电化学理论知识英文版重要
- 格式:docx
- 大小:31.23 KB
- 文档页数:3
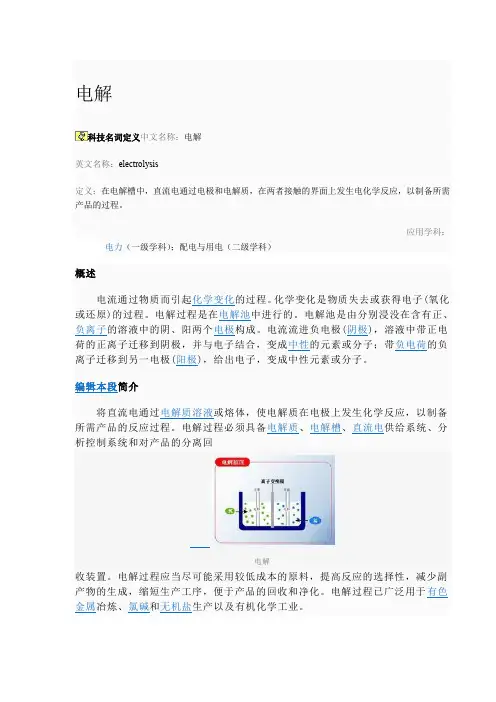
电解科技名词定义中文名称:电解英文名称:electrolysis定义:在电解槽中,直流电通过电极和电解质,在两者接触的界面上发生电化学反应,以制备所需产品的过程。
应用学科:电力(一级学科);配电与用电(二级学科)电解通电前,Cu2+和Cl-在水里自由地移动着;通电后,这些自由移动着的离子,在电场作用下,改作定向移动。
溶液中带正电的Cu2+向阴极移动,带负电的氯离子向阳极移动。
在阴极,铜离子获得电子而还原成铜原子覆盖在阴极上;在阳极,氯离子失去电子而被氧化成氯原子,并两两结合成氯分子,从阳极放出。
阴极:Cu2++2e-=Cu阳极:2Cl--2e-= Cl2↑电解CuCl2溶液的化学反应方程式:CuCl2=Cu+Cl2↑(电解)编辑本段电解质概念英文:Electrolyte电解质是指在水溶液中或熔融状态下能够导电的化合物。
例如酸、碱和盐等。
凡在上述情况下不能导电的化合物叫非电解质,例如蔗糖、酒精等。
(单质,混合物不管在水溶液中或熔融状态下能够导电与否,都不是电解质。
)电解质水溶液电解反应的综合分析在上面叙述氯化铜电解的过程中,没有提到溶液里的H+和OH-,其实H+和OH-虽少,但的确是存在的,只是他们没有参加电极反应。
也就是说在氯化铜溶液中,除Cu2+和Cl-外,还有H+和OH-,电解时,移向阴极的离子有Cu2+和H+,因为在这样的实验条件下Cu2+比H+容易得到电子,所以Cu2+在阴极上得到电子析出金属铜。
移向阳极的离子有OH-和Cl-,因为在这样的实验条件下,Cl-比OH-更容易失去电子,所以Cl-在阳极上失去电子,生成氯气。
说明①阳离子得到电子或阴离子失去电子而使离子所带电荷数目降低的过程又叫做放电。
②用石墨、金、铂等还原性很弱的材料制做的电极叫做惰性电极,理由是它们在一般的通电条件下不发生化学反应。
用铁、锌、铜、银等还原性较强的材料制做的电极又叫做活性电极,它们做电解池的阳极时,先于其他物质发生氧化反应。
电化学体系英文An electrochemical system is a system that involves the transfer of electrons between an electrode and an electrolyte. This transfer of electrons results in chemical reactions taking place at the electrode surface. Electrochemical systems are widely used in various applications such as energy storage, corrosion protection, sensors, and electroplating.In an electrochemical system, there are two main components: the electrode and the electrolyte. The electrode is the solid conductor where the electrontransfer takes place, while the electrolyte is the medium through which ions can move to maintain charge neutralityin the system.There are different types of electrochemical systems, including galvanic cells, electrolytic cells, and fuel cells. Galvanic cells, also known as voltaic cells, are devices that convert chemical energy into electrical energy through spontaneous redox reactions. Electrolytic cells, on the other hand, use electrical energy to drive non-spontaneous redox reactions. Fuel cells are electrochemicalcells that convert chemical energy from a fuel intoelectrical energy.The performance of an electrochemical system is often characterized by parameters such as the open-circuit voltage, short-circuit current, and power density. These parameters are influenced by factors such as the electrode material, electrolyte composition, and operating conditions.One key application of electrochemical systems is in energy storage, where batteries and supercapacitors play a crucial role. Batteries store energy in chemical form and can be recharged multiple times, making them ideal for portable electronics and electric vehicles. Supercapacitors, on the other hand, store energy electrostatically and can deliver high power outputs, making them suitable for applications requiring rapid energy release.Electrochemical systems are also used for corrosion protection, where sacrificial anodes are employed toprotect metal structures from corroding. By connecting a more reactive metal to the metal structure to be protected, the sacrificial anode corrodes instead of the protected metal, thus extending the lifespan of the structure.In addition to energy storage and corrosion protection, electrochemical systems are used in sensors for detecting various analytes such as glucose, pH, and heavy metals. By utilizing the specific redox reactions of the analyte of interest, electrochemical sensors can provide rapid and sensitive detection in a wide range of applications.Overall, electrochemical systems play a vital role in modern technology and have a wide range of applications in various fields. By understanding the principles of electron transfer and redox reactions, researchers and engineers can continue to develop innovative electrochemical systems for future advancements.电化学体系是涉及电极和电解质之间电子转移的系统。


Electrochemistry: Principles and Applications IntroductionElectrochemistry is a branch of chemistry that deals with the study of chemical processes involving the transfer of electrons between substances. It has both theoretical and practical aspects, with applications ranging from energy storage to corrosion prevention. This document provides an overview of the principles underlying electrochemistry and explores its various applications.1. Fundamentals of Electrochemistry1.1 Electrochemical Cells•Electrochemical cells are devices that convert chemical energy into electrical energy or vice versa.•They consist of two electrodes, an electrolyte, and a separator.•The movement of ions between the electrodes through the electrolyte allows for the flow of electrons, generating electrical current.1.2 Redox Reactions•Redox reactions involve the transfer of electrons between chemical species.•The species that loses electrons is oxidized, while the species that gains electrons is reduced.•The movement of electrons is facilitated by the presence of an electrode.1.3 Electrode Potentials•Electrode potentials measure the tendency of an electrode to gain or lose electrons.•The standard electrode potential is defined under standard conditions(1 mol/L, 298 K).•It is a measure of the driving force behind a redox reaction.2. Applications of Electrochemistry2.1 Batteries•Batteries are electrochemical devices that store and release electrical energy.•They consist of two or more electrochemical cells connected in series or parallel.•Common types of batteries include alkaline batteries, lithium-ion batteries, and lead-acid batteries.2.2 Corrosion Prevention•Corrosion is the degradation of metals due to chemical reactions with the environment.•Electrochemical methods such as cathodic protection are used to prevent corrosion.•By creating a sacrificial electrode, the metal to be protected acts as the cathode, preventing corrosion.2.3 Electroplating•Electroplating is a process that uses an electrical current to deposit a thin layer of metal onto a substrate.•It is used to enhance the appearance, corrosion resistance, and wear resistance of objects.•Common examples of electroplating include gold-plated jewelry and chrome-plated car parts.2.4 Sensors•Electrochemical sensors are widely used for the detection and measurement of analytes.•They work by utilizing the electrochemical properties of the analyte to produce a measurable signal.•Examples of electrochemical sensors include pH meters and glucose sensors.2.5 Fuel Cells•Fuel cells are electrochemical devices that convert the chemical energy of a fuel into electrical energy.•They have applications in transportation, stationary power generation, and portable electronics.•Different types of fuel cells include hydrogen fuel cells and methanol fuel cells.ConclusionElectrochemistry plays a vital role in various fields, including energy storage, corrosion prevention, and sensing. Understanding the principles of electrochemical reactions and their applications is crucial for developing innovative technologies and solving societal challenges. By harnessing the power of electrochemistry, we can create sustainable energy solutions and protect valuable assets from corrosion.。

电学英语知识点总结初中1. What is Electricity?Electricity is the flow of electric charge through a conductor. It is caused by the movement of electrons, which are negatively charged particles, through a material such as a wire. This movement of electrons can be harnessed to create electric currents, which can be used to power devices and perform work.2. Current, Voltage, and ResistanceCurrent, voltage, and resistance are the three fundamental properties of electric circuits. Current is the rate at which electric charge flows through a conductor, measured in amperes (A). Voltage is the difference in electric potential between two points in a circuit, measured in volts (V). Resistance is the opposition to the flow of electric current, measured in ohms (Ω). These t hree properties are related by Ohm's law, which states that the current flowing through a conductor is directly proportional to the voltage across it and inversely proportional to the resistance.3. Electric CircuitsAn electric circuit is a closed loop through which electric current can flow. It consists of a source of electrical energy (such as a battery or generator), a load (such as a light bulb or motor), and conductors connecting the two. There are two basic types of electric circuits: series circuits, in which the components are connected in a single path, and parallel circuits, in which the components are connected in multiple paths. Understanding