标准电极电势表(酸碱)
- 格式:doc
- 大小:266.00 KB
- 文档页数:7
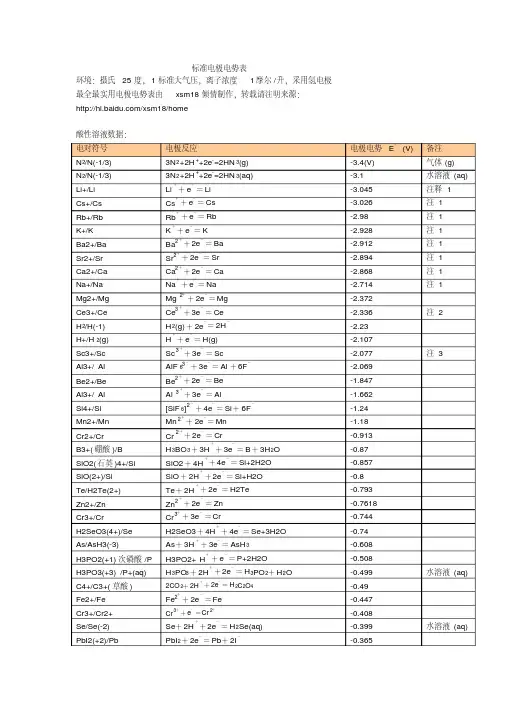

标准电极电势表维基百科,自由的百科全书标准电极电势可以用来计算化学电池或原电池的电化学势或电极电势。
本表中所给出的电极电势以标准氢电极为参比电极,溶液中离子有效浓度为1mol/L,气体分压为100kPa,温度为298K,所有离子的数据都在水溶液中测得。
[1][2][3][4][5][6][7][8][9]单击每栏上方的符号可将数据按元素符号或标准电极电势值排序。
注:(s) – 固体;(l) – 液体;(g) – 气体;(aq) – 水溶液;(Hg) – 汞齐。
Ba+ + e− Ba(s)−4.38[10][1][3] Sr+ + e− Sr(s)−4.10[11][1][3] Ca+ + e− Ca(s)−3.8[11][1][3] Pr3+ + e− Pr2+−3.1[11] N HN 3(aq)−3.09[6]Li+ + e− Li(s)−3.0401[5]N2(g) + 4 H2O + 2 e− 2 NH2OH(aq) + 2 OH−−3.04[6]Cs+ + e− Cs(s)−3.026[5]Ca(OH)2(s) + 2 e− Ca(s) + 2 OH−−3.02[11] Rb+ + e− Rb(s)−2.98[4]K+ + e− K(s)−2.931[5]Mg+ + e− Mg(s)−2.93[10] Ba2+ + 2 e− Ba(s)−2.912[5]La(OH)3(s) + 3 e− La(s) + 3OH−−2.90[5]Fr+ + e− Fr(s)−2.9[11]Sr2+ + 2 e− Sr(s)−2.899[5]Sr(OH)2(s) + 2 e− Sr(s) + 2 OH−−2.88[11] Ca2+ + 2 e− Ca(s)−2.868[5]Eu2+ + 2 e− Eu(s)−2.812[5]Ra2+ + 2 e− Ra(s)−2.8[5]Yb2+ + 2 e− Yb(s)−2.76[11][1] Na+ + e− Na(s)−2.71[5][9] Sm2+ + 2 e− Sm(s)−2.68[11][1] No2+ + 2 e− No(s)−2.50[11] HfO(OH)2(s) + H2O + 4 e− Hf(s) + 4 OH−−2.50[11]Th(OH)4(s) + 4 e− Th(s) + 4 OH−−2.48[11] Md2+ + 2 e− Md(s)−2.40[11]La + 3 e La(s)−2.379[5] Y3+ + 3 e− Y(s)−2.372[5] Mg2+ + 2 e− Mg(s)−2.372[5] ZrO(OH)2(s) + H2O + 4 e− Zr(s) + 4OH−−2.36[5] Pr3+ + 3 e− Pr(s)−2.353[11] Ce3+ + 3 e− Ce(s)−2.336[11] Er3+ + 3 e− Er(s)−2.331[11] Ho3+ + 3 e− Ho(s)−2.33[11] Al(OH)4− + 3 e− Al(s) + 4 OH−−2.33Al(OH)3(s) + 3 e− Al(s) + 3OH−−2.31Tb3+ + 3 e− Tb(s)−2.28H2(g) + 2 e− 2 H−−2.25Ac3+ + 3 e− Ac(s)−2.20Be+ + e− Be(s)−2.12[10] Cf2+ + 2 e− Cf(s)−2.12[11] Am3+ + 3 e− Am(s)−2.048[11] Cf3+ + 3 e− Cf(s)−1.94[11] Am2+ + 2 e− Am(s)−1.9[11] Be2+ + 2 e− Be(s)−1.85Rf4+ + 4 e− Rf(s)−1.67[12] U3+ + 3 e− U(s)−1.66[7] Al3+ + 3 e− Al(s)−1.66[9] Ti2+ + 2 e− Ti(s)−1.63[9] Bk2+ + 2 e− Bk(s)−1.6[11] ZrO2(s) + 4 H+ + 4 e− Zr(s) + 2 H2O−1.553[5] Hf4+ + 4 e− Hf(s)−1.55[11] Zr4+ + 4 e− Zr(s)−1.45[5]Ti + 3 e Ti(s)−1.37[13] TiO(s) + 2 H+ + 2 e− Ti(s) + H2O−1.31Ti2O3(s) + 2 H+ + 2 e− 2 TiO(s) + H2O−1.23Zn(OH)42− + 2 e− Zn(s) + 4 OH−−1.199[14] Mn2+ + 2 e− Mn(s)−1.185[14] Fe(CN)64− + 6 H+ + 2 e− Fe(s) + 4HCN(aq)−1.16[15] V2+ + 2 e− V(s)−1.175[2] Te(s) + 2 e− Te2−−1.143[2] Nb3+ + 3 e− Nb(s)−1.099Sn(s) + 4 H+ + 4 e− SnH4(g)−1.07In(OH)3(s) + 3 e− In(s) + 3 OH−−0.99[11] SiO2(s) + 4 H+ + 4 e− Si(s) + 2 H2O−0.91B(OH)3(aq) + 3 H+ + 3 e− B(s) + 3 H2O−0.89Fe(OH)2(s) + 2 e− Fe(s) + 2 OH−−0.89[15] Fe2O3(s) + 3 H2O + 2 e− 2Fe(OH)2(s) + 2 OH−−0.86[15] TiO2+ + 2 H+ + 4 e− Ti(s) + H2O−0.862 H2O + 2 e− H2(g) + 2 OH−−0.8277[5] Bi(s) + 3 H+ + 3 e− BiH3−0.8[14] Zn2+ + 2 e− Zn(Hg)−0.7628[5] Zn2+ + 2 e− Zn(s)−0.7618[5] Ta2O5(s) + 10 H+ + 10 e− 2 Ta(s) + 5 H2O−0.75Cr3+ + 3 e− Cr(s)−0.74[Au(CN)2]− + e− Au(s) + 2 CN−−0.60Ta3+ + 3 e− Ta(s)−0.6PbO(s) + H2O + 2 e− Pb(s) + 2 OH−−0.582 TiO2(s) + 2 H+ + 2 e− Ti2O3(s) + H2O−0.56Ga3+ + 3 e− Ga(s)−0.53U4+ + e− U3+−0.52[7] H3PO2(aq) + H+ + e− P(白磷[16]) + 2 H2O−0.508[5] H3PO3(aq) + 2 H+ + 2 e− H3PO2(aq) + H2O−0.499[5] H3PO3(aq) + 3 H+ + 3 e− P(红磷)[16] + 3H2O−0.454[5] Fe2+ + 2 e− Fe(s)−0.44[9] 2 CO2(g) + 2 H+ + 2 e− HOOCCOOH(aq)−0.43Cr3+ + e− Cr2+−0.42Cd2+ + 2 e− Cd(s)−0.40[9] SeO32− + 4e− + 3H2O ⇌ Se + 6OH−−0.37[17] GeO2(s) + 2 H+ + 2 e− GeO(s) + H2O−0.37Cu2O(s) + H2O + 2 e− 2 Cu(s) + 2 OH−−0.360[5] PbSO4(s) + 2 e− Pb(s) + SO42−−0.3588[5] PbSO4(s) + 2 e− Pb(Hg) + SO42−−0.3505[5] Eu3+ + e− Eu2+−0.35[7] In3+ + 3 e− In(s)−0.34[2] Tl+ + e− Tl(s)−0.34[2] Ge(s) + 4 H+ + 4 e− GeH4(g)−0.29Co2+ + 2 e− Co(s)−0.28[5] H3PO4(aq) + 2 H+ + 2 e− H3PO3(aq) + H2O−0.276[5] V3+ + e− V2+−0.26[9] Ni2+ + 2 e− Ni(s)−0.25As(s) + 3 H+ + 3 e− AsH3(g)−0.23[2] AgI(s) + e− Ag(s) + I−−0.15224[14] MoO2(s) + 4 H+ + 4 e− Mo(s) + 2 H2O−0.15Si(s) + 4 H+ + 4 e− SiH4(g)−0.14Sn2+ + 2 e− Sn(s)−0.13O2(g) + H+ + e− HO2•(aq)−0.13Pb2+ + 2 e− Pb(s)−0.13[9] WO2(s) + 4 H+ + 4 e− W(s) + 2 H2O−0.12P(红磷) + 3 H+ + 3 e− PH3(g)−0.111[5] CO2(g) + 2 H+ + 2 e− HCOOH(aq)−0.11Se(s) + 2 H+ + 2 e− H2Se(g)−0.11CO2(g) + 2 H+ + 2 e− CO(g) + H2O−0.11SnO(s) + 2 H+ + 2 e− Sn(s) + H2O−0.10SnO2(s) + 2 H+ + 2 e− SnO(s) + H2O−0.09WO3(aq) + 6 H+ + 6 e− W(s) + 3 H2O−0.09[2] P(白磷) + 3 H+ + 3 e− PH3(g)−0.063[5] Fe3+ + 3 e− Fe(s)−0.04[15] HCOOH(aq) + 2 H+ + 2 e− HCHO(aq) + H2O−0.032 H+ + 2 e− H2(g) 0.00≡ 0 AgBr(s) + e− Ag(s) + Br−+0.07133[14] S4O62− + 2 e− 2 S2O32−+0.08Fe3O4(s) + 8 H+ + 8 e− 3 Fe(s) + 4 H2O+0.085[8] N2(g) + 2 H2O + 6H+ + 6 e− 2 NH4OH(aq)+0.092HgO(s) + H2O + 2 e− Hg(l) + 2 OH−+0.0977Cu(NH3)42+ + e− Cu(NH3)2+ + 2 NH3+0.10[2] Ru(NH3)63+ + e− Ru(NH3)62++0.10[7] N2H4(aq) + 4 H2O + 2 e− 2 NH4+ + 4 OH−+0.11[6] H2MoO4(aq) + 6 H+ + 6 e− Mo(s) + 4 H2O+0.11Ge4+ + 4 e− Ge(s)+0.12C(s) + 4 H+ + 4 e− CH4(g)+0.13[2] HCHO(aq) + 2 H+ + 2 e− CH3OH(aq)+0.13S(s) + 2 H+ + 2 e− H2S(g)+0.14Sn4+ + 2 e− Sn2++0.15Cu2+ + e− Cu++0.159[2] HSO4− + 3 H+ + 2 e− SO2(aq) + 2 H2O+0.16UO22+ + e− UO2++0.163[7] SO42− + 4 H+ + 2 e− SO2(aq) + 2 H2O+0.17TiO2+ + 2 H+ + e− Ti3+ + H2O+0.19Bi3+ + 2e− Bi++0.2SbO+ + 2 H+ + 3 e− Sb(s) + H2O+0.20AgCl(s) + e− Ag(s) + Cl−+0.22233[14] H3AsO3(aq) + 3 H+ + 3 e− As(s) + 3 H2O+0.24GeO(s) + 2 H+ + 2 e− Ge(s) + H2O+0.26UO2+ + 4 H+ + e− U4+ + 2 H2O+0.273[7] At2 + e− 2 At-+0.3[11] Re3+ + 3 e− Re(s)+0.300Bi3+ + 3 e− Bi(s)+0.32VO2+ + 2 H+ + e− V3+ + H2O+0.34Cu2+ + 2 e− Cu(s)+0.340[2] [Fe(CN)6]3− + e− [Fe(CN)6]4−+0.36Tc2+ + 2 e− Tc(s)+0.40[11] O2(g) + 2 H2O + 4 e− 4 OH−(aq)+0.40[9] H2MoO4 + 6 H+ + 3 e− Mo3+ + 2 H2O+0.43Ru2+ + 2 e− Ru(s)+0.455[11] Bi+ + e− Bi(s)+0.50CH3OH(aq) + 2 H+ + 2 e− CH4(g) + H2O+0.50SO2(aq) + 4 H+ + 4 e− S(s) + 2 H2O+0.50Cu+ + e− Cu(s)+0.520[2] CO(g) + 2 H+ + 2 e− C(s) + H2O+0.52I3− + 2 e− 3 I−+0.53[9] I2(s) + 2 e− 2 I−+0.54[9] [AuI4]− + 3 e− Au(s) + 4 I−+0.56H3AsO4(aq) + 2 H+ + 2 e− H3AsO3(aq) + H2O+0.56[AuI2]− + e− Au(s) + 2 I−+0.58MnO4− + 2 H2O + 3 e− MnO2(s) + 4 OH−+0.59Rh+ + e− Rh(s)+0.600[11] S2O32 − + 6 H+ + 4 e− 2 S(s) + 3 H2O+0.60Fc+ + e− Fc(s)+0.641[18]Ag + −+0.643[11]H2MoO4(aq) + 2 H+ + 2 e− MoO2(s) + 2 H2O+0.65+ 2 H+ + 2 e−H2O2(aq)+0.70Tl3+ + 3 e− Tl(s)+0.72PtCl62− + 2 e− PtCl42− + 2 Cl−+0.726[7] H2SeO3(aq) + 4 H+ + 4 e− Se(s) + 3 H2O+0.74Rh3+ + 3 e− Rh(s)+0.758[11] PtCl42− + 2 e− Pt(s) + 4 Cl−+0.758[7] Fe3+ + e− Fe2++0.77Ag+ + e− Ag(s)+0.7996[5] Hg22+ + 2 e− 2 Hg(l)+0.80NO3−(aq) + 2 H+ + e− NO2(g) + H2O+0.80FeO42− + 5 H2O + 6 e− Fe2O3(s) + 10 OH−+0.81[15] H2(g) + 2 OH− 2 H2O + 2 e−+0.828[19] [AuBr4]− + 3 e− Au(s) + 