倍他米松378-44-9
- 格式:pdf
- 大小:181.13 KB
- 文档页数:4

【药品名】倍他米松/二丙酸倍他米松【英文名】Betamethasone/Betamethasone Dipropionate【别名】得宝松;培地米松;培地美松;倍氟美松;倍他美松;β-美松;Betadexamethasone;Betnesol;Betnovate;Celestone;Flubenisolone【剂型】1.倍他米松片:0.5mg;2.倍他米松软膏:4mg/4g,10mg/10g;3.倍他米松醋酸酯注射液:1.5mg/ml;4.倍他米松磷酸钠注射液:5.26mg/ml(相当于倍他米松4mg);5.得宝松注射液(Diprospan):1ml(二丙酸倍他米松5mg,倍他米松磷酸二钠2mg)。
【药理作用】倍他米松为地塞米松的差向异构体,后者C位甲基为α位,倍他米松则为β位。
两者糖皮质激素和盐皮质激素活性相似,0.75mg倍他米松的抗炎活性约相当于5mg泼尼松龙。
【药动学】本品为地塞米松同分异构体,半衰期大于300min。
【适应症】同地塞米松。
较多用于治疗活动性风湿病、类风湿性关节炎、红斑狼疮、严重支气管哮喘、急性白血病及眼、耳、鼻过敏性和炎性疾病。
【禁忌症】禁忌证同地塞米松。
【注意事项】【不良反应】长期大剂量应用皮质激素可出现多种不良反应,可引起电解质平衡紊乱、钠水潴留、水肿、高血压、低血钾症,人工合成的衍生物则较少发生。
其他尚可引起骨质疏松、自发性骨折、糖尿、伤口愈合迟缓、易诱发感染或使原有疾病复发或恶化、儿童生长迟缓。
妊娠期大量使用可引起胎儿或新生儿肾上腺抑制。
也引起库欣综合征,如满月脸、多毛、水牛背、皮下淤血、皮肤萎缩和变薄、痤疮等。
皮肤局部大面积应用,可因吸收量大而引起内源性肾上腺皮质激素合成受抑制的全身性不良影响。
眼局部应用,大量可引起角膜溃疡、眼内压增高、视觉功能降低。
药理剂量皮质激素可反馈性抑制垂体前叶A C TH分泌,引起肾上腺皮质萎缩。
突然撤药或降低剂量或遇感染、意外、外科创伤刺激等皮质激素需要量增加时,可突发急性肾上腺皮质功能减退,症状表现为不适、肌肉软弱、关节疼痛、呼吸困难、畏食、恶心、呕吐、发热、低血糖、低血压及脱水等,严重者可引起死亡,因此不宜突然撤药,应逐步递减剂量,缓慢撤药。


克霉唑倍他米松乳膏Kemeizuo Beitamisong RugaoClotrimazole and Betamethasone Dipropionate Cream本品含克霉唑(C22H17ClN2)与二丙酸倍他米松以倍他米松(C22H29FO5)计算,均应为标示量的90.0%~110.0%。
【处方】克霉唑 10g二丙酸倍他米松 0.643g(相当于倍他米松0.5g)基质适量全量 1000g【性状】本品为白色乳膏。
【鉴别】在含量测定项下记录的色谱图中,供试品溶液两主峰的保留时间应与对照品溶液中相应的两主峰的保留时间一致。
【检查】二苯基-(2-氯苯基)甲醇取本品适量(相当于克霉唑10mg),精密称定,置50ml量瓶中,加甲醇28ml,置50℃水浴中加热,时时振摇使溶解,然后取出强烈振摇5分钟,加水12ml,摇匀,迅速冷至室温,用水-甲醇(3:7)稀释至刻度,摇匀,用0.45μm滤膜滤过,取续滤液作为供试品溶液;另取二苯基-(2-氯苯基)甲醇对照品,精密称定,用水-甲醇(3:7)稀释制成每1ml中含4μg的溶液,作为对照品溶液。
除检测波长为215nm外,照含量测定项下的色谱条件,精密量取对照品溶液与供试品溶液各10μl,分别注入液相色谱仪,记录色谱图至供试品溶液主成分峰保留时间的1.5倍,供试品溶液色谱图中如有与二苯基-(2-氯苯基)甲醇对照品溶液主峰保留时间相应的色谱峰,按外标法以峰面积计算,二苯基-(2-氯苯基)甲醇不得大于克霉唑标示量的2.0%。
其他应符合乳膏剂项下有关的各项规定(附录ⅠF)。
【含量测定】照高效液相色谱法(附录V D)测定。
色谱条件与系统适用性试验用十八烷基硅烷键合硅胶为填充剂;以0.05mol/L磷酸二氢钾溶液-甲醇(30:70,用10%磷酸调节pH值至5.7~5.8)为流动相;检测波长为240nm。
理论板数按克霉唑峰计算不低于4000,克霉唑峰、二丙酸倍他米松峰、二苯基-(2-氯苯基)甲醇峰和其它杂质峰之间的分离度均应符合要求。

0000
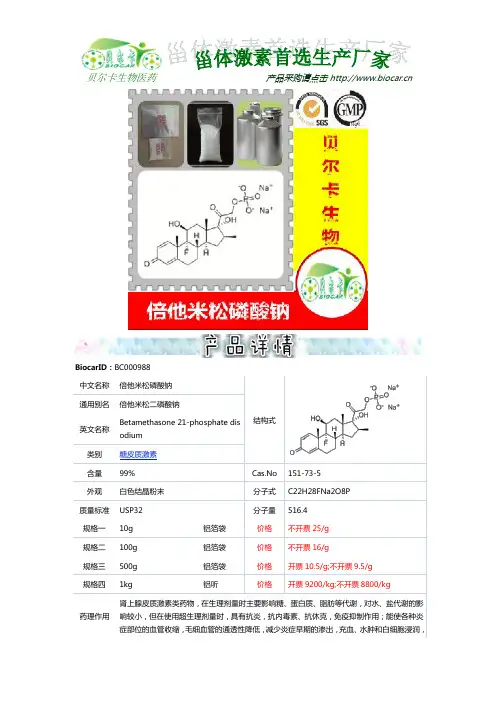
倍他米松磷酸钠详细信息0000
本文详细介绍了倍他米松磷酸钠的产品信息,包括中英文名称、别名、cas号、分子结构等基本信息,以及产品的物化性质、产品用途、产品上下游产品等综合信息,为广大化学品研究、化工产品生产制造从业者提供专业的产品信息。
本文所有信息来源化工字典。
00000
产品介绍:0000
中文名称:倍他米松磷酸钠00000
中文别名: 16beta-甲基-11beta,17alpha,21-三羟基-9alpha-氟-孕甾-1,4-二烯-3,20-二酮-21-磷酸二钠盐;倍他米松二磷酸钠;21-磷酸钠倍他米松0000
英文名称:betamethasone21-phosphatesodium0000
英文别名:0000
CAS:151-73-50000
分子结构式:0000
EINECS:205-797-00000
分子式:C22H28FNa2O8P0000
分子量:516.40460000
风险术语:R22:;00000
安全术语:S36:;00000
物化性质:0000
熔点:0000
相对密度:0000
溶解性:0000
用途:激素类药,具有抗炎作用0000
上游原料:0000
下游产品:0000
参考资料0000
倍他米松磷酸钠 /detail-倍他米松磷酸钠.html0000 0000。

倍他米松(β-类松)【药理与适用症】: 倍他米松为地塞米松的同分异构体,作用与用途同醋酸地塞米松,其钠、水潴留作用及剂量都比后者为小。
【注意事项】: 同醋酸地塞米松。
【用法与用量】: 口服:每日1.5―2mg,分3―4次,维持量每日0.5―1mg;小儿:每日O.06―0.16mg/kg。
【分子式】: C22H29O5F【性状】: 倍他米松为白色或乳白色结晶性粉末;无臭,味苦。
几乎不溶于水,略溶于乙醇,极微溶于氯仿。
【副作用与毒性】: 同醋酸地塞米松,但有时可引起轻度厌食及体重减轻。
【贮藏】: 避光,密闭保存。
【包装】: 片剂:每片0.25mg、0.5mg。
倍他米松磷酸钠注射液Injectiobe―tamethasoniPhospgatisNatrici:为无色澄明的灭菌水溶液,pH8―9,适用于急救。
对急性肾上腺功能不全者,肌注或静注,1日10―80mg(按倍他米松计算,倍他米松1.3mg相当倍他米松lmg),一般初量4―12mg,关节腔注射,1次0.2―1ml。
每支1ml含倍他米松4mg。
倍他米松水泥悬注射液InjectioBe。
tamethasoniAquosuspensa:为灭菌混悬液,pH6.8―7.2。
每支5ml含倍他米松磷酸钠15mg和醋酸倍他米松15mg。
肌注:每次1ml,每3―7日1次。
关节腔注射:每次0.25-2 ml。
倍他米松磷酸钠滴剂GuttaeBetahletha―soniPhosphatisNatrici:0.1%溶液,用于治疗眼、耳、鼻、皮肤的过敏性类症。

倍他米松乳膏(利康达宁)的说明书皮肤病的传染性是很强的,对于自身的健康也不利,许多皮肤病是由于过敏引起的,经常洗澡也不一定能保证皮肤健康。
