分子间作用力(2011)
- 格式:ppt
- 大小:1.24 MB
- 文档页数:19

分子之间的作用力
首先,范德华力(Van der Waals forces)是由于分子之间的偶极矩
和/或极化引起的吸引力。
偶极矩是由于电子云在分子内部不对称分布而
产生的。
当分子靠近时,偶极矩会相互作用,从而产生吸引力。
极化则是
由外部电场引起电子云的不均匀分布,形成暂时的偶极矩。
这些吸引力的
大小取决于分子中的电荷分布和分子间的距离。
其次,静电力是由于分子之间的电荷引力而产生的相互作用力。
当分
子中存在正电荷和负电荷时,它们会相互吸引形成静电力。
例如,正负电
荷分别位于两个分子之间时,它们之间的静电力会把两个分子吸引在一起。
静电力的大小取决于电荷的多少和分子之间的距离。
最后,氢键是一种特殊的静电力。
它是由于氢原子与具有较强电负性
的原子(如氧、氮和氟)之间形成的相互作用力。
在氢键中,氢原子共价
结合到一个原子上,而另一个原子上存在一个较强的电负性。
这样,氢原
子的电子会更倾向于位于具有较强电负性的原子附近,而形成一个偏正电荷。
这个偏正电荷会与具有部分负电荷的原子形成静电相互作用力,从而
形成氢键。
氢键的强度通常比范德华力和普通的静电力强,因此它在许多
化学和生物分子的结构和性质中起着重要的作用。
总结起来,分子之间的作用力分为范德华力、静电力和氢键。
这些作
用力的大小和属性取决于分子中的电荷分布、电子云的构成和分子之间的
距离。
通过这些作用力,分子可以相互吸引,并在化学反应、溶解和分子
间相互作用等方面发挥重要作用。
分子间的作用力上面已经讨论了三种基本类型的化学键,它们都是分子内部原子间较强的结合力,是决定分子化学性质的主要因素。
在分子与分子之间还存在着较弱的作用力,它是决定物质的沸点、熔点、溶解度等物理性质的重要因素。
为了更好地说明分子间作用力,先谈一下分子极化的问题。
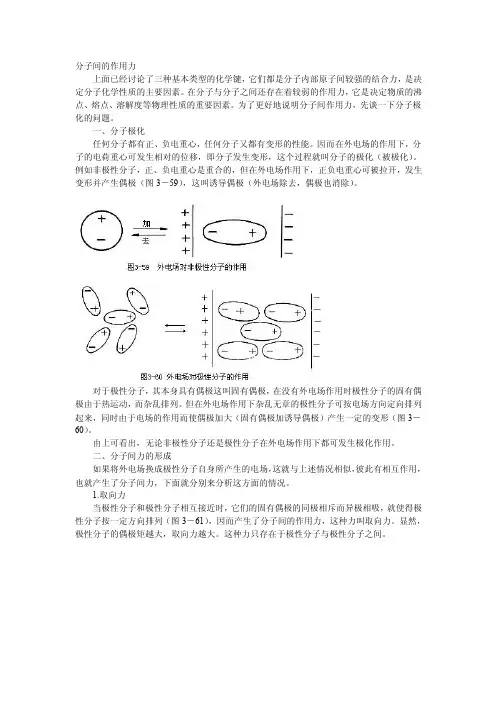
一、分子极化任何分子都有正、负电重心,任何分子又都有变形的性能。
因而在外电场的作用下,分子的电荷重心可发生相对的位移,即分子发生变形,这个过程就叫分子的极化(被极化)。
例如非极性分子,正、负电重心是重合的,但在外电场作用下,正负电重心可被拉开,发生变形并产生偶极(图3-59),这叫诱导偶极(外电场除去,偶极也消除)。
对于极性分子,其本身具有偶极这叫固有偶极,在没有外电场作用时极性分子的固有偶极由于热运动,而杂乱排列。
但在外电场作用下杂乱无章的极性分子可按电场方向定向排列起来,同时由于电场的作用而使偶极加大(固有偶极加诱导偶极)产生一定的变形(图3-60)。
由上可看出,无论非极性分子还是极性分子在外电场作用下都可发生极化作用。
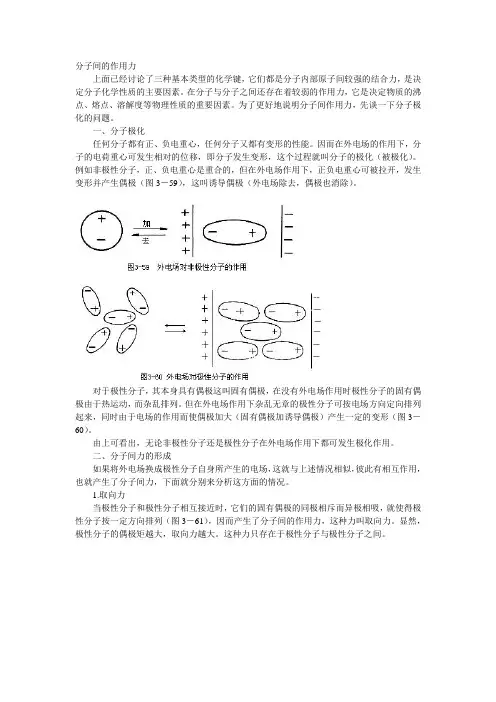
二、分子间力的形成如果将外电场换成极性分子自身所产生的电场,这就与上述情况相似,彼此有相互作用,也就产生了分子间力,下面就分别来分析这方面的情况。
1.取向力当极性分子和极性分子相互接近时,它们的固有偶极的同极相斥而异极相吸,就使得极性分子按一定方向排列(图3-61),因而产生了分子间的作用力,这种力叫取向力。
显然,极性分子的偶极矩越大,取向力越大。
这种力只存在于极性分子与极性分子之间。
2.诱导力当极性分子和非极性分子相接近时,非极性分子在极性分子的固有偶极的作用下,发生极化,而产生诱导偶极,然后诱导偶极与极性分子固有偶极相互吸引(图3——62)。
这种由于诱导偶极而产生的作用力,称为诱导力。
这种力产生于极性分子与非极性分子之间,当然极性分子与极性分子之间也互相诱导,因而也有这种力。
3.色散力非极性分子与非极性分子之间有无作用力?实验指出,N2、O2、H2……等气体,只要充分降温,都可以转变成液态和固态。

分⼦间作⽤⼒简叙分⼦间的静电⼒和van der Waals⼒的异同静电⼒:静电⼒是由极性基团的永久偶极相互作⽤引起的。
这种作⽤⼒取决于极性基团偶极矩的⼤⼩,定向程度,分⼦间的距离和环境温度。
静电⼒的能量范围在12—20KJ/Mol之间。
⼀些极性⾼分⼦聚合物,如聚甲基丙烯酸甲脂、聚⼄烯醇、聚脂等的Van Der Waals⼒为静电⼒。
分⼦间的Van.der.Waals⼒也是静电⼒,只不过它是偶极⼦之间的相互吸引⼒(静电⼒)、诱导偶极性与偶极⼦之间的吸引⼒(诱导⼒)、瞬时偶极性与诱导偶极性之间的作⽤⼒(⾊散⼒)三者的总和⽽范德华⼒包括引⼒和斥⼒,引⼒和距离的6次⽅成反⽐,排斥⼒与距离的12 次⽅成反⽐。
他们都是静电⼒在不同层次的涌现⾼分⼦之间的分⼦间作⽤⼒不仅发⽣在不同的分⼦链之间,还发⽣在于分⼦链相连的基团之间。
同低分⼦物质⼀样,分⼦间的作⽤⼒为低于共价化学键1~2量级的Van Der Waals⼒和氢键⼒。
Van Der Waals⼒不但存在于不同分⼦之间,⽽且存在于同⼀分⼦内的⾮键合原⼦之间。
依照分⼦基团间相互作⽤原理的的不同。
Van Der Waals⼜分为:静电⼒,诱导⼒,⾊散⼒1.静电⼒静电⼒是由极性基团的永久偶极相互作⽤引起的。
这种作⽤⼒取决于极性基团偶极矩的⼤⼩,定向程度,分⼦间的距离和环境温度。
对于偶极矩分别为µ1和µ2,距离为R的两种极性分⼦,温度为T(绝对温度)时,分⼦间的作⽤⼒k为Boltzman常数。
静电⼒的能量范围在12—20KJ/Mol之间。
⼀些极性⾼分⼦聚合物,如聚甲基丙烯酸甲脂、聚⼄烯醇、聚脂等的Van Der Waals⼒为静电⼒。
2.诱导⼒诱导⼒为极性分⼦的永久偶极对其他分⼦诱导,⽣成偶极,并互相吸引所产⽣的作⽤⼒。
对于偶极矩分别为µ1和µ2,分⼦极化率为α1、α2,距离为R的两个分⼦,分⼦间的作⽤⼒3.⾊散⼒⾊散⼒是由于分⼦间由于⾮极性分⼦的正负电荷中⼼瞬间不重合⽽导致的瞬间偶极相互作⽤所产⽣的分⼦作⽤⼒。

分子间作用力——范德华力范德华力是指分子之间的作用力,这种作用力主要是由极性分子之间的电荷分布和氢键作用引起的。
它是最常见的分子间作用力,可以解释许多分子物理和化学特性。
范德华力从来没有被经典物理学所解释,它是由古典力学所忽视的,但是它是促使分子间作用的主要力量。
范德华力的发现是在20世纪30年代的荷兰物理学家Peter D. van der Waals的工作中的。
他发现了一种分子间的调整力,与其其他分子间力不同,这种力被称之为范德华力。