4 Br−+0.85Hg2+ + 2 e− Hg(l)+0.85MnO4− + H+ + e− HMnO4−+0.902 Hg2+ + 2 e− Hg22++0.91[2] Pd2+ + 2 e− Pd(s)+0.915[7] [AuCl4]− + 3 e− Au(s) + 4 Cl−+0.93MnO2(s) + 4 H+ + e− Mn3+ + 2 H2O+0.95[AuBr2]− + e− Au(s) + 2 Br−+0.96 [HXeO6]3− + 2 H2O + 2 e− + [HXeO4]− + 4 OH−+0.99[20] HNO2 + H+ + e- = NO(g) + H2O+0.996H6TeO6(aq) + 2 H+ + 2 e− TeO2(s) + 4 H2O+1.02[21] Br2(l) + 2 e− 2 Br−+1.07Br2(aq) + 2 e− 2 Br−+1.09[9] NO2(g) + H+ + e- = HNO2+1.093IO3− + 5 H+ + 4 e− HIO(aq) + 2 H2O+1.13[AuCl2]− + e− Au(s) + 2 Cl−+1.15HSeO4− + 3 H+ + 2 e− H2SeO3(aq) + H2O+1.15Ir3+ + 3 e− Ir(s)+1.156[11] Ag2O(s) + 2 H+ + 2 e− 2 Ag(s) + H2O+1.17ClO3− + 2 H+ + e− ClO2(g) + H2O+1.18 [HXeO6]3− + 5 H2O + 8 e− Xe(g) + 11 OH−+1.18[20] Pt2+ + 2 e− Pt(s)+1.188[7] ClO2(g) + H+ + e− HClO2(aq)+1.192 IO3− + 12 H+ + 10 e− I2(s) + 6 H2O+1.20ClO4− + 2 H+ + 2 e− ClO3− + H2O+1.20O2(g) + 4 H+ + 4 e− 2 H2O+1.229[9] MnO2(s) + 4 H+ + 2 e− Mn2+ + 2H2O+1.23 [HXeO4]− + 3 H2O + 6 e− Xe(g) + 7 OH−+1.24[20]Tl3+ + 2 e− Tl++1.25Cr2O72 − + 14 H+ + 6 e− 2 Cr3+ + 7 H2O+1.33Cl2(g) + 2 e− 2 Cl−+1.36[9] CoO2(s) + 4 H+ + e− Co3+ + 2 H2O+1.422 NH3OH+ + H+ + 2 e− N2H5+ + 2 H2O+1.42[6] 2 HIO(aq) + 2 H+ + 2 e− I2(s) + 2 H2O+1.44Ce4+ + e− Ce3++1.44BrO3− + 5 H+ + 4 e− HBrO(aq) + 2 H2O+1.45β-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O+1.460[2]α-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O+1.468[2] 2 BrO3− + 12 H+ + 10 e− Br2(l) + 6 H2O+1.482ClO3− + 12 H+ + 10 e− Cl2(g) + 6 H2O+1.49HO2 + H+ + e− H2O2+1.495[11] MnO4− + 8 H+ + 5 e− Mn2+ + 4 H2O+1.51HO2• + H+ + e− H2O2(aq)+1.51Au3+ + 3 e− Au(s)+1.52NiO2(s) + 4 H+ + 2 e− Ni2+ + 2 OH−+1.592 HClO(aq) + 2 H+ + 2 e− Cl2(g) + 2 H2O+1.63Ag2O3(s) + 6 H+ + 4 e− 2 Ag+ + 3 H2O+1.67HClO2(aq) + 2 H+ + 2 e− HClO(aq) + H2O+1.67Pb4+ + 2 e− Pb2++1.69[2] MnO4− + 4 H+ + 3 e− MnO2(s) + 2 H2O+1.70AgO(s) + 2 H+ + e− Ag+ + H2O+1.77 H2O2(aq) + 2 H+ + 2 e− 2 H2O+1.776Co3+ + e− Co2++1.82Au+ + e− Au(s)+1.83[2] BrO4− + 2 H+ + 2 e− BrO3− + H2O+1.85半反应E° (V)[注 1]来源Ag2+ + e− Ag++1.98[2]S2O82− + 2 e− 2 SO42−+2.07O3(g) + 2 H+ + 2 e− O2(g) + H2O+2.075[7]HMnO4− + 3 H+ + 2 e− MnO2(s) + 2 H2O+2.09XeO3(aq) + 6 H+ + 6 e− Xe(g) + 3 H2O+2.12[20]H4XeO6(aq) + 8 H+ + 8 e− Xe(g) + 6 H2O+2.18[20]FeO42− + 3 e− + 8 H+ Fe3+ + 4 H2O+2.20[22]XeF2(aq) + 2 H+ + 2 e− Xe(g) + 2HF(aq)+2.32[20]H4XeO6(aq) + 2 H+ + 2 e− XeO3(aq) + H2O+2.42[20]F2(g) + 2 e− 2 F−+2.87[2][9]F2(g) + 2 H+ + 2 e− 2 HF(aq)+3.05[2]Tb4+ + e− Tb3++3.05[11]1.^ Clicking on this column to re-sort by potential didn’t work in the Safari webbrowser in v. 4.0.3 or earlier (but works in v. 4.0.5). In this case just reload the page to restore the original order.参考资料1.^ 1.01.11.21.31.41.5 Milazzo, G., Caroli, S., and Sharma, V. K. (1978). Tables ofStandard Electrode Potentials (Wiley, Chichester).2.^ 2.002.012.022.032.042.052.062.072.082.092.102.112.122.132.142.152.162.172.182.19 Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).3.^ 3.03.13.23.3 Bratsch, S. G. (1989). Journal of Physical Chemistry Reference DataVol. 18, pp. 1–21.4.^ 4.04.1 Vanýsek, Petr (2006). "Electrochemical Series," in Handbook of Chemistry and Physics: 87th Edition (/) (Chemical RubberCompany).^ 5.005.015.025.035.045.055.065.075.085.095.105.115.125.135.145.155.165.175.185.195.205.215.22 5.5.235.245.255.265.275.285.295.30 Vanýsek, Petr (2007). “Electrochemical Series”(/articles/08_08_88.pdf) , in Handbook of Chemistryand Physics: 88th Edition (/) (Chemical RubberCompany).6.^ 6.06.16.26.36.4 Greenwood, N. N.; Earnshaw, A.. Chemistry of the Elements. 2ndEdition. Oxford:Butterworth-Heinemann. 1997. ISBN0-7506-3365-4.^ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 Bard, A.J., Faulkner, L.R.(2001). Electrochemical Methods. Fundamentals and Applications , 2nd edition (John Wiley and Sons Inc).7.^ 8.0 8.1 Marcel Pourbaix (1966). Atlas of Electrochemical Equilibria in Aqueous Solutions (NACE International, Houston, Texas; Cebelcor, Brussels).8.^ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 Peter Atkins (1997). Physical Chemistry , 6th edition (W.H. Freeman and Company, New York).9.^ 10.0 10.1 10.2 Ca Sr Ba 一价[11]与两价间的标准电极电势正好有规律关系,因此可以估计近似值10.^ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 11.21 11.22 11.23 11.24 11.25 11.26 11.27 11.28 11.29 11.30 11.31 Standard Redox Potential Table (/time-to-wake-up/docs/electrochemical_redox_potential)11.^ Ti Zr Hf 的标准电极电势变化较规律,因此可估计Rf 的标准电极电势12.^ Gordon Aylward & Tristan Findlay (2008). "SI Chemical Data", 6th edition (John Wiley & Sons, Australia), ISBN 9780470816387.13.^ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Vanýsek, Petr (2007). “Electrochemical Series”, in Handbook of Chemistry and Physics: 88th Edition (Chemical Rubber Company).14.^ 15.0 15.1 15.2 15.3 15.4 WebElements Periodic Table of the Elements | Iron | compounds information (/iron/compounds.html)15.^ 16.0 16.1 由−0.454和(2×−0.499 + −0.508) ÷ 3 = −0.502推算出。
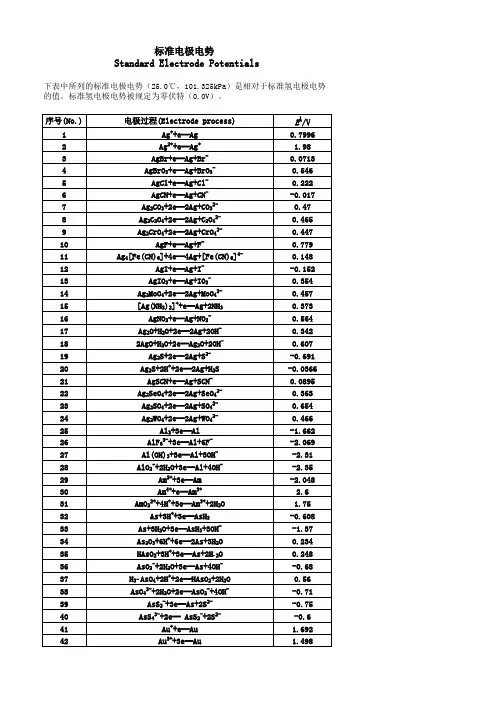
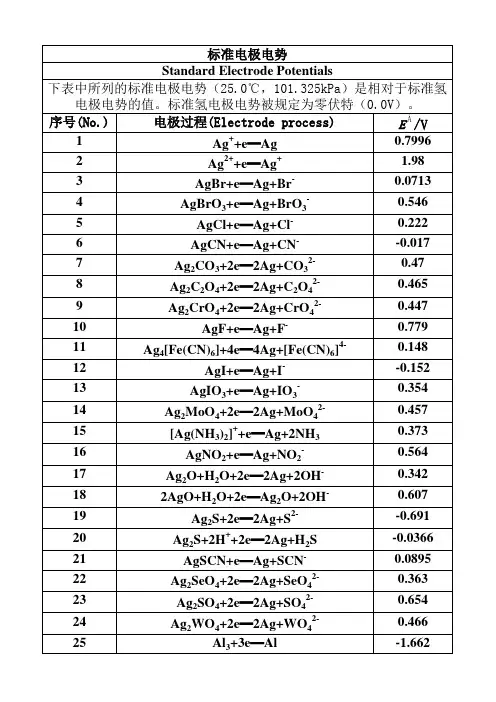
标准电极电势Standard Electrode Potentials下表中所列的标准电极电势(25.0℃,101.325kPa)是相对于标准氢电极电势的值。
标准氢电极电势被规定为零伏特(0.0V)。
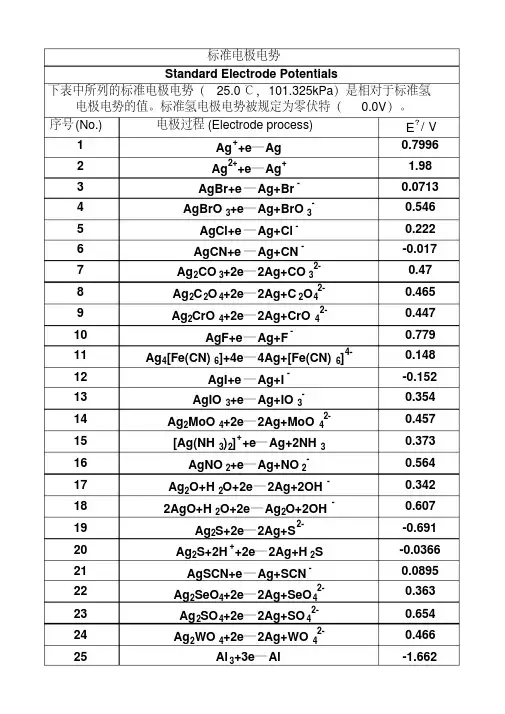
序号(No.)电极过程(Electrode process)EÅ/V 1Ag++e═Ag0.79962Ag2++e═Ag+ 1.983AgBr+e═Ag+Br-0.07134AgBrO3+e═Ag+BrO3-0.5465AgCl+e═Ag+Cl-0.2226AgCN+e═Ag+CN--0.0177Ag2CO3+2e═2Ag+CO32-0.478Ag2C2O4+2e═2Ag+C2O42-0.4659Ag2CrO4+2e═2Ag+CrO42-0.44710AgF+e═Ag+F-0.77911Ag4[Fe(CN)6]+4e═4Ag+[Fe(CN)6]4-0.14812AgI+e═Ag+I--0.152 13AgIO3+e═Ag+IO3-0.35414Ag2MoO4+2e═2Ag+MoO42-0.45715[Ag(NH3)2]++e═Ag+2NH30.37316AgNO2+e═Ag+NO2-0.56417Ag2O+H2O+2e═2Ag+2OH-0.342182AgO+H2O+2e═Ag2O+2OH-0.60719Ag2S+2e═2Ag+S2--0.691 20Ag2S+2H++2e═2Ag+H2S-0.0366 21AgSCN+e═Ag+SCN-0.0895 22Ag2SeO4+2e═2Ag+SeO42-0.36323Ag2SO4+2e═2Ag+SO42-0.65424Ag2WO4+2e═2Ag+WO42-0.46625Al3+3e═Al-1.662 26AlF63-+3e═Al+6F--2.069 27Al(OH)3+3e═Al+3OH--2.3128AlO2-+2H2O+3e═Al+4OH--2.3529Am3++3e═Am-2.048 30Am4++e═Am3+ 2.631AmO22++4H++3e═Am3++2H2O 1.7532As+3H++3e═AsH3-0.608 33As+3H2O+3e═AsH3+3OH--1.3734As2O3+6H++6e═2As+3H2O0.23435HAsO2+3H++3e═As+2H2O0.24836AsO2-+2H2O+3e═As+4OH--0.6837H3AsO4+2H++2e═HAsO2+2H2O0.5638AsO43-+2H2O+2e═AsO2-+4OH--0.7139AsS2-+3e═As+2S2--0.7540AsS43-+2e═ AsS2-+2S2--0.641Au++e═Au 1.69242Au3++3e═Au 1.49843Au3++2e═Au+ 1.401 44AuBr2-+e═Au+2Br-0.959 45AuBr4-+3e═Au+4Br-0.854 46AuCl2-+e═Au+2Cl- 1.15 47AuCl4-+3e═Au+4Cl- 