Characteristic of β-glucosidase from Sicilian blood oranges in relation to anthocyanin degradation
- 格式:pdf
- 大小:527.43 KB
- 文档页数:6

华中科技大学2002年硕士研究生入学考试试题一、选择题1.膜蛋白的功能不包括(D)A.作为信号受体B.作为离子通道C.作为酶D.储藏能量2.蛋白聚糖中的糖包括(C)A.肽聚糖B.几丁质C.透明质酸D.葡聚糖3.蛋白质中提供糖基化位点的侧链来自下列哪一种氨基酸残基(C)A.甘氨酸B.天冬氨酸C.丝氨酸D.色氨酸4.鞘氨醇不是下列什么物质的组成成分(B)A.神经酰胺B.磷脂酰乙醇胺C.神经节苷脂5.在pH7 条件下电泳,下列什么物质会向阴极移动(B)A.糖胺聚糖B.磷脂酰胆碱C.多聚谷氨酸D.多聚腺苷酸6.动植物来源的脂肪酸多是(D)A.奇数脂肪酸B.20C-30C之间C.反式脂肪酸D.偶数脂肪酸7.下列哪一种试剂可以用于标记蛋白质的N端(B)A.甲醛B.丹磺酰氯C.二异苯基磷酸P385D.TPCK P3688.属于同一蛋白质家族成员,其氨基酸序列中的哪些氨基酸出现差异可能性最小(A)A.形成功能部位(活性部位)的氨基酸B.和底物专一性或配基专一性有关的氨基酸C.与种属来源有关的氨基酸9.认为肽键是平面结构的重要依据是(D)A.肽键的键长0.132nm,等于碳碳双键之长B.肽键的键长0.132nm,等于碳氮双键之长C.肽键的键长0.132nm,介于碳碳双键和碳碳单键之间D.肽键的键长0.132nm,介于碳氮双键和碳氮单键之间10.不属于还原糖的糖有(A)A.蔗糖B.麦芽糖C.葡萄糖D.乳糖11.提取细菌膜蛋白时使用去污剂(表面活性剂)的作用是(B)A.破坏二硫键B.破坏疏水作用,使非极性基团暴露于水中C.破坏离子键D.去掉细胞壁12.以下结合蛋白质多肽链与非肽链部分不以共价键相连的是(A)A.脂蛋白B.胶原蛋白,一种糖蛋白C.琥珀酸脱氢酶,一种含有FAD的黄素蛋白13.蛋白质不具有下述哪种功能(F)A.提供能量B.运输C.防御D.加速化学反应E.调节F.储存遗传信息14.下列哪种肽段易于形成α -螺旋(C)A.多聚甘氨酸B.多聚脯氨酸C.多聚(丙氨酸-缬氨酸)15.如果胰蛋白酶不能消化某种蛋白质,说明该蛋白质在组成上不含(C)A.甘氨酸B.缬氨酸C.精氨酸D.苯丙氨酸16.β转角中肽链主链出现(C)度回折A.80B.60C.180D.15017.判断2,3-二磷酸甘油酸是血红蛋白别构调节物的证据可能是该物质(A)A.使血红蛋白的氧合曲线更加S 型B.使血红蛋白的氧合曲线变成双曲线型C.自身与血红蛋白结合曲线为S 型18.NAD+/NADH+H+辅酶在反应中转移的电子应表示成(C)A.H-B.HC.2H19.阳光维生素的意思是阳光的能量使动物的某种维生素原变为维生素,这种维生素原是(B)A.β -胡萝卜素B.7-脱氢胆固醇C.视紫红质D.色氨酸20.TPCK 抑制胰凝乳蛋白酶的根本原因是(C)A.TPCK类似酶的底物B.TPCK与酶的丝氨酸残基反应,使该丝氨酸不能作为广义酸碱催化剂C.TPCK与酶的组氨酸残基反应,使该组氨酸不能作为广义酸碱催化剂D.TPCK改变了酶的一级结构21.作为典型催化剂的酶具有下列哪一种能量效应(B)A.增加活化能B.降低活化能C.提高产物能量水平D.降低反应物能量水平22.修饰碱基主要存在于下列哪种核酸中(C)A.rRNAB.mRNAC.tRNAD.核DNAE.线粒体DNA23.在溴化乙锭-氯化铯密度梯度离心时,相同分子量的DNA,沉降最快的是(A)A.闭环DNAB.开环DNAC.双链线形DNA24.DNA的Tm不与什么有关(A)A.DNA的分子量B.DNA的GC含量C.DNA的纯度D.缓冲液组成25.ACTH 作用肾上腺时,导致的细胞响应包括(D)A.受体获得酪氨酸激酶活性B.受体激活引起细胞膜钾离子通道改变C.G蛋白激活,抑制腺苷酸环化酶D.cAMP与钙离子浓度升高26.生物膜中最丰富的脂类是(A)A.磷脂B.胆固醇C.糖脂D.三酰甘油27.Ribozyme是(B)A.蛋白质B.核酸C.糖D.脂28.胰岛素与受体结合后,受体细胞质区域(D)A.具有丝氨酸/苏氨酸激酶活性B.结合G蛋白C.激活磷脂酶D.具有酪氨酸激酶活性29.被丝氨酸蛋白激酶作用的蛋白质将(B)A.去掉磷酸丝氨酸上的磷酸基团B.从ATP 获得一个磷酸基团,使丝氨酸磷酸化C.从GTP 获得一个磷酸基团,使丝氨酸磷酸化D.水解ATP而获得磷酸基团30.限制性内切酶EcoRⅠ作用对象是(D)A.蛋白质B.RNAC.单链DNAD.双链DNA二、分析题1、某同学在提取酶的过程中发现酶已经失活,一个原因是由于操作不当引起酶的变性,请问有哪些因素会引起变性?还有什么因素可能引起失活?如果认为酶的失活不是由于变性引起的,请提出一种推测,并设计一个实验路线予以证明。
福建农林大学半期考试试卷参考答案2009——2010学年第学期课程名称:分子生物学考试时间120分钟专业年级班学号姓名一、名词解释(每小题2分,共30分)1、Splitting gene:真核基因的编码序列被非编码序列所隔断的现象。
2、Semi-discontinuous replication:DNA复制时,一条链的合成是连续的,另一条链的合成是不连续的。
3、Restricition endonuclease:能识别特异碱基序列,并在特定位点切断核酸双链的核酸内切酶。
4、Nucleosome:构成真核生物染色质的基本结构单位,是DNA和蛋白质构成的紧密结构形式。
其核心为H2A、H2B、H3、H4各2分子组成八聚体,DNA在其表面上缠绕1.75圈,约146bp,H1结合在连接相邻核小体之间的DNA上。
核小体DNA的长度平均为200bp。
5、Operon:原核基因表达调控的单位,包括启动子、操纵基因和一系列功能相关的结构基因。
6、Frameshift mutations:DNA分子中插入或缺失一个或少数几个核苷酸,使整个阅读框改变而引起的突变。
7、Induction:培养基中添加了某些物质(常常是底物),导致某些酶的合成。
8、Synonymous codon:编码同一氨基酸的密码子。
9、cis-acting element:顺式作用元件,是同一DNA分子中具有转录调节功能的特异DNA 序列。
按功能特性,真核基因顺式作用元件包括启动子、增强子及沉默子等。
10、Ribosome binding site:核糖体结合位点,由Shine和Dalgarno发现原核生物起始密码子上游8~13个nt的保守多嘌呤序列,与核糖体16S小亚基的特定序列碱基配对,相对于起始密码子正确定位核糖体,保证翻译的准确。
11、Exon:外显子,在mRNA剪接后仍会被保存下来,并可在蛋白质生物合成过程中被表达为蛋白质。
12、Leucine zipper:亮氨酸拉链,是含有4个或5个亮氨酸残基, 彼此之间精确的相距7个氨基酸残基。
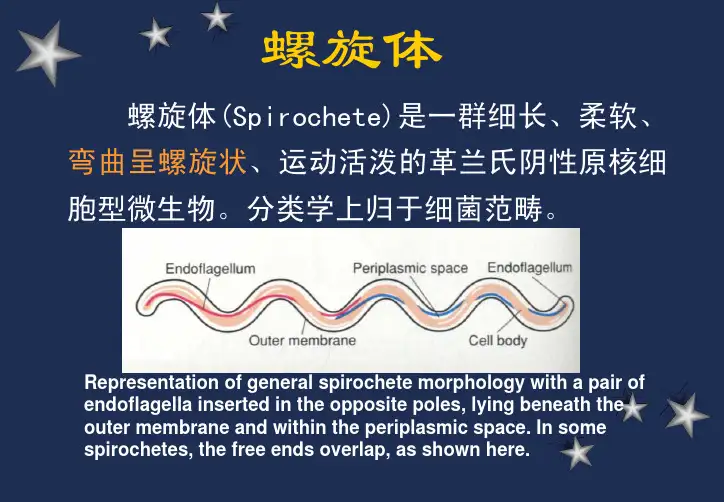
螺旋体螺旋体(Spirochete)是一群细长、柔软、弯曲呈螺旋状、运动活泼的革兰氏阴性原核细胞型微生物。
分类学上归于细菌范畴。
Representation of general spirochete morphology with a pair ofendoflagella inserted in the opposite poles, lying beneath the outer membrane and within the periplasmic space. In some spirochetes, the free ends overlap, as shown here.分类:根据抗原、螺旋的大小、数目与规则程度及两螺旋间距离。
1、疏螺旋体属:螺旋稀疏、不规则,呈波纹状回归热螺旋体Borrelia has 3-10 loose, irregualr coils.2、密螺旋体属:螺旋细密、规则梅毒螺旋体(雅司、品他)Treponema has 8-20 evenly spaced coils.3、钩端螺旋体属:螺旋更细密、规则,一端或两端弯曲呈钩状钩端螺旋体Leptospira has numerous fine, regularcoils and one or both curved ends.三属螺旋体形态模式图梅毒螺旋体蒋介石与第三任妻子陈洁如合影于黄浦军校。
在蒋与陈蜜月旅行之后,陈洁如发现她身上出了疹子。
“试用各种油膏涂抹,不但无效,而且越来越糟。
后来,突然发现腿上也出现疹块,手腕按脉处也出现两块红疤。
它们虽不痒,但看起来很不好,我很发愁,我一生从未得过这种病。
”单采花,男,某厂产品推销员,经常出差去南方。
不久前,他在旅途中与一位浓妆艳抹的女性亲热过一次,事后追悔莫及。
3周后阴茎冠状沟外发生无痛性硬结,触之硬感如软骨,逐渐扩大,约3-4天前表面发生糜烂、破溃,并无疼痛。
双侧腹股沟淋巴结肿大如蚕豆大小,不与皮肤粘连,无红肿热痛表现。

刺梨多糖提取物对小鼠Ⅱ型糖尿病的干预研究作者:伍勇韦艾骥杨堃李金桂董思思刘大利侯春兰刘红玲程驰薛飞龙刘松青来源:《广西植物》2023年第11期摘要:为探究刺梨多糖(Rosa roxbunghii Tratt. polysaccharide, RRTP)与刺梨不溶性膳食纤维(Rosa roxbunghii Tratt. insoluble dietary fiber, RTIDF)在降血糖功能上是否存在协同作用,该研究对RRTP和RTIDF进行提取、分离和纯化,通过体外实验与体内小鼠Ⅱ型糖尿病的干预实验,测定其抗氧化、降血糖活性,分析体内降血糖功能与小鼠肠道菌群结构关系。
结果表明:(1)体外实验发现,RRTP有较好的自由基清除力,能对α-葡萄糖苷酶、α-淀粉酶起显著抑制作用,IC50值分别为0.293、4.251 mg·mL-1,而RTIDF仅表现对α-淀粉酶活性具有一定的抑制作用。
(2)刺梨多糖提取物干预模型小鼠后,逆转了肥胖小鼠继续消瘦的趋势,与CK组(生理盐水)相比,RTIDF和RRTP+RTIDF干预的小鼠的血糖水平显著下调,血清中过氧化氢酶(CAT)活力明显增强,RRTP+RTIDF优于RTIDF。
(3)RRTP和RTIDF 干预可降低肝脏的炎症因子,缓解细胞肿胀程度,增加盲肠的吸收细胞数目,恢复肠壁黏膜层。
(4)分析肠道菌群发现,RRTP+RTIDF可降低拟杆菌门与厚壁菌门的比例,增加醋酸杆菌属等有益菌的种群丰度,RTIDF对于种群的调节作用更加显著。
综上认为,RRTP与RTIDF 在糖尿病小鼠的血糖干预中具有一定的协同作用,或可共同作为改善Ⅱ型糖尿病的干预剂。
关键词:刺梨多糖,膳食纤维素,分离纯化,Ⅱ型糖尿病,血糖干预中图分类号: Q946 文献标识码: A 文章编号: 1000-3142(2023)11-2120-11Intervention study of Rosa roxbunghii polysaccharide xtracts on Type Ⅱ diabetes in miceWU Yong WEI Aiji YANG Kun LI Jingui DONG Sisi LIU Dali HOU ChunlanLIU Hongling CHENG Chi XUE Feilong LIU Songqing1,2*( 1. College of Chemistry and Life Sciences, Chengdu Normal University, Chengdu 611130, China; 2. Sichuan Provincial Key Laboratoryfor Development and Utilization of Characteristic Horticultural Biological Resources, Chengdu 611130, China )Abstract: To investigate the synergistic effect of Rosa roxbunghii Tratt. polysaccharide (RRTP) and Rosa roxbunghii Tratt. insoluble dietary fiber(RTIDF) on hypoglycemic function. In this study, RRTP and RTIDF were extracted, isolated and purified, the antioxidant and hypoglycemic activities of polysaccharides were measured by in vitro experiment and the relationship between hypoglycemic function and intestinal microbial community structure in mice was analyzed in vivo intervention experiment of Type Ⅱ diabetes in mice. The results were as follows:(1) RRTP had a good free radical scavenging ability in vitro,and could significantly inhibit α-glucosidase and α-amylase activities with IC50 values of 0.293 and 4.251 mg·mL- respectively. RTIDF only showed certain inhibitory activity on α-amylase activity. (2) After the intervention of the extracts in the model mice, the tendency of the obese mice to continue to lose weight was reversed. Compared withCK group (physiological saline), the blood glucose level of RTIDF and RRTP+RTIDF mice were significantly down-regulated, and the activity of CAT enzyme in serum was significantly enhanced. RRTP+RTIDF group was superior to RTIDF group. (3) The extract intervention could reduce the inflammatory factors in the liver, relieve the degree of cell swelling, increase the number of absorbing cells in the cecum, and restore the intestinal wall mucosal layer. (4) Further analysis of intestinal microbial community showed that RTIDF+RRTP could reduce the proportion of Bacteroidetes and Firmicutes, increase the abundance of beneficial bacteria such as Acetobacter,but RTIDF had more significant regulation effect on the population. Therefore, based on in vitro hypoglycemic simulation and in vivo intervention results, RRTP and RTIDF have a certain synergistic effect on glucose intervention in diabetic mice, it may be used together as an intervention to improve Type Ⅱ diabetes.Key words: Rosa roxbunghii Tratt. polysaccharide, dietary cellulose, isolation and purification, Type Ⅱ diabetes, blood glucose interventionⅡ型糖尿病作为现代人群高发的代谢性疾病,严重威胁着人类的生命健康。

Chapter 10qRT-PCR of Small RNAsErika Varkonyi-Gasic and Roger P. HellensAbstractPlant small RNAs are a class of 19- to 25-nucleotide (nt) RNA molecules that are essential for genome stability, development and differentiation, disease, cellular communication, signaling, and adaptive responses to biotic and abiotic stress. Small RNAs comprise two major RNA classes, short interfering RNAs (siRNAs) and microRNAs (miRNAs). Efficient and reliable detection and quantification of small RNA expression has become an essential step in understanding their roles in specific cells and tissues. Here we provide protocols for the detection of miRNAs by stem-loop RT-PCR. This method enables fast and reliable miRNA expression profiling from as little as 20 pg of total RNA extracted from plant tissue and is suitable for high-throughput miRNA expression analysis. In addition, this method can be used to detect other classes of small RNAs, provided the sequence is known and their GC contents are similar to those specific for miRNAs.Key words: Small RNA, miRNA, RT, Stem-loop RT, qPCR, SYBR Green I assay, UPL probe assay 1. I ntroductionSmall RNAs are 19–25 nucleotide long noncoding RNA moleculesthat include short interfering RNAs (siRNAs), implicated in post-transcriptional and transcriptional gene silencing (1), and microR-NAs (miRNAs), implicated in processes ranging from developmentalpatterning to stress responses (2–5). While siRNAs arise from longdouble-stranded RNA precursors, miRNAs are derived from largerprecursors with a characteristic hairpin secondary structure. Similarto siRNAs that target perfect complementary sequences, plant miR-NAs repress gene expression by acting on near-perfect complemen-tary sequences in target mRNAs to guide cleavage and translationalrepression (6–9), or on DNA to guide chromatin modification (10).The majority of plant miRNA targets are developmentally importanttranscription factors (11, 12) and stress-regulated genes (13, 14).Igor KovaIchuk and Franz Zemp (eds.), Plant Epigenetics: Methods and Protocols,Methods in Molecular Biology,vol. 631,DOI 10.1007/978-1-60761-646-7_10, © Springer Science + Business Media, LLC 2010109110Varkonyi-Gasic and HellensDue to the miRNA action, these targets are either eliminated completely during cell-fate changes (12, 15, 16), or are reduced to appropriate levels of expression in tissues, where both the miRNA and the target mRNA are co-expressed (17, 18). In addition, a pos-sible long-distance signaling role was proposed for some miRNAs (19, 20), in contrast to miRNAs with demonstrated cell-autonomous expression and effects (21, 22).This complexity in miRNA modes of action demonstrates that reliable detection and quantification of miRNA expression in spe-cific tissues is an essential first step for better understanding of miRNA-mediated gene regulation. Although miRNAs represent a relatively abundant class of transcripts, their expression levels can vary dramatically between cells and tissues and they often escape detection by conventional technologies such as cloning, northern hybridization, and microarray analysis because of low abundance combined with high complexity of the small RNA population in plants (11, 23). High sensitivity and specificity of reverse transcription-polymerase chain reaction (RT-PCR) detec-tion methods provide a superior detection and quantification method over the conventional technologies. Stem-loop reverse transcription primers were shown to provide better specificity and sensitivity than linear primers (24), and a pulsed reverse transcrip-tion (RT) reaction further increases the sensitivity of miRNA detection (25). These features were utilized to derive a two-step miRNA detection method. First, the stem-loop RT primer is hybridized to the miRNA molecule and then reverse transcribed in a pulsed RT reaction. Next, the RT product is amplified using a miRNA-specific forward primer and the universal reverse primer. The product can be visualized by gel-electrophoresis upon a set number of PCR cycles or monitored in real-time using a SYBR Green I assay or a UPL probe assay that involves a dual labeled hydrolysis probe to increase specificity (Fig. 1).In addition to expression analysis of endogenous miRNAs, this method is amenable for the detection and quantification of other small RNAs, including artificial miRNAs and synthetic siRNAs.1. Plant tissue collected into liquid nitrogen and handled accord-ing to standard practices to prevent degradation of RNA.1. TRIzol reagent for isolation of total RNA (Invitrogen, Carlsbad, CA) (see Note 1).2. Solutions listed in the TRIzol protocol: chloroform, isopro-panol, 75% ethanol, water to resuspend the RNA pellet (see Note 2).2. M aterials2.1. Plant Material 2.2. Isolation and Gel-Electrophoresis of RNA111qRT -PCR of Small RNAs3. 12.3 M formaldehyde-containing 1% agarose gel. CAUTION: Formaldehyde is toxic through skin contact and inhalation of vapours. Manipulations involving formaldehyde should be done in a chemical fume hood.5’-UGACAGAAGAGAGUGAGCAC-3’5’-UGACAGAAGAGAGUGAGCAC GTTGGCTCTGGTGC 3’-CTCGTG CAaccgagacCACG A G G G T C C G A G GT A T T C 5’-GTTGGCTCTGGTGC 3’-CTCGTG CAaccgagacCACG A G G G T C C G A G G T A T TC5’-UGACAGAAGAGAGUGAGCACGTTGGCTCTGGTGC 3’-ACTGTCTTCTCTCACTCGTG CAaccgagacCACG A G G G T C CG A G G T A T T C CAaccgagacCACGCTTATGGAGCCTGGGACGTGGTCTCGGTTG-5’5’-GCGGCGG TGACAGAAGAGAGT-3’miR156stem-loop RT primer forward primer 5’-GCGGCGG TGACAGAAGAGAGT-3’3’-ACTGTCTTCTCTCACTCGTG CAaccgagacCACGCTTATGGAGCCTGGGACGTGGTCTCGGTTG-5’3’-ACTGTCTTCTCTCACTCGTG CAaccgagacCACGCTTATGGAGCCTGGGACGTGGTCTCGGTTG-5’5’-GCGGCGG TGACAGAAGAGAGTGAGCAC GTTGGCTCTGGTGCGAATACCTCGGACCCTGCACCAGAGCCAAC-3’by the quenching label 5’-GCGGCGG TGACAGAAGAGAGTGAGCAC GTTGGCTCTGGTGCGAATACCTCGGACCCTGCACCAGAGCCAAC-3’3’-CGCCGCC ACTGTCTTCTCTCACTCGTG CAaccgagacCACGCTTATGGAGCCTGGGACGTG-5’5’-tggctctg-3’+5’-GTGCAGGGTCCGAGGT-3’3’-ACTGTCTTCTCTCACTCGTG CAaccgagacCACGCTTATGGAGCCTGGGACGTGGTCTCGGTTG-5’5’-GCGGCGG TGACAGAAGAGAGTGAGCAC GTTGGCTCTGGTGCGAATACCTCGGACCCTGCACCAGAGCCAAC-3’3’-TGGAGCCTGGGACGTG-5’5’-GCGGCGG TGACAGAAGAGAGTGAGCAC GTTGGCTCTGGTGCGAATACCTCGGACCCTGCACCAGAGCCAAC-3’3’-CGCCGCC F 5’-tggctctg-3’Q 3’-CGCCGCC “unquenched”signal universal reverse primerSYBR Green I21435687910RTPCR UPLprobe SYBR Green I Fig. 1. Schematic showing the primer design and RT-qPCR process using the example of miR156. A stem-loop RT primer binds to the 3¢ portion of the miRNA, initiating reverse transcription. Then, the RT product is amplified using a miRNA specific forward primer and the universal reverse primer. Quantification is achieved either through SYBR Green I incorporation during amplification, or by the fluorescence generated upon cleavage of the UPL probe. Sequences related to miR156 are presented in grey. Sequences related to UPL probe #21 are in lower case. (1) Annealing, (2) Pulsed RT, (3) Denaturation,(4) Annealing, (5) Extension, (6) Denaturation, (7) Annealing, (8) Extension, (9) Hybridisation, (10) Cleavage112Varkonyi-Gasic and Hellens4. 10× MOPS buffer: 0.4 M MOPS, pH 7.0, 0.1 M sodium acetate, 0.01 M EDTA.5. Formaldehyde Load Dye (Ambion, Austin, TX).6. Ethidium bromide to final 10 m g/ml. CAUTION: Ethidium bromide is a strong mutagen and should be handled with extreme care .7. Molecular weight markers, e.g. 0.5–10 Kb RNA Ladder (Invitrogen). 1. Stem-loop RT primers. Prepare 100 m M stocks for long-term storage and 1 m M dilutions for immediate use. 2. 10 mM dNTP mix. Prepare by mixing dATP, dCTP, dGTP, and dCTP stock solutions, aliquot out and store at −20°C. 3. Reverse transcriptase, e.g. SuperScript III RT, 200 units/m l that is supplied with the First-Strand buffer for cDNA synthesis and 0.1 M DTT (Invitrogen). 4. RNase inhibitor such as RNaseOUT, 40 units/m l (Invitrogen). 5. Nuclease free water, e.g. UltraPure DEPC-treated Water (Invitrogen). 1. LightCycler FastStart SYBR Green I master mix (Roche Diagnostics, Mannheim, Germany), prepared according to manufacturer’s instructions. 2. Universal reverse primer. Prepare 100 m M stock for long-term storage and 10 m M dilution for immediate use. 3. Forward miRNA-specific primer. Prepare 100 m M stock for long-term storage and 10 m M dilution for im mediate use. 4. 10 mM dNTP mix as above. 5. Nuclease free water. 1. LightCycler TaqMan master mix (Roche Diagnostics) prepared according to manufacturer’s instructions. 2. UPL probe #21 prepared as 10 m M stock (Roche Diagnostics). 