他发现,当分子破坏温和氢键时,一种弱小的但仍然可观测的力量将产生作用。
范德华力是一种范德华弛豫作用。
它是由分子间反应活动引起的,涉及到分子内和分子间能量的调整,这样可以使分子以最低的总能量排列在一起。
由于分子间的排斥力不够大,因此范德华力就发挥作用了。
还有一些其他的因素也可以影响范德华力的大小,比如空间气压。
范德华力是弱的电磁作用,只有在非常小的距离(几个原子半径)内才可以引起作用。
因此,范德华力可以看作是分子的低能状态的关键,它影响分子的结构和性质。
范德华力可以解释许多分子物理和化学特性,比如粘性、表面张力、溶解度等等。
这些特性可以归因于分子间的范德华力。
例如,范德华力使得液体有粘性,因为液体分子之间存在一种粘合作用;表面张力也是由范德华力引起的,当液体分子聚集在一起时,它们之间就会有一种粘合力,从而使表面变得紧密和被张力约束;溶解度也是范德华力的结果,因为溶质的分子在溶剂的分子之间存在一种扩散性的弱电荷,这样就会减小溶质分子的结合能,从而使溶质溶于溶剂。
范德华力也被认为是催化反应中的关键作用力。
催化剂的分子间可能有一种特殊的范德华弛豫作用,可以稳定反应产物,使反应的速率大大提高。
这种作用力可以促进反应的发生,也就是催化的过程。
范德华力也被用于生物物理学中,被认为是凝胶结构形成的原因之一。
一些生物物质,比如蛋白质和糖类,可以通过范德华力形成凝胶样结构,这种结构对于生物体的维持和维护有着很重要的作用。


分子间的作用力上面已经讨论了三种基本类型的化学键,它们都是分子内部原子间较强的结合力,是决定分子化学性质的主要因素。
在分子与分子之间还存在着较弱的作用力,它是决定物质的沸点、熔点、溶解度等物理性质的重要因素。
为了更好地说明分子间作用力,先谈一下分子极化的问题。
一、分子极化任何分子都有正、负电重心,任何分子又都有变形的性能。
因而在外电场的作用下,分子的电荷重心可发生相对的位移,即分子发生变形,这个过程就叫分子的极化(被极化)。
例如非极性分子,正、负电重心是重合的,但在外电场作用下,正负电重心可被拉开,发生变形并产生偶极(图3-59),这叫诱导偶极(外电场除去,偶极也消除)。
对于极性分子,其本身具有偶极这叫固有偶极,在没有外电场作用时极性分子的固有偶极由于热运动,而杂乱排列。
但在外电场作用下杂乱无章的极性分子可按电场方向定向排列起来,同时由于电场的作用而使偶极加大(固有偶极加诱导偶极)产生一定的变形(图3-60)。
由上可看出,无论非极性分子还是极性分子在外电场作用下都可发生极化作用。
二、分子间力的形成如果将外电场换成极性分子自身所产生的电场,这就与上述情况相似,彼此有相互作用,也就产生了分子间力,下面就分别来分析这方面的情况。
1.取向力当极性分子和极性分子相互接近时,它们的固有偶极的同极相斥而异极相吸,就使得极性分子按一定方向排列(图3-61),因而产生了分子间的作用力,这种力叫取向力。
显然,极性分子的偶极矩越大,取向力越大。
这种力只存在于极性分子与极性分子之间。
2.诱导力当极性分子和非极性分子相接近时,非极性分子在极性分子的固有偶极的作用下,发生极化,而产生诱导偶极,然后诱导偶极与极性分子固有偶极相互吸引(图3——62)。
这种由于诱导偶极而产生的作用力,称为诱导力。
这种力产生于极性分子与非极性分子之间,当然极性分子与极性分子之间也互相诱导,因而也有这种力。
3.色散力非极性分子与非极性分子之间有无作用力?实验指出,N2、O2、H2……等气体,只要充分降温,都可以转变成液态和固态。


分子间的作用力
分子间作用力的类型有:氢键、范德华力、卤键。
其中范德华力又可以分为三种作用力:取向力、诱导力和色散力。
极性分子与极性分子之间,取向力、诱导力、色散力都存在。
极性分子与非极性分子之间,则存在诱导力和色散力。
非极性分子与非极性分子之间,则只存在色散力。
(1)取向力:发生在极性分子与极性分子之间。
由于极性分子的电性分布不均匀,一端带正电,一端带负电,形成偶极。
因此,当两个极性分子相互接近时,由于它们偶极的同极相斥,异极相吸,二个分子必将发生相对转动。
这种偶极子的相互转动,就使偶极子的相反的极相对,叫做“取向”。
这种由于极性分子的取向而产生的分子间的作用力,叫做取向力。
(2)诱导力:发生在极性分子与非极性分子之间以及极性分子之间。
在极性分子和非极性分子间,由于极性分子的影响,会使非极性分子的电子云与原子核发生相对位移,产生诱导偶极,与原极性分子的固有偶极相互吸引,这种诱导偶极间产生的作用力叫诱导力。
同样地极性分子间既具有取向力,又具有诱导力。
(3)色散力:当非极性分子相互接近时,由于每个分
子的电子不断运动和原子核的不断振动,经常发生电子云和原子核之间的瞬时相对位移,产生瞬时偶极。
而这种瞬时偶极又会诱导邻近分子也产生和它相吸引的瞬时偶极。
由于瞬时偶极间的不断重复作用,使得分子间始终存在着引力,因其计算公式与光色散公式相似而称为色散力。

分子间作用力(Intermolecular force)Intermolecular forceHelp edit an encyclopediaIntermolecular forceThe intermolecular force, also known as the Fan Dehua force, is, in essence, an electrical attraction, so the origin of the intermolecular force is investigated by studying the electrical and molecular structures of the molecules of matter.See the wonderful AtlasCatalogClassification of intermolecular forcesDistance philosophy from Fan Dehua's forceRelationship with hydrogen bondsFactors affecting intermolecular forceSize and physical propertiesOpenClassification of intermolecular forcesDistance