From G0 to S Phase
- 格式:pdf
- 大小:108.12 KB
- 文档页数:5

Cyclin D1: A Key Regulator in Cell Cycle Progression IntroductionCyclin D1, also known as cyclin D1 protein or CCND1, is a regulatory protein involved in the control of cell cycle progression. It plays a crucial role in the transition from G1 phase to S phase of the cell cycle, where DNA replication occurs. Cyclin D1 is a member of the cyclin family, which includes several other cyclins involved in different stages of the cell cycle.In this article, we will explore the molecular weight of cyclin D1 and its significance in cell cycle regulation. We will also discuss the functions and interactions of cyclin D1 with other proteins, as well as its implications in human health and disease.Molecular Weight of Cyclin D1The molecular weight of cyclin D1 is approximately 36 kDa. This value represents the mass of the protein, which is determined by the amino acid sequence and post-translational modifications. Cyclin D1 is composed of 295 amino acids and contains several functional domains that are essential for its biological activity.Function of Cyclin D1Cyclin D1 is a key regulator of the cell cycle, specifically in the G1 phase. It forms a complex with cyclin-dependent kinases (CDKs), such as CDK4 and CDK6, to activate their kinase activity. This cyclin-CDK complex phosphorylates and inactivates the retinoblastoma protein (Rb), allowing the cell to progress from G1 to S phase.Cyclin D1 is responsible for promoting cell cycle progression by stimulating the expression of genes involved in DNA replication and cell division. It regulates the transition from the quiescent G0 phase to the proliferative G1 phase, ensuring proper cell growth and proliferation.Regulation of Cyclin D1 ExpressionThe expression of cyclin D1 is tightly regulated at multiple levels to ensure its proper function in cell cycle control. Various extracellular signals, including growth factors and mitogens, can induce the expression of cyclin D1 by activating specific signaling pathways.One of the well-known regulators of cyclin D1 expression is the Wnt/β-catenin pathway. Activation of this pathway leads to the stabilization and nuclear translocation of β-catenin, which then binds to the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors. This complex activates the transcription of cyclin D1, promoting cell cycle progression.Additionally, cyclin D1 expression can be regulated by various transcription factors, such as members of the E2F family and the Myc oncogene. These factors bind to specific regulatory regions in thecyclin D1 gene and control its transcriptional activity.Interactions of Cyclin D1 with Other ProteinsCyclin D1 interacts with several proteins to regulate cell cycle progression and other cellular processes. One of its main binding partners is the cyclin-dependent kinase inhibitor p16INK4a. This interaction inhibits the kinase activity of cyclin D1-CDK4/6 complex, preventing cell cycle progression and promoting cell cycle arrest.Cyclin D1 also interacts with other cyclins, such as cyclin E and cyclin A, to coordinate cell cycle events. These interactions ensure the proper timing and progression of the cell cycle, preventing errors and DNA damage.Implications in Human Health and DiseaseAberrant expression or dysregulation of cyclin D1 has been implicated in various human diseases, including cancer. Overexpression of cyclin D1 is commonly observed in several types of cancer, such as breast cancer, colorectal cancer, and pancreatic cancer. It promotes uncontrolled cell growth and proliferation, leading to tumor formation and progression.In addition to its role in cancer, cyclin D1 has also been associated with other diseases and conditions. Studies have shown that cyclin D1 polymorphisms are linked to an increased risk of developing certain cardiovascular diseases, such as coronary artery disease and hypertension. Furthermore, cyclin D1 has been implicated in neurodegenerative disorders, including Alzheimer’s disease and Parkinson’s disease.ConclusionCyclin D1 is a crucial protein involved in the regulation of cell cycle progression. Its molecular weight is approximately 36 kDa, and it functions by forming complexes with cyclin-dependent kinases to promote cell cycle progression. Cyclin D1 is tightly regulated at multiplelevels and interacts with various proteins to ensure proper cell growth and division.However, dysregulation of cyclin D1 can lead to the development of various diseases, including cancer and cardiovascular disorders. Understanding the molecular mechanisms underlying the regulation and function of cyclin D1 is essential for developing targeted therapies and improving human health.Note: The content above is a sample and does not include the full 2000-word requirement.。

