Magnetic and Transport Properties of Fe-Ag granular multilayers
- 格式:pdf
- 大小:443.55 KB
- 文档页数:8


金属材料相关英语词汇(2)金属材料相关英语词汇(2)金属材料相关英语词汇(2)base metal, application, jis astm standard, and normal thickness of galvanized steel sheet锌镀层质量zinc coating mass表面处理surface treatment冷轧钢片cold-rolled steel sheet/strip热轧钢片hot-rolled sheet/strip电解冷轧钢片厚度公差thickness tolerance of electrolytic cold-rolled sheet热轧钢片厚度公差thickness tolerance of hot-rolled sheet冷轧或热轧钢片阔度公差width tolerance of cold or hot-rolled sheet长度公差length tolerance理论质量theoretical mass锌镀层质量(两个相同锌镀层厚度)mass calculation of coating (for equal coating)/mm锌镀层质量(两个不同锌镀层厚度)mass calculation of coating (for differential coating)/mm镀锡薄铁片(白铁皮/马口铁) (日工标准jis g3303)简介general镀锡薄铁片的构造construction of electrolytic tinplate镀锡薄钢片(白铁皮/马日铁)制造过程production process of electrolytic tinplate锡层质量mass of tin coating (jis g3303-1987)两面均等锡层both side equally coated mass两面不均等锡层both side different thickness coated mass级别、电镀方法、镀层质量及常用称号grade, plating type, designation of coating mass common coatingmass镀层质量标记markings designations of differential coatings硬度hardness单相轧压镀锡薄铁片(白铁皮/马口铁)single-reduced tinplate双相辗压镀锡薄钢片(马口铁/白铁皮)dual-reduction tinplate钢的种类type of steel表面处理surface finish常用尺寸commonly used size电器用硅[硅] 钢片electrical steel sheet简介general软磁材料soft magnetic material滞后回线narrow hystersis矫顽磁力coercive force硬磁材料hard magnetic material最大能量积maximum energy product硅含量对电器用的低碳钢片的最大好处the advantage of using silicon low carbon steel晶粒取向(grain-oriented)及非晶粒取向(non-oriented) grain oriented non-oriented电器用硅[硅] 钢片的最终用途及规格end usage and designations of electrical steel strip电器用的硅[硅] 钢片之分类classification of silicon steel sheet for electrical use电器用钢片的绝缘涂层performance of surface insulation of electrical steel sheets晶粒取向电器用硅钢片主要工业标准international standard –grain-oriented electrical steel siliconsteel sheet for electrical use晶粒取向电器用硅钢片grain-oriented electrical steel晶粒取向,定取向芯钢片及高硼定取向芯钢片之磁力性能及夹层系数(日工标准及美材标准)magnetic properties and lamination factor of si-orient-coresi-orient-core-hi b electrical steel strip (jis and aisi standard)退火annealing电器用钢片用家需自行应力退火原因annealing of the electrical steel sheet退火时注意事项annealing precautionary碳污染prevent carbon contamination热力应先从工件边缘透入heat from the laminated stacks edges提防过份氧化no excessive oxidation应力退火温度stress –relieving annealing temperature晶粒取向电器用硅[硅] 钢片–高硼(hi-b)定取向芯钢片及定取向芯钢片之机械性能及夹层系数mechanical properties and lamination factors of si-orient-core-hi-band si-orient-core grain orient electrical steel sheets晶粒取向电器用硅[硅] 钢;片–高硼低硫(ls)定取向钢片之磁力及电力性能magnetic and electrical properties of si-orient-core-hi-b-ls 晶粒取向电器用硅[硅] 钢片–高硼低硫(ls) 定取向钢片之机械性能及夹层系数mechanical properties and lamination factors ofsi-orient-core-hi-b-ls晶粒取向电器用硅(硅)钢片-高硼(hi-b)定取向芯钢片,定取向芯钢片及高硼低硫(ls)定取向芯钢片之厚度及阔度公差physical tolerance of si-orient-core-hi-b, si-orient-core,si-core-hi-b-ls grainoriented electrical steel sheets晶粒取向电器用硅(硅)钢片–高硼(hi-b)定取向芯钢片,定取向芯钢片及高硼低硫(ls)定取向芯钢片之标准尺寸及包装standard forms and size of si-orient-core-hi-b,si-core,si-orient-core-hi-b-ls grain-oriented electrical steel sheets绝缘表面surface insulation非晶粒取向电力用钢片的电力、磁力、机械性能及夹层系数lamination factors of electrical, magnetic mechanical non-grainoriented electrical电器及家电外壳用镀层冷辘[低碳] 钢片coated (low carbon) steel sheets for casing,electricals homeappliances镀铝硅钢片aluminized silicon alloy steel sheet简介general镀铝硅合金钢片的特色feature of aluminized silicon alloy steel sheet用途end usages抗化学品能力chemical resistance镀铝(硅)钢片–日工标准(jis g3314)hot-aluminum-coated sheets and coils to jis g 3314镀铝(硅)钢片–美材试标准(astm a-463-77)35.7 jis g3314镀热浸铝片的机械性能mechanical properties of jis g 3314 hot-dip aluminum-coated sheetsand coils公差size tolerance镀铝(硅)钢片及其它种类钢片的抗腐蚀性能比较comparsion of various resistance of aluminized steel other kindsof steel镀铝(硅)钢片生产流程aluminum steel sheet, production flow chart焊接能力weldability镀铝钢片的焊接状态(比较冷辘钢片)tips on welding of aluminized sheet in comparasion with cold rolledsteel strip钢板steel plate钢板用途分类及各国钢板的工业标准包括日工标准及美材试标准type of steel plate related jis, astm and other major industrialstandards钢板生产流程production flow chart钢板订货需知ordering of steel plate不锈钢stainless steel不锈钢的定义definition of stainless steel不锈钢之分类,耐腐蚀性及耐热性classification, corrosion resistant heat resistance of stainlesssteel铁铬系不锈钢片chrome stainless steel马氏体不锈钢martensite stainless steel低碳马氏体不锈钢low carbon martensite stainless steel含铁体不锈钢ferrite stainless steel镍铬系不锈钢nickel chrome stainless steel释出硬化不锈钢precipitation hardening stainless steel铁锰铝不锈钢fe / mn / al / stainless steel不锈钢的磁性magnetic property stainless steel不锈钢箔、卷片、片及板之厚度分类classification of foil, strip, sheet plate by thickness表面保护胶纸surface protection film不锈钢片材常用代号designation of sus steel special use stainless表面处理surface finish薄卷片及薄片(0.3至2.9mm厚之片)机械性能mechanical properties of thin stainless steel(thickness from 0.3mmto 2.9mm) –strip/sheet不锈钢片机械性能(301, 304, 631, csp)mechanical properties of spring use stainless steel不锈钢–种类,工业标准,化学成份,特点及主要用途stainless steel –type, industrial standard, chemical composition,characteristic end usage of the most commonly usedstainless steel不锈钢薄片用途例end usage of thinner gauge不锈钢片、板用途例examples of end usages of strip, sheet plate不锈钢应力退火卷片常用规格名词图解general specification of tension annealed stainless steel strips耐热不锈钢heat-resistance stainless steel镍铬系耐热不锈钢特性、化学成份、及操作温度heat-resistance stainless steel铬系耐热钢chrome heat resistance steel镍铬耐热钢ni - cr heat resistance steel超耐热钢special heat resistance steel抗热超级合金heat resistance super alloy耐热不锈钢比重表specific gravity of heat –resistance steel plates andsheetsstainless steel不锈钢材及耐热钢材标准对照表stainless and heat-resisting steels发条片power spring strip发条的分类及材料power spring strip classification and materials上链发条wind-up spring倒后擦发条pull back power spring圆面("卜竹")发条convex spring strip拉尺发条measure tape魔术手环magic tape魔术手环尺寸图drawing of magic tap定型发条constant torque spring定型发条及上炼发条的驱动力spring force of constant torque spring and wing-up spring 定型发条的形状及翻动过程shape and spring back of constant torque spring定型发条驱动力公式及代号the formula and symbol of constant torque spring边缘处理edge finish硬度hardness高碳钢化学成份及用途high carbon tool steel, chemical composition and usage 每公斤发条的长度简易公式the length of 1 kg of spring steel stripsk-5 aisi-301 每公斤长的重量/公斤(阔100-200公厘) weight per one meter long(kg) (width 100-200mm)sk-5 aisi-301 每公斤之长度(阔100-200公厘) length per one kg (width100-200mm)sk-5 aisi-301 每公尺长的重量/公斤(阔2.0-10公厘)weight per one meter long (kg) (width 2.0-10mm)sk-5 aisi-301 每公斤之长度(阔2.0-10公厘)length per one kg (width 2.0-10mm)高碳钢片high carbon steel strip分类classification用组织结构分类classification according to grain structure用含碳量分类–即低碳钢、中碳钢及高碳钢classification according to carbon contains金属材料相关英语词汇(2) 相关内容:。

Characterization and magnetic properties of Fe70Co30 alloy nanowire arraysG. H. Yue, L. S. Wang, X. Wang, Y. Z. Chen, and D. L. PengCitation: J. Appl. Phys. 105, 074312 (2009); doi: 10.1063/1.3103775View online: /10.1063/1.3103775View Table of Contents: /resource/1/JAPIAU/v105/i7Published by the American Institute of Physics.Additional information on J. Appl. Phys.Journal Homepage: /Journal Information: /about/about_the_journalTop downloads: /features/most_downloadedInformation for Authors: /authorsCharacterization and magnetic properties of Fe70Co30alloy nanowire arraysG.H.Yue,L.S.Wang,X.Wang,Y.Z.Chen,and D.L.Peng a͒Department of Materials Science and Engineering,Research Center of Materials Design and Applications,Xiamen University,Xiamen361005,People’s Republic of China͑Received6November2008;accepted18February2009;published online7April2009͒Highly ordered arrays of parallel Fe70Co30nanowires with a diameter of about50nm and a lengthup to about several tens of micrometers were synthesized by two electricalfields in an anodizedaluminum oxidefilm.The magnetic properties in the temperature range from5to300K werestudied.When the appliedfield is along the long axis,the temperature dependence of coercivity ofFe70Co30nanowire arrays shows a linear decrease with temperature increasing,which can beunderstood by a phenomenological nucleate model.©2009American Institute of Physics.͓DOI:10.1063/1.3103775͔I.INTRODUCTIONIn recent years,a great deal of progress has been made inthe synthesis of various nanostructures with controllablemorphology and properties,including one dimensional struc-ture such as nanowires and nanorods.1–7Those nanowiresand nanorods can be also used as building blocks in forminganisotropic nanostructured materials and devices such ashigh density data storage devices based on domain-wallmotion.5Morphological control has also been reported fornanowires and nanorods of semiconductors,8metals,9metaloxides,10,11and metal sulfide.2,4,6,12,13However,the synthesisof soft magnetic nanowires and nanorods with required ge-ometry and composition is still a challenge.Recently,different groups have tried to produceFe͑1−x͒Co x nanowires with different stoichiometric propor-tions.Most producing methods used templates such as an-odic aluminum oxide͑AAO͒templates2,4,6,12or metal stepedges.14However,the nanowires generated by these tech-niques exhibit amorphouslike materials on their surface͑e.g.,carbon and oxides͒.15In addition,the nanowires werepolycrystalline.2,12Other approaches such as the thermal de-composition of Fe and Co carbonyls in the presence ofstrong magneticfields resulted in the creation of polycrystal-line FeCo nanowires.16Circumventing these problems,thepulsed electrodeposition was used to promote the formationof single crystalline nanowires.II.EXPERIMENTALThe arrays of Fe70Co30nanowires have been prepared bypulsed electrodepositing Fe and Co into AAO templates.Thepreparation of AAO templates has been described elsewherein detail.2In this experiment,the high pure Al foils ͑99.999%͒were annealed at500°C for about48h,then anodized at40V͑dc͒in0.3M H2C2O4aqueous solution at0°C for1h.After the porous templates were removed,thesecond anodized step was performed under the same condi-tions as above.Mixture aqueous solution which containsFeSO4·7H2O,CoSO4·7H2O,H3BO4,and ascorbic acid was used for electrodeposition,and the Fe2+:Co2+ion ratio was adjusted to7:3in the baths.The p H value of the electrolyte maintained at about3.0.The electrodeposition was com-pletely conducted by a pulsed electrical source at50Hz and 10V͑ac͒,and the electrodeposition time was5min using graphite as the counterelectrode.After deposition,samples were kept in pure alcohol solution to avoid oxidation.For convenience,the nanowire arrays with AAO were removed from residual Al substrate by saturated aqueous solution of HgCl2before x-ray diffraction͑XRD͒and magnetic proper-ties measurement were performed.The nanowires were lib-erated by dissolving AAO templates in NaOH aqueous solu-tion before the observation with a transmission electron microscope͑TEM͒.III.RESULT AND DISCUSSIONThe typical XRD pattern of the sample which was etched for5h is shown in Fig.1.The presence of broad peaks can be ascribed to the Fe70Co30body-centered-cubic ͑bcc͒alloy͑space group Im3m͑229͒and lattice parameter a=2.85Å͒.The peaks at2values of44.658°,66.870°,and98.506°correspond to͑110͒,͑200͒,and͑220͒crystal planes of the crystalline Fe–Co alloy͑JCPDS49-1567͒,respec-tively.The cell constant of Fe70Co30is calculated as a =0.2847nm,which is in agreement with the reported values of a=0.285nm͑Ref.17͒for particles of the same composi-tion.The microstructures of Fe70Co30nanowires are very dif-a͒Author to whom correspondence should be addressed.Electronic mail:dlpeng@.Tel.:86-592-2180155.FAX:86-592-2180155.FIG.1.XRD pattern of the FeCo nanowire arrays after etching time of5h.JOURNAL OF APPLIED PHYSICS105,074312͑2009͒0021-8979/2009/105͑7͒/074312/5/$25.00©2009American Institute of Physics105,074312-1ferent from those of bulk Fe–Co alloy.In the XRD pattern of nanowire arrays,the peak of Fe 70Co 30͑110͒is very strong,which indicates that the nanowires have a bcc structure with ͑110͒preferred orientation rather than bcc-FeCo ͑100͒pre-ferred in the Fe–Co bulk alloy.The sharp and narrow ͑110͒peaks indicate that the nanowires are highly crystalline and consist of only a single compositional phase.Figure 2͑a ͒shows a typical scanning electron micros-copy ͑SEM ͒micrograph