3. Universal reverse oligo. Prepare 100 m M stock for long-term storage and 10 m M dilution for immediate use. 4. Forward miRNA-specific oligonucleotide. Prepare 100 m M stock for long-term storage and 10 m M dilution for immediate use. 5. 10 mM dNTP mix as above. 6. Nuclease free water. 1. Standard laboratory equipment for isolation of RNA (fume hood, centrifuge, tubes, pipettes, and tips).2.3. Stem-LoopedPulsed ReverseTranscription2.4. qPCR2.4.1. miRNA SYBR GreenAssay2.4.2. miRNA UPL ProbeAssay2.5. Equipment113qRT -PCR of Small RNAs 2. A spectrophotometer for quantification of RNA, e.g. NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE) (see Note 3).3. Standard gel electrophoresis equipment (casting trays, gel tanks, power supply, UV transilluminator).4. A thermal cycler for pulsed reverse transcription. Our reverse transcription reactions and end-point PCR analyses were per-formed on the Mastercycler (Eppendorf, Hamburg, Germany).5. A real-time thermal cycler for qPCRs. All our real-time PCR anal-yses were performed on LightCycler 1.5 (Roche Diagnostics).The primers are designed according to Chen et al. (24) with some modifications (26) (Fig. 1). The stem-loop RT primers have a universal backbone and a specific extension. The universal back-bone sequence is as follows:5¢-GTTGGCTCTG GTGCAGGGTCCGAGGT ATTCGCACcagagccaAC-3¢.This backbone sequence can form a stem-loop structure because of the complementarity between the nucleotides in the 5¢ and 3¢ end; it includes the reverse complement of the UPL probe #21 (in lower case) and the universal reverse primer site in the loop region (in bold).The specificity of a stem-loop RT primer to an individual miRNA is conferred by a six-nucleotide extension at the 3¢ end; this extension is a reverse complement of the last six-nucleotides at the 3¢ end of the miRNA. In an miR156 example, the miRNA sequence is as follows (last six nucleotides are underlined):5¢-UGACAGAAGAGAGUGAGCAC-3¢.Thus, the miR156 stem-loop RT primer sequence is as fol-lows (last six nucleotides that provide specificity are underlined):5¢-GTTGGCTCTG GTGCAGGGTCCGAGGT ATTC GCACcagagccaACGTGCTC-3¢.Forward primers are specific to the miRNA sequence but exclude the last six nucleotides at the 3¢ end of the miRNA. A 5¢ extension of 5–7 nucleotides is added to each forward primer to increase the length and the melting temperature; these sequences were chosen randomly and are relatively GC-rich, bringing the GC content of the forward primer to 50–60%. In an miR156 example, the forward primer sequence is as follows (the GC-rich 5¢ extension is underlined):5¢-GCGGCGGTGACAGAAGAGAGT-3¢.3. M ethods3.1. Primer Design114Varkonyi-Gasic and HellensWe provide an example of a method for isolation, quantification, and evaluation of RNA. Other methods may be used (see Notes 1–3). 1. Isolate RNA from the plant tissue snap-frozen in liquid nitrogen using the TRIzol reagent, according to manufacturer’s instructions. 2. Determine concentration by spectrophotometric analysis. Use an aliquot (200 ng–1 m g) to assess quality by gel electro-phoresis. Store the remaining RNA on ice or at −20°C. 3. Determine RNA quality by gel-electrophoresis. Prepare the gel by heating 1 g agarose in 72 ml water until dissolved, and then cool slightly. Add 10 ml 10× MOPS running buffer and mix. Add 18 ml 37% formaldehyde (12.3 M). If required, top up with water to 100 ml. Pour the gel and wait until set. 4. Assemble the gel in the tank. Add 1× MOPS running buffer to cover the gel by a few millimetres. 5. Prepare the RNA sample by adding 3× volumes Formaldehyde Load Dye to 200 ng–1 m g RNA. Add ethidium bromide to the Formaldehyde Load Dye at a final concentration of 10 µg/ml. 6. Prepare the molecular weight marker in the same manner. 7. Heat denature samples at 65°C for 5–15 min. Load the gel and electrophorese at 5–6 V/cm. 8. Stop the run when the bromophenol blue dye has migrated as far as 70% of the length of the gel. 9. Visualize the RNA on a UV transilluminator. High quality RNA will have clearly visible rRNA bands. 10. Adjust RNA concentration with nuclease free water to 20 ng/m l.The most reproducible results are obtained with 2–20 ng of total RNA per reaction, but abundant miRNAs can be detected from as little as 20 pg of total RNA. The protocol is designed to evalu-ate expression of a specific miRNA in a large number of samples or expression of a large number of miRNAs in one sample. If test-ing many RNA samples for one miRNA, prepare a “no RNA” master mix; if testing for many different miRNAs in one sample, prepare a “no RT primer” master mix. Include 10% excess to cover pipetting errors. At least three replicates per RT reaction are recommended. Also prepare “minus RT” controls by omit-ting reverse transcriptase from the reactions and “no template” controls by adding nuclease-free water in place of RNA. It is important to keep the reactions on ice and work in the cold room if handling large number of samples. 1. Prepare the “no RNA” master mix by scaling the volumes for an individual RT reaction to the desired number of RT reactions. Prepare an individual reaction by adding the following compo-nents to a nuclease-free microcentrifuge tube:3.2. Isolation andGel-Electrophoresisof RNA3.3. Stem-Loop PulsedReverse TranscriptionProtocol3.3.1. RT Reaction WhenTesting Many RNASamples for One miRNA115qRT -PCR of Small RNAs 0.5 m l 10 mM dNTP mix,11.15 m l nuclease-free water and1 m l of appropriate stem-loop RT primer (1 m M). 2. Heat mixture to 65°C for 5 min and incubate on ice for2 min. 3. Centrifuge briefly to bring solution to the bottom of the tube. 4. Add the following:4 m l 5× First-Strand buffer,2 m l 0.1 M DTT,0.1 m l RNaseOUT (40 units/m l) and 0.25 m l SuperScript III RT (200 units/m l). 5. Mix gently and centrifuge to bring solution to the bottom of the tube. 6. Assemble the RT reaction by aliquoting 19 m l of the “no RNA” master mix and adding 1 m l RNA template (see Note 4). 7. Mix gently and centrifuge to bring solution to the bottom of the tube. 1. Prepare the “no RT primer” master mix by scaling the volumes for an individual RT reaction to the desired number of RT reactions. Prepare an individual reaction by adding the follow-ing components to a nuclease-free microcentrifuge tube:0.5 m l 10 mM dNTP mix,11.15 m l nuclease-free water and 1 m l of appropriate RNA template (see Note 4). 2. Add the following:4 ml 5× First-Strand buffer,2 ml 0.1 M DTT,0.1 ml RNaseOUT (40 units/ml) and 0.25 ml SuperScript III RT (200 units/m l). 3. Mix gently and centrifuge to bring solution to the bottom of the tube. 4. Assemble the RT reaction by aliquoting 19 m l of the “no RT primer” master mix and adding 1 m l of appropriate stem-loop RT primer (1 m M) previously denatured by heating to 65°C for 5 min. 5. Mix gently and centrifuge to bring solution to the bottom of the tube. 1. Load thermal cycler and incubate for 30 min at 16°C, followed by pulsed RT of 60 cycles at 30°C for 30 s, 42°C for 30 s and 50°C for 1 s.2. Incubate at 85°C for 5 min to inactivate the reverse transcriptase.3.3.2. RT Reaction WhenTesting One RNA Samplefor Many miRNAs3.3.3. Pulsed RT Reaction116Varkonyi-Gasic and HellensProtocols are provided for the SYBR Green I Assay and the UPL probe assay. SYBR Green I assay provides good specificity, if the number of PCR cycles is limited to the maximum of 35 to mini-mize nonspecific amplification. At this number of cycles, highly and moderately abundant miRNAs can be easily quantified (Fig. 2). For miRNA sequences that are expressed at low levels or when a particular set of primers produces background amplification, the UPL probe assay provides higher specificity (Fig. 3).3.4. qPCR Fig. 2. The sensitivity of the stem-loop RT-PCR assay. (a ) RT-PCR analysis of miR159 expression visualized on agarose gel stained with ethidium bromide. Very little non-specific amplification was detected with negative control reactions (−RT, minus RT, and NTC, “no template” control) at 35 cycles. The amount of RNA used for reverse transcrip-tion reactions are indicated on the top. PCR cycle numbers are indicated on the left. Size markers are indicated on the right. (b ) qPCR analysis of the same sample using the SYBR Green I assay at 35 cycles117qRT -PCR of Small RNAs 1. Prepare 5× LightCycler FastStart SYBR Green I master mix(Roche Diagnostics) according to manufacturer’s instructions.2. Prepare a PCR master mix by scaling the volumes listed below to the desired number of amplification reactions. Include 10% excess to cover pipetting errors. For a single reaction, add the following components to a nuclease-free microcentrifuge tube:12 m l nuclease-free water4 m l SYBR Green I master mix 3.4.1. miRNA SYBR Green IAssay Fig. 3. Improved specificity of the miRNA UPL probe assay. (a ) SYBR Green I assay PCR for miR166. Negative control reactions (−RT, minus RT, and NTC, “no template” control) produced detectable amplicons after 40 cycles. (b ) UPL probe assay PCR for miR166. No fluorescence was detected in the negative control reactions after 45 cycles. (c ) UPL probe assay amplification products for miR166 separated by gel electrophoresis on 4% agarose, showing specific and nonspecific amplification products above, below and in the size-range of specific products, obtained after 45 cycles of PCR. Arrowhead indi-cates the expected size of specific amplicons118Varkonyi-Gasic and Hellens1 m l forward (miRNA specific) primer (10 m M) and 1 m l reverse (universal) primer (10 m M) 3. Mix gently and centrifuge to bring solution to the bottom of the tube. 4. Store in cooling block or on ice. 5. Place required number of LightCycler capillaries in precooled centrifuge adapters. 6. Pipette 18 m l master mix into each LightCycler capillary. 7. Add2 m l RT product. 8. Seal each capillary with a stopper. 9. Place capillaries into the LightCycler carousel and spin in the carousel centrifuge. 10. Incubate the samples at 95°C for 5 min, followed by 35–40 cycles of 95°C for 5 s and 60°C for 10 s. 11. For melting curve analysis, denature samples at 95°C, then cool to 65°C at 20°C per second. Collect fluorescence signals at 530 nm wavelength continuously from 65°C to 95°C at 0.2°C per second. 1. Prepare 5× LightCycler TaqMan master mix (Roche Diagnostics)according to manufacturer’s instructions. 2. Prepare a PCR master mix by scaling the volumes listed below to the desired number of amplification reactions. Include 10% excess to cover pipetting errors. For a single reaction, add the following components to a nuclease-free microcentrifuge tube:11.8 m l nuclease-free water,4 m l TaqMan master mix,1 m l forward (miRNA specific) primer (10 m M) and1 m l reverse (universal) primer (10 m M) and0.2 m l UPL probe #21 (10 m M).3. Mix gently and centrifuge to bring solution to the bottom of the tube.4. Store in cooling block or on ice.5. Place required number of LightCycler capillaries in precooled centrifuge adapters.6. Pipette 18 m l master mix into each LightCycler capillary.7. Add 2 m l RT product.8. Seal each capillary with a stopper.9. Place capillaries into the LightCycler carousel and spin in the carousel centrifuge.10. Incubate samples at 95°C for 5 min, followed by 35–45 cycles of 95°C for 5 s and 60°C for 10 s.3.4.2. miRNA UPL ProbeAssay119qRT -PCR of Small RNAs The qPCR data can be analysed and presented as absolute or relative values. Relative quantification is the preferred method because it takes into account the potential errors due to variation in RNA input and RT efficiency. The most accurate method to correct these potential errors is normalization to endogenous control genes. An ideal endogenous control generally demonstrates gene expression that is relatively constant and highly abundant across tissues and cell-types. In addition, a suitable control for normalization of miRNA expression would have similar properties to miRNAs in terms of size and stability and would be amenable to the miRNA assay design. Some classes of small noncoding RNAs (ncRNAs) other than miRNAs are often expressed in an abundant and stable manner. Several human and mouse snRNAs and snoRNAs were tested across the range of tissues and experimental conditions and confirmed as suitable endogenous controls for quantification of miRNA expression levels (Applied Biosystems). No such analysis was performed with plant tissues yet, and a large-scale study is required to evaluate suitability of different plant ncRNAs for miRNA quantification. Therefore, plant researchers have to select a set of controls individually and screen under appropriate conditions or select a specific miRNA that demonstrates the least variability across tissues or experimental conditions under consideration. Either way, the consistency of expression should be confirmed under the specific conditions of the experiment (see Note 5).Here, we provide general instructions for data analysis using the LightCycler Software 4.05. If using a different instrument or software, refer to the appropriate instrument user manual for instructions on how to analyze data.1. This analysis is done after the SYBR Green I Assay to deter-mine that each of the primer pairs amplified a single predomi-nant product with a distinct melting temperature (Tm).2. Follow the instrument user manual for instructions forMelting Curve Analysis and Tm calling. 3. If a single melting peak is observed for a particular primer pair, itis likely that a single product with a distinct Tm was amplified. 4. Evaluate by gel-electrophoresis (see Note 6).1. Relative Quantification analysis compares two ratios: the ratioof the target gene to a reference gene sequence in an unknown sample is compared with the ratio of the same two sequences in a standard sample called a Calibrator. 