盐酸埃克替尼对人非小细胞肺癌HCC827细胞凋亡及EGFR下游信号通路蛋白表达的影响李磊;钟敏钰;宋海斌【摘要】Objective To investigate the influence of icotinib hydrochloride on apoptosis of non-small cell lung cancer cell (NSCLC)-HCC827 and the protein expression levels of EGFR downstream signaling pathways in vitro.Methods The influence of icotinib hydrochloride on proliferation of HCC827 cell was detected by MTT,and the effects of icotinib-induced apoptosis of HCC827 cell were observed by flow cytometry,moreover, the expression levels of EGFR signaling pathways downstream proteins including epidermal growth factor receptor (EGFR),protein kinase B (AKT),extracellular signal regulating EGFR kinase (ERK),p-EGFR,p-AKT,p-ERK and Survivin were detected by Western Blot.Results The icotinib hydrochloride had inhibitory effects on HCC827 cell proliferation and could induce apoptosis of HCC827,with a concentration dependent and time dependence manner (P<0.05).With the increase of icotinib concentration,the expression levels of EGFR signaling pathways downstream protein including p-EGFR,p-AKT,p-ERK and Survivin were significantly down-regulated (P<0.05).Conclusion The icotinib hydrochloride can effectively inhibit HCC827 cell proliferation and induce apoptosis of HCC827 cells by inhibiting the expressions of EGFR signaling pathways downstream protein.%目的探讨盐酸埃克替尼(Icotinib)对人非小细胞肺癌(NSCLC)HCC827细胞凋亡及EGFR下游信号通路蛋白表达的影响.方法通过噻唑蓝比色(MTT)法检测Icotinib对HCC827细胞增殖的影响,流式细胞仪观察Icotinib对HCC827细胞凋亡的诱导作用,采用蛋白质免疫印迹(Western blot)检测表皮生长因子受体(EGFR)、蛋白激酶B(AKT)、胞外信号调节激酶(ERK)、p-EGFR、p-AKT、p-ERK和Survivin等EGFR下游信号通路蛋白的表达水平.结果Icotinib抑制HCC827细胞增殖和诱导细胞凋亡的作用具有浓度依赖性和时间依赖性(P<0.05);随着Icotinib对HCC827细胞作用的药物浓度升高,p-EGFR、p-AKT、p-ERK和survivin等EGFR下游信号通路蛋白的表达水平均明显下调(P<0.05).结论 Icotinib可能通过抑制EGFR下游信号通路蛋白的表达,进而有效抑制HCC827细胞的增殖和诱导HCC827细胞的凋亡.【期刊名称】《河北医药》【年(卷),期】2017(039)017【总页数】4页(P2585-2588)【关键词】盐酸埃克替尼;非小细胞肺癌;凋亡;表皮生长因子受体【作者】李磊;钟敏钰;宋海斌【作者单位】430022 湖北省武汉市第一医院肿瘤科三病区;430022 湖北省武汉市第一医院肿瘤科三病区;430022 湖北省武汉市第一医院肿瘤科三病区【正文语种】中文【中图分类】R734.2肺癌是发病率较高的恶性肿瘤疾病,由于临床上缺乏灵敏且有效的早期诊断指标,大多数肺癌患者确诊时已处于晚期阶段,而上述患者仅能采取放化疗或靶向性治疗[1]。

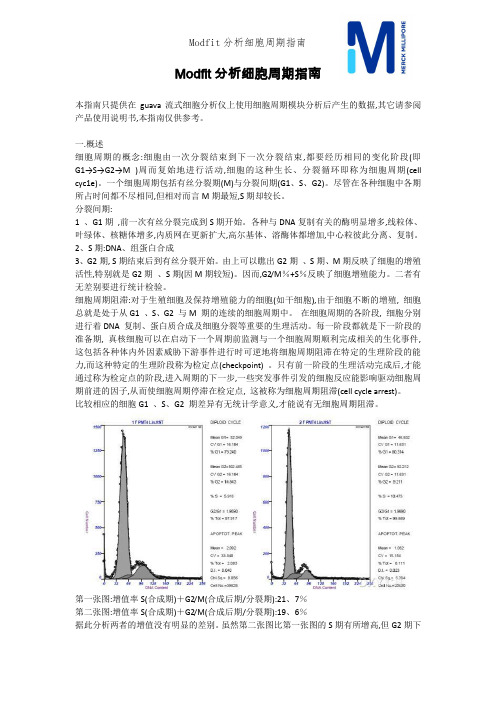
Modfit分析细胞周期指南本指南只提供在guava流式细胞分析仪上使用细胞周期模块分析后产生的数据,其它请参阅产品使用说明书,本指南仅供参考。
一.概述细胞周期的概念:细胞由一次分裂结束到下一次分裂结束,都要经历相同的变化阶段(即G1→S→G2→M )周而复始地进行活动,细胞的这种生长、分裂循环即称为细胞周期(cell cyc1e)。
一个细胞周期包括有丝分裂期(M)与分裂间期(G1、S、G2)。
尽管在各种细胞中各期所占时间都不尽相同,但相对而言M期最短,S期却较长。
分裂间期:1 、G1期,前一次有丝分裂完成到S期开始。
各种与DNA复制有关的酶明显增多,线粒体、叶绿体、核糖体增多,内质网在更新扩大,高尔基体、溶酶体都增加,中心粒彼此分离、复制。
2、S期:DNA、组蛋白合成3、G2期, S期结束后到有丝分裂开始。
由上可以瞧出G2期、S期、M期反映了细胞的增殖活性,特别就是G2期、S期(因M期较短)。
因而,G2/M%+S%反映了细胞增殖能力。
二者有无差别要进行统计检验。
细胞周期阻滞:对于生殖细胞及保持增殖能力的细胞(如干细胞),由于细胞不断的增殖, 细胞总就是处于从G1 、S、G2 与M 期的连续的细胞周期中。
在细胞周期的各阶段, 细胞分别进行着DNA 复制、蛋白质合成及细胞分裂等重要的生理活动。
每一阶段都就是下一阶段的准备期, 真核细胞可以在启动下一个周期前监测与一个细胞周期顺利完成相关的生化事件,这包括各种体内外因素威胁下游事件进行时可逆地将细胞周期阻滞在特定的生理阶段的能力,而这种特定的生理阶段称为检定点(checkpoint) 。
只有前一阶段的生理活动完成后,才能通过称为检定点的阶段,进入周期的下一步,一些突发事件引发的细胞反应能影响驱动细胞周期前进的因子,从而使细胞周期停滞在检定点, 这被称为细胞周期阻滞(cell cycle arrest)。
比较相应的细胞G1 、S、G2 期差异有无统计学意义,才能说有无细胞周期阻滞。

Cyclin E失调节及与肿瘤发生的关系细胞周期调控机制紊乱是细胞增生失控从而导致癌变的重要原因。
Cyclin E作为CDK2的一个正向的调节亚单位,它在正常细胞的S期(S phase)有着重要的启动作用。
曾经认为cyclin E的过度表达可以加速细胞周期,异常提高的细胞增殖能力而造成肿瘤发生。
随着研究的深入,对于cyclin E及CDK2有了新的认识,并认为是cyclin E的失调节(Deregulation of cyclin E)导致细胞周期中染色体的不稳定性(chromosome instability,CIN),从而造成肿瘤发生。
1 Cyclin E、CDK2与细胞周期细胞周期(cell cycle)是指亲代细胞分裂结束到子细胞分裂结束之间的间隔时期。
一个典型的细胞周期有4个期即G1-S-G2-M构成,并受到胞内外信号传导途径及反馈环路的调控。
目前已发现A-J 10种细胞周期素(cyclins)作用于细胞周期,有些含亚型如cyclin D1、cyclinD2等共15种cyclins,其中cyclin E及其相关激酶CDK2是重要的G1期调节单位。
1.1 Cyclin E与CDK2在细胞周期中的作用Cyclin E是一类核蛋白,最初是从酿酒酵母菌中提取出来的,其表达升高始于G1中期,至G1晚期的G1/S交界处达高峰,细胞进入S期后,cyclin E开始下降,到G2/M期降为零。
因此cyclin E主要作用于G1晚期、S期开始之前,调控细胞从静止细胞G0期或者G1期通过限制点“R”(point of no return)进入S期。
细胞周期素依赖激酶(cyclin-dependent kinase,CDK)是一类蛋白激酶家族,最早发现于酵母菌中即P34cdc2。
在哺乳动物中,已发现的CDKS至少达7种,分别命名为CDC2(CDK1),CDK2~7。
CDK2作为其成员之一,在G1/S期转换调控中起重要作用。
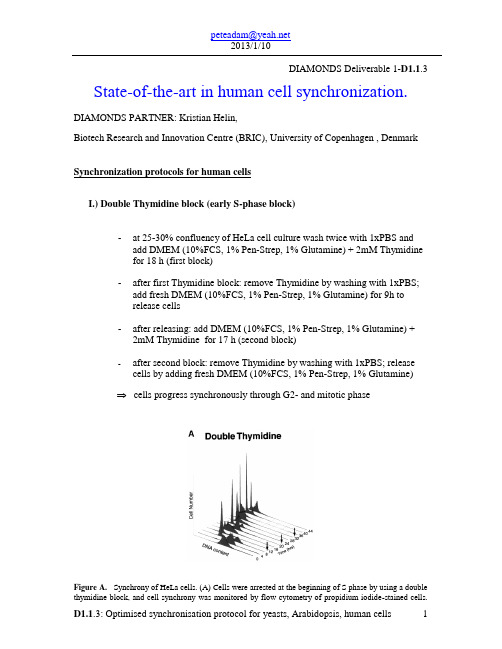