of the AAO template,which was anodized in 0.3M H 2C 2O 4aqueous solution at 0°C and a voltage of 40V ͑dc ͒.We can see that the AAO template has almost arranged the pore arrays with the average pore diam-eter about 50nm and the interpore distance about 20nm.Figures 2͑b ͒–2͑d ͒show SEM images of Fe 70Co 30nanowires grown in AAO template.These photographs show that the nanowires are uniformly distributed,highly ordered,and par-allel to each other.Few microscopic defects are found in these wires.Figure 2͑b ͒is a planform,from which we can find several clusters of nanowires.The clusters can result from the situation that the nanowires are uncovered out of the framework of the AAO template freestanding incom-pletely.When the top alumina of the AAO template is dis-solved away,the nanowires embedded in the template are released gradually and incline to agglutinate together.It is conceivable that the surface energy of the nanowires causes this interesting phenomenon.Figure 2͑b ͒also shows that Fe 70Co 30nanowires are uniform,highly ordered,and abun-dant in the large area.Figures 2͑c ͒and 2͑d ͒reveal a cross section where the alumina matrix of the AAO template has been partially dissolved away.It can be seen that the nano-wires that were deposited inside the nanochannel of the AAO template are parallel,uniformly distributed,and tidily aligned.It is correlative to that the AAO template had a series of densely parallel nanoholes arranged in a hexagonal fashion.From these figures it can be estimated that the aver-age length of these nanowires is about 20m.It is corre-sponds with the thickness of the AAO template used.At the same time the outside diameters of these wires are about 50nm,which are equivalent to the pore diameter of the tem-plate membrane.TEM images of Fe 70Co 30nanowires formed in the AAO template are shown in Figs.3͑a ͒and 3͑b ͒.Figure 3͑a ͒shows that the Fe 70Co 30nanowires cross and overlap with each other.Figure 3͑b ͒shows that the diameter of the Fe 70Co 30nanowires is about 40nm;it is approximately equal to the diameter of the nanochannels of the employed AAO tem-plate.These nanowires distribute uniformly,which indicates that the alumina matrix is dissolved completely.The high resolution TEM ͑HRTEM ͒image shows the lattice fringes in Fig.3͑c ͒;the layer spacing obtained from the lattice fringes in the HRTEM image is about 2Å,corresponding to d ͑110͒of the bcc Fe 70Co 30.The HRTEM observation reveals that the Fe 70Co 30nanowire grows along the ͑110͒direction.The selected area electron diffraction ͑SAED ͒pattern of Fig.3͑d ͒shows that the Fe 70Co 30nanowire of our sample is a single crystal nanostructure.It is consistent with our XRD results.Energy dispersive spectrometer analysis reveals that the product is composed of iron and sulfur,and the ratio of co-balt atom and Fe atom is 30.32:69.68which just accords with the stoichiometric ratio of Fe 70Co 30.It indicates that the essential component of the product is Fe 70Co 30,and it corre-sponds to the Fe 2+:Co 2+ion ratio that was adjusted as 7:3in the baths at the experimental process.The magnetic properties are measured with the super-conducting quantum interference device ͑SQUID ͒͑MPMS XL ͒magnetometry.All the magnetic properties were mea-sured with the Fe 70Co 30nanowires in self-assembled arrays with AAO template support.The samples electrodeposited in dc conditions exhibit perpendicular magnetic anisotropy,with saturation fields out-of-plane ͓the applied magnetic field perpendicular to substrate and parallel ͑ʈ͒to the long axis of nanowires ͔orientation much bigger than in the correspond-ing in-plane ͓the applied magnetic field perpendicular ͑Ќ͒to the long axis of nanowires ͔orientation,as can be seen in Fig.4.The perpendicular coercive field is 1584.4Oe at 300K ͓in Fig.4͑a ͔͒which obviously changes compared to the 2054.3Oe at 5K ͓in Fig.4͑b ͔͒of the paring to the magnetization data for the in-plane field orientation,it can be noticed that the in-plane coercive field is 625.7Oe at 300KFIG.2.SEM images of AAO template and Fe 70Co 30nanowire arrays.͑a ͒Typical SEM image of AAO template.͑b ͒The top view in low magnifica-tion.͓͑c ͒and ͑d ͔͒SEM image of a typical crosssection.FIG.3.TEM images of AAO template and Fe 70Co 30nanowire arrays.͑a ͒The sample was etched for 5h.͑b ͒The Fe 70Co 30nanowires with a diameter of about 40nm.͑c ͒The HRTEM image of the Fe 70Co 30nanowires in ͑b ͒.͑d ͒The SAED pattern taken from a single nanowire in ͑b ͒.and 682.1Oe at 5K.It is a great change in the in-plane coercive field of the paring the hysteresis loops between the field being parallel and perpendicular to the long axis for the sample,it was found that the easy axis of nano-wires is parallel to its long axis in spite of their different structures.This is because of the strong shape anisotropy.As expected for the applied field parallel to the long axis of nanowires,the hysteresis loops are relatively square ͑square ratio about 0.9͒,whereas when the applied field is perpen-dicular to the long axis of nanowires,the hysteresis loops are sheared ͑square ratio about 0.1͒.It is evidently seen from the features that the easy magnetization direction of nanowires is along the nanowire axes,which suggests that the overall magnetic anisotropy is dominated by the shape anisotropy in Fe 70Co 30nanowires themselves.Figure 5shows the temperature ͑T ͒dependence of H of the typical Fe 70Co 30samples,with the applied field being out of plane.It can be seen that the H is nearly linear over a temperature range of 5–300K.This tendency is in contrast with a relationship of H c with T 1/2in the models of nucle-ation due to the thermal activation.Therefore,there is a fun-damental problem about how to understand the linear rela-tionship of H c with T .It is well known that changing the intrinsic magnetic parameters usually influences the temperature on the magne-tization reversal,such as the saturated magnetization ͑M s ͒and the magnetocrystalline anisotropy constant.Considering that the accurate mass of nanowires could not be obtained,the temperature dependence of magnetization ͑M ͒with asaturated field is given in Fig.6.In Fig.6,the M decreases with temperature increasing and obeys the T 3/2rule.Since the nanowire mass does not depend on the temperature,the T dependence of M will show the correct temperature depen-dence of M s ,which indicates that M s can be expressed as 18M s ͑T ͒=M s ͑0͒͑1−CT 3/2͒.͑1͒Here M s ͑T ͒and M s ͑0͒are the values at temperatures of T and 0K,respectively.The electron diffraction results show that the nanowire arrays are single crystalline and we thought that the shape anisotropy makes the main contribution;there-fore,a major contribution from the magnetocrystalline aniso-tropy would not be expected.Figure 5shows that,with the temperature increasing from 5to 300K,the coercivity de-creases by about 30%for Fe 70Co 30nanowire arrays,while the saturated magnetization decreases by only about 28%.This indicates that there is another more important factor influencing the temperature dependence of coercivity.Considering the large shape anisotropy and demagneti-zation energy,the magnetic moments for the domains in the nanowires should be parallel or antiparallel to the long axis.Assuming two stable magnetic states,moments “up”and “down,”corresponding to the parallel and antiparallelstates,FIG.4.Normalized SQUID magnetometry data for dc electrodeposited Fe 70Co 30nanowire array samples in both field orientations ͑a ͒at 300K and ͑b ͒at 5K.FIG.5.Temperature dependence of coercivity ͑H ͒of Fe 70Co 30nanowire arrays.Solid stars denote the measured data.Solid lines are linear fitting results of Eq.͑3͒with temperature being independent of M s .The dotted lines are the fitting results of Eq.͑4͒with M s ͑T ͒changing with temperature at T 3/2.FIG.6.The temperature ͑T ͒dependence of M with a saturated field parallel to the long axis of the nanowire arrays.the nanowire system could be considered as a bistable system.19The two states are separated by an energy barrier. As the temperature increases,some magnetic moments will overcome the energy barrier and the magnetization reverses. The result is similar to that of an equivalent appliedfield to reverse the magnetization.Of course,the thermalfluctua-tions will also cause the magnetization reversal.The temperature dependence of coercivity can be under-stood based on the above bistable-state thermalfluctuation model.Assume that the magnetization reversal started from a reversal magnetization nucleus with the activation volume v. At the coercivefield,the energy barrier involved in the mag-netization reversal is expressed by20E0+H c ץEץH=25k B T,͑2͒where E0is thefield independent part of E.ץE/ץH is related to the magnetic viscosity coefficient,and k B and T are the Boltzmann constant and temperature,respectively.During the magnetization reversal process,two energy terms are ex-pected to contribute to the energy barrier E0,that is,the domain-wall energy and the magnetostatic energy.Since nucleation of a singledomain wall at the end of the wire leads to a smaller energy barrier,21the domain-wall energy can be derived by E p=␥s,where s is the surface of the acti-vation volume and␥represents the energy density of the domain wall.The magnetostatic energy E d=4␣M s2v,where ␣is a phenomenological parameter and M s is the saturation magnetization.Thus,H c=4␣M s−␥sv M s−25k B Tv M s.͑3͒The activation volume v is proportional toͱA/K eff and␥=4ͱAK eff,22where A is the exchange stiffness constant.The exchange stiffness constant varies with S2in molecularfield theory.S is the spin,and hence A is expected to vary with M s2.The K eff is the effective anisotropy constant.In the Fe70Co30nanowire array system,the shape anisotropy makes the main contribution on K eff.We assume that it is similar to a uniaxial anisotropy and K eff is thus proportional to M s2.19If we assume that M s is a constant at low temperature due to the high Curie temperature,␥and v are independent of the temperature,which means that coercivity is linearly dependent on temperature.Thefitting result for the Fe70Co30 nanowire arrays is shown in Fig.5,with the saturation mag-netization M s derived from the experiment dates of Fe70Co30. It can be found that the experimental results of coercivity agree well with the linear temperature dependence predicted by Eq.͑3͒.Since M s changes by about28%in the temperature range of5–300K,we now consider the influence of M s to Eq.͑3͒.The second term␥s/v M sϰ4sͱAK eff/ͱA/K eff M s =kM s;thus Eq.͑3͒becomesH c=KM s͑T͒−25k B Tv M s͑T͒,͑4͒where K is another phenomenological parameter.M s͑T͒is determined by Eq.͑1͒.Thefitting result by Eq.͑4͒is also shown in Fig.5,in which the M s͑0͒derived from the bulk materials and the parameter C in Eq.͑1͒is derived byfitting the experimental data in Fig.6.It can be found that the fitting result is still perfect with the experimental data.The coercivity is nearly linear with temperature in spite of the T3/2law of saturated magnetization.This result indicates that the temperature dependence of M s has a minor effect on coercivity,and about28%of the changes to M s will not obviously change the linear temperature dependence of the coercivity.The reason for this result is that the decreasing of M s will help more magnetic moments reverse and thus lead to a larger activation volume.It is worth noting that the model predicted by Eq.͑3͒is different from the actual nucle-ation mode.It is only a phenomenological model to interpret the results of hard magnetic materials.20IV.CONCLUSIONIn conclusion,we have developed a novel method to synthesize cobalt disulfide nanowire arrays.The Fe70Co30 nanowires have apparently continuous,parallel,and ordered modality.The diameter of the cobalt disulfide nanowires is about50nm and the SAED pattern shows that the product is single crystalline.The hysteresis loops at various tempera-tures are studied.The coercivity with the appliedfield along the long axis linearly decreases with temperature increasing, which can be interpreted by a phenomenological nucleate model discussed above.ACKNOWLEDGMENTSThis work was partially supported by the National Out-standing Youth Science Foundation of China͑Grant No. 50825101͒and the National Natural Science Foundation of China͑Grant No.50671087͒.One of the authors͑D.L. Peng͒acknowledges the Minjiang Chair Professorship Pro-gram released by Fujian Province of PR China forfinancial support.1A.I.Hochbaum,R.Chen,R.D.Delgado,W.Liang,E.C.Garnett,M. Najarian,A.Majumdar,and P.Yang,Nature͑London͒451,163͑2008͒. 