组蛋白乙酰化 去乙酰化 综述
- 格式:pdf
- 大小:35.34 KB
- 文档页数:6

表观遗传的调控机制摘要: 表观遗传是非DNA 序列遗传信息的传递, 它不涉及基因序列的改变, 不符合孟德尔式的遗传方式。
表观遗传学研究的是生物可遗传的染色质修饰。
目前,其主要研究内容包括DNA 甲基化、翻译后组蛋白修饰、组蛋白组成变化。
其中DNA 甲基化是一种重要的表观遗传修饰方式, 是调节基因组功能的重要手段。
组蛋白修饰作为表观传中重要的调控机制之一, 在包括基因表达调控等多种生物学过程中起着重要作用。
组蛋白甲基转移酶和组蛋白去甲基化酶共同参与形成和维持不同的组蛋白甲基化状态, 继而通过多种分子参与对组蛋白甲基化修饰的识别而引起下游过程的发生。
组蛋白乙酰化和去乙酰化修饰也是调控表观遗传机制之一。
最近人们还发现非编码的RNA也参与了表观遗传调控。
关键词:表观遗传,DNA甲基化,组蛋白修饰,RNA调控。
一 DNA甲基化调控表观遗传经典遗传学认为,生命的遗传信息储存在 DNA的碱基序列上,几乎所有的生命活动都受基因调控。
但是,作为开放的复杂系统,生命活动从来就不是由一种因素就能完全决定的。
随着科学的发展,人们发现一些 DNA 或染色体水平的修饰也会造成基因表达模式的改变。
这种通过有丝分裂或减数分裂来传递非DNA 序列遗传信息的现象称为表观遗传(epigenetic inheritance)。
由于它不涉及基因序列的改变,不符合孟德尔式的遗传方式,因此它是一种全新的遗传机制。
表观遗传修饰有许多,其中 DNA 甲基化是基因组DNA 的一种最重要的表观遗传修饰方式,是调节基因组功能的重要手段。
在植物中,DNA 甲基化参与细胞的许多生物学过程,在植物生长发育及进化过程中起着重要的调节作用。
1 植物DNA胞嘧啶甲基转移酶植物DNA的甲基化是在 DNA 甲基转移酶(DNAMethyltransferase,DMT)的作用下,将 S- 腺苷甲硫氨酸上的甲基基团转移到 DNA 分子的胞嘧啶碱基上。
在植物细胞中广泛存在的有三类结构和功能上不同的胞嘧啶甲基转移酶[1,2]。

乙酰化和去乙酰化修饰
乙酰化修饰通常发生在蛋白质的赖氨酸残基上,通过将乙酰基添加到赖氨酸上来改变蛋白质的电荷、构象和亲疏水性质。
这可以影响蛋白质的互作、局部结构和整体折叠状态。
乙酰化修饰也可以发生在DNA上,通过将乙酰基添加到组蛋白蛋白质上来影响DNA的紧密度和可读性,从而影响基因表达。
去乙酰化修饰则是通过去除蛋白质、DNA或RNA上的乙酰基来调节它们的功能。
这种修饰方式常常由去乙酰化酶催化,是维持细胞内化学平衡的重要机制。
例如,去乙酰化修饰可以改变某些DNA结合蛋白质与DNA的亲和力,从而影响染色质的结构和转录调控。
总之,乙酰化和去乙酰化修饰是细胞内非常重要的化学修饰方式,通过调节蛋白质、DNA或RNA的功能和稳定性,维持着细胞内的正常生理活动。
- 1 -。

摘要衰老是一个普遍的生物学现象,衰老控制着生物寿命的长短,主要受遗传因子和环境因素所影响。
了解衰老的分子机制,对于延缓衰老、保持生命活力具有重要的意义。
热休克蛋白(HSP)作为高度保守的“分子伴侣”,在细胞内广泛地参与许多复杂的功能活动,可以抵制衰老过程中一些有害蛋白的发生。
其基因的表达调控是一种特殊的真核基因表达模式,包括基础水平和诱导水平的表达。
由组蛋白乙酰转移酶(HAT)和组蛋白去乙酰化酶(HDAC)催化的乙酰化反应在真核基因的表达调控中起着重要作用,这两种酶通过对核心组蛋白进行可逆修饰来调节核心组蛋白的乙酰化水平,从而调控转录的起始与延伸。
组蛋白去乙酰化酶抑制剂(HDI)可以通过抑制HDAC活性提高组蛋白乙酰化水平,是研究乙酰化修饰在真核基因表达调控中的作用的有用工具。
本论文一方面采用HDItrichostatinA<TSA)和丁酸钠(BuA)喂食果蝇,改变果蝇体内组蛋白乙酰化水平,系统地研究组蛋白乙酰化修饰、HSP的表达以及寿命调控三者之间的关系。
结果发现hsp基因在长寿果蝇中具有较高的基础表达、较快的热激诱导反应速度以及较强的高温抵抗性。
同时,不同的hsp基因在果蝇衰老过程中的作用不尽相同,hsp22的作用最为重要,hsp70次之,而hsp26的表达几乎与寿命无关。
使用HDITSA和BuA喂食果蝇可以延长其寿命,但不同的HDI的作用机制不尽相同,同一种HDI对不同寿命品系的果蝇的延长程度也不尽相同。
TSA的处理有一种时间依赖性,更长时间的TSA处理对寿命是有利的;而BuA的处理却与此不同,过长时间的处理反而加速衰老。
同样的去乙酰化酶抑制剂,同一剂量处理,在不同果蝇品系种的作用不同,它们对短寿果蝇寿命的延长程度更为明显。
另外,HDI处理还促进果蝇衰老过程中hsp基因的基础表达和诱导表达,但是随着衰老的进行,这种促进作用逐渐减弱。
同样在不同寿命的果蝇品系中,其提高hsp基因表达的程度也不一样。


组蛋白去乙酰化技术在表观遗传学研究中的
应用
随着科学技术的不断发展,表观遗传学作为一门新兴的生物学科学日渐受到科学研究人员的关注。
在表观遗传学领域中,组蛋白去乙酰化技术被广泛用于研究基因组结构和功能,其在表观遗传学研究中的应用也越来越广泛。
表观遗传学是研究遗传信息以外的遗传信息传递的一门学科,主要是研究如何通过化学修饰来调节基因表达,以及在通过遗传方式传递的特征外,如何通过表观遗传途径传递表型特征。
而组蛋白是在核酸糖苷酸骨架上重要的蛋白质,在细胞核中占有重要的地位。
乙酰化是一种常见的组蛋白修饰形式,可以影响基因的调控和表达。
因此,组蛋白去乙酰化技术成为了研究表观遗传学的重要手段之一。
通过组蛋白去乙酰化技术,研究人员可以探索组蛋白乙酰化和去乙酰化对基因表达的影响,了解细胞信号传导通路等生理生化过程的调控机制,并深入了解越来越多的疾病发生发展机理。
在研究中,组蛋白去乙酰化技术可以起到去乙酰化的作用,从而在细胞内的DNA中恢复原有的生化信息,帮助研究人员更好地理解DNA乙酰化和去乙酰化的机制。
组蛋白去乙酰化技术的应用已经从初期的对基础生命科学领域,进一步拓展到药物研发和治疗方面。
例如,在药物研发中,组蛋
白去乙酰化技术可以较好地检测药物的毒性及其可能的副作用,
为研发安全高效的药物提供了新的突破口。
此外,组蛋白去乙酰
化技术在产前诊断和妇科疾病等领域也有广泛的应用。
总之,组蛋白去乙酰化技术在表观遗传学研究中的应用及其发
展前景是非常广阔的。
未来,在不断发展的技术和工具的支持下,组蛋白去乙酰化技术将在表观遗传学的研究中扮演更加重要的角色。

组蛋白去乙酰化酶的研究进展组蛋白去乙酰化酶(HDACs)是一类重要的酶类蛋白质,在细胞核内对染色质修饰起着重要的作用,调节基因的表达。
在过去的几十年中,对HDACs的研究取得了重大进展,深化了人们对这些蛋白质的认知和理解。
一、HDACs的分类HDACs主要分为四类:I、II、III和IV类。
其中I、II类又分为A、B、C、D子型。
这四类HDACs在受体结构及功能上都有所不同。
从结构上来看,HDACs主要由三个结构域组成,包括一个催化结构域、一个辅酶A结合结构域和一个维持结构稳定的结构域。
二、HDACs的生物学功能HDACs主要通过催化组蛋白的去乙酰化反应来调节基因的表达。
在生物体内,组蛋白去乙酰化是一个非常关键的生物过程,能够帮助细胞调节基因转录的水平,从而影响细胞的生长、分化、凋亡和代谢等生物过程。