2. To perform relative quantification with an external standard,prepare standard curves for the target and reference genes by serial dilutions of external standards with a known copy num-ber (see Note 7). Use at least three points or one point per log of concentration, whichever is greater. Always use a “no template” control.3.5. Data Analysis3.5.1. Melting Curve Analysis3.5.2. Relative Quantification120Varkonyi-Gasic and Hellens3. Prepare master mix and perform qPCR as described above.Use at least three replicates per standard dilution and “notemplate” control.4. Follow the instrument user manual for instructions for theStandard Method that will automatically calculate and displaythe amplification curves and the standard curve, crossingpoints, calculated concentrations, and statistics for replicates.5. Save as an external standard curve object.6. Perform Relative quantification, Calibrator normalized, with-out efficiency correction: select Relative Quantification –Monocolor Analysis, assign a “Target Calibrator” and a“Reference Calibrator” sample, assign appropriate pairs oftarget and reference samples and perform analysis followingthe instrument user manual (see Note 8).7. Perform efficiency correction by applying an external stan-dard curve.8. Download data and present as graphs or tables.4. N otes1. Whenever possible, we used the TRIzol Reagent for isolationof RNA because of its convenience, good RNA quality andspeed. Some plant tissues may not be amenable to isolation ofRNA by this method. Other methods for isolation of RNAmay be used; however, avoid RNA purification methods thatuse RNA-binding glass-fibre filters that do not recover smallRNA species quantitatively (e.g. Qiagen RNeasy mini andmidi kits). If unfamiliar with the method for isolation of RNA,subsequent isolation, quantification, and polyacrylamide gelelectrophoresis of the low molecular weight RNA fractioncan be used to evaluate its quantity and quality.2. RNA should be handled according to standard laboratorypractices to avoid RNase contamination. All buffers and solu-tions should be nuclease-free.3. Spectrophotometry followed by gel electrophoresis is still themost widely used method for assessing the RNA yield, purity,and quality. Fluorometry (e.g. RiboGreen, Molecular Probes)can also be used to determine yield, and microfluidic systems(such as Agilent’s bioanalyzer chips) can be used to deter-mine yield and quality.4. In our hands, both nondenatured RNA and RNA denaturedby incubation at 65°C for 5 min produced similar results.However, it has been suggested that denaturation of RNAmay reduce the yield of cDNA for some miRNAs.121qRT-PCR of Small RNAs5. In general, evaluation of endogenous controls involvesdemonstration of relatively abundant and relatively constantexpression levels across the tissues and environmental condi-tions, compared with the RNA input and expression of otherhousekeeping genes.6. Melting curve analysis needs to be combined with gel electro-phoresis. Due to the small size of the fragment, a primer-dimer product generated form the “minus RT” and “notemplate” controls often has a very similar Tm to that of theappropriate miRNA amplification fragment. This becomes anissue with lowly abundant miRNAs that require a large num-ber of PCR amplification cycles. In that case, the UPL assay isrecommended.7. Alternatively, use a cDNA sample with the highest level oftarget expression and prepare serial dilutions.8. This method assumes that the efficiency of target and refer-ence gene amplification is identical and equal to 2 (the amountof PCR product doubles during each cycle). In reality, theefficiency is often lower because of a number of different fac-tors. Efficiency correction is required for more reliable data.The software calculates the efficiency from the slope of thestandard curve.References1. Hannon GJ (2002) RNA interference. Nature418:244–2512. Bartel DP (2004) MicroRNAs: genomics,biogenesis, mechanism, and function. Cell 116:281–2973. Bartel B, Bartel DP (2003) MicroRNAs: atthe root of plant development? Plant Physiol 132:709–7174. Mallory AC, Vaucheret H (2006) Functionsof microRNAs and related small RNAs in plants. Nat Genet 38(Suppl):S31–S365. Zhang B, Wang Q, Pan X (2007) MicroRNAsand their regulatory roles in animals and plants. J Cell Physiol 210:279–2896. Aukerman MJ, Sakai H (2003) Regulation offlowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 15:2730–27417. Chen X (2004) A microRNA as a translationalrepressor of APETALA2 in Arabidopsis flower development. Science 303:2022–20258. Llave C, Xie Z, Kasschau KD, Carrington JC(2002) Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297:2053–20569. Palatnik JF, Allen E, Wu X, Schommer C,Schwab R, Carrington JC et al (2003) Controlof leaf morphogenesis by microRNAs. Nature 425:257–26310. Bao N, Lye KW, Barton MK (2004)MicroRNA binding sites in Arabidopsis class III HD-ZIP mRNAs are required for methy-lation of the template chromosome. Dev Cell 7:653–66211. Llave C, Kasschau KD, Rector MA, CarringtonJC (2002) Endogenous and silencing-associ-ated small RNAs in plants. Plant Cell 14:1605–161912. Rhoades MW, Reinhart BJ, Lim LP, BurgeCB, Bartel B, Bartel DP (2002) Prediction of plant microRNA targets. Cell 110:513–520 13. Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019 14. Sunkar R, Kapoor A, Zhu JK (2006)Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18:2051–206515. Juarez MT, Kui JS, Thomas J, Heller BA,Timmermans MC (2004) microRNA-medi-ated repression of rolled leaf1 specifies maize leaf polarity. Nature 428:84–88。

β-葡萄糖苷酶的研究进展综述食品研究与开发2oo5.V oL26.No.6一葡萄糖苷酶的研究进展许晶张永忠孙艳梅东北农业大学应用化学系哈尔滨150030摘要:本文简述了B一葡萄糖苷酶的理化性质,催化反应机制,酶活性测定方法及其在食品工业中应用.关键词:B一葡萄糖苷酶;性质;反应机制;应用l83一==ISOMERESEARCHADV ANCEOFB—GLUCOSIDASEXUJingZHANGY ongzhongSUNY anmei DepartmentofAppliedChemistry,NortheastAgriculturalUniversity,Harbin,150030 Abstract:Thearticlebrieflydiscussedthephysicalandchemicalproperties,thecatalyticmec hanism,methodsofenzymeactivitydeterminationof13-glucosidaseanditsapplicationinfoodindust ry.Keywords:13-glucosidase;characteristic;reactionmechanism;applicationB一葡萄糖苷酶(.beta.一Glucosidase)系统名称为B—D一葡萄糖苷葡萄糖水解酶(.beta.一D—glucosideglucohydrolase;EC3.2.1.21).1837年,被Liebig和Wohler首次在苦杏仁中发现,后被发现存在于自然界许多植物中,还存在于一些酵母,曲霉菌,木酶菌及细菌体内[1].它起初引起人们的注意是因为它参与了纤维素材料的生物转化.B一葡萄糖苷酶是纤维素酶系中的一个组分,它主要作用于B一(1,4)糖苷国家"十五"重大科技专项"农产品深加工技术与设备研究开发"项目编号:2001BA501A02B作者简介:许晶,女,1979年9月出生,理学学士;助教,在读硕士, 研究方向:食品化学专业.键,还能作用于B一(1,1),(1,2),(1,3),(1,6)糖苷键.对于低聚葡萄糖聚合度越小,它的水解能力越强[.多年来,许多学者分别从苦杏仁,葡萄,刀豆,玉米,黑樱桃,水稻,大豆中分离纯化了B一葡萄糖苷酶[.现将B一葡萄糖苷酶的理化性质,催化反应机制,酶活性测定方法及其在食品工业中应用简述如下.113一葡萄糖苷酶的理化性质1.1相对分子量B一葡萄糖苷酶的相对分子质量一般在40000-250000之间[4].不同来源的B一葡萄糖苷酶的相对分子量由于其结构和组成不同而差异很大.例如,的营养保健食品,因而博得了产地群众的青睐.胡颓子果实,根,叶药用,收敛止泻,镇咳解毒.常见临床配方:①治疗风湿性关节炎疼痛,胡颓子根100g,黄酒6OraL,猪脚250g,加水煮1时许,取汤一碗,连同猪脚一同服食.②治疗吐血,便血,咯血,月经过多,胡颓子根30-6Og,煎服.③治疗支气管哮喘,慢陛支气管炎,胡颓子叶15g,枇杷叶15g,水煎服.④治疗咳嗽,鲜胡颓子叶30g,水煎,加白糖少许服用.⑤治疗痢疾下血,用胡颓子15g,乌梅20g,水煎服.⑥治疗月经不调,血崩,用胡颓子15g,山萸肉20g,水煎服.除此之外,胡颓子的鲜花含芳香油,可作调香原料.茎皮纤维是造纸和制纤维板的原料.植株可作园林绿化树,配植于花丛或林缘,颇有特色.胡颓子树对多种有害气体抗性强,特别适于工厂污染区的绿化.胡颓子的野生资源非常丰富,耐干旱瘠薄,适应性很强,对土壤要求不严,常生于山坡疏林下或林缘灌丛的阴湿环境,也发现于向阳山坡或路旁.繁殖非常容易,结果早,加之营养价值高,特别是氨基酸,维生素C和矿质元素含量丰富,开发利用的前景非常广阔[1].参考文献:[1]刘孟军.中国野生果树[M].北京:中国农业出版社,1998[2]张福平等.粤东地区野果植物资源[J].中国野生植物资源.2003,22(3):13-16[3]中国科学院研究所.中国高等植物图鉴(1-5册及补编1—2册)[M].北京:科学出版社,1985收稿日期:2005一O1—25一==2DD5.V o1.26.J,fO.6食品研究与ic}发ServeW.M.Kengen等人研究的古细菌Pyrococcus furiosus分泌的B一葡萄糖苷酶的分子是由四个亚基构成的四聚体,其分子量在230000左右;而中国科学院微生物研究所的曾宇成等所测出的海枣曲霉的B一葡萄糖苷酶由两个亚基构成,分子量为200000左右;由Day&Withers等人从Agrobacterium 中分离出的野生性B一葡萄糖苷酶是一种二聚体,由两个分子量质量为50000的B一葡萄糖苷酶的亚基构成.有的菌株本身含有胞内和胞外B一葡萄糖苷酶,因此,有时来源于同一菌株的B一葡萄糖苷酶,是二种不同分子量酶的混合物.1.2等电点(pI),最适pH及pH稳定性大部分B一葡萄糖苷酶的pI都在酸性范围,并且变化不大,一般在3.5-5.5之间,但最适pH可以超过7.0,而且酸碱耐受性强[.如:Paavilainen等人从Alkalophilus中就分离出细胞外B一葡萄糖苷酶, 其最适pH就在6-9之间,而在pH4.0—10.2以外还具有一定的催化活性;中国台北学者李约昆(音译)等人从Flavobacteriummeningosepticum中分离出的B一葡萄糖苷酶其pI在9.0左右,最适pH是5.0E.1.3最适温度及热稳定性B一葡萄糖苷酶的最适温度在40一ll0℃之间都有分布.一般来说,来自古细菌的B一葡萄糖苷酶其热稳定性和最适温度要高于普通微生物来源的B一葡萄糖苷酶.如古细菌Pyrococcusfuriosus的B一葡萄糖苷酶其最适温度102—105oC,100℃时的半衰期为85h;而李约昆等人分离出的B一葡萄糖苷酶最适温度在50—55℃之间,在60℃下于磷酸盐缓冲液中,其活力在15min后只余1%.对于工业应用来说,酶的热稳定性越高越有利,因此,从嗜热细菌中分离出B一葡萄糖苷酶逐渐引了人们的兴趣.至于来自嗜热性微生物的B一葡萄糖苷酶为何具有如此强的耐热稳定性还未获得共识.据MichaelW.Bauer 等人对来自嗜热性和非嗜热性B一葡萄糖苷酶的分析认为,两者在相互演化过程中发生的酶修饰作用并不改变酶的活性中心,也不改变其专一性,只是将酶蛋白结构作部分调整以适应高温环境[63.2B一葡萄糖苷酶的催化反应机制2.1反应机制[]M.W.Bauer等人对分别来自嗜热菌Pyrococcus furiosus和非嗜热菌Agrobacterium的B一葡萄糖苷酶进行研究发现,两种来源的菌催化反应时按同一种机制进行,即在催化糖苷键的裂解反应时都遵循双取代反应机制.其反应方程式如下:EsEP第一步是酶与底物键合形成米氏复合物ES(反应速率K.和K一.);第二步是酶一底物中间体(E—S)的形成(反应速率K2);酶的亲核基团按酸催化机制进攻异头碳,形成共价的糖基酶中间体(E—S).在这一过程中,B一葡萄糖苷酶的活性中心可根据不同类型的底物而相应发生一定程度的结构变化,从而使B一葡萄糖苷酶可以和多种糖类底物结合,这一步决定了B一葡萄糖苷酶具有的底物专一性;第三步是中间体的水解:由水按碱催化机制对异头碳进攻,形成B一葡萄糖基产物并使酶回复其初始的质子化态.其中,糖苷基酶中间体的形成和水解过程经历了共价结构的氧碳鲼正离子过渡态.另外,B一葡萄糖苷酶在整个反应过程中其构型严格保持不变.2.2活性中心结构[]在多数B一葡萄糖苷酶中起催化作用的残基是二个谷氨基酸残基,其中,靠近N一端的谷氨酸起酸/碱作用,另一氨基酸起亲核试剂的作用.但Grabnitz等人研究发现来自Clostridiumthermoce1.1um的B一葡萄糖苷酶的活性部分在N一端的130个氨基酸区域,该区的个性特征是氨基酸序列中心基团His—Asn—Glu—Pro,存在于该区域的具有催化作用的残基是相隔35—55个氨基酸的His和Glu,其中质子化态的完全保持残基Hisl21作为质子供体与Glu166协同稳定氧碳鲼正离子.而高度保持的c一端附近的残基也许参与了酶与糖苷基底物的键合,其中在该区的一些微小差异与不同B一葡萄糖苷酶的底物特异性有关.2.3底物特异性[8]几乎所有的B一葡萄糖苷酶对底物的糖基部分结构的专一性较差,能袭解C一0糖苷键,c—S键,c—N键,C—F键等;有些对糖基部分的C和C:构形也不专一,能同时水解B一葡萄糖苷键和B一半乳糖苷糖,有些甚至c位的专一性也不高,能水解木糖.但在所有底物中,B一葡萄糖苷酶对纤维二糖的活性最强.2.4反应抑制剂[9]Kempton等人研究发现Agrobacterium13一葡萄糖苷酶的一系列有机物抑制剂都与底物和过渡态结构相似,并且所有的抑制剂直接与底物竞争.有相似的过渡态结构即意味着带有相同的正电荷和综述食品研究与拜发2oo5.V oL26.NO.6相似的半椅状结构,这些抑制剂能与酶键合得更为紧密.比如,最好的抑制剂是反应过程中有相似过渡态结构的gluconolactone和gluconopheylurethane 而不同位置正电荷(如1-dexynojirimycin)的抑制剂与酶的亲和力就相对较弱,非半椅状结构(如椅式构形的is0pr0pyl—p—D—thi0uc0pyran0side和船式构形的l,6一anhydro—p-glucopyranose)的抑制剂与酶的亲和力更弱.这些抑制剂都直接与底物有竞争作用.在无机抑制剂Ag对B一葡萄糖苷酶有强的抑制作用,Hg及4mol/L脲也有较强的抑制作用,而Cu",Pb",SDS及EDTA等常见抑制剂对该酶活力无明显影响.3B一葡萄糖苷酶活性的测定方法加]1.Bamsh和swiain法:它以水杨苷作底物,酶解产物用4一氨基安替比林作显色剂,使释放出来的水杨醇显色,再用分光光度法比色测定.2.荧光法:利用伞形酮(7一轻基香豆素)与4一甲基伞形酮具强烈荧光的特点,将它们生为无荧光的底物,以此测定.3.以对硝基苯基一p—D一葡糖苷(P—NPG)作为底物进行酶解,底物水解后释放出来的配基对硝基苯酚可直接在400~420am之间测定.4B一葡萄糖苷酶的应用B一葡萄糖苷酶能参与生物体的糖代谢["],对维持生物体的正常生理功能起着重要作用.如它在酶解纤维素时就起着至关重要的作用,它把由纤维素酶降解生成的纤维二糖和三糖转化成可发酵的葡萄糖.B一葡萄糖苷酶的生物学功能使它在生产中有着广泛的应用.4.1作为食品风味酶应用[12目前,B一葡萄糖苷酶的主要应用是在食品工业中.随着食品工业的发展,风味化学的研究也引人关注.近年来,人们着重研究水果风味物质在水果中存在的前体,包括一些二级代谢产物,如糖苷类物质.这一研究领域在风味研究中被认为"前体分析".法国的Cordonnier等人1974年首次提出葡萄中可能存在键合态的不挥发的萜烯类化合物.80年代澳大利亚的Williams等人对葡萄做了比较深人细致的工作,发现葡萄中的一些风味物质如萜烯醇和芳香醇等不但以游离的形式存在,而且还以糖苷键合态的形式存在,并指出,糖苷键合态化合物的含量大大超过其游离态的含量.Engel和Tressl等经研究指出,许多水果中含有单萜烯糖苷,C(13)降异185==I类萜糖苷,倍半萜烯糖苷以及其它的糖苷,几乎所有的天然糖苷是B一糖苷,所以可以利用B一葡萄糖苷酶水解水果中的风味前体物一糖苷,释放出挥发性糖苷配基,用以增强葡萄酒等果酒,果汁香气.因此B一葡萄糖苷酶作为水果风味增香酶最合适. Shoseyov等(1990)报道分离到一株产内切B一葡萄糖苷酶的黑曲霉,用该酶来处理玫瑰红葡萄酒及西番莲果汁,通过GC—MS分析及感官评定,结果表明:葡萄酒中的单萜,苹果醇,苯乙醛等风味物质有明显提高.J.L.Iborra等(1992)则研究了甜杏B一葡萄糖苷酶水解苦藏花素,产生藏花醛(可作食品风味添加剂)的过程.可以预见,随着研究的不断深人和发展,运用这一生物技术手段提高果制品的天然风味的研究有着广泛的应用前景.4.2在青梅脱苦中应用B一葡萄糖苷酶在青梅脱苦中也有重要作用[13].梅果中有人体所需的多种氨基酸和有机酸,但也含有大量的苦味物质,主要是苦杏仁苷.据方祖达和五德裕(1988)报道,约七分成熟的新鲜梅子含苦杏仁苷高达784ppm,若是九分成熟者约含260~270ppm,约为前者三分之一.由此可见,愈成熟的果实愈不苦,但成熟的梅子制得的果汁仍然苦涩难以接受,这就是梅子过去都没有被利用做成纯天然果汁饮料的最大原因.而B一葡萄糖苷酶却可以把苦杏仁苷分解为苯甲醛及氢氰酸和两分子葡萄糖,而使苦味大大减少.虽然分解出的氢氰酸有时可使人食后中毒,但氢氰酸可在加热过程中蒸发掉,而且据七十年代《美国农业联合会》报道,一定剂量的苯甲醛和氢氰酸混合物具有防癌效果.4.3在降解纤维素中应用B一葡萄糖苷酶的另一主要应用是用于降解纤维素[14,15].纤维素酶转化纤维成葡萄糖的过程细节和作用机理还不清楚或未有定论.一般认为由内切葡聚糖作用于微纤维的非结晶区,纤维二糖水解酶再从非还原端依次分解产生纤维二糖和三糖,后者再由B一葡萄糖苷酶水解成葡萄糖.纤维素是葡萄糖以B一1,4糖苷键结合的聚合物,为植物细胞壁的构成成分,占植物干重的1/2—1/3.全球一年间由光合作用生产的纤维素达1000亿吨,是最丰富的可再生资源.将植物纤维应用于发酵食品工业原料,对人类将是一个重大的贡献,可以使我们摆脱对谷物粮食的绝对依赖,缓和世界资源紧缺.4.4在水解大豆异黄酮中应用一,,1862oo5.V o1.26.NO.6食品研究与并发综述我国水果果皮的利用现状和前景鲍金勇赵国建杨公明1.华南农业大学食品学院广州510642;2.西北农林科技大学食品科学与工程学院杨凌712100摘要:本文简述了水果果皮的结构性质和我国水果果皮加工的现状,分析了水果果皮在利用中所存在的问题,并提出相应的解决措施.关键词:水果果皮;利用现状;展望THEUTILIZINGCURRENTSITUATIONOFCHINESEFRUITPEELANDTHEPROS PECTBAOJinyongZHAOGuojianYANGGongming1.CollegeofFoodScience,SouthChinaAgriculturalUniversity,Guangzhou,510642B一葡萄糖苷酶还可以用来水解大豆异黄酮,制备高活性的大豆异黄酮苷元产品[16].酶水解条件温和,多采用弱酸性的缓冲溶液,大豆异黄酮苷元不易变性,是工业上制备富含大豆异黄酮苷元产品的十分有前途的途径.不过目前高活性的大豆异黄酮糖苷水解酶还在研制阶段,也没有进行工业化.大豆自身含有的内源B一葡萄糖苷酶水解活性不强,水解效率只有22-29%[73.添加足量的高活性酶(如苦杏仁和酵母中提取B一葡萄糖苷酶[1)可使水解达到100%.4.5其它应用[1.6.2]B一葡萄糖苷酶还可应用于乳品工业来分解乳糖,与其它酶协同作用生产葡萄糖与单细胞蛋白.多酚化合物是一类较好的天然抗氧化剂,它们在植物体中主要是以糖苷的形式存在,而苷元(即游离多酚)的抗氧化活性和防癌功效比其糖苷大得多.应用B一葡萄糖苷酶催化水解这类糖苷是一类生产天然抗氧化剂的好方法.应用p一葡萄糖苷酶可以生产天然色素.如栀子蓝色素(Gardeniablue)就是以茜草科植物栀子(GardeniaJasminoidesEllis)为原料,通过p一葡萄糖苷酶的作用而产生的一种天然色素.通过基因突变改变p一葡萄糖苷酶的氨基酸序列中活性中心中的氨基酸残基,可使p一葡萄糖苷酶的催化水解作用变为催化糖苷键生成的酶.如Withers用基因突变的方法[191把Agrobacterium3-葡萄糖苷酶358位上的谷氨酸残基用一丙氨酸替代使其成为合成低聚糖的酶.参考文献:[1]李远华.B一葡萄糖苷酶的研究进展.安徽农业大学, 2002,29(4):421—425[2]DriskillL.E.;BauerM.W.;KellyR.M.BiotechnologyandBioengineering.1999,66(1),51~6o[3]孙艳梅,张永忠.大豆B一葡萄糖苷酶的提取及其酶学性质的研究.食品工业科技,2004(1)[4]MichaelW.Bauer,RobertM.Kelly.Biochemistry,1998,37: 17170~17178[5]Julieb.Kempton,StephenG.Withes.Biochemistry,1992,31: 9961—9969[6]彭喜春等.B一葡萄糖苷酶的研究现状及应用前景.江苏食品与发酵,2001(12)[7]NamehukM.N.,andWitherss.G..Biochemistry,1995,34, 16194~16202[8]YiQ.BiotechnolBioen,1998,6o(3):385-390[9]Pitsons.M..Enzyme&MicrobialTichnology,1997,210): 183—190[10]王华夫,游小青.茶叶中B一葡萄糖苷酶活性的测定.中国茶叶,1996(3):16—17[11]LiY aw—kuen.J.Chn.Chem.Soc.(Taipei),1998,269(26): 17537—17541[12]顾卫民.B一葡萄糖苷酶的特性及其在食品工业中的应用. 江苏食品与发酵,2003(1)[13]陶宁萍.苦杏仁苷酶在纯天然青梅果汁中的应用.南京农业大学硕士论文,1993[14]周晓云.酶技术.石油工业出版社,1995[15]陈驹声.酶制剂生产技术,1994[16]孙艳梅,张永忠.水解制备大豆异黄酮苷元研究进展.食品研究与开发,2002(3)[17]MatsuuraM.,ObataA.J.FoodSci.,1993.58(1),144—147[18]PandiatanN.,HettiarachchyN.JuZ…YFoodChem.and Toxicology.2000,65(3),403—407『19]22Withers,eta1.UnitedStatesPatent5716812 收稿日期:2005-03-07。

基因相关词汇专业英语翻译Aactivation domain 活化结构域adapters 连接物adenine 腺嘌呤adenosine 腺ADP (adenosine diphosphate) 腺二磷酸affinity column 亲和柱AFLP (amplified fragment length polymorphisms) 增值性断片长度多态现象agrobacterium 农杆菌属alanine 丙氨酸allele 等位基因amber mutation 琥珀型突变AMP (adenosine monophosphate) 腺一磷酸ampicillin 氨青霉素anchor primer 锚状引物annealing 退火annealing temperature 退火温度anticodon 反密码子AP-PCR (arbitrarily primed PCR) 任意引物聚合链反应arbitrary primer 任意引物ATP (adenosine triphosphate) 腺三磷酸autosome 常染色体Bbaculovirus 杆状病毒base pair 基对base sequence 基顺序beta-galactosidase β-半乳糖beta-glucuronidase β-葡糖醛酸糖bioluminescence 生物发光bioremediation 生物降解biotechnology 生物技术blotting 印迹法blue-white selection 蓝白斑筛选blunt end 平(整末)端Ccatalyst 催化剂cDNA library 反向转录DNA库centromere 着丝体centrosome 中心体chemiluminescence 化学发光chiasma 交叉chromomere 染色粒chromoplast 有色体chromosomal aberration 染色体畸变chromosomal duplication 染色体复制chromosomal fibre 染色体牵丝chromosome 染色体chromosome complement 染色体组chromosome map 染色体图chromosome mutation 染色体突变clone 克隆cloning 无性繁殖系化codon 密码子codon degeneracy 密码简并codon usage 密码子选择cohesive end 黏性末端complementary DNA (cDNA) 反向转录DNA complementary gene 互补基因consensus sequence 共有序列construct 组成cosmids 黏性质粒crossing over 互换cyclic AMP (cAMP) 环腺酸cytosine 胞嘧啶Ddark band 暗带deamination 脱氨基作用decarboxylation 脱羧基作用degenerate code 简并密码degenerate PCR 退化性聚合链反应dehydrogenase 脱氢denaturation 变性deoxyribonucleoside diphospahte 脱氧核糖核一磷酸deoxyribonucleoside monophospahte 脱氧核糖核二磷酸deoxyribonucleoside triphospahte 脱氧核糖核三磷酸deoxyribose 去(脱)氧核糖dicarboxylic acid 二羧酸digoxigenin 洋地黄毒diploid 二倍体DNA (deoxyribonucleic acid) 去(脱)氧核糖核酸DNA binding domain DNA结合性结构域DNA fingerprinting DNA指纹图谱DNA helicase DNA解螺旋DNA kinase DNA激DNA ligase DNA连接DNA polymer DNA聚合物DNA polymerase DNA聚合double helix 双螺旋double-strand 双链Eelectroporation 电穿孔endonuclease 内切核酸enhancer 增强子enterokinase 肠激episome 游离基因ethidium bromide 溴乙锭eukaryotic 真核生物的euploid 整倍体exonuclease 外切核酸expressed-sequence tags 表达的序列标记片段extron 外含子FF factor F因子FAD (flavine adenine dinucleotide) 黄素腺嘌呤二(双)核酸feedback control 反馈控制feedback inhibition 反馈抑制feedback mechanism 反馈机制first filial (F1) generation 第一子代FISH (fluoresence in situ hybridization) 荧光原位杂交forward mutation 正向突变F-pilus F纤毛functional complementation 功能性互补作用fusion protein 融合蛋白Ggel electrophoresis 凝胶电泳gene 基因gene cloning 基因克隆gene conversion 基因转变gene duplication 基因复制gene flow 基因流动gene gun 基因枪gene interaction 基因相互作用gene locus 基因位点gene mutation 基因突变gene regulation 基因调节gene segregation 基因分离gene therapy 基因治疗geneome 基因组/ 染色体组genetic map 基因图genetic modified foods (GM foods) 基因食物genetics 遗传学genetypic ratio 基因型比/ 基因型比值genome 基因组/ 染色体组genomic library 基因组文库genotype 基因型giant chromosome 巨染色体globulin 球蛋白glucose-6-phosphate dehydrogenase 6-磷酸葡萄糖脱氢GP (glycerate phosphate) 磷酸甘油酸脂GTP (guanine triphosphate) 鸟三磷酸guanine 鸟嘌呤Hhaploid 单倍体haploid generation 单倍世代heredity 遗传heterochromatin 异染色质Hfr strain 高频重组菌株holoenzyme 全homologous 同源的housekeeping gene 家务基因hybridization 杂交Iimmunoglobulin 免疫球蛋白in vitro 在体外/ 在试管内in vivio 在体内independent assortment 独立分配induced mutation 诱发性突变induction 诱导initiation codon 起始密码子inosine 次黄insert 插入片段insertional inactivation 插入失活interference 干扰intergenic 基因间的interphase 间期intragenic 基因内的intron 内含子inversion 倒位isocaudarner 同尾酸isoschizomer 同切点JKkanamycin 卡那毒素klenow fragment 克列诺夫片段Llac operon 乳糖操纵子ligase 连接ligation 连接作用light band 明带linker 连接体liposome 脂质体locus 位点Mmap distance 图距离map unit 图距单位mature transcript 成熟转录物metaphase 中期methylase 甲基化methylation 甲基化作用microarray 微列microinjection 微注射missense mutation 错差突变molecular genetics 分子遗传学monoploid 单倍体monosome 单染色体messenger RNA (mRNA) 信使RNAmultiple alleles 复(多)等位基因mutagen 诱变剂mutagenesis 诱变mutant 突变体mutant gene 突变基因mutant strain 突变株mutation 突变mutation rate 突变率muton 突变子NNAD (nicotinamide adenine dinucleotide) 烟醯胺腺嘌呤二核酸NADP (nicotinamide adenine dinucleotide phosphate) 烟醯胺腺嘌呤二核酸磷酸nicking activity 切割活性nonsense codon 无意义密码子nonsense mutation 无意义突变Northern blot Northern印迹法nuclear DNA 核DNAnuclear gene 核基因nuclease 核酸nucleic acid 核酸nucleoside 核nucleoside triphosphate 核三磷酸nucleotidase 核酸nucleotide 核酸nucleotide sequence 核酸序列Ooligonucleotide 寡核酸one gene one polypeptide hypothesis 一个基因一种学说operon 操纵子oxidative decarboxylation 氧化脱羧作用oxidative phosphorylation 氧化磷酸化作用PPCR (polymerase chain reaction) 聚合链反应peptidepeptide bond 键phagemids 噬菌粒phosphorylation 磷酸化作用physical map 物理图谱plasmid 质粒point mutation 点突变poly(A) tail poly(A)尾polymerase 聚合polyploid 多倍体positional cloning 位置性无性繁殖系化primary transcript 初级转录物primer 引物probe 探针prokaryotic 原核的promoter 启动子protease 蛋白purine 嘌呤pyrimidine 嘧啶QRrandom segregation 随机分离RAPD (rapid amplified polymorphic DNA) 快速扩增多态DNAreading frame 阅读码框recessive gene 隐性基因recombinant 重组体recombinant DNA technology 重组DNA技术recombination 重组regulator (gene) 调控基因replica 复制物/ 印模replica plating 复制平皿(板)培养法replication 复制replication origin 复制起点reporter gene 报道基因repression 阻遏repressor 阻遏物repressor gene 阻遏基因resistance strain 抗药性菌株restriction 限制作用restriction enzyme 限制性内切restriction mapping 限制性内切图谱retrovirus 反转录病毒reverse transcription 反转录作用RFLP (restricted fragment length polymorphisms) 限制性断片长度多态现象ribonucleotide 核糖核酸ribose 核糖ribosomal RNA (rRNA) 核糖体RNAribosome 核糖体RNA (ribonucleic acid) 核糖核酸RNA polymerase I RNA聚合IRNA polymerase II RNA聚合IIRNA polymerase III RNA聚合IIIR-plasmid R质粒/ 抗药性质粒Ssecond filial (F2) generation 第二子代self-ligation 自我连接作用shuttle vectors 穿梭载体sigma factor σ因子single nucleotide polymorphism 单核酸多态性single-stranded DNA 单链DNAsister chromatid 姊妹染色单体sister chromosome 姊妹染色体site-directed mutagenesis 定点诱变somatic cell 体细胞Southern blot Southern印迹法splice 拼接star activity 星号活性stationary phase 静止生长期sticky end 黏性末端stop codon 终止密码子structural gene 结构基因supernatant 上层清液supressor 抑制基因Ttelophase 末期template 模板terminator 终止子tetracycline 四环素thymine 胸腺嘧啶tissue culture 组织培养transcription 转录作用transfer RNA (tRNA) 转移RNA transformation 转化作用transgene 转基因translation 翻译/ 平移transmembrane 跨膜triplet 三联体triplet code 三联体密码triploid 三倍体UVvector 载体WWestern blot Western印迹法Aalternative splicing -- Eukaryotic genes are composed of exons and introns, the latter being removed by RNA splicing before transcribed mRNA leaves the nucleus. Commonly, a single gene can encode several different mRNA transcripts, caused by cell- or tissue-specific combination of different exons. This is known as alternative splicing.Annealing -- The time- and temperature-dependent process by which two complementary single-stranded polynucleotides associate to form a double helix (see also hybridization)Antisense strand -- the DNA strand of a gene which, during transcription, is used as a template by RNA polymerase to synthesize a complementary RNA strand.反股-- 意指一股DN**段为基因之所在,因此可用来当做模版使得RNA反转录脢在转录RNA时,可以合成和此DN**段完全结合的RN**段。