1.002 48AuI+e═Au+I-0.5 49Au(SCN)4-+3e═Au+4SCN-0.66 50Au(OH)3+3H++3e═Au+3H2O 1.45 51BF4-+3e═B+4F--1.04 52H2BO3-+H2O+3e═B+4OH--1.79 53B(OH)3+7H++8e═BH4-+3H2O-0.0481 54Ba2++2e═Ba-2.912 55Ba(OH)2+2e═Ba+2OH--2.99 56Be2++2e═Be-1.847 57Be2O32-+3H2O+4e═2Be+6OH--2.63 58Bi++e═Bi0.5 59Bi3++3e═Bi0.308 60BiCl4-+3e═Bi+4Cl-0.16 61BiOCl+2H++3e═Bi+Cl-+H2O0.16 62Bi2O3+3H2O+6e═2Bi+6OH--0.46 63Bi2O4+4H++2e═2BiO++2H2O 1.593 64Bi2O4+H2O+2e═ Bi2O3+2OH-0.56 65Br2(水溶液,aq)+2e═2Br- 1.087 66Br2(液体)+2e═2Br- 1.066 67BrO-+H2O+2e═Br-+2OH0.761 68BrO3-+6H++6e═Br-+3H2O 1.423 69BrO3-+3H2O+6e═Br-+6OH-0.61 702BrO3-+12H++10e═Br2+6H2O 1.482 71HBrO+H++2e═Br-+H2O 1.331 722HBrO+2H++2e═Br2(水溶液,aq)+2H2O 1.574 73CH3OH+2H++2e═CH4+H2O0.59 74HCHO+2H++2e═CH3OH0.19 75CH3COOH+2H++2e═CH3CHO+H2O-0.12 76(CN)2+2H++2e═2HCN0.373 77(CNS)2+2e═2CNS-0.77 78CO2+2H++2e═CO+H2O-0.12 79CO2+2H++2e═HCOOH-0.199 80Ca2++2e═Ca-2.868 81Ca(OH)2+2e═Ca+2OH--3.02 82Cd2++2e═Cd-0.403 83Cd2++2e═Cd(Hg)-0.352 84Cd(CN)42-+2e═Cd+4CN--1.09 85CdO+H2O+2e═Cd+2OH--0.783 86CdS+2e═Cd+S2--1.17 87CdSO4+2e═Cd+SO42--0.246 88Ce3++3e═Ce-2.336 89Ce3++3e═Ce(Hg)-1.437 90CeO2+4H++e═Ce3++2H2O 1.4 91Cl2(气体)+2e═2Cl- 1.35892ClO-+H2O+2e═Cl-+2OH-0.89 93HClO+H++2e═Cl-+H2O 1.482 942HClO+2H++2e═Cl2+2H2O 1.611 95ClO2-+2H2O+4e═Cl-+4OH-0.76 962ClO3-+12H++10e═Cl2+6H2O 1.47 97ClO3-+6H++6e═Cl-+3H2O 1.451 98ClO3-+3H2O+6e═Cl-+6OH-0.62 99ClO4-+8H++8e═Cl-+4H2O 1.38 1002ClO4-+16H++14e═Cl2+8H2O 1.39 101Cm3++3e═Cm-2.04 102Co2++2e═Co-0.28 103[Co(NH3)6]3++e═[Co(NH3)6]2+0.108 104[Co(NH3)6]2++2e═Co+6NH3-0.43 105Co(OH)2+2e═Co+2OH--0.73 106Co(OH)3+e═Co(OH)2+OH-0.17 107Cr2++2e═Cr-0.913 108Cr3++e═Cr2+-0.407 109Cr3++3e═Cr-0.744 110[Cr(CN)6]3-+e═[Cr(CN)6]4--1.28 111Cr(OH)3+3e═Cr+3OH--1.48 112Cr2O72-+14H++6e═2Cr3++7H2O 1.232 113CrO2-+2H2O+3e═Cr+4OH--1.2 114HCrO4-+7H++3e═Cr3++4H2O 1.35 115CrO42-+4H2O+3e═Cr(OH)3+5OH--0.13 116Cs++e═Cs-2.92 117Cu++e═Cu0.521 118Cu2++2e═Cu0.342 119Cu2++2e═Cu(Hg)0.345 120Cu2++Br-+e═CuBr0.66 121Cu2++Cl-+e═CuCl0.57 122Cu2++I-+e═CuI0.86 123Cu2++2CN-+e═[Cu(CN)2]- 1.103 124CuBr2-+e═Cu+2Br-0.05 125CuCl2-+e═Cu+2Cl-0.19 126CuI2-+e═Cu+2I-0 127Cu2O+H2O+2e═2Cu+2OH--0.36 128Cu(OH)2+2e═Cu+2OH--0.222 1292Cu(OH)2+2e═Cu2O+2OH-+H2O-0.08 130CuS+2e═Cu+S2--0.7 131CuSCN+e═Cu+SCN--0.27 132Dy2++2e═Dy-2.2 133Dy3++3e═Dy-2.295 134Er2++2e═Er-2 135Er3++3e═Er-2.331 136Es2++2e═Es-2.23 137Es3++3e═Es-1.91 138Eu2++2e═Eu-2.812 139Eu3++3e═Eu-1.991 140F2+2H++2e═2HF 3.053190IO3-+2H2O+4e═IO-+4OH-0.15 191IO3-+3H2O+6e═I-+6OH-0.26 1922IO3-+6H2O+10e═I2+12OH-0.21 193H5IO6+H++2e═IO3-+3H2O 1.601 194In++e═In-0.14 195In3++3e═In-0.338 196In(OH)3+3e═In+3OH--0.99 197Ir3++3e═Ir 1.156 198IrBr62-+e═ IrBr63-0.99 199IrCl62-+e═IrCl63-0.867 200K++e═K-2.931 201La3++3e═La-2.379 202La(OH)3+3e═La+3OH--2.9 203Li++e═Li-3.04 204Lr3++3e═Lr-1.96 205Lu3++3e═Lu-2.28 206Md2++2e═Md-2.4 207Md3++3e═Md-1.65 208Mg2++2e═Mg-2.372 209Mg(OH)2+2e═Mg+2OH--2.69 210Mn2++2e═Mn-1.185 211Mn3++3e═Mn 1.542 212MnO2+4H++2e═Mn2++2H2O 1.224 213MnO4-+4H++3e═MnO2+2H2O 1.679 214MnO4-+8H++5e═Mn2++4H2O 1.507 215MnO4-+2H2O+3e═MnO2+4OH-0.595 216Mn(OH)2+2e═Mn+2OH--1.56 217Mo3++3e═Mo-0.2 218MoO42-+4H2O+6e═Mo+8OH--1.05 219N2+2H2O+6H++6e═2NH4OH0.092 2202NH3OH++H++2e═N2H5++2H2O 1.42 2212NO+H2O+2e═N2O+2OH-0.76 2222HNO2+4H++4e═N2O+3H2O 1.297 223NO3-+3H++2e═HNO2+H2O0.934 224NO3-+H2O+2e═NO2-+2OH-0.01 2252NO3-+2H2O+2e═N2O4+4OH--0.85 226Na++e═Na-2.713 227Nb3++3e═Nb-1.099 228NbO2+4H++4e═Nb+2H2O-0.69 229Nb2O5+10H++10e═2Nb+5H2O-0.644 230Nd2++2e═Nd-2.1 231Nd3++3e═Nd-2.323 232Ni2++2e═Ni-0.257 233NiCO3+2e═Ni+CO32--0.45 234Ni(OH)2+2e═Ni+2OH--0.72 235NiO2+4H++2e═Ni2++2H2O 1.678 236No2++2e═No-2.5 237No3++3e═No-1.2 238Np3++3e═Np-1.856239NpO2+H2O+H++e═Np(OH)3-0.962 240O2+4H++4e═2H2O 1.229 241O2+2H2O+4e═4OH-0.401 242O3+H2O+2e═O2+2OH- 1.24 243Os2++2e═Os0.85 244OsCl63-+e═Os2++6Cl-0.4 245OsO2+2H2O+4e═Os+4OH--0.15 246OsO4+8H++8e═Os+4H2O0.838 247OsO4+4H++4e═OsO2+2H2O 1.02 248P+3H2O+3e═PH3(g)+3OH--0.87 249H2PO2-+e═P+2OH--1.82 250H3PO3+2H++2e═H3PO2+H2O-0.499 251H3PO3+3H++3e═P+3H2O-0.454 252H3PO4+2H++2e═H3PO3+H2O--0.276 253PO43-+2H2O+2e═HPO32-+3OH--1.05 254Pa3++3e═Pa-1.34 255Pa4++4e═Pa-1.49 256Pb2++2e═Pb-0.126 257Pb2++2e═Pb(Hg)-0.121 258PbBr2+2e═Pb+2Br--0.284 259PbCl2+2e═Pb+2Cl--0.268 260PbCO3+2e═Pb+CO32--0.506 261PbF2+2e═Pb+2F--0.344 262PbI2+2e═Pb+2I--0.365 263PbO+H2O+2e═Pb+2OH--0.58 264PbO+4H++2e═Pb+H2O0.25 265PbO2+4H++2e═Pb2+2H2O 1.455 266HPbO2-+H2O+2e═Pb+3OH--0.537 267PbO2+SO42-+4H++2e═PbSO4+2H2O 1.691 268PbSO4+2e═Pb+SO42--0.359 269Pd2++2e═Pd0.915 270PdBr42-+2e═Pd+4Br-0.6 271PdO2+H2O+2e═PdO+2OH-0.73 272Pd(OH)2+2e═Pd+2OH-0.07 273Pm2++2e═Pm-2.2 274Pm3++3e═Pm-2.3 275Po4++4e═Po0.76 276Pr2++2e═Pr-2 277Pr3++3e═Pr-2.353 278Pt2++2e═Pt 1.18 279[PtCl6]2-+2e═[PtCl4]2-+2Cl-0.68 280Pt(OH)2+2e═Pt+2OH-0.14 281PtO2+4H++4e═Pt+2H2O1 282PtS+2e═Pt+S2--0.83 283Pu3++3e═Pu-2.031 284Pu5++e═Pu4+ 1.099 285Ra2++2e═Ra-2.8 286Rb++e═Rb-2.98 287Re3++3e═Re0.3337Th4++4e═Th-1.899 338Ti2++2e═Ti-1.63 339Ti3++3e═Ti-1.37 340TiO2+4H++2e═Ti2++2H2O-0.502 341TiO2++2H++e═Ti3++H2O0.1 342Tl++e═Tl-0.336 343Tl3++3e═Tl0.741 344Tl3++Cl-+2e═TlCl 1.36 345TlBr+e═Tl+Br--0.658 346TlCl+e═Tl+Cl--0.557 347TlI+e═Tl+I--0.752 348Tl2O3+3H2O+4e═2Tl++6OH-0.02 349TlOH+e═Tl+OH--0.34 350Tl2SO4+2e═2Tl+SO42--0.436 351Tm2++2e═Tm-2.4 352Tm3++3e═Tm-2.319 353U3++3e═U-1.798 354UO2+4H++4e═U+2H2O-1.4 355UO2++4H++e═U4++2H2O0.612 356UO22++4H++6e═U+2H2O-1.444 357V2++2e═V-1.175 358VO2++2H++e═V3++H2O0.337 359VO2++2H++e═VO2++H2O0.991 360VO2++4H++2e═V3++2H2O0.668 361V2O5+10H++10e═2V+5H2O-0.242 362W3++3e═W0.1 363WO3+6H++6e═W+3H2O-0.09 364W2O5+2H++2e═2WO2+H2O-0.031 365Y3++3e═Y-2.372 366Yb2++2e═Yb-2.76 367Yb3++3e═Yb-2.19 368Zn2++2e═Zn-0.7618 369Zn2++2e═Zn(Hg)-0.7628 370Zn(OH)2+2e═Zn+2OH--1.249 371ZnS+2e═Zn+S2--1.4 372ZnSO4+2e═Zn(Hg)+SO42--0.799。
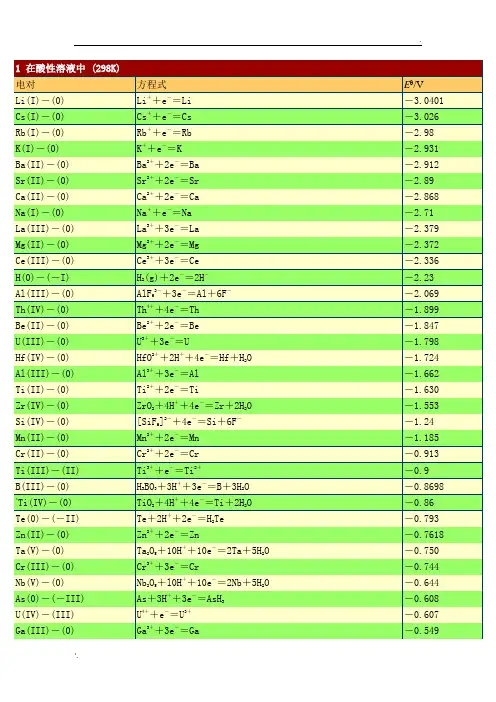
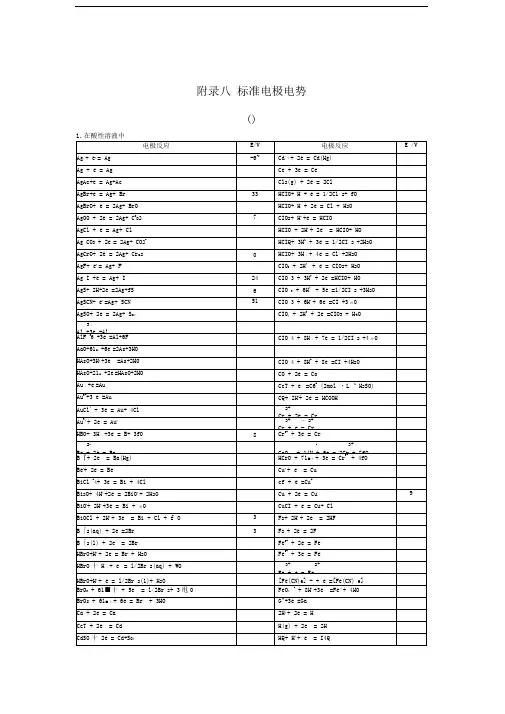
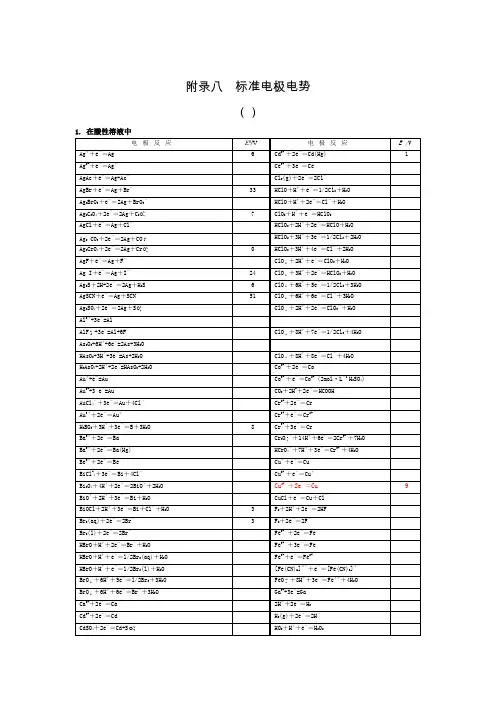