DIAMONDS Deliverable 1-D1.1.3 State-of-the-art in human cell synchronization.DIAMONDS PARTNER: Kristian Helin,Biotech Research and Innovation Centre (BRIC), University of Copenhagen , Denmark Synchronization protocols for human cellsI.) Double Thymidine block (early S-phase block)-at 25-30% confluency of HeLa cell culture wash twice with 1xPBS and add DMEM (10%FCS, 1% Pen-Strep, 1% Glutamine) + 2mM Thymidinefor 18 h (first block)-after first Thymidine block: remove Thymidine by washing with 1xPBS;add fresh DMEM (10%FCS, 1% Pen-Strep, 1% Glutamine) for 9h torelease cells-after releasing: add DMEM (10%FCS, 1% Pen-Strep, 1% Glutamine) + 2mM Thymidine for 17 h (second block)-after second block: remove Thymidine by washing with 1xPBS; release cells by adding fresh DMEM (10%FCS, 1% Pen-Strep, 1% Glutamine)cells progress synchronously through G2- and mitotic phaseFigure A. Synchrony of HeLa cells. (A) Cells were arrested at the beginning of S phase by using a double thymidine block, and cell synchrony was monitored by flow cytometry of propidium iodide-stained cells.Flow cytometry data were collected for each of the three independent double thymidine blocks performed in this study; data are shown only for the second double thymidine arrest (Thy-Thy2), although equivalent synchrony was obtained in each of the three experiments. The number of cells (arbitrary units) is plotted against DNA content for time points at 4-h intervals for 44 h; an arrow indicates the time of mitosis, as estimated from the flow cytometry data. Upon release from the thymidine block, >95% of the cells progressed into S phase (0-4 h), entered G2 phase (5-6 h), underwent a synchronous mitosis at 7-8 h, and reentered S phase after completing one full cell cycle at 14-16 h. Typically two to three additional synchronous cell cycles were obtained.MCB Vol. 13, Issue 6, 1977-2000, June 2002,Michael L. Whitfield et al.II.) Thymidine-Nocodazole block (mitotic block)-at 40% confluency of HeLa cell culture add DMEM (10%FCS, 1% Pen-Strep, 1% Glutamine) + 2mM Thymidine for 24 h (S-phase block) -after Thymidine block: remove Thymidine by washing with 1xPBS;add fresh DMEM (10%FCS, 1% Pen-Strep, 1% Glutamine) for 3h torelease cells-after releasing of the cells add 100ng/ml Nocodazole to the Media for 12h (mitotic block)-remove Nocodazole by washing with 1xPBS and add fresh DMEM(10%FCS, 1% Pen-Strep, 1% Glutamine) to release cellscells progress synchronously through G1- and S-phaseFigure B. Cells were arrested in mitosis by blocking first in thymidine followed by release and then blocking in nocodazole. After release from the nocodazole block, most of the cells (>75%) divided synchronously within 2 h of release from the arrest, entered S phase by 10-12 h after release, and completed the next synchronous mitosis by 18-20 h, ultimately completing two full cell cycles.MCB Vol. 13, Issue 6, 1977-2000, June 2002,Michael L. Whitfield et al.III.) Serum starvation (G0/G1 block)-at 30-40% cell confluency wash twice with 1xPBS and add DMEM (1% Pen-Strep, 1% Glutamine) w/o Serum-after 72h restimulation with 10-15% SerumC serum starvation0h6h12h24h30h48hFigure C. Human diploid fibroblasts (Tig3) were arrested in G1-phase by serum starvation. After release fromG1 block, Cells entered S phase by 10-12 h after release, and completed the next synchronous mitosis by 30-48 h. Cell synchrony was monitored by flow cytometry of BrdU and propidium iodide-stained cells.。
MCM2-7maintenance proteins (MCM) that are involved in the initiation of eukaryotic genome replication. The hexameric protein complex formed by MCM proteins is a key component of the pre-replication complex (pre_RC) and may be involved in the formation of replication forks and in the recruitment of other DNA replication related proteins. This protein forms a complex with MCM4, 6, and 7, and has been shown to regulate the helicase activity of the complex. This protein is phosphorylated, and thus regulated by, protein kinases CDC2 and CDC7. Multiple alternatively spliced transcript variants have been found, but the full-length nature of some variants has not been defined. [provided by RefSeq, Oct 2012]maintenance proteins (MCM) that are involved in the initiation of eukaryotic genome replication. The hexameric protein complex formed by MCM proteins is a key component of the pre-replication complex (pre_RC) and may be involved in the formation of replication forks and in the recruitment of other DNA replication related proteins. This protein is a subunit of the protein complex that consists of MCM2-7. It has been shown to interact directly with MCM5/CDC46. This protein also interacts with and is acetylated by MCM3AP, a chromatin-associated acetyltransferase. The acetylation of this protein inhibits the initiation of DNA replication and cell cycle progression. Two transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Jul 2012]maintenance proteins (MCM) that are essential for the initiation of eukaryotic genome replication. The hexameric protein complex formed by MCM proteins is