HDACs通过去掉组蛋白的乙酰基,使其变得更加紧密结合,从而抑制相应的基因表达。
三、HDACs在疾病治疗上的应用近年来,越来越多的研究表明,在很多重要的疾病中,包括癌症、炎症和神经系统疾病等,HDACs扮演着非常重要的角色。
特别是在抗癌治疗方面,HDACs已经成为了一个非常重要的领域。
很多针对HDACs的抗癌药物,如美罗华(Merck)、法诺司汀(Novartis)等,已经被广泛使用,并取得了很好的治疗效果。
四、HDACs与DNA甲基化的关系HDACs在细胞内还与DNA甲基化过程密切相关。
DNA甲基化是一种常见的表观修饰形式,在基因表达、DNA复制和细胞分化等生物过程中起着非常重要的作用。
通过调节HDACs的表达和活性,可以进一步影响细胞内DNA甲基化修饰,从而调节基因的表达。
总结起来,HDACs是一类非常重要的酶类蛋白质,对于细胞核内染色质修饰和基因转录调节起着重要的作用。
在细胞内,通过调节HDACs的表达和活性,可以进一步影响基因的表达和细胞的生物过程,具有广泛的研究和应用前景。

组蛋白去乙酰化酶(HDACs)的研究进展【摘要】在肿瘤的表观遗传学研究中,组蛋白的乙酰化修饰对肿瘤的发生发展起重要作用。
正常细胞体一旦出现核内组蛋白乙酰化与去乙酰化的失衡,即会导致正常的细胞周期与细胞代谢行为的改变而诱发肿瘤。
组蛋白去乙酰化酶(histone deacetylases,HDACs)催化组蛋白的去乙酰化,维系组蛋白乙酰化与去乙酰化的平衡状态,与癌相关基因转录表达、细胞增殖分化及细胞凋亡等诸多过程密切相关。
从组蛋白去乙酰化酶HDACs的结构分类及其与肿瘤发生发展关系两方面对HDACs做一综述。
【关键词】组蛋白去乙酰化酶(HDACs);肿瘤;表观遗传学Abstract:The modification for histone acetylation is of great importance for formulation and development oftumors in the epigenetic study of tumors. The disequilibrium of histone acetylation and deacetylation may cause some changes of cell cycle and cell metabolism. Histone deacetylases (HDACs) catalyze the deacetylation of histones,and maintain the equilibrium between histone acetylation and deacetylation as well. They are related to many regulation processes containing transcription of oncogene,cell cycle,apoptosis and so on. The structure classification of HDACs and the relationship between the HDACs and the formation and advancement of tumor were reviewed in this paper.Key words:histone feacetylases (HDACs); tumor; epigenetics肿瘤的发生是一个复杂的病理过程,受多重因素的影响,包括个体遗传因素、环境因素、物理化学因素、分子生物学因素等等。

组蛋白去乙酰化
乙酰化是多种有机体重要的信号传导过程之一,它可以促进细胞内蛋白质元件以及多只仓库中的蛋白质的功能表达,从而帮助调节该细胞的活动和发育期蛋白质的稳定性。
乙酰化是通过乙酰转移酶介导的,它们把乙酰基转移至从未乙酰过的蛋白质或者脯氨酸上,从而调节蛋白质的结构,细胞信号转导和其他的功能,乙酰化无疑是信号转导的重要环节,它能够发挥重要作用,有助于调节分子级别和细胞状态的变化。
乙酰化蛋白去乙酰化技术是一种能够反向地去乙酰化蛋白质的技术,它使用一种叫做乙酰羧化酶的酶辅助去乙酰化蛋白,此酶可以通过去乙酰化蛋白的羧基来失去乙酰基,或者它可以追踪乙酰蛋白的结构,从而提供有关乙酰化蛋白质的相关信息。
去乙酰化蛋白技术具有广泛的应用,它可以用于探索乙酰化蛋白的信号转导机制,可以用于分析和鉴定乙酰化蛋白质,也可以用于鉴定特定的乙酰蛋白,从而将其作为药物靶点。
此外,它还可以被用于检测乙酰蛋白质与细胞进化和发育,炎症反应以及免疫应答等相关联的调节因子。
综上所述,乙酰化是细胞的重要信号传递过程,去乙酰化蛋白技术被广泛用于探索蛋白乙酰化的信号转导机制,也可以用于分析和鉴定乙酰化蛋白质,有助于细胞进化发育和免疫应答等行为的调控。
它对细胞发育和机体功能具有十分重要的意义,也是未来研究和治疗疾病的热点与重点。

组蛋白去乙酰化酶的
组蛋白去乙酰化酶是一类重要的调节蛋白活性的酶,它们可以通过去
乙酰化组蛋白来调节蛋白的活性。
组蛋白去乙酰化酶是一类多功能的酶,它们可以调节细胞内的各种生物学过程,如细胞凋亡、细胞周期、细胞增殖、细胞迁移、细胞分化等。
组蛋白去乙酰化酶可以通过去乙
酰化组蛋白来调节蛋白的活性,从而调节细胞内的各种生物学过程。
组蛋白去乙酰化酶的结构主要由两部分组成,即蛋白质结构和酶结构。
蛋白质结构主要由蛋白质结构域和蛋白质结构域组成,而酶结构主要
由酶结构域和酶结构域组成。
组蛋白去乙酰化酶的活性受到蛋白质结
构域和酶结构域的调节,而蛋白质结构域和酶结构域又受到细胞内的
各种因素的调节。
组蛋白去乙酰化酶在细胞内的作用是非常重要的,它们可以调节细胞
内的各种生物学过程,如细胞凋亡、细胞周期、细胞增殖、细胞迁移、细胞分化等。
组蛋白去乙酰化酶可以调节细胞内的各种信号通路,从
而调节细胞的生长、分化和凋亡。
此外,组蛋白去乙酰化酶还可以调
节细胞内的蛋白质稳定性,从而调节细胞的表观遗传学。
组蛋白去乙酰化酶的研究可以帮助我们更好地理解细胞内的各种生物
学过程,从而更好地治疗疾病。
近年来,组蛋白去乙酰化酶的研究取
得了很大的进展,已经发现了许多新的组蛋白去乙酰化酶,并且发现
了它们在细胞内的作用机制。
未来,组蛋白去乙酰化酶的研究将会取
得更多的进展,为我们更好地治疗疾病提供更多的帮助。

说明组蛋白乙酰化和去乙酰化影响基因转录
的机制
组蛋白乙酰化和去乙酰化是两种基本的表观遗传学调控方式,可以影响基因的转录活性,从而决定生物的发育和生命过程。
组蛋白是核小体中的主要蛋白质,而核小体是染色体基本单位的组成部分。
组蛋白可以被翻译修饰,其中包括乙酰化和去乙酰化。
乙酰化增加了组蛋白的乙酰基(醋酸基)含量,从而使组蛋白的阳离子性减少,DNA与组蛋白的结合力减弱,导致染色体更加松散,更容易读取DNA上的基因序列,因此可以促进基因转录。
而去乙酰化则有相反的作用,可以增加组蛋白阳离子性,增强DNA与组蛋白的结合力,使染色体更加紧密,阻碍基因转录。
组蛋白乙酰化和去乙酰化通常通过两种方式发挥作用。
一种是直接作用于转录因子,乙酰化使这些转录因子更容易与DNA结合,同时降低了转录因子与组蛋白之间的相互作用力,从而促进基因转录。