1电对方程式E /VLi(I)-(0) Li++e-=Li -3.0401 Cs(I)-(0) Cs++e-=Cs -3.026 Rb(I)-(0) Rb++e-=Rb -2.98 K(I)-(0) K++e-=K -2.931 Ba(II)-(0) Ba2++2e-=Ba -2.912 Sr(II)-(0) Sr2++2e-=Sr -2.89 Ca(II)-(0) Ca2++2e-=Ca -2.868 Na(I)-(0) Na++e-=Na -2.71 La(III)-(0) La3++3e-=La -2.379 Mg(II)-(0) Mg2++2e-=Mg -2.372 Ce(III)-(0) Ce3++3e-=Ce -2.336 H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069 Th(IV)-(0) Th4++4e-=Th -1.899 Be(II)-(0) Be2++2e-=Be -1.847 U(III)-(0) U3++3e-=U -1.798Hf(IV)-(0) HfO2++2H++4e-=Hf+H2O -1.724 Al(III)-(0) Al3++3e-=Al -1.662 Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0) ZrO2+4H++4e-=Zr+2H2O -1.553Si(IV)-(0) [SiF6]2-+4e-=Si+6F--1.24 Mn(II)-(0) Mn2++2e-=Mn -1.185 Cr(II)-(0) Cr2++2e-=Cr -0.913 Ti(III)-(II) Ti3++e-=Ti2+-0.9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0.8698*Ti(IV)-(0) TiO2+4H++4e-=Ti+2H2O -0.86Te(0)-(-II) Te+2H++2e-=H2Te -0.793 Zn(II)-(0) Zn2++2e-=Zn -0.7618Ta(V)-(0) Ta2O5+10H++10e-=2Ta+5H2O -0.750Cr(III)-(0) Cr3++3e-=Cr -0.744Nb(V)-(0) Nb2O5+l0H++10e-=2Nb+5H2O -0.644As(0)-(-III) As+3H++3e-=AsH3-0.608 U(IV)-(III) U4++e-=U3+-0.607 Ga(III)-(0) Ga3++3e-=Ga -0.549P(I)-(0) H3PO2+H++e-=P+2H2O -0.508P(III)-(I) H3PO3+2H++2e-=H3PO2+H2O -0.499*C(IV)-(III) 2CO2+2H++2e-=H2C2O4-0.49Fe(II)-(0) Fe2++2e-=Fe -0.447Cr(III)-(II) Cr3++e-=Cr2+-0.407 Cd(II)-(0) Cd2++2e-=Cd -0.4030 Se(0)-(-II) Se+2H++2e-=H2Se(aq) -0.399Pb(II)-(0) PbI2+2e-=Pb+2I--0.365 Eu(III)-(II) Eu3++e-=Eu2+-0.36Pb(II)-(0) PbSO4+2e-=Pb+SO42--0.3588In(III)-(0) In3++3e-=In -0.3382 Tl(I)-(0) Tl++e-=Tl -0.336 Co(II)-(0) Co2++2e-=Co -0.28P(V)-(III) H3PO4+2H++2e-=H3PO3+H2O -0.276Pb(II)-(0) PbCl2+2e-=Pb+2Cl--0.2675 Ni (II)-(0) Ni2++2e-=Ni -0.257 V(III)-(II) V3++e-=V2+-0.255Ge(IV)-(0) H2GeO3+4H++4e-=Ge+3H2O -0.182Ag(I)-(0) AgI+e-=Ag+I--0.15224 Sn(II)-(0) Sn2++2e-=Sn -0.1375 Pb(II)-(0) Pb2++2e-=Pb -0.1262*C(IV)-(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0)-(-III) P(white)+3H++3e-=PH3(g) -0.063Hg(I)-(0) Hg2I2+2e-=2Hg+2I--0.0405Fe(III)-(0) Fe3++3e-=Fe -0.037 H(I)-(0) 2H++2e-=H20.0000 Ag(I)-(0) AgBr+e-=Ag+Br-0.07133S(II.V)-(II) S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III) TiO2++2H++e-=Ti3++H2O 0.1S(0)-(-II) S+2H++2e-=H2S(aq) 0.142 Sn(IV)-(II) Sn4++2e-=Sn2+0.151Sb(III)-(0) Sb2O3+6H++6e-=2Sb+3H2O 0.152Cu(II)-(I) Cu2++e-=Cu+0.153 Bi(III)-(0) BiOCl+2H++3e-=Bi+Cl-+H2O 0.1583S(VI)-(IV) SO42-+4H++2e-=H2SO3+H2O 0.172Sb(III)-(0) SbO++2H++3e-=Sb+H2O 0.212Ag(I)-(0) AgCl+e-=Ag+Cl-0.22233As(III)-(0) HAsO2+3H++3e-=As+2H2O 0.248Hg(I)-(0) Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl) 0.26808Bi(III)-(0) BiO++2H++3e-=Bi+H2O 0.320U(VI)-(IV) UO22++4H++2e-=U4++2H2O 0.327C(IV)-(III) 2HCNO+2H++2e-=(CN)2+2H2O 0.330V(IV)-(III) VO2++2H++e-=V3++H2O 0.337 Cu(II)-(0) Cu2++2e-=Cu 0.3419Re(VII)-(0) ReO4-+8H++7e-=Re+4H2O 0.368Ag(I)-(0) Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0) H2SO3+4H++4e-=S+3H2O 0.449Cu(I)-(0) Cu++e-=Cu 0.521I(0)-(-I) I2+2e-=2I-0.5355I(0)-(-I) I3-+2e-=3I-0.536As(V)-(III) H3AsO4+2H++2e-=HAsO2+2H2O 0.560Sb(V)-(III) Sb2O5+6H++4e-=2SbO++3H2O 0.581Te(IV)-(0) TeO2+4H++4e-=Te+2H2O 0.593U(V)-(IV) UO2++4H++e-=U4++2H2O 0.612**Hg(II)-(I) 2HgCl2+2e-=Hg2Cl2+2Cl-0.63Pt(IV)-(II) [PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68O(0)-(-I) O2+2H++2e-=H2O20.695Pt(II)-(0) [PtCl4]2-+2e-=Pt+4Cl-0.755*Se(IV)-(0) H2SeO3+4H++4e-=Se+3H2O 0.74Fe(III)-(II) Fe3++e-=Fe2+0.771 Hg(I)-(0) Hg22++2e-=2Hg 0.7973 Ag(I)-(0) Ag++e-=Ag 0.7996Os(VIII)-(0) OsO4+8H++8e-=Os+4H2O 0.8N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803Hg(II)-(0) Hg2++2e-=Hg 0.851Si(IV)-(0) (quartz)SiO2+4H++4e-=Si+2H2O 0.857Cu(II)-(I) Cu2++I-+e-=CuI 0.86N(III)-(I) 2HNO2+4H++4e-=H2N2O2+2H2O 0.86Hg(II)-(I) 2Hg2++2e-=Hg22+0.920N(V)-(III) NO3-+3H++2e-=HNO2+H2O 0.934Pd(II)-(0) Pd2++2e-=Pd 0.951N(V)-(II) NO3-+4H++3e-=NO+2H2O 0.957N(III)-(II) HNO2+H++e-=NO+H2O 0.983I(I)-(-I) HIO+H++2e-=I-+H2O 0.987V(V)-(IV) VO2++2H++e-=VO2++H2O 0.991V(V)-(IV) V(OH)4++2H++e-=VO2++3H2O 1.00Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002Te(VI)-(IV) H6TeO6+2H++2e-=TeO2+4H2O 1.02N(IV)-(II) N2O4+4H++4e-=2NO+2H2O 1.035N(IV)-(III) N2O4+2H++2e-=2HNO21.065I(V)-(-I) IO3-+6H++6e-=I-+3H2O 1.085Br(0)-(-I) Br2(aq)+2e-=2Br- 1.0873Se(VI)-(IV) SeO42-+4H++2e-=H2SeO3+H2O 1.151Cl(V)-(IV) ClO3-+2H++e-=ClO2+H2O 1.152Pt(II)-(0) Pt2++2e-=Pt 1.18Cl(VII)-(V) ClO4-+2H++2e-=ClO3-+H2O 1.189I(V)-(0) 2IO3-+12H++10e-=I2+6H2O 1.195Cl(V)-(III) ClO3-+3H++2e-=HClO2+H2O 1.214Mn(IV)-(II) MnO2+4H++2e-=Mn2++2H2O 1.224O(0)-(-II) O2+4H++4e-=2H2O 1.229Tl(III)-(I) T13++2e-=Tl+ 1.252Cl(IV)-(III) ClO2+H++e-=HClO21.277N(III)-(I) 2HNO2+4H++4e-=N2O+3H2O 1.297**Cr(VI)-(III) Cr2O72-+14H++6e-=2Cr3++7H2O 1.33Br(I)-(-I) HBrO+H++2e-=Br-+H2O 1.331Cr(VI)-(III) HCrO4-+7H++3e-=Cr3++4H2O 1.350Cl(0)-(-I) Cl2(g)+2e-=2Cl- 1.35827Cl(VII)-(-I) ClO4-+8H++8e-=Cl-+4H2O 1.389Cl(VII)-(0) ClO4-+8H++7e-=1/2Cl2+4H2O 1.39Au(III)-(I) Au3++2e-=Au+ 1.401Br(V)-(-I) BrO3-+6H++6e-=Br-+3H2O 1.423I(I)-(0) 2HIO+2H++2e-=I2+2H2O 1.439Cl(V)-(-I) ClO3-+6H++6e-=Cl-+3H2O 1.451Pb(IV)-(II) PbO2+4H++2e-=Pb2++2H2O 1.455Cl(V)-(0) ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I) HClO+H++2e-=Cl-+H2O 1.482Br(V)-(0) BrO3-+6H++5e-=l/2Br2+3H2O 1.482Au(III)-(0) Au3++3e-=Au 1.498Mn(VII)-(II) MnO4-+8H++5e-=Mn2++4H2O 1.507Mn(III)-(II) Mn3++e-=Mn2+ 1.5415Cl(III)-(-I) HClO2+3H++4e-=Cl-+2H2O 1.570Br(I)-(0) HBrO+H++e-=l/2Br2(aq)+H2O 1.574N(II)-(I) 2NO+2H++2e-=N2O+H2O 1.591I(VII)-(V) H5IO6+H++2e-=IO3-+3H2O 1.601Cl(I)-(0) HClO+H++e-=1/2Cl2+H2O 1.611Cl(III)-(I) HClO2+2H++2e-=HClO+H2O 1.645Ni(IV)-(II) NiO2+4H++2e-=Ni2++2H2O 1.678Mn(VII)-(IV) MnO4-+4H++3e-=MnO2+2H2O 1.679Pb(IV)-(II) PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913Au(I)-(0) Au++e-=Au 1.692 Ce(IV)-(III) Ce4++e-=Ce3+ 1.72N(I)-(0) N2O+2H++2e-=N2+H2O 1.766O(-I)-(-II) H2O2+2H++2e-=2H2O 1.776Co(III)-(II) Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83Ag(II)-(I) Ag2++e-=Ag+ 1.980S(VII)-(VI) S2O82-+2e-=2SO42- 2.010O(0)-(-II) O3+2H++2e-=O2+H2O 2.076O(II)-(-II) F2O+2H++4e-=H2O+2F- 2.153Fe(VI)-(III) FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II) O(g)+2H++2e-=H2O 2.421F(0)-(-I) F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99La(III)-(0) La(OH)3+3e-=La+3OH--2.90Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36Al(III)-(0) H AlO-+H O+3e-=Al+OH--2.33P(I)-(0) H2PO2-+e-=P+2OH--1.82B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48*Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2Te(0)-(-I) Te+2e-=Te2--1.143P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584*S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13*Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076Ag(I)-(0) AgCN+e-=Ag+CN--0.017N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373O(0)-(-II) O2+2H2O+4e-=4OH-0.401I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490Mn(VII)-(VI) MnO4-+e-=MnO42-0.558Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607。