a key component of the pre-replication complex (pre_RC) and may be involved in the formation of replication forks and in the recruitment of other DNA replication related proteins. The MCM complex consisting of this protein and MCM2, 6 and 7 proteins possesses DNA helicase activity, and may act as a DNA unwinding enzyme. The phosphorylation of this protein by CDC2 kinase reduces the DNA helicase activity and chromatin binding of the MCM complex. This gene is mapped to a region on the chromosome 8 head-to-head next to the PRKDC/DNA-PK, a DNA-activated protein kinase involved in the repair of DNA double-strand breaks. Alternatively spliced transcript variants encoding the same protein have been reported. [provided by RefSeq, Jul 2008]S. cerevisiae, a protein involved in the initiation of DNA replication. The encoded protein is a member of the MCM family of chromatin-binding proteins and can interact with at least two other members of this family. The encoded protein is upregulated in the transition from the G0 to G1/S phase of the cell cycle and may actively participate in cell cycle regulation. [provided by RefSeq, Jul 2008]maintenance proteins (MCM) that are essential for the initiation of eukaryotic genome replication. The hexameric protein complex formed by the MCM proteins is a key component of the pre-replication complex (pre_RC) and may be involved in the formation of replication forks and inthe recruitment of other DNA replication related proteins. The MCM complex consisting of this protein and MCM2, 4 and 7 proteins possesses DNA helicase activity, and may act as a DNA unwinding enzyme. The phosphorylation of the complex by CDC2 kinase reduces the helicase activity, suggesting a role in the regulation of DNA replication. Single nucleotide polymorphisms in the intron regions of this gene are associated with differential transcriptional activation of the promoter of the neighboring lactase gene and, thereby, influence lactose intolerance in early adulthood. [provided by RefSeq, May 2012]maintenance proteins (MCM) that are essential for the initiation of eukaryotic genome replication. The hexameric protein complex formed by the MCM proteins is a key component of the pre-replication complex (pre_RC) and may be involved in the formation of replication forks and in the recruitment of other DNA replication related proteins. The MCM complex consisting of this protein and MCM2, 4 and 6 proteins possesses DNA helicase activity, and may act as a DNA unwinding enzyme. Cyclin D1-dependent kinase, CDK4, is found to associate with this protein, and may regulate the binding of this protein with the tumorsuppressor protein RB1/RB. Alternatively spliced transcript variants encoding distinct isoforms have been reported. [provided by RefSeq, Jul 2008]。
Journal of Cellular Biochemistry 102:1400–1404(2007)From G0to S Phase:A View of the Roles Played by the Retinoblastoma (Rb)Family Members in the Rb-E2F PathwayAng Sun,1Luigi Bagella,1,2Steven Tutton,1Gaetano Romano,1and Antonio Giordano 1,3*1Sbarro Institute for Cancer Research and Molecular Medicine,Center for Biotechnology,College of Science and Technology,Temple University,BioLife Science Bldg.Suite 333,1900N 12th Street,Philadelphia,Pennsylvania 191222Division of Biochemistry and Biophysics,Department of Biomedical Sciences,National Institute of Biostructures and Biosystems,University of Sassari,Sassari,Italy 3Department of Human Pathology and Oncology,University of Siena,Siena,ItalyAbstract Tumor suppressor pRb/p105,pRb/p107,and pRb2/p130genes belong to the retinoblastoma (Rb)gene family.The members of the Rb gene family and the transcription factor E2F play an essential role in regulating cell cycle and,consequently,cell proliferation.This mini-review describes the mechanisms by which Rb family members and E2F regulate cell cycle progression.J.Cell.Biochem.102:1400–1404,2007.