α helix α螺旋A helical secondary structure in proteins.Pl. α helices. 蛋白质中一种螺旋形的二级结构。
复数:α helices。
α-amanitin α鹅膏蕈碱A toxin that inhibits the three eukaryotic RNA polymerases to different extents. Name derives from mushroom of genus Amanita in which toxin is found. 一种能不同程度地抑制三种真核生物RNA聚合酶的毒素。
名称来自于产生此毒素的Amanita属蘑菇。
β-galactosidase β-半乳糖苷酶Enzyme that cleaves lactose into galactose and glucose. Name origin: the bond cut by this enzyme is called a β-galactosidic bond. 将乳糖分解为半乳糖和葡萄糖的酶。
名称来源:该酶切割的键称为β-半乳糖苷键。
β sheet β折叠A secondary structure in proteins, relatively flat and formed hydrogen bonding between two parallel or anti-parallel stretches of polypeptide. 蛋白质的一种二级结构,相对平坦,在两条平行的或反向平行的肽段之间形成氢键。
σ subunit σ亚基Component of prokaryotic RNA polymerase holoenzyme. Required for recognition of promoters. 原核生物RNA聚合酶全酶的组成成分。


㊃综述㊃通信作者:崔轶霞,E m a i l :y i x i a _c yx @126.c o m 类风湿关节炎合并骨质疏松症发病机制和相关治疗药物对骨质疏松症影响的研究进展邹 琳,崔轶霞,张娜娜,陈思荣(延安大学附属医院风湿免疫科,陕西延安716000) 摘 要:类风湿关节炎是一种常见而复杂的自身免疫性疾病,临床上表现为滑膜炎症㊁血管翳形成㊁软骨退化和局灶性骨侵蚀,可累及多个系统,伴有许多关节外共病,包括骨质疏松症㊂骨质疏松症导致的关节疼痛和畸形可引起机体相关功能障碍㊁运动缺乏,从而造成严重不良后果㊂本文对类风湿关节炎合并骨质疏松症的发病机制和类风湿关节炎相关治疗药物对骨质疏松症的影响的研究进展进行综述,旨在为临床诊治提供参考㊂关键词:关节炎,类风湿;骨质疏松;发病机制;药物影响中图分类号:R 593.22 文献标志码:A 文章编号:1004-583X (2023)03-0279-06d o i :10.3969/j.i s s n .1004-583X.2023.03.016 类风湿关节炎(r h e u m a t o i da r t h r i t i s ,R A )是一种以慢性㊁侵蚀性㊁对称性多关节炎为主要临床表现的自身免疫性疾病㊂流行病学研究显示,R A 可发生于任何年龄段,其发病率随着年龄的增加呈增长趋势,在40~50岁人群中好发,女性发病率约为男性的3~5倍,全球发病率为0.5%~1%[1]㊂骨质疏松症(o s t e o po r o s i s ,O P )是一种以骨量降低和骨组织微结构破坏为特征的代谢性骨病,为R A 常见的并发症之一㊂O P 给R A 患者带来严重不良预后,使其生活质量下降,从而大大提高了R A 的致残率和病死率㊂本文对R A 合并O P 的发病机制和R A 相关治疗药物对O P 的影响的研究进展进行综述,旨在为临床诊治提供参考㊂1 R A 合并O P 的发病机制1.1 信号转导通路1.1.1 无翅型MMT V 整合位点家族/β-连环蛋白(w i n g l e s st y p e MMT V i n t e g r a t i o n s i t ef a m i l y /β-c a t e n i n ,W n t /β-c a t e n i n )信号通路 W n t 信号通路在体内参与细胞的增殖㊁迁移,组织稳态㊁再生,胚胎发育等多种生物学过程㊂W n t 蛋白与卷曲蛋白受体及低密度脂蛋白受体相关蛋白5/6(l o w -d e n s i t y l i p o p r o t e i nr e c e p t o r -r e l a t e d p r o t e i n5/6,L R P 5/6)结合,触发经典W n t /β-c a t e n i n 通路,参与R A 的发病,使间充质前体细胞和骨软骨祖细胞分化为成骨细胞,参与成骨细胞的增殖和分化㊂β-c a t e n i n 转移到细胞核中,调节O s t e r i x 信号轴及R u n x 家族转录因子2(R u n x f a m i l y t r a n s c r i pt i o n f a c t o r 2,R u n x 2)的表达[2]㊂W n t 信号传导受到卷曲蛋白受体相关蛋白㊁W n t 抑制因子1㊁硬化素和D i c k k o p f (D K K )家族蛋白等抑制剂的调节㊂硬化素拮抗L R P 5/6下游信号传导,进而抑制W n t 信号传导,增强成骨细胞凋亡,D K K -1与L R P 5/6及单通道跨膜受体K r e m e n 1/2结合,导致复合物的内化,进而抑制W n t信号传导,下调骨保护素(o s t e o p r o t e ge r i n ,O P G )和上调核因子κB (n u c l e a rf a c t o rk a p p a -B ,N F -κB )受体活化因子配体(r e c e pt o r a c t i v a t o r o f N F -κB l i ga n d ,R A N K L )的表达[3]㊂1.1.2 骨形态发生蛋白(b o n e m o r p h o ge n e t i c pr o t e i n ,B M P )/果蝇与秀丽隐杆线虫蛋白同源物(h o m o l o g u e s o ft h e d r o s o p h i l a p r o t e i n ,m o t h e r s a g a i n s t d e c a p e n t a p l e gi c a n d t h e c a e n o r h a b d i t i s e l e ga n s p r o t e i n ,S m a d )信号通路 B M P 是转化生长因子-β(t r a n s f o r m i n gg r o w t hf a c t o r -β,T G F -β)家族中最大的亚类,可刺激软骨细胞的增殖和分化,S m a d 蛋白将T G F -β与其受体结合产生的信号从细胞质传递到细胞核,从而调节成骨细胞和破骨细胞分化,在骨骼发育㊁骨形成和骨稳态过程发挥作用[4]㊂B M P 以S m a d 1/5/8(B -S m a d s )为信号传导器,与细胞表面具有丝氨酸㊁苏氨酸激酶活性的受体B M P 受体Ⅰ/Ⅱ结合磷酸化,磷酸化的B -S m a d s 与S m a d 4形成复合物在细胞核中积累,与转录辅助因子或抑制因子结合后再与目标基因的调控元件结合进行转录调控[5]㊂经典B M P -S m a d 信号通路的每一步都受到正性或负性因子调控,骨形成蛋白和激活蛋白膜结合抑制物(b o n em o r p h o ge n e t i c p r o t e i na n d a c t i v a t i n g p r o t e i n m e m b r a n e b i n d i n g in h i b i t o r ,B AM B I )被称为T G F -β伪受体,与B M P 受体Ⅰ结构相似但缺乏丝氨酸/苏氨酸磷激酶信号传导结构域,㊃972㊃‘临床荟萃“ 2023年3月20日第38卷第3期 C l i n i c a l F o c u s ,M a r c h20,2023,V o l 38,N o .3Copyright ©博看网. All Rights Reserved.B AM B I与B M P受体Ⅱ结合后形成异构复合物,阻止B M P-S m a d信号传导[6]㊂1.1.3磷脂酰肌醇-3-激酶(p h o s p h a t i d y l i n o s i t o l-3-k i n a s e,P I3K)/蛋白激酶B(p r o t e i nk i n a s eB,A k t)信号通路 P I3K-A k t信号通路受生长因子和细胞因子的调节,通过与受体酪氨酸激酶和G-蛋白偶联受体结合后磷酸化胞内的酪氨酸残基发挥作用[7]㊂P I3K-A k t信号通路调控成骨细胞和破骨细胞的存活和分化,进而维持骨稳态,并且与调控成骨分化的通路有密切联系㊂W n t/β-c a t e n i n信号通路受到小鼠糖原合成酶激酶-3β形成的降解复合物的调节, A k t通过磷酸化小鼠糖原合成酶激酶-3β使其失活丧失蛋白功能而影响W n t/β-c a t e n i n通路[8]㊂此外, A k t可以负调控B M P-S m a d通路中S m a d,抑制活化的S m a d1和S m a d5在细胞核聚集,抑制B M P-S m a d 信号传导[9]㊂1.1.4J a n u s激酶信号转导和转录激活因子(J a n u s k i n a s e/s i g n a l t r a n s d u c e r a n d a c t i v a t o r o f t r a n s c r i p t i o n,J A K/S T A T)信号通路J A K/S T A T 信号通路是多种细胞因子㊁激素和生长因子的重要下游介质,在骨发育㊁代谢和愈合中起重要作用[10]㊂白细胞介素(i n t e r l e u k i n,I L)-6通过糖蛋白130激活糖蛋白130相关的J A K,从而诱导S T A T3激活的J A K/S T A T通路上调R A N K L,导致破骨细胞分化,骨吸收增强[11]㊂W a n g等[12]研究发现,降低I L-6水平抑制了J A K/S T A T信号通路相关基因的m R N A 水平,减少了S T A T1蛋白表达并增加了成骨分化,相反增强I L-6的表达增加了S T A T1蛋白表达及J A K/S T A T信号通路相关基因的m R N A水平,降低成骨细胞的分化㊂1.1.5神经源性位点缺口同源蛋白(n e u r o g e n i c l o c u sn o t c h h o m o l o gp r o t e i n,N o t c h)信号通路N o t c h信号通路主要由N o t c h受体㊁N o t c h配体㊁转录因子及N o t c h信号调节分子组成,也包括N o t c h 的4种受体蛋白(N o t c h-1㊁2㊁3㊁4)及5种配体蛋白(D e l t a-1㊁3㊁4,J a g g e d-1㊁2),在先天性和获得性免疫的识别㊁调节中发挥作用,与T细胞㊁B细胞及巨噬细胞等免疫细胞作用调节炎症反应,参与R A[13-14]㊂此外,N o t c h通路在调控与维持骨稳态方面发挥重要的作用,参与破骨细胞的分化㊁成熟,影响软骨形成㊁骨形成和骨吸收㊂然而,不同N o t c h受体-配体结合激活的N o t c h通路存在不同的调控作用,如N o t c h1/ J a g g e d1轴抑制破骨细胞生成,而N o t c h2/D e l t a1轴促进破骨细胞生成[15]㊂1.1.6 H e d g e h o g信号通路 H e d g e h o g信号通路由H e d g e h o g信号蛋白㊁P t c h e d㊁S m o o t h e n e d特异性受体㊁G l i蛋白和下游靶基因4部分构成,H e d g e h o g 信号蛋白分为S h h㊁I h h和D h h,其中S h h分布最广,阳性表达率高,相关研究最多[16]㊂经典的H e d g e h o g 信号通路参与R A的发病㊂有研究发现,R A患者的S h h水平与类风湿因子(r h e u m a t o i d f a c t o r,R F)及抗环瓜氨酸肽(c y c l i cc i t r u l l i n a t e d p e p t i d e,C C P)抗体呈正相关,S h h信号通过细胞外信号调节丝裂原活化蛋白激酶信号介导R A成纤维样滑膜细胞的增殖和迁移[17]㊂此外,H e d g e h o g信号通路在骨髓间充质干细胞向成骨细胞分化及调控成骨细胞的增殖㊁分化㊁活性中发挥重要作用[18]㊂1.1.7新兴的骨质重塑调节因素微小R N A (m i c r o R N A,m i R N A)是非编码的单链R N A分子,在骨细胞内发挥转录后的调控作用,长链非编码R N A和环状R N A参与骨代谢的调节[19]㊂一些m i R N A针对W n t信号级联的关键成分,通过靶向部分互补序列的m R N A来转录调节基因表达,导致W n t信号的减弱或增强,例如,m i R-37c㊁m i R-23a㊁m i R-30e通过靶向W n t受体或共同受体(W n t3㊁L R P5/6)使W n t信号传导减少,使成骨细胞分化减少,相反,m i R-27a㊁m i R-142㊁m i R-29a㊁m i R-218㊁m i R-98㊁m i R-335㊁m i R-542通过靶向卷曲蛋白受体相关蛋白㊁D K K-1㊁硬化素等W n t抑制剂和负调控因子诱导W n t信号传导和成骨分化[20]㊂此外,虽然2022年L i a n g等[21]针对m i R N A对骨细胞活动的影响进行了探讨,涉及W n t信号通路㊁R u n x2㊁B M P/ S m a d s通路㊁R A N K L通路等多条信号通路,虽然这些研究仅涉及动物模型和队列研究,转化为临床有待测试,却对发现O P的潜在治疗靶点具有重要意义㊂1.2炎症因子众所周知,炎症因子的过度表达可增加R A患者发生O P的风险,以肿瘤坏死因子-α(t u m o rn e c r o s i s f a c t o rα,T N F-α)㊁I L-6和I L-1为代表的促炎细胞因子诱导R A N K L的表达刺激破骨细胞分化,增强骨吸收,打破骨形成和骨吸收间的平衡㊂1.2.1 T N F-α T N F-α在R A中的骨吸收作用已经被证实,推测其可能通过上调破骨细胞前体N F-κB受体活化因子和巨噬细胞集落刺激因子受体的表达,进而促进破骨细胞增殖,增强破骨细胞活性,影响破骨细胞分化[22]㊂然而,T N F-α不是破骨细胞形成㊁发生侵蚀性关节炎和骨溶解所必须的,这些改变在T N F-α缺乏的条件下也可发生㊂Z o u等[23]认为, T N F-α是破骨细胞生成的自分泌因子,在R A N K L㊃082㊃‘临床荟萃“2023年3月20日第38卷第3期 C l i n i c a l F o c u s,M a r c h20,2023,V o l38,N o.3Copyright©博看网. All Rights Reserved.的诱导下,破骨细胞生成增加并且随着T N F-α的增加而增加㊂1.2.2I L-1和I L-6I L-1直接作用于破骨细胞表面的I L-1受体,激活N F-κB,在T N F-α诱导破骨细胞形成的过程中发挥桥梁作用,且通过作用于前列腺素E2的合成影响破骨细胞形成,参与多核破骨细胞形成[24]㊂I L-6在R A患者的滑膜中大量表达,诱导关节炎症和损伤,软骨退化,关节侵蚀和变窄,I L-6与I L-6受体结合由J A K/S T A T信号通路介导直接促进R A患者成纤维细胞样滑膜细胞中R A N K L的表达,并且是T N F-α和I L-17诱导R A N K L所必需的[25]㊂此外,I L-6水平与慢性滑膜炎和关节破坏的严重程度相关,且I L-6水平降低可作为患者良好预后的标志[26]㊂1.2.3自身抗体瓜氨酸化蛋白抗体(a n t i-c i t r u l l i n a t ed p r o te i na n t i b o d i e s,A C P A s)通过与细胞表面瓜氨酸波形蛋白结合或形成免疫复合物调节细胞功能,A C P A s存在于R A发病的早期,在R A患者血清和关节滑膜内与破骨细胞前体细胞结合,诱导T N F-α的表达和释放,刺激破骨细胞分化,直接诱导骨丢失[27]㊂与A C P A s阴性的R A患者相比, A C P A s阳性的R A患者关节周围骨的破坏程度更明显,骨小梁结构改变㊁体积变小㊁数量变少㊁网络不均匀性增加,并且A C P A s与R F产生叠加效应,与R A 侵蚀关节数目及严重程度有关,A C P A s和R F均阳性的R A患者关节侵蚀程度更重[28-29]㊂E n g d a h l 等[30]研究发现,瓜氨酸波形蛋白所诱发关节炎较甲基化牛血清白蛋白轻,但关节周围骨丢失更明显,可能与抗瓜氨酸波形蛋白作用于关节周围骨髓中破骨细胞前体和增加成熟破骨细胞的浸润而触发关节周围骨丢失有关,这解释了在R A患者早期炎症发作前为什么骨量已经改变,甚至在A C P A s阳性而未确诊的R A患者中就已经发生骨量减少㊂1.3维生素D缺乏与肌少症维生素D缺乏与自身免疫性疾病的发病机制有关,低维生素D水平被认为是R A发病的危险因素,可抑制单核细胞向树突状细胞分化,减少了抗原提呈细胞向T细胞分化,且作用于B细胞,从而使免疫球蛋白的产生减少[31]㊂约有半数R A患者存在25-羟基维生素D缺乏(<20 n g/m l),中老年人低25-羟基维生素D水平与低骨量的相关性已被证明,25-羟基维生素D除了参与骨骼和钙代谢,还在免疫调节中也发挥重要作用㊂有研究报道,R A患者血清25-羟基维生素D水平明显降低,且与R A易感性有关,与疾病活动呈负相关[32]㊂肌少症是一种以肌肉质量㊁力量和功能低下为特征的全身性疾病,增加患者身体残疾㊁生活质量低下和死亡的风险㊂缺乏物理锻炼被认为是肌少症的首要危险因素,除此之外,激素和细胞因子失衡也会导致肌肉质量和力量的损失,极端肌肉损失发生由与年龄相关的激素浓度下降及促炎细胞因子(T N F-α㊁I L-6)所介导[33]㊂L i a n等[34]招募了549名来自中国的R A患者和158名对照受试者,并对其进行骨密度和骨骼肌质量测量,结果显示肌少症在R A患者中的发生率为61.7%,患有肌少症R A患者的年龄㊁病程㊁血沉㊁C反应蛋白水平㊁28个关节疾病活动度评分和健康评估问卷-残疾指数显著升高㊂并且C h u 等[35]研究显示,肌少症和维生素D缺乏对R A患者椎体骨质疏松性骨折具有协同作用,可能与维生素D 不足损害肌肉功能有关㊂2R A相关治疗药物对O P的影响2.1激素糖皮质激素(g l u c o c o r t i c o i d s,G C)在R A的治疗中运用广泛,长期大剂量G C对骨骼产生的负面影响是众所周知的,然而短期小剂量G C治疗的作用仍存在争议㊂全身性应用G C对骨形成和骨吸收有很大的抑制作用,特别是长期大剂量给药可促进破骨细胞形成,抑制成骨细胞活性,且促进肾脏对钙的排泄,抑制肠道对钙的吸收,减少性激素分泌,导致肌肉萎缩和步态障碍,进而增加骨质流失和骨折风险,即使在G C停药后,骨折风险仍可持续1年[36]㊂实际上,小剂量G C(<10m g/d)可以减少R A患者的全身炎症反应,对骨的保护作用能够抵消其对骨的不良影响,处于疾病早期和活动期的R A 患者使用小剂量G C不会出现显著的骨密度改变,尤其是泼尼松剂量<5m g/d[37-38]㊂然而,临床上G C 的不规范使用并不少见,受文化水平及诊疗条件等影响,大多R A患者将G C当做灵丹妙药而长期大剂量口服,糖皮质激素性骨质疏松患者大量出现,骨质疏松性骨折也越来越多㊂一项基于中国人的队列研究显示,R A使用过G C组O P的发生率为41.6%,明显高于未使用G C组(29.4%),并与使用疗程有关,而与G C每日剂量无关,且骨量丢失多发生在G C治疗的第一年,在最初治疗的3~6个月,糖皮质激素性骨质疏松的患病率没有明显增加,维持在30.0%左右,但在治疗6~12个月迅速上升,12个月后随着G C疗程的延长而向上波动[39]㊂为了最大限度地降低骨折风险,临床实践指南建议,对于预计在3~6个月内每天接受相当于5~7.5m g泼尼松治疗的患者,应给予抗O P药物治疗[40]㊂2.2传统合成改善病情抗风湿药(d i s e a s e-㊃182㊃‘临床荟萃“2023年3月20日第38卷第3期 C l i n i c a l F o c u s,M a r c h20,2023,V o l38,N o.3Copyright©博看网. All Rights Reserved.m o d i f y i n g a n t i r h e u m a t i cd r u g s,D MA R D s)临床上普遍使用G C联合传统合成D MA R D s治疗R A,目前常用的传统合成D MA R D s包括甲氨蝶呤(m e t h o t r e x a t e,MT X)㊁羟氯喹(h y d r o x y c h l o r o q u i n e,H C Q)㊁来氟米特㊁柳氮磺吡啶和新型小分子抗风湿药艾拉莫德,这些药物不仅在治疗R A中具有突出作用,对骨密度也具有显著影响㊂低剂量MT X(ɤ25m g/周)是欧洲风湿病防治联合会(E U L A R)指南推荐治疗R A的一线用药, MT X通过上调血清O P G,降低R A N K L的表达,竞争性抑制R A N K和R A N K L的结合以及调节R A N K L/R A N K/O P G通路来抑制骨破坏,与G C联合使用可预防骨侵蚀和关节破坏[41]㊂然而,近年来越来越多长期使用MT X的患者出现了所谓的 MT X骨病 ,发生多发性跟骨/胫骨远端和近端以及股骨远端干骺端不全性骨折,可能与MT X抑制原始骨髓基质细胞增殖,损害成骨细胞增殖及活性有关[42]㊂H C Q可能影响成骨细胞和破骨细胞形成,体外研究表明,H C Q降低了成熟破骨细胞的细胞内p H值,抑制破骨细胞的形成,导致骨吸收减少[43]㊂来氟米特抑制基质金属蛋白酶(MM P-1㊁MM P-9)的表达,从而抑制关节骨的破坏,是唯一一个与腰椎骨密度显著增加相关的传统合成D MA R D s[44-45]㊂柳氮磺吡啶可能会增加氧化应激,通过减少R A N K L 的表达㊁增加O P G的表达来抑制破骨细胞的形成[46]㊂艾拉莫德抑制环氧合酶-2而起到抗炎作用,抑制骨吸收,促进骨形成[47]㊂临床上MT X单一疗法失败的R A患者常联合传统合成D MA R D s进行治疗,C a r b o n e等[48]研究发现,单独或联合使用MT X㊁H C Q㊁柳氮磺吡啶对骨折风险没有显著影响㊂2.3生物制剂随着生物制剂的问世,R A的治疗有了重大突破,以T N F-α拮抗剂(英夫利昔单抗㊁阿达木单抗㊁依那西普)为代表的早期生物制剂作为治疗R A的第四代药物,具有高选择性药理作用及低毒副作用的特点,较传统合成D MA R D s起效更快㊁作用更强㊂T N F-α拮抗剂已经被证实可减轻R A患者关节骨侵蚀㊁全身骨量减少及滑膜炎,英夫利昔单抗不仅可以缓解关节炎症,还可以减少全身骨损失和抑制肌腱炎症[49]㊂阿达木单抗减轻脊柱和髋关节骨质流失,特别是在开始治疗的第一年,而手部和髋部的骨密度分别在治疗1年和4年后持续下降[50]㊂I L-6抑制剂托珠单抗能够有效控制R A炎症反应,延缓关节损伤,并且托珠单抗联合MT X消除了疾病活动和关节损伤间的相关性[51]㊂P o u t o g l i d o u等[52]通过构建C I A模型发现,托珠单抗可使肌腱组织中T N F-α和I L-23显著降低,并且明显改善小梁微结构,改善肌腱形态㊂靶向小分子药物J A K抑制剂托法替尼抑制J A K/S T A T信号传导进而减弱I L-6功能,促进成骨分化,降低破骨细胞的分化和活性,并且不损害软骨分化[53]㊂新型生物制剂阿巴西普是治疗R A的第五代药物,直接抑制T细胞活化,对股骨颈骨密度的增加有显著影响[54]㊂3结论综上,R A和O P的发病在信号转导通路及炎症因子间相互作用㊁相互联系,并且与维生素D水平及物理锻炼息息相关,R A治疗药物对O P的影响也不容小觑㊂进一步探究这些因素与R A合并O P的关系可提供更有效的治疗方式,减少R A患者骨折的发生风险,改善R A患者生活质量㊂参考文献:[1] T a n a k aY.M a n a g i n g o s t e o p o r o s i s a n d j o i n t d a m a g e i n p a t i e n t sw i t hr h e u m a t o i d a r t h r i t i s:A n o v e r v i e w[J].J C l i n M e d,2021,10(6):1241.[2] M i a oC G,Y a n g Y Y,H eX,e t a l.W n t s i g n a l i n gp a t h w a y i nr h e u m a t o i da r t h r i t i s,w i t hs p e c i a le m p h a s i so nt h ed i f f e r e n tr o l e s i ns y n o v i a l i n f l a mm a t i o na n db o n er e m o d e l i n g[J].C e l lS i g n a l,2013,25(10):2069-2078.[3] C i c iD,C o r r a d o A,R o t o n d o C,e ta l.W n ts i g n a l i n g a n db i o l o g ic a l t h e r a p y i n r h e u m a t o id a r t h r i t i s a n d s p o n d y l o a r t h r i t i s[J].I n t JM o l S c i,2019,20(22):5552.[4] Z o u M L,C h e nZ H,T e n g Y Y,e ta l.T h eS m a dd e p e n d e n tT G F-βa n d B M Ps i g n a l i n gp a t h w a y i n b o n er e m o d e l i n g a n dt h e r a p i e s[J].F r o n tM o l B i o s c i,2021,8:593310. [5] B a l Z,K u s h i o k a J,K o d a m a J,e t a l.B M Pa n dT G Fβu s e a n dr e l e a s e i n b o n e r e g e n e r a t i o n[J].T u r k JM e d S c i,2020,50(S I-2):1707-1722.[6] T o d d GM,G a o Z,H y vön e n M,e t a l.S e c r e t e d B M Pa n t a g o n i s t s a n d t h e i r r o l e i nc a n c e ra n db o n e m e t a s t a s e s[J].B o n e,2020,137:115455.[7] K i m E H,S u r e s h M.R o l eo fP I3K/A k t s i g n a l i n g i n m e m o r yC D8Tc e l l d i f f e r e n t i a t i o n[J].F r o n t I mm u n o l,2013,4:20.[8] C a s eN,M a M,S e n B,e ta l.B e t a-c a t e n i nl e v e l si n f l u e n c er a p i dm e c h a n i c a lr e s p o n s e s i no s t e o b l a s t s[J].JB i o lC h e m,2008,283(43):29196-29205.[9] A g a sD,S a b b i e t iM G,M a r c h e t t iL,e ta l.F G F-2e n h a n c e sR u n x-2/S m a d s n u c l e a r l o c a l i z a t i o n i n B M P-2c a n o n i c a ls i g n a l i n g i no s t e o b l a s t s[J].JC e l lP h y s i o l,2013,228(11):2149-2158.[10] D a m e r a u A,G a b e r T,O h r n d o r f S,e t a l.J A K/S T A Ta c t i v a t i o n:A g e n e r a l m e c h a n i s m f o rb o n e d e v e l o p m e n t,h o m e o s t a s i s,a n dr e g e n e r a t i o n[J].I n tJ M o lS c i,2020,21(23):9004.[11]S i m s N A.T h e J A K1/S T A T3/S O C S3a x i s i n b o n ed e v e l o p m e n t,p h y s i o l o g y,a n d p a t h o l o g y[J].E x p M o lM e d,2020,52(8):1185-1197.㊃282㊃‘临床荟萃“2023年3月20日第38卷第3期 C l i n i c a l F o c u s,M a r c h20,2023,V o l38,N o.3Copyright©博看网. All Rights Reserved.[12] W a n g H,L i L,Z h a n g N,e t a l.V i t a m i n K2i m p r o v e so s t e o g e n i cd i f f e r e n t i a t i o nb y i n h i b i t i n g S T A T1v i at h eB c l-6a n d I L-6/J A Ki n C3H10T1/2c l o n e8c e l l s[J].N u t r i e n t s,2022,14(14):2934.[13] Z h o uB,L i n W,L o n g Y,e ta l.N o t c hs i g n a l i n gp a t h w a y:A r c h i t e c t u r e,d i s e a s e,a n d t h e r a p e u t i c s[J].S i g n a lT r a n s d u c tT a r g e tT h e r,2022,7(1):95.[14]吴晶艺,陆欣辰,陈广洁.N o t c h信号通路在类风湿关节炎发病机制中的研究进展[J].现代免疫学,2023,43(2):144-149.