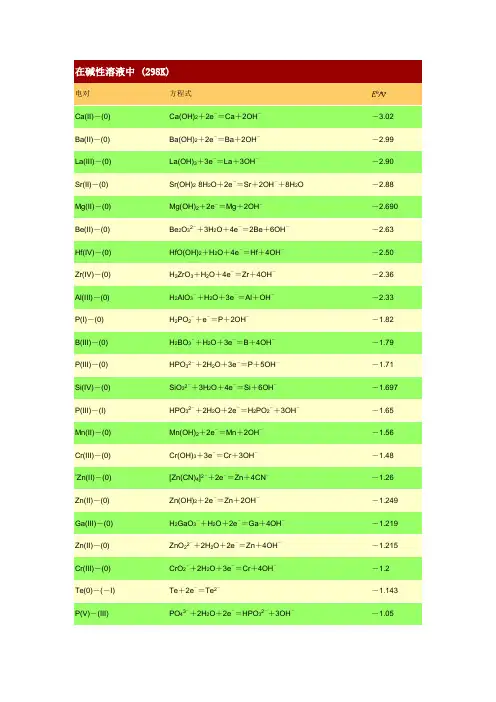
电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02 Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99 La(III)-(0) La(OH)3+3e-=La+3OH--2.90 Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88 Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690 Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63 Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50 Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36 Al(III)-(0) H2AlO3-+H2O+3e-=Al+OH--2.33 P(I)-(0) H2PO2-+e-=P+2OH--1.82 B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79 P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71 Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697 P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65 Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56 Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48 *Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26 Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249 Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219 Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215 Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2 Te(0)-(-I) Te+2e-=Te2--1.143 P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924 Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277 Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809 Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691 As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584 *S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627 Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46 *Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422 Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366 Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360 Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31 Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222 Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13 *Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12 O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076 Ag(I)-(0) AgCN+e-=Ag+CN--0.017 N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01 Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05 Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08 Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977 Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108 Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14 Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17 Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247 I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33 Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342 Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358 Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373 O(0)-(-II) O2+2H2O+4e-=4OH-0.401 I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485 *Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490 Mn(VII)-(VI) MnO4-+e-=MnO42-0.558 Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595 Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60 Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607 Br(V)-(-I) BrO3-+3H2O+6e-=Br-+6OH-0.61 Cl(V)-(-I) ClO3-+3H2O+6e-=Cl-+6OH-0.62 Cl(III)-(I) ClO2-+H2O+2e-=ClO-+2OH-0.66 I(VII)-(V) H3IO62-+2e-=IO3-+3OH-0.7 Cl(III)-(-I) ClO2-+2H2O+4e-=Cl-+4OH-0.76 Br(I)-(-I) BrO-+H2O+2e-=Br-+2OH-0.761 Cl(I)-(-I) ClO-+H2O+2e-=Cl-+2OH-0.841 *Cl(IV)-(III) ClO2(g)+e-=ClO2-0.95。

--标准--标准电极电势表--1在酸性溶液中(298K)电对方程式 E /VLi(l) —(0) Li + + e— = Li —3.0401Cs(l) —(0) Cs + + e — = Cs —3.026 Rb(l) —(0) Rb + + e — = Rb —2.98 K(l) —(0) K + + e— = K —2.931 Ba(ll) —(0) Ba2+ + 2e — = Ba —2.912 Sr(ll) —(0) Sr2+ + 2e — = Sr —2.89 Ca(ll) —(0) Ca2+ + 2e — = Ca —2.868 Na(l) —(0) Na + + e — = Na —2.71 La(lll) —(0) La3 + + 3e — = La —2.379 Mg(ll) —(0) Mg 2+ + 2e — = Mg —2.372 Ce(lll) —(0) Ce3 + + 3e — = Ce —2.336 H(0) —(—l) H2(g) + 2e — = 2H ——2.23 Al(lll) —(0) AIF63 — + 3e — = Al + 6F ——2.069 Th(IV) —(0) Th4+ + 4e — = Th —1.899 Be(ll) —(0) Be2+ + 2e — = Be —1.847 U(lll) —(0) U3+ + 3e — = U —1.798 Hf(IV) —(0) HfO 2+ + 2H ++ 4e — = Hf + H 20 —1.724 Al(lll) —(0) Al3+ + 3e —= Al —1.662 Ti(ll) —(0) Ti2+ + 2e — = Ti —1.630Zr(IV) -(0) ZrO 2+ 4H + + 4e 一= Zr + 2H 2O -1.553 Si(IV) -(0) [SiF6]2- + 4e - = Si + 6F --1.24 Mn(II) -(0) Mn 2 + + 2e 一= Mn -1.185 Cr(II) -(0) Cr2+ + 2e -= Cr -0.913 Ti(III) -(II) Ti3+ + e -= Ti2+-0.9B(III) -(0) H 3BO 3+ 3H + + 3e 一= B + 3H 2O-0.8698*Ti(IV) -(0) TiO 2 + 4H + + 4e 一= Ti + 2H 2O -0.86 Te(0) -(-II)Te + 2H + + 2e - = H2Te -0.793 Zn(II) -(0) Zn2 + + 2e —= Zn -0.7618 Ta(V) -(0) Ta2O5 + 10H + + 10e 一= 2Ta + 5H 2O -0.750 Cr(III) -(0) Cr3 + + 3e —= Cr -0.744 Nb(V) -(0) Nb 2O5+ 10H + + 10e —= 2Nb + 5H 2O -0.644 As(0) -(-III) As + 3H + + 3e —= AsH 3 -0.608 U(IV) -(III) U4+ + e-= U3+-0.607 Ga(III) -(0) Ga3 + + 3e 一= Ga -0.549 P(I) -(0) H3PO2 + H + + e —= P + 2H 2O -0.508 P(III) -(I) H3PO3 + 2H + + 2e 一= H3PO2 + H2O -0.499 *C(IV) -(III) 2CO2 + 2H + + 2e H2C2O4 -0.49 Fe(II) -(0) Fe2 ++ 2e —= Fe -0.447 Cr(III) -(II) Cr3 + + e- = Cr2 +-0.407 Cd(II) -(0) Cd2 + + 2e - = Cd -0.4030 Se(0) -(-II)Se + 2H + + 2e 一= H2Se(aq) -0.399Pb(II) -(0) Pbl 2+ 2e —= Pb + 2I —-0.365 Eu(III) -(II) Eu3+ + e- = Eu2+-0.36Pb(II) -(0) PbSO 4+ 2e - = Pb + SO42--0.3588 In(III) -(0) ln3+ + 3e —= In -0.3382 Tl(I) -(0) Tl ++ e —= Tl -0.336 Co(II) -(0) Co2 + + 2e - = Co -0.28P(V) -(III) H3PO4 + 2H + + 2e 一= H3PO3 + H2O -0.276 Pb(II) -(0) PbCI 2 + 2e —= Pb + 2CI —-0.2675 Ni (II) -(0) Ni2 + + 2e —= Ni -0.257V(III) -(II) V3 + + e- = V2+-0.255 Ge(IV) -(0) H 2GeO 3 + 4H + + 4e 一= Ge + 3H 2 O -0.182 Ag(I) -(0) Agl + e _= Ag + I —-0.15224 Sn(II) -(0) Sn2++2e-=Sn -0.1375 Pb(II) -(0) Pb2++2e-=Pb -0.1262**C(IV) -(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0) -( -III) P(white) +3H++3e-=PH3(g) -0.063 Hg(I) -(0) Hg2I2+2e-=2Hg +2I--0.0405 Fe(III) -(0) Fe3+ + 3e -= Fe -0.037H(I) -(0) 2H + + 2e -= H2 0.0000 Ag(I) -(0) AgBr + e-= Ag + Br -0.07133 S(II.V) -(II) S4O62-+2e-=2S2O32-0.08*Ti(IV) -(III) TiO2++2H++e-=Ti3++H2O 0.1S(0) -( -II) S+ 2H + + 2e —= H2S(aq) 0.142 Sn(IV) -(II) Sn4+ + 2e —= Sn2+0.151 Sb(III) -(0) Sb2O3 + 6H + + 6e 一= 2Sb + 3H 20 0.152 Cu(II) -(I) Cu2+ + e- = Cu +0.153 