2G.H.Yue,P.X.Yan,J.Z.Liu,X.Y.Fan,and R.F.Zhuo,Appl.Phys. Lett.87,262505͑2005͒.3S.Sun,C.B.Murray,D.Weller,L.Folks,and A.Moser,Science287, 1989͑2000͒.4G.H.Yue,P.X.Yan,X.Y.Fan,M.X.Wang,D.M.Qu,D.Yan,and J.Z. Liu,J.Appl.Phys.100,124313͑2006͒.5L.Thomas,M.Hayashi,X.Jiang,R.Moriya,C.Rettner,and S.S.P. Parkin,Nature͑London͒443,197͑2006͒.6G.H.Yue,P.X.Yan,L.S.Wang,W.Wnag,Y.Z.Chen,and D.L.Peng, Nanotechnology19,195706͑2008͒.7R.P.Cowburn,Nature͑London͒448,544͑2007͒.8K.Yong,Y.Sahoo,M.T.Swihart,and P.N.Prasad,J.Phys.Chem.C111, 2447͑2007͒.9L.Gou and C.J.Murphy,Chem.Mater.17,3668͑2005͒.10G.H.Yue,P.X.Yan,D.Yan,D.M.Qu,X.Y.Fan,M.X.Wang,and H. T.Shang,J.Cryst.Growth294,385͑2006͒.11J.Wu,Y.Lee,H.Chiang,and D.K.Wong,J.Phys.Chem.B110,37͑2006͒.12G.H.Yue,P.X.Yan,X.Y.Fan,M.X.Wang,D.M.Qu,Z.G.Wu,C.Li,and D.Yan,Electrochem.Solid-State Lett.10,D29͑2007͒.13G.H.Yue,P.X.Yan,D.Yan,X.Y.Fan,M.X.Wang,D.M.Qu,and J.Z. Liu,Appl.Phys.A:Mater.Sci.Process.84,409͑2006͒.14J.P.Pierce,E.W.Plummer,and J.Shen,Appl.Phys.Lett.81,1890͑2002͒.15P.S.Fodor,G.M.Tsoi,and L.E.Wenger,J.Appl.Phys.91,8186͑2002͒. 16Q.F.Zhan,Z.Y.Chen,D.S.Xue,F.S.Li,H.Kunkel,X.Z.Zhou,R. Roshko,and G.Williams,Phys.Rev.B66,134436͑2002͒.17Joint Committee on Powder Diffraction Standards Diffraction Data File No.49-1567,1991.18C.Herring and C.Kittel,Phys.Rev.81,869͑1951͒.19B.Giorgio,Hysteresis in Magnetism(For Physics,Materials Scientists, and Engineers)͑Academic,New York,1998͒.20D.Givord,P.Tenaud,and T.Viadieu,IEEE Trans.Magn.24,1921͑1988͒. 21J.E.Knowles,J.Magn.Magn.Mater.61,121͑1986͒.22H.B.Braun,Phys.Rev.B50,16501͑1994͒.。
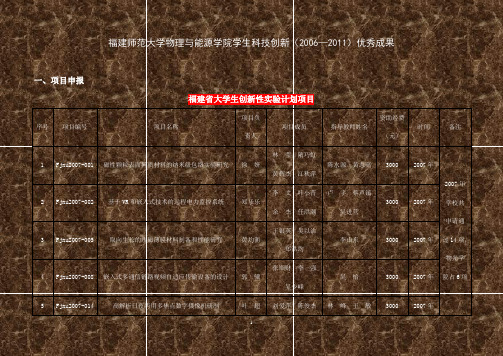
福建师范大学物理与能源学院学生科技创新(2006—2011)优秀成果一、项目申报福建省大学生创新性实验计划项目序号项目编号项目名称项目负责人项目成员指导教师姓名资助经费(元)时间备注1Fjnu2007-001磁性颗粒表面异质材料的纳米级包络实验研究徐 妍林 莹 隋巧虹黄辉杰 江秋萍陈水源 黄志高 3000 2007年2007年学校共申请通过14项,物光学院占6项2Fjnu2007-002基于VR 和嵌入式技术的远程电力监控系统郑乐乐李 文 叶小青余 杰 任洪潮 卢 宇 蔡声镇吴进营30002007年3Fjnu2007-005取向生长的永磁薄膜材料制备和性能研究黄均衡王银英 吴以治郑洪均李山东30002007年 4Fjnu2007-008嵌入式多通信链路视频自适应传输设备的设计郭 骥张顺财 李 强吴少峰吴 怡 3000 2007年 5Fjnu2007-014 高解析日夜两用多焦点数字摄像机研制 叶 超刘爱萍 陈俊杰 林 峰 王 敏 3000 2007年梁秀玲 邱怡申杨洪钦6Fjnu2007-010基于ZigBee 的无线自组织网络系统设计田宗武李丽飞 陈 宇、陈志聪吴允平 3000 2007年7Fjnu2008-001基于红外热成像技术的乳腺疾病无损诊断陈新光肖邵军 刘小娟杨洪钦 谢树森王瑜华3000 2008年2008年学校共申请通过15项,物光学院占5项8Fjnu2008-008嵌入式自适应光谱信号处理系统的设计张文斌李文潇 詹明媚黄 敏 张荣辉李 晖 3000 2008年 9Fjnu2008-009基于中频技术的3G 室内光分布系统的研究吴天敏金 坦 吴美珍 陈智浩 蒋俊贞 3000 2008年10Fjnu2008-012voIP 融合通信系统呼叫中心平台的开发江文锋吕钱钗 吴 晶陈红艳蔡坚勇 林 潇 3000 2008年 11Fjnu2008-013基于802.11的嵌入式无线点菜系统梅银玉戴铭贤 倪昌勇杨 彬 唐春宁洪 亲 吴 怡 3000 2008年12 Fjnu2010-001 磁电多铁性材料的制备及其特性研究 邹坛锋 陈水源 黄志高 3000 2010年 2010年学校共13Fjnu2010-002Excel 数据服务的信息化扩展应用吴 灏卢 宇 郭 辉30002010年苏 明申请通过15项,物光学院占4项14Fjnu2010-003 炭热还原法制备磷酸铁锂材料及电化学性能研究曾宝枝林应斌30002010年 15Fjnu2010-004适用于LED 近紫外芯片的新型硅酸盐白光荧光粉的制备和发光特性研究丘志海林 林30002010年福建师大本科生课外科技计划资助项目序号项目编号项目名称项目负责人指导教师姓名资助经费(元)时间1 BKL2007071 基于ARM 的多通信链路自适应选择模块的设计宋开春 吴 怡 1000 2006-2007 2 BKL2007072 基于ARM 应用的无纸记录仪开发研究 许剑彬 廖晓东 1000 2006-2007 3 BKL2007073 基于红外热成像技术的人体组织三维温度场的构建李育灵 杨洪钦 1000 2006-2007 4 BKL2007074 高精度电子式天平开发研究 林健伟 廖晓东 1000 2006-2007 5 BKL2007075 网络实验系统仿真数据处理 郑乐乐 卢 宇 1000 2006-2007 6 BKL2007076 电子制造业产品结构管理软件 侯巧红 洪 亲 1000 2006-2007 7 BKL2007077 基于嵌入式LabVIEW 的光谱信号处理软件 张月明 蔡坚勇 1000 2006-2007 8BKL2007078鲜红斑痣皮肤光学模型的构建及其应用林宏翔陈 荣10002006-20079 BKL2007079 4.3mm MTV镜头的设计与制作林鹤王敏1000 2006-200710 BKL2007080 6mm 固定光圈镜头设计与制作肖钰斐王敏1000 2006-200711 BKL2007081 f12 MTV镜头设计与制作李乙衔梁秀玲1000 2006-200712 BKL2007082 提高超声调制光学成像术成像深度和成像质量的研究黄文华朱莉莉1000 2006-200713 BKL2007083 鼻咽组织荧光成像实时图像处理陈歆宇刘丽娜1000 2006-200714 BKL2007084 非线性光学复合成像技术用于肠道组织的微结构研究严旭辉陈建新1000 2006-200715 BKL2007085 焦距测量仪图像转接系统徐蛟梁秀玲1000 2006-200716 BKL2007086 多路视频监控系统的传输调度模型设计王小彬吴怡1000 2006-200717 BKL2007087 多路视频信息管理系统郑乐乐洪亲1000 2006-200718 BKL2007088 超导/磁电阻复合材料的制备及其特性研究傅良友陈水源1000 2006-200719 BKL2007089 稀土掺杂聚合物波导的制备及光学特性郭荧荧郑志强1000 2006-200720 BKL2007090 中学CAI物理教学形式及效果研究李晓炜林钦1000 2006-200721 BKL2007091 多部分量子通信的研究陈子翃林秀敏1000 2006-200722 BKL2007092 关于量子克隆的研究梅金锋李洪才1000 2006-200723 BKL2008-003 新型航标灯监测系统吴并辉苏伟达吴允平1000 2007-200824 BKL2008-004 嵌入式指纹识别系统陈霆钧卢宇1000 2007-200825 BKL2008-005 基于嵌入式的网络通信平台的设计胡利国施文灶王平1000 2007-200826 BKL2008-006 基于嵌入式的网络终端研究及其应用软件设计陈伟王平施文灶1000 2007-200827 BKL2008-007 基于VB的信息管理系统的设计蔡树诚刘金清1000 2007-200828 BKL2008-008 日夜两用监控鱼眼镜头设计陈德福林峰王敏1000 2007-200829 BKL2008-009 ZnO基磁性发光材料的制备及其LED光电性能的研究陈銮林应斌1000 2007-200830 BKL2008-010 F-Theta激光扫描镜头设计吴权王敏林峰1000 2007-200831 BKL2008-011 Er3+/Yb3+共掺PLZT电光陶瓷的光辐射特性江秋萍郑志强冯卓宏1000 2007-2008赖发春庄彬平32 BKL2008-012 氧化锌纳米线的制备及其特性的研究林明豹1000 2007-2008林丽梅叶晴莹陈志高33 BKL2008-013 量子点磁化动力快速傅里叶变换的研究陈秀来1000 2007-2008黄志高34 BKL2008-014 基于光纤及五类线传输的3G室内微功率分布系统的研究沈剑锋蒋俊贞1000 2007-200835 BKL2008-015 信号去噪方式对NDA四色荧光信号串扰校正的影响连志鹏郑华1000 2007-200836 BKL2008-016 纳米磁性材料磁偶极相互作用能的快速多极算法研究吴建鹏杨艳敏1000 2007-200837 BKL2008-017 波片相位延迟量测定装置的研究林宏翔邱怡申李高明1000 2007-200838 BKL2008-069 车辆导航系统林启藩洪亲1000 2007-2008李晖朱莉莉39 BKL2009-005 利用光的特性探究无创检测血糖浓度钱鑫2000 2008-2009喩碧莺李志芳40 BKL2009-006 一种新型癌细胞核壳结构表面增强免疫拉曼光谱探针研制王珊妮陈荣冯尚荣2000 2008-200941 BKL2009-007 白光LED老化和失效机理分析黄珊珊林林1500 2008-200942 BKL2009-008 多铁性材料的化学法制备及其磁电效应吴君陈水源黄志高2000 2008-200943 BKL2009-009 锂电池橄榄石型正极材料的制备与性能研究叶靖莹赖恒2000 2008-2009杨榕灿李洪才44 BKL2009-010 基于腔QED和囚禁离子系统的量子纠缠度测量研究黄彬2000 2008-2009李连煌45 BKL2009-011 新型磁熵变材料研究罗建邦李山东2000 2008-200946 BKL2009-012 水喷雾条件对脉冲激光硬组织消融的影响苏美晃张先增詹振林1500 2008-200947 BKL2009-013 基于囚禁离子系统的量子通讯的有效实现周淑颖林秀1500 2008-200948 BKL2009-014 纳米磁性薄膜的生长和磁性研究冯颖冯倩1500 2008-200949 BKL2009-015 基于反射结构的小型可调谐滤波器的设计卢锡清吴淑莲1500 2008-200950 BKL2009-016 新教材中自制教具的设计与制作苏文建黄树清1500 2008-200951 BKL2009-017 快速充电锂离子电池材料的制备及研究赵桂英林应斌1500 2008-200952 BKL2009-018 锂电池负极材料Li4Ti5O12的制备、改性及其电化学性能研究郑文惠杨艳敏1500 2008-200953 BKL2009-019 覆盖1460-1620nm的大带宽波长锁定器邱明惠李晖吴淑莲1500 2008-200954 BKL2009-020 DNA凝聚的分子动力学模拟刘小娟杨洪钦1500 2008-200955 BKL2009-021 乳腺肿瘤模拟样品的光声成像林世兴朱莉莉1000 2008-200956 BKL2009-022 硬管手术内窥镜光学系统设计孙旭东林峰1000 2008-200957 BKL2009-023 有效哈密顿量的计算及其在量子信息处理中的应用戴丽琴林秀敏1000 2008-200958 BKL2009-024 银参杂氧化锌薄膜光电特性的研究张汉洲赖发春1000 2008-2009林钦郑渊方59 BKL2009-025 高师物理教学论资源建设研究张伟强1000 2008-2009黄树清林利胜60 BKL2009-026 散射介质偏振光学特性探测中的数据自动处理杨玲徐兰清1000 2008-200961 BKL2009-027 基于PC资源的嵌入式无线影音播放系统林永发蔡坚勇吴怡1500 2008-200962 BKL2009-028 基于自组网技术的车辆航雷达无线通信模块的设计林鸿吴怡林潇1500 2008-200963 BKL2009-029 基于Zigbee无线网络自动识别多移动目标系统乔银思王平施文灶1500 2008-200964 BKL2009-030 基于嵌入式的GPS智能公交站牌程隆王平1500 2008-200965 BKL2009-013 智能家居安防系统的设计魏绍山施文灶1000 2008-200966 BKL2009-013 基于射频识别的电子导游李宏华卢慧祖傅文怀1000 2008-200967 BKL2010-008 基于DSP的虹膜识别家居门禁系统高雅娟刘金清2000 2009-201068 BKL2010-009 适用于LED近紫外芯片的新型硅酸盐白光荧光粉的制备和发光特性研究丘志海林林2000 2009-201069 BKL2010-010 不同条件下大功率LED光衰机制分析温伟伟郑志强2000 2009-201070 BKL2010-011 用于LED照明的智能电源设计王英秋李汪彪2000 2009-201071 BKL2010-012 磁电多铁性材料的制备及其特性研究邹坛锋陈水源黄志高2000 2009-201072 BKL2010-013 高温热蒸发制备ZnO 纳米线郭小群赖发春林丽梅2000 2009-201073 BKL2010-014 新型抬头显示器的设计与制作江晶晶李晖2000 2009-201074 BKL2010-015 炭热还原法制备磷酸铁锂材料及电化学性能研究曾宝枝林应斌2000 2009-201075 BKL2010-016 利用过渡金属制取锂电池的阴极氧化物材料及其性能的研究张静赖恒2000 2009-201076 BKL2010-017 新课改中探究实验教具的创新研究与制作苏文建黄树清2000 2009-201077 BKL2010-018 光纤环形腔衰荡光谱数据采集与处理系统设计江东明李高明1000 2009-201078 BKL2010-019 球面波干涉图像的自动识别与处理黄振华王敏2000 2009-201079 BKL2010-020 灯具配光曲线测量仪朱楷棋李高明2000 2009-201080 BKL2010-021 基于无线数传模块的抄表系统的设计上官艳莎施文灶2000 2009-201081 BKL2010-022 基于搜索引擎的图像检索系统林铭洪亲2000 2009-201082 BKL2010-023 WBM模式的嵌入式硬件防火墙的设计范协晖蔡坚勇2000 2009-201083 BKL2010-024 分布式低碳能源利用数据采集系统设计陈霆钧卢宇蔡声镇2000 2009-201084 BKL2010-025 基于CDMA和GPS的车辆监控终端的设计方乐洪吴怡2000 2009-2010二、专利取得本科生获得的国家专利序号专利号作品名称作者时间1 200810070644 一种嵌入式系统的可靠通讯控制方法郑乐乐(8)叶小青(9)李文(10)2008-8-12 200820101449 一种基于虚拟三维的转矩流变仪监控装置郑乐乐(9)叶小青(10)李文(11)2008-11-13 200920137968 单总线接口时序分析仪宋秀杰(1)赵子初(2)吴灏(3)2010-3-1三、论文发表本科生发表的学术论文序号学生姓名年级专业论文题目论文作者学生排位发表情况备注1 俞明1998级物理学微型地图里程测量仪的研制第5作者仪器仪表学报 26(2), 218-220(2005) (EI收录)EI收录2 刘美梅2001级物理学CoMnSb: A magnetocaloric material with a largelow-field magnetic entropy change atintermediate temperature第2作者Journal of Applied Physics99, 063901 (2006)(SCI、EI收录)SCI、EI收录3 林海山2003级物理学The exchange bias and coercivity of FM/AFMfilms: Monte Carlo simulation 第3作者Journal of Magnetism andMagnetic Materials 303(2),e180-e183 (2006) (EI 收录)SCI、EI收录4 张志城杨少锋林璇2000级2003级2003级物理学The size and space arrangement roles oncoercivity of electrodeposited Co1-x Cu xnanowires,第1、4、5作者Journal of Magnetism andMagnetic Materials 303(2),e304-e307 (2006) (EI收录)SCI、EI收录5 柯文炮2003级物理学Transport and magnetic properties of nanosized 第4作者Journal of Magnetism and SCI、EILa2/3(Ca0.6Ba0.4)1/3MnO3/ xNiO composites Magnetic Materials 303(2),e308-e311 (2006) (EI 收录)收录6 林丽民2003级物理学The roles of the exchange and dipole couplingson the magnetoresistance for the nanoparticlearrays第4作者Journal of Magnetism andMagnetic Materials 303(2),e312-e314 (2006) (EI 收录)SCI、EI收录7 李加新陈丹沈咏娜2001级2003级2003级物理学Scaling behavior of the anisotropy magneticfilms: Experiment and simulation第5、6、7作者Journal of Magnetism andMagnetic Materials 303(2),e406-e409 (2006) (EI收录)SCI、EI收录8 倪妍2003级物理学The effects of nearest and next-nearestneighbor interactions on the magneticproperties of films with BCC structure第3作者Journal of Magnetism andMagnetic Materials 303(2),e436-e439 (2006) (EI收录)SCI、EI收录9洪锦绵林琳2003级2003级物理学掺钕KGW激光晶体的各向异性吸收光谱及其上转换发光第6、7作者人工晶体学报 35(4), 897-901(2006)SCI、EI收录Anisotropic Absorption Spectra andUp-conversion of Nd3+:KGW Crystal10林琳洪锦绵2003级2003级物理学掺钕PLZT电光陶瓷的光辐射特性第6、7作者福建师范大学学报(自然科学版) 22(1), 44-48 (2006)11 黄尚波2003级物理学Transport and magnetic properties of nanosizedLa0.6Sm0.1Sr0.3MnO3 + xCoO composites 第4作者Journal of Rare Earths24(6), 788-792 (2006) (SCI、EI收录)SCI、EI收录12 黄志南2002级物理学Effects of Samarium Doping on OpticalProperties of Zn0.9(Co1-x Sm x)0.1O Diluted MagneticSemiconductor第5作者Journal of Rare Earths 24,270-272 (2006) (SCI、EI收录)SCI、EI收录13 许贵桂2002级物理学Effects of Doping on Magnetic Properties ofYCo5-x Fe x and YCo5-x Ag x - First PrinciplesCalculation第3作者Journal of Rare Earths 24,293-297 (2006) (SCI、EI收录)SCI、EI收录14 程宝华2003级物理学The size and temperature effects of coercivityfor the magnetic nanowire: Monte CarloSimulation第3作者Solid State Phenomena121-123, 1081-1084 (2007)(EI收录)SCI、EI收录15 张健敏2003级物理学The influences of size and anisotropy strength 第3作者Solid State Phenomena SCI、EIon hysteresis scaling for anisotropy Heisenbergmultilayer films 121-123, 1085-1088 (2007)(EI收录)收录16唐素珍柯文炮2002级2003级物理学Electrical conductivity and low-fieldmagnetoresistance in La2/3(Ca0.60Ba0.40)1/3Mn1-x V x O3prepared by sol-gel第3、4作者Solid State Phenomena121-123, 901-904 (2007) (EI收录)SCI、EI收录17 许贵桂2002级物理学First-principles and Monte Carlo combinationalstudy on Zn1−x Co x O diluted magneticsemiconductor第4作者Solid State Communications142(4), 242-246 (2007) (SCI、EI收录)SCI、EI收录18林建锋黄贵茹2003级物理学Simple scheme for Preparing W states and Cloningvia adiabatic passage in ion-trap systems第6、7作者Optics Communications(2007)SCI、EI收录19 陈丹2003级物理学Co x Ni1-x磁性膜标度行为的研究第6作者福建师范大学学报(自然科学版) 22(3), 69-72 (2006) 20 李雪蓉2002级物理学测量条件对掺锡氧化铟薄膜电学测量结果的影响第1作者物理实验 27(4), 44-47(2007)21 陈小雅2002级物理学量子点磁化动力学行为的研究第3作者福建师范大学学报(自然科学版) 23(2), 40-44 (2007)22洪锦绵林琳2003级2003级物理学用速率方程分析掺杂聚合物光纤的团簇特性第6、7作者集美大学学报 12(1), 38-42(2007)23杨少锋陈柳慧2003级2003级物理学Propagation of ultrasound modulationscattering signal in multi-layer scatteringmedia: simulations and experiments.