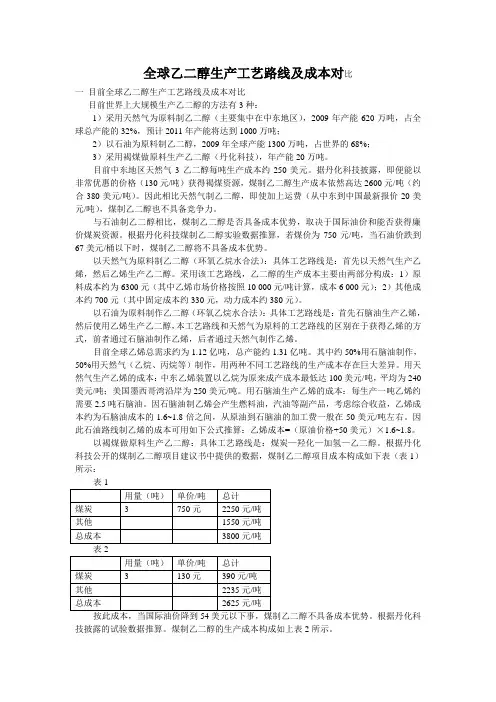
全球乙二醇生产工艺路线及成本对比一目前全球乙二醇生产工艺路线及成本对比目前世界上大规模生产乙二醇的方法有3种:1)采用天然气为原料制乙二醇(主要集中在中东地区),2009年产能620万吨,占全球总产能的32%,预计2011年产能将达到1000万吨;2)以石油为原料制乙二醇,2009年全球产能1300万吨,占世界的68%;3)采用褐煤做原料生产乙二醇(丹化科技),年产能20万吨。
目前中东地区天然气3乙二醇每吨生产成本约250美元。
据丹化科技披露,即便能以非常优惠的价格(130元/吨)获得褐煤资源,煤制乙二醇生产成本依然高达2600元/吨(约合380美元/吨)。
因此相比天然气制乙二醇,即使加上运费(从中东到中国最新报价20美元/吨),煤制乙二醇也不具备竞争力。
与石油制乙二醇相比,煤制乙二醇是否具备成本优势,取决于国际油价和能否获得廉价煤炭资源。
根据丹化科技煤制乙二醇实验数据推算,若煤价为750元/吨,当石油价跌到67美元/桶以下时,煤制乙二醇将不具备成本优势。
以天然气为原料制乙二醇(环氧乙烷水合法):具体工艺路线是:首先以天然气生产乙烯,然后乙烯生产乙二醇。
采用该工艺路线,乙二醇的生产成本主要由两部分构成:1)原料成本约为6300元(其中乙烯市场价格按照10 000元/吨计算,成本6 000元);2)其他成本约700元(其中固定成本约330元,动力成本约380元)。
以石油为原料制作乙二醇(环氧乙烷水合法):具体工艺路线是:首先石脑油生产乙烯,然后使用乙烯生产乙二醇,本工艺路线和天然气为原料的工艺路线的区别在于获得乙烯的方式,前者通过石脑油制作乙烯,后者通过天然气制作乙烯。
目前全球乙烯总需求约为1.12亿吨,总产能约1.31亿吨。
其中约50%用石脑油制作,50%用天然气(乙烷、丙烷等)制作,用两种不同工艺路线的生产成本存在巨大差异。
用天然气生产乙烯的成本:中东乙烯装置以乙烷为原来成产成本最低达100美元/吨,平均为240美元/吨;美国墨西哥湾沿岸为250美元/吨。


摘要乙二醇在國民經濟中有著極其重要的地位,廣泛用於生產聚酯纖維、薄膜、容器瓶類等聚酯系列產品和汽車防凍劑,但國內乙二醇的產量一直無法滿足國內市場的強勁需求。
因此,本設計以乙二醇精製為中心和重點,經過嚴密的計算和論證,得到了肯定的結果。
關鍵字:乙二醇;環氧乙烷;水合法。
目錄前言 (1)1文獻綜述...........................................................................1.1 乙二醇工業的發展[1][2]........................................前言乙二醇在國民經濟中有著極其重要的地位,是大宗有機化工產品。
廣泛用於生產聚酯纖維、薄膜、容器瓶類等聚酯系列產品和汽車防凍劑,還可用於除冰劑、表面塗料、表面活性劑、增塑劑、不飽和聚酯樹脂以及合成乙二醇醚、乙二醛、乙二酸等化工產品的原料,雖然乙二醇產品用途極廣,但國內乙二醇的產量一直無法滿足國內市場的強勁需求,乙二醇自給率不足50%,如圖1有相當大的部分需要進口,易受國際市場供求關係的影響。
因此,發展和技術改造乙二醇工藝設計對我國經濟發展有著重要的意義。
隨著我國市場經濟的發展,以前那種單純*增大原料和能源的消耗來提高產量的做法已逐漸被淘汰,繼續這種做法的企業已經瀕臨破產倒閉;現在只有依*科技的力量,通過技術的改造來降低能源的消耗,同時使各種生產資料得到優化的配置,才是擺脫困境最有效的方法。
乙二醇工藝設計中,乙二醇的精製是整個工藝流程的核心部分,關係著乙二醇產品的品質和產量。
因此,本設計以乙二醇精製為中心和重點,經過嚴密的計算和論證,得到了肯定的結果。
該技術具有世界共同發展趨向的節能性,是生產乙二醇工藝的重大突破。
圖1 我國近些年乙二醇的供需情況年份產量萬噸/年進口量萬噸/年需求量萬噸/年自給率%2000 2001 2002 2003 2004 2005 2006 2007 2008 9080909694110156174214105160214251339400406480522195240304347433510562654736463330282221282729第1章文獻綜述1.1乙二醇工業的發展[1][2]乙二醇是最簡單和最重要的脂肪族二元醇,它在有機化工生產中是一種重要的基本原料,尤其廣泛用於聚酯纖維、聚酯塑膠的生產。

乙二醇制备工艺选择乙二醇的制备工艺根据原料来源主要可以分为石油路线、非石油路线两种,每种路线又包括多种具体的工艺,下面进行详细的描述。
1.石油路线合成乙二醇石油路线的基本原料是乙烯和氧气,在银催化剂、甲烷或氮气致稳剂、氯化物抑制剂存在条件下,将乙烯直接氧化生成环氧乙烷,然后将环氧乙烷制得乙二醇,具体的工艺又可以分为环氧乙烷直接水合法、环氧乙烷催化水合法、碳酸乙烯酯法,下面予以详述。