philosophy from Fan Dehua's forceRelationship with hydrogen bondsFactors affecting intermolecular forceSize and physical propertiesOpenEdit this paragraphClassification of intermolecular forcesIntermolecular force refers to the force acting between molecules and molecules or functional groups of molecules within polymers, referred to as intermolecular forces. It mainly consists of:Fan Dehua force: originally proposed in order to correct the Fan Dehua equation. Ubiquitous in solids, liquids, gases, any particles, and inversely proportional to the distance between the six. According to different sources can be divided into: the dispersion force (en:London dispersion force): the electrical attraction between the instantaneous dipole orientation; force (dipole-dipole force): electric dipole attraction between the electrically induced force; induced dipole and dipole attraction between hydrogen bonds: X-H... Y type of force. In addition, novel intermolecular forces have also been reported, including double hydrogen bonds and gold bonds.Definition: Fan Dehua force (also called molecular force) arises from electrostatic interaction between molecules or atoms. The empirical equation for calculation of energy: U =B/r 12- A/r (6 to 2 carbon atoms, the parameter values for B =11.5 * 10-6 kJnm^12/mol * 10-3; A=5.96 kJnm^6/mol; A B, different atoms have different values) when the two atoms are close to each other near the electronic cloud overlap, has strong rejection, rejection force and distance is inversely proportional to the square of 12 times. The low point in the figure is the distance Fan Dehua forces maintain and the maximum force is called the Fan Dehua radius.Vander Ed Ley can be divided into three kinds of forces: induction force, dispersion force and orientation force.Dispersion forceAtomic interior model of particlesDispersion force, also known as the force of London, all molecules or atoms exist. Is the force between the instantaneous dipole, that is due to the movement of electrons, the instant electronic position is the asymmetry of atomic nuclei, i.e. positive charge center and negative charge center instantaneous do not overlap, resulting in instantaneous dipole. The dispersion force and the deformation of the interacting molecules are related. The larger the deformation (the greater the general molecular weight, the greater the deformability), the greater the dispersion force. The dispersion force is related to the ionization potential of the interacting molecules. The lower the ionization potential ofthe molecule (the more electrons there are in the molecule), the greater the dispersion force. The interaction of the dispersion force varies with 