标准电极电势表(全)
- 格式:doc
- 大小:236.00 KB
- 文档页数:8
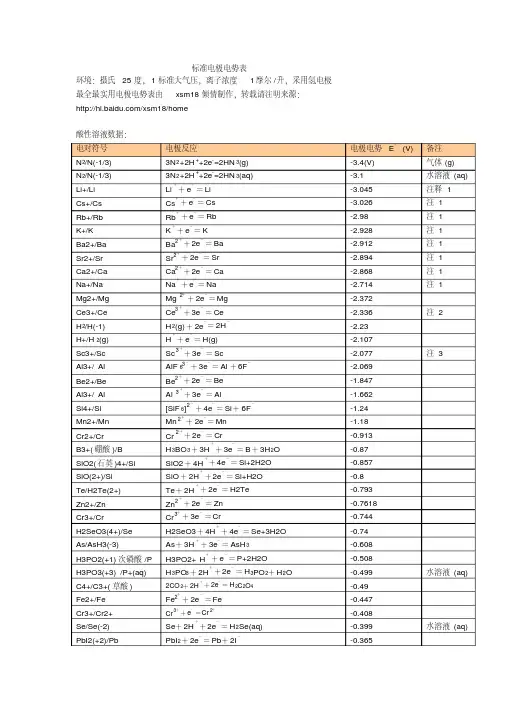

标准电极电势表标准电极电势可以用来计算化学电池或原电池的电化学势或电极电势。
本表中所给出的电极电势以标准氢电极为参比电极,溶液中离子有效浓度为1mol/L,气体分压为100kPa,温度为298K,所有离子的数据都在水溶液中测得。
[1][2][3][4][5][6][7][8][9]单击每栏上方的符号可将数据按元素符号或标准电极电势值排序。
注:(s) –固体;(l) –液体;(g) –气体;(aq) –水溶液;(Hg) –汞齐。
半反应E°(V)[注 1]来源Ba++ e−Ba(s) −4.38 [10][1][3]Sr++ e−Sr(s) −4.10 [11][1][3] Ca++ e−Ca(s) −3.8 [11][1][3]Pr3++ e−Pr2+−3.1 [11] N2(g) + H++ e−HN3(aq) −3.09 [6]Li++ e−Li(s) −3.0401 [5]N2(g) + 4 H2O + 2 e−2 NH2OH(aq) + 2 OH−−3.04 [6]Cs++ e−Cs(s) −3.026 [5]Ca(OH)2(s) + 2 e−Ca(s) + 2 OH−−3.02 [11]Rb++ e−Rb(s) −2.98 [4]K++ e−K(s) −2.931 [5]半反应E°(V)[注 1]来源Mg++ e−Mg(s) −2.93 [10] Ba2++ 2 e−Ba(s) −2.912 [5]La(OH)3(s) + 3 e−La(s) + 3OH−−2.90 [5]Fr++ e−Fr(s) −2.9 [11] Sr2++ 2 e−Sr(s) −2.899 [5]Sr(OH)2(s) + 2 e−Sr(s) + 2 OH−−2.88 [11] Ca2++ 2 e−Ca(s) −2.868 [5] Eu2++ 2 e−Eu(s) −2.812 [5] Ra2++ 2 e−Ra(s) −2.8 [5] Yb2++ 2 e−Yb(s) −2.76 [11][1] Na++ e−Na(s) −2.71 [5][9] Sm2++ 2 e−Sm(s) −2.68 [11][1] No2++ 2 e−No(s) −2.50 [11] HfO(OH)2(s) + H2O + 4 e−Hf(s) + 4 OH−−2.50 [11]半反应E°(V)[注 1]来源Th(OH)4(s) + 4 e−Th(s) + 4 OH−−2.48 [11] Md2++ 2 e−Md(s) −2.40 [11] La3++ 3 e−La(s) −2.379 [5]Y3++ 3 e−Y(s) −2.372 [5] Mg2++ 2 e−Mg(s) −2.372 [5] ZrO(OH)2(s) + H2O + 4 e−Zr(s) + 4OH−−2.36 [5]Pr3++ 3 e−Pr(s) −2.353 [11] Ce3++ 3 e−Ce(s) −2.336 [11] Er3++ 3 e−Er(s) −2.331 [11] Ho3++ 3 e−Ho(s) −2.33 [11]Al(OH)4−+ 3 e−Al(s) + 4 OH−−2.33Al(OH)3(s) + 3 e−Al(s) + 3OH−−2.31Tb3++ 3 e−Tb(s) −2.28半反应E°(V)[注 1]来源H2(g) + 2 e−2 H−−2.25Ac3++ 3 e−Ac(s) −2.20Be++ e−Be(s) −2.12 [10] Cf2++ 2 e−Cf(s) −2.12 [11] Am3++ 3 e−Am(s) −2.048 [11] Cf3++ 3 e−Cf(s) −1.94 [11] Am2++ 2 e−Am(s) −1.9 [11] Be2++ 2 e−Be(s) −1.85Rf4++ 4 e−Rf(s) −1.67 [12]U3++ 3 e−U(s) −1.66 [7]Al3++ 3 e−Al(s) −1.66 [9]Ti2++ 2 e−Ti(s) −1.63 [9]Bk2++ 2 e−Bk(s) −1.6 [11]半反应E°(V)[注 1]来源ZrO2(s) + 4 H++ 4 e−Zr(s) + 2 H2O −1.553 [5]Hf4++ 4 e−Hf(s) −1.55 [11]Zr4++ 4 e−Zr(s) −1.45 [5]Ti3++ 3 e−Ti(s) −1.37 [13] TiO(s) + 2 H++ 2 e−Ti(s) + H2O −1.31Ti2O3(s) + 2 H++ 2 e−2 TiO(s) + H2O −1.23Zn(OH)42−+ 2 e−Zn(s) + 4 OH−−1.199 [14] Mn2++ 2 e−Mn(s) −1.185 [14] Fe(CN)64−+ 6 H++ 2 e−Fe(s) + 4HCN(aq) −1.16 [15]V2++ 2 e−V(s) −1.175 [2]Te(s) + 2 e−Te2−−1.143 [2] Nb3++ 3 e−Nb(s) −1.099Sn(s) + 4 H++ 4 e−SnH4(g) −1.07半反应E°(V)[注 1]来源In(OH)3(s) + 3 e−In(s) + 3 OH−−0.99 [11] SiO2(s) + 4 H++ 4 e−Si(s) + 2 H2O −0.91B(OH)3(aq) + 3 H++ 3 e−B(s) + 3 H2O −0.89Fe(OH)2(s) + 2 e−Fe(s) + 2 OH−−0.89 [15] Fe2O3(s) + 3 H2O + 2 e−2Fe(OH)2(s) + 2 OH−−0.86 [15] TiO2++ 2 H++ 4 e−Ti(s) + H2O −0.862 H2O+ 2 e−H2(g) + 2 OH−−0.8277 [5]Bi(s) + 3 H++ 3 e−BiH3−0.8 [14] Zn2++ 2 e−Zn(Hg) −0.7628 [5]Zn2++ 2 e−Zn(s) −0.7618 [5]Ta2O5(s) + 10 H++ 10 e−2 Ta(s) + 5 H2O −0.75Cr3++ 3 e−Cr(s) −0.74[Au(CN)2]−+ e−Au(s) + 2 CN−−0.60半反应E°(V)[注 1]来源Ta3++ 3 e−Ta(s) −0.6PbO(s) + H2O + 2 e−Pb(s) + 2 OH−−0.582 TiO2(s) + 2 H++ 2 e−Ti2O3(s) + H2O −0.56Ga3++ 3 e−Ga(s) −0.53U4++ e−U3+−0.52 [7]H3PO2(aq) + H++ e−P(白磷[16]) + 2 H2O −0.508 [5]H3PO3(aq) + 2 H++ 2 e−H3PO2(aq) + H2O −0.499 [5]H3PO3(aq) + 3 H++ 3 e−P(红磷)[16]+ 3H2O −0.454 [5]Fe2++ 2 e−Fe(s) −0.44 [9]2 CO2(g) + 2 H++ 2 e−HOOCCOOH(aq) −0.43Cr3++ e−Cr2+−0.42Cd2++ 2 e−Cd(s) −0.40 [9] SeO32−+ 4e−+ 3H2O ⇌Se + 6OH−−0.37 [17]半反应E°(V)[注 1]来源GeO2(s) + 2 H++ 2 e−GeO(s) + H2O −0.37Cu2O(s) + H2O + 2 e−2 Cu(s) + 2 OH−−0.360 [5] PbSO4(s) + 2 e−Pb(s) + SO42−−0.3588 [5] PbSO4(s) + 2 e−Pb(Hg) + SO42−−0.3505 [5] Eu3++ e−Eu2+−0.35 [7]In3++ 3 e−In(s) −0.34 [2]Tl++ e−Tl(s) −0.34 [2] Ge(s) + 4 H++ 4 e−GeH4(g) −0.29Co2++ 2 e−Co(s) −0.28 [5]H3PO4(aq) + 2 H++ 2 e−H3PO3(aq) + H2O −0.276 [5]V3++ e−V2+−0.26 [9]Ni2++ 2 e−Ni(s) −0.25As(s) + 3 H++ 3 e−AsH3(g) −0.23 [2]半反应E°(V)[注 1]来源AgI(s) + e−Ag(s) + I−−0.15224 [14] MoO2(s) + 4 H++ 4 e−Mo(s) + 2 H2O −0.15Si(s) + 4 H++ 4 e−SiH4(g) −0.14Sn2++ 2 e−Sn(s) −0.13O2(g) + H++ e−HO2•(aq) −0.13Pb2++ 2 e−Pb(s) −0.13 [9] WO2(s) + 4 H++ 4 e−W(s) + 2 H2O −0.12P(红磷) + 3 H++ 3 e−PH3(g) −0.111 [5] CO2(g) + 2 H++ 2 e−HCOOH(aq) −0.11Se(s) + 2 H++ 2 e−H2Se(g) −0.11CO2(g) + 2 H++ 2 e−CO(g) + H2O −0.11SnO(s) + 2 H++ 2 e−Sn(s) + H2O −0.10半反应E°(V)[注 1]来源SnO2(s) + 2 H++ 2 e−SnO(s) + H2O −0.09WO3(aq) + 6 H++ 6 e−W(s) + 3 H2O −0.09 [2]P(白磷) + 3 H++ 3 e−PH3(g) −0.063 [5]Fe3++ 3 e−Fe(s) −0.04 [15] HCOOH(aq) + 2 H++ 2 e−HCHO(aq) + H2O −0.032 H++ 2 e−H2(g)0.00 ≡0 AgBr(s) + e−Ag(s) + Br−+0.07133 [14]S4O62−+ 2 e−2 S2O32−+0.08Fe3O4(s) + 8 H++ 8 e−3 Fe(s) + 4 H2O +0.085 [8]N2(g) + 2 H2O + 6H++ 6 e−2 NH4OH(aq) +0.092HgO(s) + H2O + 2 e−Hg(l) + 2 OH−+0.0977Cu(NH3)42++ e−Cu(NH3)2++ 2 NH3+0.10 [2]Ru(NH3)63++ e−Ru(NH3)62++0.10 [7]半反应E°(V)[注 1]来源N2H4(aq) + 4 H2O + 2 e−2 NH4++ 4 OH−+0.11 [6]H2MoO4(aq) + 6 H++ 6 e−Mo(s) + 4 H2O +0.11Ge4++ 4 e−Ge(s) +0.12C(s) + 4 H++ 4 e−CH4(g) +0.13 [2] HCHO(aq) + 2 H++ 2 e−CH3OH(aq) +0.13S(s) + 2 H++ 2 e−H2S(g) +0.14Sn4++ 2 e−Sn2++0.15Cu2++ e−Cu++0.159 [2] HSO4−+ 3 H++ 2 e−SO2(aq) + 2 H2O +0.16UO22++ e−UO2++0.163 [7] SO42−+ 4 H++ 2 e−SO2(aq) + 2 H2O +0.17TiO2++ 2 H++ e−Ti3++ H2O +0.19半反应E°(V)[注 1]来源Bi3++ 2e−Bi++0.2SbO++ 2 H++ 3 e−Sb(s) + H2O +0.20AgCl(s) + e−Ag(s) + Cl−+0.22233 [14]H3AsO3(aq) + 3 H++ 3 e−As(s) + 3 H2O +0.24GeO(s) + 2 H++ 2 e−Ge(s) + H2O +0.26UO2++ 4 H++ e−U4++ 2 H2O +0.273 [7]At2+ e−2 At-+0.3 [11] Re3++ 3 e−Re(s) +0.300Bi3++ 3 e−Bi(s) +0.32VO2++ 2 H++ e−V3++ H2O +0.34Cu2++ 2 e−Cu(s) +0.340 [2] [Fe(CN)6]3−+ e−[Fe(CN)6]4−+0.36半反应E°(V)[注 1]来源Tc2++ 2 e−Tc(s) +0.40 [11]O2(g) + 2 H2O + 4 e−4 OH−(aq) +0.40 [9]H2MoO4+ 6 H++ 3 e−Mo3++ 2 H2O +0.43Ru2++ 2 e−Ru(s) +0.455 [11]Bi++ e−Bi(s) +0.50CH3OH(aq) + 2 H++ 2 e−CH4(g) + H2O +0.50SO2(aq) + 4 H++ 4 e−S(s) + 2 H2O +0.50Cu++ e−Cu(s) +0.520 [2] CO(g) + 2 H++ 2 e−C(s) + H2O +0.52I3−+ 2 e−3 I−+0.53 [9]I2(s) + 2 e−2 I−+0.54 [9] [AuI4]−+ 3 e−Au(s) + 4 I−+0.56半反应E°(V)[注 1]来源H3AsO4(aq) + 2 H++ 2 e−H3AsO3(aq) + H2O +0.56[AuI2]−+ e−Au(s) + 2 I−+0.58MnO4−+ 2 H2O + 3 e−MnO2(s) + 4 OH−+0.59Rh++ e−Rh(s) +0.600 [11]S2O32 −+ 6 H++ 4 e−2 S(s) + 3 H2O +0.60Fc++ e−Fc(s) +0.641 [18]+0.643 [11]+ e−Ag + −H2MoO4(aq) + 2 H++ 2 e−MoO2(s) + 2 H2O +0.65+0.6992 [14] + 2 H++ 2 e−O2(g) + 2 H++ 2 e−H2O2(aq) +0.70Tl3++ 3 e−Tl(s) +0.72半反应E°(V)[注 1]来源PtCl62−+ 2 e−PtCl42−+ 2 Cl−+0.726 [7]H2SeO3(aq) + 4 H++ 4 e−Se(s) + 3 H2O +0.74Rh3++ 3 e−Rh(s) +0.758 [11] PtCl42−+ 2 e−Pt(s) + 4 Cl−+0.758 [7]Fe3++ e−Fe2++0.77Ag++ e−Ag(s) +0.7996 [5] Hg22++ 2 e−2 Hg(l) +0.80NO3−(aq) + 2 H++ e−NO2(g) + H2O +0.80FeO42−+ 5 H2O + 6 e−Fe2O3(s) + 10 OH−+0.81 [15]H2(g) + 2 OH−2 H2O + 2 e−+0.828 [19] [AuBr4]−+ 3 e−Au(s) + 4 Br−+0.85Hg2++ 2 e−Hg(l) +0.85半反应E°(V)[注 1]来源MnO4−+ H++ e−HMnO4−+0.902 Hg2++ 2 e−Hg22++0.91 [2] Pd2++ 2 e−Pd(s) +0.915 [7] [AuCl4]−+ 3 e−Au(s) + 4 Cl−+0.93MnO2(s) + 4 H++ e−Mn3++ 2 H2O +0.95[AuBr2]−+ e−Au(s) + 2 Br−+0.96[HXeO6]3−+ 2 H2O + 2 e−+ [HXeO4]−+ 4 OH−+0.99 [20] HNO2+ H++ e-= NO(g)+ H2O +0.996H6TeO6(aq) + 2 H++ 2 e−TeO2(s) + 4 H2O +1.02 [21] Br2(l) + 2 e−2 Br−+1.07Br2(aq) + 2 e−2 Br−+1.09 [9] NO2(g) + H++ e-= HNO2+1.093半反应E°(V)[注 1]来源IO3−+ 5 H++ 4 e−HIO(aq) + 2 H2O +1.13[AuCl2]−+ e−Au(s) + 2 Cl−+1.15HSeO4−+ 3 H++ 2 e−H2SeO3(aq) + H2O +1.15Ir3++ 3 e−Ir(s) +1.156 [11] Ag2O(s) + 2 H++ 2 e−2 Ag(s) + H2O +1.17ClO3−+ 2 H++ e−ClO2(g) + H2O +1.18[HXeO6]3−+ 5 H2O + 8 e−Xe(g) + 11 OH−+1.18 [20]Pt2++ 2 e−Pt(s) +1.188 [7] ClO2(g) + H++ e−HClO2(aq) +1.192 IO3−+ 12 H++ 10 e−I2(s) + 6 H2O +1.20ClO4−+ 2 H++ 2 e−ClO3−+ H2O +1.20O2(g) + 4 H++ 4 e−2 H2O+1.229 [9]半反应E°(V)[注 1]来源MnO2(s) + 4 H++ 2 e−Mn2++ 2H2O +1.23[HXeO4]−+ 3 H2O + 6 e−Xe(g) + 7 OH−+1.24 [20]Tl3++ 2 e−Tl++1.25Cr2O72 −+ 14 H++ 6 e−2 Cr3++ 7 H2O +1.33Cl2(g) + 2 e−2 Cl−+1.36 [9] CoO2(s) + 4 H++ e−Co3++ 2 H2O +1.422 NH3OH++ H++ 2 e−N2H5++ 2 H2O +1.42 [6]2 HIO(aq) + 2 H++ 2 e−I2(s) + 2 H2O +1.44Ce4++ e−Ce3++1.44BrO3−+ 5 H++ 4 e−HBrO(aq) + 2 H2O +1.45β-PbO2(s) + 4 H++ 2 e−Pb2++ 2 H2O +1.460 [2]α-PbO2(s) + 4 H++ 2 e−Pb2++ 2 H2O +1.468 [2]半反应E°(V)[注 1]来源2 BrO3−+ 12 H++ 10 e−Br2(l) + 6 H2O +1.482ClO3−+ 12 H++ 10 e−Cl2(g) + 6 H2O +1.49HO2+ H++ e−H2O2+1.495 [11] MnO4−+ 8 H++ 5 e−Mn2++ 4 H2O +1.51HO2•+ H++ e−H2O2(aq) +1.51Au3++ 3 e−Au(s) +1.52NiO2(s) + 4 H++ 2 e−Ni2++ 2 OH−+1.592 HClO(aq) + 2 H++ 2 e−Cl2(g) + 2 H2O +1.63Ag2O3(s) + 6 H++ 4 e−2 Ag++ 3 H2O +1.67HClO2(aq) + 2 H++ 2 e−HClO(aq) + H2O +1.67Pb4++ 2 e−Pb2++1.69 [2] MnO4−+ 4 H++ 3 e−MnO2(s) + 2 H2O +1.70半反应E°(V)[注 1]来源AgO(s) + 2 H++ e−Ag++ H2O +1.77 H2O2(aq) + 2 H++ 2 e−2 H2O +1.776Co3++ e−Co2++1.82Au++ e−Au(s) +1.83 [2] BrO4−+ 2 H++ 2 e−BrO3−+ H2O +1.85Ag2++ e−Ag++1.98 [2]S2O82−+ 2 e−2 SO42−+2.07O3(g) + 2 H++ 2 e−O2(g) + H2O +2.075 [7] HMnO4−+ 3 H++ 2 e−MnO2(s) + 2 H2O +2.09XeO3(aq) + 6 H++ 6 e−Xe(g) + 3 H2O +2.12 [20]H4XeO6(aq) + 8 H++ 8 e−Xe(g) + 6 H2O +2.18 [20] FeO42−+ 3 e−+ 8 H+Fe3++ 4 H2O +2.20 [22] XeF2(aq) + 2 H++ 2 e−Xe(g) + 2HF(aq) +2.32 [20]半反应E°(V)[注 1]来源H4XeO6(aq) + 2 H++ 2 e−XeO3(aq) + H2O +2.42 [20]F2(g) + 2 e−2 F−+2.87 [2][9] F2(g) + 2 H++ 2 e−2 HF(aq) +3.05 [2]Tb4++ e− Tb3++3.05 [11]。