Bi(III) -(0) BiOCI + 2H ++ 3e 一= Bi + Cl 一+ H20 0.1583 S(VI) -(IV) S04_+ 4H + + 2e —= H2SO3 + H20 0.172 Sb(III) -(0) SbO + + 2H ++ 3e 一= Sb + H20 0.212 Ag(I) -(0) AgCI + e = Ag + Cl 0.22233 As(III) -(0) HAsO 2 + 3H + + 3e 一= As + 2H 20 0.248 Hg(I) -(0) Hg 2CI2+ 2e-= 2Hg + 2CI -(饱和KCI) 0.26808 Bi(III) -(0) BiO ++ 2H ++ 3e -= Bi + H 2O 0.320U(VI) -(IV) UO 22++ 4H ++ 2e -= U4++ 2H 2O 0.327C(IV) -(III) 2HCNO + 2H ++ 2e -= (CN) 2+ 2H 2O 0.330V(IV) -(III) VO 2++ 2H ++ e-= V3++ H 2O 0.337 Cu(II) -(0) Cu 2++ 2e -= Cu 0.3419 Re(VII) -(0) ReO 4-+ 8H ++7e -= Re+ 4H 2O 0.368 Ag(I) -(0) Ag 2CrO 4+ 2e -= 2Ag + CrO 42-0.4470 S(IV) -(0) H 2SO 3+ 4H + + 4e -= S+ 3H 2O 0.449 Cu(I) -(0) Cu + + e -= Cu 0.521I(0) -(-I) I2+ 2e -= 2I-0.5355 I(0) -(-I) I3 -+ 2e -= 3I -0.536 As(V) -(III) H 3AsO 4+ 2H ++ 2e -= HAsO 2+ 2H 2O 0.560Sb(V)(III) Sb2O5 + 6H + + 4e 一= 2SbO 十+ 3H 20 0.581 -Te(IV) -(0) TeO 2+ 4H + + 4e 一= Te + 2H 20 0.593 U(V) -(IV) UO2 ++ 4H + + e —= U4+ + 2H 2O 0.612**** Hg(II) -(I) 2HgCI 2 + 2e 一= Hg 2CI2 + 2CI —0.63 Pt(IV) -(II) [PtCI 6]2- + 2e —= [PtCI 4]2- + 2CI —0.68O(0) - (-I) O2+ 2H + + 2e —= H2O2 0.695 Pt(II) -(0) [PtCI 4]2- + 2e - = Pt + 4CI -0.755 *Se(IV) -(0) H 2SeO 3 + 4H ++ 4e 一= Se + 3H 2O 0.74 Fe(III) -(II) Fe3++ e —= Fe2+0.771 Hg(I) -(0) Hg 22+ + 2e 一= 2Hg 0.7973 Ag(I) -(0) Ag + + e -= Ag 0.7996 Os(VIII) -(0) OsO 4 + 8H + + 8e 一= Os + 4H 2O 0.8N(V) -(IV) 2NO 3- + 4H + + 2e - = N2O4 + 2H 2O 0.803 Hg(II) -(0) Hg 2+ + 2e _ = Hg 0.851Si(IV)(0) (quartz)SiO 2 + 4H + + 4e 一= Si + 2H 2O 0.857 -Cu(II)(I) Cu2 ++厂 + e-= CuI 0.86 -N(III) -(I) 2HNO 2 + 4H + + 4e 一= H2N2O2 + 2H 2O 0.86 Hg(II) -(I) 2Hg 2 + + 2e - = Hg 22+0.920 N(V) -(III) NO 3- + 3H + + 2e HNO 2+ H2O 0.934Pd(II)(0) Pd2 + + 2e —= Pd 0.951 -N(V) -(II) NO 3一+ 4H ++ 3e 一= NO + 2H2O 0.957 N(III) -(II) HNO 2+ H + + e —= NO + H2O 0.983I(I) -(-I) HIO + H + + 2e —=厂+ H2O 0.987 V(V) -(IV) VO 2 + + 2H + + e - = VO2+ + H2O 0.991 V(V) -(IV) V(OH) 4 + + 2H + + e —= VO2十+ 3H 2O 1.00 Au(III) -(0) [AuCI 4] 一+ 3e —= Au + 4Cl — 1.002 Te(VI) -(IV) H6TeO 6 + 2H ++ 2e —= TeO 2 + 4H 2O 1.02N(IV) -(II) N2O4 + 4H + + 4e —= 2NO + 2H 2O 1.035 N(IV) -(III) N2O4 + 2H + + 2e 一= 2HNO 2 1.065 I(V) -( -I) IO 3- + 6H + + 6e —=厂+ 3H 2O 1.085 Br(0) -(-I) Br2(aq) + 2e —= 2Br — 1.0873 Se(VI) -(IV) SeO42- + 4H + + 2e 一= H2SeO3 + H 2O 1.151 Cl(V) -(IV) CIO 3一+ 2H + + e —= ClO2 + H2O 1.152 Pt(II) -(0) Pt2+ + 2e —= Pt 1.18 Cl(VII) -(V) CIO 4- + 2H + + 2e - = CIO 3-+ H2O 1.189 I(V) -(0) 2IO 3- + 12H + + 10e —= I2+ 6H 2O 1.195 Cl(V) -(III) ClO 3- + 3H + + 2e —= HClO 2+ H2O 1.214 Mn(IV) -(II) MnO 2 + 4H + + 2e 一= Mn 2++ 2H 2O 1.224 O(0) -(-II) O2+ 4H + + 4e —= 2H 2O 1.229 Tl(III) -(I) T13+ + 2e - = Tl + 1.252 Cl(IV) -(III) ClO 2 + H + + e -= HClO 2 1.277 N(III) -(I) 2HNO 2 + 4H + + 4e 一= N2O + 3H 2O 1.297**Cr2O7_ + 14H + + 6e 一= 2Cr3+ + 7H 2O 1.33 **Cr(VI) -(III)Br(I) -(-I) HBrO + H + + 2e 一= BL+ H2O 1.331Cr(VI) -(III) HCrO 4- + 7H + + 3e -=Cr3 ++ 4H2O 1.350 Cl(0) -( -I) Cl2(g) + 2e - = 2CI - 1.35827Cl(VII) -(-I)ClO 4-+ 8H+ + 8e -=CIT 4H 2O 1.389Cl(VII) -(0)ClO 4-+ 8H+ + 7e -=1/2CI 2+ 4H 2O 1.39Au(III) -(I)Au 3 ++ 2e —= Au + 1.401Br(V) -(-I) BrO 3 -+ 6H + + 6e -=BL + 3H 2O 1.423 I(I) -(0) 2HIO + 2H+ + 2e —= I2+ 2H 2O 1.439Cl(V) -(-I) ClO 3-+ 6H + + 6e -=CI- + 3H 2O 1.451 Pb(IV) -(II) PbO 2 + 4H + + 2e —Pb 2++ 2H 2O 1.455Cl(V) -(0) ClO 3-+ 6H + + 5e -=1/2CI 2+ 3H 2O 1.47 Cl(I) -( -I) HClO + H + + 2e —=CI -+ H 2O 1.482 Br(V) -(0) BrO 3 -+ 6H + + 5e -=I/2Br 2 + 3H 2O 1.482 Au(III) -(0) Au3 ++ 3e —= Au 1.498 Mn(VII) -(II) MnO 4 -+ 8H ++ 5e -=Mn 2+ + 4H 2O 1.507 Mn(III) -(II) Mn 3+ + e —= Mn 2+ 1.5415 Cl(III) -( -I) HClO 2+ 3H ++ 4e -=CU 2H 2O 1.570 Br(I) -(0) HBrO + H + + e —= I/2Br 2(aq) + H2O 1.574 N(II) -(I) 2NO + 2H ++ 2e —N2O+H2O 1.591 I(VII) -(V) H 5IO 6 + H + + 2e —= IO 3-+ 3H 2O 1.601 Cl(I) -(0) HClO + H + + e —= 1/2CI 2 + H2O 1.611 Cl(III) -(I) HClO 2+ 2H ++ 2e -=HCIO + H2O 1.645 Ni(IV) -(II) NiO 2+ 4H + + 2e 一=Ni2++2H2O 1.678Mn (VII) —(IV) MnO 4 — + 4H + + 3e — = MnO 2 + 2H 20 1.679 Pb(IV) —(II) PbO 2 + SO42— + 4H + + 2e — = PbSO 4+2H 2O 1.6913 Au(I) —(0) Au + + e — = Au 1.692 Ce(IV) —(III) Ce4+ + e — = Ce3+ 1.72N(I) —(0) N2O + 2H + + 2e — = N2+ H2O 1.766O( —I) —( —H2O2 + 2H + + 2e —= 2H 2O 1.776 II)Co(III) —(II) Co3 + + e — = Co2 +(2mol •—1 H 2SO 4) 1.83 Ag(II) —(I) Ag 2 + + e— = Ag + 1.980 S(VII) —(VI) S2O82 — + 2e — = 2SO42— 2.010 0(0) —(—II) O3+ 2H + + 2e — = O2+ H2O 2.076 O(II) —(—II) F2O + 2H + + 4e — = H2O + 2F — 2.153 Fe(VI) —(III) FeO42 — + 8H + + 3e —= Fe3+ + 4H 2O 2.200(0) —(—II) O(g) + 2H + + 2e — = H2O 2.421 F(0) —( —I) F2 + 2e — = 2F — 2.866F2 + 2H + + 2e — = 2HF 3.053 2在碱性溶液中(298K)电对方程式 E /V Ca(II) —(0) Ca(OH) 2+ 2e — = Ca + 2OH ——3.02 Ba(II) —(0) Ba(OH) 2+ 2e — = Ba + 2OH ——2.99 La(III) —(0) La(OH) 3+ 3e —= La + 3OH ——2.90 Sr(II) —(0) Sr(OH) 2 • 8HO + 2e — = Sr + 2OH — + 8H 2O — 2.88Mg(II) —(0) Mg(OH) 2 + 2e — = Mg + 2OH ——2.690Be(II) -(0) Be 2O32_+ 3H 20 + 4e —= 2Be + 60H ——2.63Hf(IV) -(0)HfO(OH) 1 2+ H 20 + 4e —= Hf + 40H ——2.50Zr(IV) -(0) H 2ZrO 3 + H20 + 4e — = Zr + 40H ——2.36Al(III) -(0) H 2AlO 3 —+ H20 + 3e — = Al + 0H ——2.33P(I) -(0) H 2PO 2-+ e — = P+ 20H ——1.82B(III) -(0) H 2BO 3-+ H20 + 3e — = B + 40H ——1.79P(III) -(0)HPO 32-+ 2H 20 + 3e —= P+ 50H ——1.71 Si(IV) -(0) SiO 32-+ 3H 2O + 4e- = Si + 60H -—1.697 P(III) -(I) HPO 32-+ 2H 20 + 2e —= H2PO2— + 30H ——1.65 Mn(II) -(0) Mn(OH) 2 + 2e — = Mn + 20H ——1.56 Cr(III) -(0) Cr(OH) 3 + 3e —= Cr + 30H ——1.48 *Zn(II) -(0) [Zn(CN) 4]2-+ 2e — = Zn + 4CN ——1.26 Zn(II) -(0) Zn(OH) 2+2e—=Zn+20H ——1.249 Ga(III) -(0) H 2GaO 3—+H20+2e—=Ga+40H ——1.219 Zn(II) -(0) ZnO 22-+2H 20+2e—=Zn +40H ——1.215 Cr(III) -(0) CrO 2一+ 2H 20 + 3e 一= Cr + 40H ——1.2Te(0) -(-I)Te +2e—= Te2——1.143P(V)-(III) PO43- + 2H 20 + 2e - = HPO 32- + 30H -—1.05*Zn(II) -(0) [Zn(NH 3)4]2++2e—=Zn+4NH 3 —1.04*W(VI) -(0) WO42-+4H20+6e—=W+80H ——1.01**Ge(IV) -(0) HGeO 3 —+2H20+4e—=Ge+50H ——1.0Sn(IV) -(II) [Sn(OH) 6]2—+2e—=HSn0 2—+H20+30H—0.93S(VI) -(IV) S04—+ H20 + 2e-= SO32- + 2OH --0.93Se(0) -(-II) Se + 2e - = Se —-0.924 Sn(II) -(0) HSnO 2一+ H2O + 2e 一= Sn + 3OH —-0.909P(0) -( -III) P+ 3H 2O + 3e —= PH 3(g) + 3OH —-0.87N(V) -(IV) 2NO 3 - + 2H2O + 2e 一= N2O4+ 4OH —-0.85H(I) -(0) 2H2O + 2e〜H2 + 2OH —-0.8277 Cd(II) -(0) Cd(OH) 2+ 2e —= Cd(Hg) + 2OH —-0.809 Co(II) -(0) Co(OH) 2+ 2e - = Co + 2OH --0.73Ni(II) -(0) Ni(OH) 2+ 2e 一= Ni + 2OH —-0.72As(V) -(III) AsO 43- + 2H 2O + 2e 一= AsO 2- + 4OH —-0.71Ag(I) -(0) Ag 2S+ 2e 一= 2Ag + S2—-0.691 As(III) -(0) AsO 2 一+ 2H 20 + 3e 一= As + 4OH —-0.68Sb(III) -(0) SbO 2- + 2H 2O + 3e - = Sb + 4OH --0.66*Re(VII) -(IV) ReO4一+ 2H 2O+ 3e 一= ReO 2 + 4OH —-0.59**Sb(V) -(III) SbO 3-+ H2O + 2e 一= SbO 2- + 2OH —-0.59Re(VII) -(0) ReO 4一+ 4H 2O+ 7e 一= Re + 8OH —-0.584**S(IV) -(II) 2SO 32- + 3H 2O + 4e - = S2O32+ 6OH --0.58Te(IV) -(0) TeO 32- + 3H 2O + 4e - = Te + 6OH --0.57Fe(III) -(II) Fe(OH) 3+ e 一= Fe(OH) 2+ OH —-0.56S(0) -( -II) S+ 2e - = S2--0.47627 Bi(III) -(0) Bi2O3 + 3H 2O + 6e 一= 2Bi + 6OH —-0.46N(III) -(II) NO 2 一+ H2O + e —= NO + 2OH —-0.46*Co(II) -C(0) [Co(NH3)6]2++ 2e —= Co + 6NH 3 -0.422 Se(IV) -(0) SeO 32- + 3H 2 O + 4e -= Se + 6OH --0.366 Cu(I) -(0) Cu2O + H2O + 2e -= 2Cu + 2OH —-0.360 Tl(I) -(0) Tl(OH) + e-= Tl + OH —-0.34 *Ag(I) -(0) [Ag(CN) 2]- + e-= Ag + 2CN —-0.31 Cu(II) -(0) Cu(OH) 2+ 2e- = Cu + 2OH —-0.222 Cr(VI) -(III) CrO 42 - + 4H 2O + 3e- = Cr(OH) 3 + 5OH —-0.13 *Cu(I) -(0) [Cu(NH 3)2] + + e- = Cu + 2NH 3 -0.12 O(0) -(-I) O2+ H2O + 2e- = HO 2- + OH —-0.076 Ag(I) -(0) AgCN + e - = Ag + CN —-0.017 N(V) -(III) NO 3 - + H2O + 2e - = NO 2 - + 2OH —0.01 Se(VI) -(IV) SeO42- + H2O + 2e -= SeO32- + 2OH -0.05 Pd(II) -(0) Pd(OH) 2+ 2e - = Pd + 2OH —0.07S(II,V) -(II) S4O62-+ 2e - = 2S2O32-0.08 Hg(II) -(0) HgO + H2O + 2e- = Hg + 2OH —0.0977 Co(III) -(II) [Co(NH 3)6]3++ e- = [Co(NH 3)6]2+0.108 Pt(II) -(0) Pt(OH) 2+ 2e -= Pt + 2OH —0.14 Co(III) -(II) Co(OH) 3+ e -= Co(OH) 2 + OH -0.17 Pb(IV) -(II) PbO 2 + H2O + 2e -= PbO + 2OH -0.247 I(V) -( -I) IO3- + 3H 2O + 6e- = l- + 6OH —0.26 Cl(V) -(III) CIO 3- + H2O + 2e - = ClO 2—+ 2OH —0.33Ag(I) -(0) Ag 2O + H2O + 2e - = 2Ag + 2OH —0.342Fe(III) -(II) [Fe(CN) 6]3一+ e一= [Fe(CN) 6]4—0.358 Cl(VII) -(V) CIO 4一+ H 20 + 2e —= ClO 3—+ 2OH —0.36*Ag(I) -(0) [Ag(NH 3)2] + + e一= Ag + 2NH 3 0.373 O(0) -(-II) 02+ 2H 20 + 4e —= 40H —0.401 I(I) -(-I) I0 一+ H20 + 2e —=厂 + 20H —0.485 *Ni(IV) -(II) NiO 2+ 2H 20 + 2e 一= Ni(0H) 2 + 20H —0.490 Mn(VII) -(VI) Mn0 4- + e —= Mn0 42-0.558 Mn(VII) -(IV) Mn0 4- + 2H 20 + 3e 一= Mn0 2 + 40H —0.595 Mn(VI) -(IV) Mn0 42一+ 2H 20 + 2e 一= Mn0 2+ 40H —0.60 Ag(II) -(I) 2Ag0 + H20 + 2e一= Ag 20 + 20H —0.607 Br(V) -(-I) Br0 3一+ 3H 20 + 6e 一= BL + 60H —0.61 Cl(V) -(-I) CI03—+ 3H 20 + 6e 一= Cl_+ 60H —0.62 Cl(III) -(I) Cl02-+H20+2e-=Cl0-+20H -0.66 I(VII) -(V) H3I062-+2e-=I03-+30H-0.7 Cl(III) -( -I) CI02-+2H20+4e-=CI-+40H-0.76 Br(I) -(-I) Br0-+H20+2e-=Br-+20H-0.761 Cl(I) -( -I) CI0-+H20+2e-=CI-+20H -0.841 *Cl(IV) -(III) CI0 2(g) +e-= CI0 2-0.95O(0) -(-II) 03+H20+2e-=02+20H - 1.24 R.Lide, Handbook of Chemistry and Physics, 8 -25 -8 -30, 78th. edition, 1997 -1998 Dean Ed,Lange ' s Handbook of Chemistry, 13th. edition, 1985 参考书.。
标准电极电势表 --酸性溶液中(298K)>>> 碱性溶液中(298K)>>>1电对方程式E /VLi(I)-(0)Li++e-=Li-3.0401 Cs(I)-(0)Cs++e-=Cs-3.026 Rb(I)-(0)Rb++e-=Rb-2.98 K(I)-(0)K++e-=K-2.931 Ba(II)-(0)Ba2++2e-=Ba-2.912 Sr(II)-(0)Sr2++2e-=Sr-2.89 Ca(II)-(0)Ca2++2e-=Ca-2.868 Na(I)-(0)Na++e-=Na-2.71 La(III)-(0)La3++3e-=La-2.379 Mg(II)-(0)Mg2++2e-=Mg-2.372 Ce(III)-(0)Ce3++3e-=Ce-2.336 H(0)-(-I)H2(g)+2e-=2H--2.23 Al(III)-(0)AlF63-+3e-=Al+6F--2.069 Th(IV)-(0)Th4++4e-=Th-1.899 Be(II)-(0)Be2++2e-=Be-1.847 U(III)-(0)U3++3e-=U-1.798 Hf(IV)-(0)HfO2++2H++4e-=Hf+H2O-1.724 Al(III)-(0)Al3++3e-=Al-1.662 Ti(II)-(0)Ti2++2e-=Ti-1.630 Zr(IV)-(0)ZrO2+4H++4e-=Zr+2H2O-1.553 Si(IV)-(0)[SiF6]2-+4e-=Si+6F--1.24 Mn(II)-(0)Mn2++2e-=Mn-1.185 Cr(II)-(0)Cr2++2e-=Cr-0.913 Ti(III)-(II)Ti3++e-=Ti2+-0.9B(III)-(0)H3BO3+3H++3e-=B+3H2O-0.8698 *Ti(IV)-(0)TiO2+4H++4e-=Ti+2H2O-0.86 Te(0)-(-II)Te+2H++2e-=H2Te-0.793 Zn(II)-(0)Zn2++2e-=Zn-0.7618 Ta(V)-(0)Ta2O5+10H++10e-=2Ta+5H2O-0.750 Cr(III)-(0)Cr3++3e-=Cr-0.744 Nb(V)-(0)Nb2O5+l0H++10e-=2Nb+5H2O-0.644 As(0)-(-III)As+3H++3e-=AsH3-0.608 U(IV)-(III)U4++e-=U3+-0.607 Ga(III)-(0)Ga3++3e-=Ga-0.549 P(I)-(0)H3PO2+H++e-=P+2H2O-0.508 P(III)-(I)H3PO3+2H++2e-=H3PO2+H2O-0.499 *C(IV)-(III)2CO2+2H++2e-=H2C2O4-0.49 Fe(II)-(0)Fe2++2e-=Fe-0.447Cr(III)-(II)Cr3++e-=Cr2+-0.407 Cd(II)-(0)Cd2++2e-=Cd-0.4030 Se(0)-(-II)Se+2H++2e-=H2Se(aq)-0.399 Pb(II)-(0)PbI2+2e-=Pb+2I--0.365 Eu(III)-(II)Eu3++e-=Eu2+-0.36 Pb(II)-(0)PbSO4+2e-=Pb+SO42--0.3588 In(III)-(0)In3++3e-=In-0.3382 Tl(I)-(0)Tl++e-=Tl-0.336 Co(II)-(0)Co2++2e-=Co-0.28P(V)-(III)H3PO4+2H++2e-=H3PO3+H2O-0.276 Pb(II)-(0)PbCl2+2e-=Pb+2Cl--0.2675 Ni (II)-(0)Ni2++2e-=Ni-0.257 V(III)-(II)V3++e-=V2+-0.255 Ge(IV)-(0)H2GeO3+4H++4e-=Ge+3H2O-0.182 Ag(I)-(0)AgI+e-=Ag+I--0.15224 Sn(II)-(0)Sn2++2e-=Sn-0.1375 Pb(II)-(0)Pb2++2e-=Pb-0.1262 *C(IV)-(II)CO2(g)+2H++2e-=CO+H2O-0.12P(0)-(-III)P(white)+3H++3e-=PH3(g)-0.063 Hg(I)-(0)Hg2I2+2e-=2Hg+2I--0.0405 Fe(III)-(0)Fe3++3e-=Fe-0.037 H(I)-(0)2H++2e-=H20.0000 Ag(I)-(0)AgBr+e-=Ag+Br-0.07133 S(II.V)-(II)S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III)TiO2++2H++e-=Ti3++H2O0.1S(0)-(-II)S+2H++2e-=H2S(aq)0.142Sn(IV)-(II)Sn4++2e-=Sn2+0.151Sb(III)-(0)Sb2O3+6H++6e-=2Sb+3H2O0.152Cu(II)-(I)Cu2++e-=Cu+0.153Bi(III)-(0)BiOCl+2H++3e-=Bi+Cl-+H2O0.1583S(VI)-(IV)SO42-+4H++2e-=H2SO3+H2O0.172Sb(III)-(0)SbO++2H++3e-=Sb+H2O0.212Ag(I)-(0)AgCl+e-=Ag+Cl-0.22233 As(III)-(0)HAsO2+3H++3e-=As+2H2O0.248Hg(I)-(0)Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl)0.26808 Bi(III)-(0)BiO++2H++3e-=Bi+H2O0.320U(VI)-(IV)UO22++4H++2e-=U4++2H2O0.327C(IV)-(III)2HCNO+2H++2e-=(CN)2+2H2O0.330V(IV)-(III)VO2++2H++e-=V3++H2O0.337Cu(II)-(0)Cu2++2e-=Cu0.3419 Re(VII)-(0)ReO4-+8H++7e-=Re+4H2O0.368Ag(I)-(0)Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0)H2SO3+4H++4e-=S+3H2O0.449Cu(I)-(0)Cu++e-=Cu0.521I(0)-(-I)I2+2e-=2I-0.5355 I(0)-(-I)I3-+2e-=3I-0.536 As(V)-(III)H3AsO4+2H++2e-=HAsO2+2H2O0.560 Sb(V)-(III)Sb2O5+6H++4e-=2SbO++3H2O0.581 Te(IV)-(0)TeO2+4H++4e-=Te+2H2O0.593 U(V)-(IV)UO2++4H++e-=U4++2H2O0.612 **Hg(II)-(I)2HgCl2+2e-=Hg2Cl2+2Cl-0.63 Pt(IV)-(II)[PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68 O(0)-(-I)O2+2H++2e-=H2O20.695 Pt(II)-(0)[PtCl4]2-+2e-=Pt+4Cl-0.755 *Se(IV)-(0)H2SeO3+4H++4e-=Se+3H2O0.74 Fe(III)-(II)Fe3++e-=Fe2+0.771 Hg(I)-(0)Hg22++2e-=2Hg0.7973 Ag(I)-(0)Ag++e-=Ag0.7996 Os(VIII)-(0)OsO4+8H++8e-=Os+4H2O0.8N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803 Hg(II)-(0)Hg2++2e-=Hg0.851 Si(IV)-(0)(quartz)SiO2+4H++4e-=Si+2H2O0.857 Cu(II)-(I)Cu2++I-+e-=CuI0.86 N(III)-(I)2HNO2+4H++4e-=H2N2O2+2H2O0.86 