1/r6. Its formula is:I1 and I2 are the ionization energies of two interacting molecules, respectively. Alpha 1 and alpha 2 are their polarizability.Inductive forceInductive force (induction, force) has an inductive force between polar molecules and nonpolar molecules, and between polar molecules and polar molecules. Due to the polar molecular dipole generated by the electric field on the non-polar molecules, the nonpolar molecule electron cloud deformation (i.e. the electron cloud is attracted to the polar molecular dipole positive pole), the non polar molecules and electron cloud nuclei occur relative displacement, have non positive and negative focus in charge of polar molecules is coincidence, the relative displacement will no longer coincide, the non-polar molecules produced a dipole. The relative displacement of the charge center is called deformation. The dipole produced by deformation is called the induced dipole, which is distinguished from the intrinsic dipole in the polar molecule. The induced dipole and the intrinsic dipole attract each other, and the force induced by the dipole is called the induced force. In polar molecules and polar molecules, in addition to the orientational force, each molecule deforms and induces an induced dipole due to the interaction of polar molecules. As a result, the dipole distance increases with both the orientational force and the induced force. An induced force isalso found between cations and anions.The induced force is proportional to the square of the dipole moment of the polar molecule. The induced force is proportional to the deformation of the induced molecule. The larger the outer shell of the nucleus in each molecule (the more heavy atoms), the more easily it becomes deformed under the influence of external electrostatic force. The interaction changes with 1/r6, and the induction force is independent of temperature. Its formula:Alpha polarizability.Orientation forceThe orientational force (orientation, force) of the orientational force occurs between the polar molecule and the polar molecule. Because of the uneven distribution of the molecules of the polar molecule, one end is positively charged and one end is negatively charged, forming a dipole. Thus, when the two polar molecules are close to each other,Because of their dipole poles repel, heteropolar, relative rotation of two molecules will occur. The dipole rotates so that the opposing poles of the dipole are called "orientations"". Because the opposite pole is close, very far, the gravity is bigger than the repulsion, two molecules near, when close to a certain distance, attraction and repulsion balance. The intermolecular forces produced by the