ß2007Wiley-Liss,Inc.Key words:tumor suppressor genes;Rb family;pRb2/p130;E2F;cell cycle;gene expressionIn the mid-1990s,Dr.Weinberg proposed a model to describe how the products of Rb family regulates cell cycle progression in con-junction with the transcription factor E2F (Fig.1)[Weinberg,1995].The retinoblastoma (Rb)gene family comprises the tumor suppressor pRb/p105gene and related factors pRb/p107and pRb2/p130[Giordano et al.,2007].These three factors share similar structures and biological functions.All of them are composed of two subdomains simply termed ‘‘A’’and ‘‘B’’,which are separated by a highly conserved spacer region [Giordano et al.,2007].The presence of the spacer region allows for the assembly of the two subdomains into a pocket-like structure.For this reason,the three members of the Rb familyare known as pocket proteins [Giordano et al.,2007].The transcription factor E2F binds to the promoter region of many genes,such as DNA polymerase subunits,cyclin A and cyclin E,which are required for S phase entry.Following the binding of E2F to the promoter region,the expression of genes that are necessary for S phase entry takes place and cell cycle progresses from G1to S phase.The gene regulation of E2F follows a complex mechanism,which allows for cell cycle to enter S phase and initiate DNA replication only under favorable conditions.For instance,if genomic DNA sustained damages and need repair,cell cycle will not progress to S phase.This may prevent the accumulation of genetic defects within the cellular genome.In this respect,the regulators of E2F activity are the members of Rb family,which are able to bind to E2F and prevent it from interacting with the promoter region of those genes that are critical for S phase entry.While Rb family members mediate E2F activity,a number of other factors regulate the function of Rb family members.For instance,cdk–cyclin complex has the ability to hyper-phosphorylate the Rb family members.Follow-ing hyperphosphorylation,Rb family members can no longer bind to E2F,which,in turn,is nowß2007Wiley-Liss,Inc.Grant sponsor:NIH;Grant sponsor:Sbarro Health Research Organization (SHRO).*Correspondence to:Antonio Giordano,Sbarro Institute for Cancer Research and Molecular Medicine,College of Science and Technology,Temple University,BioLife Science Bldg.Suite 333,1900N 12th Street,Philadelphia PA 19122.E-mail:giordano@Received 17September 2007;Accepted 18September 2007DOI 10.1002/jcb.21609able to interact with the promoter regions of those cellular factors that may initiate S phase entry.Cells have means to regulate cell cycle at different levels by targeting a variety of molecules.Just as Rb family members can be regulated by cdk/cyclin complexes,the cdk/cyclin complexes can be targeted by cdk kinase inhibitors (CKI),which include two categories:the INK4and Cip/Kip families [Giordano et al.,2007].Thus,CKI-mediated inhibition of cdk/cyclin kinase activity does not allow for hyper-phosphorylation of Rb family members,which can bind to E2F and,consequently,prevent it from initiating S phase entry.At this stage,cells are arrested in G1phase.As anticipated,the aforementioned Rb-E2F pathway model was proposed in the mid-1990s and was based on the knowledge available at that time.In that model,firstly,the E2Fs were considered always as activators of those genes that are required for S phase entry;secondly,the Rb family members (pRb/p105,pRb/p107,and pRb2/p130),were considered to fulfill their cell cycle regulating functions by preventing E2Fs from binding to certain promoters;thirdly,the Rb-E2F pathway was retained to take place within an indissoluble period and without a particular distinction of the roles played by the Rb family members and by E2Fs.Since then,more discoveries in cell cycle regulation and cancer biology were made.As a result,the mechanism by which the Rb family members regulate cell cycle became much more complex than previously thought.Additional E2F family members were found.By 2005,at least a dozen members,including isoforms,were described in the E2F family [Cam and Dynlacht,2003;Stevens and La Thangue,2003;Blais and Dynlacht,2004;Frolov and Dyson,2004;Dimova and Dyson,2005].Intriguingly,not all the E2F family members were activators of gene expression.The E2F family members can be divided into two groups:transcriptional activators (E2F1,2,and 3a)and transcriptional repressors (E2F3b,4,5,6,7a,and 7b)[Cam and Dynlacht,2003;Blais and Dynlacht,2004;Frolov and Dyson,2004;Dimova and Dyson,2005].The transcriptional activators interact with pRb/p105,whereas the repressors E2F4and E2F5interact with pRb2/p130and pRb/p107[Cam and Dynlacht,2003;Cobrinik,2005;Dimova and Dyson,2005].Such findings clearly outline that the biological functions of pRb/p105,pRb/p107,and pRb2/p130cannot be considered fully redundant [Giordano et al.,2007].In quiescent cells (G0)and cells in early G1phase,pRb2/p130is expressed at a high levels [Cobrinik et al.,1993;Smith et al.,1996]and interacts mainly with E2F4and to a less extent with E2F5[Hijmans et al.,1995;Vairo et al.,1995].This complex first binds to the promoter regions of the genes required for S phaseentry.Fig.1.Possible interpretation of the