how electric circuits work is essential for designing and troubleshooting electrical systems.4. Electric PowerElectric power is the rate at which electric energy is transferred by an electric circuit. It is measured in watts (W) and is calculated using the formula P = VI, where P is power, V is voltage, and I is current. Power is an important concept in electrical engineering, as it determines the performance of electrical devices and the cost of electricity consumption.5. Magnetism and ElectromagnetismMagnetism is the force exerted by magnets, which can attract or repel other magnetic materials. It is caused by the alignment of the magnetic dipoles in a material and plays a crucial role in the operation of electric motors, generators, and other electrical devices. Electromagnetism is the interaction of electric currents with magnetic fields and is the basis for the operation of transformers, inductors, and other electrical components.6. Capacitance and InductanceCapacitance is the ability of a component to store electric charge, while inductance is the ability to store magnetic energy. These properties are essential in the design of electroniccircuits and devices, as they determine how electric fields and magnetic fields interact with components. Understanding capacitance and inductance is crucial for designing efficient and reliable electrical systems.7. Electrical SafetyElectrical safety is a critical consideration when working with electricity. It is important to follow proper procedures and use the right equipment to prevent electrical hazards and accidents. This includes wearing protective gear, properly insulating and grounding electrical circuits, and following safety standards and regulations.In conclusion, electricity is a fascinating and essential field of study with a wide range of practical applications. Understanding the key concepts in electrical theory and their practical applications is crucial for anyone working in the field of electrical engineering or technology. By applying this knowledge, we can design and maintain efficient and reliable electrical systems that power the modern world.。

电化学方法与原理英文Electrochemical Methods and PrinciplesElectrochemistry is a fundamental branch of chemistry that deals with the relationship between electrical and chemical phenomena. It encompasses the study of various processes, such as the generation of electricity from chemical reactions, the use of electrical energy to drive chemical transformations, and the behavior of materials in electrochemical systems. Electrochemical methods have a wide range of applications, from energy production and storage to corrosion protection and analytical techniques.One of the core principles of electrochemistry is the understanding of oxidation and reduction reactions, also known as redox reactions. In these reactions, electrons are transferred between chemical species, resulting in changes in their oxidation states. The driving force behind these electron transfers is the difference in the ability of the participating species to attract and release electrons, known as their reduction potential. By harnessing and controlling these redox processes, electrochemists can design and optimize various electrochemical devices and processes.Electrochemical cells are the fundamental building blocks of electrochemical systems. These cells consist of two electrodes, an anode and a cathode, immersed in an electrolyte solution. The anode is where oxidation occurs, and the cathode is where reduction takes place. The electrolyte provides the necessary ionic conduction between the two electrodes, allowing the flow of ions and the completion of the overall electrochemical reaction.One of the most widely recognized applications of electrochemistry is energy conversion and storage. Electrochemical cells, such as batteries and fuel cells, convert the chemical energy stored in fuels or reactants directly into electrical energy. Batteries, for example, use the principle of redox reactions to generate a flow of electrons, which can then be used to power various electronic devices. Fuel cells, on the other hand, generate electricity by combining fuel (such as hydrogen) and an oxidant (such as oxygen) in an electrochemical reaction.In addition to energy applications, electrochemical methods are also used in a variety of analytical techniques. Electroanalytical methods, such as potentiometry, voltammetry, and electrochemical sensors, utilize the principles of electrochemistry to detect and quantify the presence of specific chemical species in a sample. These techniques are widely used in fields like environmental monitoring, healthcare, and chemical analysis.Corrosion is another area where electrochemistry plays a crucial role. Corrosion is an electrochemical process that involves the deterioration of materials, usually metals, due to their interaction with the surrounding environment. Understanding the electrochemical principles underlying corrosion enables the development of effective strategies for corrosion prevention and mitigation, such as the use of protective coatings, cathodic protection, and the selection of corrosion-resistant materials.Electrochemistry also finds applications in the synthesis and processing of materials. Electrochemical techniques, such as electroplating and electrodeposition, are used to deposit thin filmsor coatings of various materials onto a substrate. These processes are employed in the production of electronic components, decorative finishes, and protective coatings.The field of electrochemistry is constantly evolving, with new developments and applications emerging as our understanding of the underlying principles expands. Researchers continue to explore innovative electrochemical technologies, such as energy storage systems, fuel cells, and electrochemical sensors, to address pressing global challenges related to energy, the environment, and healthcare.In conclusion, electrochemical methods and principles arefundamental to a wide range of scientific and technological fields. From energy conversion and storage to analytical techniques and material processing, the principles of electrochemistry underpin numerous important processes that shape our modern society. As we continue to push the boundaries of scientific knowledge, the importance of electrochemistry will only grow, making it a crucial area of study for scientists and engineers alike.。

电气专业英语词汇电压源 voltage source电流源 current source理想电压源 ideal voltage source理想电流源 ideal current source伏安特性 volt-ampere characteristic 电动势 electromotive force电压 voltage电流 current电位 potential电位差 potential difference欧姆 Ohm伏特 Volt安培 Ampere瓦特 Watt焦耳 Joule电路 circuit电路元件 circuit elemen t电阻 resistance电阻器 resistor电感 inductance电感器 inductor电容 capacitance电容器 capacitor电路模型 circuit model参考方向 reference direction 参考电位 reference potential欧姆定律 Ohm’s law基尔霍夫定律 Kirchhoff’s law基尔霍夫电压定律 Kirchhoff’s voltage law(KVL)基尔霍夫电流定律 Kirchhoff’s current law(KCL)结点 node支路 branch回路 loop网孔 mesh支路电流法 branch current analysis网孔电流法 mesh current analysis结点电位法 node voltage analysis电源变换 source transformations叠加原理 superposition theorem网络 network无源二端网络 passive two-terminal network有源二端网络 active two-terminal network戴维宁定理 Thevenin’s theorem诺顿定理 Norton’s theorem开路(断路)open circuit短路 short circuit开路电压 open-circuit voltage短路电流 short-circuit current直流电路 direct current circuit (dc) 交流电路 alternating current circuit (ac)正弦交流电路 sinusoidal a-c circuit 平均值 average value有效值 effective value均方根值root-mean-squire value (rms) 瞬时值 instantaneous value电抗 reactance感抗 inductive reactance容抗 capacitive reactance法拉 Farad亨利 Henry阻抗 impedance复数阻抗 complex impedance相位 phase初相位 initial phase相位差 phase difference相位领先 phase lead相位落后 phase lag倒相,反相 phase inversion频率 frequency角频率 angular frequency赫兹 Hertz相量 phasor相量图 phasor diagram有功功率 active power无功功率 reactive power视在功率 apparent power功率因数 power factor 功率因数补偿 power-factor compensation 串联谐振 series resonance并联谐振 parallel resonance谐振频率 resonance frequency频率特性 frequency characteristic幅频特性amplitude-frequency response characteristic相频特性 phase-frequency response characteristic截止频率 cutoff frequency品质因数 quality factor通频带 pass-band带宽 bandwidth (BW)滤波器 filter一阶滤波器 first-order filter二阶滤波器 second-order filter低通滤波器 low-pass filter高通滤波器 high-pass filter带通滤波器 band-pass filter带阻滤波器 band-stop filter转移函数 transfer function波特图 Bode diagram傅立叶级数 Fourier series三相电路 three-phase