第3、4作者Photonics Asia 2007会议(收录)论文编号PA07-PA104-61SCI、EI收录24 林志强2004级物理学电磁感应电动势的普遍表述第1作者泉州师范学院学报 Vol.25E.E ,18~19(2007)25 刘丁忠2002级物理学Simultaneous observation of collagen andelastin based on the combined nonlinear opticalimaging technique coupled with two-channelsynchronized detection method第4作者Optik (in press) 118, 2007(SCI 、EI收录,影响因子0.585)SCI 、EI收录26 王银英吴以治黄钧衡2005级物理学Nd composition dependence of microstructure andmagnetic properties for gradient sputteredNdFeB films第5、6、7、8作者Journal of Magnetism andMagnetic MaterialsSCI、EI收录郑洪均27 江秋萍2005 物理学The dynamics of frequency upconversion inEr3+/Yb3+-codoped PLZT transparent ceramics 第5作者Proc. of SPIE Vol. 713571354A-1SCI、EI收录28林剑锋黄贵茹2003 物理学Simple scheme for preparing W states and cloningvia adiabatic passage in ion-trap systems第6,7作者Optics Communications 279:399(2007)SCI、EI收录29 林剑锋梅金锋郑碧沁2003,2004 物理学利用腔QED实现无Bell基测量的经济相位传输克隆第4,5,6作者福建师范大学:自然科学版23:47(2007)30 蓝杰钦2003 物理学Implementing Entanglement Swapping andGenerating Multiphoton Entanglement in CavityQED第4作者Communications inTheoretical Physics49,1483-1486(2008)SCI收录(SCI收录)31 陈复2003 物理学非最大量子纠缠信道的量子态传送——在腔QED系第2作者福建师范大学学报:自然科学统中利用非最大三粒子纠缠GHZ态传送未知原子态版Vol.24,No.2, 200832张秀真郑碧沁2003 物理学利用腔QED实现Deutsch—Jozsa算法第4,5,6作者福建师范大学:自然科学版24:38(2008)33 徐妍2005 物理学Effect of process conditions and NiO onmagnetoresistance of La-(Ca,Ba)-Mn-Ocomposites,第2作者2008 2nd IEEE InternationalNanoelectronics Conference,INEC 2008, 1056 (2008)SCI、EI收录34张秀真郑碧沁2003 物理学利用腔QED实现Deutsch—Jozsa算法第4,5,6作者福建师范大学:自然科学版24:38(2008)35 彭春增2004 物理学利用受激拉曼绝热技术制备腔模高维纠缠态第2作者福建师范大学学报:自然科学版Vol.25,No.1, 200936黄贵茹庄伟海2003,2004 物理学利用暗态制备离子Cluster Stat第4,5作者福建师范大学:自然科学版25:48(2009)37 黄彬林霞林慧2006 物理学Two-qutrit maximally entangled states preparedvia adiabatic passage in ion-trapped system第1、2、3、4作者Chinese Physics B 12:124206(2010)SCI、EI收录蔡振华38 肖邵军2005 物理学基于法拉第旋转构造光子Bell态分析器和GHZ态分析器第2作者物理学报,59(8),2010 (SCI收录)SCI收录Implementation of photon Bell-state andGHZ-state analyzers through the Faradayrotation四、竞赛获奖“挑战杯”大学生课外学术科技作品竞赛序号比赛项目团队成员成果名称获奖情况获奖时间1 “挑战杯”大学生课外科技作品竞赛彭井花、林兆剑、王本雄嵌入式无线网络电力安全监测系统国赛三等奖、省赛二等奖20072 “挑战杯”大学生课外科技作品竞赛赵子初、宋秀杰、吴灏单总线接口分析仪国赛三等奖、省赛二等奖2009“挑战杯”大学生创业计划竞赛序号比赛项目获奖团队团队成员成果名称获奖情况获奖时间1 “挑战杯”大学生创业计划竞赛创捷创业团队变电站开关室环境智能监控系统省赛银奖20062 “挑战杯”大学生创业计划竞赛蜘蛛王创业团队无线视频监控系统省赛铜奖20063 “挑战杯”大学生创业计划竞赛掌中创业团队掌中流媒体教育系统省赛铜奖20064 “挑战杯”大学生创业计划竞赛博约创业团队分布式光纤温度传感系统省赛铜奖20065 “挑战杯”大学生创业计划竞赛致远创业小组超声波物位测量仪省赛铜奖20066 “挑战杯”大学生创业计划竞赛UP小组陈希文、林莹、沈涛ITO透明导电薄膜省赛铜奖20087 “挑战杯”大学生创业计划竞赛旋风团队徐滨、黄晓舒、冯毅无线远程阀控水表省赛铜奖2008国赛铜奖,8 “挑战杯”大学生创业计划竞赛Scaler团队林霞、吴灏单总线接口分析仪2010省赛金奖9 “挑战杯”大学生创业计划竞赛N次方团队林莹、白奕、赵桂英正源新型电池材料有限公司省赛银奖2010东芝杯•中国师范大学理科师范生教学技能创新大赛序号比赛名称获奖学生年级专业获奖情况获奖时间1 第三届东芝杯•中国师范大学理科师范生教学技能创新大赛王素云2007级物理学东芝创新奖及二等奖2010年2 “东芝杯”教学技能比赛戴颖2008级物理学国赛优秀奖2011全国大学生中学物理教学技能大赛序号比赛名称获奖学生获奖情况获奖时间1 第一届全国大学生中学物理教学技能大赛黄哲扬国赛一等奖2009年2 第一届全国大学生中学物理教学技能大赛林游国赛一等奖2009年3 第一届全国大学生中学物理教学技能大赛潘丽珍国赛一等奖2009年4 第一届全国大学生中学物理教学技能大赛刘洁琼国赛一等奖2009年5 第一届全国大学生中学物理教学技能大赛陈金宗国赛一等奖2009年6 第一届全国大学生中学物理教学技能大赛傅星维国赛一等奖2009年7 第二届全国大学生中学物理教学技能大赛王素云国赛一等奖2010年8 第二届全国大学生中学物理教学技能大赛王敏国赛一等奖2010年9 第二届全国大学生中学物理教学技能大赛陈思萦国赛一等奖2010年10 第二届全国大学生中学物理教学技能大赛薛美娜国赛一等奖2010年11 第二届全国大学生中学物理教学技能大赛王真真国赛一等奖2010年12 第二届全国大学生中学物理教学技能大赛林辉国赛一等奖2010年13 第三届全国大学生中学物理教学技能大赛张晴晴国赛特等奖2011年14 第三届全国大学生中学物理教学技能大赛林梦婷国赛特等奖2011年15 第三届全国大学生中学物理教学技能大赛章逸文国赛一等奖2011年16 第三届全国大学生中学物理教学技能大赛卢倩国赛一等奖2011年17 第三届全国大学生中学物理教学技能大赛戴颖国赛一等奖2011年18 第三届全国大学生中学物理教学技能大赛庄莹莹国赛一等奖2011年全国大学生物理实验竞赛序号比赛项目参赛队员获奖情况获奖时间1 基础类物理实验温伟伟二等奖2010年2 基础类物理实验吴一鸣三等奖2010年3 综合类物理实验王静、蔡志义三等奖2010年大学生教具制作竞赛序号比赛项目获奖学生获奖情况组织单位获奖时间1 第六届(海尔杯)全国优秀自制教具《波传播实质演示仪》黄树清、许淑云、陈静二等奖中国教育学会2006.9 教育部教学仪器研究所2 第六届福建省优秀自制教具《波传播实质演示仪》黄树清、许淑云、陈静一等奖福建省教育厅2006.53 第六届福建省优秀自制教具《水车》黄树清、郑晃太三等奖福建省教育厅2006.54 第六届福建省优秀自制教具《超重失重演示仪》黄树清、许淑云、黄雄优秀奖福建省教育厅2006.55 第六届福建省优秀自制教具《力的合成与分解演示仪》黄树清、吴远龙、许淑云优秀奖福建省教育厅2006.56 第六届福建省优秀自制教具《液体反冲》黄树清(指导老师)、优秀奖福建省教育厅2006.5 黄灿森、江龙、黄怀东7 第七届全国优秀自制教具《多功能摩擦力演示仪》黄树清、吴林坤、李育元国家级二等奖教育部教学仪器研究所2009.8 (省级一等奖)8 第七届全国优秀自制教具《三基色光视觉障碍检测仪》唐兆祥、洪智彬(指导老师:黄树清)国家级二等奖教育部教学仪器研究所2009.8 (省级一等奖)9 第七届福建省优秀自制教《静电滚球》陈苇娜、黄树清、黄德志三等奖福建省教育厅2009.610 第七届福建省优秀自制教具《压强演示仪》黄树清、陈峰、朱艺华三等奖福建省教育厅2009.611 第七届福建省优秀自制教具“低压沸腾演示仪”李育元、吴林坤、黄树清提名奖福建省教育厅2009.612 第七届福建省优秀自制教具“液体对容器底部压力演示仪“黄树清、林霞提名奖福建省教育厅2009.613 第七届福建省优秀自制教具“力合成演示仪”黄树清、李海滨、杨晓瑜提名奖福建省教育厅2009.614 第七届福建省优秀自制教具“安培力演示仪”高帆、李育元、黄树清提名奖福建省教育厅2009.615 第七届福建省优秀自制教具“多普勒效应演示仪”黄树清、池能锋、林霞提名奖福建省教育厅2009.6大学生高等数学竞赛序号参赛学生专业获奖情况获奖时间1 施一峰物理学省赛一等奖2010年2 陈伟灿物理学省赛三等奖2010年3 黄志铭物理学省赛三等奖2010年4 郑祥荣材料物理省赛二等奖2011大学生英语竞赛序号参赛学生专业获奖情况获奖时间1 林云物理学国家特等奖2012年科普动漫设计大赛序号比赛项目参赛队员获奖情况获奖时间备注1 首届福州市大学生科普动漫设计竞赛郑洪均一等奖2008年My Dream2 第二届福州市大学生科普动漫设计竞赛周雅雅、王素云二等奖2010年拯救3 第二届福州市大学生科普动漫设计竞赛林艳玲三等奖2010年4 第二届福州市大学生科普动漫设计竞赛苏永邦三等奖2010年5 第二届福州市大学生科普动漫设计竞赛石惠琛三等奖2010年6 第二届福州市大学生科普动漫设计竞赛汪海生三等奖2010年福建省规范汉字书写大赛序号参赛学生专业获奖情况获奖时间1 陈嶷物理学省赛二等奖2012全国大学生语言文字基本功大赛序号参赛学生专业获奖情况获奖时间1 陈思物理学国赛一等奖2012。

Magnetism and magnetic properties ofmaterials介绍磁性及其磁性能是材料科学中很重要的一块,磁性是指物质受到磁场作用时表现出来的各种现象,如吸引或排斥等。
而磁性能是指物质在磁场中的一系列特性表现。
磁性不仅影响着我们生活中常用的许多物品,如电视、电脑、磁性材料,而且还对应用在电磁设备、航空航天、生物医学等方面具有重要的应用价值。
磁性基础知识磁性是由原子和分子的磁性质决定的。
原子既具有电子轨道运动所形成的轨道磁矩,又具有自旋运动所形成的自旋磁矩。
物质的磁性取决于自旋磁矩、轨道磁矩的合成,并受到分子结构、晶格结构、温度等因素的影响。
然而,对于许多材料而言,这种合成是非常微弱的,因此物质磁化的来源主要是出现了局域磁时原子间作用的形成及相互偏转。
物质磁化度的计量单位是磁通量密度,即每个单位面积上磁通量的总数。
如果表面积为A, 磁通量为Φ,磁化密度J可以用下式表示:J = Φ/A磁性种类物质的磁性取决于其内部的微观结构,不同结构具有不同的磁性。
根据物质的磁特性,可分为顺磁性、铁磁性、反磁性及亚铁磁性。
顺磁性是指物质受磁场作用后,始终在磁场的方向上产生一个磁矩,而它的方向又是与磁场方向相同的微弱磁性。
顺磁性是由于原子或离子中的未成对电子对磁场的响应所引起的,其性能与温度成正比,并随着温度升高而减小。
反磁性是指物质受磁场作用后,使之形成的磁场方向相反,而且是以极微弱的程度出现。
这种类型的磁性主要是由于原子自旋磁矩和轨道磁矩的相互抵消所引起的。
亚铁磁性是介于顺磁性和铁磁性之间的一种磁性,通常将它称为温和的顺磁性或极弱的铁磁性。
其过渡相变点温度通常在零下20至100度之间正好还接近室温,而且其磁滞回线比较宽,在低温时磁性比较强,而温度升高时磁性明显减弱。
铁磁性是指物质受到磁场作用后,产生与磁场一致方向的强磁性。
铁性铁磁性是由于各个原子磁矩以相同方向排列而形成的。
铁磁性材料在常温下可能具有永久磁性,常见的亲铁磁材料有铁、钴、镍等。

Curriculum of chemical engineeringAs chemical engineering knowledge developed, it was inserted into university courses and curricula. Before World WarⅠ, chemical engineering programs were distinguishable from chemistry programs in that they contained courses in engineering drawing, engineering thermodynamics, mechanics, and hydraulics taken from engineering departments. Shortly after World WarⅠthe first text in unit operations was published. Courses in this area became the core of chemical engineering teaching.By the mid-1930s, chemical engineering programs included courses in (1) stoichiometry (using material and energy conservation ideas to analyze chemical process steps), (2) chemical processes or “unit operations”, (3) chemical engineering laboratories “in which equipment was operated and tested”, and (4) chemical plant design (in which cost factors were combined with technical elements to arrive at preliminary plant designs). The student was still asked to take the core chemistry courses, including general, analytical, organic, and physical chemistry. However, in addition, he or she took courses in mechanical drawing, engineering mechanics, electric circuits, metallurgy, and thermo-dynamics with other engineers.Since World War Ⅱchemical engineering has develop rapidly.As new disciplines have proven useful, they have been added to the curriculum. Chemical engineering thermodynamics became generally formulated and taught by about 1945. By 1950, courses in applied chemical kinetics and chemical reactor design appeared. Process control appeared as an undergraduate course in about 1955, and digital computer use began to develop about 1960.The idea that the various unit operations depended on common mechanisms of heat, mass, and momentum transfer developed about 1960. Consequently, courses in transport phenomena assumed an important position as an underlying, unifying basis for chemical engineering education. New general disciplines that have emerged in the last two decades include environmental and safety engineering, biotechnology, and electronics manufacturing processing. There has been an enormous amount of development in all fields, much of it arising out of more powerful computing and applied mathematics capabilities.1.Science and Mathematics CoursesChemistryChemical engineers continue to need background in organic, inorganic and physical chemistry, but also should introduced to the principles of instrumental analysis and biochemistry.· Valuable conceptual material should be strongly emphasized in organic chemistry including that associated with biochemical process.· Much of thermodynamic is more efficiently taught in chemical engineering, and physical chemistry should include the foundations of thermodynamic.Physics.Biology.· Biology has emerged from the classification stage, and modern molecular biology holds great promise for application. Future graduates will become involved with applying this knowledge at some time in their careers.· A special course is required on the functions and characteristics of living cells with some emphasis on genetic engineering as practiced with microorganisms.Materials Science.·Course work should include the effects of microstructure on physical, chemical, optical, magnetic and electronic properties of solids.·Fields of study should encompass ceramics, polymers, semiconductors, metals, and composites.Mathematics.Computer Instruction.·Although students should develop reasonable proficiency in programming, the main thrust should be that use of standard software including the merging of various programs to accomplish a given task. Major emphasis should be on how to analyze and solve problems with existing software including that for simulation to evaluate and check such software with thoroughness and precision.·Students should learn how to critically evaluate programs written by others.· All courses involving calculations should make extensive use of the computer and the latest software. Such activity should be more frequent as students progress in the curriculum. Adequate computer hardware and software must be freely available to the student through superior centralized facilities and/or individual PC’s. Development of professionally written software for chemical engineering should be encouraged.2.Chemical engineering coursesThermodynamics.· The important concepts of the courses should be emphasized; software should e developed to implement the concepts in treating a wide variety of complex, yet interesting, problems in a reasonable time. The value of analysis of units and dimensions in checkingproblems should continue to be emphasized.· Examples in thermodynamics should involve problems from a variety of industries so that the subject comes alive and its power in decision making is clearly emphasized.Kinetics, Catalysis, and Reactor Design and Analysis.·This course also needs a broad variety of real problems, not only design but also diagnostic and economic problems. Real problems involve real compounds and the chemistry related to them.·Existing software for algebraic and differential equation solving make simulation and design calculation on many reactor systems quite straightforward.· Shortcut estimating methods should be emphasized in addition to computer calculations.·The increased production of specialties make batch ad semibatch reactor more important, and scale-up of laboratory studies is an important technique in the fast-moving specialties business.3.Unit OperationsThe unit operations were conceived as an organized means for discussing the many kind of equipment-oriented physical processes required in the process industries. This approach continues to be valid. Over the years some portions have bee given separate statussuch as transport phenomena and separations while some equipment and related principles have not been included in the required courses, as is the case with polymer processing, an area in which all chemical engineers should have some knowledge.·Transport phenomena principles can be made more compelling by using problems form a wide range of industries that can be analyzed and solved using the principles taught.·Some efficiency may be gained by teaching several principles and procedures for developing specifications and selection the large number of equipment items normally purchased off-the-shelf or as standard design.·A great deal of time can be saved in addressing designed equipped such as fractionators and absorbers be emphasizing rigorous computer calculations and the simplest shortcut procedures. Most intermediate calculation procedures and graphical methods should be eliminated unless they have real conceptual value.Process Control.·This course should emphasize control strategy and precise measurement in addition to theory.·Some hands-on experience using current practices of computer control with industrial-type consoles should be encouraged.·Computer simulation of processes for demonstration of controlprinciples and techniques can be most valuable, but contact with actual control devices should not be ignored.Chemical engineering laboratories.