orientation of polar molecules are called orientational forces. The orientational force is proportional to the square of the dipole moment of themolecule, i.e., the greater the polarity of the molecule, the greater the orientation force. The orientation force is inversely proportional to the absolute temperature. The higher the temperature, the weaker the orientation force and the change of the interaction with the 1/r6. Its formula is:1, mu 2 is the dipole moment of two molecules; R is the distance between the molecular center of mass; K is Boltzmann constant; T is thermodynamic temperature; negative value means energy decrease.The relation of three forcesBetween polar molecules and polar molecules, orientation force, induced force, dispersion force exists; between polar and non-polar molecule, is induced and dispersive; between non polar and non-polar molecules, then there is only the dispersion force. The size of these three types of forces depends on the polarity and deformability of the interacting molecules. The greater the polarity, the more important the orientation force; the greater the deformation, the more important the dispersion force; the induction force is related to these two factors. But for most molecules, dispersion is the main force. Experiments have shown that for most molecules, the dispersion force is the main; only the dipole moment of large molecules (such as water), the orientation force is the main; and the induction force is usually very small. The polarizability alpha reflects whether the electron cloud in the molecule is susceptible to deformation. Although van Edward's force is only 0.4 - 4.0kJ/mol, the interaction between a large number of macromolecules can be very stable. For example, C -H in benzene, van Edward force has 7 kJ/mol, and in lysozyme and sugar bound substrate van, Edward force has 60kJ/mol, van Edward force has additivity.The molecular forces, ionic bonds, salt bonds, and covalent bonds are all electrostatic attraction. Why is the gap so great?So the real keyword is "distance", and we can consider the molecular force and the ionic bond together.Types of action energy and distance relationsElectrostatic interaction of charged groups 1/rIon dipole 1/r2Ion induced dipole 1/r4Dipole dipole 1/r6 alignment force chemistryDipole induced dipole 1/r6 induced forceInduced dipole induced dipole 1/r6 dispersion forceNon - bond exclusive 1/r12 - 1/r6In secondary school, we studied ionic bonds, and the crystal configuration of six typical compounds, NaCl, CsCl, CaF2, cubic ZnS, six party ZnS and rutile TiO2, is a strong force.In biology, the focus is on understanding the ionicinteractions of organic molecules. Ion organic