circuit三相电源 three-phase source对称三相电源 symmetrical three-phase source对称三相负载 symmetrical three-phase load相电压 phase voltage相电流 phase current线电压 line voltage线电流 line current三相三线制 three-phase three-wire system三相四线制 three-phase four-wire system三相功率 three-phase power星形连接 star connection(Y-connection) 三角形连接 triangular connection(D- connection ,delta connection)中线 neutral line暂态 transient state稳态 steady state暂态过程,暂态响应 transient response 换路定理 low of switch一阶电路 first-order circuit三要素法 three-factor method时间常数 time constant积分电路 integrating circuit微分电路 differentiating circuit磁路与变压器磁场magnetic field磁通 flux磁路 magnetic circuit磁感应强度 flux density磁通势 magnetomotive force磁阻 reluctance 直流电动机 dc motor交流电动机 ac motor异步电动机 asynchronous motor同步电动机 synchronous motor三相异步电动机 three-phase asynchronous motor单相异步电动机 single-phase asynchronous motor旋转磁场 rotating magnetic field定子 stator转子 rotor转差率 slip起动电流 starting current起动转矩 starting torque额定电压 rated voltage额定电流 rated current额定功率 rated power机械特性 mechanical characteristic按钮 button熔断器 fuse 开关 switch行程开关 travel switch继电器 relay接触器 contactor常开(动合)触点 normally open contact 常闭(动断)触点 normally closed contact 时间继电器 time relay热继电器 thermal overload relay中间继电器 intermediate relay可编程控制器 programmable logic controller语句表 statement list梯形图 ladder diagram本征半导体intrinsic semiconductor掺杂半导体doped semiconductorP型半导体 P-type semiconductorN型半导体 N--type semiconductor自由电子 free electron空穴 hole载流子 carriersPN结 PN junction扩散 diffusion漂移 drift二极管 diode硅二极管 silicon diode锗二极管 germanium diode阳极 anode阴极 cathode发光二极管 light-emitting diode (LED) 光电二极管 photodiode稳压二极管 Zener diode晶体管(三极管) transistorPNP型晶体管 PNP transistorNPN型晶体管 NPN transistor发射极 emitter集电极 collector 基极 base电流放大系数 current amplification coefficient场效应管 field-effect transistor (FET) P沟道 p-channelN沟道 n-channel结型场效应管 junction FET(JFET)金属氧化物半导体 metal-oxide semiconductor (MOS)耗尽型MOS场效应管 depletion mode MOSFET(D-MOSFET)增强型MOS场效应管 enhancement mode MOSFET(E-MOSFET)源极 source栅极 grid漏极 drain跨导 transconductance夹断电压 pinch-off voltage热敏电阻 thermistor开路 open短路 shorted放大器 amplifier正向偏置 forward bias反向偏置 backward bias静态工作点 quiescent point (Q-point) 等效电路 equivalent circuit电压放大倍数 voltage gain总的电压放大倍数 overall voltage gain 饱和 saturation截止 cut-off放大区 amplifier region饱和区 saturation region截止区 cut-off region失真 distortion饱和失真 saturation distortion截止失真 cut-off distortion零点漂移 zero drift正反馈 positive feedback负反馈 negative feedback串联负反馈 series negative feedback 并联负反馈 parallel negative feedback 共射极放大器 common-emitter amplifier 射极跟随器 emitter-follower共源极放大器 common-source amplifier 共漏极放大器 common-drain amplifier 多级放大器 multistage amplifier阻容耦合放大器 resistance-capacitance coupled amplifier直接耦合放大器 direct- coupled amplifier输入电阻 input resistance输出电阻 output resistance负载电阻 load resistance动态电阻 dynamic resistance负载电流 load current旁路电容 bypass capacitor耦合电容 coupled capacitor 直流通路 direct current path交流通路 alternating current path直流分量 direct current component交流分量 alternating current component 变阻器(电位器)rheostat电阻(器)resistor电阻(值)resistance电容(器)capacitor电容(量)capacitance电感(器,线圈)inductor电感(量),感应系数 inductance正弦电压 sinusoidal voltage差动放大器 differential amplifier运算放大器 operationalamplifier(op-amp)失调电压 offset voltage失调电流 offset current共模信号 common-mode signal差模信号 different-mode signal共模抑制比 common-mode rejection ratio (CMRR)积分电路 integrator(circuit)微分电路 differentiator(circuit)有源滤波器 active filter低通滤波器 low-pass filter高通滤波器 high-pass filter带通滤波器 band-pass filter带阻滤波器 band-stop filter波特沃斯滤波器 Butterworth filter切比雪夫滤波器 Chebyshev filter贝塞尔滤波器 Bessel filter截止频率 cut-off frequency上限截止频率 upper cut-off frequency 下限截止频率 lower cut-off frequency 中心频率 center frequency带宽 Bandwidth开环增益 open-loop gain闭环增益 closed-loop gain共模增益 common-mode gain输入阻抗 input impedance电压跟随器 voltage-follower电压源 voltage source电流源 current source单位增益带宽 unity-gain bandwidth频率响应 frequency response频响特性(曲线)response characteristic 波特图 the Bode plot稳定性stability补偿 compensation比较器 comparator迟滞比较器 hysteresis comparator阶跃输入电压 step input voltage仪表放大器 instrumentation amplifier 隔离放大器 isolation amplifier对数放大器 log amplifier 反对数放大器 antilog amplifier反馈通道 feedback path反向漏电流 reverse leakage current相位phase相移 phase shift锁相环 phase-locked loop(PLL)锁相环相位监测器 PLL phase detector和频 sum frequency差频 difference frequency振荡器 oscillator RC振荡器 RC oscillatorLC振荡器 LC oscillator正弦波振荡器 sinusoidal oscillator三角波发生器 triangular wave generator 方波发生器square wave generator幅度 magnitude电平level饱和输出电平(电压) saturated output level功率放大器 power amplifier交越失真 cross-over distortion甲类功率放大器 class A power amplifier 乙类推挽功率放大器class B push-pull power amplifier OTL功率放大器 output transformerless power amplifier OCL功率放大器 output capacitorless power amplifier半波整流 full-wave rectifier全波整流 half-wave rectifier电感滤波器 inductor filter电容滤波器 capacitor filter串联型稳压电源 series (voltage) regulator开关型稳压电源 switching (voltage) regulator集成稳压器 IC (voltage) regulator晶闸管及可控整流电路晶闸管 thyristor 单结晶体管 unijunction transistor(UJT)可控整流 controlled rectifier可控硅 silicon-controlled rectifier 峰点 peak point谷点 valley point控制角 controlling angle导通角 turn-on angle二进制 binary二进制数 binary number十进制 decimal十六进制 hexadecimal二-十进制 binary coded decimal (BCD)门电路 gate三态门tri-state gate与门 AND gate或门 OR gate非门 NOT gate与非门 NAND gate或非门 NOR gate 异或门 exclusive-OR gate反相器 inverter布尔代数 Boolean algebra真值表 truth table卡诺图 the Karnaugh map逻辑函数 logic function逻辑表达式 logic expression组合逻辑电路 combination logic circuit 译码器 decoder编码器 coder比较器 comparator半加器 half-adder全加器 full-adder七段显示器 seven-segment display时序逻辑电路 sequential logic circuit R-S 触发器 R-S flip-flopD触发器 D flip-flopJ-K触发器 J-K flip-flop主从型触发器 master-slave flip-flop置位 set复位 reset直接置位端direct-set terminal直接复位端direct-reset terminal寄存器 register移位寄存器 shift register双向移位寄存器bidirectional shift register计数器 counter同步计数器 synchronous counter异步计数器asynchronous counter加法计数器 adding counter减法计数器 subtracting counter定时器 timer清除(清0)clear载入 load时钟脉冲 clock pulse触发脉冲 trigger pulse上升沿 positive edge下降沿 negative edge时序图 timing diagram波形图 waveform单稳态触发器 monostable flip-flop双稳态触发器 bistable flip-flop无稳态振荡器 astable oscillator晶体 crystal555定时器 555 timer模拟信号 analog signal数字信号 digital signalAD转换器analog -digital converter (ADC)DA转换器 digital-analog converter (DAC) 半导体存储器只读存储器 read-only memory(ROM)随机存取存储器 random-access memory (RAM)可编程ROM programmable ROM(PROM)laminated core 叠片铁芯short-circuiting ring 短路环squirrel cage 鼠笼rotor core 转子铁芯cast-aluminum rotor 铸铝转子bronze 青铜horsepower 马力random-wound 散绕insulation 绝缘ac motor 交流环电动机end ring 端环alloy 合金coil winding 线圈绕组form-wound 模绕performance characteristic 工作特性 frequency 频率revolutions per minute 转/分motoring 电动机驱动generating 发电per-unit value 标么值breakdown torque 极限转矩breakaway force 起步阻力overhauling 检修wind-driven generator 风动发电机revolutions per second 转/秒number of poles 极数speed-torque curve 转速力矩特性曲线 plugging 反向制动synchronous speed 同步转速percentage 百分数locked-rotor torque 锁定转子转矩full-load torque 满载转矩prime mover 原动机inrush current 涌流magnetizing reacance 磁化电抗line-to-neutral 线与中性点间的staor winding 定子绕组leakage reactance 漏磁电抗no-load 空载full load 满载Polyphase 多相(的)iron-loss 铁损complex impedance 复数阻抗rotor resistance 转子电阻leakage flux 漏磁通locked-rotor 锁定转子chopper circuit 斩波电路separately excited 他励的compounded 复励dc motor 直流电动机de machine 直流电机speed regulation 速度调节shunt 并励series 串励armature circuit 电枢电路optical fiber 光纤interoffice 局间的wave guide 波导波导管bandwidth 带宽light emitting diode 发光二极管silica 硅石二氧化硅regeneration 再生, 后反馈放大coaxial 共轴的,同轴的high-performance 高性能的carrier 载波mature 成熟的Single Side Band(SSB) 单边带coupling capacitor 结合电容propagate 传导传播modulator 调制器demodulator 解调器line trap 限波器shunt 分路器Amplitude Modulation(AM 调幅Frequency Shift Keying(FSK) 移频键控tuner 调谐器attenuate 衰减incident 入射的two-way configuration 二线制generator voltage 发电机电压dc generator 直流发电机polyphase rectifier 多相整流器boost 增压time constant 时间常数forward transfer function 正向传递函数error signal 误差信号regulator 调节器stabilizing transformer 稳定变压器 time delay 延时direct axis transient time constant 直轴瞬变时间常数transient response 瞬态响应solid state 固体buck 补偿operational calculus 算符演算gain 增益pole 极点feedback signal 反馈信号dynamic response 动态响应voltage control system 电压控制系统 mismatch 失配error detector 误差检测器excitation system 励磁系统field current 励磁电流transistor 晶体管high-gain 高增益boost-buck 升压去磁feedback system 反馈系统reactive power 无功功率feedback loop 反馈回路automatic Voltage regulator(AVR)自动电压调整器 reference Voltage 基准电压magnetic amplifier 磁放大器amplidyne 微场扩流发电机self-exciting 自励的limiter 限幅器manual control 手动控制block diagram 方框图linear zone 线性区potential transformer 电压互感器 stabilization network 稳定网络stabilizer 稳定器air-gap flux 气隙磁通saturation effect 饱和效应saturation curve 