1.1环氧乙烷直接水合法环氧乙烷直接水合法是在2.23MPa、190~200℃条件下,在管式反应器中进行如下反应:生成的乙二醇水溶液中乙二醇质量分数大约在10%左右,同时副产一缩二乙二醇、三缩三乙二醇和多缩聚乙二醇,反应所得乙二醇稀溶液经薄膜蒸发器浓缩,再经脱水、精制得到合格的乙二醇产品及副产品。
环氧乙烷直接水合法是目前国内外工业化生产乙二醇的主要方法,目前,这种生产技术基本上由Shell、Halcon-SD以及UCC三家公司垄断,他们的工艺技术和工艺流程基本上相似,三家公司的专利技术主要区别体现在一些技术细节上。
由于反应液中含有大量的水,需要设置多个蒸发器脱水,造成工艺流程长,设备多,能耗高,直接影响乙二醇的生产成本,这也是现行乙二醇工业生产方法的主要缺点。
1.2环氧乙烷催化水合法环氧乙烷催化水合法是针对目前直接水合法生产乙二醇工艺中水比高的缺点,为了提高选择性,降低水比,同时保证降低反应温度和能耗。
目前,Shell公司、UCC公司、莫斯科门捷列夫化工学院、上海石油化工研究院等机构已经发表了一些环氧乙烷催化水合法制乙二醇的专利文献,其关键是催化剂的研制与开发,大致可分为均相催化水合法和非均相催化水合法两大类其中最有代表性的生产方法是Shell公司的非均相催化水合法和UCC公司的均相催化水合法。
Shell公司1994年报道了季胺型酸式碳酸盐阴离子交换树脂作为催化剂进行环氧乙烷催化水合的工艺,环氧乙烷转化率达到95%~98%,乙二醇选择性为97%~98%。

煤化工知识点之:乙二醇工艺方案的选择1石油路线工艺1.1环氧乙烷直接水合法1859年Wurtz首次将乙二醇二乙酸酯与氢氧化钾作用制得乙二醇。
1860年,又由环氧乙烷直接水合制得,其后经过不断技术改进,环氧乙烷直接水合法不断衍生出氯乙醇法、直接氧化法(空气氧化法、氧气氧化法)等工艺,最新技术为氧气氧化法,其工艺原理为环氧乙烷氧化反应原料乙烯和纯氧与循环气混合后,进入固定床环氧乙烷反应器,在入口温度约200℃,压力约2.OMPa的条件下,在高选择性银催化剂的作用下发生乙烯氧化反应,主反应生成环氧乙烷,氧化反应包括选择氧化和深度氧化,其反应过程:主反应(选择氧化):C2H4+1/202→C2H40+105.5kJ/mol并列副反应(深度氧化):C2H4+302→2C02+2H20+1422.6kJ/mol并列副反应(深度氧化):C2H4O+5/2O2→2CO2+2H2O+1316.4kJ/mol目前此工艺技术全部掌握在外资手中,Shell、DOW(陶式化学公司)和SD二家技术的生产能力合计占总生产能力的91%,其中Shell占38%,SD 占31%,DOW占22%,余下的9%主要为德国的BASF、日本的触媒公司、意大利的SNAM等公司占有。
由于反应中环氧乙烷与水以l:20-22(摩尔比)混合,需要大量的水,并且水大量过剩,产物中乙二醇的浓度较低,因此为了提纯出产品需蒸发除去大量的水分,生产工艺流程长、设备多、能耗高、成本较高。
1.2环氧乙烷催化水合法针对环氧乙烷直接水合法生产乙二醇工艺中存在的不足,为了提高选择性,降低用水量,降低反应温度和能耗,世界上许多公司进行了环氧乙烷催化水合生产乙二醇技术的研究和开发工作。
其技术的关键是催化剂的生产,生产方法可分为均相催化水合法和非均相催化水合法两种,其中最有代表性的生产方法是Shell公司的非均相催化水合法和UCC公司的均相催化水合法。
尽管许多公司在环氧乙烷催化水合生产乙二醇技术方面做了大量的工作,大大降低了水比,提高了环氧乙烷的转化率和乙二醇的选择性,但在催化剂制备、再生和寿命方面还存在一定的问题.因而采用该方法进行大规模工业化生产还待时日。
摘要乙二醇在国民经济中有着极其重要的地位,广泛用于生产聚酯纤维、薄膜、容器瓶类等聚酯系列产品和汽车防冻剂,但国内乙二醇的产量一直无法满足国内市场的强劲需求。
因此,本设计以乙二醇精制为中心和重点,经过严密的计算和论证,得到了肯定的结果。
关键词:乙二醇;环氧乙烷;水合法。
目录前言 (1)1文献综述...........................................................................1.1 乙二醇工业的发展[1][2]........................................前言乙二醇在国民经济中有着极其重要的地位,是大宗有机化工产品。
广泛用于生产聚酯纤维、薄膜、容器瓶类等聚酯系列产品和汽车防冻剂,还可用于除冰剂、表面涂料、表面活性剂、增塑剂、不饱和聚酯树脂以及合成乙二醇醚、乙二醛、乙二酸等化工产品的原料,虽然乙二醇产品用途极广,但国内乙二醇的产量一直无法满足国内市场的强劲需求,乙二醇自给率不足50%,如图1有相当大的部分需要进口,易受国际市场供求关系的影响。
因此,发展和技术改造乙二醇工艺设计对我国经济发展有着重要的意义。
随着我国市场经济的发展,以前那种单纯*增大原料和能源的消耗来提高产量的做法已逐渐被淘汰,继续这种做法的企业已经濒临破产倒闭;现在只有依*科技的力量,通过技术的改造来降低能源的消耗,同时使各种生产数据得到优化的配置,才是摆脱困境最有效的方法。