而去乙酰化则有相反的作用,可以阻碍转录因子与DNA的结合,导致基因转录受阻。
另一种方式是通过影响染色质结构来发挥作用。
乙酰化可以直接降低组蛋白和组蛋白之间的相互作用力,从而使染色体更加松散,容易转录。
去乙酰化则可以更加稳定染色体结构,从而阻碍转录因子的结合和基因的转录。
总之,组蛋白乙酰化和去乙酰化是控制基因转录的重要机制。
不同类型的细胞和生物在基因调控方面都存在着独特的表观遗传学调控
机制,而组蛋白乙酰化和去乙酰化则是其中的重要代表。
对于研究基因转录调控及其表观遗传学机制,深入了解组蛋白乙酰化和去乙酰化作用机理是非常必要的。
同时,通过在实验室中甄别组蛋白乙酰化和去乙酰化酶的活性、开发相关的抑制剂和激动剂等措施,也有助于治疗一些基因和表观表达异常相关的疾病。
修正版分子机制研究套路(九)组蛋白(去)乙酰化课题:组蛋白乙酰转移酶A在B基因转录调控中的作用1.K念介绍:表观遗传学是研究基因的核甘酸序列不发生改变的情况下基因表达了可遗传的变化的一门遗传学分支学科。
表观遗传的现象很多主要包括DNA甲基化、组蛋白修饰和非编码RNA。
染色质的基本组成单位是核小体,核小体是由146bp碱基对缠绕由H2A、H2B、H3、H4各2个组成的组蛋白八聚体构成的,各个核小体之间由H1连接,最终组成染色质。
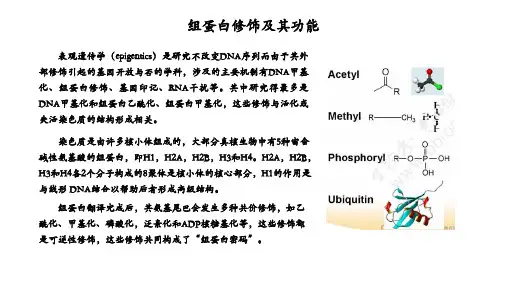
组蛋白修饰指对组蛋白N端尾部氨基酸的修饰,包括乙酰化、磷酸化、甲基化和泛素化等。
在组蛋白的各种修饰方式中,组蛋白乙酰化的研究较为透彻。
组蛋白的乙酰化主要发生在赖氨酸残基上,赖氨酸侧链含有氨基,在生理条件下带正电荷,从而能够和含有磷酸基团的DNA紧密结合,被乙酰化后使得正电荷被中和,无法和DNA紧密结合使得染色质结构松散,促进基因的表达。
组蛋白的乙酰化动态平衡由大量不同的组蛋白去乙酰转移酶HDAC)和组蛋白乙酰化酶(HAT)调节。
如前所述,组蛋白的乙酰化能够促进某些基因的激活,因此通过使用HDAC抑制剂相对提高组蛋白乙酰化的程度,能够显著上调大量具有保护作用的基因,达到治疗某些疾病的目的。
目前临床使用的HDAC抑制剂包括丙戊酸(VPA1曲古菌素A、T酸苯酯等。
早期,人们认为组蛋白修饰只是提供一个信号,指导与染色体功能相关的非组蛋白与染色体的结合。
随着研究的深入,人们越来越清楚地认识到这些修饰的某种组合对转录调控具有极其深刻的影响,"组蛋白修饰密码”假说诞生了。
这个假说推测特定的组蛋白修饰是同一个组蛋白或另一个组蛋白分子进一步修饰的决定因素,特定的共价修饰,修饰发生的顺序特征以及各种修饰的组合模式形成了复杂的"组蛋白密码",这种密码决定了基因的转录状态,修正版 这种密码会被调控染色体结构和基因转录的蛋白质解读。
• urn Lvl/ulinji • 111^1 II* U I II HII • phu^|>>iriij< y I IA ii«in 图2 :核心组蛋白八聚体的结构和组蛋白尾部可能的共价修饰3.研究思路:3.1 HDAC 抑制剂使B 基因mRNA 上调,且使B 基因启动子组蛋白H4乙酰化水平升高..33.2 组蛋白乙酰转移酶A 过表达增强B 基因启动子的转录活性 (3)3.38 基因启动子上有A 蛋白的结合,过表达A 蛋白能升高细胞内源性B 基因mRNA 水平.43.48 基因启动子上的两个Sp1结合位点被确认 ..................................... .43.49 酰转移酶A 和转录因子GATA-1协同激活B 基因启动子转录,B 基因启动子上两个 Histone acety^atianH&AC inhrNcofS(VfA, bulMS T5A)图1 :组蛋白乙酰化的动态调节过程3 H修正版GATA-1位点对于A的功能是必要的 (5)3.50酰转移酶A与转录因子GATA-1和Sp1协同增强B基因启动子的转录活性 (7)3.1HDAC抑制剂使B基因mRNA上调,且使8基因启动子组蛋白H4乙酰化水平升高为弄清楚组蛋白乙酰化是否与B基因的转录调控有关,用HDAC抑制剂丁酸钠aBu和TSA 处理cell-1细胞培养24h后进行RT-PCR分析。
百泰派克生物科技
组蛋白的乙酰化
组蛋白是真核生物染色质中的一种碱性蛋白质,可与DNA双螺旋形成DNA-组蛋白
复合物。
在不同的组蛋白酶作用下,组蛋白会发生不同的修饰,如甲基化、乙酰化、磷酸化和泛素化等。
组蛋白修饰在一定程度上会导致转录激活或基因沉默,从而调控基因表达,影响免疫系统和免疫反应,甚至导致肿瘤等疾病的发生。
在乙酰化转移酶(HAT)的作用下,组蛋白的N端碱性氨基酸集中区的特定赖氨酸
残基可以共价结合乙酰基发生乙酰化修饰,从而激活转录反应;乙酰化修饰与磷酸化修饰一样,是可逆的修饰过程,在组蛋白去乙酰化酶(HDAC)催化下,组蛋白能发生去乙酰化,抑制基因表达。
百泰派克生物科技采用Thermo Fisher的Q ExactiveHF质谱平台结合Nano-LC,
提供组蛋白翻译后修饰分析服务技术包裹,可对各种组蛋白修饰如乙酰化、甲基化、泛素化、磷酸化和ADP核糖基化等进行定性和定量鉴定,还可根据需求提供定制化的检测方案,欢迎免费咨询。
the genetics and biochemistry of chromatin remodeling complexes: large, multisubunit catalytic entities perform the work of histone modification that leads either to transcriptional activation or repression of target genes. Here, promoter selectivity for sequence-specific DNA binding proteins must guide the assembly of these big chromatin-modifying machines,yet the genetic regulatory elements must also be able to respond rapidly to changing transcriptional requirements. Active investigation of chromatin remodeling continues in many laboratories, from the level of sequence-specific modification of specific histones to the level of multiprotein complex assembly.A particular protein motif called a “bromodomain” has been noticed in many of the proteins that compose the chromatin modifying machinery. It was first identified in 1992 as a 61 – 63amino acid signature (14). Although it lacked a known