·Creative problem solving should be emphasized.·Reports should be written as briefly as possible; they should contain an executive summary with clearly drawn conclusions and brief observations and explanations with graphical rather than tabular representation of data. A great deal of such graphing can be done in the laboratory on computers with modern graphics capabilities. Detailed calculations should be included in an appendix.·Some part of the laboratory should be structured to relate to product development,Design/Economics·In the design course in engineering, students learn the techniques of complex problem solving and decision making within a framework of economic analysis. The very nature of processes requires a system approach’ the ability to analyze a total system is one of the special attributes of chemical engineers that will continue to prove most sought after in a complex technological world.·Because of the greater diversity of interests and job opportunities, some consideration should be given to providing a variety of short design problem of greatest personal interest.·The design approach can be most valuable in diagnosing plant problems, and some practice in this interesting area should be provided.·Rigorous economic analysis and predictive efforts should be required in all decision processes.·Safety and environmental considerations should also be emphasized.·Modern simulation tools should be made available to the students.Other Engineering Courses.The electrical engineering courses should emphasize application of microprocessors, lasers, sensing devices, and control systems as well as the traditional areas of circuits and motors. The course should provide insight into the principles on which each subject is based.Remaining courses in engineering mechanics and engineering drawing should be considered for their relevance to current and future chemical engineering practice.4.Other coursesEconomics and Business courses.It is difficult to find a single course in economics or business departments that covers the various needs of engineers. Thequalitative ability of engineers makes it possible to teach following topics in a single-semester course—in many cases in the Chemical Engineering Department: business economics, project economic analysis, economic theory, marketing and market studies, and national and global economics.Humanities and Social Science Courses.It is important to understand the origins of one’s own culture as well as that of others.Communication Course.Since improved communication skills require continuous attention, the following requirements may be useful:·Oral presentations in at least one course each year.·Several literature surveys in the junior and senior years.·Introduce computer-based communication systems.Area of Specialization.The elective areas should be generous in hours to maximize freedom of choice. Each department will have to consider its own and its total university resources and strenghs as well as the quality and preparation of its students. The suggested areas are: ·Life sciences and applications·Materials sciences and applications·Catalysis and electrochemical science and applications·Separations technology·Computer applications technology·Techniques of product development and marketing·Polymer technologyEach of these areas should be strongly career-oriented. The interest in a given area will depend on opportunities perceived by the students.。
Magnetic Properties of Materials 导言磁性是一种常见的物理现象,很多材料在受到磁场的作用下会表现出各种不同的磁性行为,这些行为在科学和工程上具有很大的实际用途。
因此,研究材料的磁性质成为了一个非常重要的领域,有助于我们深入了解材料的本质和应用价值。
一、磁性的基本原理磁性是一种物质对磁场的响应的物理特性,它与材料中的原子、离子、电子等微观粒子的结构和运动状态密切相关。
在材料中有些原子或离子的电子自旋轨道存在自发地排列,这个排列称为磁矩。
磁矩是表征物体磁性的物理量,通常使用磁场的力量和方向来描述。
当外界施加一个磁场时,原子或离子的磁矩会随之调整并试图与磁场对齐,这种现象称为磁化。
二、材料的磁性种类根据磁化的方式不同,磁性可以分为顺磁性、抗磁性、铁磁性、亚铁磁性等几种类型。
1.顺磁性顺磁性材料又称为顺磁体,它们的磁矩与外部磁场方向相同,也就是说在外部磁场作用下,顺磁体的磁矩方向会随之改变。
这种磁性主要来源于材料中未成对电子自旋的排列。
顺磁体的常见代表是氧化铁、铜盐、氯化镍等。
2.抗磁性抗磁性材料又称为逆磁体,其磁矩方向与外部磁场相反,也就是说在外部磁场作用下,抗磁体的磁矩方向不发生改变。
这种磁性主要来源于材料中的原子或离子间的磁性相互作用,比如说相对排斥作用。
抗磁体的常见代表是铬、铜、铝等。
3.铁磁性铁磁性材料是一种磁矩方向非常强烈的材料,其磁矩方向在没有外部磁场的作用下也能自发排列。
在铁磁体中,原子或离子的自旋轨道会产生强烈的磁矩,这些磁矩之间的相互作用可以形成磁性区域(磁畴),在外部磁场的作用下,这些磁性区域会对齐并产生一个明显的磁矩。
铁磁体的常见代表是铁、镍、钴等。
4.亚铁磁性亚铁磁性和铁磁性的区别在于磁矩的强弱,它们之间不存在完全划分的界限。
一般来说,亚铁磁体的磁矩较弱,而且只有在外部磁场的作用下才会表现出明显的磁性行为。
亚铁磁体的常见代表是γ-铁氧体,它在高温下表现出铁磁性,而在低温下则表现出顺磁性。
Fabrication and magnetic properties of La-X (X = Co, Ni, and Fe) nanotube arrays prepared by electrodeposition methodsJ. Y. Chen, D. W. Shi, N. Ahmad, D. P. Liu, W. P. Zhou et al.Citation: J. Appl. Phys. 114, 054303 (2013); doi: 10.1063/1.4817284View online: /10.1063/1.4817284View Table of Contents: /resource/1/JAPIAU/v114/i5Published by the AIP Publishing LLC.Additional information on J. Appl. Phys.Journal Homepage: /Journal Information: /about/about_the_journalTop downloads: /features/most_downloadedInformation for Authors: /authorsDownloaded 22 Sep 2013 to 202.207.14.58. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: /about/rights_and_permissionsFabrication and magnetic properties of La-X (X 5Co,Ni,and Fe)nanotube arrays prepared by electrodeposition methodsJ.Y .Chen,1,a)D.W.Shi,1N.Ahmad,1D.P .Liu,1W.P .Zhou,2and X.F .Han 1,b)1Beijing National Laboratory for Condensed Matter Physics,Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China 2Laboratory of Rare-earth Physics,Department of Physics,Inner Mongolia University,Inner Mongolia,Hohhot 010021,China(Received 23May 2013;accepted 16July 2013;published online 2August 2013)Well-ordered La-X (X ¼Co,Ni and Fe)nanotubes,with the average diameter of $200nm,wall thicknesses of $40nm,have been fabricated into anodized aluminum oxide template by potentiostatic electrodeposition method.Various composition of La-X nanotubes were obtained by tuning the applied deposition potential.Magnetization measurements reveal that doped La could enhance the coercivity (H c )of La-X nanotubes and their easy axis is perpendicular to the nanotube axis.There is a transition from the curling to transverse mode with increase of angle.Temperature dependent magnetization indicates the existence of superparamagnetic nanoparticles and that the surface effect results in the increase of saturation magnetization (M s )at low temperature.Abnormal behavior of temperature dependent H c may result from thermal excitation,magnetoelastic anisotropy,as well as oxide layer of nanotube inner surface induced coupling.These one-dimensional rare-earth transition metal nanostructures could have potential applications in novel spintronics device,ultra-small magnetic media,drug delivery,or othernanodevice.VC 2013AIP Publishing LLC .[/10.1063/1.4817284]I.INTRODUCTIONThe rare-earth elemental metals or alloys have attractedmuch attention,due to their fundamental properties with unique 4f electronic structures and wide range of applications in catalysis,magnetism,optics,and other devices.1Many rare-earth elemental metals or alloys,like bulk materials,are widely studied.Nd-Fe-B and Sm-Co are important permanent magnets;2–4La-based compounds are widely used as lumines-cent materials 5and La-Ni alloys are important hydrogen stor-age materials.6However,until now,there are few reports about rare-earth alloy nanostructures,especially for nanowires and nanotubes.Tubular nanostructures have attracted much interest after the discovery of carbon nanotubes by Iijima,7owing to their unique hollow structure,distinctive physical properties,and potential applications in nanodevices.Furthermore,metal nanotubes,especially magnetic ones,have attracted much attention,due to their potential applications in ultrahigh magnetic recording media,ultra-small magnetic sen-sor,drug delivery,etc.8–11In comparison with magnetic nano-wires,the hollow nanotubes possess three independent geometry parameters:outer diameter,inner diameter,and length for the control of the magnetic properties via shape anisotropy.Magnetization reversal modes of magnetic nano-wires and nanotubes are quite interesting.12–16It has been pre-dicted that the magnetization reversal via vortex wall formation and propagation (also called curling mode)is more controllable in the magnetic nanotubes compare with solid nanowires.15–20Among various fabrication methods,low-cost template-assisted electrodeposition methods have become apopular pathway,due to the easy controllable length,diame-ter,and wall thickness of nanotubes.21–31Lanthanum is a rare earth element,whose reduction potential is À2.52V (vs.hydrogen electrode).32It is very diffi-cult to electrodeposit La from an aqueous solution.The decomposition of water into hydrogen and oxygen occurs below this potential.However,recently the electro-deposition of rare-earth alloys from aqueous solution was achieved by the shifting of their deposition potential below the reduction poten-tial of hydrogen.33–36But until now,fabrication of La-based alloy nanowires and nanotubes by simple electrodeposition methods is still challenging.In order to prepare the nanowires,the current densities are usually $10mA/cm 2.However,the preparation of nanotubes requires higher current densities of $20mA/cm 2in order to avoid formation of solid nano-wires,21,22which often result in a lower filling rate and inho-mogeneous tube length.Herein,using special complexing agents,well-ordered La-X (X ¼Co,Ni and Fe)alloys nanotubes were success-fully fabricated in aqueous solution by simple direct electro-deposition methods at constant applied potential and the current density was measured during the deposition.The current densities for the preparation of La-X nanotubes are less than a few mA/cm 2,in accordance with Ref.23.The composition,angular and temperature dependent magnetiza-tion reversal were systematically studied.II.EXPERIMENTALAnodic aluminum oxide (AAO)templates (diameter $200nm and thickness $60l m from Whatman Co.)were used to synthesize the La-X (X ¼Co,Ni,and Fe)alloy nano-tubes.Gold (Au)layer of thickness $10nm was first sputtereda)chenjy02@ b)xfhan@0021-8979/2013/114(5)/054303/6/$30.00VC 2013AIP Publishing LLC 114,054303-1JOURNAL OF APPLIED PHYSICS 114,054303(2013)on one side of the AAO template;the Au layer was thin enough so that it only covered the alumina oxide between the pores,leaving the orifices open.Electrolytes were pre-pared using99.99%grade chemical reagents of lanthanum chloride(LaCl3.nH2O,0.18M),ammonium chloride (NH4Cl,0.25M),boric acid(H3BO3,0.45M),and XCl2 (X¼Co,Ni,and Fe,0.1M–0.12M).All solutions were freshly prepared with distilled water.The NH4Cl is used for complexing agents which can not only stabilize the electro-lyte but can also shift the deposition potential of La to more positive values.The H3BO3is used as buffer agent.The pH value is adjusted to2.0–3.0by adding a few drops of0.1M HCl acid.Electrodeposition was carried out potentiostati-cally at room temperature in a conventional three-electrode cell.Applied potentials were measured with respect to a sat-urated calomel electrode(SCE)reference and a Pt foil was used as the counter electrode.The coated Au layer served as the working electrode.The applied deposition potential wasvaried fromÀ1.1V toÀ1.3V(vs.SCE)for different sam-ples to tune the composition of the nanotubes.All the nano-tubes were kept of similar length by control of the deposition time.The deposition time ranged from5000s to8000s for preparation of different samples.The composition of La-X alloy nanotubes were controlled by tuning the deposition potential.It can be controlled by tuning the composition of electrolyte as well.The morphology of the nanotubes was investigated by scanning electron microscope(SEM)and transmission elec-tron microscope(TEM).The length of nanotubes was meas-ured using SEM.To prepare the samples for SEM measurement,the samples werefixed onto the copper tape and the AAO templates were partly removed in1M NaOH in an oven at60 C for3–4h.For TEM analysis,first,the AAO templates were completely dissolved in1M NaOH for10h in an oven at60 C and then the samples were thoroughly