因此皮肤病的药物就至关重要了,倍他米松乳膏(利康达宁)就是目前治疗皮肤病最好的药物,它的功效有哪些您知道吗?它的具体用药原则和效果我们在如下内容为您介绍。
【药品名称】通用名称:倍他米松乳膏商品名称:倍他米松乳膏(利康达宁)拼音全码:BeiTaMiSongRuGao(LiKangDaNing)【主要成份】复方制剂,其组分为每克含倍他米松戊酸酯以倍他米松计为1毫克及硫酸新霉素3500单位。
化学名:16β-甲基-11β,17α,21-三羟基-9α-孕甾-1,4-二烯-3,20-二酮分子式:C22H29FO5分子量:392.461【性状】本品为乳剂型基质的白色软膏。
【适应症/功能主治】用于过敏性皮炎、湿疹、神经性皮炎、脂溢性皮炎及瘙痒症等。
【规格型号】15g【用法用量】外用:一日2-4次,涂于患处,并轻揉片刻。
【不良反应】长期使用可引起局部皮肤萎缩,毛细血管扩张、色素沉着、毛囊炎、口周皮炎以及继发感染。
【禁忌】1.禁用于感染性皮肤病,如脓疱病、体癣、股癣等。
2.对本品过敏者禁用。
【注意事项】1.不宜长期使用,并避免全身大面积使用。
2.用药一周后症状未缓解,应向医师咨询。
3.涂布部位如有灼烧感、瘙痒、红肿等,应停止用药,洗净。
必要时向医师咨询。
4.当药品性状发生改变时,禁止使用。
【儿童用药】儿童对本品敏感,故忌用。
【老年患者用药】老年患者慎用。
【孕妇及哺乳期妇女用药】孕妇使用本品的安全性尚未确立,故不能长期大量用于孕妇。
哺乳期妇女慎用。
【药物相互作用】尚不明确。
【药物过量】症状:长期外用皮质激素可抑制垂体-肾上腺功能继发性肾上腺功能减退。
外用后新霉素的过度吸收有潜在的耳毒性,还可能发生急性肾功能衰竭。

【药品名称】通用名称:倍他米松磷酸钠注射液英文名称:Betamethasone Sodium Phosphate Injection 汉语拼音:Beitamisong LinsuannaZhusheye【成份】本品主要成份为:倍他米松磷酸钠。
其化学名称为:16β-甲基-11β,17α,21-三羟基-9α氟-孕甾-1,4-二烯-3,20-二酮-21-磷酸二钠盐。
化学结构式:分子式:C22H28FNa2O8P 分子量:516.41 辅料为:亚硫酸氢钠、5%氢氧化钠溶液、丙二醇、注射用水。
【性状】本品为无色的澄明液体。
【适应症】主要用于过敏性与自身免疫性炎症性疾病。
现多用于活动性风湿病、类风湿性关节炎、红斑狼疮、严重支气管哮喘、严重皮炎、急性白血病等,也用于某些感染的综合治疗。
【规格】1ml:5.26mg(相当于倍他米松4mg) 【用法用量】肌注或静脉注射:一日2~20mg(0.38~3.8支),分次给药。
【不良反应】糖皮质激素在应用生理剂量替代治疗时无明显不良反应,不良反应多发生在应用药理剂量时,而且与疗程、剂量、用药种类、用法及给药途径等有密切关系。
常见不良反应有以下几类。
(1)长程使用可引起以下副作用。
医源性库欣综合征面容和体态、体重增加、下肢浮肿、紫纹、易出血倾向、创口愈合不良、痤疮、月经紊乱、肱或股骨头缺血性坏死、骨质疏松及骨折(包括脊椎压缩性骨折、长骨病理性骨折)、肌无力、肌萎缩、低血钾综合征、胃肠道刺激(恶心、呕吐)、胰腺炎、消化性溃疡或穿孔,儿童生长受到抑制、青光眼、白内障、良性颅内压升高综合征、糖耐量减退和糖尿病加重。
(2)患者可出现精神症状:欣快感、激动、谵妄、不安、定向力障碍,也可表现为抑制。
精神症状由易发生与患慢性消耗性疾病的人及以往有过精神不正常者。
(3)并发感染为肾上腺皮质激素的主要不良反应。
以真菌、结核菌、葡萄球菌、变形杆菌、铜绿假单胞菌和各种疱疹病毒为主。
(4)糖皮质激素停药综合征。

·病例报告·复方倍他米松注射液致过敏性休克1例艾菁1,2, 肖阳娜1, 袁立燕1, 王天晶1, 谷梅1, 刘红芳1(1.广东省皮肤病医院,广东广州 510091;2.广东医学院,广东湛江 524023)[摘要] 报告1例肌肉注射复方倍他米松注射液致过敏性休克的病例。
患者男,26岁,因“全身红斑、脱屑伴痒2月余”入院,初步诊断:1.药疹?2.成人特应性皮炎?予复方倍他米松注射液抗过敏治疗,5分钟后患者出现头晕、烦躁、血压下降等过敏性休克的表现,予常规抗过敏性休克的急救措施治疗后,患者休克得以纠正。
医护人员应注意患者个体因素的复杂性,对于高度敏感或有药物过敏史的患者,在诊治中应提高警惕,防患于未然。
[关键词] 复方倍他米松; 过敏性休克[中图分类号] R593.1 [文献标识码] BDOI:10.3969/j.issn.1674-8468.2015.05.007复方倍他米松注射液是一种倍他米松酯类的复方制剂,为强效糖皮质激素,具有强效抗炎、抗过敏、抗风湿作用,因其疗效显著,故临床应用范围不断扩大。
该药不良反应报道极少,引起过敏性休克更是罕见,现将我院1例因肌肉注射复方倍他米松注射液后发生过敏性休克病例报告如下。
1 病例资料患者男,26岁,因“全身红斑、脱屑伴痒2月余”入院。
患者3月余前因患“精神分裂症”,口服“阿普唑仑片、派罗匹隆片、曲唑酮片、氯氮平片、苯海索片”等药物治疗1个月后,全身开始出现红斑、脱屑伴瘙痒,并逐渐加重,入我院后初步诊断为:1.药疹?2.成人特应性皮炎?辅助检查:血常规示全血单核细胞绝对值0.65↑×109/L、嗜酸细胞绝对值2.61↑×109/L、嗜碱细胞绝对值0.07↑×109/L;血清IgE定量2363.0↑IU/mL;余无明显异常。
入院后即予常规止痒、抗过敏治疗,患者症状缓解不明显,采用文献经验[1],予复方倍他米松注射液(批准文号:国药准字J20080062,上海先灵葆雅制药有限公司生产)7mg抗过敏治疗,肌肉注射5分钟后患者出现头晕、心悸、情绪烦躁等症状,查体:患者意识尚清,情绪烦躁,心率136次/分,血压69/29mmHg,呼吸19次/分,血氧饱和度85%。
东康源糖皮质激素类原料药简介1.醋酸地塞米松醋酸地塞米松又叫做醋酸氟甲强的松龙,国际上叫做Dexamethasone-17-acetate,属于糖皮质激素类原料药,具有抗炎、抗过敏作用,适用于类风湿性关节炎和其他胶原性疾病等,可用于生化研究。
醋酸地塞米松的CAS登入号是1177-87-3,分子式是C24H31FO6,分子量为434.5。
外表为白色或类白色结晶性粉末,无臭,味微苦。
醋酸地塞米松在丙酮中易溶,在甲醇或乙醇中溶解,在乙醇或氯仿中略溶,在乙mi中极微溶解,在水中不溶。
熔点215-227℃。
2.泼尼松龙磷酸钠泼尼松龙磷酸钠又叫做氢化泼尼松磷酸钠,国际上叫做Prednisolone phosphate sodium,属于糖皮质激素类原料药,具有抗炎、抗过敏作用,可用于生化研究。
泼尼松龙磷酸钠的CAS登入号是125-02-0,分子式是C21H27Na2O8P,分子量为484.39。
外表为白色或类白色结晶性粉末,无臭。
3.地塞米松磷酸钠地塞米松磷酸钠又叫做21-磷酸地塞米松,国际上叫做Dexamethasone 21-phosphate disodium salt,属于糖皮质激素类原料药,具有抗炎、抗过敏、抗毒素、抗休克四大作用,可用于生化研究。
地塞米松磷酸钠的CAS登入号是2392-39-4,分子式是C22H28FNa2O8P,分子量为516.4。
外表为白色或类白色结晶性粉末,无臭。