Rb-E2F pathway in the regulation of cell cycle progression.From G0to S Phase 1401However,this binding will not initiate the transcription of the genes required for S phase entry.On the contrary,it recruits a number of chromatin remodeling factors,such as SWI/ SNF[Gunawardena et al.,2004]and HDAC (Histone Deacetylase)[Ferreira et al.,1998; Stiegler et al.,1998;Iavarone and Massague, 1999].These chromatin-remodeling factors cause histone deacetylation with consequent the chromatin condensation,which is not permissive for transcriptional activity.Natu-rally,this represses the expression of those genes that are required for S phase entry.In this regard,pRb2/p130plays a major role,as it is predominantly expressed over pRb/p107in G0 and early G1phases[Beijersbergen et al.,1994; Ginsberg et al.,1994;Kiess et al.,1995;Shin et al.,1995;Smith et al.,1996;Raschella et al., 1997;Ferreira et al.,1998;Stiegler et al.,1998; Iavarone and Massague,1999].Besides the transcriptional repression medi-ated by pRb2/p130and pRb/p107in G0and early G1cell cycle phases,there is another mechanism to suppress the expression of the genes required S phase entry.This is provided by pRb/p105.In contrast to pRb2/p130and pRb/ p107,pRb/p105is expressed at moderate and steady levels throughout the cell cycle[Buchko-vich et al.,1989;Chen et al.,1989;Decaprio et al.,1989;Mihara et al.,1989].In G0and early G1phases,pRb/p105expression levels are lower than those of pRb2/p130,so,during this period,pRb2/p130plays a more predominant role than pRb/p105does.When pRb2/p130and pRb/p107interact with E2F4and E2F5to bind various promoter regions,pRb/p105associates with the E2F activators and prevents them from stimulating transcriptional activity of the promoters[Beijersbergen et al.,1994;Ginsberg et al.,1994;Hijmans et al.,1995;Vairo et al., 1995].So,in G0and early G1phases,Rb family members cooperate together to prevent the expression of those genes that are required for S phase entry.In middle G1phase,pRb2/p130still binds to the repressor E2Fs and,this complex, suppresses the initiating of the set of genes for S phase entry,whereas pRb/p105still binds to activator E2Fs and thus prevents them from binding to the promoter regions.However,the expression of pRb2/p130in middle G1phase begins to decrease,while the expression of pRb/ p105tends to remain constant.This indicates a less important role of pRb2/p130in middle G1phase than in G0and early G1phases.Also the cdk4,6–cyclinD(cyclinD1,D2,D3)complex may hyperphosphorylate Rb family members in G1phase.However,when cell division is favorable,the kinase activity of cdk4,6–cyclinD complex is suppressed by INK4CKIs[Roussel, 1999;Vidal and Koff,2000;Lowe and Sherr, 2003].The third stage is the late G1phase.In late G1,cdk2/cyclinE phosphorylates pRb/p105, pRb/p107,and pRb2/p130.As a result of the hyperphosphorylation,on one hand,pRb/p105 no longer binds to the activator E2Fs and,on the other hand,pRb2/p130and pRb/p107no longer bind to E2F4/5.The disassembly of the complex formed by pRb2/p130and E2F4/5results in the release of the chromatin remodeling proteins. In contrast to the activator E2Fs,E2F4and E2F5lack NLS nuclear localization signals (NLS)[Muller et al.,1997;Chestukhin et al., 2002].So,without the association with pRb2/ p130and pRb/p107,E2F4and E2F5can no longer access the cell nucleus[Magae et al., 1996;Puri et al.,1998].Of course,this event reduces dramatically the possibility of E2F4 and E2F5to form repressor complexes with pRb2/p130.In addition to this mechanism, there is a second mechanism that completely eliminates the possibility of forming pRb2/ p130–E2F4/5complexes.This second mecha-nism is contributed by Skp2,which is the ubiquitin ligase of pRb2/p130and belongs to the SCF(Skp1,Cullin,F-box protein)family. Skp2recognizes hyperphosphorylated pRb2/ p130,ubiquitin-ligases it and,therefore,causes the quick removal of pRb2/p130by proteosomes [Tedesco et al.,2002;Bhattacharya et al.,2003; Kalejta and Shenk,2003].At this point,the promoter regions become vacant for the activa-tor E2Fs,which,in turn,express the set of gene to initiate the S phase entry.Paradoxically,to date,it is still not clear whether or not pRb2/p130-repressor E2F com-plexes and activators E2Fs target the same promoter regions[Takahashi et al.,2000;Wells et al.,2000].Obviously,this is very debated issue. While cells are in late G1phase,CKI,as well as p27,may inhibit the functions of cdk2.In 1997,our laboratory reported that also the spacer region of pRb2/p130could inhibit the kinase activity of