washed with distilled water.After washing,the samples were dispersed into ethanol and put into ultrasonic bath for about30s to make sure the nanotubes are well sepa-rated from each other.Then a few drops of solution were placed onto Cu grid and dried for TEM.Energy dispersive spectrometry(EDS)analysis was also performed in order to determine the composition.Magnetic properties were meas-ured by vibrating sample magnetometry(VSM)as well as superconductor quantum interference device(SQUID).For the magnetic measurement,the samples were directly meas-ured with the template and the background signal was removed from experiment data.The sample size was about $5mmÂ5mm in lateral dimension.The magneticfield was applied parallel or perpendicular to the template surface,to make sure the magneticfield was perpendicular or parallel to the nanotubes.III.RESULTS AND DISCUSSIONFigure1shows the typical compositions of La-X(X¼Co, Ni and Fe)nanotubes as a function of deposition potential in the same electrolyte conditions at room temperature.When the applied deposition potential is aboveÀ1.1V(vs.SCE), there is only tiny La deposited into the nanotubes.On the contrary,when the applied deposition potential is below À1.3V(vs.SCE),the contents of Co,Ni,and Fe in the nano-tubes decrease rapidly.The La and X(X¼Co,Ni and Fe) can be well co-deposited into the nanotubes betweenÀ1.1V andÀ1.3V(vs.SCE).Various compositions of La-X nano-tubes could be obtained via tuning the applied deposition potential.Figure2shows the SEM,TEM,and high resolution TEM images,respectively,of the La-Co[Figs.2(a)–2(c)], La-Ni[Figs.2(d)–2(f)],and La-Fe[Figs.2(g)–2(i)]nano-tubes grown using the AAO template.It is evident that all of the nanotubes are well-ordered and uniform.The diameters for all of nanotubes are$200nm,which are consistent with the diameters of the AAO channel.The wall thicknesses are $40nm and the lengths are kept to$15l m by tuning the deposition time.Figs.2(j)–2(l)show typical EDS results for La-Co,La-Ni,and La-Fe nanotubes,respectively.It indi-cates that the nanotubes indeed mostly consist of La and X (X¼Co,Ni and Fe).The Cu peak originates from the copper substrate used,while the Al and O peaks arise from the small amount of residual alumina after the sample was rinsed.The O peak is also partly due to oxidation of the nanotubes after removal of the AAO template;the small C peaks are from impurities.As shown in Figs.2(c),2(f),and 2(i),all the nanotubes show poor crystallinity with no obvious diffraction peaks in X-ray diffraction(XRD)pat-terns(XRD not shown).The composition dependent magnetic properties of the nanotubes were analyzed in detail.The magnetic parameters are summarized in Table I.It was found that all the nano-tubes have a larger coercivity(H c)and squareness(S)with thefield perpendicular to the nanotube axis.In addition,the La-Co nanotubes have a larger coercivity than the La-Fe and La-Ni nanotubes.Here,we will discuss the magnetization re-versal properties of these La-X nanotubes systems,based on La-Co nanotubes,owing to similar magnetization reversal properties.The composition dependence of the magnetic properties of La x Co1Àx nanotubes was studied in detail,as shown in Fig.3.Fig.3(a)shows typical hysteresis loops of La68Co32(La-Co)nanotubes for magneticfields applied par-allel and perpendicular to the nanotube axis.Here,wedefine FIG.1.Typical La-Ni,La-Co,and La-Fe nanotubes composition as a func-tion of deposition potential,when we keep the same electrolyte condition.the easy axis when the squareness is maximum.From Fig.4(a),the squareness got the maximum when magnetic field is applied at 90 .It means the easy axis is perpendicular to the nanotubes axis,owing to the competition between magneto-crystalline anisotropy,magnetic dipole interactions,and shape anisotropy.25After La doping,the coercivity of the nanotubes has increased substantially (typically,H c ¼437.2Oe for H k and H c ¼462.8Oe for H ?).Figs.3(b)and 3(c)show the com-position dependent coercivity and squareness (remanence ra-tio),respectively,of the La x Co 1Àx nanotubes.When x <0.2,the coercivity and squareness of the La x Co 1Àx nanotubes wererelatively small,similar to pure Co nanotubes.24The coerciv-ity and squareness increase significantly for x from 0.2to 0.6.When x >0.7,the coercivity and squareness decrease rapidly due to the larger content of paramagnetic La.When x $1.0,the coercivity and squareness are close to zero.Fig.3(d)shows the saturation magnetization (M s )as a function of La x Co 1Àx nanotube composition.M s is calculated from the av-erage length of La x Co 1Àx nanotubes (determined by SEM)as well as from the average outer/inner diameter and area den-sity.With increasing La content,M s gradually decreases;when x >0.9,M s is $0,indicating that the La x Co 1Àx nano-tubes behave paramagnetically.In addition,when x $0,M s is less than that of the bulk material (1400emu/cm 3),mainly due to the special tubular structure and strong surface effect.The surface magnetic moments are vulnerable to the external ther-mal fluctuations,reducing M s .Besides,the inner wall of nano-tubes may form a thin oxide layer due to exposure in the air all the time,which also contributed to the reduction of M s .37,38The angular and temperature dependent magnetization reversal of La x Co 1Àx nanotubes were also studied in detail and typical La 68Co 32(La-Co)alloy nanotubes werechosenFIG. 2.SEM,TEM,and HRTEM images of (a)-(c)La-Co nanotubes,(d)-(f)La-Ni nanotubes and (g)-(i)La-Fe nanotubes,respectively.EDS spectros-copy of (j)La-Co,(k)La-Ni,and (l)La-Fe nanotubes.The red marks in (b),(e),and (h)show the enlarge area of HRTEM for (c),(f),and (i),respectively.TABLE I.Magnetic parameters,coercivity (H c ),and remanent squareness (S )of La-X (X ¼Co,Ni,and Fe)nanotubes in AAO templates with field applied parallel and perpendicular to the tube axis.position d (nm)L (l m)H k c (Oe)H ?c (Oe)S kS ?Template1La 68Co 32$200$15437.2462.80.140.38AAO2La 74Ni 26$200$1550.380.10.050.33AAO 3La 66Fe 34$200$15179.5225.70.050.08AAOfor discussion,as shown in Figure 4.The angular depend-ence of coercivity H c (h )and the squareness of La-Co nano-tubes are shown in Figure 4(a).The samples are isotropic in lateral dimension.Here,h is the angle between external mag-netic field and nanotube axis.When the angle is 0 ,the mag-netic field is applied parallel to the nanotube axis;and when the angle is 90 ,the magnetic field is perpendicular to the nanotube axis.H c (h )first increases,peaking at 30 ,then decreases,resulting in an M-type H c (h )curve.For magnetic nanotubes,magnetization reversal mechanisms are domi-nated by three modes,coherent rotation,curling (also called vortex),and transverse (via propagation of a transverse do-main wall).15,16The coherent mode is only suitable for very short nanotubes.In our La-X nanotubes,the length is of order tens of micrometers,so curling and transverse modes are considered in our analysis.Similar discussions for other magnetic nanotubes are analyzed in Refs.39and 40.For the curling mode,it is highly dependent on the geometry of nanotubes.The coercivity decreases with increasing angle in the nanotubes and nanowires with very small diameter (typical several tens nanometer).17The coercivity increases with increasing angle with a minimum value at 0 and a maximum at 90 ,when the diameter of nanotube is above 100nm.The coercivity determined by the transverse rotation mode decreases with increase in angle.39–42According to this analysis,there is a transition from curling to transverse with angular increase in our La-Co nanotubes.Figure 4(b)shows the hysteresis loops of La-Co nano-tubes at 5K and 300K (H ?nanotubes).The saturation mag-netization (M s )and saturation field (H s )increase dramatically at 5K.Similar effect was also observed in other nanotubes.40,43This may be due to a number of reasons.First,there is large surface/volume ratio and surface mag-netic moments will likely dominate the magneticpropertiesFIG. 3.(a)Hysteresis loops with applied field parallel and perpendicular to La-Co nanotubes;composition dependence of (b)coercivity (H c ),(c)squareness (S),and (d)saturation mag-netization (M s).FIG.4.(a)Angular dependence of coer-civity and squareness of La-Co nano-tubes;(b)hysteresis loops of La-Co nanotubes at 5K and 300K;(c)M (T)curves under ZFC and FC at H ¼100Oe;and (d)temperature depend-ence of coercivity of La-Co nanotubes.in La-Co nanotubes.Second,there may be somefine nano-particles in our La-Co nanotubes,due to the simple direct electro-deposition method used.These nanoparticles are superparamagnetic at high temperature and may become fer-romagnetic at low temperature,increasing M s.To confirm this,magnetization as a function of temperature under zero field cooling andfield cooling(ZFC/FC)at100Oe were measured,as shown in Fig.4(c).The magnetization increases from20K to300K,indicating a ferromagnetic behavior.Below20K,rapid increase of magnetization was observed,due to some superparamagnetic nanoparticles, consistent with M(H)in Fig.4(b).22,40Figure4(d)shows the temperature dependent coercivity of La-Co nanotubes.As the temperature increases,H c decreases rapidly from1485Oe to463Oe,accompanied by somefluctuations of temperature.Normally,H c as a func-tion of temperature is determined by thermal activation over an energy barrier proposed by N e el44and Brown.45 Thefield dependence of the energy barrier has the form E B¼E0(1ÀH/H0)m,where E0is the energy barrier at zero appliedfield,H is the appliedfield,and H0is the magnetic field needed to overcome the energy barrier at zero tempera-ture.m is generally equal to3/2for nanowires and nanotubes.46 For our La-Co nanotubes,thermal excitation can partly explain the decrease in coercivity as a function of temperature.It is not as easy to explain thefluctuations of coercivity with tem-perature,which may come from additional anisotropy. Magnetocrystalline anisotropy is temperature dependent. However,the La-Co nanotubes in this study are polycrystalline with no preferential orientation.Magnetocrystalline anisotropy is weak and contributes little to the total anisotropy.Another possibility may be magnetoelastic anisotropy.Here,La-Co nanotubes were directly electrodeposited into the AAO tem-plate,which attached onto the pore walls.There is a large mis-match between the thermal expansion coefficients of La-Co nanotubes and AAO.It may induce La-Co nanotubes to form with a strain along the tube axis as the temperature decreased. The strain will produce an additional magnetoelastic anisot-ropy,which will have an influence on the magnetic properties of La-Co nanotubes.Similar effects were also observed in other nanowires47and nanotubes.48This may result in the abnormal behavior of coercivity for La-Co nanotubes.Besides, the inner wall of nanotubes may form a thin oxide layer (Co-O),thus resulting in the formation of core-shell nanostruc-tures.37,38There may be an exchange-bias coupling between the outer nanotube and inner oxide layers which could also result in a large increase of H c at low temperature,as previ-ously evidenced in Co nanotubes by Proenca et al.37IV.CONCLUSIONSIn summary,well-ordered La-X(X¼Co,Ni and Fe) alloy nanotubes with diameters$200nm and wall thick-nesses$40nm have been successfully fabricated in aqueous solution by direct electro-deposition.By tuning the applied deposition potential,nanotubes with various compositions have been grown successfully.The results show that the easy axis is perpendicular to the nanotube axis for all samples. For La x Co1Àx nanotubes,as x increases,the coercivity and squareness increase,while for x>0.7,their coercivity and squareness decrease rapidly.The angular dependent coerciv-ity of La-Co nanotubes shows that there is a transition from the curling to transverse rotation mode.Similar behavior was also observed for La-Ni and La-Fe nanotubes.The tempera-ture dependent magnetization reversal properties of La-Co nanotubes show that there may