乙二醇工艺设计中,乙二醇的精制是整个工艺流程的核心部分,关系着乙二醇产品的质量和产量。
因此,本设计以乙二醇精制为中心和重点,经过严密的计算和论证,得到了肯定的结果。
该技术具有世界共同发展趋向的节能性,是生产乙二醇工艺的重大突破。
图1 我国近些年乙二醇的供需情况年份产量万吨/年进口量万吨/年需求量万吨/年自给率%2000 2001 2002 2003 2004 2005 2006 2007 2008 9080909694110156174214105160214251339400406480522195240304347433510562654736463330282221282729第1章文献综述1.1乙二醇工业的发展[1][2]乙二醇是最简单和最重要的脂肪族二元醇,它在有机化工生产中是一种重要的基本原料,尤其广泛用于聚酯纤维、聚酯塑料的生产。
全球乙二醇生产工艺路线及成本对比一目前全球乙二醇生产工艺路线及成本对比目前世界上大规模生产乙二醇的方法有3种:1)采用天然气为原料制乙二醇(主要集中在中东地区),2009年产能620万吨,占全球总产能的32%,预计2011年产能将达到1000万吨;2)以石油为原料制乙二醇,2009年全球产能1300万吨,占世界的68%;3)采用褐煤做原料生产乙二醇(丹化科技),年产能20万吨。
目前中东地区天然气3乙二醇每吨生产成本约250美元。
据丹化科技披露,即便能以非常优惠的价格(130元/吨)获得褐煤资源,煤制乙二醇生产成本依然高达2600元/吨(约合380美元/吨)。
因此相比天然气制乙二醇,即使加上运费(从中东到中国最新报价20美元/吨),煤制乙二醇也不具备竞争力。
与石油制乙二醇相比,煤制乙二醇是否具备成本优势,取决于国际油价和能否获得廉价煤炭资源。
根据丹化科技煤制乙二醇实验数据推算,若煤价为750元/吨,当石油价跌到67美元/桶以下时,煤制乙二醇将不具备成本优势。
以天然气为原料制乙二醇(环氧乙烷水合法):具体工艺路线是:首先以天然气生产乙烯,然后乙烯生产乙二醇。
采用该工艺路线,乙二醇的生产成本主要由两部分构成:1)原料成本约为6300元(其中乙烯市场价格按照10 000元/吨计算,成本6 000元);2)其他成本约700元(其中固定成本约330元,动力成本约380元)。
以石油为原料制作乙二醇(环氧乙烷水合法):具体工艺路线是:首先石脑油生产乙烯,然后使用乙烯生产乙二醇,本工艺路线和天然气为原料的工艺路线的区别在于获得乙烯的方式,前者通过石脑油制作乙烯,后者通过天然气制作乙烯。
目前全球乙烯总需求约为1.12亿吨,总产能约1.31亿吨。
其中约50%用石脑油制作,50%用天然气(乙烷、丙烷等)制作,用两种不同工艺路线的生产成本存在巨大差异。
用天然气生产乙烯的成本:中东乙烯装置以乙烷为原来成产成本最低达100美元/吨,平均为240美元/吨;美国墨西哥湾沿岸为250美元/吨。
乙二醇生产反应器操作规程一.工艺流程简述1.反应原理在乙二醇反应器中,来自精制塔底的环氧乙烷和来自循环水排放物流的水反应形成乙二醇水溶液。
其反应机理如下:主反应:乙二醇副反应:二乙二醇2.工艺流程简述精制塔塔底物料在流量控制下同循环水排放物流以1:22的摩尔比混合。
混合后通过在线混合器进入乙二醇反应器。
反应为放热反应,反应温度为200℃时,每生成一摩尔乙二醇放出热量为8.315×104J。
来自循环水排放浓缩器的水,是在同精制塔塔底物料的流量比控制下进入乙二醇反应器上游的在线混合器的。
混合物流通过乙二醇反应器在此反应形成乙二醇。
反应器的出口压力是通过维持背压来控制的。
从乙二醇反应器流出的乙二醇-水物流进入干燥塔。
其反应流程图如图1所示:图 1乙二醇生产工艺流程图二.操作1.开车前的检查和准备A、把循环水排放流量控制器置于手动,开始由循环水排放浓缩器底部向反应器进水。
在乙二醇反应器进口排放这些水直到清洁为止。
B、关闭进口倒淋阀并开始向反应器充水,打开出口倒淋阀,关闭乙二醇反应器压力控制阀。
当反应器出口倒淋阀排水干净时关闭它。
C、来自精制塔塔底泵的热水用泵通过在线混合器送到乙二醇反应器。
各种联锁报警均应校验。
D、当乙二醇反应器出口倒淋排放清洁时,把水送到干燥塔。
E、运行乙二醇反应器压力控制器,调节乙二醇反应器压力使之接近设计条件。
F、干燥塔在运行前,干燥塔喷射系统应试验。
后面的所有喷射系统都遵循这个一般程序。
为了在尽可能短的时间内进行试验,关闭冷凝器和喷射器之间的阀门,因此在试验期间,塔不必排泄。
G、检查所有喷射器的倒淋和插入热井底部水封的尾管。
用水充满热井所有喷射器冷凝器,并密封管线。
H、打开喷射器系统的冷却水流量。
稍开高压蒸汽管线过滤器的倒淋阀,然后稍开到喷射泵的蒸汽阀。
关闭倒淋阀,然后慢慢打开蒸汽阀。
I、使喷射器运行直到压力减少到正常操作压力。
在这个试验期间应切断塔的压力控制系统。
隔离切断阀下游喷射系统和相关设备,在24小时内,最大允许压力上升速度为33.3Pa/h。