饱和曲线flux linkage 磁链per unit value 标么值shunt field 并励磁场magnetic circuit 磁路load-saturation curve 负载饱和曲线 air-gap line 气隙磁化线polyphase rectifier 多相整流器circuit components 电路元件circuit parameters 电路参数electrical device 电气设备electric energy 电能primary cell 原生电池energy converter 电能转换器conductor 导体heating appliance 电热器direct-current 直流time invariant 时不变的self-inductor 自感mutual-inductor 互感the dielectric 电介质storage battery 蓄电池e.m.f = electromotive force 电动势generator 发电机gas insulated substation GIS 气体绝缘变电站turbogenerator 汽轮发电机neutral point 中性点hydrogenerator 水轮发电机moving contact 动触头hydraulic turbine 水轮机fixed contact 静触头steam turbine 汽轮机arc-extinguishing chamber 灭弧室dynamo 直流发电机stray capacitance 杂散电容motor 电动机stray inductance 杂散电感stator 定子sphere gap 球隙rotor 转子bushing tap grounding wire 套管末屏接地线power transformer 电力变压器electrostatic voltmeter 静电电压表variable transformer 调压变压器 ammeter 电流表taped transformer 多级变压器grounding capacitance 对地电容step up (down) transformer 升(降)压变压器voltage divider 分压器circuit breaker CB 断路器surge impedance 波阻抗dead tank oil circuit breaker 多油断路器Schering bridge 西林电桥live tank oil circuit breaker 少油断路器Rogowski coil 罗可夫斯基线圈vacuum circuit breaker 真空断路器oscilloscope 示波器sulphur hexafluoride breaker SF6 断路器peak voltmeter 峰值电压表potential transformer PT 电压互感器conductor 导线current transformer CT 电流互感器cascade transformer 串级变压器disconnector 隔离开关coupling capacitor 耦合电容earthing switch 接地开关test object 被试品synchronous generator 同步发电机detection impedance 检测阻抗asynchronous machine 异步电机substation 变电站Insulator 绝缘子hydro power station 水力发电站lightning arrester 避雷器thermal power station 火力发电站metal oxide arrester MOA 氧化锌避雷器 nuclear power station 核电站bus bar 母线il-filled power cable 充油电力电缆overhead line 架空线mixed divider (阻容)混合分压器transmission line 传输线XLPE cable 交链聚乙烯电缆(coaxial) cable (同轴)电缆relay 继电器iron core 铁芯tuned circuit 调谐电路winding 绕组suspension insulator 悬式绝缘子bushing 套管porcelain insulator 陶瓷绝缘子front(tail) resistance 波头(尾)电阻 glass insulator 玻璃绝缘子inverter station 换流站flash counter 雷电计数器steel-reinforced aluminumconductor钢芯铝绞线charging(damping) resistor 充电(阻尼)电阻tank 箱体point plane gap 针板间隙earth(ground) wire 接地线exciting winding 激磁绕组grading ring 均压环trigger electrode 触发电极highvoltage engineering 高电压工程glow discharge 辉光放电highvoltage testing technology 高电压试验技术harmonic 谐波Power electronics 电力电子Automatic control 自动控制Principles of electric circuits 电路原理Digital signal processing 数字信号处理电气工程专业英语词汇表 2power system 电力系统impulse current 冲击电流power network 电力网络impulse flashover 冲击闪络insulation 绝缘inhomogenous field 不均匀场overvoltage 过电压insulation coordination 绝缘配合aging 老化internal discharge 内部放电alternating current 交流电lightning stroke 雷电波AC transmission system 交流输电系统lightning overvoltage 雷电过电压arc discharge 电弧放电loss angle (介质)损耗角attachment coefficient 附着系数magnetic field 磁场attenuation factor 衰减系数mean free path 平均自由行程anode (cathode) 阳极(阴极)mean molecular velocity 平均分子速度breakdown (电)击穿negative ions 负离子bubble breakdown 气泡击穿non-destructive testing 非破坏性试验cathode ray oscilloscope 阴极射线示波器non-uniform field 不均匀场cavity 空穴,腔partial discharge 局部放电corona 电晕peak reverse voltage 反向峰值电压composite insulation 组合绝缘photoelectric emission 光电发射critical breakdown voltage 临界击穿电压photon 光子Discharge 放电phase-to-phase voltage 线电压Dielectric 电介质,绝缘体polarity effect 极性效应dielectric constant 介质常数 power capacitor 电力电容dielectric loss 介质损耗quasi-uniform field 稍不均匀场direct current 直流电radio interference 无线干扰divider ratio 分压器分压比rating of equipment 设备额定值grounding 接地routing testing 常规试验electric field 电场residual capacitance 残余电容electrochemical deterioration 电化学腐蚀shielding 屏蔽electron avalanche 电子崩short circuit testing 短路试验electronegative gas 电负性气体space charge 空间电荷epoxy resin 环氧树脂streamer breakdown 流注击穿expulsion gap 灭弧间隙surface breakdown 表面击穿field strength 场强sustained discharge 自持放电field stress 电场力switching overvoltage 操作过电压field distortion 场畸变thermal breakdown 热击穿field gradient 场梯度treeing 树枝放电field emission 场致发射uniform field 均匀场flashover 闪络wave front(tail) 波头(尾)gaseous insulation 气体绝缘withstand voltage 耐受电压Prime mover 原动机Power factor 功率因数Torque 力矩Distribution automation system 配电网自动化系统Servomechanism 伺服系统Automatic meter reading 自动抄表Boiler 锅炉Armature 电枢Internal combustion engine 内燃机Brush 电刷Deenergize 断电Commutator 换向器Underground cable 地下电缆Counter emf 反电势电气工程专业英语词汇表 3Loop system 环网系统Demagnetization 退磁,去磁Distribution system 配电系统Relay panel 继电器屏Trip circuit 跳闸电路Tertiary winding 第三绕组Switchboard 配电盘,开关屏 Eddy current 涡流Instrument transducer 测量互感器Copper loss 铜损Oil-impregnated paper 油浸纸绝缘Iron loss 铁损Bare conductor 裸导线Leakage flux 漏磁通Reclosing 重合闸Autotransformer 自耦变压器Distribution dispatch center 配电调度中心Zero sequence current 零序电流Pulverizer 磨煤机Series (shunt) compensation 串(并)联补偿Drum 汽包,炉筒estriking 电弧重燃Superheater 过热器Automatic oscillograph 自动录波仪Peak-load 峰荷Tidal current 潮流Prime grid substation 主网变电站Trip coil 跳闸线圈Reactive power` 无功功率Synchronous condenser 同步调相机Active power 有功功率Main and transfer busbar 单母线带旁路Shunt reactor 并联电抗器Feeder 馈电线Blackout 断电、停电Skin effect 集肤效应Extra-high voltage (EHV) 超高压Potential stress 电位应力(电场强度) Ultra-high voltage (UHV) 特高压Capacitor bank 电容器组Domestic load 民用电crusher 碎煤机Reserve capacity 备用容量pulverizer 磨煤机Fossil-fired power plant 火电厂baghouse 集尘室Combustion turbine 燃气轮机Stationary (moving) blade 固定(可动)叶片Right-of-way 线路走廊Shaft 转轴Rectifier 整流器Kinetic(potential) energy 动(势)能Inductive (Capacitive) 电感的(电容的) Pumped storage power station 抽水蓄能电站Reactance (impedance) 电抗(阻抗)Synchronous condenser 同步调相机Reactor 电抗器Light(boiling)-water reactor轻(沸)水反应堆Reactive 电抗的,无功的Stator(rotor) 定(转)子Phase displacement (shift) 相移Surge 冲击,过电压Salient-pole 凸极Retaining ring 护环Slip ring 滑环Carbon brush 炭刷Arc suppression coil 消弧线圈Short-circuit ratio 短路比Primary(backup) relaying 主(后备)继电保护Induction 感应Phase shifter 移相器Autotransformer 自藕变压器Power line carrier(PLC) 电力线载波(器) Bushing 套管Line trap 线路限波器Turn (turn ratio) 匝(匝比,变比) Uninterruptible power supply 不间断电源Power factor 功率因数Spot power price 实时电价Tap 分接头Time-of-use(tariff) 分时(电价) Recovery voltage 恢复电压XLPE(Cross LinkedPolyethylene )交联聚乙烯(电缆)Arc reignition 电弧重燃Rms (root mean square) 均方根值Operation mechanism 操动机构RF (radio frequency) 射频电气工程专业英语词汇表 4Pneumatic(hydraulic) 气动(液压)Rpm (revolution per minute) 转/分Nameplate 铭牌LAN (local area network) 局域网Independent pole operation 分相操作LED (light emitting diode) 发光二极管Malfunction 失灵Single (dual, ring) bus 单(双,环形)母线Shield wire 避雷线IC (integrated circuit) 集成电路Creep distance 爬电距离FFT (fast Fourier transform) 快速傅立叶变换Silicon rubber 硅橡胶Telemeter 遥测Composite insulator 合成绝缘子Load shedding 甩负荷Converter (inverter) 换流器(逆变器) Lateral 支线Bus tie breaker 母联断路器Power-flow current 工频续流Protective relaying 继电保护sparkover 放电Transfer switching 倒闸操作Silicon carbide 碳化硅Outgoing (incoming) line 出(进)线Zinc oxide 氧化锌Phase Lead(lag) 相位超前(滞后) Withstand test 耐压试验Static var compensation (SVC) 静止无功补偿Dispatcher 调度员Flexible AC transmission system(FACTS) 灵活交流输电系统Supervisory control and data acquisition (SCADA) 监控与数据采集EMC (electromagnetic compatibility) 电磁兼ISO (international standardization organization) 国际标准化组织GIS (gas insulated substation, geographic information system) 气体绝缘变电站地理信息系统IEC (international Electrotechnical Commission) 国际电工(技术)委员会IEEE (Institute of Electrical and Electronic Engineers)电气与电子工程师学会(美)IEE (Institution of Electrical Engineers) 电气工程师学会(英)scale 刻度,量程calibrate 校准rated 额定的terminal 接线端子fuse 保险丝,熔丝humidity 湿度resonance 谐振,共振moisture 潮湿,湿气analytical 解析的operation amplifier 运算放大器numerical 数字的amplitude modulation (AM) 调幅frequency-domain 频域frequency modulation (FM) 调频time-domain 时域operation amplifier 运算放大器octal 八进制active filter 有源滤波器decimal 十进制passive filter 无源滤波器hexadecimal 十六进制。