molecules, electronegativity difference is not so large, unlike the interaction of these typical ionic compounds such as ionic bond, so called ion interaction; but they have in common are electrostatically formation.NaCl, CsCl, CaF2 crystal, cubic ZnS, six party ZnS, rutile TiO2 six typical compounds of the ionic bond is an energy and distance is inversely proportional to the square, the interaction between Mg2+ and ATP, the interaction between the zwitterionic amino acids. The ion dipole decreases with the two side of the distance, and the ion induced dipole decreases with the 4 side of the distance. Therefore, the interaction of ions in biological molecules (also called salt bonds) is a weak interaction, and decreases with the 1/r2 - 1/r4.Interaction of ATP with magnesium ionThe van Edward force includes gravity and repulsion, gravity and distance of the 6 side is inversely proportional to the repulsion force and the 12 side of the distance is inversely proportional. They are static electricity, springing up at different levels.Edit this paragraphDistance philosophy from Fan Dehua's forceVan Edward's force is well understood, which is different from the quark's progressive freedom. In Confucius's words, "near is rude, far away."." Between people need to have a certaindistance between the mind, far away will be lonely, need to draw close to each other; near, the contradiction will intensify. For the atom, this paradox is inversely proportional to the 12, and the attraction is inversely proportional to the 6. The distance between atoms can be approximately measured, but the distance between man and mind is not measurable......There is also a safe distance between people. Duncan, a master of American psychology, said: "1.2 meters is the safe distance between people.". Unless it's someone you trust, know or get close to, it's going to make you feel insecure, whether it's talking or other communication. On the street to and fro stream of people, two people, a group, three people, a group of, before and after the distance between each other are mostly kept within a certain distance,It's also a subconscious security precaution; there's one meter safety line in front of the atm. Take care of yourself and you'll find the safe distance is with usEdges are visible everywhere. Some people say that marriage is a siege, the people who do not go in want to go in; people who go in and want to come out. The distance between people is often greater than the life of the "Fan Dehua radius", less trust each other.After entering the marriage besieged city, they longed for each other's heart distance to be closer, became no longer lonely, also was precisely because crossed