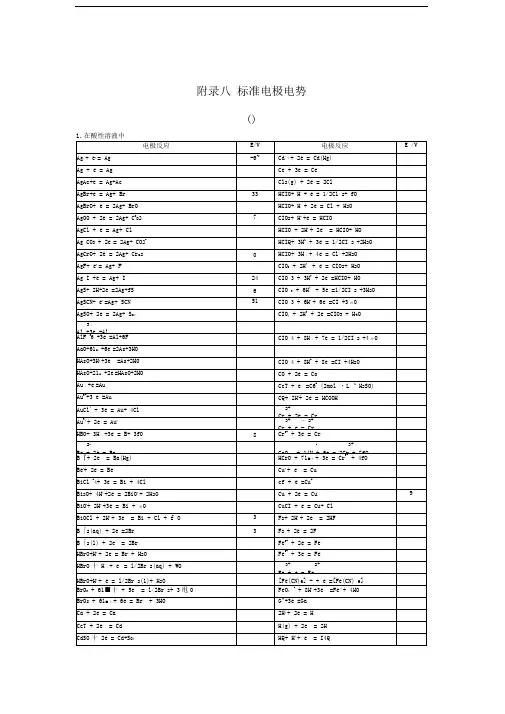
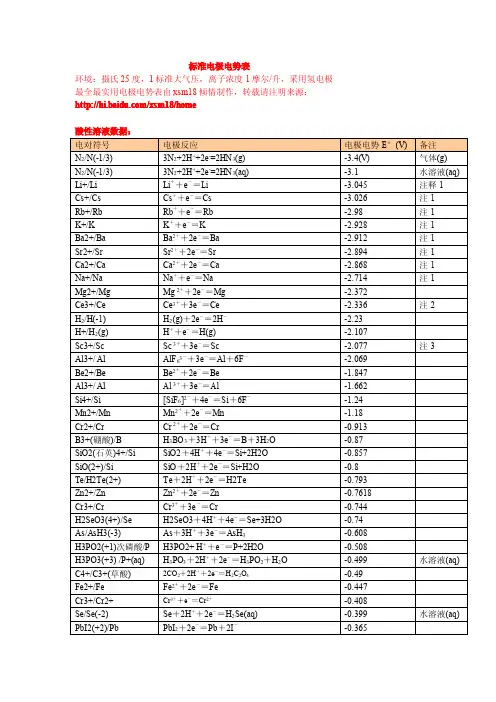
标准电极电势表环境:摄氏25度,1标准大气压,离子浓度1摩尔/升,采用氢电极最全最实用电极电势表由xsm18倾情制作,转载请注明来源:/xsm18/homePbSO4(+2)/Pb PbSO4+2e-=Pb+SO42--0.3588PbBr2(+2)/Pb PbBr2+2e-=Pb+2Br--0.284Co2+/Co Co2++2e-=Co-0.28H3PO4/H3PO3H3PO4+2H++2e-=H3PO3+H2O-0.276PbCl2(+2)/Pb PbI2+2e-=Pb+2I--0.2675Ni2+/Ni Ni2++2e-=Ni-0.257CO2/HCOOH(甲酸)CO2(g)+2H++2e-=HCOOH(aq)-0.199CuI(+1)/Cu CuI+e-=Cu+I-0.1852AgI(+1)/Ag AgI+e-=Ag+I-0.15224Sn2+/Sn Sn2++2e-=Sn-0.1375Pb2+/Pb Pb2++2e-=Pb-0.1262C4+/C2+CO2(g)+2H++2e-=CO+H2O-0.12P/PH3P(白磷)+3H++3e-=PH3(g)-0.063气体(g)Hg2I2(+1)/Hg Hg2I2+2e-=2Hg+2I--0.0405Fe3+/Fe Fe3++3e-=Fe-0.037Ag2S(+1)/Ag Ag2S+2H++2e-=2Ag+H2S-0.0366H+/H22H++2e-=H20.00CuBr(+1)/Cu CuBr+e-=Cu+Br-0.033AgBr(+1)/Ag AgBr+e-=Ag+Br-0.07133Si/SiH4Si+4H++4e-=SiH40.102C(石墨)/CH4C+4H++4e-=CH40.1316CuCl(+1)/Cu CuCl+e-=Cu+Cl-0.137Hg2Br2(+1)/Hg Hg2Br2+2e-=2Hg+2Br-0.13923S/H2S(aq)S+2H++2e-=H2S(aq)0.142水溶液Sn4+/Sn2+Sn4++2e-=Sn2+0.151Cu2+/Cu+Cu2++e-=Cu+0.153S6+/S4+SO42-+4H++2e-=H2SO3+H2O0.172AgCl(+1)/Ag AgCl+e-=Ag+Cl-0.2223As3+/As(亚砷酸)HAsO2(aq)+3H++3e-=As+2H2O0.2476HAsO2.H2O Hg2Cl2/Hg Hg2Cl2+2e-=2Hg+2Cl-0.268Bi3+/Bi Bi3++3e-=Bi0.308Cu2+/Cu Cu2++2e-=Cu0.337AgIO3/Ag AgIO3+e-=Ag+IO3-0.354S6+/S SO42-+8H++6e-=S+4H2O0.3572Ag2CrO4/Ag Ag2CrO4+2e-=2Ag+CrO42-0.447铬酸银S4+/S H2SO3+4H++4e-=S+3H2O0.449Ag2C2O4/Ag Ag2C2O4+2e-=2Ag+C2O42-0.4647草酸银Cu+/Cu Cu++e-=Cu0.521I2/I-I2+2e-=2I-0.5355AgBrO3/Ag AgBrO3+e-=Ag+BrO3-0.546As5+/As3+H3AsO4(aq)+2H++2e-=HAsO2+2H2O0.56水溶液AgNO2/Ag AgNO2+e-=Ag+NO2-0.564Te4+/Te TeO2+4H++4e-=Te+2H2O0.593Hg2SO4/Hg Hg2SO4+2e-=2Hg+SO42-0.614Ag2SO4/Ag Ag2SO4+2e-=2Ag+SO42-0.654Pt4+(氯铂酸)/Pt2+[PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68O2/O-O2+2H++2e-=H2O20.695Pt2+/Pt(二氯化铂)[PtCl4]2-+2e-=Pt+4Cl-0.73Se4+/Se H2SeO3+4H++4e-=Se+3H2O0.74Fe3+/Fe2+Fe3++e-=Fe2+0.771AgF/Ag AgF+e-=Ag+F-0.779Hg+/Hg Hg22++2e-=2Hg0.788Ag+/Ag Ag++e-=Ag0.7991N5+/N4+(硝酸)2NO3-+4H++2e-=N2O4(g)+2H2O0.803气体(g) Hg2+/Hg Hg2++2e-=Hg(lq)0.853液态(水银) Si4+(石英)/Si SiO2+4H++4e-=Si+2H2O0.857Hg2+/Hg+2Hg2++2e-=Hg22+0.92N5+/N3+(亚硝酸)NO3-+3H++2e-=HNO2+H2O0.934Pd2+/Pd Pd2++2e-=Pd0.951N5+/N2+NO3-+4H++3e-=NO+2H2O0.957Au3+/Au(三溴化金)AuBr2-+e-=Au+2Br-0.959N3+/2+HNO2+H++e-=NO+H2O0.983Au3+/Au(三氯化金)[AuCl4]-+3e-=Au+4Cl- 1.002Te6+/Te4+H6TeO6+2H++2e-=TeO2+4H2O 1.02N4+/N2+N2O4+4H++4e-=2NO+2H2O 1.03Pt4+/Pt PtO2+4H++4e-=Pt+2H2O 1.045Br2(lq)/Br-Br2(lq)+2e-=2Br- 1.0652液溴(lq)N4+/N3+N2O4+2H++2e-=2HNO2 1.07Br2(aq)/Br-Br2(aq)+2e-=2Br- 1.087水溶液(aq) Se6+/Se4+SeO42-+4H++2e-=H2SeO3+H2O 1.151Cl5+/Cl4+ClO3-+2H++e-=ClO2+H2O 1.152O2/H2O(g)O2+4H++4e-=2H2O(g) 1.185水蒸汽(g) Pt2+/Pt Pt2++2e-=Pt 1.188Cl7+/Cl5+ClO4-+2H++2e-=ClO3-+H2O 1.189I5+/I22IO3-+12H++10e-=I2(s)+6H2O 1.195碘单质(s) Cl5+/Cl3+ClO3-+3H++2e-=HClO2+H2O 1.21Mn4+/Mn2+MnO2+4H++2e-=Mn2++2H2O 1.224O2/H2O(液态水)O2+4H++4e-=2H2O 1.229常温水S+(S2Cl2)/S S2Cl2+2e-=2S+2Cl- 1.23Fe3O4/Fe2+Fe3O4+8H++2e-=3Fe2++4H2O 1.23Tl3+/Tl+T13++2e-=Tl+ 1.25注4Cl4+/Cl3+ClO2+H++e-=HClO2 1.277N3+/N+2HNO2(aq)+4H++4e-=N2O(g)+3H2O 1.297Cr6+/Cr3+Cr2O72-+14H++6e-=2Cr3++7H2O 1.33重铬酸根Br+/Br-HBrO+H++2e-=Br-+H2O 1.331Cr6+/Cr3+HCrO4-+7H++3e-=Cr3++4H2O 1.35铬酸根Cl2/Cl-Cl2(g)+2e-=2Cl- 1.358(g)氯气Au2O3(+3)/Au Au2O3+6H++6e-=2Au+3H2O 1.36Cl7+/Cl-ClO4-+8H++8e-=Cl-+4H2O 1.388Cl7+/Cl2ClO4-+8H++7e-=1/2Cl2+4H2O 1.392Au3+/Au+Au3++2e-=Au+ 1.41Br5+/Br-BrO3-+6H++6e-=Br-+3H2O 1.424I+/I22HIO+2H++2e-=I2+2H2O 1.439Cl5+/Cl-ClO3-+6H++6e-=Cl-+3H2O 1.451Pb4+/Pb2+PbO2+4H++2e-=Pb2++2H2O 1.455Cl5+/Cl2ClO3-+6H++5e-=1/2Cl2+3H2O 1.47CrO2(+4)/Cr3+CrO2+4H++e-=Cr3++2H2O 1.48二氧化铬Cl+/Cl-HClO+H++2e-=Cl-+H2O 1.482Au3+/Au Au3++3e-=Au 1.498Mn7+/Mn2+MnO4-+8H++5e-=Mn2++4H2O 1.507Cl4+/Cl-ClO2+4H++5e-=Cl-+2H2O 1.511Br5+/Br2BrO3-+6H++5e-=l/2Br2+3H2O 1.52Mn3+/Mn2+Mn3++e-=Mn2+ 1.5415注5Cl3+/Cl-HClO2+3H++4e-=Cl-+2H2O 1.57N2+/N+2NO+2H++2e-=N2O+H2O 1.59Br+/Br2HBrO+H++e-=l/2Br2(aq)+H2O 1.595I7+/I5+H5IO6+H++2e-=IO3-+3H2O 1.603注6Cl+/Cl2HClO+H++e-=1/2Cl2+H2O 1.611Cl3+/Cl2HClO2+3H++2e-=1/2Cl2+2H2O 1.628Cl3+/Cl+HClO2+2H++2e-=HClO+H2O 1.645Ni4+/Ni2+NiO2+4H++2e-=Ni2++2H2O 1.678Mn7+/Mn4+MnO4-+4H++3e-=MnO2+2H2O 1.68注7Pb4+/Pb2+PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.69Au+/Au Au++e-=Au 1.691Ce4+/Ce3+Ce4++e-=Ce3+ 1.74注8Br7+/Br5+BrO4-+2H++2e-=BrO3-+H2O 1.763注9N+/N2N2O+2H++2e-=N2+H2O 1.766O-(H2O2)/O2-(H2O)H2O2+2H++2e-=2H2O 1.776NiO42-/NiO2NiO42-+4H++2e-=NiO2+2H2O 1.8Co3+/Co2+Co3++e-=Co2+ 1.808Co3+/Co2+Co3++e-=Co2+ 1.83稀硫酸中Co3+/Co2+Co3++e-=Co2+ 1.92稀高氯酸Ag2+/Ag+Ag2++e-=Ag+ 1.93稀硝酸Ag2+/Ag+Ag2++e-=Ag+ 1.98稀硫酸Ag2+/Ag+Ag2++e-=Ag+ 2.0稀高氯酸S2O82-/SO42-S2O82-+2e-=2SO42- 2.01Bi5+/Bi3+BiO3-+2H++2e-=Bi3++H2O 2.03铋酸盐O3/O2-O3+2H++2e-=O2+H2O 2.076XeO3/Xe XeO3+6H++6e-=Xe+3H2O 2.1最全最实用电极电势表由xsm18倾情制作,转载请注明来源:/xsm18/home注释1:碱族和从钙开始的碱土元素均和水发生反应,其电极电势数值为理论计算值注释2:铈元素(Ce)属于镧系元素,有稳定的+3,+4价,+3价有很强的还原性,+4有很强的氧化性,该元素有很好的代表性。
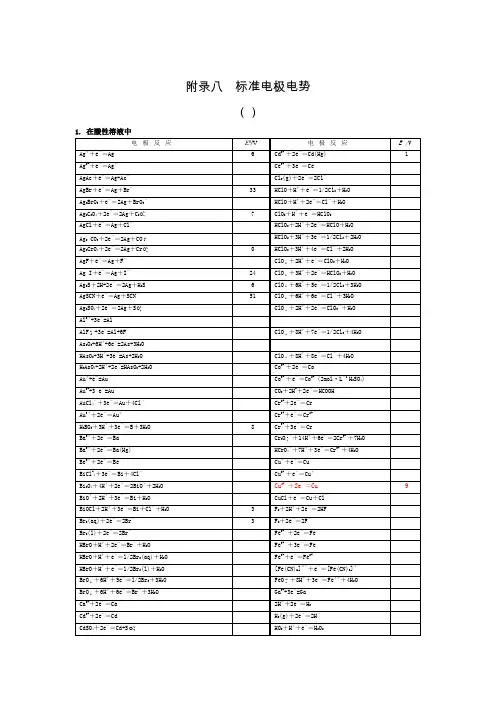

标准电极电势表维基百科,自由的百科全书标准电极电势可以用来计算化学电池或原电池的电化学势或电极电势。
本表中所给出的电极电势以标准氢电极为参比电极,溶液中离子有效浓度为1mol/L,气体分压为100kPa,温度为298K,所有离子的数据都在水溶液中测得。
[1][2][3][4][5][6][7][8][9]单击每栏上方的符号可将数据按元素符号或标准电极电势值排序。
注:(s) – 固体;(l) – 液体;(g) – 气体;(aq) – 水溶液;(Hg) – 汞齐。
Ba+ + e− Ba(s)−4.38[10][1][3] Sr+ + e− Sr(s)−4.10[11][1][3] Ca+ + e− Ca(s)−3.8[11][1][3] Pr3+ + e− Pr2+−3.1[11] N HN 3(aq)−3.09[6]Li+ + e− Li(s)−3.0401[5]N2(g) + 4 H2O + 2 e− 2 NH2OH(aq) + 2 OH−−3.04[6]Cs+ + e− Cs(s)−3.026[5]Ca(OH)2(s) + 2 e− Ca(s) + 2 OH−−3.02[11] Rb+ + e− Rb(s)−2.98[4]K+ + e− K(s)−2.931[5]Mg+ + e− Mg(s)−2.93[10] Ba2+ + 2 e− Ba(s)−2.912[5]La(OH)3(s) + 3 e− La(s) + 3OH−−2.90[5]Fr+ + e− Fr(s)−2.9[11]Sr2+ + 2 e− Sr(s)−2.899[5]Sr(OH)2(s) + 2 e− Sr(s) + 2 OH−−2.88[11] Ca2+ + 2 e− Ca(s)−2.868[5]Eu2+ + 2 e− Eu(s)−2.812[5]Ra2+ + 2 e− Ra(s)−2.8[5]Yb2+ + 2 e− Yb(s)−2.76[11][1] Na+ + e− Na(s)−2.71[5][9] Sm2+ + 2 e− Sm(s)−2.68[11][1] No2+ + 2 e− No(s)−2.50[11] HfO(OH)2(s) + H2O + 4 e− Hf(s) + 4 OH−−2.50[11]Th(OH)4(s) + 4 e− Th(s) + 4 OH−−2.48[11] Md2+ + 2 e− Md(s)−2.40[11]La + 3 e La(s)−2.379[5] Y3+ + 3 e− Y(s)−2.372[5] Mg2+ + 2 e− Mg(s)−2.372[5] ZrO(OH)2(s) + H2O + 4 e− Zr(s) + 4OH−−2.36[5] Pr3+ + 3 e− Pr(s)−2.353[11] Ce3+ + 3 e− Ce(s)−2.336[11] Er3+ + 3 e− Er(s)−2.331[11] Ho3+ + 3 e− Ho(s)−2.33[11] Al(OH)4− + 3 e− Al(s) + 4 OH−−2.33Al(OH)3(s) + 3 e− Al(s) + 3OH−−2.31Tb3+ + 3 e− Tb(s)−2.28H2(g) + 2 e− 2 H−−2.25Ac3+ + 3 e− Ac(s)−2.20Be+ + e− Be(s)−2.12[10] Cf2+ + 2 e− Cf(s)−2.12[11] Am3+ + 3 e− Am(s)−2.048[11] Cf3+ + 3 e− Cf(s)−1.94[11] Am2+ + 2 e− Am(s)−1.9[11] Be2+ + 2 e− Be(s)−1.85Rf4+ + 4 e− Rf(s)−1.67[12] U3+ + 3 e− U(s)−1.66[7] Al3+ + 3 e− Al(s)−1.66[9] Ti2+ + 2 e− Ti(s)−1.63[9] Bk2+ + 2 e− Bk(s)−1.6[11] ZrO2(s) + 4 H+ + 4 e− Zr(s) + 2 H2O−1.553[5] Hf4+ + 4 e− Hf(s)−1.55[11] Zr4+ + 4 e− Zr(s)−1.45[5]Ti + 3 e Ti(s)−1.37[13] TiO(s) + 2 H+ + 2 e− Ti(s) + H2O−1.31Ti2O3(s) + 2 H+ + 2 e− 2 TiO(s) + H2O−1.23Zn(OH)42− + 2 e− Zn(s) + 4 OH−−1.199[14] Mn2+ + 2 e− Mn(s)−1.185[14] Fe(CN)64− + 6 H+ + 2 e− Fe(s) + 4HCN(aq)−1.16[15] V2+ + 2 