[15]S e k i n e C,K o y a n a g i A,K o y a m a N,e t a l.D i f f e r e n t i a lr e g u l a t i o n o fo s t e o c l a s t o g e n e s i sb y N o t c h2/D e l t a-l i k e1a n dN o t c h1/J a g g e d1a x e s[J].A r t h r i t i sR e sT h e r,2012,14(2): R45.[16]S uY,X i n g H,K a n g J,e t a l.R o l e o f t h eh e d g e h o g s i g n a l i n gp a t h w a y i n r h e u m a t i c d i s e a s e s:A n o v e r v i e w[J].F r o n tI mm u n o l,2022,13:940455.[17] Q i nS,S u nD,L iH,e ta l.T h ee f f e c to fS HH-G l i s i g n a l i n gp a t h w a y o n t h e s y n o v i a l f i b r o b l a s t p r o l i f e r a t i o n i nr h e u m a t o i da r t h r i t i s[J].I n f l a mm a t i o n,2016,39(2):503-512.[18]王雨荷,刘红,李艳,等.H e d g e h o g-G l i信号通路在骨质疏松发生中作用的研究进展[J].中国骨质疏松杂志,2021,27(10):1550-1553.[19] Y a n g T L,S h e n H,L i u A,e t a l.A r o a d m a p f o ru n d e r s t a n d i n g m o l e c u l a r a n d g e n e t i c d e t e r m i n a n t s o f o s t e o p o r o s i s[J].N a tR e vE n d o c r i n o l,2020,16(2):91-103.[20] B e l l a v i aD,D eL u c aA,C a r i n aV,e t a l.D e r e g u l a t e dm i R N A si nb o n eh e a l t h:E p i g e n e t i cr o l e si n o s t e o p o r o s i s[J].B o n e,2019,122:52-75.[21] L i a n g B,B u r l e y G,L i nS,e ta l.O s t e o p o r o s i s p a t h o g e n e s i sa n d t r e a t m e n t:E x i s t i n g a n de m e r g i n g a v e n u e s[J].C e l lM o lB i o l L e t t,2022,27(1):72.[22] Z h a L,H e L,L i a n g Y,e t a l.T N F-αc o n t r i b u t e s t op o s t m e n o p a u s a l o s t e o p o r o s i s b y s y n e r g i s t i c a l l y p r o m o t i n gR A N K L-i n d u c e d o s t e o c l a s t f o r m a t i o n[J].B i o m e dP h a r m a c o t h e r,2018,102:369-374.[23] Z o u W,A m c h e s l a v s k y A,T a k e s h i t a S,e ta l.T N F-a l p h ae x p r e s s i o n i s t r a n s c r i p t i o n a l l y r e g u l a t e d b y R A N K l i g a n d[J].JC e l l P h y s i o l,2005,202(2):371-378.[24]颜廷鑫,王诗军,姜俊杰,等.I L-1与骨质疏松研究进展[J].中国骨质疏松杂志,2022,28(3):460-464.[25] H a s h i z u m eM,H a y a k a w aN,M i h a r aM.I L-6t r a n s-s i g n a l l i n gd i re c t l y i n d u c e sR A N K Lo nf i b r o b l a s t-l i k e s y n o v i a l c e l l s a n d i si n v o l v e d i n R A N K Li n d u c t i o nb y T N F-a l p h aa n dI L-17[J].R h e u m a t o l o g y(O x f o r d),2008,47(11):1635-1640. [26] Y i p R M L,Y i m C W.R o l eo f i n t e r l e u k i n6i n h i b i t o r s i nt h em a n a g e m e n to fr h e u m a t o i da r t h r i t i s[J].JC l i n R h e u m a t o l, 2021,27(8):e516-e524.[27] C a t r i n aA,K r i s h n a m u r t h y A,R e t h iB.C u r r e n tv i e wo nt h ep a t h o g e n i c r o l e o f a n t i-c i t r u l l i n a t e d p r o t e i n a n t i b o d i e s i n r h e u m a t o i da r t h r i t i s[J].RM D O p e n,2021,7(1):e001228.[28] H e c h tC,E n g l b r e c h tM,R e c h J,e t a l.A d d i t i v e e f f e c t o f a n t i-c i t r u l l i n a t ed p r o te i na n t i b o d i e s a n dr h e u m a t o i df a c t o ro nb o n ee r o s i o n s i n p a t i e n t sw i t hR A[J].A n nR h e u m D i s,2015,74(12):2151-2156.[29] K o c i j a n R,H a r r e U,S c h e t t G.A C P A a n d b o n el o s si nr h e u m a t o i da r t h r i t i s[J].C u r rR h e u m a t o l R e p,2013,15(10): 366.[30] E n g d a h l C,B a n g H,D i e t e l K,e t a l.P e r i a r t i c u l a r b o n e l o s s i na r t h r i t i si s i n d u c e db y a u t o a n t i b o d i e s a g a i n s tc i t r u l l i n a t e dv i m e n t i n[J].JB o n eM i n e rR e s,2017,32(8):1681-1691.[31] G a t e n b y P,L u c a sR,S w a m i n a t h a n A.V i t a m i nDd e f i c i e n c ya n d r i s kf o rr h e u m a t i cd i s e a s e s:A n u p d a t e[J].C u r r O p i nR h e u m a t o l,2013,25(2):184-191.[32] L e eY H,B a eS C.V i t a m i nD l e v e l i n r h e u m a t o i d a r t h r i t i s a n di t s c o r r e l a t i o n w i t ht h ed i s e a s ea c t i v i t y:A m e t a-a n a l y s i s[J].C l i nE x p R h e u m a t o l,2016,34(5):827-833.[33] D h i l l o n R J,H a s n i S.P a t h o g e n e s i s a n d m a n a g e m e n t o fs a r c o p e n i a[J].C l i nG e r i a t rM e d,2017,33(1):17-26.[34] L i a nL,W a n g J X,X u Y C,e ta l.S a r c o p e n i a m a y b ear i s kf a c t o r f o ro s t e o p o r o s i si n C h i n e s e p a t i e n t s w i t hr h e u m a t o i da r t h r i t i s[J].I n t JG e n M e d,2022,15:2075-2085.[35] C h uY R,X uS Q,W a n g J X,e t a l.S y n e r g y o f s a r c o p e n i aa n dv i t a m i n D d e f i c i e n c y i n v e r t e b r a l o s t e o p o r o t i cf r a c t u r e si n r h e u m a t o i d a r t h r i t i s[J].C l i nR h e u m a t o l,2022,41(7):1979-1987.[36] A m i c h e MA,A b t a h iS,D r i e s s e n J HM,e ta l.I m p a c to fc u m u l a t i v e e x p o s u r e t o h i g h-d o se o r a l g l u c o c o r t i c o i d s o nf r a c t u r er i s ki n D e n m a r k:A p o p u l a t i o n-b a s e d c a s e-c o n t r o ls t u d y[J].A r c hO s t e o p o r o s,2018,13(1):30.[37] B l a v n s f e l d tA G,d e T h u r a h A,T h o m s e n M D,e ta l.T h ee f f e c to f g l u c o c o r t i c o i d so n b o n e m i n e r a ld e n s i t y i n p a t i e n t sw i t hr h e u m a t o i d a r t h r i t i s:A s y s t e m a t i cr e v i e w a n d m e t a-a n a l y s i so fr a n d o m i z e d,c o n t r o l l e dt r i a l s[J].B o n e,2018,114:172-180.[38] G u a nY,H a o Y,G u a n Y,e ta l.T h ee f f e c to fv i t a m i n Ds u p p l e m e n t a t i o n o n r h e u m a t o i d a r t h r i t i s p a t i e n t s:As y s t e m a t i c r e v i e w a n d m e t a-a n a l y s i s[J].F r o n t M e d(L a u s a n n e),2020,7:596007.[39] M aC C,X uS Q,G o n g X,e ta l.P r e v a l e n c ea n dr i s kf a c t o r sa s s o c i a t e dw i t h g l u c o c o r t i c o i d-i n d u c e do s t e o p o r o s i s i nC h i n e s ep a t i e n t sw i t h r h e u m a t o i d a r t h r i t i s[J].A r c hO s t e o p o r o s,2017, 12(1):33.[40] G r o s s m a nJ M,G o r d o n R,R a n g a n a t h V K,e ta l.A m e r i c a nC o l l e g e o f R h e u m a t o l o g y2010r e c o mm e n d a t i o n s f o r t h ep r e v e n t i o n a n d t r e a t m e n t o f g l u c o c o r t i c o i d-i n d u c e do s t e o p o r o s i s[J].A r t h r i t i s C a r e R e s(H o b o k e n),2010,62(11):1515-15126.[41] K a n a g a w aH,M a s u y a m aR,M o r i t a M,e ta l.M e t h o t r e x a t ei n h i b i t s o s t e o c l a s t o g e n e s i s b y d e c r e a s i n g R A N K L-i n d u c e dc a l c i u mi n f l u xi n t oo s t e o c l a s t p r o g e n i t o r s[J].JB o n e M i n e rM e t a b,2016,34(5):526-531.[42] R u f f e rN,K r u s c h e M,B e i lF T,e ta l.C l i n i c a lf e a t u r e so fm e t h o t r e x a t e o s t e o p a t h y i n r h e u m a t i c m u s c u l o s k e l e t a ld i se a s e:A s y s t e m a t i cr e v i e w[J].S e m i n A r t h r i t i s R h e u m,2022,52:151952.[43] B o t h T,Z i l l i k e n s M C,S c h r e u d e r s-K o e d a m M,e t a l.㊃382㊃‘临床荟萃“2023年3月20日第38卷第3期 C l i n i c a l F o c u s,M a r c h20,2023,V o l38,N o.3Copyright©博看网. All Rights Reserved.H y d r o x y c h l o r o q u i n ea f f e c t sb o n er e s o r p t i o nb o t h i nv i t r oa n di nv i v o[J].JC e l l P h y s i o l,2018,233(2):1424-1433.[44] L i t i n s k y I,P a r a n D,L e v a r t o v s k y D,e ta l.T h ee f f e c t so fl e f l u n o m i d eo nc l i n i c a l p a r a m e t e r sa n ds e r u ml e v e l so f I L-6,I L-10,MM P-1a n d MM P-3i n p a t i e n t s w i t h r e s i s t a n tr h e u m a t o i da r t h r i t i s[J].C y t o k i n e,2006,33(2):106-110.[45] K w o n O C,O hJ S,H o n g S,e ta l.C o n v e n t i o n a ls y n t h e t i cd i se a s e-m o d if y i ng a n t i rh e u m a ti c d r u g s a n d b o n e m i n e r a ld e n s i t y i n r h e u m a t o i d a r t h r i t i s p a t i e n t s w i t h o s t e o p o r o s i s:P o s s i b l e b e n e f i c i a l e f f e c t o f l e f l u n o m i d e[J].C l i n E x pR h e u m a t o l,2019,37(5):813-819.[46] L e eC K,L e e E Y,C h u n g S M,e ta l.E f f e c t s o f d i s e a s e-m o d i f y i n g a n t i r h e u m a t i c d r u g s a n d a n t i i n f l a mm a t o r y c y t o k i n e s o nh u m a no s t e o c l a s t o g e n e s i s t h r o u g h i n t e r a c t i o nw i t h r e c e p t o ra c t i v a t o r o f n u c l e a r f a c t o r k a p p a B,o s t e o p r o t e g e r i n,a n dr e c e p t o r a c t i v a t o r o f n u c l e a r f a c t o r k a p p a B l i g a n d[J].A r t h r i t i sR h e u m,2004,50(12):3831-3843.[47]王静,赵庆杰,卓小斌,等.类风湿性关节炎的治疗药物研究进展[J].药学实践杂志,2019,37(6):485-490. [48] C a r b o n eL,V a s a n S,E l a m R,e ta l.T h e a s s o c i a t i o n o fm e t h o t r e x a t e,s u l f a s a l a z i n e,a n dh y d r o x y c h l o r o q u i n eu s ew i t hf r a c t u r e i n p o s t m e n o p a u s a lw o m e nw i t hr h e u m a t o i da r t h r i t i s:F i n d i n g s f r o mt h eW o m e n'sH e a l t hI n i t i a t i v e[J].J B M RP l u s,2020,4(10):e10393.[49] P o u t o g l i d o uF,P o u r z i t a k i C,M a n t h o u M E,e t a l.I n f l i x i m a bp r e v e n t s s y s t e m i c b o n e l o s s a n d s u p p r e s s e s t e n d o ni n f l a mm a t i o ni n ac o l l a g e n-i n d u c e d a r t h r i t i sr a t m o d e l[J].I n f l a mm o p h a r m a c o l o g y,2021,29(3):661-672.[50] K r i e c k a e r t C L,N u r m o h a m e dM T,W o l b i n kG,e t a l.C h a n g e si n b o n e m i n e r a l d e n s i t y d u r i n g l o n g-t e r m t r e a t m e n t w i t ha d a l i m u m ab i n p a t i e n t s w i t hr h e u m a t o i da r t h r i t i s:Ac o h o r ts t u d y[J].R h e u m a t o l o g y(O x f o r d),2013,52(3):547-553.[51]S m o l e n J S,A v i l a J C,A l e t a h a D.T o c i l i z u m a b i n h i b i t sp r o g r e s s i o no f j o i n t d a m a g e i n r h e u m a t o i d a r t h r i t i s i r r e s p e c t i v e o fi t s a n t i-i n f l a mm a t o r y e f f e c t s:D i s a s s o c i a t i o n o ft h el i n kb e t w e e n i n f l a mm a t i o na n dd e s t r uc t i o n[J].A n n R h e u m D i s,2012,71(5):687-693.[52] P o u t o g l i d o u F,P o u r z i t a k i C,M a n t h o u M E,e t a l.T h ei n h i b i t o r y e f f e c t o ft o c i l i z u m a b o n s y s t e m i c b o n el o s s a n dt e n d o n i n f l a mm a t i o n i n a j u v e n i l e c o l l a g e n-i n d u c e d a r t h r i t i s r a tm o d e l[J].C o n n e c tT i s s u eR e s,2022,63(6):577-589. [53] G a b e rT,B r i n k m a n A C K,P i e n c z i k o w s k i J,e t a l.I m p a c to fj a n u s k i n a s e i n h i b i t i o n w i t h t o f a c i t i n i b o n f u n d a m e n t a l p r o c e s s e s o f b o n e h e a l i n g[J].I n t JM o l S c i,2020,21(3):865.[54] T a d aM,I n u iK,S u g i o k aY,e t a l.A b a t a c e p tm i g h t i n c r e a s eb o n e m i n e r a l d e n s i t y a t f e m o r a l n ec k f o r p a t i e n t s w i t hr h e u m a t o i d a r t h r i t i si n c l i n i c a l p r a c t i c e:A I R T I G H T s t u d y[J].R h e u m a t o l I n t,2018,38(5):777-784.收稿日期:2022-10-31编辑:王晶璇㊃482㊃‘临床荟萃“2023年3月20日第38卷第3期 C l i n i c a l F o c u s,M a r c h20,2023,V o l38,N o.3Copyright©博看网. All Rights Reserved.。

Lesson One 细胞器的结构和功能Actin:肌动蛋白,是微丝的结构蛋白, 以两种形式存在, 即单体和多聚体。
basal body::基体,真核细胞的纤毛或鞭毛基底部由微管及其相关蛋白质构成的短筒状结构,是纤毛和鞭毛的微管组织中心。
centriole:中心粒,动物、某些藻类和菌类细胞中的圆筒状细胞器,位于间期细胞核附近或有丝分裂细胞的纺锤体极区中心。
chemotaxis:趋化性,即由介质中化学物质的浓度差异形成的刺激所引起的趋向性。
chloroplast:叶绿体,绿色植物细胞内进行光合作用的结构,是一种质体。
chromosome:染色体,实质是脱氧核甘酸,为细胞核内由核蛋白组成、能用碱性染料染色、有结构的线状体,是遗传物质基因的载体。
cilia:纤毛,从一些原核细胞和真核细胞表面伸出的、能运动的突起。
cytoplasm:胞质,由细胞质基质、内膜系统、细胞骨架和包涵物组成。
cytoskeleton:细胞骨架,真核细胞中与保持细胞形态结构和细胞运动有关的纤维网络。
包括微管、微丝和中间丝。
dynein:动力蛋白,即纤毛中的一种具有ATP酶活性的巨大的蛋白质复合体。
endoplasmic reticulum:内质网,指细胞质中一系列囊腔和细管,彼此相通,形成一个隔离于细胞质基质的管道系统。
flagella:鞭毛,在某些细菌菌体上具有细长而弯曲的丝状物,是细菌的运动器官。
Golgi complex:高尔基复合体,由许多扁平的囊泡构成的以分泌为主要功能的细胞器。
lysosome:溶酶体,真核细胞中一种膜包围的异质的消化性细胞器。
是细胞内大分子降解的主要场所。
microfilament:微丝,由肌动蛋白分子螺旋状聚合成的纤丝,又称肌动蛋白丝,是细胞骨架的主要成分之一。
microtubule:微管,由微管蛋白原丝组成的不分支的中空管状结构,是细胞骨架成分,与细胞支持和运动有关。
mitochondrion:线粒体,真核细胞中由双层高度特化的单位膜围成的细胞器。
第一章绪论一简答题1. 21世纪是生命科学的世纪。
20世纪后叶分子生物学的突破性成就,使生命科学在自然科学中的位置起了革命性的变化。
试阐述分子生物学研究领域的三大基本原则,三大支撑学科和研究的三大主要领域?答案:(1)研究领域的三大基本原则:构成生物大分子的单体是相同的;生物遗传信息表达的中心法则相同;生物大分子单体的排列(核苷酸,氨基酸)导致了生物的特异性。
(2)三大支撑学科:细胞学,遗传学和生物化学。
(3)研究的三大主要领域:主要研究生物大分子结构与功能的相互关系,其中包括DNA和蛋白质之间的相互作用;激素和受体之间的相互作用;酶和底物之间的相互作用。
2. 分子生物学的概念是什么?答案:有人把它定义得很广:从分子的形式来研究生物现象的学科。
但是这个定义使分子生物学难以和生物化学区分开来。
另一个定义要严格一些,因此更加有用:从分子水平来研究基因结构和功能。
从分子角度来解释基因的结构和活性是本书的主要内容。
3 二十一世纪生物学的新热点及领域是什么?答案:结构生物学是当前分子生物学中的一个重要前沿学科,它是在分子层次上从结构角度特别是从三维结构的角度来研究和阐明当前生物学中各个前沿领域的重要学科问题,是一个包括生物学、物理学、化学和计算数学等多学科交叉的,以结构(特别是三维结构)测定为手段,以结构与功能关系研究为内容,以阐明生物学功能机制为目的的前沿学科。
这门学科的核心内容是蛋白质及其复合物、组装体和由此形成的细胞各类组分的三维结构、运动和相互作用,以及它们与正常生物学功能和异常病理现象的关系。
分子发育生物学也是当前分子生物学中的一个重要前沿学科。
人类基因组计划,被称为“21世纪生命科学的敲门砖”。
“人类基因组计划”以及“后基因组计划”的全面展开将进入从分子水平阐明生命活动本质的辉煌时代。
目前正迅速发展的生物信息学,被称为“21世纪生命科学迅速发展的推动力”。
尤应指出,建立在生物信息基础上的生物工程制药产业,在21世纪将逐步成为最为重要的新兴产业;从单基因病和多基因病研究现状可以看出,这两种疾病的诊断和治疗在21世纪将取得不同程度的重大进展;遗传信息的进化将成为分子生物学的中心内容”的观点认为,随着人类基因组和许多模式生物基因组序列的测定,通过比较研究,人类将在基因组上读到生物进化的历史,使人类对生物进化的认识从表面深入到本质;研究发育生物学的时机已经成熟。
部分招生单位研究生入学历年考试真题2004年北京师范大学攻读硕士研究生入学考试试题一、名词解释(每题3分,共45分)1.Ames试验 2.包膜 J.胞吞作用 4.不亲和性 5.Col质粒 6.端粒7.附加体 8.感染复数 9.回文结构 10.末端重复 11.轻链 12.噬菌体展示 13.卫星RNA 14.致育因子 15.原毒素二、简要回答题(每题5分,共45分)1.说明控制微生物生长繁殖的主要方法及原理。
2.SASR病毒粒子及其基因组的基本结构是什么?3.以简要的图示和文字说明酿酒酵母菌的生活史。
4.溶源性细菌有哪些特性?5.什么是细菌群体的生长曲线?它在生产上有哪些应用?6.病毒壳体结构有哪几种对称形式?病毒粒子主要结构类型有哪些?7.固氮微生物中大多为好氧菌,它们如何保证固氮酶既不被氧灭活,又能提供必要的氧产生ATP进行固氮?8.说明红硫细菌,枯草杆菌,硝化细菌的营养及获能方式。
9.什么是病毒的一步生长曲线?该曲线中各时期的特点是什么?三、试验设计(每题15分,共30分)1.设计一个实验程序,以确保在对未知菌进行革兰氏染色时操作正确,结果可靠。
2.设计一套从自然界筛选分离一株对聚氯联苯类农药降解能力高的菌株的方案。
四、问答题(每题15分,共30分)1.什么是营养缺陷型?如何从诱变菌株中筛选出营养缺陷型。
2.光合细菌有哪几类?细菌的光合作用与绿色植物的光合作用之间有什么不同?2005年北京师范大学攻读硕士研究生入学考试试题一、名词解释(每题3分,共45分)1.半抗原 2.表型 3.病毒入胞 4.病毒因子 5.超敏反应 6.反向末端重复 7.分段基因组 8.富集培养 9.干扰素 IO.感受态细胞 11.核壳12.类囊体 13.免疫原性 14.原养型 15.微生物传感器二、简要回答题(每题5分,共45分)1.RNA是微生物的遗传物质吗?为什么?2.HIV病毒粒子中的逆转录酶的生物学功能是什么?3.以简要的图示和文字说明路德类酵母菌的生活史。
AAbundance (mRNA 丰度):指每个细胞中mRNA 分子的数目。
Abundant mRNA(高丰度mRNA):由少量不同种类mRNA组成,每一种在细胞中出现大量拷贝。
Acceptor splicing site (受体剪切位点):内含子右末端和相邻外显子左末端的边界。
Acentric fragment(无着丝粒片段):(由打断产生的)染色体无着丝粒片段缺少中心粒,从而在细胞分化中被丢失。
Active site(活性位点):蛋白质上一个底物结合的有限区域。
Allele(等位基因):在染色体上占据给定位点基因的不同形式。
Allelic exclusion(等位基因排斥):形容在特殊淋巴细胞中只有一个等位基因来表达编码的免疫球蛋白质。
Allosteric control(别构调控):指蛋白质一个位点上的反应能够影响另一个位点活性的能力。
Alu-equivalent family(Alu 相当序列基因):哺乳动物基因组上一组序列,它们与人类Alu家族相关。
Alu family (Alu家族):人类基因组中一系列分散的相关序列,每个约300bp长。
每个成员其两端有Alu 切割位点(名字的由来)。
α-Amanitin(鹅膏覃碱):是来自毒蘑菇Amanita phalloides 二环八肽,能抑制真核RNA聚合酶,特别是聚合酶II 转录。
Amber codon (琥珀MM子):核苷酸三联体UAG,引起蛋白质合成终止的三个MM子之一。
Amber mutation (琥珀突变):指代表蛋白质中氨基酸MM子占据的位点上突变成琥珀MM子的任何DNA 改变。
Amber suppressors (琥珀抑制子):编码tRNA的基因突变使其反MM子被改变,从而能识别UAG MM子和之前的MM子。
Aminoacyl-tRNA (氨酰-tRNA):是携带氨基酸的转运RNA,共价连接位在氨基酸的NH2基团和tRNA 终止碱基的3¢或者2¢-OH 基团上。