4.醋酸曲安奈德醋酸曲安奈德又叫做曲安奈德醋酸酯,国际上叫做Triamcinolone acetonide 21-acetate,属于糖皮质激素类原料药,为曲安西龙(去炎松)的衍生物,作用与曲安西龙相似,具有抗炎、抗过敏、抗瘙痒和收缩毛细血管的作用,其水钠潴留作用微弱,而抗炎、抗过敏作用均比氢化可的松(10~30倍)、泼尼松强而持久,气雾吸入治疗支气管哮喘,作用强且持久。
对局部作用疗效较曲安西龙好。
醋酸曲安奈德的CAS登入号是3870-07-3,分子式是C26H33FO7,分子量为476.5344232。

倍他米松片说明书兽用处方药【兽药名称】通用名称:倍他米松片商品名称:英文名称:Betamethasone Tablets汉语拼音:Beitamisong Pian【主要成分】倍他米松【性状】本品为白色片。
【药理作用】本品具有抗炎、抗过敏、抗毒素、抗休克作用,但其抗炎作用与糖原异生作用较地塞米松强,为氢化可的松的30 倍,钠潴留作用稍弱于地塞米松。
本品内服易吸收,在体内广泛分布。
【药物相互作用】(1)苯巴比妥等肝药酶诱导剂可促进本品的代谢,使药效降低。
(2)可使水杨酸盐的消除加快、疗效降低。
(3)噻嗪类利尿药能促进钾排泄,与本品合用时可导致低血钾,应注意补钾。
【作用与用途】糖皮质激素类药。
有抗炎、抗过敏和影响糖代谢等作用。
主要用于炎症性、过敏性疾病等的治疗。
【用法与用量】内服:一次量,犬、猫0.5~2 片。
【不良反应】(1)有较强的水、钠潴留和排钾作用。
(2)有较强的免疫抑制作用。
(3)妊娠后期大剂量使用可引起流产。
(4)大剂量或长期使用易引起肾上腺皮质功能衰退。
【注意事项】(1)严重肝功能不良、骨软症、骨折治疗期、创伤修复期、疫苗接种期动物禁用。
(2)妊娠早期及后期禁用。
(3)严格掌握适应证,防止滥用。
(4)对细菌性感染应与抗菌药合用。
(5)长期用药不能突然停药,应逐渐减量,直至停药。
【休药期】无需制定。
【规格】0.5mg【包装】【马苗苗APP 宠物行业商家一站式采购平台】【贮藏】遮光,密封保存。
【有效期】【批准文号】【生产企业】。
注射用帕尼培南倍他米隆说明书注射用帕尼培南倍他米隆是一个复方制剂,在临床上的应用广泛,常用于一些细菌引起的感染症。
下面是店铺整理的注射用帕尼培南倍他米隆说明书,欢迎阅读。
注射用帕尼培南倍他米隆商品介绍通用名:注射用帕尼培南倍他米隆生产厂家: 日本三共株式会社平冢工厂批准文号:注册证号H20020329药品规格:500mg/500mg(冻干粉)药品价格:¥205元注射用帕尼培南倍他米隆说明书注射用帕尼培南倍他米隆商品名称克倍宁,主要成分为复方制剂,其组份为帕尼培南和倍他米隆。
帕尼培南的化学名称:(5R,6S)-6-[(1R)-1-羟乙基]-3-[(3S)-1-(1-亚胺基乙基)吡咯烷-3-基硫烷基]-7-氧代-1-氮杂双环[3.2.0]庚-2-烯-2-羧酸,分子式为C15H21N3O4S,分子量:39.41。
倍他米隆的化学名称:3-苯甲酰氨丙酸,分子式为C10H11NO3,分子量:193.20。
辅料:氯化钠、氢氧化钠、碳酸氢钠、盐酸。
本品上层为淡黄色至黄褐色冻干之块状物或粉末;下层为白色冻干之块状物或粉末,有引湿性。
药代动力学血药浓度:肾功能正常的健康成年人志愿者和儿童单次静脉滴注后,帕尼培南血药浓度随时间而变化。
血药峰浓度及血药浓度-时间曲线下面积随剂量成比例增加。
消除半衰期与给药剂量无关,健康成人志愿者帕尼培南的半衰期约为70分钟,倍他米隆约为40分钟。
儿童帕尼培南的半衰期约为60分钟,倍他米隆约为30分钟。
分布:给药后本品分布于痰液、前列腺、胆汁、子宫/卵巢/输卵管、骨盆腔液、前房水、皮肤、中耳/上颌窦粘膜/扁桃体组织、口腔组织、唾液、脑脊液等各种组织和体液中。
代谢和排泄:无论何种给药途径帕尼培南均主要经肾脏排泄。
5名健康成年受试者静脉滴注本品500mg(效价)/500mg滴注时间60分钟,0-24小时中尿液中帕尼培南的回收率约为30%,β-内酰胺环开环的代谢物回收率约为50%。
肾功能损害时的药动学情况:各种肾功能障碍的患者使用本品500mg(效价)/500mg,静脉滴注时间60分钟,肾功能障碍越严重,帕尼培南在体内的滞留时间越长,半衰期越长,尿液中的排泄过程延迟。
复方倍他米松针肌注及口服强的松片治疗亚急性甲状腺炎疗效比较魏春荣摘要】:目的观察分析复方倍他米松针肌注及口服强的松片治疗亚急性甲状腺炎的临床疗效。
方法选择我院2015年8月-2017年9月收治的亚急性甲状腺炎患者92例,随机分为对照组和观察组,每组46例。
对照组患者口服强的松片进行治疗,观察组患者肌肉注射复方倍他米松针进行治疗。
比较两组患者的临床症状体征消失时间以及治疗前后的血沉水平。
结果与对照组比较,观察组患者的退热时间、甲状腺疼痛消失时间以及甲状腺肿大消失时间均明显降低,差异有统计学意义(P<0.05)。
与治疗前比较,两组患者治疗后1周、4周、8周的血沉水平均明显降低,差异有统计学意义(P<0.05)。
观察组患者治疗后1周、4周、8周的血沉水平均明显低于对照组相同时间点的水平,差异有统计学意义(P<0.05)。
结论复方倍他米松针臀部肌肉注射可以快速的改善急性甲状腺炎患者的临床症状和体征,降低机体炎症反应,值得进行临床的推广。
【关键词】:甲状腺炎;免疫调节;复发倍他米松;强的松;临床疗效亚急性甲状腺炎是自愈性的自限性疾病,一种自身免疫性甲状腺疾病,又称肉芽肿性甲状腺炎。
其病因尚未完全明确,多认为由病毒感染所致,轻症患者经适当休息、对症治疗及非甾体抗炎药治疗可缓解;如甲状腺局部或全身症状严重,则需糖皮质激素治疗。
糖皮质激素是治疗亚急性甲状腺炎的主要方法闻,口服糖皮质激素有疗程长、用量大、复发率高等缺点,会出现向心性肥胖、多血质、紫纹、痤疮、多毛、高血压、继发性糖尿病、肌萎缩、骨质疏松、性功能障碍、电解质和酸碱失衡等皮质醇增多症表现,因此目前倾向于对炎症具有导向性、局部药物浓度高且疗效更强的长效激素注射免疫调节治疗。
本研究对比分析复方倍他米松针剂臀部注射及口服强的松片治疗亚急性甲状腺炎的疗效。
现报道如下。
1 资料与方法1.1一般资料选择我院2015年8月-2017年9月收治的亚急性甲状腺炎患者92例,随机分为对照组和观察组,每组46例。
第一部分化学品及企业标识
化学品中文名:倍他米松磷酸钠
化学品英文名:Betamethasone 21-phosphate disodium
CAS No.:151-73-5
分子式:C22H28FNa2O8P
产品推荐及限制用途:主要用于过敏性和自身免疫性疾病,现多用于活动性风湿病、类风湿性关节炎、红斑狼疮、严重支气管炎、严重皮炎、急Chemicalbook性白血病等。
也用于某些危重感染的综合治疗。
局部外用治疗过敏性皮炎、湿疹、神经性皮炎、脂溢性皮炎及瘙痒症等。
第二部分危险性概述
紧急情况概述
吞咽有害。
GHS危险性类别
急性经口毒性类别 4
标签要素:
象形图:
警示词:警告
危险性说明:
H302 吞咽有害
防范说明
●预防措施:
—— P264 作业后彻底清洗。
—— P270 使用本产品时不要进食、饮水或吸烟。
●事故响应:
—— P301+P312 如误吞咽:如感觉不适,呼叫解毒中心/医生。
—— P330 漱口。
●安全储存:
—— P403+P233 存放在通风良好的地方。
保持容器密闭。
—— P405 存放处须加锁。
●废弃处置:
—— P501 按当地法规处置内装物/容器。
物理和化学危险:无资料。