e− V(s)−1.175[2] Te(s) + 2 e− Te2−−1.143[2] Nb3+ + 3 e− Nb(s)−1.099Sn(s) + 4 H+ + 4 e− SnH4(g)−1.07In(OH)3(s) + 3 e− In(s) + 3 OH−−0.99[11] SiO2(s) + 4 H+ + 4 e− Si(s) + 2 H2O−0.91B(OH)3(aq) + 3 H+ + 3 e− B(s) + 3 H2O−0.89Fe(OH)2(s) + 2 e− Fe(s) + 2 OH−−0.89[15] Fe2O3(s) + 3 H2O + 2 e− 2Fe(OH)2(s) + 2 OH−−0.86[15] TiO2+ + 2 H+ + 4 e− Ti(s) + H2O−0.862 H2O + 2 e− H2(g) + 2 OH−−0.8277[5] Bi(s) + 3 H+ + 3 e− BiH3−0.8[14] Zn2+ + 2 e− Zn(Hg)−0.7628[5] Zn2+ + 2 e− Zn(s)−0.7618[5] Ta2O5(s) + 10 H+ + 10 e− 2 Ta(s) + 5 H2O−0.75Cr3+ + 3 e− Cr(s)−0.74[Au(CN)2]− + e− Au(s) + 2 CN−−0.60Ta3+ + 3 e− Ta(s)−0.6PbO(s) + H2O + 2 e− Pb(s) + 2 OH−−0.582 TiO2(s) + 2 H+ + 2 e− Ti2O3(s) + H2O−0.56Ga3+ + 3 e− Ga(s)−0.53U4+ + e− U3+−0.52[7] H3PO2(aq) + H+ + e− P(白磷[16]) + 2 H2O−0.508[5] H3PO3(aq) + 2 H+ + 2 e− H3PO2(aq) + H2O−0.499[5] H3PO3(aq) + 3 H+ + 3 e− P(红磷)[16] + 3H2O−0.454[5] Fe2+ + 2 e− Fe(s)−0.44[9] 2 CO2(g) + 2 H+ + 2 e− HOOCCOOH(aq)−0.43Cr3+ + e− Cr2+−0.42Cd2+ + 2 e− Cd(s)−0.40[9] SeO32− + 4e− + 3H2O ⇌ Se + 6OH−−0.37[17] GeO2(s) + 2 H+ + 2 e− GeO(s) + H2O−0.37Cu2O(s) + H2O + 2 e− 2 Cu(s) + 2 OH−−0.360[5] PbSO4(s) + 2 e− Pb(s) + SO42−−0.3588[5] PbSO4(s) + 2 e− Pb(Hg) + SO42−−0.3505[5] Eu3+ + e− Eu2+−0.35[7] In3+ + 3 e− In(s)−0.34[2] Tl+ + e− Tl(s)−0.34[2] Ge(s) + 4 H+ + 4 e− GeH4(g)−0.29Co2+ + 2 e− Co(s)−0.28[5] H3PO4(aq) + 2 H+ + 2 e− H3PO3(aq) + H2O−0.276[5] V3+ + e− V2+−0.26[9] Ni2+ + 2 e− Ni(s)−0.25As(s) + 3 H+ + 3 e− AsH3(g)−0.23[2] AgI(s) + e− Ag(s) + I−−0.15224[14] MoO2(s) + 4 H+ + 4 e− Mo(s) + 2 H2O−0.15Si(s) + 4 H+ + 4 e− SiH4(g)−0.14Sn2+ + 2 e− Sn(s)−0.13O2(g) + H+ + e− HO2•(aq)−0.13Pb2+ + 2 e− Pb(s)−0.13[9] WO2(s) + 4 H+ + 4 e− W(s) + 2 H2O−0.12P(红磷) + 3 H+ + 3 e− PH3(g)−0.111[5] CO2(g) + 2 H+ + 2 e− HCOOH(aq)−0.11Se(s) + 2 H+ + 2 e− H2Se(g)−0.11CO2(g) + 2 H+ + 2 e− CO(g) + H2O−0.11SnO(s) + 2 H+ + 2 e− Sn(s) + H2O−0.10SnO2(s) + 2 H+ + 2 e− SnO(s) + H2O−0.09WO3(aq) + 6 H+ + 6 e− W(s) + 3 H2O−0.09[2] P(白磷) + 3 H+ + 3 e− PH3(g)−0.063[5] Fe3+ + 3 e− Fe(s)−0.04[15] HCOOH(aq) + 2 H+ + 2 e− HCHO(aq) + H2O−0.032 H+ + 2 e− H2(g) 0.00≡ 0 AgBr(s) + e− Ag(s) + Br−+0.07133[14] S4O62− + 2 e− 2 S2O32−+0.08Fe3O4(s) + 8 H+ + 8 e− 3 Fe(s) + 4 H2O+0.085[8] N2(g) + 2 H2O + 6H+ + 6 e− 2 NH4OH(aq)+0.092HgO(s) + H2O + 2 e− Hg(l) + 2 OH−+0.0977Cu(NH3)42+ + e− Cu(NH3)2+ + 2 NH3+0.10[2] Ru(NH3)63+ + e− Ru(NH3)62++0.10[7] N2H4(aq) + 4 H2O + 2 e− 2 NH4+ + 4 OH−+0.11[6] H2MoO4(aq) + 6 H+ + 6 e− Mo(s) + 4 H2O+0.11Ge4+ + 4 e− Ge(s)+0.12C(s) + 4 H+ + 4 e− CH4(g)+0.13[2] HCHO(aq) + 2 H+ + 2 e− CH3OH(aq)+0.13S(s) + 2 H+ + 2 e− H2S(g)+0.14Sn4+ + 2 e− Sn2++0.15Cu2+ + e− Cu++0.159[2] HSO4− + 3 H+ + 2 e− SO2(aq) + 2 H2O+0.16UO22+ + e− UO2++0.163[7] SO42− + 4 H+ + 2 e− SO2(aq) + 2 H2O+0.17TiO2+ + 2 H+ + e− Ti3+ + H2O+0.19Bi3+ + 2e− Bi++0.2SbO+ + 2 H+ + 3 e− Sb(s) + H2O+0.20AgCl(s) + e− Ag(s) + Cl−+0.22233[14] H3AsO3(aq) + 3 H+ + 3 e− As(s) + 3 H2O+0.24GeO(s) + 2 H+ + 2 e− Ge(s) + H2O+0.26UO2+ + 4 H+ + e− U4+ + 2 H2O+0.273[7] At2 + e− 2 At-+0.3[11] Re3+ + 3 e− Re(s)+0.300Bi3+ + 3 e− Bi(s)+0.32VO2+ + 2 H+ + e− V3+ + H2O+0.34Cu2+ + 2 e− Cu(s)+0.340[2] [Fe(CN)6]3− + e− [Fe(CN)6]4−+0.36Tc2+ + 2 e− Tc(s)+0.40[11] O2(g) + 2 H2O + 4 e− 4 OH−(aq)+0.40[9] H2MoO4 + 6 H+ + 3 e− Mo3+ + 2 H2O+0.43Ru2+ + 2 e− Ru(s)+0.455[11] Bi+ + e− Bi(s)+0.50CH3OH(aq) + 2 H+ + 2 e− CH4(g) + H2O+0.50SO2(aq) + 4 H+ + 4 e− S(s) + 2 H2O+0.50Cu+ + e− Cu(s)+0.520[2] CO(g) + 2 H+ + 2 e− C(s) + H2O+0.52I3− + 2 e− 3 I−+0.53[9] I2(s) + 2 e− 2 I−+0.54[9] [AuI4]− + 3 e− Au(s) + 4 I−+0.56H3AsO4(aq) + 2 H+ + 2 e− H3AsO3(aq) + H2O+0.56[AuI2]− + e− Au(s) + 2 I−+0.58MnO4− + 2 H2O + 3 e− MnO2(s) + 4 OH−+0.59Rh+ + e− Rh(s)+0.600[11] S2O32 − + 6 H+ + 4 e− 2 S(s) + 3 H2O+0.60Fc+ + e− Fc(s)+0.641[18]Ag + −+0.643[11]H2MoO4(aq) + 2 H+ + 2 e− MoO2(s) + 2 H2O+0.65+ 2 H+ + 2 e−H2O2(aq)+0.70Tl3+ + 3 e− Tl(s)+0.72PtCl62− + 2 e− PtCl42− + 2 Cl−+0.726[7] H2SeO3(aq) + 4 H+ + 4 e− Se(s) + 3 H2O+0.74Rh3+ + 3 e− Rh(s)+0.758[11] PtCl42− + 2 e− Pt(s) + 4 Cl−+0.758[7] Fe3+ + e− Fe2++0.77Ag+ + e− Ag(s)+0.7996[5] Hg22+ + 2 e− 2 Hg(l)+0.80NO3−(aq) + 2 H+ + e− NO2(g) + H2O+0.80FeO42− + 5 H2O + 6 e− Fe2O3(s) + 10 OH−+0.81[15] H2(g) + 2 OH− 2 H2O + 2 e−+0.828[19] [AuBr4]− + 3 e− Au(s) + 4 Br−+0.85Hg2+ + 2 e− Hg(l)+0.85MnO4− + H+ + e− HMnO4−+0.902 Hg2+ + 2 e− Hg22++0.91[2] Pd2+ + 2 e− Pd(s)+0.915[7] [AuCl4]− + 3 e− Au(s) + 4 Cl−+0.93MnO2(s) + 4 H+ + e− Mn3+ + 2 H2O+0.95[AuBr2]− + e− Au(s) + 2 Br−+0.96 [HXeO6]3− + 2 H2O + 2 e− + [HXeO4]− + 4 OH−+0.99[20] HNO2 + H+ + e- = NO(g) + H2O+0.996H6TeO6(aq) + 2 H+ + 2 e− TeO2(s) + 4 H2O+1.02[21] Br2(l) + 2 e− 2 Br−+1.07Br2(aq) + 2 e− 2 Br−+1.09[9] NO2(g) + H+ + e- = HNO2+1.093IO3− + 5 H+ + 4 e− HIO(aq) + 2 H2O+1.13[AuCl2]− + e− Au(s) + 2 Cl−+1.15HSeO4− + 3 H+ + 2 e− H2SeO3(aq) + H2O+1.15Ir3+ + 3 e− Ir(s)+1.156[11] Ag2O(s) + 2 H+ + 2 e− 2 Ag(s) + H2O+1.17ClO3− + 2 H+ + e− ClO2(g) + H2O+1.18 [HXeO6]3− + 5 H2O + 8 e− Xe(g) + 11 OH−+1.18[20] Pt2+ + 2 e− Pt(s)+1.188[7] ClO2(g) + H+ + e− HClO2(aq)+1.192 IO3− + 12 H+ + 10 e− I2(s) + 6 H2O+1.20ClO4− + 2 H+ + 2 e− ClO3− + H2O+1.20O2(g) + 4 H+ + 4 e− 2 H2O+1.229[9] MnO2(s) + 4 H+ + 2 e− Mn2+ + 2H2O+1.23 [HXeO4]− + 3 H2O + 6 e− Xe(g) + 7 OH−+1.24[20]Tl3+ + 2 e− Tl++1.25Cr2O72 − + 14 H+ + 6 e− 2 Cr3+ + 7 H2O+1.33Cl2(g) + 2 e− 2 Cl−+1.36[9] CoO2(s) + 4 H+ + e− Co3+ + 2 H2O+1.422 NH3OH+ + H+ + 2 e− N2H5+ + 2 H2O+1.42[6] 2 HIO(aq) + 2 H+ + 2 e− I2(s) + 2 H2O+1.44Ce4+ + e− Ce3++1.44BrO3− + 5 H+ + 4 e− HBrO(aq) + 2 H2O+1.45β-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O+1.460[2]α-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O+1.468[2] 2 BrO3− + 12 H+ + 10 e− Br2(l) + 6 H2O+1.482ClO3− + 12 H+ + 10 e− Cl2(g) + 6 H2O+1.49HO2 + H+ + e− H2O2+1.495[11] MnO4− + 8 H+ + 5 e− Mn2+ + 4 H2O+1.51HO2• + H+ + e− H2O2(aq)+1.51Au3+ + 3 e− Au(s)+1.52NiO2(s) + 4 H+ + 2 e− Ni2+ + 2 OH−+1.592 HClO(aq) + 2 H+ + 2 e− Cl2(g) + 2 H2O+1.63Ag2O3(s) + 6 H+ + 4 e− 2 Ag+ + 3 H2O+1.67HClO2(aq) + 2 H+ + 2 e− HClO(aq) + H2O+1.67Pb4+ + 2 e− Pb2++1.69[2] MnO4− + 4 H+ + 3 e− MnO2(s) + 2 H2O+1.70AgO(s) + 2 H+ + e− Ag+ + H2O+1.77 H2O2(aq) + 2 H+ + 2 e− 2 H2O+1.776Co3+ + e− Co2++1.82Au+ + e− Au(s)+1.83[2] BrO4− + 2 H+ + 2 e− BrO3− + H2O+1.85半反应E° (V)[注 1]来源Ag2+ + e− Ag++1.98[2]S2O82− + 2 e− 2 SO42−+2.07O3(g) + 2 H+ + 2 e− O2(g) + H2O+2.075[7]HMnO4− + 3 H+ + 2 e− MnO2(s) + 2 H2O+2.09XeO3(aq) + 6 H+ + 6 e− Xe(g) + 3 H2O+2.12[20]H4XeO6(aq) + 8 H+ + 8 e− Xe(g) + 6 H2O+2.18[20]FeO42− + 3 e− + 8 H+ Fe3+ + 4 H2O+2.20[22]XeF2(aq) + 2 H+ + 2 e− Xe(g) + 2HF(aq)+2.32[20]H4XeO6(aq) + 2 H+ + 2 e− XeO3(aq) + H2O+2.42[20]F2(g) + 2 e− 2 F−+2.87[2][9]F2(g) + 2 H+ + 2 e− 2 HF(aq)+3.05[2]Tb4+ + e− Tb3++3.05[11]1.^ Clicking on this column to re-sort by potential didn’t work in the Safari webbrowser in v. 4.0.3 or earlier (but works in v. 4.0.5). In this case just reload the page to restore the original order.参考资料1.^ 1.01.11.21.31.41.5 Milazzo, G., Caroli, S., and Sharma, V. K. (1978). Tables ofStandard Electrode Potentials (Wiley, Chichester).2.^ 2.002.012.022.032.042.052.062.072.082.092.102.112.122.132.142.152.162.172.182.19 Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).3.^ 3.03.13.23.3 Bratsch, S. G. (1989). Journal of Physical Chemistry Reference DataVol. 18, pp. 1–21.4.^ 4.04.1 Vanýsek, Petr (2006). "Electrochemical Series," in Handbook of Chemistry and Physics: 87th Edition (/) (Chemical RubberCompany).^ 5.005.015.025.035.045.055.065.075.085.095.105.115.125.135.145.155.165.175.185.195.205.215.22 5.5.235.245.255.265.275.285.295.30 Vanýsek, Petr (2007). “Electrochemical Series”(/articles/08_08_88.pdf) , in Handbook of Chemistryand Physics: 88th Edition (/) (Chemical RubberCompany).6.^ 6.06.16.26.36.4 Greenwood, N. N.; Earnshaw, A.. Chemistry of the Elements. 2ndEdition. Oxford:Butterworth-Heinemann. 1997. ISBN0-7506-3365-4.^ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 Bard, A.J., Faulkner, L.R.(2001). Electrochemical Methods. Fundamentals and Applications , 2nd edition (John Wiley and Sons Inc).7.^ 8.0 8.1 Marcel Pourbaix (1966). Atlas of Electrochemical Equilibria in Aqueous Solutions (NACE International, Houston, Texas; Cebelcor, Brussels).8.^ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 Peter Atkins (1997). Physical Chemistry , 6th edition (W.H. Freeman and Company, New York).9.^ 10.0 10.1 10.2 Ca Sr Ba 一价[11]与两价间的标准电极电势正好有规律关系,因此可以估计近似值10.^ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 11.21 11.22 11.23 11.24 11.25 11.26 11.27 11.28 11.29 11.30 11.31 Standard Redox Potential Table (/time-to-wake-up/docs/electrochemical_redox_potential)11.^ Ti Zr Hf 的标准电极电势变化较规律,因此可估计Rf 的标准电极电势12.^ Gordon Aylward & Tristan Findlay (2008). "SI Chemical Data", 6th edition (John Wiley & Sons, Australia), ISBN 9780470816387.13.^ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Vanýsek, Petr (2007). “Electrochemical Series”, in Handbook of Chemistry and Physics: 88th Edition (Chemical Rubber Company).14.^ 15.0 15.1 15.2 15.3 15.4 WebElements Periodic Table of the Elements | Iron | compounds information (/iron/compounds.html)15.^ 16.0 16.1 由−0.454和(2×−0.499 + −0.508) ÷ 3 = −0.502推算出。
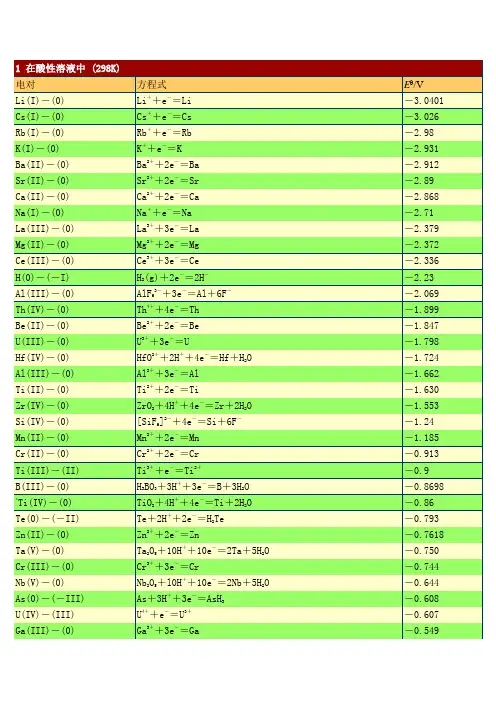
1电对方程式E /VLi(I)-(0) Li++e-=Li -3.0401 Cs(I)-(0) Cs++e-=Cs -3.026 Rb(I)-(0) Rb++e-=Rb -2.98 K(I)-(0) K++e-=K -2.931 Ba(II)-(0) Ba2++2e-=Ba -2.912 Sr(II)-(0) Sr2++2e-=Sr -2.89 Ca(II)-(0) Ca2++2e-=Ca -2.868 Na(I)-(0) Na++e-=Na -2.71 La(III)-(0) La3++3e-=La -2.379 Mg(II)-(0) Mg2++2e-=Mg -2.372 Ce(III)-(0) Ce3++3e-=Ce -2.336 H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069 Th(IV)-(0) Th4++4e-=Th -1.899 Be(II)-(0) Be2++2e-=Be -1.847 U(III)-(0) U3++3e-=U -1.798Hf(IV)-(0) HfO2++2H++4e-=Hf+H2O -1.724 Al(III)-(0) Al3++3e-=Al -1.662 Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0) ZrO2+4H++4e-=Zr+2H2O -1.553Si(IV)-(0) [SiF6]2-+4e-=Si+6F--1.24 Mn(II)-(0) Mn2++2e-=Mn -1.185 Cr(II)-(0) Cr2++2e-=Cr -0.913 Ti(III)-(II) Ti3++e-=Ti2+-0.9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0.8698*Ti(IV)-(0) TiO2+4H++4e-=Ti+2H2O -0.86Te(0)-(-II) Te+2H++2e-=H2Te -0.793 Zn(II)-(0) Zn2++2e-=Zn -0.7618Ta(V)-(0) Ta2O5+10H++10e-=2Ta+5H2O -0.750Cr(III)-(0) Cr3++3e-=Cr -0.744Nb(V)-(0) Nb2O5+l0H++10e-=2Nb+5H2O -0.644As(0)-(-III) As+3H++3e-=AsH3-0.608 U(IV)-(III) U4++e-=U3+-0.607 Ga(III)-(0) Ga3++3e-=Ga -0.549P(I)-(0) H3PO2+H++e-=P+2H2O -0.508P(III)-(I) H3PO3+2H++2e-=H3PO2+H2O -0.499*C(IV)-(III) 2CO2+2H++2e-=H2C2O4-0.49Fe(II)-(0) Fe2++2e-=Fe -0.447Cr(III)-(II) Cr3++e-=Cr2+-0.407 Cd(II)-(0) Cd2++2e-=Cd -0.4030 Se(0)-(-II) Se+2H++2e-=H2Se(aq) -0.399Pb(II)-(0) PbI2+2e-=Pb+2I--0.365 Eu(III)-(II) Eu3++e-=Eu2+-0.36Pb(II)-(0) PbSO4+2e-=Pb+SO42--0.3588In(III)-(0) In3++3e-=In -0.3382 Tl(I)-(0) Tl++e-=Tl -0.336 Co(II)-(0) Co2++2e-=Co -0.28P(V)-(III) H3PO4+2H++2e-=H3PO3+H2O -0.276Pb(II)-(0) PbCl2+2e-=Pb+2Cl--0.2675 Ni (II)-(0) Ni2++2e-=Ni -0.257 V(III)-(II) V3++e-=V2+-0.255Ge(IV)-(0) H2GeO3+4H++4e-=Ge+3H2O -0.182Ag(I)-(0) AgI+e-=Ag+I--0.15224 Sn(II)-(0) Sn2++2e-=Sn -0.1375 Pb(II)-(0) Pb2++2e-=Pb -0.1262*C(IV)-(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0)-(-III) P(white)+3H++3e-=PH3(g) -0.063Hg(I)-(0) Hg2I2+2e-=2Hg+2I--0.0405Fe(III)-(0) Fe3++3e-=Fe -0.037 H(I)-(0) 2H++2e-=H20.0000 Ag(I)-(0) AgBr+e-=Ag+Br-0.07133S(II.V)-(II) S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III) TiO2++2H++e-=Ti3++H2O 0.1S(0)-(-II) S+2H++2e-=H2S(aq) 0.142 Sn(IV)-(II) Sn4++2e-=Sn2+0.151Sb(III)-(0) Sb2O3+6H++6e-=2Sb+3H2O 0.152Cu(II)-(I) Cu2++e-=Cu+0.153 Bi(III)-(0) BiOCl+2H++3e-=Bi+Cl-+H2O 0.1583S(VI)-(IV) SO42-+4H++2e-=H2SO3+H2O 0.172Sb(III)-(0) SbO++2H++3e-=Sb+H2O 0.212Ag(I)-(0) AgCl+e-=Ag+Cl-0.22233As(III)-(0) HAsO2+3H++3e-=As+2H2O 0.248Hg(I)-(0) Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl) 0.26808Bi(III)-(0) BiO++2H++3e-=Bi+H2O 0.320U(VI)-(IV) UO22++4H++2e-=U4++2H2O 0.327C(IV)-(III) 2HCNO+2H++2e-=(CN)2+2H2O 0.330V(IV)-(III) VO2++2H++e-=V3++H2O 0.337 Cu(II)-(0) Cu2++2e-=Cu 0.3419Re(VII)-(0) ReO4-+8H++7e-=Re+4H2O 0.368Ag(I)-(0) Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0) H2SO3+4H++4e-=S+3H2O 0.449Cu(I)-(0) Cu++e-=Cu 0.521I(0)-(-I) I2+2e-=2I-0.5355I(0)-(-I) I3-+2e-=3I-0.536As(V)-(III) H3AsO4+2H++2e-=HAsO2+2H2O 0.560Sb(V)-(III) Sb2O5+6H++4e-=2SbO++3H2O 0.581Te(IV)-(0) TeO2+4H++4e-=Te+2H2O 0.593U(V)-(IV) UO2++4H++e-=U4++2H2O 0.612**Hg(II)-(I) 2HgCl2+2e-=Hg2Cl2+2Cl-0.63Pt(IV)-(II) [PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68O(0)-(-I) O2+2H++2e-=H2O20.695Pt(II)-(0) [PtCl4]2-+2e-=Pt+4Cl-0.755*Se(IV)-(0) H2SeO3+4H++4e-=Se+3H2O 0.74Fe(III)-(II) Fe3++e-=Fe2+0.771 Hg(I)-(0) Hg22++2e-=2Hg 0.7973 Ag(I)-(0) Ag++e-=Ag 0.7996Os(VIII)-(0) OsO4+8H++8e-=Os+4H2O 0.8N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803Hg(II)-(0) Hg2++2e-=Hg 0.851Si(IV)-(0) (quartz)SiO2+4H++4e-=Si+2H2O 0.857Cu(II)-(I) Cu2++I-+e-=CuI 0.86N(III)-(I) 2HNO2+4H++4e-=H2N2O2+2H2O 0.86Hg(II)-(I) 2Hg2++2e-=Hg22+0.920N(V)-(III) NO3-+3H++2e-=HNO2+H2O 0.934Pd(II)-(0) Pd2++2e-=Pd 0.951N(V)-(II) NO3-+4H++3e-=NO+2H2O 0.957N(III)-(II) HNO2+H++e-=NO+H2O 0.983I(I)-(-I) HIO+H++2e-=I-+H2O 0.987V(V)-(IV) VO2++2H++e-=VO2++H2O 0.991V(V)-(IV) V(OH)4++2H++e-=VO2++3H2O 1.00Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002Te(VI)-(IV) H6TeO6+2H++2e-=TeO2+4H2O 1.02N(IV)-(II) N2O4+4H++4e-=2NO+2H2O 1.035N(IV)-(III) N2O4+2H++2e-=2HNO21.065I(V)-(-I) IO3-+6H++6e-=I-+3H2O 1.085Br(0)-(-I) Br2(aq)+2e-=2Br- 1.0873Se(VI)-(IV) SeO42-+4H++2e-=H2SeO3+H2O 1.151Cl(V)-(IV) ClO3-+2H++e-=ClO2+H2O 1.152Pt(II)-(0) Pt2++2e-=Pt 1.18Cl(VII)-(V) ClO4-+2H++2e-=ClO3-+H2O 1.189I(V)-(0) 2IO3-+12H++10e-=I2+6H2O 1.195Cl(V)-(III) ClO3-+3H++2e-=HClO2+H2O 1.214Mn(IV)-(II) MnO2+4H++2e-=Mn2++2H2O 1.224O(0)-(-II) O2+4H++4e-=2H2O 1.229Tl(III)-(I) T13++2e-=Tl+ 1.252Cl(IV)-(III) ClO2+H++e-=HClO21.277N(III)-(I) 2HNO2+4H++4e-=N2O+3H2O 1.297**Cr(VI)-(III) Cr2O72-+14H++6e-=2Cr3++7H2O 1.33Br(I)-(-I) HBrO+H++2e-=Br-+H2O 1.331Cr(VI)-(III) HCrO4-+7H++3e-=Cr3++4H2O 1.350Cl(0)-(-I) Cl2(g)+2e-=2Cl- 1.35827Cl(VII)-(-I) ClO4-+8H++8e-=Cl-+4H2O 1.389Cl(VII)-(0) ClO4-+8H++7e-=1/2Cl2+4H2O 1.39Au(III)-(I) Au3++2e-=Au+ 1.401Br(V)-(-I) BrO3-+6H++6e-=Br-+3H2O 1.423I(I)-(0) 2HIO+2H++2e-=I2+2H2O 1.439Cl(V)-(-I) ClO3-+6H++6e-=Cl-+3H2O 1.451Pb(IV)-(II) PbO2+4H++2e-=Pb2++2H2O 1.455Cl(V)-(0) ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I) HClO+H++2e-=Cl-+H2O 1.482Br(V)-(0) BrO3-+6H++5e-=l/2Br2+3H2O 1.482Au(III)-(0) Au3++3e-=Au 1.498Mn(VII)-(II) MnO4-+8H++5e-=Mn2++4H2O 1.507Mn(III)-(II) Mn3++e-=Mn2+ 1.5415Cl(III)-(-I) HClO2+3H++4e-=Cl-+2H2O 1.570Br(I)-(0) HBrO+H++e-=l/2Br2(aq)+H2O 1.574N(II)-(I) 2NO+2H++2e-=N2O+H2O 1.591I(VII)-(V) H5IO6+H++2e-=IO3-+3H2O 1.601Cl(I)-(0) HClO+H++e-=1/2Cl2+H2O 1.611Cl(III)-(I) HClO2+2H++2e-=HClO+H2O 1.645Ni(IV)-(II) NiO2+4H++2e-=Ni2++2H2O 1.678Mn(VII)-(IV) MnO4-+4H++3e-=MnO2+2H2O 1.679Pb(IV)-(II) PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913Au(I)-(0) Au++e-=Au 1.692 Ce(IV)-(III) Ce4++e-=Ce3+ 1.72N(I)-(0) N2O+2H++2e-=N2+H2O 1.766O(-I)-(-II) H2O2+2H++2e-=2H2O 1.776Co(III)-(II) Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83Ag(II)-(I) Ag2++e-=Ag+ 1.980S(VII)-(VI) S2O82-+2e-=2SO42- 2.010O(0)-(-II) O3+2H++2e-=O2+H2O 2.076O(II)-(-II) F2O+2H++4e-=H2O+2F- 2.153Fe(VI)-(III) FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II) O(g)+2H++2e-=H2O 2.421F(0)-(-I) F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99La(III)-(0) La(OH)3+3e-=La+3OH--2.90Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36Al(III)-(0) H AlO-+H O+3e-=Al+OH--2.33P(I)-(0) H2PO2-+e-=P+2OH--1.82B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48*Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2Te(0)-(-I) Te+2e-=Te2--1.143P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584*S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13*Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076Ag(I)-(0) AgCN+e-=Ag+CN--0.017N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373O(0)-(-II) O2+2H2O+4e-=4OH-0.401I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490Mn(VII)-(VI) MnO4-+e-=MnO42-0.558Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607。