the humanSelf boundaries will have suspicion, mistrust,misunderstanding, longing for each other to pay more, worry about personal gains and losses, more demanding to each other, and ultimately like quarks and fermions as to the "asymptotic freedom" to constitute a society now divorce. If people are too far away from each other, people will be lonely, unable to rely on, will become no longer trust others, and then be excluded from society. Everyone has to find the inner integrity and keep the right distance from different people. This is the "Fan Dehua radius" of life, which is the philosophy of molecules, and also the philosophy of man.Edit this paragraphRelationship with hydrogen bondsThe essence of hydrogen bonding is the electrostatic attraction between a hydrogen nucleus with a strong polar bond (A-H) and an electronegative electric atom containing an unpaired electron pair and an atom with partial negative charge B. Hydrogen atoms can be combined with 2 electrically electronegative atoms with small atomic radii (such as O, N, F, etc.). In X - H... Y, X, and Y are atoms with large electronegativity, small atomic radii, and electrons with no shared electrons. X H, X has a strong electronegativity, the electron density of X - H bond bias in the X end, and H shows a partial positive charge; another molecule in Y but also a concentration of the electron cloud and significantly negative, it and H with static electricity combined, this is the nature of hydrogen bonds. Therefore, the electrostatic attraction of hydrogen bonds is usually called the Fan Dehua force, and the difference is that it is saturated and directional. This forceis generally below 40kJ/mol, much smaller than the average bond energy.From the point of view of physics and mechanics, it can be divided into gravitation and repulsionGravitation:When an external force tries to stretch an object, a large number of molecules that make up the object will show gravity to resist the pull of the outside.Although there are gaps between molecules, a large number of molecules can cluster together to form solid and liquid, indicating the existence of gravitational attraction between molecules.Solids hold a definite shape to indicate gravitational attraction between molecules.Repulsion:When an external force tries to compress an object, a large number of molecules forming an object will exhibit repulsion to resist the compression of the outside.There are gravitational forces between molecules, but molecules do not stick together, but there are gaps, indicating that there is repulsion between molecules.Edit this paragraphFactors affecting intermolecular forceHydrogen bond, polarity of bond and relative