355 JP XV O‹cial Monographs/BetamethasoneSystem performance:When the procedure is run with20m L of the standard solution under the above operating condi-tions,the number of theoretical plates and the symmetry fac-tor of the peak of betahistine are not less than2000and not more than1.5,respectively.System repeatability:When the test is repeated6times with 20m L of the standard solution under the above operating conditions,the relative standard deviation of the peak area of betahistine is not more than2.0z.Assay Weigh accurately the mass of not less than20Beta-histine Mesilate Tablets,and powder.Weigh accurately a portion of the powder,equivalent to about20mg of beta-histine mesilate(C8H12N2.2CH4O3S),add40mL of0.1 mol/L hydrochloric acid TS,agitate for10minutes with the aid of ultrasonic waves,and add0.1mol/L hydrochloric acid TS to make exactly50mL.Centrifuge,and use the super-natant liquid as the sample solution.Separately,weigh ac-curately about0.1g of betahistine mesilate for assay,previ-ously dried under reduced pressure with phosphorous(V) oxide at709C for24hours,and dissolve in0.1mol/L hydrochloric acid TS to make exactly50mL.Pipet10mL of this solution,add0.1mol/L hydrochloric acid TS to make exactly50mL,and use this solution as the standard solution. Perform the test with exactly5m L each of the sample solu-tion and standard solution as directed under Liquid Chro-matography<2.01>,according to the following conditions, and determine the peak areas,A T and A S,of betahistine.Amount(mg)of betahistine mesilate(C8H12N2.2CH4O3S)=W S×(A T/A S)×(1/5)W S:Amount(mg)of betahistine mesilate for assayOperating conditions—Detector:An ultraviolet absorption photometer (wavelength:261nm).Column:A stainless steel column 4.6mm in inside di-ameter and15cm in length,packed with octadecylsilanized silica gel for liquid chromatography(5m m in particle di-ameter).Column temperature:A constant temperature of about 359C.Mobile phase:To5mL of diethylamine and20mL of acet-ic acid(100)add water to make1000mL.In630mL of this solution dissolve2.3g of sodium lauryl sulfate,and add370 mL of acetonitrile.Flow rate:Adjust the‰ow rate so that the retention time of betahistine is about5minutes.System suitability—System performance:When the procedure is run with5m L of the standard solution under the above operating condi-tions,the number of theoretical plates and the symmetry fac-tor of the peak of betahistine are not less than2000and not more than1.5,respectively.System repeatability:When the test is repeated6times with 5m L of the standard solution under the above operating con-ditions,the relative standard deviation of the peak area of be-tahistine is not more than1.0z.Containers and storage Containers—Tight containers.BetamethasoneベタメタゾンC22H29FO5:392.469-Fluoro-11b,17,21-trihydroxy-16b-methylpregna-1,4-diene-3,20-dione[378-44-9]Betamethasone,when dried,contains not less than 96.0z and not more than103.0z of C22H29FO5. Description Betamethasone occurs as a white to pale yel-lowish white,crystalline powder.It is sparingly soluble in methanol,in ethanol(95)and in acetone,and practically insoluble in water.Melting point:about2409C(with decomposition).Identiˆcation(1)Proceed10mg of Betamethasone as directed under Oxygen Flask Combustion Method<1.06>,us-ing a mixture of0.5mL of0.01mol/L sodium hydroxide TS and20mL of water as an absorbing liquid,and prepare the test solution:the test solution so obtained responds to the Qualitative Tests<1.09>for‰uoride.