function at the time, it has subsequently been identified in transcription factors, co-activators and other proteins that are important in transcription or chromatin remodeling and its boundaries have been expanded to about 110 amino acids. The number of such proteins was about forty at last report (15,16)and several important additions to the family have been made since then. The first described bromodomain protein, yeast Gcn5 (17), was shown to be necessary for amino acid metabolism and was characterized as a transcriptional co-activator (18). It provides a histone acetylation (19) component of the ADA (Adapter) and SAGA (Spt-Ada-Gcn5acetyltransferase) transcription complexes (20), which is fundamental and essential for viability (21). Gcn5 is also structurally related to the mammalian proteins CBP, p300 and Hat1 (22). In mammals, CBP and p300 also have intrinsic HAT activity (23,24) and interact with many important transcription factors as co-activators of transcription. Virtually all of the nuclear histone acetyltransferases (HATs) contain bromodomains (16), but not all bromodomain proteins are HATs. For example, other classes of bromodomain proteins include MLL, a putative transcription factor (25,26) that interacts with the SWI/SNF chromatin remodeling complex (27); Spt7, an acidic transcriptional activator and component of the SAGA complex (28); and a helicase superfamily that includes Snf2, Rsc1/Rsc2 andSth1, components of the SWI/SNF (29) and RSC complexes (30); Brg1, which binds RB(31,32); and brahma , which also contacts RB, is related to Swi2/Snf2 (33,34) and hashomeotic functions in Drosophila (35–37). The role of bromodomains in transcriptioncomplexes has been controversial because their deletion has widely different consequences:in yeast, bromodomain deletion of Spt7 has no phenotype, of Snf2 causes slow growth, butdeletion of Sth1, Rsc1 and Rsc2 causes lethality (16). Much of the apparent significance ofbromodomain proteins lies in their either having intrinsic HAT activity, or being associatedwith promoter-bound complexes that contain HAT or histone deacetylase (HDAC) activity.Bromodomain proteins are thereby potentially important players in the transcriptionalcontrol of a wide variety of eukaryotic genes, including those that control growth.The bromodomain proteins that interact with RB highlight an important duality intranscriptional control: the need also to turn promoters off. In particular, the transcriptionalcontrol of E2F-regulated mammalian cell cycle genes is essential for proper progressionthrough each stage of the cell cycle. Whereas transcriptional activation of one set of genes isnecessary to enter a stage of the cell cycle, repression of certain other genes associated withthe previous stage is necessary to exit from that stage. RB (and its family members p107 andp130) bind to E2F proteins and block their transcription activation function (38,39).Recent evidence has revealed that in addition to this direct repression, RB also recruits ahistone deacetylase (40,41), as do p107 and p130 (42), through cooperation with