Hg(II)-(I)2Hg2++2e-=Hg22+0.920 N(V)-(III)NO3-+3H++2e-=HNO2+H2O0.934 Pd(II)-(0)Pd2++2e-=Pd0.951 N(V)-(II)NO3-+4H++3e-=NO+2H2O0.957 N(III)-(II)HNO2+H++e-=NO+H2O0.983 I(I)-(-I)HIO+H++2e-=I-+H2O0.987 V(V)-(IV)VO2++2H++e-=VO2++H2O0.991 V(V)-(IV)V(OH)4++2H++e-=VO2++3H2O 1.00 Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002 Te(VI)-(IV)H6TeO6+2H++2e-=TeO2+4H2O 1.02 N(IV)-(II)N2O4+4H++4e-=2NO+2H2O 1.035 N(IV)-(III)N2O4+2H++2e-=2HNO2 1.065 I(V)-(-I)IO3-+6H++6e-=I-+3H2O 1.085 Br(0)-(-I)Br2(aq)+2e-=2Br- 1.0873 Se(VI)-(IV)SeO42-+4H++2e-=H2SeO3+H2O 1.151 Cl(V)-(IV)ClO3-+2H++e-=ClO2+H2O 1.152 Pt(II)-(0)Pt2++2e-=Pt 1.18 Cl(VII)-(V)ClO4-+2H++2e-=ClO3-+H2O 1.189 I(V)-(0)2IO3-+12H++10e-=I2+6H2O 1.195 Cl(V)-(III)ClO3-+3H++2e-=HClO2+H2O 1.214 Mn(IV)-(II)MnO2+4H++2e-=Mn2++2H2O 1.224 O(0)-(-II)O2+4H++4e-=2H2O 1.229 Tl(III)-(I)T13++2e-=Tl+ 1.252 Cl(IV)-(III)ClO2+H++e-=HClO2 1.277N(III)-(I)2HNO2+4H++4e-=N2O+3H2O 1.297 **Cr(VI)-(III)Cr2O72-+14H++6e-=2Cr3++7H2O 1.33Br(I)-(-I)HBrO+H++2e-=Br-+H2O 1.331 Cr(VI)-(III)HCrO4-+7H++3e-=Cr3++4H2O 1.350 Cl(0)-(-I)Cl2(g)+2e-=2Cl- 1.35827 Cl(VII)-(-I)ClO4-+8H++8e-=Cl-+4H2O 1.389 Cl(VII)-(0)ClO4-+8H++7e-=1/2Cl2+4H2O 1.39 Au(III)-(I)Au3++2e-=Au+ 1.401 Br(V)-(-I)BrO3-+6H++6e-=Br-+3H2O 1.423 I(I)-(0)2HIO+2H++2e-=I2+2H2O 1.439 Cl(V)-(-I)ClO3-+6H++6e-=Cl-+3H2O 1.451 Pb(IV)-(II)PbO2+4H++2e-=Pb2++2H2O 1.455 Cl(V)-(0)ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I)HClO+H++2e-=Cl-+H2O 1.482 Br(V)-(0)BrO3-+6H++5e-=l/2Br2+3H2O 1.482 Au(III)-(0)Au3++3e-=Au 1.498 Mn(VII)-(II)MnO4-+8H++5e-=Mn2++4H2O 1.507 Mn(III)-(II)Mn3++e-=Mn2+ 1.5415 Cl(III)-(-I)HClO2+3H++4e-=Cl-+2H2O 1.570 Br(I)-(0)HBrO+H++e-=l/2Br2(aq)+H2O 1.574 N(II)-(I)2NO+2H++2e-=N2O+H2O 1.591 I(VII)-(V)H5IO6+H++2e-=IO3-+3H2O 1.601 Cl(I)-(0)HClO+H++e-=1/2Cl2+H2O 1.611 Cl(III)-(I)HClO2+2H++2e-=HClO+H2O 1.645 Ni(IV)-(II)NiO2+4H++2e-=Ni2++2H2O 1.678 Mn(VII)-(IV)MnO4-+4H++3e-=MnO2+2H2O 1.679 Pb(IV)-(II)PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913 Au(I)-(0)Au++e-=Au 1.692 Ce(IV)-(III)Ce4++e-=Ce3+ 1.72N(I)-(0)N2O+2H++2e-=N2+H2O 1.766 O(-I)-(-II)H2O2+2H++2e-=2H2O 1.776 Co(III)-(II)Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83 Ag(II)-(I)Ag2++e-=Ag+ 1.980 S(VII)-(VI) S2O82-+2e-=2SO42- 2.010 O(0)-(-II)O3+2H++2e-=O2+H2O 2.076 O(II)-(-II)F2O+2H++4e-=H2O+2F- 2.153 Fe(VI)-(III)FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II)O(g)+2H++2e-=H2O 2.421 F(0)-(-I)F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532电对方程式E /V Ca(II)-(0)Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0)Ba(OH)2+2e-=Ba+2OH--2.99 La(III)-(0)La(OH)3+3e-=La+3OH--2.90 Sr(II)-(0)Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O-2.88 Mg(II)-(0)Mg(OH)2+2e-=Mg+2OH--2.690 Be(II)-(0)Be2O32-+3H2O+4e-=2Be+6OH--2.63 Hf(IV)-(0)HfO(OH)2+H2O+4e-=Hf+4OH--2.50 Zr(IV)-(0)H2ZrO3+H2O+4e-=Zr+4OH--2.36 Al(III)-(0)H2AlO3-+H2O+3e-=Al+OH--2.33 P(I)-(0)H2PO2-+e-=P+2OH--1.82 B(III)-(0)H2BO3-+H2O+3e-=B+4OH--1.79 P(III)-(0)HPO32-+2H2O+3e-=P+5OH--1.71 Si(IV)-(0)SiO32-+3H2O+4e-=Si+6OH--1.697 P(III)-(I)HPO32-+2H2O+2e-=H2PO2-+3OH--1.65 Mn(II)-(0)Mn(OH)2+2e-=Mn+2OH--1.56 Cr(III)-(0)Cr(OH)3+3e-=Cr+3OH--1.48 *Zn(II)-(0)[Zn(CN)4]2-+2e-=Zn+4CN--1.26 Zn(II)-(0)Zn(OH)2+2e-=Zn+2OH--1.249 Ga(III)-(0)H2GaO3-+H2O+2e-=Ga+4OH--1.219 Zn(II)-(0)ZnO22-+2H2O+2e-=Zn+4OH--1.215 Cr(III)-(0)CrO2-+2H2O+3e-=Cr+4OH--1.2 Te(0)-(-I)Te+2e-=Te2--1.143 P(V)-(III)PO43-+2H2O+2e-=HPO32-+3OH--1.05 *Zn(II)-(0)[Zn(NH3)4]2++2e-=Zn+4NH3-1.04 *W(VI)-(0)WO42-+4H2O+6e-=W+8OH--1.01 *Ge(IV)-(0)HGeO3-+2H2O+4e-=Ge+5OH--1.0 Sn(IV)-(II)[Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93 S(VI)-(IV)SO42-+H2O+2e-=SO32-+2OH--0.93 Se(0)-(-II)Se+2e-=Se2--0.924 Sn(II)-(0)HSnO2-+H2O+2e-=Sn+3OH--0.909 P(0)-(-III)P+3H2O+3e-=PH3(g)+3OH--0.87 N(V)-(IV)2NO3-+2H2O+2e-=N2O4+4OH--0.85 H(I)-(0)2H2O+2e-=H2+2OH--0.8277 Cd(II)-(0)Cd(OH)2+2e-=Cd(Hg)+2OH--0.809 Co(II)-(0)Co(OH)2+2e-=Co+2OH--0.73 Ni(II)-(0)Ni(OH)2+2e-=Ni+2OH--0.72 As(V)-(III)AsO43-+2H2O+2e-=AsO2-+4OH--0.71 Ag(I)-(0)Ag2S+2e-=2Ag+S2--0.691 As(III)-(0)AsO2-+2H2O+3e-=As+4OH--0.68 Sb(III)-(0)SbO2-+2H2O+3e-=Sb+4OH--0.66 *Re(VII)-(IV)ReO4-+2H2O+3e-=ReO2+4OH--0.59 *Sb(V)-(III)SbO3-+H2O+2e-=SbO2-+2OH--0.59 Re(VII)-(0)ReO4-+4H2O+7e-=Re+8OH--0.584 *S(IV)-(II)2SO32-+3H2O+4e-=S2O32-+6OH--0.58 Te(IV)-(0)TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II)Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II)S+2e-=S2--0.47627 Bi(III)-(0)Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II)NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0)[Co(NH3)6]2++2e-=Co+6NH3-0.422 Se(IV)-(0)SeO32-+3H2O+4e-=Se+6OH--0.366 Cu(I)-(0)Cu2O+H2O+2e-=2Cu+2OH--0.360 Tl(I)-(0)Tl(OH)+e-=Tl+OH--0.34*Ag(I)-(0)[Ag(CN)2]-+e-=Ag+2CN--0.31 Cu(II)-(0)Cu(OH)2+2e-=Cu+2OH--0.222 Cr(VI)-(III)CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13*Cu(I)-(0)[Cu(NH3)2]++e-=Cu+2NH3-0.12O(0)-(-I)O2+H2O+2e-=HO2-+OH--0.076 Ag(I)-(0)AgCN+e-=Ag+CN--0.017 N(V)-(III)NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV)SeO42-+H2O+2e-=SeO32-+2OH-0.05Pd(II)-(0)Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II)S4O62-+2e-=2S2O32-0.08Hg(II)-(0)HgO+H2O+2e-=Hg+2OH-0.0977 Co(III)-(II)[Co(NH3)6]3++e-=[Co(NH3)6]2+0.108Pt(II)-(0)Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II)Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II)PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I)IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III)ClO3-+H2O+2e-=ClO2-+2OH-0.33Ag(I)-(0)Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II)[Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358Cl(VII)-(V)ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0)[Ag(NH3)2]++e-=Ag+2NH30.373O(0)-(-II)O2+2H2O+4e-=4OH-0.401I(I)-(-I)IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II)NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490Mn(VII)-(VI)MnO4-+e-=MnO42-0.558Mn(VII)-(IV)MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV)MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I)2AgO+H2O+2e-=Ag2O+2OH-0.607Br(V)-(-I)BrO3-+3H2O+6e-=Br-+6OH-0.61Cl(V)-(-I)ClO3-+3H2O+6e-=Cl-+6OH-0.62Cl(III)-(I)ClO2-+H2O+2e-=ClO-+2OH-0.66I(VII)-(V)H3IO62-+2e-=IO3-+3OH-0.7Cl(III)-(-I)ClO2-+2H2O+4e-=Cl-+4OH-0.76Br(I)-(-I)BrO-+H2O+2e-=Br-+2OH-0.761Cl(I)-(-I)ClO-+H2O+2e-=Cl-+2OH-0.841*Cl(IV)-(III)ClO2(g)+e-=ClO2-0.95。