(2)Dissolve 1.0mg of Betamethasone in10mL of ethanol(95).Mix 2.0mL of the solution with10mL of phenylhydrazinium hydrochloride TS,heat in a water bath at 609C for20minutes,and cool the solution.Determine the absorption spectrum of the solution as directed under Ultrav-iolet-visible Spectrophotometry<2.24>,using as the blank the solution prepared with2.0mL of ethanol(95)in the same manner as the former solution,and compare the spectrum with the Reference Spectrum or the spectrum of a solution of Betamethasone Reference Standard prepared in the same manner as the sample solution:both spectra exhibit similar intensities of absorption at the same wavelengths.(3)Determine the infrared absorption spectrum of Be-tamethasone,previously dried,as directed in the potassium bromide disk method under Infrared Spectrophotometry <2.25>,and compare the spectrum with the Reference Spec-trum or the spectrum of previously dried Betamethasone Reference Standard:both spectra exhibit similar intensities of absorption at the same wave numbers.If any diŠerence appears between the spectra,dissolve Betamethasone and Be-tamethasone Reference Standard in acetone,respectively, then evaporate the acetone to dryness,and repeat the test on the residues.Optical rotation<2.49>[a]20D:+118–+1269(after drying, 0.1g,methanol,20mL,100mm).Purity(1)Heavy metals<1.07>—Proceed with0.5g of Betamethasone according to Method2,and perform the test. Prepare the control solution with1.5mL of Standard Lead Solution(not more than30ppm).(2)Related substances—Dissolve10mg of Betametha-sone in5mL of a mixture of chloroform and methanol(9:1), WUHAN XINXINJIALI BIO-TECH CO.,LTD.Betamethasone CAS NO.:378-44-9come from356JP XV Betamethasone Tablets/O‹cial Monographsand use this solution as the sample solution.Pipet1mL ofthe sample solution,add a mixture of chloroform and methanol(9:1)to make exactly100mL,and use this solution as the standard solution.Perform the test with these solu-tions as directed under Thin-layer Chromatography<2.03>. Spot5m L each of the sample solution and standard solution on a plate of silica gel with‰uorescent indicator for thin-layer chromatography.Develop the plate with a mixture of dichloromethane,diethyl ether,methanol and water (385:75:40:6)to a distance of about12cm,and air-dry the plate.Examine under ultraviolet light(main wavelength:254 nm):the spots other than the principal spot from the sample solution are not more intense than the spot from the standard solution.Loss on drying<2.41>Not more than0.5z(0.5g,in vacu-um,phosphorus(V)oxide,4hours).Residue on ignition<2.44>Not more than0.5z(0.1g, platinum crucible).Assay Dissolve about20mg each of Betamethasone and Betamethasone Reference Standard,previously dried and ac-curately weighed,in methanol to make exactly50mL.Pipet5 mL each of these solutions,add exactly5mL each of the in-ternal standard solution,then add methanol to make50mL, and use these solutions as the sample solution