电学英语知识点总结Basic Concepts of ElectricityTo start with, it is important to understand the basic concepts of electricity before delving into more complex topics. Electricity is the flow of electric charge, typically through a conductor such as a wire. It can take the form of either direct current (DC), where the electric charge flows in one direction, or alternating current (AC), where the flow of electric charge alternates direction periodically. Voltage is the difference in electric potential between two points and is measured in volts. Current is the flow of electric charge and is measured in amperes. Resistance is the opposition to the flow of electric charge and is measured in ohms.Ohm's LawOhm's Law is a fundamental principle in electrical engineering and states that the current flowing through a conductor between two points is directly proportional to the voltage across the two points and inversely proportional to the resistance between them. This can be expressed as the formula I = V/R, where I is the current in amperes, V is the voltage in volts, and R is the resistance in ohms.Kirchhoff's LawsKirchhoff's laws are two fundamental laws in electrical engineering that are used to analyze complex electrical circuits. Kirchhoff's current law states that the total current entering a junction in a circuit is equal to the total current leaving the junction. Kirchhoff's voltage law states that the total voltage around a closed loop in a circuit is equal to the sum of the voltage drops across the individual components in the loop.Series and Parallel CircuitsSeries and parallel circuits are two fundamental types of electrical circuits. In a series circuit, the components are connected in a daisy chain, with the same current flowing through each component. In a parallel circuit, the components are connected across common points, with the same voltage applied to each component. Understanding the behavior of series and parallel circuits is essential for analyzing and designing electrical circuits.Capacitance and InductanceCapacitance is the ability of a component to store electric charge, and is measured in farads. Inductance is the ability of a component to store energy in a magnetic field, and is measured in henrys. Both capacitance and inductance are important in electrical engineering and are used in a wide range of applications, including energy storage, filtering, and power factor correction.ResonanceResonance is a phenomenon that occurs in electrical circuits when the inductive and capacitive elements in the circuit are balanced, leading to the transfer of energy back and forth between the inductive and capacitive elements. Understanding resonance is important for designing circuits that operate at specific frequencies and for avoiding unwanted resonant effects in circuits.TransformersTransformers are electrical devices that are used to transfer electrical energy between two or more circuits through electromagnetic induction. They are commonly used to step up or step down the voltage in AC power transmission and distribution systems, and are essential for the efficient and safe operation of electrical power systems.Electric Motors and GeneratorsElectric motors and generators are devices that convert electrical energy into mechanical energy and vice versa. Understanding the principles of operation of electric motors and generators is essential for anyone working in the field of electrical engineering, as they are used in a wide range of applications, including industrial machinery, transportation, and power generation.Power SystemsPower systems are complex networks of electrical components that are used to generate, transmit, and distribute electrical power. Understanding the behavior of power systems, including factors such as voltage regulation, power factor correction, and system stability, is essential for the safe and efficient operation of electrical power systems.Electrical SafetyFinally, it is important to understand the principles of electrical safety, as working with electricity can be hazardous if proper precautions are not taken. This includes understanding the principles of electrical shock, arc flash, and fire hazards, as well as the importance of using proper personal protective equipment and safe work practices when working with electricity.In conclusion, electricity is a fundamental part of our modern world, and understanding the principles of electrical engineering and the basics of electricity is essential for anyone working in the field. The concepts covered in this article, including Ohm's Law, Kirchhoff's laws, series and parallel circuits, capacitance and inductance, transformers, electric motors and generators, power systems, and electrical safety, provide a comprehensive overview of the key knowledge points in electrical engineering and electrical principles. By understanding these concepts, anyone can develop a solid foundation in electrical engineering and apply this knowledge to a wide range of practical applications.。

学标准电极电位数据手册 《Surface scie nee studies of model fue cell electrocatalysts 》 《标准电极电势》 《Fuel Cells - Green Power 》Handbook of Batteries 燃料电池 《Fuel cell systemsexpla ined 》 《Electrochemistry in light water reactors 》 《Build your own Fuel Cells 》Fuel cell Handbook 第 7 版《经典专著 化学电源》《Battery Separators 〉电池用铝合金阳极材料研Fuel_Cell_Electronics_Packaging EIS 在电池研发中的应用 化学与能源 一本燃料电池的书籍----教你制做燃料电 池碳材料电化学电容器综述 微生物燃料电池 第一本书(Logan , 2008)研究表征和评价化学电源的传统电化学技术Fuel Cell Tech no logy PEM Fuel《电化学测试技术》 《Impeda nee Spectroscopy, Theory Experime ntand Applications 》 《Techniques and Mechanisms in Electrochemistry) 《实验电化学》 《电化学阻抗谱导论》 标准电极电位数据手册 电 化学阻抗谱-浙江大字-张鉴清 《Electrochemistry principles, methods, and applications 》《交流阻抗综述》电导率的测定资料专著实验电化学原理及应用 08年新书,Electrochemical Impedanee Spectroscopy 《标准电极电势》electrochemical instruments introduction电极 的维护和保养 极化曲线的原理及应用 天津大学电化学测试方法 PPT Basics of Electrochemical Impeda nee Spectroscopy Ag/AgCl 软件极化曲线拟合软件 循环伏安法模拟软件 电化学测定方法.腾岛.昭.等著.陈震等译 voltammetry and polarography (PPT form) 电化学仪器(美国)其他 电池 究的新进展专著Cells modeling solid oxide fuel cell动力电池论坛参比电极的制备zview 阻抗使用说明(含交流阻抗)不同型号CHI工作站官方资料电化学阻抗谱EIS简介.电化学暂态研究技l.ppt电化学测试技术电化学阻抗谱导论电化学拉曼《金属腐蚀与防护》《腐蚀电化学》赵世伟标准电极电位数据手册金《Electrochemistry prin ciples, methods, and applicati onS〉《Corrosi on 属Dictio nary》《Chemical Processes on Solid Surface》电导率的测腐定资料《标准电极电势》仪器分析录像片[14]电化学系统金属电化学保护参比电极阴极保护简明手册(德一贝克曼)金属腐蚀学原理(高校用书)腐蚀电化学蚀分析实验金属腐蚀电化学测试技术简介Ag/AgCI参比电极的制备电极的维护和保养材料选择不当造成的腐蚀破坏案例ASTMG5-94(1999)e1作静电位标记和动电位阳与极极化测量的基准测试方法厦门大学金属防腐课件腐蚀电化学分析腐蚀与防护全书--热喷涂腐蚀与防护全书--肖纪美--腐蚀总论——材料的腐蚀及其控制方法腐蚀防与防护全书--周静好--防锈技术腐蚀与防护全书--自然环境的腐蚀与防护腐蚀与防护全书--工业冷却水系统中金属的腐蚀与防护腐蚀与防护全书--肖纪美--应力作用护下的金属腐蚀.pdf腐蚀与防护全书--腐蚀试验的统计分析方法--曹楚南腐蚀与防护全书-腐蚀电化学研究方法腐蚀与防护全书--曹楚南--腐蚀电化学腐蚀与防护全书-不锈钢(王正樵编著化学工业出版社1991年版)NACE standard RP0775-05 NACE 阴极保护培训课程教案金属腐蚀电化学热力学一一电位一PH图及其应用。

电学英语知识点归纳在电学领域中,有许多与电相关的术语和概念是由英语单词或短语表示的。
了解这些电学的英语知识点对于学习和理解电学原理非常重要。
下面是一些常见的电学英语知识点的介绍。
1. Electricity(电):Electricity是指电荷(Charge)通过导体(Conductor)流动时产生的能量形式。
电学中的基本单位是Coulomb(库仑),通常用C表示。
2. Circuit(电路):Circuit是指由导线、电源和负载组成的一个完整的路径,使得电流可以流动。
Circuit中的电流通常用Ampere(安培)来表示。
3. Current(电流):Current是指电荷通过导体的流动。
电流的方向是从正电荷流向负电荷。
电流的单位是Ampere,简称A。
4. Voltage(电压):Voltage是指电流在电路中的推动力或压力。
电压的单位是Volt (伏特),简称V。
5. Resistance(电阻):Resistance是指电流在电路中遇到的阻力,它限制了电流的流动。
电阻的单位是Ohm(欧姆),简称Ω。
6. Ohm's Law(欧姆定律):Ohm's Law是指电压、电流和电阻之间的关系。
根据欧姆定律,电压等于电流乘以电阻,用公式表示为V = I * R。
7. Power(功率):Power是指电流在电路中产生或消耗的能量。
功率的单位是Watt(瓦特),简称W。
功率可以通过电压和电流的乘积来计算,用公式表示为P = V * I。
8. Series Circuit(串联电路):Series Circuit是指电路中的负载(电阻或电器)依次连接在一起,共享相同的电流。
串联电路中的电压等于各个负载电压的总和。
9. Parallel Circuit(并联电路):Parallel Circuit是指电路中的负载以多个分支并联连接,每个负载之间有相同的电压,但电流会分流。
并联电路中的总电流等于各个分支电流之和。
2. Types of EESSs and their working principlesThe working mechanism of any EESS relies on an inherent potential difference between two electrodes known as the operating voltage. The operating voltage of the device is dictated by the differences in redox potential of the positive and negative electrode. The potential difference is used to drive electrochemical reactions on either electrode when they are connected through an external circuit. This creates a flow of electrons from the negative electrode to the positive electrode. The flow of electrons induces oxidation reactions on the negative electrode (anode) and reduction reactions on the positive electrode (cathode) when discharging. The charged electrodes are balanced by a concomitant flow of counter-ions. EESSs are grouped into a number of different categories depending on the composition of the electrodes, the counter-ions, and the nature of the redox reactions (Fig. 1).2.1 Solid electrode batteries2.1.1 Metal-ion battery working principle. Batteries operate with a constant voltage defined, approximately, by the potential difference between the anode and cathode. Because of this, In a galvanostatic charge/discharge experiment the potential of the electrode or device ideally remains constant until the active material has been fully reduced (oxidized). In a cyclic voltammogram experiment, one observes a reversible, sharp redox peak when a redox event occurs (inset Fig. 1a–c).Metal-ion batteries are the most common type of EESSs. They are typically composed of an anode (negative electrode), a cathode (positive electrode), electrolyte (either aqueous, organic, solid-state,18or polymeric10,19), a