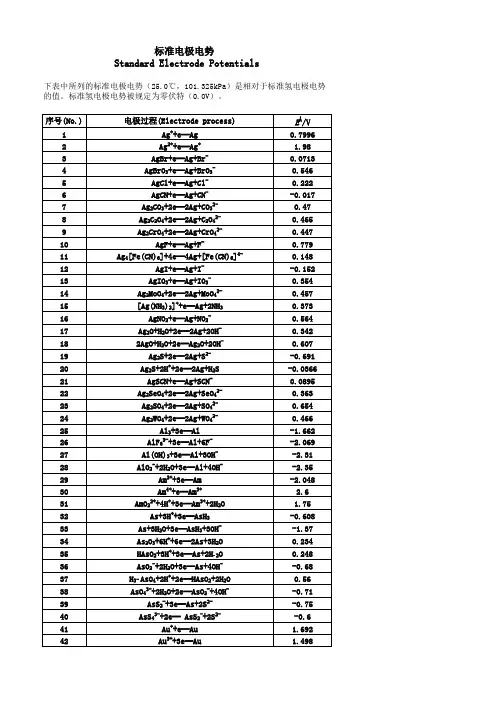
标准电极电势Standard Electrode Potentials下表中所列的标准电极电势(25.0℃,101.325kPa)是相对于标准氢电极电势的值。
标准氢电极电势被规定为零伏特(0.0V)。
序号(No.)电极过程(Electrode process)EÅ/V 1Ag++e═Ag0.79962Ag2++e═Ag+ 1.983AgBr+e═Ag+Br-0.07134AgBrO3+e═Ag+BrO3-0.5465AgCl+e═Ag+Cl-0.2226AgCN+e═Ag+CN--0.0177Ag2CO3+2e═2Ag+CO32-0.478Ag2C2O4+2e═2Ag+C2O42-0.4659Ag2CrO4+2e═2Ag+CrO42-0.44710AgF+e═Ag+F-0.77911Ag4[Fe(CN)6]+4e═4Ag+[Fe(CN)6]4-0.14812AgI+e═Ag+I--0.152 13AgIO3+e═Ag+IO3-0.35414Ag2MoO4+2e═2Ag+MoO42-0.45715[Ag(NH3)2]++e═Ag+2NH30.37316AgNO2+e═Ag+NO2-0.56417Ag2O+H2O+2e═2Ag+2OH-0.342182AgO+H2O+2e═Ag2O+2OH-0.60719Ag2S+2e═2Ag+S2--0.691 20Ag2S+2H++2e═2Ag+H2S-0.0366 21AgSCN+e═Ag+SCN-0.0895 22Ag2SeO4+2e═2Ag+SeO42-0.36323Ag2SO4+2e═2Ag+SO42-0.65424Ag2WO4+2e═2Ag+WO42-0.46625Al3+3e═Al-1.662 26AlF63-+3e═Al+6F--2.069 27Al(OH)3+3e═Al+3OH--2.3128AlO2-+2H2O+3e═Al+4OH--2.3529Am3++3e═Am-2.048 30Am4++e═Am3+ 2.631AmO22++4H++3e═Am3++2H2O 1.7532As+3H++3e═AsH3-0.608 33As+3H2O+3e═AsH3+3OH--1.3734As2O3+6H++6e═2As+3H2O0.23435HAsO2+3H++3e═As+2H2O0.24836AsO2-+2H2O+3e═As+4OH--0.6837H3AsO4+2H++2e═HAsO2+2H2O0.5638AsO43-+2H2O+2e═AsO2-+4OH--0.7139AsS2-+3e═As+2S2--0.7540AsS43-+2e═ AsS2-+2S2--0.641Au++e═Au 1.69242Au3++3e═Au 1.49843Au3++2e═Au+ 1.401 44AuBr2-+e═Au+2Br-0.959 45AuBr4-+3e═Au+4Br-0.854 46AuCl2-+e═Au+2Cl- 1.15 47AuCl4-+3e═Au+4Cl- 1.002 48AuI+e═Au+I-0.5 49Au(SCN)4-+3e═Au+4SCN-0.66 50Au(OH)3+3H++3e═Au+3H2O 1.45 51BF4-+3e═B+4F--1.04 52H2BO3-+H2O+3e═B+4OH--1.79 53B(OH)3+7H++8e═BH4-+3H2O-0.0481 54Ba2++2e═Ba-2.912 55Ba(OH)2+2e═Ba+2OH--2.99 56Be2++2e═Be-1.847 57Be2O32-+3H2O+4e═2Be+6OH--2.63 58Bi++e═Bi0.5 59Bi3++3e═Bi0.308 60BiCl4-+3e═Bi+4Cl-0.16 61BiOCl+2H++3e═Bi+Cl-+H2O0.16 62Bi2O3+3H2O+6e═2Bi+6OH--0.46 63Bi2O4+4H++2e═2BiO++2H2O 1.593 64Bi2O4+H2O+2e═ Bi2O3+2OH-0.56 65Br2(水溶液,aq)+2e═2Br- 1.087 66Br2(液体)+2e═2Br- 1.066 67BrO-+H2O+2e═Br-+2OH0.761 68BrO3-+6H++6e═Br-+3H2O 1.423 69BrO3-+3H2O+6e═Br-+6OH-0.61 702BrO3-+12H++10e═Br2+6H2O 1.482 71HBrO+H++2e═Br-+H2O 1.331 722HBrO+2H++2e═Br2(水溶液,aq)+2H2O 1.574 73CH3OH+2H++2e═CH4+H2O0.59 74HCHO+2H++2e═CH3OH0.19 75CH3COOH+2H++2e═CH3CHO+H2O-0.12 76(CN)2+2H++2e═2HCN0.373 77(CNS)2+2e═2CNS-0.77 78CO2+2H++2e═CO+H2O-0.12 79CO2+2H++2e═HCOOH-0.199 80Ca2++2e═Ca-2.868 81Ca(OH)2+2e═Ca+2OH--3.02 82Cd2++2e═Cd-0.403 83Cd2++2e═Cd(Hg)-0.352 84Cd(CN)42-+2e═Cd+4CN--1.09 85CdO+H2O+2e═Cd+2OH--0.783 86CdS+2e═Cd+S2--1.17 87CdSO4+2e═Cd+SO42--0.246 88Ce3++3e═Ce-2.336 89Ce3++3e═Ce(Hg)-1.437 90CeO2+4H++e═Ce3++2H2O 1.4 91Cl2(气体)+2e═2Cl- 1.35892ClO-+H2O+2e═Cl-+2OH-0.89 93HClO+H++2e═Cl-+H2O 1.482 942HClO+2H++2e═Cl2+2H2O 1.611 95ClO2-+2H2O+4e═Cl-+4OH-0.76 962ClO3-+12H++10e═Cl2+6H2O 1.47 97ClO3-+6H++6e═Cl-+3H2O 1.451 98ClO3-+3H2O+6e═Cl-+6OH-0.62 99ClO4-+8H++8e═Cl-+4H2O 1.38 1002ClO4-+16H++14e═Cl2+8H2O 1.39 101Cm3++3e═Cm-2.04 102Co2++2e═Co-0.28 103[Co(NH3)6]3++e═[Co(NH3)6]2+0.108 104[Co(NH3)6]2++2e═Co+6NH3-0.43 105Co(OH)2+2e═Co+2OH--0.73 106Co(OH)3+e═Co(OH)2+OH-0.17 107Cr2++2e═Cr-0.913 108Cr3++e═Cr2+-0.407 109Cr3++3e═Cr-0.744 110[Cr(CN)6]3-+e═[Cr(CN)6]4--1.28 111Cr(OH)3+3e═Cr+3OH--1.48 112Cr2O72-+14H++6e═2Cr3++7H2O 1.232 113CrO2-+2H2O+3e═Cr+4OH--1.2 114HCrO4-+7H++3e═Cr3++4H2O 1.35 115CrO42-+4H2O+3e═Cr(OH)3+5OH--0.13 116Cs++e═Cs-2.92 117Cu++e═Cu0.521 118Cu2++2e═Cu0.342 119Cu2++2e═Cu(Hg)0.345 120Cu2++Br-+e═CuBr0.66 121Cu2++Cl-+e═CuCl0.57 122Cu2++I-+e═CuI0.86 123Cu2++2CN-+e═[Cu(CN)2]- 1.103 124CuBr2-+e═Cu+2Br-0.05 125CuCl2-+e═Cu+2Cl-0.19 126CuI2-+e═Cu+2I-0 127Cu2O+H2O+2e═2Cu+2OH--0.36 128Cu(OH)2+2e═Cu+2OH--0.222 1292Cu(OH)2+2e═Cu2O+2OH-+H2O-0.08 130CuS+2e═Cu+S2--0.7 131CuSCN+e═Cu+SCN--0.27 132Dy2++2e═Dy-2.2 133Dy3++3e═Dy-2.295 134Er2++2e═Er-2 135Er3++3e═Er-2.331 136Es2++2e═Es-2.23 137Es3++3e═Es-1.91 138Eu2++2e═Eu-2.812 139Eu3++3e═Eu-1.991 140F2+2H++2e═2HF 3.053190IO3-+2H2O+4e═IO-+4OH-0.15 191IO3-+3H2O+6e═I-+6OH-0.26 1922IO3-+6H2O+10e═I2+12OH-0.21 193H5IO6+H++2e═IO3-+3H2O 1.601 194In++e═In-0.14 195In3++3e═In-0.338 196In(OH)3+3e═In+3OH--0.99 197Ir3++3e═Ir 1.156 198IrBr62-+e═ IrBr63-0.99 199IrCl62-+e═IrCl63-0.867 200K++e═K-2.931 201La3++3e═La-2.379 202La(OH)3+3e═La+3OH--2.9 203Li++e═Li-3.04 204Lr3++3e═Lr-1.96 205Lu3++3e═Lu-2.28 206Md2++2e═Md-2.4 207Md3++3e═Md-1.65 208Mg2++2e═Mg-2.372 209Mg(OH)2+2e═Mg+2OH--2.69 210Mn2++2e═Mn-1.185 211Mn3++3e═Mn 1.542 212MnO2+4H++2e═Mn2++2H2O 1.224 213MnO4-+4H++3e═MnO2+2H2O 1.679 214MnO4-+8H++5e═Mn2++4H2O 1.507 215MnO4-+2H2O+3e═MnO2+4OH-0.595 216Mn(OH)2+2e═Mn+2OH--1.56 217Mo3++3e═Mo-0.2 218MoO42-+4H2O+6e═Mo+8OH--1.05 219N2+2H2O+6H++6e═2NH4OH0.092 2202NH3OH++H++2e═N2H5++2H2O 1.42 2212NO+H2O+2e═N2O+2OH-0.76 2222HNO2+4H++4e═N2O+3H2O 1.297 223NO3-+3H++2e═HNO2+H2O0.934 224NO3-+H2O+2e═NO2-+2OH-0.01 2252NO3-+2H2O+2e═N2O4+4OH--0.85 226Na++e═Na-2.713 227Nb3++3e═Nb-1.099 228NbO2+4H++4e═Nb+2H2O-0.69 229Nb2O5+10H++10e═2Nb+5H2O-0.644 230Nd2++2e═Nd-2.1 231Nd3++3e═Nd-2.323 232Ni2++2e═Ni-0.257 233NiCO3+2e═Ni+CO32--0.45 234Ni(OH)2+2e═Ni+2OH--0.72 235NiO2+4H++2e═Ni2++2H2O 1.678 236No2++2e═No-2.5 237No3++3e═No-1.2 238Np3++3e═Np-1.856239NpO2+H2O+H++e═Np(OH)3-0.962 240O2+4H++4e═2H2O 1.229 241O2+2H2O+4e═4OH-0.401 242O3+H2O+2e═O2+2OH- 1.24 243Os2++2e═Os0.85 244OsCl63-+e═Os2++6Cl-0.4 245OsO2+2H2O+4e═Os+4OH--0.15 246OsO4+8H++8e═Os+4H2O0.838 247OsO4+4H++4e═OsO2+2H2O 1.02 248P+3H2O+3e═PH3(g)+3OH--0.87 249H2PO2-+e═P+2OH--1.82 250H3PO3+2H++2e═H3PO2+H2O-0.499 251H3PO3+3H++3e═P+3H2O-0.454 252H3PO4+2H++2e═H3PO3+H2O--0.276 253PO43-+2H2O+2e═HPO32-+3OH--1.05 254Pa3++3e═Pa-1.34 255Pa4++4e═Pa-1.49 256Pb2++2e═Pb-0.126 257Pb2++2e═Pb(Hg)-0.121 258PbBr2+2e═Pb+2Br--0.284 259PbCl2+2e═Pb+2Cl--0.268 260PbCO3+2e═Pb+CO32--0.506 261PbF2+2e═Pb+2F--0.344 262PbI2+2e═Pb+2I--0.365 263PbO+H2O+2e═Pb+2OH--0.58 264PbO+4H++2e═Pb+H2O0.25 265PbO2+4H++2e═Pb2+2H2O 1.455 266HPbO2-+H2O+2e═Pb+3OH--0.537 267PbO2+SO42-+4H++2e═PbSO4+2H2O 1.691 268PbSO4+2e═Pb+SO42--0.359 269Pd2++2e═Pd0.915 270PdBr42-+2e═Pd+4Br-0.6 271PdO2+H2O+2e═PdO+2OH-0.73 272Pd(OH)2+2e═Pd+2OH-0.07 273Pm2++2e═Pm-2.2 274Pm3++3e═Pm-2.3 275Po4++4e═Po0.76 276Pr2++2e═Pr-2 277Pr3++3e═Pr-2.353 278Pt2++2e═Pt 1.18 279[PtCl6]2-+2e═[PtCl4]2-+2Cl-0.68 280Pt(OH)2+2e═Pt+2OH-0.14 281PtO2+4H++4e═Pt+2H2O1 282PtS+2e═Pt+S2--0.83 283Pu3++3e═Pu-2.031 284Pu5++e═Pu4+ 1.099 285Ra2++2e═Ra-2.8 286Rb++e═Rb-2.98 287Re3++3e═Re0.3337Th4++4e═Th-1.899 338Ti2++2e═Ti-1.63 339Ti3++3e═Ti-1.37 340TiO2+4H++2e═Ti2++2H2O-0.502 341TiO2++2H++e═Ti3++H2O0.1 342Tl++e═Tl-0.336 343Tl3++3e═Tl0.741 344Tl3++Cl-+2e═TlCl 1.36 345TlBr+e═Tl+Br--0.658 346TlCl+e═Tl+Cl--0.557 347TlI+e═Tl+I--0.752 348Tl2O3+3H2O+4e═2Tl++6OH-0.02 349TlOH+e═Tl+OH--0.34 350Tl2SO4+2e═2Tl+SO42--0.436 351Tm2++2e═Tm-2.4 352Tm3++3e═Tm-2.319 353U3++3e═U-1.798 354UO2+4H++4e═U+2H2O-1.4 355UO2++4H++e═U4++2H2O0.612 356UO22++4H++6e═U+2H2O-1.444 357V2++2e═V-1.175 358VO2++2H++e═V3++H2O0.337 359VO2++2H++e═VO2++H2O0.991 360VO2++4H++2e═V3++2H2O0.668 361V2O5+10H++10e═2V+5H2O-0.242 362W3++3e═W0.1 363WO3+6H++6e═W+3H2O-0.09 364W2O5+2H++2e═2WO2+H2O-0.031 365Y3++3e═Y-2.372 366Yb2++2e═Yb-2.76 367Yb3++3e═Yb-2.19 368Zn2++2e═Zn-0.7618 369Zn2++2e═Zn(Hg)-0.7628 370Zn(OH)2+2e═Zn+2OH--1.249 371ZnS+2e═Zn+S2--1.4 372ZnSO4+2e═Zn(Hg)+SO42--0.799。
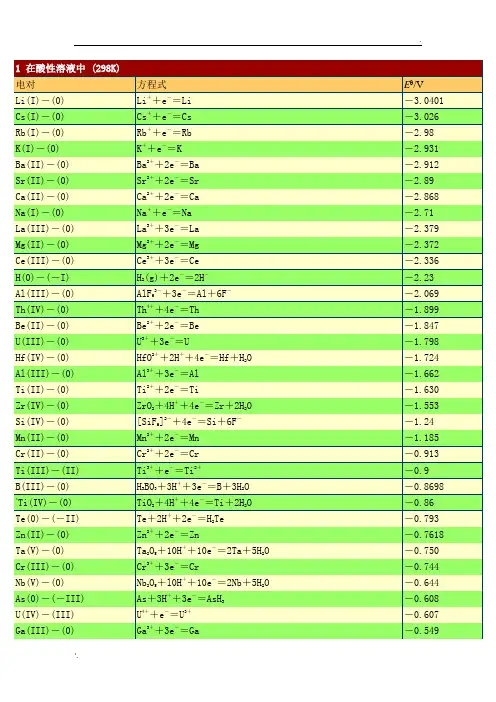