and standard solution,respectively.Perform the test with10m L each of these solutions as directed under Liquid Chromatography <2.01>according to the following conditions,and calculate the ratios,Q T and Q S,of the peak area of betamethasone to that of the internal standard,respectively.Amount(mg)of C22H29FO5=W S×(Q T/Q S)W S:Amount(mg)of Betamethasone Reference Standard Internal standard solution—A solution of butyl parahydrox-ybenzoate in methanol(2in3500).Operating conditions—Detector:An ultraviolet absorption photometer (wavelength:240nm).Column:A stainless steel column about4.0mm in inside diameter and15cm in length,packed with octadecylsilanized silica gel for liquid chromatography(5m m in particle di-ameter).Column temperature:A constant temperature of about 259C..Mobile phase:A mixture of water and acetonitrile(3:2).Flow rate:Adjust the‰ow rate so that the retention time of betamethasone is about4minutes.System suitability—System performance:When proceed the test with10m L of the standard solution under the above operating conditions, betamethasone and the internal standard are eluted in this order with the resolution between these peaks being not less than10.System repeatability:When the test is repeated6times with 10m L of the standard solution under the above operating conditions,the relative standard deviation of the ratio of the peak area of betamethasone to that of the internal standard is not more than1.0z.Containers and storage Containers—Tight containers.Storage—Light-resistant.Betamethasone Tabletsベタメタゾン錠Betamethasone Tablets contain not less than90.0z and not more than107.0z of the labeled amount of betamethasone(C22H29FO5:392.46).Method of preparation Prepare as directed under Tablets, with Betamethasone.Identiˆcation Pulverize Betamethasone Tablets.To a por-tion of the powder,equivalent to2mg of Betamethasone ac-ceding to the labeled amount,add20mL of methanol,shake for5minutes,andˆlter.Evaporate theˆltrate on a water bath to dryness,dissolve the residue after cooling in2mL of methanol,ˆlter if necessary,and use this as the sample solu-tion.Separately,dissolve2mg of Betamethasone Reference Standard in2mL of methanol,and use this solution as the standard solution.Perform the test with these solutions as directed under Thin-layer Chromatography<2.03>.Spot5m L each of the sample solution and standard solution on a plate of silica gel with‰uorescent indicator for thin-layer chro-matography,develop with a mixture of1-butanol,water and acetic anhydride(3:1:1)to a distance of about10cm,and air-dry the plate.Examine under ultraviolet light(main wavelength:254nm):the principal spot obtained with the sample solution and the spot with the standard solution show the same R f value.Uniformity of dosage units<6.02>Perform the test accord-ing to the following method:it meets the requirements of the Content uniformity test.To1tablet of Betamethasone Tablets add V mL of water so that each mL contains about50m g of betamethasone(C22 H29FO5)according to the labeled amount,add exactly an amount of the internal standard solution equivalent to2mL per50m g of betamethasone,shake vigorously for10minutes, centrifuge,and use the