基金项目:上海市农业科学院作物所创新基金(编号:作创字2021 C X G04);上海市农产品保鲜加工专业技术服务平台(编号:21D Z 2292200)作者简介:陈冰洁,女,上海市农业科学院助理研究员,硕士.通信作者:乔勇进(1967 ),男,上海市农业科学院研究员,博士.E Gm a i l :y j qi a o 2002@126.c o m 收稿日期:2022G11G23㊀㊀改回日期:2023G05G02D O I :10.13652/j .s p jx .1003.5788.2022.81093[文章编号]1003G5788(2023)07G0165G07金瓜多糖不同分级组分的抗氧化和降血糖活性A n t i o x i d a n t a n dh y p o g l y c e m i ca c t i v i t i e s o f d i f f e r e n t gr a d e d f r a c t i o n s o f p o l ys a c c h a r i d e f r o m g o l d e n m e l o n 陈冰洁1,2C H E N B i n g Gj i e 1,2㊀乔勇进1,2Q I A OY o n g Gj i n 1,2㊀王㊀晓1,2WA N G X i a o 1,2㊀王春芳1,2WA N GC h u n Gf a n g 1,2㊀刘贵阁1,2L I UG u i Gge 1,2(1.上海市农业科学院作物育种栽培研究所,上海㊀201403;2.上海农产品保鲜加工工程技术研究中心,上海㊀201403)(1.I n s t i t u t e o f C r o p B r e e d i n g a n dC u l t i v a t i o n ,S h a n g h a iA c a d e m y o f A g r i c u l t u r eS c i e n c e s ,S h a n g h a i 201403,C h i n a ;2.A g r i GF o o dS t o r a g e a n dP r o c e s s i n g E n g i n e e r i n g T e c h n o l o g y R e s e a r c hC e n t e r o fS h a n g h a i ,S h a n gh a i 201403,C h i n a )摘要:目的:研究金瓜多糖不同分级组分的理化特性㊁结构㊁抗氧化性和降血糖活性.方法:采用水提醇沉法分级纯化得到4种金瓜多糖(C P G40㊁C P G60㊁C P G70㊁C P G80),通过高效液相色谱㊁傅里叶变换红外光谱等对其分子量㊁单糖组成㊁基团构成㊁抗氧化和降血糖活性进行研究.结果:金瓜多糖组分随着乙醇体积分数的增加,总糖含量升高,糖醛酸含量降低.4种多糖分子量不同,单糖组成相同但组成比例不同,且均具有多糖类物质的特征吸收峰.金瓜多糖体外抗氧化能力和降血糖活性存在明显的量 效关系,C P G70对D P P H 自由基㊁羟自由基和超氧阴离子自由基清除作用高于其他组,抗氧化性最强,C P G80对αG葡萄糖苷酶和αG淀粉酶抑制作用最强,降血糖活性最佳.结论:金瓜多糖具有良好的抗氧化性和降血糖活性.关键词:金瓜;多糖;体外抗氧化性;降血糖活性A b s t r a c t :O b je c t i v e :T h e s t u d y a i m e d t o r e s e a r c h t h e p h y s i c o c h e m i c a l p r o p e r t i e s ,s t r u c t u r e ,a n t i o x i d a n ta c t i v i t y a n d h y p o g l y c e m i ca c t i v i t y o fd if f e r e n t f r a c t i o n so f p o l ys a c c h a r i d eo f go l d e n m e l o n .M e t h o d s :F o u rk i n d so fgo l d e n m e l o n p o l y s a c c h a r i d e s (C P G40,C P G60,C P G70,C P G80)w e r eo b t a i n e d b y gr a d e d p u r i f i c a t i o n o f w a t e r e x t r a c t i o n a n d a l c o h o l i c p r e c i pi t a t i o n .T h e i r m o l e c u l a rw e i gh t ,m o n o s a c c h a r i d ec o m p o s i t i o n ,g r o u p c o m p o s i t i o n ,a n t i o x id a n ta n dh y p o g l y ce m i c a c t i v i t i e s w e r e i n v e s t i g a t e d b y h i g h p e rf o r m a n c e l i qu i d c h r o m a t o g r a p h y a n d f o u r i e r t r a n s f o r m i n f r a r e d s p e c t r o s c o p y .R e s u l t s :W i t ht h e i n c r e a s eo f e t h a n o l v o l u m e f r a c t i o n ,t h et o t a ls u g a r c o n t e n t i n c r e a s e d a n d t h e g l u c u r o n i c a c i d c o n t e n t d e c r e a s e d .T h e m o l e c u l a r w e i g h t s o f t h e s e f o u r p o l ys a c c h a r i d e s w e r e d i f f e r e n t ,w h i c h i n d i c a t e d b y t h e s a m e c o m p o s i t i o n o f m o n o s a c c h a r i d e sb u td i f f e r e n tc o m p o s i t i o nr a t i o s ,a n dt h e y al l h a d t h ec h a r a c t e r i s t i ca b s o r p t i o n p e a k so f p o l y s a c c h a r i d e s .T h e a n t i o x i d a n t c a p a c i t y a n d h y p o g l y c e m i c a c t i v i t y of t h e p o l y s a c c h a r i d e s i nv i t r o h a d o b v i o u s q u a n t i t y Ge f f e c t r e l a t i o n s h i p s ,C P G70h a dt h es t r o n ge s ta n t i o x i d a n tef f e c to n D P P H r a d i c a l s ,h y d r o x y l r a d i c a l sa n ds u p e r o x i d ea n i o n ss c a v e ng i n g th a n o t h e r g r o u p s ,w hi l eC P G80h a dt h eb e s th y p o g l y c e m i ca c t i v i t y s i n c e i t h a d t h e s t r o n g e s t i n h i b i t o r y e f f e c t s o n αGg l u c o s i d a s e a n d αGa m y l a s e .C o n c l u s i o n :T h e p o l y s a c c h a r i d eo f g o l d e n m e l o n h a d g o o d a n t i o x i d a n t p r o p e r t i e s a n dh y p o g l y c e m i c a c t i v i t y .K e yw o r d s :g o l d e n m e l o n ;p o l y s a c c h a r i d e ;a n t i o x i d a n t a c t i v i t y ;h y p o g l y c e m i c a c t i v i t y金瓜(C u c u r b i t a p e po L .v a r .m e d u l l o s a A l e f .)又名金丝瓜㊁搅瓜和面条瓜,是葫芦科南瓜属美洲南瓜(C u c u r b i t a p e po L .)的一个变种,因果皮呈金黄色而得名[1].金丝瓜在欧美地区及东南亚地区均有栽培,目前中国南北各地均广泛种植,其中以上海市崇明县的金瓜最著名[2].金瓜和中国南瓜同为南瓜属,南瓜(C u c u r b i t am o s c h a t a )多糖是公认的降糖活性物质且具有较好的抗氧化功能[3-4],但目前并未针对性地对金瓜多糖及其相关功能特性进行研究,不利于金瓜的开发利用.乙醇体积分数会直接影响所获得多糖的结构及其性质.研究拟采用梯度醇沉的方法对金瓜多糖进行分级处理,考察乙醇体积分数对多糖理化性质㊁分子量㊁单糖组成㊁基团构成㊁抗氧化和降血糖活性等的影响,阐释金瓜多糖的结构561F O O D &MA C H I N E R Y 第39卷第7期总第261期|2023年7月|Copyright ©博看网. All Rights Reserved.特征与活性之间的相关性,旨在为制备具有特定营养活性的金瓜多糖提供理论依据.1㊀材料与方法1.1㊀材料与试剂金瓜:崇金3号,上海崇明生产基地;1,1G二苯基G2G三硝基苯肼(D P P H )㊁甘露糖㊁鼠李糖㊁葡萄糖醛酸㊁葡萄糖和半乳糖:光谱级,美国S i gm a 公司;透析袋:3500D ,上海源叶生物科技有限公司;其他试剂均为分析纯.1.2㊀仪器与设备电热恒温鼓风干燥箱:L D 0O G101G3型,上海龙跃仪器设备有限公司;冷冻干燥机:T e l s t a r (L y o Q u e s t )型,西班牙T e l s t a r 公司;酶标仪:MQ X 200型,美国百特仪器有限公司;高效液相色谱:A g i l e n t 1100型,美国安捷伦公司;傅里叶红外光谱仪:N I C O L E T G380型,美国N i c o l e t公司.1.3㊀方法1.3.1㊀金瓜多糖的提取及分级醇沉㊀根据刘继攀等[5]的方法修改,选取肉质呈橘黄色的金瓜,除去果皮和籽粒后将果肉切成薄片,50ħ烘干12h,粉碎机粉碎成粉末.称取500g 的金瓜粉加入20倍的蒸馏水,于85ħ提取3次,提取时间分别为3,2,2h .收集滤液,8000r /m i n 离心5m i n ,收集上清液,真空浓缩,加入95%的乙醇醇沉12h 以上,离心得沉淀物并透析(M w c u t Go f f 3500D a )3d ,浓缩,冷冻干燥得粗多糖.粗多糖配成10%的多糖溶液后,与无水乙醇混合使乙醇体积分数达到40%,醇沉12h ,离心㊁沉淀冷冻干燥得到40%醇沉组分,上述上清液与乙醇溶液混合使乙醇体积分数达到50%,重复醇沉干燥过程得到50%醇沉多糖,以此类推得到60%,70%,80%醇沉多糖,得到金瓜多糖的5种组分分别命名为:C P G40㊁C P G50㊁C P G60㊁C P G70㊁C P G80.称重并按式(1)计算其得率.y =m 1m 2ˑ100%,(1)式中:c 得率,%;m 1 各组分质量,g;m 2 粗多糖质量,g.1.3.2㊀金瓜多糖理化性质测定(1)总糖含量:采用苯酚 硫酸法[6],以葡萄糖为标准品.(2)蛋白质含量:采用考马斯亮蓝法[7],以牛血清白蛋白为标准品.(3)葡萄糖醛酸含量:采用硫酸 咔唑法[8],以葡萄糖醛酸为标准品.1.3.3㊀金瓜多糖分子量测定㊀采用H P G P C 法[9].1.3.4㊀金瓜多糖的单糖组成分析㊀采用高效液相色谱法.根据文献[10],修改如下:多糖样品(10m g )与三氯乙酸(4m L ,2m o l /L ,T F A )混匀后加入密封管90ħ下水解4h ,用氮吹仪吹干T F A 后加入甲醇溶液重复操作2~3次.N a OH 溶液调节至中性,进行1G苯基G3G甲基G5G吡唑啉酮(P M P )衍生.1.3.5㊀金瓜多糖红外光谱(F T GI R )分析㊀将样品与K B r 按m 样品ʒm K B r =1ʒ100的比例混合,研磨均匀,压成薄片后在4000~500c m -1范围内用F T GI R 仪扫描.1.3.6㊀体外抗氧化能力测定(1)D P P H 自由基清除能力:根据文献[11].(2)羟自由基清除能力:根据文献[12].(3)超氧阴离子自由基清除能力:根据文献[13].(4)还原力:根据文献[14].1.3.7㊀降血糖活性测定㊀根据文献[15].1.4㊀数据处理所有数据均以3次重复后的平均值ʃ标准差(S D )表示.样品间差异的显著性通过单因素方差分析(O r i gi n P r o 8.5)进行.由O r i gi n 软件制作图表.2㊀结果与分析2.1㊀理化性质金瓜粗多糖分别经体积分数40%,50%,60%,70%,80%的乙醇醇沉后得到5种多糖组分:C P G40㊁C P G50㊁C P G60㊁C P G70㊁C P G80.由于C P G50提取率极低,故不做后续研究.由表1得出,多糖的得率和组成随醇沉时乙醇体积分数的不同而变化.各组分多糖的提取率存在显著差异(P <0.05),C P G40的提取率较高,达51.1%,远远高于其他组分.随着乙醇体积分数的增加,金瓜多糖的总糖含量升高,说明多糖含量随乙醇体积分数的升高而增加,表1㊀多糖的理化性质†T a b l e 1㊀P h y s i c o c h e m i c a l p r o p e r t i e s o f p o l ys a c c h a r i d e s %组分提取率总糖含量葡萄糖醛酸含量蛋白质含量C P G4051.10ʃ0.11d45.08ʃ0.21a21.26ʃ0.33d1.22ʃ0.06C P G604.90ʃ0.08a 45.99ʃ0.17b 16.12ʃ0.11c 1.03ʃ0.04C P G705.80ʃ0.07b 57.42ʃ0.22c 14.16ʃ0.09b 1.06ʃ0.03C P G8013.80ʃ0.09c61.52ʃ0.08d 10.14ʃ0.14a 1.14ʃ0.09㊀㊀㊀㊀㊀㊀㊀㊀㊀†㊀同列字母不同表示差异显著(P <0.05).661营养与活性N U T R I T I O N &A C T I V I T Y 总第261期|2023年7月|Copyright ©博看网. All Rights Reserved.可能与高浓度的乙醇能沉淀更多小分子的多糖有关.葡萄糖醛酸含量随醇沉浓度的增大而降低,4种多糖中均含有一定量的糖醛酸,说明金瓜多糖各组分均是酸性多糖.同时,各醇沉组分均含有少量蛋白质,推测金瓜多糖中可能含有部分糖蛋白.2.2㊀分子量如图1所示,C PG40㊁C PG60为单一对称峰(其中21.575处为溶剂峰),说明在乙醇体积分数为40%和60%的条件下沉淀所得的金瓜多糖纯度较高,根据葡聚糖标准曲线y=-0.341x+10.685(R2=0.996),得出C PG40和C PG60的相对分子量分别为26.8,11.5k D a.而C PG70㊁C PG80除了溶剂峰外,还有1个不对称的峰,说明存在不同相对分子量的金瓜多糖组分,其中C PG70㊁C PG80中占比较大的多糖的分子量为10.0,8.1k D a,表明体积分数较低的乙醇醇沉的是大分子量的多糖,而体积分数较高的乙醇醇沉的主要是小分子量的多糖,与蔡冰洁等[16]的研究结果一致.2.3㊀单糖组成由表2和图2可知,C PG40㊁C PG60㊁C PG70㊁C PG80的单糖组成一样,均含有甘露糖㊁鼠李糖㊁葡萄糖醛酸㊁葡萄糖和半乳糖,但组成比例不同.2.4㊀红外分析如图3所示,3400c m-1处较宽的吸收峰(O H键伸缩振动)和2900c m-1处的吸收峰(C H键伸缩振动)被认为是多糖的特征吸收峰.1732c m-1处的吸收峰为果胶类多糖中甲氧基的特征吸收峰[17],证明了多糖中甲氧基的存在.1600,1415c m-1处的两个吸收峰来自于羧基的不对称和对称伸缩振动,证明多糖中糖醛酸的存在[18],与表1测定结果一致.1013c m-1处的吸收峰可能是由吡喃环伸缩振动引起的,这也是多糖的典型吸收图1㊀多糖分子量检测F i g u r e1㊀D e t e r m i n a t i o no fm o l e c u l a rw e i g h t o f p o l y s a c c h a r i d e表2㊀多糖单糖组成T a b l e2㊀M o n o s a c c h a r i d e c o m p o s i t i o no f p o l y s a c c h a r i d e s%组分单糖组成(以摩尔百分比表示)甘露糖鼠李糖葡萄糖醛酸葡萄糖半乳糖C PG4010.848.1220.367.538.81C PG607.865.3411.9322.4716.94C PG7011.788.1618.2112.308.26C PG804.713.568.2832.5830.40峰[19].推测出不同浓度乙醇沉淀所得4种金瓜多糖都具有多糖的典型基团,且所含基团无明显差异,与周慧吉等[20]研究的结果相似,分级醇沉对多糖红外光谱影响较小.2.5㊀多糖的抗氧化活性如图4(a)所示,随着多糖质量浓度的增加,D P P H自由基的清除活性逐渐增加,表现出良好的剂量依赖关系.其中C PG70的D P P H自由基清除作用最强,明显高于其他组分,当多糖质量浓度为0.90m g/m L时,C PG70对761|V o l.39,N o.7陈冰洁等:金瓜多糖不同分级组分的抗氧化和降血糖活性Copyright©博看网. All Rights Reserved.M a n .甘露糖㊀R h a .鼠李糖㊀G l c u a .葡萄糖醛酸㊀G l c .葡萄糖㊀G a l .半乳糖图2㊀单糖组成色谱图F i g u r e 2㊀C h r o m a t o g r a mo fm o n o s a c c h a r i d e c o m po s i t i on 图多糖红外光谱扫描图F i g u r e 3㊀I n f r a r e d s p e c t r u mo f p o l ys a c c h a r i d e s D P P H 自由基的清除能力达76.47%,低于阳性对照V C (97.44%),可见金瓜多糖C P G70具有明显清除D P P H 自由基的能力.C P G70和V C 的I C 50分别是0.118,0.482m g /m L .㊀㊀从图4(b )可以看出,4种多糖对羟自由基的清除能力呈一定的剂量依赖性,当多糖质量浓度为0.9m g/m L 时,C P G40㊁C P G60㊁C P G70和C P G80的羟自由基清除率分别为28.02%,32.74%,35.65%,26.66%,明显小于阳性对照V C 的,说明金瓜多糖有一定的羟自由基清除作用,且C P G70的清除能力略高于其他组分.由图4(c)可得出,金瓜多糖的还原能力低于V C ,随着多糖质量浓度的增加,其相应的还原力逐渐增加,但不同醇沉组分还原力不同,C P G70的还原力高于其他组分,当多糖质量浓度为0.9m g /m L 时,还原力表现为C P G70>C P G60>C P G80>C P G40.这与王佳等[21]报道指出的不同体积分数乙醇(30%,50%,70%,90%)分级沉淀得到虎杖多糖(P C P G30㊁P C P G50㊁P C P G70㊁P C P G90)中P C P G70还原力最强的结果相似.从图4(d )可以看出,4种多糖和V C 清除超氧阴离子自由基能力具有浓度依赖性,且清除作用大小为:V C >C P G70>C P G80>C P G40>C P G60,V C 清除超氧阴离子自由基能力显著高于各组分多糖,且金瓜多糖醇沉组分间抑制作用差异不显著.多糖的分子量和多糖理化性质是影响多糖抗氧化活性的重要因素,研究[22]表明,低分子量的多糖具有更好的抗氧化活性;也有研究[23]证实,糖醛酸含量越高的多糖表现出越强的抗氧化活性,由此得出金瓜多糖C P G70较强的抗氧化性与其分子量和糖醛酸含量密切相关,但可能还受多糖结构和构象的影响,这有待后续的深入研究.综合以上抗氧化性指标可知,金瓜多糖具有较好的清除D P P H 自由基能力和还原能力,以及一定的清除羟自由基及超氧阴离子自由基的能力.通过对比发现,金瓜多糖的抗氧化性高于南瓜多糖,金瓜多糖(C P G70)质量浓度为0.9m g /m L 时,D P P H 自由基清除率为76.47%,而南瓜纯化组分S L W P P G3质量浓度为1.0m g/m L 时,861营养与活性N U T R I T I O N &A C T I V I T Y 总第261期|2023年7月|Copyright ©博看网. All Rights Reserved.图4㊀多糖的抗氧化能力F i g u r e 4㊀A n t i o x i d a n t c a p a c i t y o f p o l ys a c c h a r i d e D P P H 自由基清除率为43.7%[24].同时,金瓜清除超氧阴离子自由基能力[25]及还原力[26]也高于南瓜多糖.说明金瓜多糖具有良好的抗氧化活性,可作为一种天然的抗氧化剂.2.6㊀多糖的降血糖活性如图5所示,各组分多糖在试验浓度范围内对αG葡萄糖苷酶㊁αG淀粉酶均有显著的抑制作用(P <0.05),且随着乙醇体积分数的增加,抑制能力增强,即C P G80>C P G70>C P G60>C P G40,但阿卡波糖的降血糖作用优于金瓜多糖.此外,在试验浓度范围内,种多糖组分对αG葡萄糖苷酶的抑制作用优于αG淀粉酶.通过比较得出:当多糖质量浓度为2m g/m L 时,C P G80的αG葡萄糖苷酶和αG淀粉酶抑制率分别为47.11%,44.02%,而南瓜多糖对αG葡萄糖苷酶和αG淀粉酶抑制率分别为17.8%,30.4%,证实金瓜多糖的降血糖活性优于南瓜多糖的降血糖活性[4].魏鑫悦等[15]研究证实,多糖的降血糖活性与其分子量相关,在合适的范围内,分子量越低的多糖组分对αG葡萄糖苷酶和αG淀粉酶抑制活性的抑制效果越高,这与C P 具有较小分子量且具有较高的降血糖活性结果一图5㊀多糖的降血糖能力F i g u r e 5㊀H y p o g l y c e m i c e f f e c t o f p o l ys a c c h a r i d e 961|V o l .39,N o .7陈冰洁等:金瓜多糖不同分级组分的抗氧化和降血糖活性Copyright ©博看网. All Rights Reserved.致.同时,有研究[27]证明,多糖抑制αG葡萄糖苷酶的活性与多糖的组成有关,当多糖主要单糖组成由G a l㊁G l c㊁M a n构成时,具有较强的αG葡萄糖苷酶抑制活性.试验中C PG80的G a l㊁G l c和M a n所占比例高于其他组分,因此对αG葡萄糖苷酶的抑制活性最强.3㊀结论试验研究了不同体积分数乙醇沉淀对金瓜多糖理化性质㊁结构和活性的影响,发现随着乙醇体积分数的增加,多糖的总糖含量升高,糖醛酸含量降低;不同醇沉组分的分子量不同,单糖组成(组成比例存在差异)及所含基团相同;金瓜多糖组分C PG70具有较好的抗氧化作用,金瓜多糖组分C PG80具有较好的降血糖作用.金瓜多糖优良的抗氧化和降血糖活性有助于其在医药㊁保健品及化妆品中发挥作用,筛选出活性较高的组分.研究对多糖的理化性质㊁抗氧化和降血糖活性进行了分析,但是对其结构表征较少,后续研究可在多糖分离纯化的基础上采用核磁㊁甲基化㊁圆二色谱技术等对多糖结构进行分析.同时,可以通过细胞及动物模型深入研究金瓜多糖的抗氧化及降血糖的作用机理.参考文献[1]刘晨霞,乔勇进,王晓,等.崇明金瓜贮藏保鲜技术研究进展[J].农产品加工,2020(3):69G72,78.LIU C X,QIAO Y J,WANG X,et al.Advances in research on quality changes and storage and preservation techniques of postharvest Chongming Goldenmelon[J].Farm Products Processing, 2020(3):69G72,78.[2]沙勤,朱爱萍,乔勇进,等.金瓜果实贮藏特性评价指标的确定与应用[J].中国瓜菜,2020(6):46G49.SHA Q,ZHU A P,QIAO Y J,et al.Determination and application of evaluation indexes for storage characteristics of goldenGmelon[J]. Chinese Melon and Vegetable,2020(6):46G49.[3]WANG S,LU A,ZHANG L,et al.Extraction and purification ofpumpkin polysaccharides and their hypoglycemic effect[J].International Journal of Biological Macromolecules,2017,98: 182G187.[4]LI F,WEI Y L,LIANG L,et al.A novel lowGmolecularGmasspumpkin polysaccharide:Structural characterization,antioxidant activity,and hypoglycemic potential[J].Carbohydrate Polymers, 2021,251:117090.[5]刘继攀,李佳欢,张紫华,等.分级醇沉对真姬菇多糖抗氧化活性的影响[J].食品科技,2018,43(1):210G214,221.LIU J P,LI J H,ZHANG Z H,et al.Effect of step alcohol precipitation on antioxidant properties of Hyspizygus marmoreus polysaccharides[J].Food Science and Technology,2018,43(1):210G214,221.[6]DUBOIS M,GILLES K A,HAMILTON J K,et al.Colorimetricmethod for determination of sugars and related substances[J]. Analytical Chemistry,1956,28(3):350G356.[7]BRADFORD M M.A rapid and sensitive method for thequantitation of microgram quantities of protein utilizing the principle of proteinGdye binding[J].Analytical Biochemistry,1976, 72(1/2):248G254.[8]杨钊,周星彤,于甜甜,等.海藻酸钠中糖醛酸含量测定方法的研究[J].中国卫生检验杂志,2018,28(9):1049G1050,1053.YANG Z,ZHOU X T,YU T T,et al.Study on determination method of uronic acid content in sodium alginate[J].Chinese Journal of Health Laboratory T echnology,2018,28(9):1049G1050,1053.[9]蒋茂婷,冉艳红,刘娜,等.蒜皮水溶性多糖的制备及结构表征[J].食品科学,2022,43(6):57G65.JIANG M T,RAN Y H,LIU N,et al.Preparation and structural characterization of waterGsoluble polysaccharide from Garlic skin[J]. Food Science,2022,43(6):57G65.[10]戴军,朱松,汤坚,等.PMP柱前衍生高效液相色谱法分析杜氏盐藻多糖的单糖组成[J].分析测试学报,2007,26(2): 206G210.DAI J,ZHU S,TANG J,et al.Analysis of monosaccharide compositions in polysaccharides from D.Salina by preGcolumn derivatization high performance liquid chromatography[J].Journal of Instrumental Analysis,2007,26(2):206G210.[11]HU X Q,WANG J L,JING Y S,et al.Structural elucidation and invitro antioxidant activities of a new heteropolysaccharide from Litchi chinensis[J].Drug Discoveries&Therapeutics,2015,9(2): 116G122.[12]CHEN Y X,LIU X Y,XIAO Z,et al.Antioxidant activities ofpolysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations[J].International Journal of Biological Macromolecules,2016,430(22):29G38.[13]CHEN B J,SHI M J,CUI S,et al.Improved antioxidant and antiGtyrosinase activity of polysaccharide from Sargassum fusiforme by degradation[J].International Journal of Biological Macromolecules,2016,92:715G722.[14]KUMARAN A,JOEL KARUNAKRAN R.Antioxidant and freeradical scavenging activity of an aqueous extract of Coleus aromaticus[J].Food Chemistry,2006,97(1):109G114.[15]魏鑫悦,陈克保,关统伟.攀枝花黑松露多糖的抗氧化和降血糖活性[J].现代食品科技,2022,38(3):1G7.WEI X Y,CHEN K B,GUAN T W.Antioxidant and hypoglycemic activity of polysaccharide from Panzhihua Black Truffle[J]. Modern Food Science and Technology,2022,38(3):1G7.[16]蔡冰洁,汪苗苗,刘咏.枳椇多糖的分级醇沉及其免疫调节活性的研究[J].合肥工业大学学报,2018,41(6):708G714.CAI B J,WANG M M,LIU Y.Graded ethanol precipitation and immunoregulatory activity of polysaccharide from Hovenia dulcis peduncles[J].Journal of Hefei University of Technology,2018,41 (6):708G714.071营养与活性N U T R I T I O N&A C T I V I T Y总第261期|2023年7月|Copyright©博看网. All Rights Reserved.[17]CHYLINSKA M,SZYMANSKAGCHARGOT M,ZDUNEK A.FTGIR and FTGRaman characterization of nonGcellulosic polysaccharides fractions isolated from plant cell wall[J]. Carbohydrate polymers,2016,154:48G54.[18]赵婧.南瓜酸性多糖的结构解析及其与功能蛋白的相互作用[D].北京:中国农业大学,2017:20G21.ZHAO J.Structural elucidation of pumpkin acidic polysaccharides and their interactions with functional proteins[D].Beijing:China Agricultural University,2017:20G21.[19]COBSGROSAS M,CONCHAGOLMOS J,WEINSTEINGOPPENHEIMER C,et al.Assessment of antiproliferative activity of pectic substances obtained by different extraction methods from rapeseed cake on cancer cell lines[J].Carbohydrate Polymers, 2015,117:923G932.[20]周慧吉,马海乐,郭丹钊,等.不同体积分数乙醇沉淀桑黄胞内多糖的理化性质及抗氧化活性[J].食品科学,2015,36(19): 34G38.ZHOU H J,MA H L,GUO D Z,et al.Physicochemical properties and antioxidant activity of intracellular polysaccharides from Phellinus igniarius precipitated by different ethanol concentrations [J].Food Sciences,2015,36(19):34G38.[21]王佳,李进霞,张慧芝,等.虎杖多糖乙醇分级纯化及其抗氧化性[J].食品工业科技,2019,40(1):92G95.WANG J,LI J X,ZHANG H Z,at al.Ethanol fractional purification and antioxidant activities of polysaccharides from Polygonum cuspidatum[J].Science and Technology of Food Industry,2019,40(1):92G95.[22]张梦洁,易阳,闵婷,等.不同浓度醇沉级分的莲藕多糖成分分析与对RAW264.7细胞免疫活性的影响[J].食品科技,2021, 46(8):162G170.ZHANG M J,YI Y,MIN T,at position analysis and in vitro evaluation of some activities of RAW264.7of Lotus Root polysaccharide extracted by ethanol precipitation with different concentrations[J].Food Science and Technology,2021,46(8): 162G170.[23]康文艺,蒙丽君,王莉,等.花中多糖化学组成与生物活性研究进展[J].食品科学,2022,43(1):1G13.KANG W Y,MENG L J,WANG L,at al.Chemical composition and biological activity of polysaccharides from flowers:A review [J].Food Science,2022,43(1):1G13.[24]CHEN L,HUANG G L.Antioxidant activities of sulfated pumpkinpolysaccharides[J].International Journal of Biological Macromolecules,2019,126:743G746.[25]SONG Y,NI Y Y,HU X S,et al.Effect of phosphorylation onantioxidant activities of pumpkin(Cucurbita pepo,Lady godiva) polysaccharide[J].International Journal of Biological Macromolecules,2015,81:41G48.[26]ZHANG H X,ZHAO J C,SHANG H M,at al.Extraction,purification,hypoglycemic and antioxidant activities of red clover (Trifolium pratense L.)polysaccharides[J].International Journal of Biological Macromolecules,2020,148:750G760.[27]赵婧,袁驰,周春丽,等.南瓜多糖降血糖作用研究进展[J].食品研究与开发,2014,35(7):108G110.ZHAO J,YUAN C,ZHOU C L,at al.Research advance in hypoglycemic effects of pumpkin polysaccharides[J].Food Research and Development,2014,35(7):108G110.(上接第28页)[20]张健,颜伟,王佳晨,等.探讨血糖代谢障碍对心率变异性的影响[J].解放军医学院学报,2022,43(3):277G283,353.ZHANG J,YAN W,WANG J C,et al.Exploring the effect of impaired glucose metabolism on heart rate variability[J].Academic Journal of Chinese PLA Medical School,2022,43(3):277G283,353.[21]孔令琴,陈飞,赵跃进,等.融合心率变异性与表情的非接触心理压力检测[J].光学学报,2021,41(3):68G77. KONG L Q,CHEN F,ZHAO Y J,et al.NonGcontact psychological stress detection by fusing heart rate variability with expression[J].Acta Optica Sinica,2021,41(3):68G77.[22]史波林,赵镭,汪厚银,等.智能感官分析技术在茶叶品质检测中的应用[J].食品科学,2009,30(19):351G355.SHI B L,ZHAO L,WANG H Y,et al.Application of intelligent sensory analysis technology in tea quality testing[J].Food Science, 2009,30(19):351G355.[23]余顺园.信号与系统中时域频域的对称性[J].科技视界,2012 (25):192G193.YU S Y.Symmetry in the time and frequency domains of signals and systems[J].Science&Technology Vision,2012(25):192G193.(上接第156页)[31]TANG Y,FAN Z,YANGM,et al.Low concentrations of theantidepressant venlafaxine affect courtship behaviour and alter serotonin and dopamine systems in zebrafish(Danio rerio)[J]. Aquatic Toxicology,2022,244(10):60G82.[32]KOPSCHINA FELTES P,DOORDUIN J,KLEIN HC,et al.AntiGinflammatory treatment for major depressive disorder:Implicationsfor patients with an elevated immune profile and nonGrespondersto standard antidepressant therapy[J].Journal of Psychopharmacology,2017,31(9):1149G1165.[33]NGUYEN M,STEWART A M,KALUEFF A V.Aquatic blues:Modeling depression and antidepressant action in zebrafish[J]. Progress in NeuroGPsychopharmacology and Biological Psychiatry, 2014,55(12):26G39.171|V o l.39,N o.7陈冰洁等:金瓜多糖不同分级组分的抗氧化和降血糖活性Copyright©博看网. All Rights Reserved.。