1电对方程式E /VLi(I)-(0) Li++e-=Li -3.0401 Cs(I)-(0) Cs++e-=Cs -3.026 Rb(I)-(0) Rb++e-=Rb -2.98 K(I)-(0) K++e-=K -2.931 Ba(II)-(0) Ba2++2e-=Ba -2.912 Sr(II)-(0) Sr2++2e-=Sr -2.89 Ca(II)-(0) Ca2++2e-=Ca -2.868 Na(I)-(0) Na++e-=Na -2.71 La(III)-(0) La3++3e-=La -2.379 Mg(II)-(0) Mg2++2e-=Mg -2.372 Ce(III)-(0) Ce3++3e-=Ce -2.336 H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069 Th(IV)-(0) Th4++4e-=Th -1.899 Be(II)-(0) Be2++2e-=Be -1.847 U(III)-(0) U3++3e-=U -1.798Hf(IV)-(0) HfO2++2H++4e-=Hf+H2O -1.724 Al(III)-(0) Al3++3e-=Al -1.662 Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0) ZrO2+4H++4e-=Zr+2H2O -1.553Si(IV)-(0) [SiF6]2-+4e-=Si+6F--1.24 Mn(II)-(0) Mn2++2e-=Mn -1.185 Cr(II)-(0) Cr2++2e-=Cr -0.913 Ti(III)-(II) Ti3++e-=Ti2+-0.9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0.8698*Ti(IV)-(0) TiO2+4H++4e-=Ti+2H2O -0.86Te(0)-(-II) Te+2H++2e-=H2Te -0.793 Zn(II)-(0) Zn2++2e-=Zn -0.7618Ta(V)-(0) Ta2O5+10H++10e-=2Ta+5H2O -0.750Cr(III)-(0) Cr3++3e-=Cr -0.744Nb(V)-(0) Nb2O5+l0H++10e-=2Nb+5H2O -0.644As(0)-(-III) As+3H++3e-=AsH3-0.608 U(IV)-(III) U4++e-=U3+-0.607 Ga(III)-(0) Ga3++3e-=Ga -0.549P(I)-(0) H3PO2+H++e-=P+2H2O -0.508P(III)-(I) H3PO3+2H++2e-=H3PO2+H2O -0.499*C(IV)-(III) 2CO2+2H++2e-=H2C2O4-0.49Fe(II)-(0) Fe2++2e-=Fe -0.447Cr(III)-(II) Cr3++e-=Cr2+-0.407 Cd(II)-(0) Cd2++2e-=Cd -0.4030 Se(0)-(-II) Se+2H++2e-=H2Se(aq) -0.399Pb(II)-(0) PbI2+2e-=Pb+2I--0.365 Eu(III)-(II) Eu3++e-=Eu2+-0.36Pb(II)-(0) PbSO4+2e-=Pb+SO42--0.3588In(III)-(0) In3++3e-=In -0.3382 Tl(I)-(0) Tl++e-=Tl -0.336 Co(II)-(0) Co2++2e-=Co -0.28P(V)-(III) H3PO4+2H++2e-=H3PO3+H2O -0.276Pb(II)-(0) PbCl2+2e-=Pb+2Cl--0.2675 Ni (II)-(0) Ni2++2e-=Ni -0.257 V(III)-(II) V3++e-=V2+-0.255Ge(IV)-(0) H2GeO3+4H++4e-=Ge+3H2O -0.182Ag(I)-(0) AgI+e-=Ag+I--0.15224 Sn(II)-(0) Sn2++2e-=Sn -0.1375 Pb(II)-(0) Pb2++2e-=Pb -0.1262*C(IV)-(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0)-(-III) P(white)+3H++3e-=PH3(g) -0.063Hg(I)-(0) Hg2I2+2e-=2Hg+2I--0.0405Fe(III)-(0) Fe3++3e-=Fe -0.037 H(I)-(0) 2H++2e-=H20.0000 Ag(I)-(0) AgBr+e-=Ag+Br-0.07133S(II.V)-(II) S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III) TiO2++2H++e-=Ti3++H2O 0.1S(0)-(-II) S+2H++2e-=H2S(aq) 0.142 Sn(IV)-(II) Sn4++2e-=Sn2+0.151Sb(III)-(0) Sb2O3+6H++6e-=2Sb+3H2O 0.152Cu(II)-(I) Cu2++e-=Cu+0.153 Bi(III)-(0) BiOCl+2H++3e-=Bi+Cl-+H2O 0.1583S(VI)-(IV) SO42-+4H++2e-=H2SO3+H2O 0.172Sb(III)-(0) SbO++2H++3e-=Sb+H2O 0.212Ag(I)-(0) AgCl+e-=Ag+Cl-0.22233As(III)-(0) HAsO2+3H++3e-=As+2H2O 0.248Hg(I)-(0) Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl) 0.26808Bi(III)-(0) BiO++2H++3e-=Bi+H2O 0.320U(VI)-(IV) UO22++4H++2e-=U4++2H2O 0.327C(IV)-(III) 2HCNO+2H++2e-=(CN)2+2H2O 0.330V(IV)-(III) VO2++2H++e-=V3++H2O 0.337 Cu(II)-(0) Cu2++2e-=Cu 0.3419Re(VII)-(0) ReO4-+8H++7e-=Re+4H2O 0.368Ag(I)-(0) Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0) H2SO3+4H++4e-=S+3H2O 0.449Cu(I)-(0) Cu++e-=Cu 0.521I(0)-(-I) I2+2e-=2I-0.5355I(0)-(-I) I3-+2e-=3I-0.536As(V)-(III) H3AsO4+2H++2e-=HAsO2+2H2O 0.560Sb(V)-(III) Sb2O5+6H++4e-=2SbO++3H2O 0.581Te(IV)-(0) TeO2+4H++4e-=Te+2H2O 0.593U(V)-(IV) UO2++4H++e-=U4++2H2O 0.612**Hg(II)-(I) 2HgCl2+2e-=Hg2Cl2+2Cl-0.63Pt(IV)-(II) [PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68O(0)-(-I) O2+2H++2e-=H2O20.695Pt(II)-(0) [PtCl4]2-+2e-=Pt+4Cl-0.755*Se(IV)-(0) H2SeO3+4H++4e-=Se+3H2O 0.74Fe(III)-(II) Fe3++e-=Fe2+0.771 Hg(I)-(0) Hg22++2e-=2Hg 0.7973 Ag(I)-(0) Ag++e-=Ag 0.7996Os(VIII)-(0) OsO4+8H++8e-=Os+4H2O 0.8N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803Hg(II)-(0) Hg2++2e-=Hg 0.851Si(IV)-(0) (quartz)SiO2+4H++4e-=Si+2H2O 0.857Cu(II)-(I) Cu2++I-+e-=CuI 0.86N(III)-(I) 2HNO2+4H++4e-=H2N2O2+2H2O 0.86Hg(II)-(I) 2Hg2++2e-=Hg22+0.920N(V)-(III) NO3-+3H++2e-=HNO2+H2O 0.934Pd(II)-(0) Pd2++2e-=Pd 0.951N(V)-(II) NO3-+4H++3e-=NO+2H2O 0.957N(III)-(II) HNO2+H++e-=NO+H2O 0.983I(I)-(-I) HIO+H++2e-=I-+H2O 0.987V(V)-(IV) VO2++2H++e-=VO2++H2O 0.991V(V)-(IV) V(OH)4++2H++e-=VO2++3H2O 1.00Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002Te(VI)-(IV) H6TeO6+2H++2e-=TeO2+4H2O 1.02N(IV)-(II) N2O4+4H++4e-=2NO+2H2O 1.035N(IV)-(III) N2O4+2H++2e-=2HNO21.065I(V)-(-I) IO3-+6H++6e-=I-+3H2O 1.085Br(0)-(-I) Br2(aq)+2e-=2Br- 1.0873Se(VI)-(IV) SeO42-+4H++2e-=H2SeO3+H2O 1.151Cl(V)-(IV) ClO3-+2H++e-=ClO2+H2O 1.152Pt(II)-(0) Pt2++2e-=Pt 1.18Cl(VII)-(V) ClO4-+2H++2e-=ClO3-+H2O 1.189I(V)-(0) 2IO3-+12H++10e-=I2+6H2O 1.195Cl(V)-(III) ClO3-+3H++2e-=HClO2+H2O 1.214Mn(IV)-(II) MnO2+4H++2e-=Mn2++2H2O 1.224O(0)-(-II) O2+4H++4e-=2H2O 1.229Tl(III)-(I) T13++2e-=Tl+ 1.252Cl(IV)-(III) ClO2+H++e-=HClO21.277N(III)-(I) 2HNO2+4H++4e-=N2O+3H2O 1.297**Cr(VI)-(III) Cr2O72-+14H++6e-=2Cr3++7H2O 1.33Br(I)-(-I) HBrO+H++2e-=Br-+H2O 1.331Cr(VI)-(III) HCrO4-+7H++3e-=Cr3++4H2O 1.350Cl(0)-(-I) Cl2(g)+2e-=2Cl- 1.35827Cl(VII)-(-I) ClO4-+8H++8e-=Cl-+4H2O 1.389Cl(VII)-(0) ClO4-+8H++7e-=1/2Cl2+4H2O 1.39Au(III)-(I) Au3++2e-=Au+ 1.401Br(V)-(-I) BrO3-+6H++6e-=Br-+3H2O 1.423I(I)-(0) 2HIO+2H++2e-=I2+2H2O 1.439Cl(V)-(-I) ClO3-+6H++6e-=Cl-+3H2O 1.451Pb(IV)-(II) PbO2+4H++2e-=Pb2++2H2O 1.455Cl(V)-(0) ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I) HClO+H++2e-=Cl-+H2O 1.482Br(V)-(0) BrO3-+6H++5e-=l/2Br2+3H2O 1.482Au(III)-(0) Au3++3e-=Au 1.498Mn(VII)-(II) MnO4-+8H++5e-=Mn2++4H2O 1.507Mn(III)-(II) Mn3++e-=Mn2+ 1.5415Cl(III)-(-I) HClO2+3H++4e-=Cl-+2H2O 1.570Br(I)-(0) HBrO+H++e-=l/2Br2(aq)+H2O 1.574N(II)-(I) 2NO+2H++2e-=N2O+H2O 1.591I(VII)-(V) H5IO6+H++2e-=IO3-+3H2O 1.601Cl(I)-(0) HClO+H++e-=1/2Cl2+H2O 1.611Cl(III)-(I) HClO2+2H++2e-=HClO+H2O 1.645Ni(IV)-(II) NiO2+4H++2e-=Ni2++2H2O 1.678Mn(VII)-(IV) MnO4-+4H++3e-=MnO2+2H2O 1.679Pb(IV)-(II) PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913Au(I)-(0) Au++e-=Au 1.692 Ce(IV)-(III) Ce4++e-=Ce3+ 1.72N(I)-(0) N2O+2H++2e-=N2+H2O 1.766O(-I)-(-II) H2O2+2H++2e-=2H2O 1.776Co(III)-(II) Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83Ag(II)-(I) Ag2++e-=Ag+ 1.980S(VII)-(VI) S2O82-+2e-=2SO42- 2.010O(0)-(-II) O3+2H++2e-=O2+H2O 2.076O(II)-(-II) F2O+2H++4e-=H2O+2F- 2.153Fe(VI)-(III) FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II) O(g)+2H++2e-=H2O 2.421F(0)-(-I) F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99La(III)-(0) La(OH)3+3e-=La+3OH--2.90Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36Al(III)-(0) H AlO-+H O+3e-=Al+OH--2.33P(I)-(0) H2PO2-+e-=P+2OH--1.82B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48*Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2Te(0)-(-I) Te+2e-=Te2--1.143P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584*S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13*Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076Ag(I)-(0) AgCN+e-=Ag+CN--0.017N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373O(0)-(-II) O2+2H2O+4e-=4OH-0.401I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490Mn(VII)-(VI) MnO4-+e-=MnO42-0.558Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607。
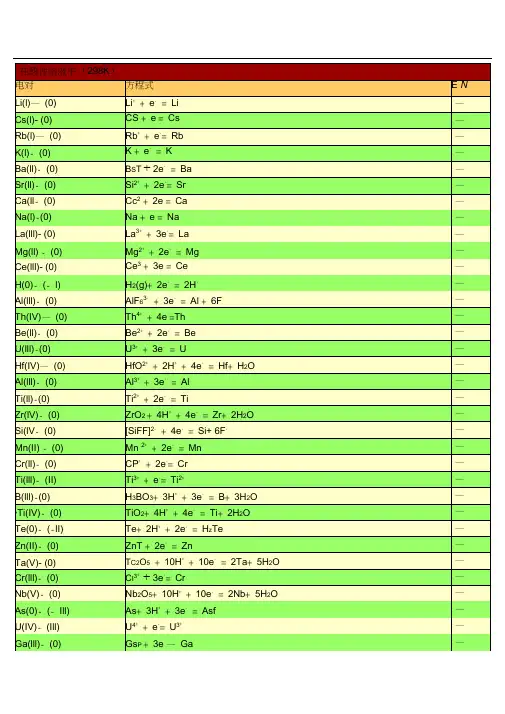
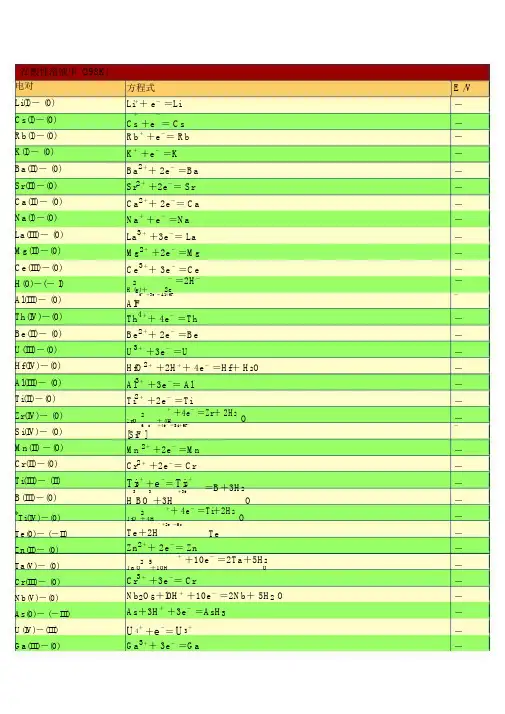