优秀毕业论文(设计)5万吨乙二醇生产工艺初步设计石河子大学毕业设计题目: 5万吨/年乙二醇生产工艺初步设计5万吨/年乙二醇生产工艺初步设计摘要乙二醇是一种重要的石油化工基本有机原料,主要用于生产聚酯纤维、不饱和聚酯树脂、防冻剂等。
目前.国内乙二醇的工业生产方法是环氧乙烷直接水合法。
我国目前拥有大小不等的环氧乙烷/乙二醇(EO/EG)装置11套,但是相比于国外的同类装置,这些装置的工艺落后,能耗和水耗都较高,因此研究EO/EG工艺优化以降低生产成本,具有非常现实的意义。
本设计主要是针对乙二醇工艺的缺点,采用比较新的反应精馏工艺,利用化工模拟软件ASPEN PLUS对过程进行模拟优化,最后得到即可达到产品标准又可满足设计任务的结果。
同时还对主要设备进行了选型,绘制了PID工艺流程图。
关键词:环氧乙烷;乙二醇;反应精馏;ASPEN PLUS;EO/EG50000 tons/year glycol production processpreliminary designAbstractThe glycol is an important basic organic raw materials of petrochemical, mainly for the production ofpolyester fiber, unsaturated polyester resins, antifreeze, etc.. The present. The methods of industrialproduction of the domestic ethylene glycol is ethylene oxide legitimate directwater.China currently has a size ranging from EO / EG set 11 sets, but compared to similar devices in theforeign and backward technology of these devices, energy and water consumption are higher, so theresearchers EO / EG process optimization to reduce production costs. a very realsense.This design is for the shortcomings of the ethylene glycol process, using the new reactive distillationprocess, the use of chemical process simulation software ASPEN PLUS process simulation andoptimization, and finally get to meet product standards and to meet the design task results. Of majorequipment selection, draw the diagram of the PID process.Key words: ethylene oxide; glycol; reactive distillation; ASPEN PLUS; EO /EG目录第一章 文献综述 (5)1.1 简介 (5)1.1.1 乙二醇物理性质 (5)1.1.2 乙二醇的用途 (5)1.2 国内乙二醇生产现状 (6)1.3 乙二醇生产技术现状及进展 (8)1.3.1 乙烯直接水合法 (9)1.3.2甲醛合成法 (9)1.3.3 环氧乙烷水合法 (10)1.3.4 碳酸乙烯酯法 (11)1.3.5合成气法 (12)1.3.6 氧化偶联法 (12)1.3.7 多元醇加氢裂解法 (13)1.3.8反应精馏技术 (13)1.4 反应精馏技术简介 (13)1.4.1反应精馏技术的应用 (14)1.4.2反应精馏的主要优点 (14)1.4.3 乙二醇反应精馏工艺流程 (15)1.5设计内容与目标 (16)第二章 工艺流程模拟 (17)2.1设计任务书 (17)2.2 物性方法的选择 (18)2.3反应精馏塔的模拟优化 (18)2.3.1反应精馏涉及反应 (18)2.3.2反应精馏塔建模与参数设置 (19)2.3.3反应精馏塔各项参数优化 (22)2.3脱水塔的模拟优化 (29)2.3.1脱水塔简捷计算 (29)2.3.2 脱水塔严格计2.3 乙二醇精制塔模拟优化 (41)2.3.1乙二醇精制塔简捷计算 (41)2.3.2乙二醇精制塔严格计算 (42)第三章 全流程模拟及生产任务校核 (52)3.1 全流程模拟及结果 (52)3.2生产任务校核 (54)第四章 设备计算及选型 (55)4.1反应精馏塔设备计算 (55)4.1.1反应段操作条件及物性参数 (55)4.1.2反应段尺寸计算 (56)4.1.3精馏段操作条件及物性参数 (57)4.1.4精馏段尺寸计算 (58)4.2 塔体尺寸计算 (67)4.2.1塔高的计算 (67)4.2.2塔体和封头选材 (68)4.2.3塔体壁厚计算 (68)4.2.4塔设备质量载荷计算 (70)4.2.5基础环设计 (72)4.3设备机械性能校核 (73)4.3.1上封头校核 (73)4.7.2下封头校核 (73)4.7.3塔体强度计算汇总表 (73)第五章 三废处理 (74)设计总结 (75)参考文献 (76)致谢 (78)附录 (79)第一章文献综述1.1 简介1.1.1 乙二醇物理性质乙二醇(Ethylene Glycol,简称EG)又名“甘醇”、“1,2-亚乙基二醇”,化学式为(HOCH2)₂,是最简单和最重要的脂肪族二元醇,同时也是一种重要的有机化工原料。
摘要乙二醇在国民经济中有着极其重要的地位,广泛用于生产聚酯纤维、薄膜、容器瓶类等聚酯系列产品和汽车防冻剂,但国内乙二醇的产量一直无法满足国内市场的强劲需求。
因此,本设计以乙二醇精制为中心和重点,经过严密的计算和论证,得到了肯定的结果。
关键词:乙二醇;环氧乙烷;水合法。
目录前言 (1)1 文献综述...........................................................................1.1 乙二醇工业的发展[1][2]........................................前言乙二醇在国民经济中有着极其重要的地位,是大宗有机化工产品。
广泛用于生产聚酯纤维、薄膜、容器瓶类等聚酯系列产品和汽车防冻剂,还可用于除冰剂、表面涂料、表面活性剂、增塑剂、不饱和聚酯树脂以及合成乙二醇醚、乙二醛、乙二酸等化工产品的原料,虽然乙二醇产品用途极广,但国内乙二醇的产量一直无法满足国内市场的强劲需求,乙二醇自给率不足50%,如图1 有相当大的部分需要进口,易受国际市场供求关系的影响。
因此,发展和技术改造乙二醇工艺设计对我国经济发展有着重要的意义。
随着我国市场经济的发展,以前那种单纯*增大原料和能源的消耗来提高产量的做法已逐渐被淘汰,继续这种做法的企业已经濒临破产倒闭;现在只有依*科技的力量,通过技术的改造来降低能源的消耗,同时使各种生产数据得到优化的配置,才是摆脱困境最有效的方法。
乙二醇工艺设计中,乙二醇的精制是整个工艺流程的核心部分,关系着乙二醇产品的质量和产量。
因此,本设计以乙二醇精制为中心和重点,经过严密的计算和论证,得到了肯定的结果。
该技术具有世界共同发展趋向的节能性,是生产乙二醇工艺的重大突破。
图 1 我国近些年乙二醇的供需情况产量进口量需求量自给率年份万吨/年万吨/年万吨/年%2000901051954620018016024033200290214304302003962513472820049433943322200511040051021200615640656228200717448065427200821452273629第1章文献综述1.1 乙二醇工业的发展[1][2]乙二醇是最简单和最重要的脂肪族二元醇,它在有机化工生产中是一种重要的基本原料,尤其广泛用于聚酯纤维、聚酯塑料的生产。
年产五十万吨乙二醇的生产工艺流程下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by theeditor. I hope that after you download them,they can help yousolve practical problems. The document can be customized andmodified after downloading,please adjust and use it according toactual needs, thank you!In addition, our shop provides you with various types ofpractical materials,such as educational essays, diaryappreciation,sentence excerpts,ancient poems,classic articles,topic composition,work summary,word parsing,copy excerpts,other materials and so on,want to know different data formats andwriting methods,please pay attention!年产五十万吨乙二醇的生产工艺流程一、准备工作阶段。