苦荞芦丁水解酶序列鉴定及在昆虫系统中的表达杜 程1,崔晓东1,王转花1,2,*(1.山西大学生物技术研究所,化学生物学与分子工程教育部重点实验室,山西太原030006;2.山西大学生命科学学院,山西太原030006)摘 要:为获得苦荞麦中芦丁水解酶基因(Fagopyrum tartaricum rutin-hydrolyzing enzyme gene,FtRHE),以云荞1号苦荞种子为材料,制备获得天然芦丁水解酶(rutin-hydrolyzing enzyme,RHE),通过基质辅助激光解吸电离飞行时间质谱鉴定天然RHE序列信息,结合转录组数据,筛选苦荞RHE基因FtRHE。
结果显示,纯化的天然RHE,分子质量约为62 kDa,肽质量指纹图谱证明其为一种β-葡萄糖苷酶,质荷比为914.428 6的肽段FSISWSR为其特征肽段。
在苦荞种子转录组数据中筛选获得FtRHE基因序列基础上,通过反转录聚合酶链式反应获得了苦荞FtRHE基因。
FtRHE开放阅读框由1 539 个碱基组成,编码512 个氨基酸,1~29位氨基酸为其信号肽。
多重序列比对发现RHE与不同物种来源的β-葡萄糖苷酶氨基酸保守性不高,同源性普遍集中在54%~62%之间。
构建Bacmid-FtRHE杆粒,转染昆虫细胞Sf9后,成功表达了具有较高活性的重组RHE。
结果表明,获得的FtRHE即为苦荞中RHE基因。
该研究为高含量芦丁及无苦涩味荞麦育种以及由芦丁生物转化生产槲皮素等的应用提供了理论支持。
关键词:苦荞麦;芦丁水解酶;氨基酸序列;昆虫表达系统;槲皮素Primary Structure Identification of Rutin-Hydrolyzing Enzyme and Its Expression in Insect SystemDU Cheng1, CUI Xiaodong1, WANG Zhuanhua1,2,*(1. Key Laboratory of Chemical Biology and Molecular Engineering, Ministry of Education, Institute of Biotechnology,Shanxi University, Taiyuan 030006, China; 2. College of Life Science, Shanxi University,Taiyuan 030006, China) Abstract: In order to obtain the gene encoding rutin-hydrolyzing enzyme in Fagopyrum tartaricum (FtRHE), the enzyme was obtained from the seeds of the tartary buckwheat cultivar ‘Yunqian 1’ and its peptide mass sequence was determined by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) and then transcriptomic analysis and gene amplification were carried out. Results showed that molecular mass of RHE was about 62 kDa, and RHE was a β-glycosidase as evident from its peptide mass fingerprint. FSISWSR was a characteristic peptide fragment of RHE with mass-to-charge ratio of 914.428 6. Based on the DNA sequences obtained from the transcriptome of tartary buckwheat seeds, FtRHE gene was obtained by reverse transcription PCR amplification using specific primers. The open reading frame of FtRHE consisted of 1 539 bases, encoding 512 amino acids with amino acid residues 1–29 making upa signal peptide.Multiple sequence alignment showed that RHE shared a 54%–62% similarity with other β-glycosidases from various plants, indicating a low level of gene conservation. Bacmid-FtRHE was constructed by DNA recombination, and then transfected into insect cell Sf9. FtRHE was successfully expressed with higher rutin hydrolysis activity, indicating that the obtained FtRHE was the gene encoding rutin-hydrolyzing enzyme in tartary buckwheat seeds. This study may provide a theoretical basis for the breeding of buckwheat cultivars with high rutin content and without bitter or astringent taste and for the biotransformation of rutin into quercetin.Keywords: tartary buckwheat; rutin-hydrolyzing enzyme (RHE); amino acid sequence; insect expression system; quercetin DOI:10.7506/spkx1002-6630-201814014中图分类号:Q556;S188 文献标志码:A 文章编号:1002-6630(2018)14-0091-08引文格式:杜程, 崔晓东, 王转花. 苦荞芦丁水解酶序列鉴定及在昆虫系统中的表达[J]. 食品科学, 2018, 39(14): 91-98.DOI:10.7506/spkx1002-6630-201814014. 收稿日期:2017-10-23基金项目:国家自然科学基金青年科学基金项目(31300653);山西省科技平台项目(2014091028)第一作者简介:杜程(1990—),男,硕士,研究方向为蛋白质化学与工程。
Enzyme and Microbial Technology41(2007)570–575Characteristic of-glucosidase from Sicilian bloodoranges in relation to anthocyanin degradationR.N.Barbagallo a,∗,R.Palmeri a,S.Fabiano a,P.Rapisarda b,G.Spagna a,∗a Dipartimento di OrtofloroArboricoltura e Tecnologie Agroalimentari(DOFATA),Via Santa Sofia98,951123Catania,Italyb CRA-Istituto Sperimentale per l’Agrumicoltura,Corso Savoia190,95024Acireale(CT),ItalyReceived5August2005;received in revised form9May2007;accepted9May2007AbstractAnthocyanin content in Sicilian sweet orange[Citrus sinensis(L)Osbeck]varieties known as blood oranges(Tarocco,Moro e Sanguinello) undergoes changes during the ripening process.Concentration increases during ripening,reaching a maximum in the fully ripe fruit.At latter stage of maturity,a decrease of these pigments is observed.This study aims to evaluate-d-glucosidase activity(G,EC3.2.1.21)in Tarocco variety, the most common Sicilian blood orange,and to determine the main physicochemical and kinetic properties of this enzyme in order to underline its role on anthocyanins degradation during ripening.The enzymatic extract was assayed on both anthocyanins extract from Tarocco juice and a synthetic substrate(p-nitrophenyl--d-glucopyranoside,p NPG).It was observed a400times higher specificity of the synthetic substrate than the natural substrate.Kinetic studies and physicochemical characterization ofG such as V max and K m(V max2.1×10−2and K m2.67×10−4using p NPG as substrate;V max3.3×10−3and K m2.1×10−1using natural substrate),optimum conditions of pH(4.5)and temperature(60◦C),fructose and glucose inhibition(competitive inhibition by glucose)and thermal stability(decimal reduction time3min at75◦C and decimal reduction temperature9.5◦C)were also performed.-d-Glucosidase activity seems involved on anthocyanins degradation duringfinal ripening stage of fruit.©2007Elsevier Inc.All rights reserved.Keywords:Tarocco orange;-d-Glucosidase;Anthocyanins;Stability;Ripening1.IntroductionCitrus fruit are important sources of health promoting con-stituents.In addition to ascorbic acid,these includeflavonoids, hydroxycinnamic acids,carotenoids and limonoids[1].Some of these compounds are present as glycosides and the type and position of glycosidic substituent has a profound effect on taste,solubility and bioavailability.Anthocyanins are glycosides belonging to theflavonoid compounds,a class of plant secondary metabolites responsible for the rind andflesh red color of some Sicilian sweet orange[Citrus sinensis(L)Osbeck]varieties known as blood oranges(Tarocco,Moro e Sanguinello).Antho-cyanin content has been considered as an important quality attribute in both fresh fruit market and processing industry due to its biological activity[2,3].Several anthocyanins have been ∗Corresponding author at:Food Biotechnology Group,DOFATA,University of Catania,Via S.Sofia98,95123Catania,Italy.Tel.:+390957580213; fax:+390957141960.E-mail addresses:rbarbaga@unict.it(R.N.Barbagallo),gspagna@unict.it(G.Spagna).identified in blood oranges juice,with cyanidin-3-glucoside and cyanidin-3-(6 -malonyl)-glucoside as the major pigments [4,5].Anthocyanins stability in fruit is promoted by copigmenta-tion with otherflavonoids or phenolic acids,self-association, and low pH[6].On the other hand,enzymatic systems present in plant tissues such as anthocyanase(anthocyanin--glucosidase) and polyphenoloxidase could play an important role in degrada-tion process of anthocyanins.Such enzymes mainly act during senescence of fruit,hydrolising and oxidizing the anthocyanin molecules.Few works have been carried out on citrus fruit-glycosidase(G).Burns[7]found a low level of-glycosidase activity in sweet orange fruit juice vesicles,while no activity of this enzyme was detected in grapefruit peel and juice vesicle [8–10].Finally,a recent study[11]put in evidence the pres-ence ofG in fruitflavedo and albedo tissue of Valencia orange variety.Despite the importance of-d-glucosidase(G,EC 3.2.1.21)activity onflavour profile of many fruit,few researches were found about its role on anthocyanins degradation.Due to loss of glycosidic moiety for hydrolysis,the aglycon(antho-cyanidin)could become extremely unstable with consequent0141-0229/$–see front matter©2007Elsevier Inc.All rights reserved. doi:10.1016/j.enzmictec.2007.05.006R.N.Barbagallo et al./Enzyme and Microbial Technology41(2007)570–575571 adverse effect on juice color and represents a good substratefor other enzymes.The present study aims to evaluate-d-glucosidase activityin Tarocco orange and to determine the main physicochemicaland kinetic properties of such enzyme in order to underline itsrole on the anthocyanins degradation during different ripeningstages.2.Materials and methods2.1.Plant materialsThe study was performed on fruit from Tarocco cultivar(“Arcimusa”clone)hand-harvested randomly fromfive trees,grafted on sour orange(C.aurantiumL.),and planted at the“Palazzelli”(Siracusa,Italy)experimental farm of theCRA–Istituto Sperimentale per l’Agrumicoltura of Acireale(Catania,Italy).Twenty fruit from each tree were collected atfive different ripening stages,from February to May,2005.Each fruit was squeezed separately using ahousehold juicer(Moulinex,Milan,Italy)and the cloudy juice obtained wascentrifuged at20,000×g for30min at5◦C,in order to separate the residualpulp from the juice.These fractions were stored singularly at−50◦C prior tophysicochemical and enzymatic analysis.2.2.Physicochemical analysesTotal soluble solid(TSS),total acidity(TA),ratio(TSS/TA)were determinedon cloudy juice according to standard methods[12].Ascorbic acid content wasdetermined on centrifuged juice by2,6-dichlorophenolindophenol titrimetricmethod modified by Rapisarda and Intelisano[13].Total anthocyanins in juice(a)and extract(b)were determined spectrophotometrically by pH differentialmethod[14,15].For determination in juice(a)an aliquot of juice(2mL)wasdiluted up to25mL with a pH1solution(125mL of0.2M KCl and375mLof0.2M HCl).A second aliquot(2mL)was diluted up to25mL with a pH4.5buffered solution(400mL of1M CH3CO2Na,240mL of1M HCl,and360mLof H2O).Absorbance of the solutions was measured at510nm.Concentrationof anthocyanins was calculated by the equation:C mg/L=(Abs pH1−Abs pH4.5)×484.82×1000 24,825×DFwhere the term in parentheses is the difference of absorbance at510nm between pH1and pH4.5solutions,484.82is the molecular mass of cyanidin-3-glucoside chloride,24,825is its molar absorptivity(ε)at510nm in the pH1solution,and DF is the dilution factor.Accuracy of results by this method is supported by independent HPLC data.For anthocyanins extraction(b),3L of Tarocco orange juice previously centrifuged were loaded on a glass column(length40cm;i.d. 2cm)filled with a nonpolar SDVB(styrene-divinylbenzene)resin Kastel S-112(Dow-Italia,Milano,Italy).After the adsorption,resin was washed with 2–3volumes of distillated water in order to obtain sugars and other soluble components elution.Anthocyanins were desorbed using1%HCl methanol(v/v) and different fraction were collected.Methanolic solution of the anthocyanins was concentrated by rotary evaporator maintaining the temperature below35◦C. This extract was used for enzymatic assay.The juice color was determined with a compact tristimulus chromameter (Minolta CR-300,Ramsey,NJ,USA)with anØ8mm viewing aperture,white plate reference(Y=94.3;x=0.3142;y=0.3211)and C illuminant(CIE,2◦observer)was used.Readings were expressed as L*,a*and b*parameters. Chroma[(a*2+b*2)1/2]and Hue angle[tan−1(b*/a*)]were calculated.2.3.Enzymes determinationAn aliquot of pulp(50g)was homogenised at4.0◦C by adding200mL of extracting solution(citrate–phosphate buffer,0.1M;NaCl,1M;dithiothreitol, 1mM;pH5.0)[16].Suspension was maintained under stirring for2h at4.0◦C, then centrifuged at10,000×g for15min at4◦C.Starting from the extraction at optimum pH conditions,surfactants PEG400and PEG4000were tested by dissolving in1/3part of the extracting solution volume and adding to the suspension with the other2/3parts of the solution before pH correction.Tested concentrations of both surfactants were up to1.5%(w/v);besides,concentrations tested for PEG4000were2.3and4.0%(w/v).In order to maximize yield,the extract was:(a)saturated with ammonium sulphate80%,kept at4.0◦C overnight andfiltrate under vacuum with a5892filter(Whatman);(b)directlyfiltrate and concentrated by tangentialflow ultrafiltration with50kDa cut-off membrane, and retentate utilised as enzymatic extract.The juice was directly analysed.G activity was determined by hydrolysis of synthetic(p-nitrophenyl--d-glucopyranoside,p NPG)and natural substrates(orange anthocyanins extract) at pH4.0and30◦C[17].An aliquot of0.05mL of diluted enzyme was added to0.45mL of0.55×10−2M p NPG dissolved in0.1M citrate–phosphate(C–P) buffer pH5.0at25◦C under continuous stirring.After1min,reaction was stopped by adding1mL sodium carbonate1M.Blank was carried out by invert-ing sodium carbonate with the enzyme solution.The absorbance of released 4-nitrophenolate ion was read at400nm and the concentration determined by usingε18,300M−1cm−1.One unit of enzyme activity was defined as the amount of enzyme releasing1mol of4-nitrophenol min−1under assay conditions.For anthocyanase determination[18]was used the cyanidin-3-glucoside extinction coefficient(ε=24,825M−1cm−1)at510nm.Enzymatic units were defined as micromoles of anthocyanins.Polyphenoloxidase(PPO,EC1.14.18.1)was assayed spectrophotometri-cally at505nm using3,4-dihydroxy phenylacetic acid(DOPAC)as phenolic substrate with MBTH(3-methyl-benzothyazolinone hydrazone)to trap the enzyme-generated ortoquinone[19,20].The absorbance was read at505nm and concentration determined by usingε25,400M−1cm−1.One unit of PPO activity was defined as the amount of enzyme which produces1mol of adduct for min at25◦C.Protein concentration was determined after precipitation in7%(w/v) trichloroacetic acid by means of Coomassie Blue G250and by employing BSA as standard[21].The solvents and reagents not expressly specified had a high degree of purity(RPE)and were supplied by Carlo Erba(Rodano,Italy).2.4.Characterization ofβGThe following physicochemical and kinetic parameters were established: optimum temperature,optimum pH,inhibitions(glucose,fructose,ethanol), V max,K m,thermo-stability and molecular weight by SDS-PAGE.2.4.1.Optimum temperatureThe enzyme was assayed at temperatures from21to90◦C(at pH5.0),with 600mL of1M sodium carbonate added in order to stop the reaction.2.4.2.Optimum pHCitric–citrate buffer solutions(0.1M)were prepared under different pH con-ditions(from2.5to8.0at30◦C)in such a way as to assess the enzyme behaviour even in very different conditions to those of orange juices.2.4.3.Glucose and fructose inhibitionsGlucose and fructose inhibition tests were performed using sugar concen-trations not exceeding25g L−1(at pH5.0and30◦C).The enzyme assay was carried out as previously described except as regards the sugar and buffer con-centrations,added in proportional