molecular weight. The radius (similar to the ion compound), the greater the radius, the farther the distance, the weaker the ionic bond, the lower the boiling pointEdit this paragraphSize and physical propertiesMaterial composition and structure similarity, the higher relative molecular mass and the Fan Dehua stress is, to overcome the intermolecular force of the material melting and vaporization on the need for more energy, the higher the melting and boiling points. But there are hydrogen bonds of boiling point and melting point of molecular crystals are abnormally high. The distance between gas molecules is large, so the small molecular interactions; liquid and solid, it is proved that intermolecular attraction effect; and the liquid and solid to compression, and that rejection showed in near distance between molecules.The solid is difficult to stretch, intermolecular force performance, so the A of B; the liquidity is not in liquid illustrated with gravitation and repulsion, its reason is the chemical bond effect; C, the gas molecules even without compression or repulsion, but smaller, D that the steel intermolecular voids, the oil overflows from the tube, is the result of external factors, but not on steel. The molecularrepulsion of oil molecules to [1]。

分子之间的作用力一、范德华力(Van der Waals力)范德华力是分子之间的吸引力,分为三种类型:弥散力、取向力和诱导力。
1.弥散力:一组非极性分子(如氢气、氮气和甲烷等)在接近时,由于电子云的瞬态偏移,使得一个分子在一些时刻稍微带有正电荷,而其他分子在该时刻稍微带有负电荷。
这种瞬态的偶极矩引起了分子间的吸引力,称为弥散力。
2.取向力:当带有极性的分子(如HCl和H2O等)接近时,由于其正负电荷分布的非球对称性,会引起一种电荷分布不均匀,从而带来吸引力,称为取向力。
3.诱导力:弥散力和取向力的作用促使分子中的电子云发生重排,并使其产生一个瞬态的极化。
这种极化会影响周围的分子,并导致这些分子发生极化。
这种临时产生的极化又会引起分子之间的再次吸引力,称为诱导力。
范德华力是一种弱的力量,只能在非常近距离时产生影响,只有当分子之间的距离足够近,这种弱吸引力才能起到关键的作用。
二、静电力1.离子-离子相互作用力:这种力是指由于正离子和负离子之间的静电相互作用而引起的力。
2.离子-极性分子之间的相互作用力:这种相互作用是由于一个带正电的离子与一个带有负电部分的极性分子之间的静电引力或斥力造成的。
3.极性分子之间的相互作用力:带有极性部分的两个分子之间的静电相互作用力也会影响它们的相互作用。
静电力是一种强的力,其作用范围比范德华力大得多,能够在分子之间产生较大的影响。
三、氢键氢键是一种特殊的相互作用力,涉及到一个带有部分正电荷(δ+)的氢离子与一个带有负电荷(δ-)的原子间的相互作用。
氢键主要在带有氮、氧或氟原子的分子之间形成,并且可以在分子中产生一个强大的吸引力。
氢键对于决定蛋白质的二级结构、DNA的双螺旋结构等生物大分子的稳定性起着重要的作用。
总结:分子之间的作用力包括范德华力、静电力和氢键。
范德华力是分子之间的吸引力,可以分为弥散力、取向力和诱导力。
静电力是由于带电部分间的相互吸引或排斥引起的力。
氢键是一种特殊的相互作用力,涉及到一个带有部分正电荷的氢离子与一个带有负电荷的原子间的相互作用。
分子间作用力
分子间作用力是分子之间相互作用的力量,它对物质的性质和行为产生重要影响。
这些作用力影响着液体的表面张力、气体的压强、固体的熔点和沸点等物理性质。
在化学反应中,分子间作用力也扮演着重要角色,影响反应速率和产率。
分子间作用力可以分为几种主要类型:范德华力、氢键、离子键和共价键。
范德华力是非极性分子之间的弱作用力,它是由于电子在空间中的不均匀分布而产生的。
氢键是一种特殊的静电相互作用力,它发生在一个电负性较高的氢原子与一个电负性较低的原子之间。
离子键则是由正负电荷之间的相互吸引力产生的。
共价键则是由原子之间共享电子形成的。
这些分子间作用力的强弱决定了物质的性质。
例如,范德华力较弱,因此非极性物质通常具有较低的沸点和熔点。
氢键较强,使得水具有较高的沸点和熔点,以及较大的表面张力。
离子键较强,导致离子晶体具有高熔点,而共价键通常具有较高的强度和熔点。
在化学反应中,分子间作用力也可以影响反应的进行。
例如,在溶剂中,分子间作用力可以使溶质分子离解,促进化学反应的发生。
此外,在催化剂的作用下,分子间作用力可以调节反应的速率和选择性。
总而言之,分子间作用力是决定物质性质和化学反应过程的重要因素,它们的强弱和类型对物质的性质和行为产生重要影响。