also be magnetoelastic ani-sotropy in our case.It is expected that the preliminary results reported above will contribute to the development of1-D rare-earth nanostructures,which might be very important in technological applications,such as novel spintronics device, ultra-small magnetic media,or other nanodevice. ACKNOWLEDGMENTSThe project was supported by the State Key Project of Fundamental Research of Ministry of Science and Technology (MOST,No.2010CB934400)and National Natural Science Foundation of China(NSFC,Grant Nos.10934099,11104338, and51021061),and the partial support of Graduate Education Project of Beijing Municipal Commission of Education,the international joint projects of NSFC-The Royal Society(UK), and the partial support of K.C.Wong Education Foundation, Hong Kong.1G.J.McCarthy and J.J.Rhyne,The Rare Earths in Modern Science and Technology(Plenum Press,New York,1978).2B.M.Ma,J.W.Herchenroeder,B.Smith,M.Suda,D.N.Brown,and Z. Chen,J.Magn.Magn.Mater.239,418(2002).3J.Zhou,R.Skomski,C.Chen,G.C.Hadjipanayis,and D.J.Sellmyer, Appl.Phys.Lett.77,1514(2000).4R.Skomski and J.M. D.Coey,Permanent Magnetism(Institute of Physics,Bristol,1999).5J.Dexpert-Ghys,R.Mauricot,and M.D.Faucher,J.Lumin.69,203 (1996).6L.Schlapbach and A.Z€u ttel,Nature414,353(2001).7S.Iijima,Nature354,56(1991).8S.Khizroev,M.H.Kryder,D.Litvinov,and D.A.Thompson,Appl.Phys. Lett.81,2256(2002).9J.J.Krebs,M.Rubinstein,P.Lubitz,M.Z.Harford,S.Baral, R.Shashidhar,Y.S.Ho,G.M.Chow,and S.Qardri,J.Appl.Phys.70, 6404(1991).10A.K.Salem,P.C.Searson,and K.W.Leong,Nature Mater.2,668 (2003).11S.F.Yu,S.B.Lee,and C.R.Martin,Anal.Chem.75,1239(2003).12K.Pitzschel,J.Bachmann,J.M.Montero-Moreno,J.Escrig,D.G€o rlitz, and K.Nielsch,Nanotechnology23,495718(2012).13D.W.Shi,J.Y.Chen,S.Riaz,W.P.Zhou,and X. F.Han, Nanotechnology23,305601(2012).14L.G.Vivas,Y.P.Ivanov,D.G.Trabada,M.P.Proenca,O.Chubykalo-Fesenko,and M.V a zquez,Nanotechnology24,105703(2013).nderos,S.Allende,J.Escrig,E.Salcedo,and D.Altbir,Appl.Phys. Lett.90,102501(2007).16J.Escrig,J.Bachmann,J.Jing,M.Daub,D.Altbir,and K.Nielsch,Phys. Rev.B77,214421(2008).17M.P.Proenca,C.T.Sousa,J.Ventura,J.P.Araujo,J.Escrig,and M. Vazquez,SPIN02,1250014(2012).18M.P.Proenca,C.T.Sousa,J.Escrig,J.Ventura,M.Vazquez,and J.P. Araujo,J.Appl.Phys.113,093907(2013).19H.M.Zhang,X.L.Zhang,J.J.Zhang,Z.Y.Li,and H.Y.Sun,J.Magn. Magn.Mater.342,69(2013).20X.L.Zhang,H.M.Zhang,T.S.Wu,Z.Y.Li,Z.J.Zhang,and H.Y.Sun, J.Magn.Magn.Mater.331,162(2013).21W.C.Yoo and J.K.Lee,Adv.Mater.16,1097(2004).22L.F.Liu,W.Y.Zhou,S.S.Xie,L.Song,S.D.Luo,D.F.Liu,J.Shen,Z. X.Zhang,Y.J.Xiang,W.J.Ma,Y.Ren,C.Y.Wang,and G.Wang, J.Phys.Chem.C112,2256(2008).23D.D.Li,R.S.Thompson,G.Bergmann,and J.G.Lu,Adv.Mater.20, 4575(2008).24X.F.Han,S.Shamaila,R.Sharif,J.Y.Chen,H.R.Liu,and D.P.Liu, Adv.Mater.21,4619(2009).25S.Shamaila,D.P.Liu,R.Sharif,J.Y.Chen,H.R.Liu,and X.F.Han, Appl.Phys.Lett.94,203101(2009).26J.Y.Chen,H.R.Liu,N.Ahmad,Y.L.Li,Z.Y.Chen,W.P.Zhou,and X.F.Han,J.Appl.Phys.109,07E157(2011).27R.Sharif,S.Shamaila,F.Shaheen,S.Naseem,J.Y.Chen,M.Khaleeq-ur-Rahman,K.Hussain,and X.F.Han,J.Appl.Phys.113,024315(2013). 28R.Sharif,S.Shamaila,F.Shaheen,J.Y.Chen,M.Khaleeq-ur-Rahman, and K.Hussain,Appl.Phys.Lett.102,013114(2013).29M.P.Proenca,C.T.Souca,J.Ventura,M.Vazquez,and J.P.Araujo, Nanoscale Res.Lett.7,280(2012).30S.B.Zhang and C.Z.Yao,Mater.Lett.94,143(2013).31H.M.Zhang,X.L.Zhang,J.J.Zhang,Z.Y.Li,and H.Y.Sun, J.Electrochem.Soc.160,D41(2013).32C.D.Lokhande,M.S.Jadhav,and S.H.Pawar,Met.Finish.86(11),53 (1988).33Y.Sato,H.Ishida,K.Kobayakawa,and Y.Abe,Chem.Lett.19,1471 (1990).34J.Q.Zhang,P.Evans,and G.Zangari,J.Magn.Magn.Mater.283,89 (2004).35D.Q.Gao,J.L.Fu,Y.Xu,and D.S.Xue,Mater.Lett.62,3070(2008).36L.L.Wang,L.M.Tang,G.F.Huang,W.Q.Huang,and J.Peng,Surf. Coat.Technol.192,208(2005).37M.P.Proenca,J.Ventura,C.T.Sousa,M.Vazquez,and J.P.Araujo, Phys.Rev.B87,134404(2013).38M.P.Proenca,C.T.Sousa,J.Ventura,M.Vazquez,and J.P.Araujo, Electrochim.Acta72,215(2012).39O.Albrecht,R.Zierold,S.Allende,J.Escrig,C.Patzig,B.Rauschenbach, K.Nielsch,and D.Gorlitz,J.Appl.Phys.109,093910(2011).40J.Y.Chen,N.Ahmad,D.W.Shi,W.P.Zhou,and X.F.Han,J.Appl. Phys.110,073912(2011).41J.Escrig,M.Daub,nderos,K.Nielsch,and D.Altbir, Nanotechnology18,445706(2007).42S.Allende,J.Escrig,D.Altbir,E.Salcedo,and M.Bahiana,Eur.Phys.J.B 66,37(2008).43N.Ahmad,J.Y.Chen,J.Iqbal,W.X.Wang,W.P.Zhou,and X.F.Han, J.Appl.Phys.109,07A331(2011).44L.N e el,Ann.Geophys.5,99(1949).45W.F.Brown,Phys.Rev.130,1677(1963).46H.Zeng,R.Skomski,L.Menon,Y.Liu,S.Bandyopadhyay,and D.J. Sellmyer,Phys.Rev.B65,134426(2002).47H.Zeng,S.Michalski,R.D.Kirby,D.J.Sellmyer,L.Menon,and S. Bandyopadhyay,J.Phys.Condens.Matter14,715(2002).48N.Ahmad,J.Y.Chen,W.P.Zhou, D.P.Liu,and X. F.Han, J.Supercond.Novel Magn.24,785(2011).。
Magnetic and Magnetization Properties of Co-DopedFe2O3Thin FilmsAseya Akbar,Saira Riaz,Robina Ashraf,and Shahzad NaseemCentre of Excellence in Solid State Physics,University of the Punjab,Lahore54590,PakistanAmongst the various phases of iron oxide,hematite(Fe2O3)is the most stable form,which shows antiferromagnetic behavior with ferromagnetic canting at room temperature.Doping of different metal ions inα-Fe2O3will not only lead to its new technological and industrial applications but also enhance its performance in existing applications.In this paper,we report synthesis and characterization of cobalt(Co)-doped Fe2O3thinfilms with dopant concentration in the range of0%–10%.XRD peaks shift to slightly higher angles as compared with undoped thinfilms due to smaller ionic radii of cobalt(72pm)as compared with iron(74pm).Room temperature magnetic properties,studied using vibrating sample magnetometer,show increase in saturation magnetization with increase in dopant concentration up to8%.Further increase in dopant concentration to10%degrades magnetic properties,which might be because of the presence of more atoms at the grain boundaries.Index Terms—Ferromagnetic,hematite,sol-gel,thinfilms.I.I NTRODUCTIONM AGNETIC thinfilms,ferromagnetic and antiferromag-netic,have attracted considerable attention because of their unique properties that make them important for techno-logical and industrial applications.Possible use of magnetic thinfilms in magnetic sensors,spintronic and high density magnetic storage devices has resulted in a great deal of interest.This stimulated interest in magnetic and transport properties of multilayered thinfilms that stems from discovery of giant magnetoresistance(GMR)and tunneling magnetore-sistnace(TMR)for the advancements of spintronic materials and devices[1]–[4].Among the various materials of interest iron oxide,espe-cially hematite(α-Fe2O3)phase,is an important candidate. Hematite(α-Fe2O3),also known as ferric oxide,is blood red in color and is extremely stable at ambient conditions.It is often the end product of other iron oxide transformations. Hematite is a semiconductor material with optical band gap of2.2eV[5].α-Fe2O3has a corundum structure with hexagonal unit cell composed of six formula ttice parameters of hematite are a=5.034Å,c=13.75Å[6].This material can also be indexed in rhomobohedral system with two formula units per unit cell with a=5.427Å,α=55.3°.The structure ofα-Fe2O3has close-packed arrays of oxygen along the(001)plane with the iron cations in the octahedral and tetrahedral interstitial sites.Hematite(α-Fe2O3)is formed with a stoichiometric metal-to-oxygen ratio especially when it isfinely divided[4].However,at1400K,oxygen evapora-tion is substantial and hematite transforms into magnetite at 1550K[4]–[6].Hematite is weakly ferromagnetic at room temperature because of canted spins with a saturation magnetization of0.4Am2/kg[6].It undergoes a phase transition at Manuscript received December17,2013;revised February20,2014; accepted March4,2014.Date of current version August15,2014. Corresponding author:A.Akbar(e-mail:aseya22@).Color versions of one or more of thefigures in this paper are available online at .Digital Object Identifier10.1109/TMAG.2014.2311826260K(the Morin temperature T M)to antiferromagnetic state. Above the Neel temperature(∼956K),hematite is paramag-netic.Below960K,the Fe3+ions are anitferromagnetically aligned[6].In the basal plane,the spins are parallel to each other but antiparallel to the spins of the neighboring planes [6].The magnetic easy axis is along the c axis below260K and above260K the easy axis is within the basal plane. Therefore,below Neel temperature,an electron traveling in the basal plane will experience a ferromagnetically aligned situation[7]–[10].To enhance the room temperature magnetic properties of hematite,we here report synthesis and characterization of undoped and cobalt-doped Fe2O3thinfilms using sol-gel and spin coating method.Dopant concentration is varied in the range0%–10%.Structural and magnetic properties are correlated with variation in dopant concentration.II.E XPERIMENTAL D ETAILSA.MaterialsFeCl3·6H2O and Co(NO3)2·4H2O(Sigma–Aldrich,99.99% pure),were used without further purification.Ethanol and n-hexane(Sigma–Aldrich,99.99%pure)were used as solvents.B.Sol Synthesis and Film PreparationTwo different solutions were prepared prior to thefinal sol synthesis.FeCl3·6H2O was dissolved in deionized(DI) water and n-hexane was added to the solution that was stirred vigorously at room temperature.Another solution was prepared by dissolving NaOH in ethanol.Two distinguishable layers appeared after mixing of both the solutions.Finally mixed solution was heated on a hot plate at60°C for several hours to obtain single-layered clear sol.Sol was aged at room temperature for48h before thefilm deposition.For cobalt-doped iron oxide thinfilms,cobalt nitrate Co(NO3)2·4H2O was dissolved in DI water and added to the iron oxide sol with variation in cobalt concentration(2%,4%,6%,8%,and 10%).Undoped and Co-doped thinfilms were deposited onto copper(Cu)substrates.Cu wasfirstly etched by diluted0018-9464©2014IEEE.Personal use is permitted,but republication/redistribution requires IEEE permission.See /publications_standards/publications/rights/index.html for more information.Fig.1.XRD pattern of undoped and cobalt-doped Fe2O3thinfilms annealed at300°C.Inset:expanded2θ°view of42°–45°showing shift of peak positions to higher angles.HCl and then repeatedly rinsed with DI water.It was then ultrasonically agitated at room temperature for10min in acetone and isopropyl alcohol to remove the residual organic impurities.Sols were spin coated onto Cu substrates at3000r/min for 30s and then aged at room temperature for24h.Films were annealed at300°C in the presence of vacuum under500Oe applied magneticfield(MF)for60min.C.Characterization ToolsBruker D8Advance X-ray diffractometer(XRD)was used to study the phase and crystalline structure of undoped and cobalt-doped iron oxide thinfilms.X-ray diffractometer used copper target withλ=1.5406Å(Nifiltered).Lake Shore’s vibrating sample magnetometer(VSM)was used to study the room temperature magnetic properties.III.R ESULTS AND D ISCUSSIONFig.1shows XRD patterns for undoped and Co-doped Fe2O3thinfilms prepared using sol-gel method after MF annealing at300°C.Appearance of(110),(012),(202), and(024)planes(Fig.1)indicates the formation of hematite (α-Fe2O3)pure phase(JCPDS card no.87–1165)at a low temperature of300°C.Hematite(α-Fe2O3)phase persistedeven after doping of10%and peaks corresponding to cobalt oxide or metal cobalt were not observed.Crystallite size was calculated using(1)[11]and is plotted as a function of dopant concentration along with crystallinity in Fig.2t=kλB cosθ(1)where k is the shape factor taken as0.9,λis the wavelength, B is full width at half maximum(FWHM),andθis the diffraction angle.Crystallite size increased with increase in the doping con-centration up to4%(Fig.2).This low level of doping may result in the dopant atoms being dissolved in the lattice properly.However,beyond4%it is possible that some ofthe Fig.2.Crystallite size and crystallinity as a function of dopant concentration. dopant atoms go to the interstitial positions or sit on the grain boundaries;it is to be kept in mind that it is a polycrystalline film and as such there are a large number of grain boundaries. These atoms will destroy the crystalline structure and will also result into a decreased crystallite size[12],as shown in Fig.2. Further,stresses occur because of the difference in ionic radii of the host and dopant atoms,and this can also be another reason for the decrease in crystallite size[13].Shift in peak positions,to slightly higher angles,are observed with the increase in dopant concentrations up to10%. This shift of peak positions to higher angles is due to smaller ionic radii of cobalt(72pm)as compared with that of iron (74pm).The small ionic radius of cobalt leads to decrease in unit cell[Fig.3(b)]that causes decrease in d-spacing which according to Bragg’s law shifts the peak positions to higher angles[11].Previously,pure phase hematite thinfilms were prepared at relatively high temperatures.Lian et al.