在酸性溶液中 (298K)电对Li(I)- (0)Cs(I)-(0) Rb(I)-(0)K(I)- (0)Ba(II)- (0) Sr(II)-(0) Ca(II)- (0) Na(I)-(0)La(III)- (0) Mg(II)-(0) Ce(III)-(0) H(0)-(- I) Al(III)- (0) Th(IV)-(0) Be(II)- (0)U(III)-(0)Hf(IV)-(0)Al(III)- (0)Ti(II)-(0)Zr(IV)- (0) Si(IV)- (0) Mn(II) -(0) Cr(II)-(0)Ti(III)- (II)B(III)-(0)*T i(IV)-(0) Te(0)- (-II) Zn(II)- (0) Ta(V)- (0) Cr(III)- (0) Nb(V)-(0) As(0)- (-III) U(IV)-(III) Ga(III)-(0)方程式 E /V Li++ e-=Li-+-= Cs-Cs +eRb++e-= Rb-K++e-=K-Ba2++ 2e-=Ba-Sr2++2e-= Sr-Ca2++ 2e-= Ca-Na++e-=Na-La3++3e-= La-Mg2++2e-=Mg-Ce3++ 3e-=Ce-2-=2H--H (g)+2e63-+3e-=Al+6F--AlFTh4++ 4e-=Th-Be2++ 2e-=Be-U3++3e-=U-HfO2++2H++ 4e-=Hf+ H2O-Al3++3e-= Al-Ti2++2e-=Ti-2++4e-=Zr+ 2H2O-ZrO+ 4H62-+4e-=Si+6F--[SiF ]Mn 2++2e-=Mn-Cr2++2e-= Cr-Ti3++e-=Ti2+=B+3H2-33++3e-O-H BO +3H2++ 4e-=Ti+2H2O-TiO +4H++2e-=H2-Te+2H TeZn2++ 2e-= Zn-25++10e-=2Ta+5H2-Ta O+10H OCr3++3e-= Cr-Nb2O5+l0H++10e-=2Nb+ 5H2 O-As+3H++3e-=AsH3-U4++e-=U3+-Ga3++ 3e-=Ga-P(I)- (0)P(III)-(I)*C(IV)- (III) Fe(II)-(0) Cr(III)- (II) Cd(II)- (0) Se(0)- (-II) Pb(II)- (0) Eu(III)-(II) Pb(II)- (0) In(III)- (0) Tl(I)-(0)Co(II)- (0) P(V)-(III) Pb(II)- (0) Ni (II)- (0) V(III)-(II) Ge(IV)-(0) Ag(I)-(0) Sn(II)- (0) Pb(II)- (0)*C(IV)- (II) P(0)-(- III) Hg(I)- (0) Fe(III)-(0) H(I)- (0) Ag(I)-(0) S -(II)*T i(IV)-(III) S(0)-(- II) Sn(IV)-(II) Sb(III)-(0) Cu(II)- (I) Bi(III)-(0) S(VI)-(IV) Sb(III)-(0)32+-=P+ 2H2O-H PO +H33++2e-=H32+2-H PO +2H PO H O++2e-= H2 2 4-2CO+2H C OFe2++2e-= Fe-Cr3++e-=Cr2+-Cd2++ 2e-=Cd-++ 2e-=H2-Se+2H Se(aq)2---=Pb+2IPbI +2eEu3+-+-+ e=Eu24-= Pb+SO42--PbSO+2eIn3++3e-= In-+-=Tl-Tl + eCo2++ 2e-=Co-H3PO4+2H++2e-=H3PO3+ H2O-PbCl2+2e-=Pb+2Cl--Ni2++2e-= Ni-V3++e-=V2 +-23++ 4e-=Ge+ 3H2O-H GeO+4HAgI+ e-=Ag+I--Sn2++ 2e-= Sn-Pb2++ 2e-=Pb-2++2e-=CO+H2-CO (g)+ 2H OP(white)+ 3H++3e-=PH3(g)-Hg2 I2+ 2e-=2Hg+2I--Fe3++3e-= Fe-2H++2e-= H2--AgBr+ e =Ag+BrS4O62-+ 2e-=2S2O32-TiO2++2H++e-=Ti3++H2OS+++2e-=H2S(aq)2H4+-2+Sn+ 2e = Sn23++ 6e-=2Sb+3H2Sb O +6H OCu2++e-=Cu+++3e-= Bi+Cl-+H2BiOCl+2H OSO42-+4H++ 2e-=H2SO3+H2OSbO++2H++3e-= Sb+H2O-(0) -=Ag + Cl-Ag(I)AgCl +e-2++3e -=As + 2H 2OAs(III)(0) HAsO +3HHg(I)- (0)22-= 2Hg +2Cl-( 饱和KCl)Hg Cl +2eBi(III)- (0)BiO ++ 2H + +3e -=Bi + H 2OU(VI)-(IV)22++4H ++2e -=U 4++2H 2UOO- (III) 2HCNO +2H++2e-= (CN)2+ 2C(IV)2H OV(IV)-(III)2+ +2H + +e - =V 3+ +H2VOO-2 +-+ 2e =CuCu(II)(0)Cu--+-(0)4 +8H +7e =Re +4H 2ORe(VII) ReO- (0) 24-= 2Ag +CrO 42-Ag(I)Ag CrO +2e- 2 3 ++ 4e -=S +3H 2OS(IV) (0)H SO +4H-(0)++e -= CuCu(I)Cu=2I -I(0) - -I) 2-( I +2e I(0)- (-I) I 3 -+ 2e - =3I -As(V)- (III) H 3AsO 4 +2H + +2e -= HAsO 2+2H 2O Sb(V)- (III) Sb 2O 5+6H ++ 4e - =2SbO ++ 3H 2O Te(IV)-(0) TeO 2+ 4H + +4e -=Te +2H 2OU(V)-(IV)UO 2++4H ++e -=U4++2H 2O**H g(II)- (I)2HgCl + 2e-=Hg 2Cl 2+ 2Cl-Pt(IV)- (II) 6 2-+ 2e -=[PtCl 4 ]2-+ 2Cl -[PtCl ] + +2e - =H 2 2 O(0)-(-I)2O +2H OPt(II)-(0) 4 2-+ 2e-=Pt +4Cl -[PtCl ]* S e(IV)-(0)H 2SeO 3+4H + +4e -= Se +3H 2O Fe(III)-(II)Fe 3++e-=Fe 2+-(0) 22++2e -=2HgHg(I)Hg-(0) ++e -= AgAg(I)Ag- 4++8e -= Os +4H 2Os(VIII) (0) OsO +8HO-3-+ 4H ++2e -=N 24+ 2 N(V) (IV) 2NOO 2H O -(0)2++ 2e -=HgHg(II)HgSi(IV)- (0)2++4e -=Si +2H 2(quartz)SiO + 4HO-(I)2++ I -+e -= CuICu(II) Cu+4e -=H 2-(I)2 + 2 2+2N(III) 2HNO + 4H N O 2H OHg(II)- (I) 2Hg 2+ +2e -= Hg 22+N(V)-(III) 3 -+3H + +2e -= HNO 2+ 2 NOH O Pd(II)- (0) Pd 2++ 2e -=PdN(V)-(II)NO 3 -+4H ++3e -= NO + 2H 2ON(III)-(II)2 + +e -=NO +H 2 OHNO +HI(I)-(-I) HIO +H + +2e - =I -+H 2 OV(V)-(IV)2+ +2H + +e - =VO 2++H 2VOO-++-2++ 3H 24+2H +e =VOV(V) (IV)V(OH)O-(0)4-+3e -= Au + 4Cl -Au(III)[AuCl ] + +2e -= TeO 2+ 2- (IV)66Te(VI) H TeO +2H 4H O-(II)2 4+ +4e - =2NO +2H 2 N(IV) N O +4HO-2 4+-(III)2H +2e =2HNO 2N(IV) N O +- --+--3 +6H+6e=I +3H 2I(V) (I) IOOBr(0)-(-I) 2- =2Br-Br (aq)+2eSe(VI)-(IV)42-+ 4H ++2e -= H 23+ 2 SeOSeO H O-- +-=ClO 2+2(IV) 3 +2H+ eCl(V)ClOH O - (0)2+ +2e -= PtPt(II)PtCl(VII)-(V) ClO 4- +2H ++ 2e -= ClO 3- +H 2OI(V)- (0) 2IO 3- +12H + +10e - =I 2+6H 2OCl(V)-(III) - +3H ++ 2e -= HClO +H 2O ClO 32 Mn(IV)-(II)++ 2e -=Mn 2++2HMnO 2+4H2O--II) 2++4e -=2H 2O(0) (O +4HOTl(III)- (I) T13++ 2e -= Tl +Cl(IV)- (III)2 + +e -=HClO 2 ClO +H +4e - =N 2N(III)-(I)2+ +22HNO + 4HO 3H O ** C r(VI)- (III)Cr 2O 72- +14H + +6e -= 2Cr 3 ++ 7H 2O Br(I)-(- I)HBrO +H ++ 2e -=Br -+H 2OCr(VI)-(III)HCrO 4- +7H ++3e -= Cr 3++4H 2OCl(0)-(-I)Cl 2(g)+2e - =2Cl -Cl(VII)-(- I)4- +8H + + 8e -= Cl -+ 4H 2OClO--+8H+ - = 1/2Cl 2+2(0)4+ 7eCl(VII) ClO4H OAu(III)-(I) Au 3++ 2e - =Au +- --+--+3H 2I)3 +6H+ 6e =BrBr(V) ( BrOOI(I) - (0)++2e -= I 2+22HIO +2H2H O-+6H +--Cl(V)-(-I) 3+ 6e= Cl + 3H 2OClOPb(IV)-(II)2++2e -= Pb 2++2H 2PbO +4H O- (0) 3- +6H + + 5e -= 1/2Cl 2+ 2Cl(V)ClO+ 2e - =Cl - +H 23H O- -I)+Cl(I) ( HClO +HO Br(V)-(0) BrO 3- +6H ++ 5e -=l/2Br 2+3H 2O Au(III)-(0)Au 3++ 3e - =AuMn(VII)- (II) MnO -+ +5e - = Mn 2 ++4H 2O4 +8HMn(III) -(II) Mn 3++e-= Mn 2+Cl(III)- (-I)2++ 4e -=Cl -+2H 2OHClO +3HBr(I) - (0)++ e -=l/2Br 2 (aq) + 2HBrO +HH ON(II) -(I)2NO +++2e -=N 2+ 22HO H O-(V)5 6++ 2e -=IO 3-+ 3H 2OI(VII) H IO +H- (0)++ e -=1/2Cl 2+ 2Cl(I)HClO +HH O2+-=HClO +H 2Cl(III)- (I)+ 2eHClO +2HO2+-+Ni(IV)-(II)4H +2e=Ni 2+2H 2ONiO+--+ +3e - = MnO 2+ 2Mn(VII) (IV)MnO 4 +4H2H O-242-+4H++ 2e -= PbSO 4+2 Pb(IV) (II) PbO +SO2H O-(0)++e -=AuAu(I)AuCe(IV)-(III) Ce 4++ e -=Ce 3+N(I)- (0)N 2O +2H ++ 2e - =N 2+ H 2OO( - - - H 2O 2++ +2e -=2HI) ( II)2H 2O-3 +-+-(II)+ e=Co 2·1H 2SO 4)Co(III) Co(2mol LAg(II)- (I)Ag 2++ e -=Ag +2 2- + - 2-S(VII)- (VI) 82e=2SO4S O +2e - =O 2+O(0)-(-II) 3 + 2O +2H H OO(II)-(-II)2++4e -= H 2+-F O +2HO 2FFe(VI)-(III)42-+ 8H ++3e -= Fe 3++4H 2FeO+ 2e - =H 2OO(0)-(-II)+O(g)+2H OF(0)-(- I)F 2+2e - =2F -F 2+2H ++ 2e - =2HF2 在碱性溶液中 (298K)电对方程式E / V- (0) 2-=Ca + 2OH --Ca(II)Ca(OH)+ 2e-(0) 2- =Ba + 2OH-- Ba(II)Ba(OH)+ 2e-(0)3-= La +3OH--La(III)La(OH)+3eSr(II)-(0) 2 2-= Sr +2OH -+8H 2-Sr(OH) ·8H O + 2eOMg(II)-(0)2-=Mg +2OH --Mg(OH)+2e- =2Be +6OH --(0)2 32-+ 3H 2 +-Be(II) Be OO 4eHf(IV) -(0)2 2- =Hf + 4OH - -HfO(OH) +H O +4e--- -(0) 232= Zr +4OHZr(IV)H ZrO +H O +4e=Al + OH-Al(III)- (0) 23-+ H 2+--H AlOO 3e-2----(0)2 +e=P +2OHP(I) H PO-2- +H 2+---(0)33e =B +4OHB(III) H BO O- (0)32-+2H 2 + 3e -= P + 5OH - -P(III) HPO O- (0)32-+ 3H 2 + - =Si +6OH --Si(IV) SiO O 4e -= H 2 - +3OH --(I)32-+2H 2 + 2e2-P(III) HPO O POMn(II) -(0) 2 - =Mn + 2OH --Mn(OH)+2eCr(III)- (0)3-= Cr +3OH--Cr(OH) +3e*-42 ----(0)+2e = Zn +4CNZn(II) [Zn(CN) ]-2---(0)=Zn +2OHZn(II) Zn(OH) + 2e2-+H 2+---Ga(III)-(0)3 2e = Ga + 4OHH GaOO- (0)22- +2H 2 + 2e -= Zn +4OH --Zn(II) ZnO O- 2 -+ 2H 2 + - =Cr +4OH --Cr(III)(0)CrOO3eTe(0)- (-I) Te + 2e -=Te2-- P(V)-(III)3 -+ 2H+ - =HPO 2-+3OH--PO 4 2O 2e3*Zn(II)-(0) [Zn(NH 3) 4] 2+ +2e-=Zn +4NH-3*W(VI)-(0)WO4 2-+4H + -=W +8OH--2O6e*Ge(IV)- (0)HGeO 3 -+ 2H 2O + 4e -=Ge +5OH ---(II)6 2-+2e -= HSnO 2-+H 2+--Sn(IV) [Sn(OH) ]O 3OHS(VI)-(IV) 42-+H 2+-=SO 32-+2OH--SOO 2eSe(0)- (-II)- 2--Se + 2e =Se-=Sn + 3OH -Sn(II)- (0)2-+H 2+-HSnOO2eP(0)-(- III)2-=PH 3+--P +3H O +3e(g)3OHN(V)-(IV) 2NO 3 -+2H 2 O +2e - =N 2O 4+4OH --H(I)- (0) 2H 2O +2e -=H 2+2OH --Cd(II)- (0) 2 -=Cd(Hg)+2OH --Cd(OH)+ 2eCo(II)- (0) 2 - =Co + 2OH --Co(OH)+ 2eNi(II)-(0)2-= Ni +2OH--Ni(OH) +2eAs(V)- (III)43-+2H 2 +-=AsO 2 -+4OH--AsOO2e- (0)2 -=2Ag +S2--Ag(I) Ag S + 2e- (0) 2 -+ 2H 2 + - =As + 4OH--As(III) AsOO 3e- (0)2-+ 2H 2 + - =Sb + 4OH --Sb(III) SbO O 3e =ReO 2+*-4 -+ 2H 2+- -- Re(VII)(IV)ReO -+ H 2 O 3e 4OH*-(III)3+-=SbO 2-+2OH --Sb(V) SbOO2e =Re + 8OH --4-+ 4H 2+ --Re(VII) (0)ReOO 7e 32- +6OH -*S(IV)- (II) 2SO 3 2-+3H 2+-=S 2-O 4eOTe(IV)-(0) TeO 3 2-+3H +4e -= Te +6OH --2OFe(III)-(II)3 -=Fe(OH)2+-Fe(OH)+ eOHS(0)-(- II) S +2e - =S 2-- (0)2 3 2 -= 2Bi + 6OH-Bi(III) Bi O +3H O +6e-+H 2+ --N(III)-(II)2 e =NO +2OHNOO*Co(II)-C(0)3 62++2e -=Co +6NH 3[Co(NH ) ]=Se +6OH--(0)32-+3H 2+-Se(IV) SeOO 4e- (0) 2 2 -=2Cu +2OH-Cu(I)Cu O +H O +2eTl(I)-(0)--Tl(OH)+ e =Tl + OH*-2 ---(0)+e = Ag +2CNAg(I) [Ag(CN) ]- (0)2 - =Cu + 2OH -Cu(II) Cu(OH)+ 2e- (III)42-+4H 2+-=Cr(OH)3+-Cr(VI) CrOO3e5OH*- (0)3 2++ e -=Cu +2NH 3Cu(I) [Cu(NH ) ] - =HO 2-+ OH -- -I)22O(0) ( O + H O +2e- (0)-=Ag + CN -Ag(I) AgCN +eN(V)-(III) NO 3-+H 2O + 2e -=NO 2-+2OH --(IV) SeO 4 2-+H + -= SeO 2-+ 2OH -Se(VI) 2O 2e 3- (0)Pd(OH)2+ - =Pd + 2OH -Pd(II) 2e4 2--2-S(II,V)-(II) 6+2e=2S2 3S OOHg(II)- (0)2 -=Hg +2OH-HgO + H O +2e- (II)3 63++e -= [Co(NH 3) 6 ]2 +Co(III) [Co(NH ) ]- (0) 2 -= Pt +2OH-Pt(II) Pt(OH) +2e- (II)3-=Co(OH)2 +-Co(III) Co(OH)+ eOH-(II)PbO 2+H 2O +2e -= PbO +2OH -Pb(IV) =I - +6OH -- -I)-+ 3H 2+-I(V) ( IO 3 3- +H 2 O 6e =ClO 2- +2OH --(III)+-Cl(V) ClO O 2e- (0)2 2-= 2Ag +2OH -Ag(I) Ag O +H O +2eFe(III)-(II)63- +e -=[Fe(CN)6 4-[Fe(CN) ]]-- +H 2 +-- -(V) 4 2e =ClO 3+2OH Cl(VII) ClO O*- (0) 3 2 ] ++ e -=Ag +2NH 3Ag(I) [Ag(NH )- - II) 2 2 - =4OH -O(0) ( O + 2H O +4eI(I) - ( - I) - +H 2 + - =I - +2OH -IO O 2e* N i(IV)-(II)NiO 2+2H 2O +2e - =Ni(OH)2+ 2OH -Mn(VII)- (VI) MnO 4- +e -= MnO 42-Mn(VII)- (IV)MnO 4-+2H 2 +-= MnO 2 +-O 3e4OHMn(VI) -(IV)2-+ 2H 2+-=MnO 2+-MnO 4 O 2e4OHAg(II)- (I)2AgO +H 2O + 2e - =Ag 2O +2OH ---------------- -I)3-+ 3H 2 +-=Br - +6OH-Br(V) ( BrOO 6e--- +3H 2 + ---( I)3 6e = Cl + 6OHCl(V) ClOO-- +H 2 +-- -(I) 2 2e =ClO +2OHCl(III)ClOO-3----(V) 62 +2e= IO 3+3OHI(VII)H IO- - I)2-+2H 2+ 4e -= Cl -+ 4OH -Cl(III) ( ClOOBr(I) - -I)-+H 2 +-= Br - +2OH-( BrO O 2e- -I)- +H 2+-= Cl -+ 2OH -Cl(I) ( ClOO 2e*-2--(III)= ClO 2Cl(IV) ClO (g)+e-=O 2- -II) 3 2+-O(0) ( O + H O +2e2OH。