amounts with respect to thefinal volume of the assay.2.4.4.Kinetic parameters(V max and K m)The behaviour of the enzyme(at pH5.0and30◦C)was explained by the Michaelis–Menten equation and the kinetic parameters calculated by way of graphical extrapolation according to Lineweaver–Burk[22].Substrates were prepared increasing concentration until saturation.2.4.5.StabilityThree aliquots of extract(60mL)were lyophilized and dissolved in10mL citric–citrate buffer0.1M pH5.0.Three tests were prepared by adding0.50L to1.5mL of pasteurized(90◦C for30min)and centrifuged orange juice;the three samples were put in thermostat at temperatures of37,52and70◦C.572R.N.Barbagallo et al./Enzyme and Microbial Technology 41(2007)570–5752.4.6.SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis)The enzyme extract was performed as described by Laemmhi [23].The samples,previously partially purified by purified by ammonium sulphate pre-cipitation (80%saturation)were put in touch with a 12%(w/v)polyacrylamide gel,both were dissolved according to the protein content determined by the above cited Coomassie Blue G250and by employing BSA as standard [21].2.5.Statistical analysisAll determinations were conducted three times at least.Analysis of variance (ANOV A)of the data was evaluated by the Statistical Analysis System (SAS version 9.0).Duncan’s Multiple Range Test was employed to determine the statistical significance of the differences between the means (p ≤0.05).3.Results and discussion 3.1.Physicochemical analysesVariations of physicochemical parameters during orange mat-uration process were reported in Table 1.Total soluble solids (TSS)showed a slight increase between February and first days of May;after this period a decrease was noticed.Total acidity (TA)decreased quickly until 14March and then remained quite constant.The ratio (TSS/TA)values revealed that in March sam-ples reached the optimal maturity stage.Besides,although it was noticed an increasing of TSS and TA values at first sampling of May,ratio value remained unchanged.Ascorbic acid content was stable during maturation except than in the last stage in which a decrease was observed.The anthocyanin content reached a max-imum in 14March when fruit were fully ripe.After this stage,values decreased.That was the most evident result between last two sampling,in which biochemical senescence process is more active.In samples withdrawn on 24May,anthocyanin content reduced to half in comparison with the maximum value.L *a *b *values described changes in juice color at different stages of Sicilian blood oranges.Apart from a slight loss in lightness (L *)and green-red (a *)values after 14March,juice did not show any other significant variation of colour parameters,whereas the blue-yellow (b *)chromatism did not influence significantly the organoleptic profile.Changes in total anthocyanin amount of blood orange juice did not seem to reflect such visible changes in juice colour,since the maturation stages probably influenced stability of anthocyanins,as reported by other authors [24,25].3.2.Enzymes determinationThe analysis of raw juice showed a G activity of 1.5×10−3U g −1.Relatively to pulp,extraction tests at different pH conditions were performed (Fig.1).The maximum value of enzyme activity was noticed at pH 6.0,subsequently chosen for all extractions.A higher pH cause an increasing of dissociation rate of pulp pectins carboxylic groups;it causes an increasing of the intra and inter-molecular electrostatic repulsions between the pectin chains and,probably,even between enzyme and pectin,resulting in an easier G release in extraction solutions.On residual part of the first extraction,constituted by pulp and a gelatinous layer (calcium pectate),a second extraction was car-T a b l e 1P h y s i c o -c h e m i c a l c h a r a c t e r i s t i c s o f b l o o d o r a n g e j u i c e h a r v e s t e d a t fiv e d i f f e r e n t r i p e n i n g s t a g e sp HT S S (%)T A (%)T S S /T AA s c o r b i c a c i d (m g %)A n t h o c y a n i n s (m g /L )L *a *b *8F e b r u a r y 3.44±0.02a 11.61±0.2a 1.35±0.1a 8.60±0.7a 66.48±1.4b 89.70±11.35a 37.37±3.72a 23.89±0.58a 41.68±1.40a 14M a r c h 3.46±0.05a 11.98±0.5a 1.02±0.2a 11.75±1.6b 66.93±1.8b 156.00±12.90c 38.05±2.19a 24.76±0.19a 40.86±0.91a 11A p r i l 3.48±0.04a 12.00±0.5a 1.09±0.1a 11.01±0.8a b 65.24±0.9b 114.56±10.29b 35.31±1.03b 29.36±0.28b 37.18±2.33a 4M a y 3.48±0.02a 12.35±0.4a 1.19±0.2a 10.38±1.3a b 68.09±2.8b 79.63±7.15a 31.31±1.03b 31.36±0.28b 36.18±2.63a 24M a y 3.41±0.05a 11.13±0.5a 1.09±0.1a 10.21±0.8a b 48.46±6.7a 75.01±6.95a 29.31±1.03b31.30±0.26b 36.10±2.35a N o t e :M e a n o f s e p a r a t e 20f r u i t j u i c e s d e t e r m i n a t i o n .M e a n s i n a s a m e c o l u m n f o l l o w e d b y t h e s a m e l e t t e r a r e n o t s i g n i fic a n t l y d i f f e r e n t a t t h e p ≤0.05l e v e l a c c o r d i n g t o D u n c a n ’s m u l t i p l e r a n g e t e s t .R.N.Barbagallo et al./Enzyme and Microbial Technology 41(2007)570–575573Fig.1.Effect of pH extraction on G activity.ried out,that led to a limited activity recover (lower than 5%).Total pulp activity resulted 8–10times higher,for all samples,than the centrifuged juice activity,since the G is mostly bond to the solid fraction of fruit and,presumably,to pectin chains.In order to increase the G extraction,it was tried to add a non ionic surfactant,polyethylene glycol (PEG)at two different molecu-lar weights (400and 4000),as suggested by Gunata [16]for G extraction from grapes skin.The results showed a worsening in extraction yield with rela-tive specific activity of G extracted of 79.74and 64.26%,with PEG 400and 4000surfactants,respectively.Probably,the dif-ferent behaviour depends to the presence of a wax layer over the grapes and/or to different molecular structure of the enzyme.The use of ammonium sulphate precipitation (80%saturation)reduced the activity to less than 1/3,so ultrafiltration was pre-ferred since its higher yield (>80%).Fig.2reports anthocyanins and G changes in fruit,from February to May 2005.Anthocyanin concentrations in fruit reached a maximum at day 34and then decreased,while G showed an opposite trend,proportionally increasing its activity,with a 80%correlation coefficient (95%confidence interval).Then,it would seem that G plays a role in anthocyanins degra-dation of Tarocco fruit at the end of ripening.In fact,loss of glucosidic substituent from anthocyanins by G hydrolisis destabilizes the anthocyanins leading to adverse effects on color quality,as the aglycon is extremely unstable.The anthocyanidins may be a good substrate for oxidases such as polyphenoloxidase (PPO)in a successive enzymatic degradation process [26].PPO activity was found in Tarocco orange (with a range fromFebru-Fig.2.Anthocyanins content and -glucosidase activity during fruitripening.Fig.3.Enzyme kinetics with synthetic substrate (p NPG,3a)and natural sub-strate (anthocyanins,3b).ary to May of 2.50–2.99×10−4Ug −1),although with lower value than G (ca.1/30).Presence of the PPO activity in blood orange indicates that this enzyme could play a role on antho-cyanidin degradation.3.3.Characterization of βGThe enzyme involved in p NPG hydrolysis followed a Michaelis–Menten kinetics (Fig.3a),with K m 2.67×10−4M and V max di 2.1×10−2nmol/mg proteins min.With regard to the hydrolysis of anthocyanins obtained from Tarocco extract (Fig.3b),it was observed a six times reduc-tion of enzymatic activity and enzyme inhibition at high anthocyanins concentration (product inhibition).The enzyme behaviour on anthocyanins substrate cannot be explained by Michaelis–Menten equation.Referring to the point of maximum,apparent K m was 2.1×10−1M and V max was 3.3×10−3nmol/mg proteins min.Therefore,G specificity on synthetic substrate compared to anthocyanins,calculated from V max /K m ratios of respective substrates,resulted 400times higher.Relative activity reached maximum value at pH 4.5(Fig.4),with a 35%of relative activity at physiological pH of the fruit (3.5).This course resulted similar in G from microbial sources,as Aspergillus niger [27,28].Therefore,it could be supposed that the reaction mechanism is always an acid–base reaction with involvement of at least two carboxylic groups [28].Optimum temperature (Fig.5)resulted quite high for a vegetable source574R.N.Barbagallo et al./Enzyme and Microbial Technology 41(2007)570–575Fig.4.Effect of pH on -glucosidase relativeactivity.Fig.5.Effect of temperature on -glucosidase relative activity.enzyme (60◦C)and even in this case similar to enzyme from microbial source [29].The enzyme was not inhibited by fructose and ethanol,while glucose inhibition was noticed.Kinetic studybyFig.6.Glucose inhibition according to Lineweaver–Burk (a);thermal stability of -glucosidase(b).Fig.7.Arrhenius plot for heat inactivation of -glucosidase.Lineaweaver–Burk interpolation,allowed to establish that the inhibition was competitive,with an inhibition constant value K i ,equal to 3.08×10−2M (Fig.6a).G stability tests allowed to determine,by activity versus time plot (Fig.6b),the enzyme inactivation constants (k in )at various temperatures.By these results,an inactivation energy of 30kcal/mol K was calculated by the Arrhenius plot (Fig.7).Such results,expressed as second Bigelow law (ln D1/D2= T /z ),allowed to determine for G a decimal reduction time (D )of about 3min at 75◦C and a deci-mal reduction temperature (z )of about 9.5◦C.Then,the enzyme showed a good stability so it could play a role in thermal process of blood orange juice.SDS-polyacrylamide gel electrophoresis of enzyme partially purified by ammonium sulphate precipitation (80%saturation)was performed as shown in Fig.8.There are two major bands corresponding to the molecular weight of 65and 55kDa.In conclusion,results obtained put in evidence for the first time presence of G in blood orange cv.Tarocco .The enzyme activity in pulp resulted higher than in centrifuged juice,showing that G is mainly bond to the solid part of fruit and probably to pectin chains.Specificity resulted higher for syntheticsubstrateFig.8.SDS-polyacrylamide gel electrophoresis of pulp and partially purified ne 1:10L of protein molecular weight standard;Lane 2:10L of crude enzyme;Lane 3:10L of enzyme extract.R.N.Barbagallo et al./Enzyme and Microbial Technology41(2007)570–575575than for natural substrate,with a different kinetic behaviour.The enzyme followed Michaelis–Menten kinetics and showed some properties(optimal pH and temperature conditions,glucose competitive inhibition)similar to those found for-glucosidase from A.niger[30].Finally,relatively to fruit,it was reported a relation betweenG activity increasing and anthocyanins level decreasing,so it is possible to hypotize the enzyme is involved in anthocyanins degradation reactions duringfinal ripening stage.References[1]Widmer WW,Montanari AM.The potential for citrus phytochemicals inhypernutrition foods.In:Finley JW,Armstrong DJA,Nagy S,Robinson SF, editors.Hypernutritious foods.Auburndale,FL:Agscience,Inc.;1996.p.75–89.[2]Felgines C,Talav´e ra S,Texier O,Besson C,Fogliano V,Lamaison J-L,etal.Absorption and metabolism of red orange juice anthocyanins in rats.Br J Nutr2006;5:898–904.[3]Rapisarda P,Bellomo SE,Intrigliolo F.Anthocyanins in blood oranges:composition and biological activity.Recent Res Dev Agric Food Chem 2001;5:217–30.[4]Maccarone E,Maccarone A,Rapisarda P.Acylated anthocyanins fromoranges.Ann Chim1985;75:79–86.[5]Maccarone E,Rapisarda P,Fanella F,Arena E,Mondello L.Cianidin-3-(6 -malonyl)--glucoside.One of the mayor anthocyanins in blood orangejuice.Ital J Food Sci1998;10:367–72.[6]Maccarone E,Maccarone A,Rapisarda P.Stabilization of anthocyanins ofblood orange fruit juice.J Food Sci1985;50:901–4.[7]Burns JK.␣-and-glucosidase activities in juice vesicles of stored Valenciaoranges.Phytochemistry1990;29:2425–9.[8]Burns JK,Baldwin EA.Glycosidase activities in grapefruitflavedo,albedo and juice vesicles during maturation and senescense.Physiol Plant 1994;90:37–44.[9]Alcalde-Eon C,Escribano-Bailon MT,Santos-Buelga C,Rivas-GonzaloJ.Changes in the detailed pigment composition of red wine dur-ing maturity and ageing—a comprehensive study.Anal Chim Acta 2006;563(1–2):238–54.[10]Rivas EGP,Alcalde-Eon C,Santos-Buelga C,Rivas-Gonzalo J,Escribano-Bail´o n M.Behaviour and characterisation of the colour during red wine making and maturation.Anal Chim Acta2006;563(1–2):215–22. [11]Cameron RG,Manthey JA,Baker RA,Grohmann K.Purification and char-acterization of a beta-glucosidase from Citrus sinensis var.Valencia fruit tissue.J Agric Food Chem2001;49:4457–62.[12]AOAC.Official methods of analysis.14th ed.Virginia,USA:Associationof Official Analytical Chemists;1984.p.414–22.[13]Rapisarda P,Intelisano S.Sample preparation for vitamin C analysis ofpigmented orange juices.Ital J Food Sci1996;8:251–6.[14]Rapisarda P,Fallico B,Izzo R,Maccarone E.A simple and reliablemethod for determining anthocyanins in blood orange juices.Agrochimica 1994;38:157–64.[15]Rapisarda P,Fanella F,Maccarone E.Riability of analytical methods fordetermining anthocyanins in blood orange juices.J Agric Food Chem 2000;48:2249–52.[16]Gunata Z,Blondeel,Vallier MJ,Lepoutre,Sapis JC,Watanabe.An endoglycosidase from grape berry skin of cv M.Alexandria, hydrolysing potentially aromatic disaccaride glycosides.J Agric Food Chem1998;46:2748–53.[17]Spagna G,Barbagallo RN,Martino A,Pifferi PG.A method of purify␣-l-arabinofuranosidase and-d-glucopyranosidase from Aspergillus niger to increase the aroma wine.Ital Food Bev Technol2000;20:11–6. [18]Fu Mian C,Pifferi PG,Spagna G.Partial purification and characteriza-tion of anthocyanase(-glucosidase)from Aspergillus niger.Cerevisia 1992;17:27–35.[19]Pifferi PG,Baldassari L.A spectrophotometric method for determinationof the catecholase activity of tyrosinase by Besthorn’s hydrazone.Anal Biochem1973;52:325–35.[20]Espin JC,Morales M,Varon R,Tudela J,Garc`ıa-Canovas F.Continuousspectrophotometric method for determining monophenolase and dipheno-lase activities of pear polyphenoloxidase.J Food Sci1996;61:1177–81.[21]Bradford MM.A rapid and sensitive method for the quantification of micro-gram quantities of protein utilizing the principle of protein dye binding.Anal Biochem1976;72:248–57.[22]Lineaweaver H,Burk D.The determination of enzyme dissociation con-stants.J Am Chem Soc1934;56:658–66.[23]Laemmhi UK.Cleavage of structural proteins during the assembly of thehead of bacteriophage T4.Nature1970;227:680–5.[24]Brouillard R.Chemical structure of anthocyanins.In:Markakis P,editor.Anthocyanins as food colors.New York:Academic Press;1982.p.1–40.[25]Mazza G,Miniati E.Anthocyanins in fruit,vegetables and grains.BocaRaton,FL,USA:CRC Press;1993.p.1–6.[26]Zhang Z,Pang Xuequn X,Ji Z,Jaing Y.Role of anthocyanin degradationin litchi pericarp browning.Food Chem2001;72:217–21.[27]Martino A,Pifferi PG,Spagna G.Production of-glucosidase fromAspergillus niger using carbon sources derived from agricultural wastes.J Chem Technol Biotechnol1994;61:247–52.[28]Barbagallo RN,Spagna G,Abbate C,Azzaro G,Palmeri R.Inexpensiveisolation of-d-glucopyranosidase from␣-l-arabinofuranosidase,␣-l-rhamnopyranosidase and o-acetylesterase.Appl Biochem Biotech,Part A 2002;101:1–13.[29]Spagna G,Barbagallo RN,Greco E,Manenti I,Pifferi PG.A mistureof purified glycosidases from Aspergillus niger for oenological applica-tion immobilized by inclusion in chitosan gels.Enzyme Microb Technol 2002;30:80–9.[30]Barbagallo RN,Spagna G,Palmeri R,Restuccia C,Giudici P.Selection,characterization and comparison of-glucosidase from mold and yeasts for enological applications.Enzyme Microb Technol2004;35:58–66.。