separator (to prevent short circuiting), current collectors (to collect charge at each electrode), and a cell casing (to keep the components together and prevent exposure to the external environment). Metal-ion batteries are used for a wide variety of both portable and stationary applications for either primary or back-up power. In metal-ion batteries the charged anodes and cathodes are balanced by the metal ion in a ‘rocking-chair’ type mechanism (Fig. 1a). This is a strict requirement imposed by the definition of metal-ion batteries that should be clearly distinct from dual-ion batteries described below. Metal-ion batteries can be constructed with relatively small amounts of electrolyte because the ions balancing the charge at one electrode are constantly being replenished. Additionally, metal-ion batteries are very attractive candidates for use with solid-state electrolytes because the mobility of only one ion needs to be considered.2.1.2 Dual-ion battery working principle.In a dual-ion battery the charged anodes and cathodes are balanced by cations and anions respectively (Fig. 1b). Dual-ion batteries encompass a wide variety of electrolytes and electrodes. The anodes range from negative charge-accepting compounds to reduced metals and inorganic materials. The cathodes can also be a wide variety of materials as long as they are balanced by anions when charged. We will adhere to this definition throughout this review, but we note that others have referred to these systems as organic batteries, metal organic batteries, and radical polymer batteries.14,22Although these terms may be used to describe the electrodes, the convention of naming solid electrode batteries based on the mobile counter-ions is upheld with this nomenclature.2.1.3 Performance metrics of solid electrode batteries.A number of performance metrics need to be considered for the development of electrodematerials for solid electrode batteries. These performance metrics can be used to estimate the overall performance of the device (Tables 1–7). The theoretical capacitance (C theor) of a material is the maximum amount of charge a material can hold with respect to its mass. It is typically reported in m A h gÀ1and is calculated using eqn (1):Here, n is the maximum number of charges the compound can accept (or give up), F is Faraday’s constant, and M is the molecular weight of the compound in g molÀ1. Typically, the C theo r is used to assess how well the material could perform under optimized conditions. If the C theor is reached, then it is expected that the electrode cannot accept any more charge.The specific capacity (C sp) is the measured capacity of the electrode at a specific current density for either charging or discharging. The C sp is reported in m A h gÀ1and by measuring C sp at different rates (usually reported as a C-rate, where 1C is the amount of current it would take to collect the total charge of the C theor in 1 hour) the rate capabilities of the electrode can be determined. The C sp is typically calculated from galvanostatic charge/discharge curves using eqn (2):Here, i is the current in milliamperes, t is the time of discharging (charging) in seconds, and m is the mass of the active material in grams. If the C sp at low and high rates are similar, it can be said that the electrode has high rate capabilities. This typically depends on the electron transfer kinetics of the compound, and the electronic and ionic conductivity of the electrode and electrolyte.The coulombic efficiency (CE) is measured by dividing the C sp for discharging by the C sp for charging. This provides insight into the reversibility of the redox reactions and indicates whether any side reactions occur with the electrode and electrolyte. The CE is a good indicator of whether a stable solid electrolyte interface (SEI)is formed in the charging cycles and if the material itself will be stable upon extended cycling. If the CE is low in the first charging cycles but increases to B100% afterwards, it is typically attributed to SEI formation.The cycling stability is an important parameter that quantifies the retention of capacity upon charging and discharging the electrode multiple times. Usually this measurement is performed under galvanostatic conditions and is reported as a percentage of the initial capacity after a specified number of cycles. The current density (or C-rate) must be specified for these measurements because the rate can h ave a significant effect on the cycling stability. This effect is especially pronounced if capacity fading is due to electrode dissolution, which is a common problem with organic electrode materials.The potential at which the redox process(es) occur(s) is also a very important parameter. Combined with the capacity, the redox potential can be used to predict the energy density of the device when paired with an anode/cathode of known redox potential. To have a high energy density, the potential of cathode material should be as high as possible while that of anode material should be as low as possible within the electro-chemical window of the electrolyte, or within the electrolytes’ ability to form a stable SEI. Although an ideal battery maintains a constantvoltage while it discharges, real batteries tend to have a decreased voltage with decreasing state-of-charge (SOC).This creates a sloping voltage plateau that is especially apparent in polymeric electrodes or in electrodes with multiple redoxevents.23The reduction and oxidation peak splitting is also important to provide insight into electron-transfer kinetics, and to predict the energy efficiency of the device.While energy and power density are important parameters to gauge the performance of energy storage, we chose to exclude them from our evaluation of solid organic electrode materials since they pertain to fully assembled devices and relate to the combined performance of all aspects of the device including both the anode and cathode, the electrolyte, membrane, and resistances associated with various aspects of the device. Additionally, it is important to report the electrode formulations and procedure for electrode manufacturing, electrode morphologies, electrode thicknesses, electrolyte, and the conditions under which the experiments are being performed. All of these factors can have an enormous effect on device performance. For example, in our lab we have observed that changes in the electrolyte solvent can influence the electrochemical properties, such as the capacity, by as much as an order of magnitude. Therefore, we encourage others to report the details of electrode preparation and testing in full.。
电路原理英语知识点总结1. Electric Circuit ComponentsAn electric circuit is made up of various components that are necessary for the flow of electricity. The main components of an electric circuit include:- Voltage source: A voltage source provides the electrical energy required to initiate the flow of current in the circuit. Examples of voltage sources include batteries, generators, and power supplies.- Conductors: Conductors are materials that allow the flow of electrical current. They are usually made of copper or aluminum and are used to connect the various components of the circuit.- Resistors: Resistors are components that are used to control the flow of current in the circuit. They are made of materials that resist the flow of electricity and are used to limit the amount of current in a circuit.- Capacitors: Capacitors are components that are used to store and release electrical energy. They are made of plates separated by a dielectric material and are used to store charge in the circuit.- Inductors: Inductors are components that are used to store and release energy in the form of a magnetic field. They are made of coils of wire and are used in applications where energy storage is required.- Switches: Switches are components that are used to control the flow of current in the circuit. They can be used to open or close the circuit, allowing the flow of current to be controlled.2. Electric Circuit LawsThere are several laws and principles that govern the behavior of electric circuits. These laws are essential in understanding how electric circuits work and in analyzing and designing electrical systems. The main laws and principles of electric circuits include:- Ohm's Law: Ohm's law states that the current flowing through a conductor is directly proportional to the voltage across it and inversely proportional to its resistance. Mathematically, Ohm's law is expressed as I = V/R, where I is the current, V is the voltage, and R is the resistance.