电对方程式E/V Li(I)-(0)Li++e-=Li-Cs(I)-(0)Cs++e-=Cs-Rb(I)-(0)Rb++e-=Rb-K(I)-(0)K++e-=K-Ba(II)-(0)Ba2++2e-=Ba-Sr(II)-(0)Sr2++2e-=Sr-Ca(II)-(0)Ca2++2e-=Ca-Na(I)-(0)Na++e-=Na-La(III)-(0)La3++3e-=La-Mg(II)-(0)Mg2++2e-=Mg-Ce(III)-(0)Ce3++3e-=Ce-H(0)-(-I)H2(g)+2e-=2H--Al(III)-(0)AlF63-+3e-=Al+6F--Th(IV)-(0)Th4++4e-=Th-Be(II)-(0)Be2++2e-=Be-U(III)-(0)U3++3e-=U-Hf(IV)-(0)HfO2++2H++4e-=Hf+H2O-Al(III)-(0)Al3++3e-=Al-Ti(II)-(0)Ti2++2e-=Ti-Zr(IV)-(0)ZrO2+4H++4e-=Zr+2H2O-Si(IV)-(0)[SiF6]2-+4e-=Si+6F--Mn(II)-(0)Mn2++2e-=Mn-Cr(II)-(0)Cr2++2e-=Cr-Ti(III)-(II)Ti3++e-=Ti2+-B(III)-(0)H3BO3+3H++3e-=B+3H2O-*Ti(IV)-(0)TiO2+4H++4e-=Ti+2H2O-Te(0)-(-II)Te+2H++2e-=H2Te-Zn(II)-(0)Zn2++2e-=Zn-Ta(V)-(0)Ta2O5+10H++10e-=2Ta+5H2O-Cr(III)-(0)Cr3++3e-=Cr-Nb(V)-(0)Nb2O5+l0H++10e-=2Nb+5H2O-As(0)-(-III)As+3H++3e-=AsH3-U(IV)-(III)U4++e-=U3+-Ga(III)-(0)Ga3++3e-=Ga-P(I)-(0)H3PO2+H++e-=P+2H2O-P(III)-(I)H3PO3+2H++2e-=H3PO2+H2O-*C(IV)-(III)2CO2+2H++2e-=H2C2O4-Fe(II)-(0)Fe2++2e-=Fe-Cr(III)-(II)Cr3++e-=Cr2+-Cd(II)-(0)Cd2++2e-=Cd-Se(0)-(-II)Se+2H++2e-=H2Se(aq)-Pb(II)-(0)PbI2+2e-=Pb+2I--Eu(III)-(II)Eu3++e-=Eu2+-Pb(II)-(0)PbSO4+2e-=Pb+SO42--In(III)-(0)In3++3e-=In-Tl(I)-(0)Tl++e-=Tl-Co(II)-(0)Co2++2e-=Co-P(V)-(III)H3PO4+2H++2e-=H3PO3+H2O-Pb(II)-(0)PbCl2+2e-=Pb+2Cl--Ni (II)-(0)Ni2++2e-=Ni-V(III)-(II)V3++e-=V2+-Ge(IV)-(0)H2GeO3+4H++4e-=Ge+3H2O-Ag(I)-(0)AgI+e-=Ag+I--Sn(II)-(0)Sn2++2e-=Sn-Pb(II)-(0)Pb2++2e-=Pb-*C(IV)-(II)CO2(g)+2H++2e-=CO+H2O-P(0)-(-III)P(white)+3H++3e-=PH3(g)-Hg(I)-(0)Hg2I2+2e-=2Hg+2I--Fe(III)-(0)Fe3++3e-=Fe-H(I)-(0)2H++2e-=H2Ag(I)-(0)AgBr+e-=Ag+Br-S-(II)S4O62-+2e-=2S2O32-*Ti(IV)-(III)TiO2++2H++e-=Ti3++H2OS(0)-(-II)S+2H++2e-=H2S(aq)Sn(IV)-(II)Sn4++2e-=Sn2+Sb(III)-(0)Sb2O3+6H++6e-=2Sb+3H2OCu(II)-(I)Cu2++e-=Cu+Bi(III)-(0)BiOCl+2H++3e-=Bi+Cl-+H2OS(VI)-(IV)SO42-+4H++2e-=H2SO3+H2OSb(III)-(0)SbO++2H++3e-=Sb+H2OAg(I)-(0)AgCl+e-=Ag+Cl-As(III)-(0)HAsO2+3H++3e-=As+2H2OHg(I)-(0)Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl) Bi(III)-(0)BiO++2H++3e-=Bi+H2OU(VI)-(IV)UO22++4H++2e-=U4++2H2OC(IV)-(III)2HCNO+2H++2e-=(CN)2+2H2O V(IV)-(III)VO2++2H++e-=V3++H2OCu(II)-(0)Cu2++2e-=CuRe(VII)-(0)ReO4-+8H++7e-=Re+4H2OAg(I)-(0)Ag2CrO4+2e-=2Ag+CrO42-S(IV)-(0)H2SO3+4H++4e-=S+3H2OCu(I)-(0)Cu++e-=CuI(0)-(-I)I2+2e-=2I-I(0)-(-I)I3-+2e-=3I-As(V)-(III)H3AsO4+2H++2e-=HAsO2+2H2O Sb(V)-(III)Sb2O5+6H++4e-=2SbO++3H2O Te(IV)-(0)TeO2+4H++4e-=Te+2H2OU(V)-(IV)UO2++4H++e-=U4++2H2O**Hg(II)-(I)2HgCl2+2e-=Hg2Cl2+2Cl-Pt(IV)-(II)[PtCl6]2-+2e-=[PtCl4]2-+2Cl-O(0)-(-I)O2+2H++2e-=H2O2Pt(II)-(0)[PtCl4]2-+2e-=Pt+4Cl-*Se(IV)-(0)H2SeO3+4H++4e-=Se+3H2OFe(III)-(II)Fe3++e-=Fe2+Hg(I)-(0)Hg22++2e-=2HgAg(I)-(0)Ag++e-=AgOs(VIII)-(0)OsO4+8H++8e-=Os+4H2ON(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O Hg(II)-(0)Hg2++2e-=HgSi(IV)-(0)(quartz)SiO2+4H++4e-=Si+2H2O Cu(II)-(I)Cu2++I-+e-=CuIN(III)-(I)2HNO2+4H++4e-=H2N2O2+2H2O Hg(II)-(I)2Hg2++2e-=Hg22+N(V)-(III)NO3-+3H++2e-=HNO2+H2OPd(II)-(0)Pd2++2e-=PdN(V)-(II)NO3-+4H++3e-=NO+2H2ON(III)-(II)HNO2+H++e-=NO+H2OI(I)-(-I)HIO+H++2e-=I-+H2OV(V)-(IV)VO2++2H++e-=VO2++H2OV(V)-(IV)V(OH)4++2H++e-=VO2++3H2O Au(III)-(0) [AuCl4]-+3e-=Au+4Cl-Te(VI)-(IV)H6TeO6+2H++2e-=TeO2+4H2O N(IV)-(II)N2O4+4H++4e-=2NO+2H2ON(IV)-(III)N2O4+2H++2e-=2HNO2I(V)-(-I)IO3-+6H++6e-=I-+3H2OBr(0)-(-I)Br2(aq)+2e-=2Br-Se(VI)-(IV)SeO42-+4H++2e-=H2SeO3+H2O Cl(V)-(IV)ClO3-+2H++e-=ClO2+H2OPt(II)-(0)Pt2++2e-=PtCl(VII)-(V)ClO4-+2H++2e-=ClO3-+H2OI(V)-(0)2IO3-+12H++10e-=I2+6H2OCl(V)-(III)ClO3-+3H++2e-=HClO2+H2O Mn(IV)-(II)MnO2+4H++2e-=Mn2++2H2OO(0)-(-II)O2+4H++4e-=2H2OTl(III)-(I)T13++2e-=Tl+Cl(IV)-(III)ClO2+H++e-=HClO2N(III)-(I)2HNO2+4H++4e-=N2O+3H2O**Cr(VI)-(III)Cr2O72-+14H++6e-=2Cr3++7H2O Br(I)-(-I)HBrO+H++2e-=Br-+H2OCr(VI)-(III)HCrO4-+7H++3e-=Cr3++4H2O Cl(0)-(-I)Cl2(g)+2e-=2Cl-Cl(VII)-(-I)ClO4-+8H++8e-=Cl-+4H2OCl(VII)-(0)ClO4-+8H++7e-=1/2Cl2+4H2O Au(III)-(I)Au3++2e-=Au+Br(V)-(-I)BrO3-+6H++6e-=Br-+3H2OI(I)-(0)2HIO+2H++2e-=I2+2H2OCl(V)-(-I)ClO3-+6H++6e-=Cl-+3H2OPb(IV)-(II)PbO2+4H++2e-=Pb2++2H2OCl(V)-(0)ClO3-+6H++5e-=1/2Cl2+3H2O Cl(I)-(-I)HClO+H++2e-=Cl-+H2OBr(V)-(0)BrO3-+6H++5e-=l/2Br2+3H2O Au(III)-(0)Au3++3e-=AuMn(VII)-(II)MnO4-+8H++5e-=Mn2++4H2OMn(III)-(II)Mn3++e-=Mn2+Cl(III)-(-I)HClO2+3H++4e-=Cl-+2H2OBr(I)-(0)HBrO+H++e-=l/2Br2(aq)+H2ON(II)-(I)2NO+2H++2e-=N2O+H2OI(VII)-(V)H5IO6+H++2e-=IO3-+3H2OCl(I)-(0)HClO+H++e-=1/2Cl2+H2OCl(III)-(I)HClO2+2H++2e-=HClO+H2ONi(IV)-(II)NiO2+4H++2e-=Ni2++2H2OMn(VII)-(IV)MnO4-+4H++3e-=MnO2+2H2OPb(IV)-(II)PbO2+SO42-+4H++2e-=PbSO4+2H2OAu(I)-(0)Au++e-=AuCe(IV)-(III)Ce4++e-=Ce3+N(I)-(0)N2O+2H++2e-=N2+H2OO(-I)-(-II)H2O2+2H++2e-=2H2OCo(III)-(II)Co3++e-=Co2+(2mol·L-1 H2SO4)Ag(II)-(I)Ag2++e-=Ag+S(VII)-(VI) S2O82-+2e-=2SO42-O(0)-(-II)O3+2H++2e-=O2+H2OO(II)-(-II)F2O+2H++4e-=H2O+2F-Fe(VI)-(III)FeO42-+8H++3e-=Fe3++4H2OO(0)-(-II)O(g)+2H++2e-=H2OF(0)-(-I)F2+2e-=2F-F2+2H++2e-=2HF2电对方程式E/V Ca(II)-(0)Ca(OH)2+2e-=Ca+2OH--Ba(II)-(0)Ba(OH)2+2e-=Ba+2OH--La(III)-(0)La(OH)3+3e-=La+3OH--Sr(II)-(0)Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O-Mg(II)-(0)Mg(OH)2+2e-=Mg+2OH--Be(II)-(0)Be2O32-+3H2O+4e-=2Be+6OH--Hf(IV)-(0)HfO(OH)2+H2O+4e-=Hf+4OH--Zr(IV)-(0)H2ZrO3+H2O+4e-=Zr+4OH--Al(III)-(0)H2AlO3-+H2O+3e-=Al+OH--P(I)-(0)H2PO2-+e-=P+2OH--B(III)-(0)H2BO3-+H2O+3e-=B+4OH--P(III)-(0)HPO32-+2H2O+3e-=P+5OH--Si(IV)-(0)SiO32-+3H2O+4e-=Si+6OH--P(III)-(I)HPO32-+2H2O+2e-=H2PO2-+3OH--Mn(II)-(0)Mn(OH)2+2e-=Mn+2OH--Cr(III)-(0)Cr(OH)3+3e-=Cr+3OH--*Zn(II)-(0)[Zn(CN)4]2-+2e-=Zn+4CN--Zn(II)-(0)Zn(OH)2+2e-=Zn+2OH--Ga(III)-(0)H2GaO3-+H2O+2e-=Ga+4OH--Zn(II)-(0)ZnO22-+2H2O+2e-=Zn+4OH--Cr(III)-(0)CrO2-+2H2O+3e-=Cr+4OH--Te(0)-(-I)Te+2e-=Te2--P(V)-(III)PO43-+2H2O+2e-=HPO32-+3OH--*Zn(II)-(0)[Zn(NH3)4]2++2e-=Zn+4NH3-*W(VI)-(0)WO42-+4H2O+6e-=W+8OH--*Ge(IV)-(0)HGeO3-+2H2O+4e-=Ge+5OH--Sn(IV)-(II)[Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--S(VI)-(IV)SO42-+H2O+2e-=SO32-+2OH--Se(0)-(-II)Se+2e-=Se2--Sn(II)-(0)HSnO2-+H2O+2e-=Sn+3OH--P(0)-(-III)P+3H2O+3e-=PH3(g)+3OH--N(V)-(IV)2NO3-+2H2O+2e-=N2O4+4OH--H(I)-(0)2H2O+2e-=H2+2OH--Cd(II)-(0)Cd(OH)2+2e-=Cd(Hg)+2OH--Co(II)-(0)Co(OH)2+2e-=Co+2OH--Ni(II)-(0)Ni(OH)2+2e-=Ni+2OH--As(V)-(III)AsO43-+2H2O+2e-=AsO2-+4OH--Ag(I)-(0)Ag2S+2e-=2Ag+S2--As(III)-(0)AsO2-+2H2O+3e-=As+4OH--Sb(III)-(0)SbO2-+2H2O+3e-=Sb+4OH--*Re(VII)-(IV)ReO4-+2H2O+3e-=ReO2+4OH--*Sb(V)-(III)SbO3-+H2O+2e-=SbO2-+2OH--Re(VII)-(0)ReO4-+4H2O+7e-=Re+8OH--*S(IV)-(II)2SO32-+3H2O+4e-=S2O32-+6OH--Te(IV)-(0)TeO32-+3H2O+4e-=Te+6OH--Fe(III)-(II)Fe(OH)3+e-=Fe(OH)2+OH--S(0)-(-II)S+2e-=S2--Bi(III)-(0)Bi2O3+3H2O+6e-=2Bi+6OH--N(III)-(II)NO2-+H2O+e-=NO+2OH--*Co(II)-C(0)[Co(NH3)6]2++2e-=Co+6NH3-Se(IV)-(0)SeO32-+3H2O+4e-=Se+6OH--Cu(I)-(0)Cu2O+H2O+2e-=2Cu+2OH--Tl(I)-(0)Tl(OH)+e-=Tl+OH--*Ag(I)-(0)[Ag(CN)2]-+e-=Ag+2CN--Cu(II)-(0)Cu(OH)2+2e-=Cu+2OH--Cr(VI)-(III)CrO42-+4H2O+3e-=Cr(OH)3+5OH--*Cu(I)-(0)[Cu(NH3)2]++e-=Cu+2NH3-O(0)-(-I)O2+H2O+2e-=HO2-+OH--Ag(I)-(0)AgCN+e-=Ag+CN--N(V)-(III)NO3-+H2O+2e-=NO2-+2OH-Se(VI)-(IV)SeO42-+H2O+2e-=SeO32-+2OH-Pd(II)-(0)Pd(OH)2+2e-=Pd+2OH-S(II,V)-(II)S4O62-+2e-=2S2O32-Hg(II)-(0)HgO+H2O+2e-=Hg+2OH-Co(III)-(II)[Co(NH3)6]3++e-=[Co(NH3)6]2+Pt(II)-(0)Pt(OH)2+2e-=Pt+2OH-Co(III)-(II)Co(OH)3+e-=Co(OH)2+OH-Pb(IV)-(II)PbO2+H2O+2e-=PbO+2OH-I(V)-(-I)IO3-+3H2O+6e-=I-+6OH-Cl(V)-(III)ClO3-+H2O+2e-=ClO2-+2OH-Ag(I)-(0)Ag2O+H2O+2e-=2Ag+2OH-Fe(III)-(II)[Fe(CN)6]3-+e-=[Fe(CN)6]4-Cl(VII)-(V)ClO4-+H2O+2e-=ClO3-+2OH-*Ag(I)-(0)[Ag(NH3)2]++e-=Ag+2NH3O(0)-(-II)O2+2H2O+4e-=4OH-I(I)-(-I)IO-+H2O+2e-=I-+2OH-*Ni(IV)-(II)NiO2+2H2O+2e-=Ni(OH)2+2OH-Mn(VII)-(VI)MnO4-+e-=MnO42-Mn(VII)-(IV)MnO4-+2H2O+3e-=MnO2+4OH-Mn(VI)-(IV)MnO42-+2H2O+2e-=MnO2+4OH-Ag(II)-(I)2AgO+H2O+2e-=Ag2O+2OH-。