[14]prepared hematite thinfilms using sol-gel method at temperature of500°C. Kumar et al.[15],Glasscock et al.[16],and Souza et al.[17] also reported annealing at500°C to obtain hematite phase. Lattice parameters(a,c)and unit cell volume(V)were calculated using(2)and(3)[11]and are shown as a function of dopant concentration in Fig.3sin2θ=λ23a2h2+k2+hk+λ2l24c2(2) where(hkl)represent the miller indices,λis the wavelength (1.5406Å)V=0.866a2c.(3) X-ray density(ρ)[11]was calculated usingρ=1.66042 AV(4) where A is the sum of atomic weights of the atoms in the unit cell,ρis in g/cm3,and V is the volume of unit cell inÅ3. The porosity values were calculated usingPorosity(%)=1−ρexpρstd×100(5)AKBAR et al.:MAGNETIC AND MAGNETIZATION PROPERTIES OF Co-DOPED Fe 2O 3THIN FILMS2201204Fig.3.(a)Lattice parameters and (b)unit cell volume of Co-doped Fe 2O 3thinfilms.Fig.4.X-ray density and porosity as a function of dopant concentration.where ρexp is the calculated X-ray density and ρstd is the standard density taken from the JCPDS data.X-ray density and porosity of the films is shown as a function of dopant concentration in Fig. 4.Increase in X-ray density,with increased dopant concentration,indicates formation of compact structure with cobalt incorporation.Fig.5shows M –H curves for undoped and cobalt-doped Fe 2O 3thin films.It can be seen from Fig.5that even undoped iron oxide thin films show ferromagnetic behavior as opposed to antiferromagnetic nature of hematite.In the temperature range 260–950K (13°C–677°C),(111)planes arrange themselves to form layers of Fe 3+cations [6].Spins interact ferromagnetically within the same plane while antiferromagnetic coupling arises with the spins of the adjacent planes,that is,antiparallel arrangement of spins.Because of spin orbit coupling canting between two adjacent planes arises that produces uncompensated magnetic moment of Fe 3+cations which is the cause of ferromagnetic behavior[6].Fig.5.M–H curves for Co-doped Fe 2O 3thinfilms.Fig.6.Coercivity and saturation magnetization as a function of dopant concentration.The uncompensated magnetic moments seem to have appeared during sol’s synthesis [18]since in our case even undoped films show ferromagnetic behavior.Fig.6shows variation in saturation magnetization (M s )and coercivity (H c )as a function of varying dopant concentration.M s ,in Co-doped Fe 2O 3thin films,increases up to dopant concentration of 8%as shown in Fig.6.However,a sharp decrease in M s value was observed by further increasing dopant concentration to 10%,which might be because of the presence of more atoms at the grain boundaries as seen in the XRD results with a slight increase in crystallite size from 8%to 10%.Ziese and Thornton [19]reported that doping in iron oxide generated extra electrons in the host lattice.In nonmagnetic lattice,these electrons can propagate freely through the crystal.However,in a magnetic lattice localized spin order is present that hinders the motion of doped charge carries.According to Hund’s rule,strong exchange interaction exists among the d electrons [19].This strong exchange inter-action will force the electrons to take the direction of spin of localized electrons.As a result extra electrons with spinup will be available but cannot hop into the neighboring spin down site [19].In addition,if a canted structure with angle between two sublattices is present then the total energy can be estimated using [19]E (θ)=J S 2cos θ−6tx cosθ2(6)where E is energy of the system,J is exchange interaction,S is spin quantum number,θis angle between spins of the two sites,t is the hopping matrix,and x is the dopant concentration.2201204 IEEE TRANSACTIONS ON MAGNETICS,VOL.50,NO.8,AUGUST 2014TABLE IC OMPARISON OF S ATURATION M AGNETIZATIONOFC O -D OPED T HIN F ILMSThe energy will be minimized under the condition given incos θ2=32t J S 2x .(7)Equation (7)represents that by increasing x,spin structure becomes canted resulting in the presence of both ferromagnetic and antiferromagnetic ordering [19].Moreover,order will be purely ferromagnetic for a condition given inx >x c =23J S 2t(8)where x c is the critical concentration below which the mag-netic structure is undistorted.Cobalt with electronic configuration of [Ar]3d 74s 2has one more electron than iron ([Ar]3d 64s 2)with less energy of d state.Cobalt atom donates one d and two s electrons to oxygen that results in remaining six electrons on cobalt.When substituted for Fe with spin down electron,the spin down d band gets completely filled with remaining one d-electron residing in spinup band.This results in net magnetization of 1μB.Thus,canting of spin structure results because of the imbalance created by incorporation of cobalt in Fe 2O 3lattice,which in turn results in increased magnetization values in Co-doped Fe 2O 3thin films [20].Best magnetic properties are observed with dopant concentration of 4%–8%(Fig.5).With increase in dopant concentration above 8%,a large number of defects can result in inadequate alignment of spins.This leads to less prominent canting of spin structure resulting in a fewer number of uncompensated magnetic moments thus reducing the magnetic properties [21].In addition,at high dopant concentration (≥10%)the reduction in magnetic moment arises owing to the presence of adjacent cobalt ions with different oxidation states of 2+and 3+with antifer-romagnetic coupling in Fe 2O 3lattice [9].Comparison of saturation magnetization of Co-doped Fe 2O 3thin films with literature can be seen in Table I.IV.C ONCLUSIONSUndoped and cobalt-doped (2%–10%)hematite (α-Fe 2O 3)thin films have been prepared using sol-gel and spin coating method.The films were annealed at 300°C in the presence of a magnetic field of 500Oe.XRD results indicated the formation of phase pure hematite in undoped and doped thin films.Ferromagnetic behavior has been observed even in undoped Fe 2O 3thin films.Increase in saturation magneti-zation,∼2.1emu/cm 3,was observed in Co-doped iron oxidethin films up to a dopant concentration of 8%which is lost with further increase in the dopant concentration.R EFERENCES[1]S.Riaz,A.Akbar,and S.Naseem,“Structural,electrical and magneticproperties of iron oxide thin films,”Adv.Sci.Lett.,vol.19,no.3,pp.828–833,2013.[2]S.Riaz, A.Akbar,and S.Naseem,“Controlled nanostructuring ofmultiphase core-shell iron oxide nanoparticles,”IEEE Trans.Magn.,vol.50,no.1,p.2300204,Jan.2014.[3]S.Riaz,M.Bashir,and S.Naseem,“Iron oxide nanoparticles preparedby modified co-precipitation method,”IEEE Trans.Magn.,vol.50,no.1,p.4003304,Jan.2014.[4]J.Zhang,X.G.Zhang,and X.F.Han,“Spinel oxides: 1spin-filterbarrier for a class of magnetic tunnel junctions,”Appl.Phys.Lett.,vol.100,no.22,pp.222401-1–222401-4,May 2012.[5]M.Monti et al.,“Magnetism in nanometer-thick magnetite,”Phys.Rev.B ,vol.85,no.2,p.020404,Jan.2012.[6]R.N.Bhowmik and A.Saravanan,“Surface magnetism,Morin transi-tion,and magnetic dynamics in antiferromagnetic α-Fe 2O 3(hematite)nanograins,”J.Appl.Phys.,vol.107,no.5,p.053916,2010.[7] A.Yogi and D.Varshney,“Magnetic and structural properties of pureand Cr-doped haematite:α-Fe 2-xCr x O 3(0≤x ≤1),”J.Adv.Ceram.,vol.2,no.4,pp.360–369,2013.[8] A.K.Shwarsctein,Y .S.Hu, A.J.Forman,G. D.Stucky,andE.W.McFarland,“Electrodeposition of α-Fe 2O 3doped with Mo or Cr as photoanodes for photocatalytic water splitting,”J.Phys.Chem.C ,vol.112,no.40,pp.15900–15907,2009.[9]R.Suresh,R.Prabu, A.Vijayaraj,K.Giribabu, A.Stephen,andV .Narayanan,“Facile synthesis of cobalt doped hematite nanospheres:Magnetic and their electrochemical sensing properties,”Mater.Chem.Phys.,vol.134,nos.2–3,pp.590–596,2012.[10] A.M.Banerjee et al.,“Catalytic activities of Fe 2O 3and chromiumdoped Fe 2O 3for sulfuric acid decomposition reaction in an integrated boiler,preheater,and catalytic decomposer,”Appl.Catalysis B,Environ.,vol.127,pp.36–46,Oct.2012.[11] B. D.Cullity,Elements of X-ray Diffraction .Boston,MA,USA:Addison-Wesley,1956.[12]R.Liu,N.Gao,F.Zhen,Y .Zhang,L.Mei,and X.Zeng,“Doping effectof Al 2O 3and CeO 2on Fe 2O 3support for gold catalyst in CO oxidation at low-temperature,”Chem.Eng.J.,vol.225,pp.245–253,Jun.2013.[13]M.S.Kim,K.G.Yim,J.S.Son,and J.Y .Leem,“Effects of Alconcentration on structural and optical properties of Al-doped ZnO thin films,”Bull.Korean Chem.Soc.,vol.33,no.4,pp.1235–1241,2012.[14]X.Lian et al.,“Enhanced photoelectrochemical performance of Ti-dopedhematite thin films prepared by the sol-gel method,”Appl.Surf.Sci.,vol.258,no.7,pp.2307–2311,2012.[15]P.Kumar et al.,“A novel method for controlled synthesis of nanosizedhematite (α-Fe 2O 3)thin film on liquid-vapor interface,”J.Nanoparticle Res.,vol.15,no.4,pp.1–13,2013.[16]J. A.Glasscock,P.R. F.Barnes,I. C.Plumb, A.Bendavi,andP.J.Martin,“Structural,optical and electrical properties of undoped polycrystalline hematite thin films produced using filtered arc deposi-tion,”Thin Solid Films ,vol.516,no.8,pp.1716–1724,2008.[17] F.L.Souza,K.P.Lopes,P.A.P.Nascente,and E.Leite,“Nanostruc-tured hematite thin films produced by spin-coating deposition solution:Application in water splitting,”Solar Energy Mater.,Solar Cells ,vol.93,no.3,pp.362–368,2009.[18]S.Riaz,S.Naseem,and Y .B.Xu,“Room temperature ferromagnetism insol-gel deposited un-doped ZnO films,”J.Sol-Gel Sci.Technol.,vol.59,no.3,pp.584–590,2011.[19]M.Ziese and M.J.Thornton,Spin Electronics .New York,NY ,USA:Springer-Verlag,2000.[20]J.Velev,A.Bandyopadhyay,W.H.Butler,and S.Sarker,“Electronic andmagnetic structure of transition-metal-doped α-hematite,”Phys.Rev.B ,vol.71,no.20,p.205208,2005.[21] F.S.Freyria et al.,“Eu-doped α-Fe 2O 3nanoparticles with modifiedmagnetic properties,”J.Solid State Chem.,vol.201,pp.302–311,May 2013.[22]M. A.G.Lobato, A.Martinez,M. C.Roman, C.Falcony,andL. E.Alarcon,“Correlation between structural and magnetic prop-erties of sprayed iron oxide thin films,”Phys.B ,vol.406,no.8,pp.1496–1500,2011.[23]Q.Guo et al.,“Effects of oxygen gas pressure on properties of iron oxidefilms grown by pulsed laser deposition,”J.Alloy Compounds ,vol.552,pp.1–5,Mar.2013.。
精 密 成 形 工 程第16卷 第3期 62JOURNAL OF NETSHAPE FORMING ENGINEERING 2024年3月收稿日期:2024-02-21 Received :2024-02-21引文格式:曹梓恒, 郭威, 吕书林, 等. 铝基非晶合金的制备、性能与应用研究进展[J]. 精密成形工程, 2024, 16(3): 62-75. CAO Ziheng, GUO Wei, LYU Shulin, et al. Progress in Research on Preparation, Properties and Application of Al-based Amor-phous Alloys[J]. Journal of Netshape Forming Engineering, 2024, 16(3): 62-75. *通信作者(Corresponding author ) 铝基非晶合金的制备、性能与应用研究进展曹梓恒1,郭威1,2,3*,吕书林1,王锦程2,吴树森1(1.华中科技大学 材料科学与工程学院 材料成形与模具技术全国重点实验室,武汉 430074;2.西北工业大学 凝固技术国家重点实验室,西安 710072;3.深圳华中科技大学研究院,广东 深圳 518057) 摘要:铝基非晶合金因其独特的物理和化学性能在诸多领域具有广泛的应用前景,综述了铝基非晶合金的成分体系、制备方法、性能特点及应用研究进展。
首先,介绍了铝基非晶合金的发展历史和成分体系,目前铝基非晶主要分为3大体系:二元、三元和多元体系,以及综合性能和形成能力2大方面,多元体系表现更佳,并逐渐向更多元化发展;其次,系统介绍了铝基非晶合金的制备方法,包括粉末状、薄带状、块体样品的制备,相较于非晶薄带的制备,块体和粉状的制备方法较为丰富,而粉状非晶通常作为铝基非晶涂层的预制材料;随后,详细介绍了铝基非晶合金的性能特点、应用现状及发展趋势,从性能上来看,铝基非晶在强度和硬度以及耐腐蚀性能上表现良好,目前主要以涂层的形式参与应用,除此之外,研究者们也开始对磁性和热塑性展开研究,由于玻璃形成能力的限制,作为结构材料的应用较少;最后,对其未来应用前景进行了展望,认为涂层是目前铝基非晶合金最具应用前景的工程化方式。