mammalianbrahma and other proteins in the SWI/SNF complex (31,32). Coordinated transcriptionalactivation and repression of the key E2F-regulated mammalian cell cycle genes cyclin E ,cyclin A and cdc2 permit proper transitions between G1 and S phases, and S and G 2 phases(43). This dual nature of chromatin remodeling complexes was first suspected in yeast,NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscriptwhere SWI/SNF complexes, initially associated with transcriptional activation (44), were later linked to repression as well: more genes are activated than repressed by SWI/SNF mutations (45). It now appears that SWI/SNF function may establish a widely applicable paradigm in chromatin remodeling complexes, whereby transcriptionally active euchromatin can be converted to inactive heterochromatin and vice versa in part through the exchange of HAT and HDAC enzymes in the complex (46). This model has been refined lately with the observation in yeast that several inducible genes active during interphase can recruit HAT activity independently of SWI/SNF, whereas mitotic genes require SWI/SNF to recruit HAT activity (47). This observation emphasizes the importance of coordinated complex formation for proper transit of the cell cycle.A central development in the field of bromodomain-containing proteins came with a report of Zhou and colleagues (48), who used nuclear Overhauser enhancements to solve the solution structure of the bromodomain of p/CAF in association with N-acetylated lysine.The highly conserved structure of bromodomain proteins suggests a hypothesis that many of them will bind N-acetyl-lysine in histones, however by no means will this necessarily be true for all. The presence of bromodomains in many proteins that are known independently to possess HAT activity strongly supports the Zhou hypothesis. A looser notion that this motif is present in proteins that are involved in chromatin modification and transcription regulation is the best guide to their classification at the moment. The future discovery of bromodomains in proteins that are uninvolved in chromatin restructuring will be a test of the utility of such a classification.In this special issue, several authors have been invited to contribute their perspectives on the developing field of bromodomain proteins and associated chromatin-modifying activities.Major questions that they address continue to provoke the development of the field, and include:A.What are the number and type of histone modifications, including phosphorylation,acetylation, methylation, ADP-ribosylation and ubiquitylation, that could regulatethe recruitment of different classes of chromatin-modifying enzymes and mightthese represent a kind of combinatorial “histone code”? How do modifications ofbromodomain-containing proteins reciprocally affect histone modificationactivities?B.Should bromodomain-containing proteins be thought of as a kind of bridge orplatform that recruits diverse enzymatic activities, such as HATs, HDACs, kinasesor helicases, to chromatin? Why are these activities present in some bromodomain-containing