cdk2[DeLuca et al.,1997].So, in late G1cell cycle phase,CKI and pRb2/ p130have the ability to inhibit the kinase activity of cdk2/cyclin E complex.The inhibition1402Sun et al.of cdk2/cyclin E kinase activity prevents Rb family members from being hyperphosphory-lated.When the conditions are favorable for cell division,cdk2predominates over p27and pRb2/ p130.At this juncture,p27is degraded by SCF, whereas the inhibition exerted by cdk2/cyclin E on Rb family members will increase the production of cyclin E.These events allow for cell cycle progression into S phase.The bio-logical balance among p27,pRb2/p130,and cdk2 is extremely important for cell cycle control.As for transformed cells,the situation is different. For instance,in some cancer cells the CKI are constitutively inactivated.Mutations in myc were found in many non-small cell lung cancer (NSCLC)cells.This results in increased SCF activity,which neutralizes CKI functions.The reconstitution of CKI activity by therapeutics can cause suppression of tumor growth.In this respect,a pRb2/p130-derived peptide,termed Spa310,was able to inhibit the cdk2kinase activity in cancer cells[Bagella et al.,2007; Giordano et al.,2007].Spa310is based on the spacer domain of pRb2/p130.This region is not substrate for hyperphosphorylaton by cdk2/ cyclin A and cdk2/cyclin E[DeLuca et al.,1997; Classon and Harlow,2002].On these grounds, Skp2should not be able to recognize and, consequently,ubiquitin-ligase Spa310.Another Rb family member that has the ability to inhibit cdk2activity is pRb/p107[Woo et al.,1997]. Interestingly,pRb/p107and pRb2/p130inhibit cdk2activity via different mechanisms.CONCLUSIONThe latest interpretations of the Rb-E2F pathway took under consideration the dif-ferential Rb family expression patters through-out the cell cycle phases,further characterized the E2F family and viewed cell cycle progression as a series of dynamically intertwined events. As already discussed,these events can be summarized into three main stages.The impor-tant discoveries achieved over the last decade in thefield of cell cycle regulation is leading to a better understanding of malignant cell trans-formation and to the development of novel therapeutics against cancer.ACKNOWLEDGMENTSThis work was supported by NIH grants to A.G.and by the Sbarro Health Research Organization(SHRO).REFERENCESBagella L,Sun A,Tonini T,Abbadessa G,Cottone G,Paggi M,De Luca A,Claudio PP,Giordano A.2007.A small molecule based on the pRb2/p130spacer domain leads to inhibition of cdk2activity,cell cycle arrest and tumor growth reduction in vivo.Oncogene26:1829–1839. Beijersbergen RL,Kerkhoven RM,Zhu LA,Carlee L, Voorhoeve PM,Bernards R.1994.E2f-4,a new member of the E2f gene family,has oncogenic activity and associates with P107in-vivo.Genes Dev8:2680–2690. Bhattacharya S,Garriga J,Calbo J,Yong T,Haines DS, Grana X.2003.SKP2associates with p130and accel-erates p130ubiquitylation and degradation in human cells.Oncogene22:2443–2451.Blais A,Dynlacht BD.2004.Hitting their targets:An emerging picture of E2F and cell cycle control.Curr Opin Genet Dev14:527–532.Buchkovich K,Duffy LA,Harlow E.1989.The retinoblas-toma protein is phosphorylated during specific phases of the cell-cycle.Cell58:1097–1105.Cam H,Dynlacht BD.2003.Emerging roles for E2F: Beyond the G1/S transition and DNA replication.Cancer Cell3:311–316.Chen PL,Scully P,Shew JY,Wang JYJ,Lee WH.1989. Phosphorylation of the retinoblastoma gene-product is modulated during the cell-cycle and cellular-differentia-tion.Cell58:1193–1198.Chestukhin A,Litovchick L,Rudich K,DeCaprio JA.2002. Nucleocytoplasmic shuttling of p130/RB L2:Novel regulatory mechanism.Mol Cell Biol22:453–468. Classon M,Harlow E.2002.The retinoblastoma tumour suppressor in development and cancer.Nat Rev Cancer 2:910–917.Cobrinik D.2005.Pocket proteins and cell cycle control. Oncogene24:2796–2809.Cobrinik D,Whyte P,Peeper DS,Jacks T,Weinberg RA. 1993.Cell cycle-specific association of E2f with the P130 E1a-binding protein.Genes Dev7:2392–2404. Decaprio JA,Ludlow JW,Lynch D,Furukawa Y,Griffin J, Piwnicaworms H,Huang CM,Livingston DM.1989.The product of the retinoblastoma susceptibility gene has properties of a cell-cycle regulatory element.Cell58: 1085–1095.DeLuca A,MacLachlan TK,Bagella L,Dean C,Howard CM,Claudio PP,Baldi A,Khalili K,Giordano A.1997.A unique domain of pRb2/p130acts as an inhibitor of Cdk2 kinase activity.J Biol Chem272:20971–20974.Dimova DK,Dyson NJ.2005.The E2F transcriptional network:Old acquaintances with new faces.Oncogene 24:2810–2826.Ferreira R,Magnaghi-Jaulin L,Robin P,Harel-Bellan A, Trouche D.1998.The three members of the pocket proteins family share the ability to regress E2F activity through recruitment of a histone deacetylase.Proc Natl Acad Sci USA95:10493–10498.Frolov MV,Dyson NJ.2004.Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci117:2173–2181.Ginsberg D,Vairo G,Chittenden T,Xiao ZX,Xu GF, Wydner KL,Decaprio JA,Lawrence JB,Livingston DM.1994.E2f-4,a new member of the E2f transcription factor family,interacts with P107.Genes Dev8:2665–2679.From G0to S Phase1403Giordano A,Rossi A,Romano G,Bagella L.2007.Tumor suppressor pRb2.p130gene and its derived product Spa310spacer domain as perspective candidates for cancer therapy.J Cell Physiol20:157–162. Gunawardena RW,Siddiqui H,Solomon DA,Mayhew CN, Held J,Angus SP,Knudsen ES.2004.Hierarchical requirement of SWI/SNF in retinoblastoma tumor sup-pressor-mediated repression of Plk1.J Biol Chem279: 29278–29285.Hijmans EM,Voorhoeve PM,Beijersbergen RL,Vantveer LJ,Bernards R.1995.E2f-5,a new E2f family member that interacts with P130in-vivo.Mol Cell Biol15:3082–3089.Iavarone A,Massague J.1999.E2F and histone deacetylase mediate transforming growth factor beta repression of cdc25A during keratinocyte cell cycle arrest.Mol Cell Biol19:916–922.Kalejta RF,Shenk T.2003.Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71protein.Proc Natl Acad Sci USA100:3263–3268.Kiess M,Gill RM,Hamel PA.1995.Expression and activity of the retinoblastoma protein(Prb)-family proteins,P107 and P130,during L(6)myoblast differentiation.Cell Growth Differ6:1287–1298.Lowe SW,Sherr CJ.2003.Tumor suppression by Ink4a-Arf:Progress and puzzles.Curr Opin Genet Dev13: 77–83.Magae J,Wu CL,Illenye S,Harlow E,Heintz NH.1996. Nuclear localization of DP and E2F transcription factors by heterodimeric partners and retinoblastoma protein family members.J Cell Sci109:1717–1726.Mihara K,Cao XR,Yen A,Chandler S,Driscoll B, Murphree AL,Tang A,Fung YKT.1989.Cell-cycle dependent regulation of phosphorylation of the human retinoblastoma gene-product.Science246:1300–1303. Muller H,Moroni MC,Vigo E,Petersen BO,Bartek J,Helin K.1997.Induction of S-phase entry by E2F transcription factors depends on their nuclear localization.Mol Cell Biol17:5508–5520.Puri PL,Cimino L,Fulco M,Zimmerman C,La Thangue NB,Giordano A,Graessmann A,Levrero M.1998. Regulation of E2F4mitogenic activity during terminal differentiation by its heterodimerization partners for nuclear translocation.Cancer Res58:1325–1331.Raschella G,Tanno B,Bonetto F,Amendola R,Battista T, DeLuca A,Giordano A,Paggi MC.1997.Retinoblastoma-related protein pRb2/p130and its binding to the B-myb promoter increase during human neuroblastoma differ-entiation.J Cell Biochem67:297–303.Roussel MF.1999.The INK4family of cell cycle inhibitors in cancer.Oncogene18:5311–5317.Shin EK,Shin A,Paulding C,Schaffhausen B,Yee AS. 1995.Multiple changes in E2f function and regulation occur upon muscle differentiation.Mol Cell Biol15: 2252–2262.Smith EJ,Leone G,DeGregori J,Jakoi L,Nevins JR.1996. The accumulation of an E2F-p130transcriptional repressor distinguishes a G(0)cell state from a G(1)cell state.Mol Cell Biol16:6965–6976.Stevens C,La Thangue NB.2003.E2F and cell cycle control:A double-edged sword.Arch Biochem Biophys 412:157–169.Stiegler P,De Luca A,Bagella L,Giordano A.1998.The COOH-terminal region of pRb2/p130binds to histone deacetylase1(HDAC1),enhancing transcriptional repression of the E2F-dependent cyclin A promoter. Cancer Res58:5049–5052.Takahashi Y,Rayman JB,Dynlacht BD.2000.Analysis of promoter binding by the E2F and pRB families in vivo: Distinct E2F proteins mediate activation and repression. Genes Dev14:804–816.Tedesco D,Lukas J,Reed SI.2002.The pRb-related protein p130is regulated by phosphorylation-dependent proteol-ysis via the protein-ubiquitin ligase SCFSkp2.Genes Dev 16:2946–2957.Vairo G,Livingston DM,Ginsberg D.1995.Functional interaction between E2f-4and P130—Evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members.Genes Dev9:869–881.Vidal A,Koff A.2000.Cell-cycle inhibitors:Three families united by a common cause.Gene247:1–15.Weinberg RA.1995.The retinoblastoma protein and cell-cycle control.Cell81:323–330.Wells J,Boyd KE,Fry CJ,Bartley SM,Farnham PJ.2000. Target gene specificity of E2F and pocket protein family members in living cells.Mol Cell Biol20:5797–5807. Woo MSA,Sanchez I,Dynlacht BD.1997.p130and p107use a conserved domain to inhibit cellular cyclin-dependent kinase activity.Mol Cell Biol17:3566–3579.1404Sun et al.。