产5万吨乙二醇工艺流程成人高等教育毕业设计(论文)年产5万吨乙二醇工艺流程设计题目_________________________________学号_________________________________学生_________________________________联系电话_________________________________指导教师_________________________________教学站点_________________________________专业_________________________________完成日期_________________________________乙二醇在经济中有着极其重要的地位。
用于生产聚酯纤维、薄膜、容器瓶类等系列产品和汽车防冻剂,还可用于除冰剂、表面涂料、表面活性剂、增塑剂等化工产品的原料。
因此,发展和技术改造乙二醇工艺设计对我国经济发展有着重要的意义。
在本设计中,进行对原料、产品的说明,生产方法的选择,工艺流程介绍,根据反应原理进行原料配比。
其中,整个工艺流程及原料的投入量是本设计的核心部分,关系着乙二醇的产品的质量和产量,因此本设计以工艺流程和原料投入量为中心和重点,经过原料投入量的严密的计算和论证,得到了肯定的结果。
关键词乙二醇环氧乙烷精制温度压力AbstractThe glycol has the extremely important status in the economy. For producing polyester fiber, thin film and vessel bottle kind and other serial products and automobile antifreeze, but may also be used in the de-icing agent, superficial coating, surface active agent, plasticizer and other product of chemical industry of the raw materials. Therefore, the development and technical transformation glycol technological design has the vital significance to our country economic development. In this design, carries on to the explanation of raw material and product, the choice of production method, the technical process introduced that carries on the molar ratio of raw materials according to the reaction principal. And, the inputs of entire technical process and raw material are the core component of design, is relating glycol the quality and output of product, therefore this design centers on the technical process and raw material inputs the center, after the strict computation and proof of raw material inputs, obtained the affirmative result.Key word :Glycol oxirane Purification Temperature Pressure目录乙二醇在经济中有着极其重要的地位。
用于生产聚酯纤维、薄膜、容器瓶类等系列产品和汽车防冻剂,还可用于除冰剂、表面涂料、表面活性剂、增塑剂等化工产品的原料。
因此,发展和技术改造乙二醇工艺设计对我国经济发展有着重要的意义。
在本设计中,进行对原料、产品的说明,生产方法的选择,工艺流程介绍,根据反应原理进行原料配比。
其中,整个工艺流程及原料的投入量是本设计的核心部分,关系着乙二醇的产品的质量和产量,因此本设计以工艺流程和原料投入量为中心和重点,经过原料投入量的严密的计算和论证,得到了肯定的结果。
................................................. 5一、乙二醇工业的发展 ......................................................................................................................... 9二、国外乙二醇工业的概况 ............................................................................................................. 10三、国内乙二醇工业的概况 ............................................................................................................. 10第二章生产工艺..................................................................................................................................... 10一、产品说明..................................................................................................................................... 10二、生产乙二醇的原料说明 ............................................................................................................. 11表1环氧乙烷质量指标..................................................................................................................... 11三、乙二醇的生产方法介绍及方法的选择 ..................................................................................... 11四、生产乙二醇的反应原理 ............................................................................................................. 12五、生产乙二醇的工艺条件 ............................................................................................................. 12六、生产乙二醇的设备及反应器的选择 ......................................................................................... 13第三章生产乙二醇的工艺流程 ......................................................................................................... 13反应器的物料衡算: ............................................................................................................................ 16反应器进料中环氧乙烷和水的摩尔比为1︰18,因为环氧乙烷在反应器中基本达到全部转换,故可设为98%,乙二醇年产量为5万吨。
.............................................................................................. 16由于原料为环氧乙烷和水,设环氧乙烷的质量为M环氧乙烷,水的质量为M水 ............................ 16所以,依据已知条件N环氧乙烷︰N水=1︰18;环氧乙烷转换率为98%;乙二醇年产量为5万吨;M 环氧乙烷=44G/MOL,M水=18G/MOL........................................................................................................... 16由此可计算M环氧乙烷和M水.............................................................................................................. 16因为N环氧乙烷︰N水=1︰18 ........................................................................................................... 16M环氧乙烷︰M环氧乙烷=M水︰M水=1/18(1) .............................................................. 16M环氧乙烷︰M水×M环氧乙烷︰M水=1/18..................................................................................... 16M环氧乙烷︰M水×18/44=1/18 ........................................................................................................ 16M环氧乙烷︰M水=1/18×44/18 ........................................................................................................ 16=0.135816由于环氧乙烷转换率为98%,乙二醇年产量为5万吨,可计算得M环氧乙烷和M水: ............... 16M环氧乙烷+M水=5×107/98%(2) ................................................................ 16=5×109/98 ............................................................................................................................................ 16=5.1×107(KG) ................................................................................................................................... 16由(1)、(2)式可知: .................................................................................................................... 16M环氧乙烷=0.1358M水(3)..................................................................... 16M环氧乙烷+M水=5.0×107(KG)(4).................................................................. 16把(3)式代入(4)式可计算得: .................................................................................................... 160.1358 M水+ M水=5.1×107(5).................................................................... 161.1358 M水=5.1×107 .......................................................................................................................... 16M水=4.49×107(KG)........................................................................................................................... 16由(4)、(5)式联立可得: ............................................................................................................ 16M环氧乙烷+4.49×107=5.1×107 ........................................................................................................ 16M环氧乙烷=0.61×107(KG) ............................................................................................................. 16第六章乙二醇生产技术的展望及发展建议 ......................................................................................... 17通过这次设计让我知道了知识是综合能力的体现,不是独立的学科。