- Kirchhoff's Laws: Kirchhoff's laws are two principles that govern the behavior of electric circuits. Kirchhoff's current law states that the total current entering a junction is equal to the total current leaving the junction. Kirchhoff's voltage law states that the sum of the voltage drops around a closed loop in a circuit is equal to the sum of the voltage rises.- Thevenin's Theorem: Thevenin's theorem states that any linear electrical network can be replaced by an equivalent circuit consisting of a single voltage source and a single series resistor, where the voltage source is equal to the open-circuit voltage and the series resistor is equal to the Thevenin resistance.- Norton's Theorem: Norton's theorem is similar to Thevenin's theorem and states that any linear electrical network can be replaced by an equivalent circuit consisting of a single current source and a single parallel resistor, where the current source is equal to the short-circuit current and the parallel resistor is equal to the Norton resistance.- Maximum Power Transfer Theorem: The maximum power transfer theorem states that the maximum power is transferred from a source to a load when the load resistance is equal to the source resistance.3. Series and Parallel CircuitsElectric circuits can be connected in two ways: series and parallel. In a series circuit, the components are connected end-to-end, so that the current flows through each component in sequence. In a parallel circuit, the components are connected across each other, so that the current is divided and flows through each component simultaneously. Understanding the behavior of series and parallel circuits is important in the analysis and design of electrical systems.In a series circuit, the total resistance is the sum of the individual resistances, and the total voltage is the sum of the individual voltages. The current is the same in all components in a series circuit. In a parallel circuit, the total resistance is the reciprocal of the sum of the reciprocals of the individual resistances, and the total current is the sum of the individual currents. The voltage is the same across all components in a parallel circuit.4. Applications of Electric CircuitsElectric circuits have numerous applications in a wide range of electrical and electronics systems. Some of the key applications of electric circuits include:- Power distribution: Electric circuits are used to distribute electrical power from the source to the various loads in a system. Power distribution systems are essential in providing electricity to homes, industries, and other facilities.- Electronic devices: Electric circuits are used in the design and development of electronic devices, such as computers, smartphones, televisions, and other consumer electronics. The behavior of electric circuits is fundamental to the operation of these devices.- Control systems: Electric circuits are used in the design of control systems that are used to regulate and control the behavior of various systems. Control systems are used in industrial automation, robotics, and other applications.- Communication systems: Electric circuits are used in the design of communication systems, such as telecommunication networks, wireless communication systems, and satellite communication systems. These systems rely on the behavior of electric circuits to transmit and receive signals.- Renewable energy systems: Electric circuits are used in the design and implementation of renewable energy systems, such as solar power systems, wind power systems, and hydroelectric power systems. These systems rely on the behavior of electric circuits to convert and distribute the generated electrical energy.In conclusion, electric circuits are essential in the study and application of electrical engineering and technology. Understanding the principles of electric circuits, including their components, laws, behavior, and applications, is crucial in the design, analysis, and operation of electrical and electronics systems. Whether it is power distribution, electronic devices, control systems, communication systems, or renewable energy systems, electric circuits play a critical role in the functioning of modern electrical and electronic systems.。
第一章电化学理论基础1.1 电化学体系的基本单元1.1.1所有电化学体系至少含有浸在电解质溶液中或紧密附于电解质上的两个电极, 而且在许多情况下有必要采用隔膜将两电极分隔开。
1.1.2电极电极(electrode)是与电解质溶液或电解质接触的电子导体或半导体, 为多相体系。
电化学体系借助于电极实现电能的输入或输出, 电极是实施电极反应的场所。
一般电化学体系为三电极体系, 相应的三个电极为工作电极、参比电极和辅助电极。
化学电源一般分为正、负极;而对于电解池, 电极则分为阴、阳极。
现介绍如下。
工作电极(working electrode, 简称WE): 又称研究电极, 是指所研究的反应在该电极上发生。
一般来讲, 对于工作电极的基础要求是: 所研究的电化学反应不会因电极自身所发生的反应而受到影响, 并且能够在较大的电位区域中进行测试;电极必须不与溶剂或电解液组分发生反应;电极面积不宜太大, 电极表面最好应均一、平滑的, 且能够通过简单的方法进行表面净化等等。
工作电极可以是固体, 也可以是液体, 各式各样的能导电的固体材料均能作电极。
通常根据研究的性质来预先确定电极材料, 但最普通的“惰性”固体电极材料是玻璃、铂、金、银、铅和导电玻璃等。
采用固体电极时, 为了保证实验的重现性, 必须注意建立合适的电极预处理步骤, 以保证氧化还原、表面形貌和不存在吸附杂质的可重现状态。
在液体电极中, 汞和汞齐是最常用的工作电极, 它们都是液体, 都有可重现的均相表面, 制备和保持清洁都较容易, 同时电极上高的氢析出超电势提高了在负电位下的工作窗口, 已被广泛用于电化学分析中。
辅助电极(counter electrode,简称CE):又称对电极, 该电极和工作电极组成回路, 使工作电极上电流畅通, 以保证所研究的反应在工作电极上发生, 但必须无任何方式限制电池观测的响应。
由于工作电极发生氧化或还原反应时, 辅助电极上可以安排为气体的析出反应或工作电极反应的逆反应, 以使电解液组分不变, 即辅助电极的性能一般不显著影响研究电极上的反应。
assure 保证activity coefficient 活度系数adjacent 临近的ampere 安培anion 阴离子anodic阳极的background limits 极值电流barrier界线barrier能垒base electrolyte 基底电解质bulk phase本体溶液cadmium 镉capacitance电容cathodic阴极的cation 阳离子cell电池charged species 带电粒子chloride 氯化物circuit 回路coefficient系数component 成分conducting 传导configuration配置,结构consumed 损耗convection 对流convention 传统的convert转换correspondence相当于,一致corresponding 相应的coulombs 库伦current density 电路密度current—potential curve i-E曲线curve 曲线cyclic voltarmmetry 循环伏安法data数据defined 确定的determined测量devise设计,发明diffusion 扩散dimension 维diminish使减少distribution分布electric field 电场electrolysis 电解池electrolyte 电解液/质electrolytes 电解质electronic conductor 电子导体electrostatic 静电的element 自然环境employ采用ensuing接着发生的equation等式,方程equilibrium 平衡equivalent 相当于,等价的estimate 估量excess 过量exhibit表现experiment 实验exponential 指数extent 扩大,延伸external 外在的favorable 有利的flow 流动flux 流量function 函数fused salts 熔盐generate 形成gradient 梯度harness利用heterogeneous非均相,多相的homogeneous均匀的ideally 理想的immersed浸入impurities 杂质indefinitely无止境的inert working electrode(WE)惰性工作电极initial 初始instantaneous 瞬时的interface界面intermediate 中间物intermediate介于investigation 调查,研究ionic conductor 离子导体iR drop 欧姆压降irreversible 不可逆isolated 孤立的kinetics动力学linear 线性logarithmic对数mass transfer coefficient 传质系数mechanical 机械的mercury 汞migration 迁移minimize 最小化monitored 检测nature种类nay ionic 非离子物质negligible 可忽略non-aqueous solvent 非水溶剂normal hydrogen electrode(NHE)标准氢电极oblige迫使orbital轨道order of 10s 10的数量级oxidation current 氧化电流oxidized被氧化的parameter参数passage通路perpendicular 垂直phases 阶段plane electrode 平板电极plateau current 平顶电流polarized 极化potential可能的,电势principle原理properties性能proportion 比例的protons 质子pump 泵quadratic 二次方qualitative 定性的radical ion自由基离子rate determining step速度控制步骤rate constant 速率常数reactant 反应物reagent化学计量的reagent试剂reduction 还原region部位relative areas 相对面积resistance电阻respective分别的reversed相反的reversible可逆的rotating discelectrode 旋转圆盘电极saturated calomel electrode 饱和甘汞电极(SCE)scope 范围sink 接收slash 斜线soluble可溶的solutes 溶解species种类,物质spectroscopic 光谱学starting material 原材料steep 陡峭的stir 搅动structure结构substance物质sufficient 足够的symmetry对称terms as well as 以及thermodynamics热力学thermostat 恒温的trace 痕量transfer coefficient传递系数transient 暂态transition 过渡,转换unit activity 单位活度vacant electronic电子空位valence electron价电子vibration 震荡voltmeter 电压计。
Understanding the Electrochemistry ofBatteries在电池应用方面,了解电化学反应是必须的。
电池物理的本质就是通过电化学反应实现电能的转化。
当电池在使用过程中,化学物质的变化以及电子和离子的流动将电能转化为其他形式的能源,这种转化是反应性的,而电化学便是这种反应的研究领域。
电池中的化学反应,通常被分为两个半反应式,一个氧化反应式和一个还原反应式。
氧化反应会造成电子流出,而还原反应则会导致电子得到。
在电池使用过程中,电子的流动和氧化还原反应是紧密相关的。
例如,这或许已为您所知,许多化合物以及物质可以被氧化或还原。
许多桥梁和建筑的钢材就是被涂层的金属防止锈蚀。
因为金属锈蚀本质是它们的返回原始状态的一个过程,金属氧化导致电子流出,金属还原则导致电子得到。
电池物理的本质追求的就是这个反应的可逆性。
现代电池有几个关键的特征。
首先,它们都是由正极和负极以及离子交换层组成的。
这些部分由一个化学反应链相互作用而成,电池内部的化学反应会带来电子和离子的流动。
这些流动建立了一个带电区域,也就是一个带电场。
电自由能被定义为电场势能,并且在物质对电子和离子的流动做功时,将产生这个自由能。
这样的作用使得电子和离子回到它们对应的电极上,从而带动电池的工作。
不同的化学反应会产生不同类型的电池。
有些电池是可充电的,例如锂离子电池和镍氢电池,它们的化学反应都是在电池内部可逆的。
这些电池根据他们的化学反应、材料和操作方式不同,具有不同的构造和特性。
另外一些电池是非可充电的,例如普通的碱性干电池,它们的化学反应是不可逆的,也就是说,一个干电池工作时,其中化学反应产生的电能不可以再次用于将电池重置,然后再用电池产生电能。
这就是为什么干电池在用后需要被处理的原因。
在一些可充电电池中,例如锂离子电池,电池内部的化学反应涉及金属离子的嵌入和出离。