proteins as independently-folding domains of a single polypeptide chainand in other cases as separate proteins? Does the weak affinity constant for a singlebromodomain binding to N-acetyl-lysine (~0.1 mM) imply that bromodomains canfunction only in multiprotein complexes with multiple interaction sites?C.Do different bromodomains have different functions, including those that arepresent more than once in a single protein? For example, double bromodomains,such as those in TAF II 250 might provide mutual cooperativity for protein bindingto chromatin or might interfere with binding instead; or they might conferdifferential promoter specificity.D.Why are some bromodomains essential for enzymatic function or cell viabilitywhereas deletion of others has no apparent phenotype? Does this behavior reflectredundancy within bromodomain-containing complexes, so that for example SWI/SNF activities on some promoters can partially substitute for HAT-containingcomplexes such as SAGA?NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author ManuscriptE.What is the significance of the time order of recruitment of SWI/SNF activities andHAT activities to certain promoters? Why does SWI/SNF recruitment of HATactivity impact yeast transcriptional activation during late mitosis (47), whereasmany inducible promoters recruit HATs independently of SWI/SNF earlier in thecell cycle, and how widespread is this behavior in eukaryotes?AcknowledgmentsThe author’s work is supported by grant CA75107 from NIH.References1. Bradbury EM. Reversible histone modifications and the chromosome cell cycle. Bioessays 1992;14:9–16. [PubMed: 1312335]2. Wolffe AP, Pruss D. Targeting chromatin disruption: Transcription regulators that acetylate histones. Cell 1996;84:817–819. [PubMed: 8601304]3. Roth SY, Allis CD. Histone acetylation and chromatin assembly: a single escort, multiple dances?Cell 1996;87:5–8. [PubMed: 8858142]4. Grunstein M. Histone acetylation in chromatin structure and transcription. Nature 1997;389:349–352. [PubMed: 9311776]5. Tyler JK, Kadonaga JT. The “dark side” of chromatin remodeling: repressive effects on transcription. Cell 1999;99:443–446. [PubMed: 10589670]6. Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell 2000;103:263–271. [PubMed: 11057899]7. Orphanides G, Reinberg D. RNA polymerase II elongation through chromatin. Nature 2000;407:471–475. [PubMed: 11028991]8. Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell 2000;5:905–915. [PubMed: 10911985]9. Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, Marmorstein R, Berger SL.Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol Cell 2000;5:917–926. [PubMed: 10911986]10. Mahadevan LC, Willis AC, Barratt MJ. Rapid histone H3 phosphorylation in response to growthfactors, phorbol esters, okadaic acid and protein synthesis inhibitors. Cell 1991;65:775–783.[PubMed: 2040014]11. Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis CD.Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3.Science 1999;285:886–891. [PubMed: 10436156]12. Chadee DN, Hendzel MJ, Tylipski CP, Allis CD, Bazett-Jones DP, Wright JA, Davie JR. IncreasedSer-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mousefibroblasts. J Biol Chem 1999;274:24914–24920. [PubMed: 10455166]13. Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required forproper chromosome condensation and segregation. Cell 1999;97:99–109. [PubMed: 10199406]14. Haynes SR, Dollard C, Winston F, Beck S, Trowsdale J, Dawid IB. The bromodomain: aconserved sequence found in human, Drosophila and yeast proteins. Nucl Acids Res1992;20:2603. [PubMed: 1350857]15. Jeanmougin F, Wurtz JM, Le Douarin B, Chambon P, Losson R. The bromodomain revisited.Trends Biochem Sci 1997;22:151–153. [PubMed: 9175470]16. Winston F, Allis CD. The bromodomain: a chromatin-targeting module? Nature Struct Biol1999;6:601–604. [PubMed: 10404206]17. Hinnebusch AG, Fink GR. Positive regulation in the general amino acid control of Saccharomycescerevisiae . Proc Nat’l Acad Sci USA 1983;80:5374–5378.18. Guarente L. Transcriptional coactivators in yeast and beyond. Trends Biochem Sci 1995;20:517–521. [PubMed: 8571454]NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscript19. Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev 1998;12:599–606. [PubMed: 9499396]20. Wang L, Liu L, Berger SL. Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo . Genes Dev 1998;12:640–653. [PubMed: 9499400]21. Kuo MH, Brownell JE, Sobel RE, Ranalli TA, Cook RG, Edmondson DG, Roth SY, Allis CD.Transcription-linked acetylation by Gcn5p of histone H3 and H4 at specific lysines. Nature 1996;383:269–272. [PubMed: 8805705]22. Dutnall RN, Tafrov ST, Sternglanz R, Ramakrishnan V. Structure of the histone acetyltransferase Hat1: A paradigm for the GCN5-related N-acetyltransferase superfamily. Cell 1998;94:427–438.[PubMed: 9727486]23. Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature 1996;384:641–643. [PubMed: 8967953]24. Ogryzko VV, Schiltz OL, Russanova V, Howard BH, Nakatani Y. The transcriptional co-activators p300 and CBP are histone acetyltransferases. Cell 1996;87:953–959. [PubMed: 8945521]25. Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce CM, Canaani E. The t(4;11)chromosomal translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax , to the AF-4 gene. Cell 1992;71:701–708. [PubMed: 1423625]26. Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23chromosomal translocations in acute leukemias. Cell 1992;71:691–700. [PubMed: 1423624]27. Rozenblatt-Rosen O, Rozovskaia T, Burakov D, Sedkov Y, Tillib S, Blechman J, Nakamura T,Croce CM, Mazo A, Canaani E. The C-terminal SET domains of ALL-1 and TRITHORAX interact with the INI1 and SNR1 proteins, components of the SWI/SNF complex. Proc Nat’l Acad Sci USA 1998;95:4152–4157.28. Gansheroff LJ, Dollard C, Tan P, Winston F. The Saccharomyces cerevisiae SPT7 gene encodes a very acidic protein important for transcription in vivo . Genetics 1995;139:523–536. [PubMed:7713415]29. Peterson CL, Tamkun JW. The SWI-SNF complex: a chromatin remodeling machine? Trends Biochem Sci 1995;20:143–146. [PubMed: 7770913]30. Cairns BR, Lorch Y, Li Y, Zhang M, Lacomis L, Erdjument-Bromage H, Tempst P, Du