supernatant liquid as the sample solu-tion.Separately,weigh accurately about20mg of Be-tamethasone Reference Standard,previously dried in a desic-cator(in vacuum,phosphorus(V)oxide)for4hours,and dis-solve in acetonitrile to make exactly200mL.Pipet5mL of this solution,add exactly20mL of the internal standard so-lution,and use this solution as the standard solution.Per-form the test with50m L each of the sample solution and stan-dard solution as directed under Liquid Chromatography <2.01>according to the following conditions,and determine the ratios,Q T and Q S,of the peak area of betamethasone to that of the internal standard.Amount(mg)of betamethasone(C22H29FO5)=W S×(Q T/Q S)×(V/400)W S:Amount(mg)of Betamethasone Reference Standard Internal standard solution—A solution of butyl parahydrox-ybenzoate in acetonitrile(1in40,000)Operating conditions—Proceed as directed in the AssaySystem suitability—System performance:When the procedure is run with50 m L of the standard solution under the above operating condi-WUHAN XINXINJIALI BIO-TECH CO.,LTD.Betamethasone CAS NO.:378-44-9come from357 JP XV O‹cial Monographs/Betamethasone Tabletstions,betamethasone and the internal standard are eluted in this order with the resolution between these peaks being not less than10.System repeatability:When the test is repeated6times with 50m L of the standard solution under the above operating conditions,the relative standard deviation of the ratio of the peak area of betamethasone to that of the internal standard is not more than1.0z.Dissolution<6.10>Perform the test according the following method:it meets the requirement.Perform the test with1tablet of Betamethasone Tablets at 50revolutions per minute according to the Paddle method us-ing900mL of water as the dissolution medium.Withdraw20 mL or more of the dissolution medium30minutes after start-ing the test,andˆlter through a membraneˆlter with a pore size not exceeding0.45m m.Discard theˆrst10mL of the ˆltrate,pipet the subsequent V mL of theˆltrate,add water to make exactly V?mL so that each mL contains about0.56 m g of betamethasone(C22H29FO5)according to the labeled amount,and use this solution as the sample solution. Separately,weigh accurately about28mg of Betamethasone Reference Standard,previously dried in a desiccator(in vacu-um,phosphorus(V)oxide)for4hours,dissolve in methanol to make exactly100mL.Pipet5mL of this solution,and add water to make exactly100mL.Pipet4mL of this solution, add water to make exactly100mL,and use this solution as the standard solution.Perform the test with exactly100m L each of the sample solution and standard solution as directed under Liquid Chromatography<2.01>according to the fol-lowing conditions,and determine the peak areas,A T and A S, of betamethasone.The dissolution rate in30minutes is not less than85z.Dissolution rate(z)with respect to the labeled amount of betamethasone(C22H29FO5)=W S×(A T/A S)×(V?/V)×(1/C)×(9/5)W S:Amount(mg)of Betamethasone Reference Standard C:Labeled amount(mg)of betamethasone(C22H29FO5)in 1tabletOperating conditions—Detector:An ultraviolet absorption photometer (wavelength:241nm).Column:A stainless steel column 4.6mm in inside di-ameter and15cm in length,packed with octadecylsilanized silica gel for liquid chromatography(5m m in particle di-ameter).Column temperature:A constant temperature of about 259C.Mobile phase:A mixture of methanol and water(3:2).Flow rate:Adjust the‰ow rate so that the retention time of betamethasone is about7minutes.System suitability—System performance:When the procedure is run with100 m L of the standard solution under the above operating condi-tions,the number of theoretical plates and the symmetry fac-tor of the peak of betamethasone are not less than3000and not more than2.0,respectively.System repeatability:When the test is repeated6times with 100m L of the standard solution under the above operating conditions,the relative standard deviation of the peak area of betamethasone is not more than2.0z.Assay Weigh accurately the mass of not less than20Be-tamethasone Tablets,and powder.Weigh accurately a por-tion of the powder,equivalent to about5mg of betametha-sone(C22H29FO5),add25mL of water,then add exactly50 mL of the internal standard solution,and shake vigorously for10minutes.Filter through a membraneˆlter with pore size of not more than0.5m m,discard theˆrst5mL of the ˆltrate,and use the subsequentˆltrate as the sample solution. Separately,weigh accurately about20mg of Betamethasone Reference Standard,previously dried in a desiccator(in vacu-um,phosphorus(V)oxide)for4hours,and dissolve in acetonitrile to make exactly50mL.Pipet5mL of this solu-tion,add exactly20mL of the internal standard solution and 5mL of water,and use this solution as the standard solution. Perform the test with20m L each of the sample solution and standard solution as directed under Liquid Chromatography <2.01>according to the following conditions,and determine the ratios,Q T and Q S,of the peak area of betamethasone to that of the internal standard.Amount(mg)of betamethasone(C22H29FO5)=W S×(Q T/Q S)×(1/4)W S:Amount(mg)of Betamethasone Reference Standard Internal standard solution—A solution of butyl parahydrox-ybenzoate in acetonitrile(1in10,000).Operating conditions—Detector:An ultraviolet absorption photometer (wavelength:240nm).Column:A stainless steel column4mm in inside diameter and15cm in length,packed with octadecylsilanized silica gel for liquid chromatography(5m m in particle diameter).Column temperature:A constant temperature of about 259C.Mobile phase:A mixture of water and acetonitrile(3:2).Flow rate:Adjust the‰ow rate so that the retention time of betamethasone is about4minutes.System suitability—System performance:When the procedure is run with20 m L of the standard solution under the above operating condi-tions,betamethasone and the internal standard are eluted in this order with the resolution between these peaks being not less than10.System repeatability:When the test is repeated6times with 20m L of the standard solution under the above operating conditions,the relative standard deviation of the ratio of the peak area of betamethasone to that of the internal standard is not more than1.0z.Containers and storage Containers—Tight containers.Storage—Light-resistant.WUHAN XINXINJIALI BIO-TECH CO.,LTD.Betamethasone CAS NO.:378-44-9come from。