例如锂离子电池中,电池正极所作用的锂离子被嵌入碳基材料中,随后通过电池内部的离子交换层操作,离子向电池负极移动,而电流将经过一个外部电路。
2. Types of EESSs and their working principlesThe working mechanism of any EESS relies on an inherent potential difference between two electrodes known as the operating voltage. The operating voltage of the device is dictated by the differences in redox potential of the positive and negative electrode. The potential difference is used to drive electrochemical reactions on either electrode when they are connected through an external circuit. This creates a flow of electrons from the negative electrode to the positive electrode. The flow of electrons induces oxidation reactions on the negative electrode (anode) and reduction reactions on the positive electrode (cathode) when discharging. The charged electrodes are balanced by a concomitant flow of counter-ions. EESSs are grouped into a number of different categories depending on the composition of the electrodes, the counter-ions, and the nature of the redox reactions (Fig. 1).2.1 Solid electrode batteries2.1.1 Metal-ion battery working principle. Batteries operate with a constant voltage defined, approximately, by the potential difference between the anode and cathode. Because of this, In a galvanostatic charge/discharge experiment the potential of the electrode or device ideally remains constant until the active material has been fully reduced (oxidized). In a cyclic voltammogram experiment, one observes a reversible, sharp redox peak when a redox event occurs (inset Fig. 1a–c).Metal-ion batteries are the most common type of EESSs. They are typically composed of an anode (negative electrode), a cathode (positive electrode), electrolyte (either aqueous, organic, solid-state,18or polymeric10,19), a separator (to prevent short circuiting), current collectors (to collect charge at each electrode), and a cell casing (to keep the components together and prevent exposure to the external environment). Metal-ion batteries are used for a wide variety of both portable and stationary applications for either primary or back-up power. In metal-ion batteries the charged anodes and cathodes are balanced by the metal ion in a ‘rocking-chair’ type mechanism (Fig. 1a). This is a strict requirement imposed by the definition of metal-ion batteries that should be clearly distinct from dual-ion batteries described below. Metal-ion batteries can be constructed with relatively small amounts of electrolyte because the ions balancing the charge at one electrode are constantly being replenished. Additionally, metal-ion batteries are very attractive candidates for use with solid-state electrolytes because the mobility of only one ion needs to be considered.2.1.2 Dual-ion battery working principle.In a dual-ion battery the charged anodes and cathodes are balanced by cations and anions respectively (Fig. 1b). Dual-ion batteries encompass a wide variety of electrolytes and electrodes. The anodes range from negative charge-accepting compounds to reduced metals and inorganic materials. The cathodes can also be a wide variety of materials as long as they are balanced by anions when charged. We will adhere to this definition throughout this review, but we note that others have referred to these systems as organic batteries, metal organic batteries, and radical polymer batteries.14,22Although these terms may be used to describe the electrodes, the convention of naming solid electrode batteries based on the mobile counter-ions is upheld with this nomenclature.2.1.3 Performance metrics of solid electrode batteries.A number of performance metrics need to be considered for the development of electrodematerials for solid electrode batteries. These performance metrics can be used to estimate the overall performance of the device (Tables 1–7). The theoretical capacitance (C theor) of a material is the maximum amount of charge a material can hold with respect to its mass. It is typically reported in m A h gÀ1and is calculated using eqn (1):Here, n is the maximum number of charges the compound can accept (or give up), F is Faraday’s constant, and M is the molecular weight of the compound in g molÀ1. Typically, the C theo r is used to assess how well the material could perform under optimized conditions. If the C theor is reached, then it is expected that the electrode cannot accept any more charge.The specific capacity (C sp) is the measured capacity of the electrode at a specific current density for either charging or discharging. The C sp is reported in m A h gÀ1and by measuring C sp at different rates (usually reported as a C-rate, where 1C is the amount of current it would take to collect the total charge of the C theor in 1 hour) the rate capabilities of the electrode can be determined. The C sp is typically calculated from galvanostatic charge/discharge curves using eqn (2):Here, i is the current in milliamperes, t is the time of discharging (charging) in seconds, and m is the mass of the active material in grams. If the C sp at low and high rates are similar, it can be said that the electrode has high rate capabilities. This typically depends on the electron transfer kinetics of the compound, and the electronic and ionic conductivity of the electrode and electrolyte.The coulombic efficiency (CE) is measured by dividing the C sp for discharging by the C sp for charging. This provides insight into the reversibility of the redox reactions and indicates whether any side reactions occur with the electrode and electrolyte. The CE is a good indicator of whether a stable solid electrolyte interface (SEI)is formed in the charging cycles and if the material itself will be stable upon extended cycling. If the CE is low in the first charging cycles but increases to B100% afterwards, it is typically attributed to SEI formation.The cycling stability is an important parameter that quantifies the retention of capacity upon charging and discharging the electrode multiple times. Usually this measurement is performed under galvanostatic conditions and is reported as a percentage of the initial capacity after a specified number of cycles. The current density (or C-rate) must be specified for these measurements because the rate can h ave a significant effect on the cycling stability. This effect is especially pronounced if capacity fading is due to electrode dissolution, which is a common problem with organic electrode materials.The potential at which the redox process(es) occur(s) is also a very important parameter. Combined with the capacity, the redox potential can be used to predict the energy density of the device when paired with an anode/cathode of known redox potential. To have a high energy density, the potential of cathode material should be as high as possible while that of anode material should be as low as possible within the electro-chemical window of the electrolyte, or within the electrolytes’ ability to form a stable SEI. Although an ideal battery maintains a constantvoltage while it discharges, real batteries tend to have a decreased voltage with decreasing state-of-charge (SOC).This creates a sloping voltage plateau that is especially apparent in polymeric electrodes or in electrodes with multiple redoxevents.23The reduction and oxidation peak splitting is also important to provide insight into electron-transfer kinetics, and to predict the energy efficiency of the device.While energy and power density are important parameters to gauge the performance of energy storage, we chose to exclude them from our evaluation of solid organic electrode materials since they pertain to fully assembled devices and relate to the combined performance of all aspects of the device including both the anode and cathode, the electrolyte, membrane, and resistances associated with various aspects of the device. Additionally, it is important to report the electrode formulations and procedure for electrode manufacturing, electrode morphologies, electrode thicknesses, electrolyte, and the conditions under which the experiments are being performed. All of these factors can have an enormous effect on device performance. For example, in our lab we have observed that changes in the electrolyte solvent can influence the electrochemical properties, such as the capacity, by as much as an order of magnitude. Therefore, we encourage others to report the details of electrode preparation and testing in full.。