J, LaurentB, Kornberg RD. RSC, an essential, abundant chromatin-remodeling complex. Cell1996;87:1249–1260. [PubMed: 8980231]31. Trouche D, Le Chalony C, Muchardt C, Yaniv M, Kouzarides T. RB and hbrm cooperate torepress the activation functions of E2F1. Proc Nat’l Acad Sci USA 1997;94:11268–11273.32. Muchardt C, Yaniv M. The mammalian SWI/SNF complex and the control of cell growth. SemCell Dev Biol 1999;10:189–195.33. Tamkun JW, Deuring R, Scott MP, Kissenger M, Pattatucci AM, Kaufman TC, Kennison JA.brahma - a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SWI2/SNF2. Cell 1992;68:561–572. [PubMed: 1346755]34. Pazin MJ, Kadonaga JT. SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein-DNA interactions? Cell 1997;88:737–740. [PubMed: 9118215]35. Kennison JA, Tamkun JW. Dosage-dependent modifiers of Polycomb and Antennapedia mutationsin Drosophila . Proc Nat’l Acad Sci USA 1988;85:8136–8140.36. Elfring LK, Deuring R, McCallum CM, Peterson CL, Tamkun JW. Identification andcharacterization of Drosophila relatives of the yeast transcriptional activator SNF2/SWI2. Mol Cell Biol 1994;14:2225–2234. [PubMed: 7908117]37. Elfring LK, Daniel C, Papoulas O, Deuring R, Sarte M, Moseley S, Beek SJ, Waldrip WR,Daubresse G, DePace A, Kennison JA, Tamkun JW. Genetic analysis of brahma: the Drosophila homolog of the yeast chromatin remodeling factor SWI2/SNF2. Genetics 1998;148:251–265.[PubMed: 9475737]38. Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev 1998;12:2245–2262.[PubMed: 9694791]39. Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastomafamilies. Cell Growth Diff 1998;9:585–593. [PubMed: 9716176]NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscript40. Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 1998;391:597–601. [PubMed:9468139]41. Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F,Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 1998;391:601–605. [PubMed: 9468140]42. Ferreira R, Magnaghi-Jaulin L, Robin P, Harel-Bellan A, Trouche D. The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase. Proc Nat’l Acad Sci USA 1998;95:10493–10498.43. Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, Harbour JW, Dean DC. Exit from G1and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 2000;101:79–89. [PubMed: 10778858]44. Burns LG, Peterson CL. The yeast SWI-SNF complex facilitates binding of a transcriptional activator to nucleosomal sites in vivo . Mol Cell Biol 1997;17:4811–4819. [PubMed: 9234737]45. Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES,Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 1998;95:717–728.[PubMed: 9845373]46. Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G,Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfield MG. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 1997;387:43–48. [PubMed: 9139820]47. Krebs JE, Fry CJ, Samuels ML, Peterson CL. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 2000;102:587–598. [PubMed: 11007477]48. Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999;399:491–496. [PubMed: 10365964]NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript。