ASCE-2014(文献检索)
- 格式:ppt
- 大小:3.75 MB
- 文档页数:28


文献检索报告班级:10地下一班姓名:文树勋学号:10210123一、课题名称:天津地铁的规划及建设情况二、检索分析天津地处华北平原北部,东临渤海,北依燕山,位于海河下游,地跨海河两岸,是北京通往东北、华东地区铁路的交通咽喉和远洋航运的港口,有“河海要冲”和畿辅门户”之称。
对内腹地辽阔,辐射华北、东北、西北13个省市自治区,对外面向东北亚,是中国北方最大的沿海开放城市,是中国经济增长速度最快的几个城市之一。
伴随着经济的飞速发展,人口和车辆的增加,人们的出行方式的改变,现有的城市道路应经满足不了需求,修建地铁成为解决交通问题的金钥匙。
目前,天津规划地铁系统总长度227公里,其中地铁1号线、2号线、3号线为轨道交通骨干线;地铁4号线、5号线、6号线为轨道交通填充线;7号线、8号线为轨道交通外围线;9号线为津滨轻轨(西段)。
为了更详细的掌握天津地铁的规划建设情况,为以后的地下空间规划设计提供资料,对天津地铁的建设进行详细的检索。
需要了解的问题有:天津地铁的规划方案,天津地铁的施工情况,施工方案,技术难点。
三、检索词中文:天津地铁规划设计施工轨道交通地质线路水文施工天津地铁集团地铁站技术管线盾构网络勘察外文: tianjin metro metro engineering programme shield subway tunnel geology foundation pit dewatering construct urban railtransit四、资源检索1、查找本校纸本图书馆藏目录(1)检索途径(字段):所有字段(2)检索式(检索词、字段、检索组配算符构成):地铁and (建设),天津and (地铁),地下轨道交通(3)检中数量: 25条(4)检中结果列举①北京建工京精大房工程建设监理公司,城市轨道交通工程土建监理工作手册[J],1版,北京: 中国建筑工业出版社, 2011②高峰, 梁波, 城市地铁与轻轨工程[M].1版. 北京: 人民交通出版社, 2012③刘洪涛,北京地铁八通线 Bei jing Di tie Ba Tong Xian 勘察设计·施工监理·建设管理[M],1版,北京: 中国建筑工业出版社, 20082、查找中文数据库(1)检索数据库名称:万方数字化系统(2)检索途径:全部(3)检索式:天津地铁and (施工),地下轨道交通规划and (设计)and(施工),天津地铁规划and (建设)(4)检中数量:8(5)检中结果列举①王敏洁;地铁站综合开发与城市设计研究[D];同济大学;2006年②朱小雷;地铁车站高效空间环境的导向性和易识别性设计初探[J];南方建筑;2002③贾洪梅;国内当前地铁车站室内环境设计的方法及发展初探[D];南京林业大学;2006④郑明远.轨道交通时代的城市开发[M].北京:中国铁道出版社,2006.⑤汤青.轨道交通引导合肥市城市空间发展研究[D].合肥:合肥工业大学建筑与艺术学院,2010.3. 查找外文数据库(1)检索数据库名称:ASCE(2)检索途径: all fields(3)检索式: Tianjin and (urban rail transit) , urban rail transit or (subway tunnel geology)(4)检中数量:20(5)检中结果列举(以标准参考文献格式列出结果3个以上):①T. Litman, Evaluating rail transit criticism, Victoria TransportPolicy Institute, October 2004.②J. Richmond, A whole-system approach to evaluating urban transitinvestments, Transport Reviews 21 (2001) 141–179.③ C. Winston, C. Shirley, Alternate route: Toward efficient urbantransportation, Brookings Institution, Washington DC, 1998.④ I. Savage, Management objectives and the causes of mass transitdeficits, Transportation Research 38A (2004) 181–199.五、文献综述天津地铁始于“7047工程”,1970年4月7日,在向中央政府申请拨款建地铁未果的情况下,天津市决定自行筹款以备战通道为目的,以“天津市墙子河改造工程”的名义立项。


主要的英文文献网找一个服务的网站:不过想自己弄的话可以以下网站吧:Academic Research Library (ProQuest)【地址】原界面链接【文献类型】报纸、期刊、全文/部分全文【访问年限】1971-【描述】本数据库为综合性学术期刊数据库,收录2974种综合性期刊和报纸的文摘/索引(内含Peer Reviewed(同行评审)期刊1502种),其中2020种是全文期刊(内含全文延期上网期刊208种),包括SCI收录的核心全文刊189种,SSCI收录的核心全文...Academic Search Complete学术期刊集成全文数据库 (EBSCO)【地址】原界面链接【文献类型】报纸、多出版类型、期刊、全文/部分全文【访问年限】1965-【描述】Academic Search Premier 收录超过8230种出版物,其中3342种为全文专家评审刊。
它为 100 多种期刊提供了可追溯至 1975 年或更早年代的 PDF 过期案卷,并提供了 1000 多个标题的可检索参考文献。
涉及了几乎所有自然科学和社会科学领域,...ACLS人文科学电子图书-学术著作精选【地址】原界面链接【文献类型】全文/部分全文、图书【访问年限】【描述】《ACLS人文科学电子图书-学术著作精选》(ACLS Humanities E-Book Collection, HEB)由美国学术团体协会(American Council of LearnedSocieties, ACLS)提供。
ACLS成立于1919年,是一家非营利机构,与20个学术团体以及超过100家学术出版社合作HEB项...ACM(美国计算机学会)电子期刊及会议录(ACM总站)【地址】原界面链接【文献类型】期刊、全文/部分全文、会议论文【访问年限】【描述】ACM Digital Library数据库收录了美国计算机协会(Association for Computing Machinery)的各种电子期刊、会议录、快报等文献。
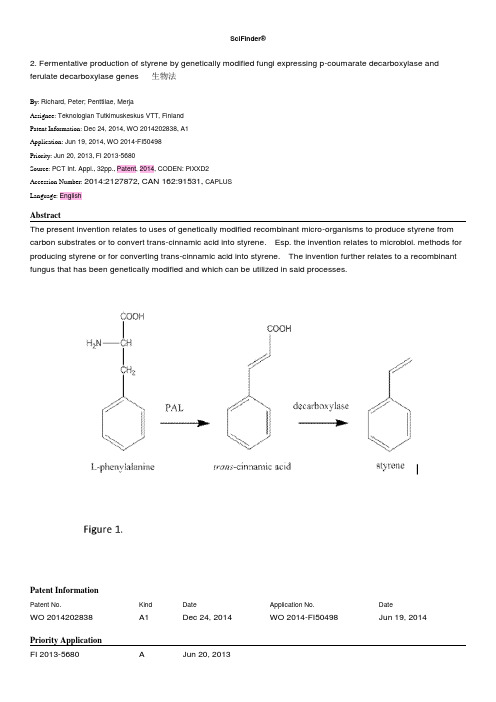
2. Fermentative production of styrene by genetically modified fungi expressing p-coumarate decarboxylase and ferulate decarboxylase genes 生物法By: Richard, Peter; Penttilae, MerjaAssignee: Teknologian Tutkimuskeskus VTT, FinlandPatent Information: Dec 24, 2014, WO 2014202838, A1Application: Jun 19, 2014, WO 2014-FI50498Priority: Jun 20, 2013, FI 2013-5680Source: PCT Int. Appl., 32pp., Patent, 2014, CODEN: PIXXD2Accession Number: 2014:2127872, CAN 162:91531, CAPLUSLanguage: EnglishAbstractThe present invention relates to uses of genetically modified recombinant micro-organisms to produce styrene from carbon substrates or to convert trans-cinnamic acid into styrene. Esp. the invention relates to microbiol. methods for producing styrene or for converting trans-cinnamic acid into styrene. The invention further relates to a recombinant fungus that has been genetically modified and which can be utilized in said processes.Patent InformationPatent No. Kind Date Application No. DateWO 2014202838 A1 Dec 24, 2014 WO 2014-FI50498 Jun 19, 2014Priority ApplicationFI 2013-5680 A Jun 20, 2013IndexingFermentation and Bioindustrial Chemistry (Section 16-5)Section cross-reference(s):10Substances50-99-7D-Glucose, processes140-10-3trans-Cinnamic acid, processes9034-32-6Hemicellulosefermentative prodn. of styrene by genetically modified fungi expressing p-coumarate decarboxylase and ferulate decarboxylase genesBiochemical process; Biological study; Process100-42-5P Styrene, preparationfermentative prodn. of styrene by genetically modified fungi expressing p-coumarate decarboxylase and ferulate decarboxylase genesBioindustrial manufacture; Biological study; Preparation1132-68-94-Fluoro-L-phenylalanine9024-28-6Phenylalanine ammonia-lyase60748-74-5Ferulate decarboxylasefermentative prodn. of styrene by genetically modified fungi expressing p-coumarate decarboxylase and ferulate decarboxylase genesBiological study, unclassified; Biological studySupplementary Termsgenetically modified fungus styrene fermn coumarate ferulate decarboxylase geneCitations1)NIELSEN DAVID ROSS; WO 2012122333 A1 20122)LEYS DAVID; WO 2013186215 A1 20133)Mukai; Journal Of Bioscience And Bioengineering 2010, 109, 5644)Schwarz; Journal Of The Institute Of Brewing 2012, 118, 2805)Curran; Metabolic Engineering 2012, 15, 556)Mckenna; Metabolic Engineering 2011, 13, 5447)Mukai; Journal Of Bioscience And Bioengineering 2014, 118, 5010. Treatment of aromatic hydrocarbon stream to remove oxygenates and olefinsBy: Bender, Timothy P.; Wang, Chong Jhoo; Heeter, Glenn A.; Pilliod, Dana L.Assignee: Exxonmobil Chemical Patents Inc., USAPatent Information: Nov 13, 2014, US 20140336436, A1Application: Mar 21, 2014, US 2014-14221764Priority: May 07, 2013, US 2013-61820286Source: U.S. Pat. Appl. Publ., 9pp., Patent, 2014, CODEN: USXXCOClassifications: US 585821000Accession Number: 2014:1909308, CAN 161:700165, CAPLUSLanguage: EnglishAbstractThe invention relates to a process, comprising: contacting at least one zeolite selected from the MWW family of zeolites with an arom. hydrocarbon stream comprising xylenes, olefins, and oxygenates, in at least one reactor, to produce a product stream from the reactor, wherein at least one condition of contacting in the reactor is adjusted in response to at least one measurement of a system comprising zeolite, arom. hydrocarbon stream, reactor, and product stream, so as to preferentially increase oxygenate removal or to preferentially increase olefin removal from the arom. hydrocarbon stream.Patent InformationPatent No. Kind Date Application No. DateUS 20140336436 A1 Nov 13, 2014 US 2014-14221764 Mar 21, 2014IndexingIndustrial Organic Chemicals, Leather, Fats, and Waxes (Section 45-4)Substances67-56-1Methanol, reactions115-10-6Dimethyl etheralkylating agent; treatment of arom. hydrocarbon stream to remove oxygenates and olefinsSciFinder®Reactant; Reactant or reagent100-42-5P Styrene, preparation108-95-2P Phenol, preparationtreatment of arom. hydrocarbon stream to remove oxygenates and olefinsByproduct; Removal or disposal; Preparation; Process106-42-3DP p-Xylene, polymerstreatment of arom. hydrocarbon stream to remove oxygenates and olefinsIndustrial manufacture; Preparation106-42-3P p-Xylene, preparationtreatment of arom. hydrocarbon stream to remove oxygenates and olefinsIndustrial manufacture; Purification or recovery; Preparation1330-20-7P Xylene, preparationtreatment of arom. hydrocarbon stream to remove oxygenates and olefinsPurification or recovery; Preparation71-43-2Benzene, reactions108-88-3Toluene, reactionstreatment of arom. hydrocarbon stream to remove oxygenates and olefinsReactant; Reactant or reagentSupplementary Termsxylene purifn arom hydrocarbon oxygenate olefin zeolite16. Process for producing styrene from benzene and acetic acid via methyl phenyl ketoneBy: Lange, Jean-PaulAssignee: Shell Internationale Research Maatschappij B.V., Neth.; Shell Oil CompanyPatent Information: Oct 16, 2014, WO 2014167025, A1Application: Apr 09, 2014, WO 2014-EP57205Priority: Apr 12, 2013, EP 2013-163486Source: PCT Int. Appl., 23pp., Patent, 2014, CODEN: PIXXD2Accession Number: 2014:1755052, CAN 161:551321, CAPLUSLanguage: EnglishAbstractThe invention relates to a process for producing styrene, comprising reacting benzene and acetic acid into Me Ph ketone and converting the Me Ph ketone into styrene.Patent InformationPatent No. Kind Date Application No. DateWO 2014167025 A1 Oct 16, 2014 WO 2014-EP57205 Apr 09, 2014Priority ApplicationEP 2013-163486 A Apr 12, 2013IndexingChemistry of Synthetic High Polymers (Section 35-2)Section cross-reference(s):25Substances75-56-9P Propylene oxide, preparationprocess for producing styrene from benzene and acetic acid via Me Ph ketoneByproduct; Preparation100-42-5P Styrene, preparationprocess for producing styrene from benzene and acetic acid via Me Ph ketoneIndustrial manufacture; Preparation98-85-1P Methyl phenyl carbinol98-86-2P Methyl phenyl ketone, preparationprocess for producing styrene from benzene and acetic acid via Me Ph ketoneIndustrial manufacture; Reactant; Preparation; Reactant or reagent64-19-7Acetic acid, reactions71-43-2Benzene, reactions115-07-1Propylene, reactions3071-32-7Ethylbenzene hydroperoxideprocess for producing styrene from benzene and acetic acid via Me Ph ketoneReactant; Reactant or reagentSupplementary Termsbenzene acetic acid methyl phenyl ketone styrene synthesisCitations1)HAWTON MALCOLM JOHN; WO 2008049823 A1 20082)NISBET TIMOTHY MICHAEL; EP 1786796 A1 20073)BUCHANAN J SCOTT; WO 2009082464 A1 20094)Groggins, P; Industrial & Engineering Chemistry, 10.1021/ie50300a024 1934, 26, 13175)Matsushita, Y; Tetrahedron Letters 2004, 45, 472320. Purification of aromatic hydrocarbon streams from olefin impurities in process for producing p-xylene by alkylation of benzene and/or tolueneBy: Heeter, Glenn A.; Ou, John D.; Tinger, Robert G.Assignee: ExxonMobil Chemical Patents Inc., USAPatent Information: Oct 02, 2014, US 20140296598, A1Application: Feb 21, 2014, US 2014-14186482Priority: Apr 01, 2013, US 2013-61807067Source: U.S. Pat. Appl. Publ., 7pp., Patent, 2014, CODEN: USXXCOClassifications: US 585446000Accession Number: 2014:1659634, CAN 161:507012, CAPLUSLanguage: EnglishAbstractThe invention relates to a process for the removal of olefin impurities from an arom. hydrocarbon feed-stream comprising p-xylene in greater than equil. amts. and olefin impurities, by contact of the feed-stream with a solid acid catalyst to produce a product stream comprising reduced olefin impurities, the improvement comprising at least one of the following conditions: (I) reduced bed temp. on startup relative to a predetd. bed temp. at normal operating conditions; and (II) increased flow rate on startup relative to a predetd. flow rate at normal operating conditions.Patent InformationPatent No. Kind Date Application No. DateUS 20140296598 A1 Oct 02, 2014 US 2014-14186482 Feb 21, 2014IndexingIndustrial Organic Chemicals, Leather, Fats, and Waxes (Section 45-4)Section cross-reference(s):25, 67Substances100-42-5P Styrene, preparationpurifn. of arom. hydrocarbon streams from olefin impurities in process for producing p-xylene by alkylation of benzene and/or tolueneByproduct; Removal or disposal; Preparation; Process106-42-3P p-Xylene, preparationpurifn. of arom. hydrocarbon streams from olefin impurities in process for producing p-xylene by alkylation of benzene and/or tolueneIndustrial manufacture; Purification or recovery; Preparation67-56-1Methanol, reactions71-43-2Benzene, reactions108-88-3Toluene, reactions115-10-6Dimethyl etherpurifn. of arom. hydrocarbon streams from olefin impurities in process for producing p-xylene by alkylation of benzene and/or tolueneReactant; Reactant or reagentSupplementary Termstoluene benzene alkylation xylene purifn styrene removal zeolite catalyst29. Method of converting lignin and uses thereof 生化By: Dadi, Ananthram Prasad; Paripati, PraveenAssignee: Suganit Systems, Inc., USAPatent Information: Sep 18, 2014, WO 2014143657, A1Application: Mar 14, 2014, WO 2014-US27374Priority: Mar 15, 2013, US 2013-61799012, Mar 14, 2014, WO 2014-US27374Source: PCT Int. Appl., 39pp., Patent, 2014, CODEN: PIXXD2Accession Number: 2014:1562643, CAN 161:448135, CAPLUSLanguage: EnglishAbstractMethod for creating valuable products from lignocellulosic biomass comprises sequential pretreatment oflignocellulosic biomass with ionic liq. followed by hydrolysis and hydrothermal processing of the lignin. Intermediate steps in conversion of the lignin of lignocellulosic biomass to chems. comprises (a) mixing biomass in an ionic liq. to swell biomass and not dissolve biomass, (b) applying radio frequency (RF) heating to the swelled biomass to heat to a target temp. range, (c) applying ultrasonics, electromagnetic, convective, conductive heating, or combinations thereof, to the swelled biomass to maintain the biomass at target temp. range, (d) washing the treated biomass, and (e) sepg. the cellulosic and lignin fractions.Patent InformationPatent No. Kind Date Application No. DateWO 2014143657 A1 Sep 18, 2014 WO 2014-US27374 Mar 14, 2014CA 2906565 A1 Sep 18, 2014 CA 2014-2906565 Mar 14, 2014Priority ApplicationUS 2013-61799012 P Mar 15, 2013WO 2014-US27374 W Mar 14, 2014IndexingCellulose, Lignin, Paper, and Other Wood Products (Section 43-5)Substances71-43-2P Benzene, preparation82-43-9P83-32-9P Acenaphthene90-05-1P90-12-0P91-10-1P91-20-3P Naphthalene, preparation91-57-6P92-52-4P Biphenyl, preparation93-51-6P95-48-7P , preparation96-48-0P Butyrolactone97-53-0P Eugenol97-54-1P Phenol, 2-methoxy-4-(1-propenyl)98-00-0P2-Furanmethanol100-42-5P Styrene, preparation108-39-4P , preparation108-88-3P Toluene, preparation108-95-2P Phenol, preparation110-82-7P Cyclohexane, preparation120-12-7P Anthracene, preparation120-80-9P Catechol, preparation121-33-5P Vanillin121-34-6P Vanillic acid123-05-7P129-00-0P Pyrene, preparation134-96-3P135-77-3P1,2,4-Trimethoxybenzene191-07-1P Coronene191-24-2P Benzo[ghi]perylene206-44-0P Fluoranthene207-08-9P Benzo[k]fluoranthene259-79-0P Biphenylene306-08-1P494-99-5P3,4-Dimethoxytoluene501-19-9P3-Allyl-6-methoxyphenol544-76-3P Hexadecane605-83-4P610-48-0P616-47-7P624-45-3PSciFinder®645-08-9P3-Hydroxy-4-methoxybenzoic acid767-58-8P Indan, 1-methyl776-99-8P3,4-Dimethoxyphenyl acetone832-69-9P934-00-9P1127-76-0P1195-09-1P2-Methoxy-5-methylphenol1330-20-7P Xylene, preparation1589-47-5P1855-47-6P1-Isopropenylnaphthalene1916-07-0P2033-89-8P2380-78-1P Homovanillyl Alcohol2475-56-1P2478-38-8P2503-46-0P2579-04-6P8-Heptadecene2785-87-7P2785-89-9P3674-69-9P4925-88-6P4-Methyl-2,5-dimethoxybenzaldehyde5077-67-8P1-Hydroxy-2-butanone5888-51-7P6232-48-0P6443-69-2P6627-88-9P Phenol, 2,6-dimethoxy-4-(2-propenyl)7507-89-3P7786-61-0P2-Methoxy-4-vinylphenol14786-82-4P60563-13-5P Ethyl homovanillate376372-00-8P Stigmast-3-ene443678-79-3Pconverting biomass to lignin to chems.Industrial manufacture; Preparation9005-53-2P Lignin, reactionsconverting biomass to lignin to chems.Industrial manufacture; Purification or recovery; Reactant; Preparation; Reactant or reagent 65039-10-31-Allyl-3-methylimidazolium chloride79917-90-11-n-Butyl-3-methylimidazolium chloride125652-55-33-Methyl-N-butylpyridinium chloride143314-17-41-Ethyl-3-methylimidazolium acetate865627-64-11-Ethyl-3-methylimidazolium propionateconverting biomass to lignin to chems.Other use, unclassified; UsesSupplementary Termsbiomass sepn lignin hydrolysis hydrothermal processing org chemCitations1)VARANASI SASIDHAR; US 20080227162 A1 20082)BRADY MICHAEL; US 20110256615 A1 20113)Sasaki; Chemical Engineering And Processing 2007, 47, 16094)Kang; Renewable And Sustainable Energy Reviews 2013, 27, 546Copyright © 2015 American Chemical Society (ACS). All Rights Reserved.32. Method for reducing energy consumption in the production of styrene monomer utilizing azeotropic water/ethylbenzene feed vaporizationBy: Welch, Vincent A.; Oleksy, Slawomir A.Assignee: Stone & Webster Process Technology, Inc., USAPatent Information: Sep 18, 2014, WO 2014142994, A1Application: Mar 15, 2013, WO 2013-US32244Priority: Mar 15, 2013, WO 2013-US32244Source: PCT Int. Appl., 25pp., Patent, 2014, CODEN: PIXXD2Accession Number: 2014:1555275, CAN 161:460121, CAPLUSLanguage: EnglishAbstractThe invention relates to a method for reducing the amt. of steam used in a dehydrogenation section of an alkenyl arom. hydrocarbon prodn. facility, the dehydrogenation section for dehydrogenating ethylbenzene to styrene monomer, the method comprising: (I) heating a feed stream comprising ethylbenzene and water as an azeotrope to provide an ethylbenzene/feed steam stream contg. vaporized ethylbenzene and feed steam having a feed steam to ethylbenzene ratio of 0.4 - 0.6; (II) mixing the ethylbenzene/feed steam stream with heating steam from a steam superheater; (III) supplying the ethylbenzene/feed steam stream and heating steam to a first reactor in the dehydrogenation section; (IV) dehydrogenating the ethylbenzene in the first reactor, a second reactor, and at least a third reactor of the dehydrogenation section to produce styrene monomer; and (V) reheating an effluent from the first reactor in at least a first reheat exchanger and an effluent from the second reactor in at least a second reheat exchanger, wherein each reheat exchanger is provided with heating steam from at least one steam superheater, the heating steam having a temp. of < 899° and the method utilizing a total heating steam to ethylbenzene ratio of < 0.65.Patent InformationPatent No. Kind Date Application No. DateWO 2014142994 A1 Sep 18, 2014 WO 2013-US32244 Mar 15, 2013Priority ApplicationWO 2013-US32244 Mar 15, 2013IndexingChemistry of Synthetic High Polymers (Section 35-2)Section cross-reference(s):48Substances37246-01-8304H60616-02-6Incoloy 800H130572-98-4Incoloy 800HTdehydrogenation reactor constructed from; method for reducing energy consumption in prodn. of styrene monomer utilizing azeotropic water/ethylbenzene feed vaporizationTechnical or engineered material use; Uses100-42-5P Styrene, preparationmethod for reducing energy consumption in prodn. of styrene monomer utilizing azeotropic water/ethylbenzene feed vaporizationIndustrial manufacture; Preparation100-41-4Ethylbenzene, reactionsmethod for reducing energy consumption in prodn. of styrene monomer utilizing azeotropic water/ethylbenzene feed vaporizationReactant; Reactant or reagentSupplementary Termsethylbenzene dehydrogenation styrene azeotropic vaporizer energy consumptionCitations1)GAMI AJAYKUMAR CHANDRAVADAN; US 20120149960 A1 20122)WILCOX RICHARD J; US 8163971 B2 20123)MERRILL JAMES; US 20110245561 A1 20114)WELCH VINCENT A; US 8084660 B2 20115)WHITTLE LESLIE F; US 4695664 A 19876)SARDINA HELION H; US 4628136 A 1986Copyright © 2015 American Chemical Society (ACS). All Rights Reserved.41. Selective hydrogenation of styrene to ethylbenzeneBy: Chen, Tan-Jen; Ou, John Di-Yi; Abichandani, Jeevan S.; Heeter, Glenn AllenAssignee: ExxonMobil Chemical Patents Inc., USAPatent Information: Aug 07, 2014, US 20140221710, A1Application: Jan 27, 2014, US 2014-14165255Priority: Feb 06, 2013, US 2013-61761402Source: U.S. Pat. Appl. Publ., 4pp., Patent, 2014, CODEN: USXXCOClassifications: US 585254000Accession Number: 2014:1289184, CAN 161:320833, CAPLUSLanguage: EnglishAbstractThe invention relates to the selective hydrogenation of styrene to ethylbenzene and more particularly to the removal of small quantities of styrene present in the product stream of a method of making para-xylene selectively by the alkylation of arom. species with an alkylating agent over a solid catalyst. A feedstream comprising para-xylene and styrene is contacted, in the presence of hydrogen, with a catalyst comprising at least one metal, selected from one or more metals selected from Groups 8-10.Patent InformationPatent No. Kind Date Application No. DateUS 20140221710 A1 Aug 07, 2014 US 2014-14165255 Jan 27, 2014IndexingBenzene, Its Derivatives, and Condensed Benzenoid Compounds (Section 25-2)Substances1344-28-1Aluminum oxide, uses7440-02-0Nickel, uses7440-05-3Palladium, uses7440-18-8Ruthenium, uses7440-22-4Silver, uses7440-44-0Carbon, uses7440-48-4Cobalt, uses7440-57-5Gold, uses7440-74-6Indium, uses7631-86-9Silica, uses13463-67-7Titanium oxide, usesselective hydrogenation of styrene to ethylbenzeneCatalyst use; Uses71-43-2Benzene, reactionsselective hydrogenation of styrene to ethylbenzeneReactant; Reactant or reagent100-42-5P Styrene, preparation108-88-3P Toluene, preparationselective hydrogenation of styrene to ethylbenzeneReactant; Synthetic preparation; Preparation; Reactant or reagent95-47-6P o-Xylene, preparation100-41-4P Ethylbenzene, preparation106-42-3P p-Xylene, preparation108-38-3P m-Xylene, preparation108-95-2P Phenol, preparationselective hydrogenation of styrene to ethylbenzeneSynthetic preparation; PreparationSupplementary Termsstyrene selective hydrogenation catalyst ethylbenzene prepn54. Method for reducing energy consumption in a process to purify styrene monomerBy: Welch, VincentAssignee: Stone & Webster Process Technology, Inc., USAPatent Information: Jun 26, 2014, WO 2014098816, A1Application: Dec 19, 2012, WO 2012-US70494Priority: Dec 19, 2012, WO 2012-US70494Source: PCT Int. Appl., 30pp., Patent, 2014, CODEN: PIXXD2Accession Number: 2014:1046536, CAN 161:112526, CAPLUSLanguage: EnglishAbstractAn energy conservation process directed to the purifn. of styrene monomer via distn. after the dehydrogenation reaction of ethylbenzene to produce crude styrene is disclosed. As practiced today, the purifn. of styrene via distn. requires large amt. energy (i.e., steam) to provide heat to the various distn. columns. The presently disclosed improved process allows for a redn. in the amt. of steam needed for this purpose.Patent InformationPatent No. Kind Date Application No. DateWO 2014098816 A1 Jun 26, 2014 WO 2012-US70494 Dec 19, 2012 KR 2015088892 A Aug 03, 2015 KR 2015-7017374 Dec 19, 2012 CN 104903280 A Sep 09, 2015 CN 2012-80077795 Dec 19, 2012IndexingIndustrial Organic Chemicals, Leather, Fats, and Waxes (Section 45-4)Section cross-reference(s):25Substances100-42-5P Styrene, preparationmethod for reducing energy consumption in process to purify styrene monomerPurification or recovery; Preparation71-43-2Benzene, processes98-83-9 -Methylstyrene, processes100-41-4Ethylbenzene, processes108-88-3Toluene, processesmethod for reducing energy consumption in process to purify styrene monomerRemoval or disposal; ProcessSupplementary Termsenergy consumption conservation redn styrene monomer column distn purifnCitations1)WELCH VINCENT A; US 6171449 B1 20012)ART BILLIE E; US 6096941 A 20003)OLEKSY SLAWOMIR A; US 20100111785 A1 20104)GRAFF RICHARD R DE; US 3558729 A 19715)KEIL THOMAS; US 5386075 A 19956)Gheshlaghi; American Journal Of Advanced Scientific Research 2012, 1, 13757. Preparation of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenationBy: Bunquin, Jeffrey Camacho; Stryker, Jeffrey MarkAssignee: Governors of the University of Alberta, Can.Patent Information: Jun 26, 2014, US 20140179954, A1Application: Dec 21, 2012, US 2012-13725595Priority: Dec 21, 2012, US 2012-13725595Source: U.S. Pat. Appl. Publ., 17pp., Patent, 2014, CODEN: USXXCOClassifications: US 568630000Accession Number: 2014:1037632, CAN 161:155939, CAPLUSLanguage: EnglishAbstractPhosphoranimide-metal catalysts and their role in C-O bond hydrogenolysis and hydrodeoxygenation are disclosed. The catalysts comprise of first row transition metals such as nickel, cobalt and iron. The catalysts have a metal to anionic phosphoranimide ratio of 1:1 and catalyze C-O bond hydrogenolysis of a range of oxygen-contg. org. compds.under lower temp. and pressure conditions than those commonly used in industrial hydrodeoxygenation. For example, styrene oxide nickel-catalyzed underwent hydrodeoxygenation to give styrene.Patent InformationPatent No. Kind Date Application No. DateUS 20140179954 A1 Jun 26, 2014 US 2012-13725595 Dec 21, 2012US 9051229 B2 Jun 09, 2015IndexingBenzene, Its Derivatives, and Condensed Benzenoid Compounds (Section 25-2)Section cross-reference(s):67, 78Substances1429325-02-9P1429325-04-1P1613520-28-7P1614254-20-4Pprepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds. Catalyst use; Synthetic preparation; Preparation; Uses60-29-7Ether, reactions93-04-92-Methoxynaphthalene96-09-3Styrene oxide101-84-8Diphenyl Ether132-64-9Dibenzofuran271-89-6Benzofuran286-99-7Cyclododecene Oxide613-37-64-Methoxybiphenyl937-30-4946-80-5Benzyl phenyl Ether6485-40-1R-Carvone7111-67-313118-91-71,3-Bis(4-methoxyphenoxy)benzene24771-62-83,4-Diphenyl-2-cyclopentenone69249-21-4Lithium tri-tert-butylphosphinimide1429325-01-8prepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds. Reactant; Reactant or reagent1333-74-0Hydrogen, reactions7646-79-9Cobalt dichloride, reactions28923-39-999611-53-7Dibromo(1,2-dimethoxyethane)ironprepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds.SciFinder®Reagent; Reactant or reagent71-43-2P Benzene, preparation90-43-7P Biphenyl-2-ol91-20-3P Naphthalene, preparation92-52-4P Biphenyl, preparation92-69-3P Biphenyl-4-ol100-42-5P Styrene, preparation100-66-3P Methoxybenzene, preparation105-05-5P1,4-Diethylbenzene108-95-2P Phenol, preparation135-19-3P2-Naphthalenol, preparation138-86-3P4-Isopropenyl-1-methylcyclohex-1-ene150-76-5P4-Methoxyphenol792-68-7P1,2-Dibenzylbenzene1501-82-2P Cyclododecene1655-69-2P4-Methoxydiphenyl ether3454-07-7P4-Ethylstyrene18457-38-0P20357-70-4P24018-98-2P1-(4-Methoxyphenoxy)-3-phenoxybenzene35115-46-9P1,5-Diphenyl-1-cyclopentene74753-01-8P1,5-Diphenylcyclopentadiene173370-68-8P2,3-Di(4-ethylphenyl)butane1616251-16-1Pprepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds. Synthetic preparation; PreparationSupplementary Termsether hydrodeoxygenation nickel cobalt; cycloalkene arom hydrocarbon prepn; nickel cobalt hydrodeoxygenation catalystCopyright © 2015 American Chemical Society (ACS). All Rights Reserved.59. Preparation of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenationBy: Bunquin, Jeffrey Camacho; Stryker, Jeffrey MarkAssignee: Governors of the University of Alberta, Can.Patent Information: Jun 21, 2014, CA 2799356, A1Application: Dec 21, 2012, CA 2012-2799356Priority: Dec 21, 2012, CA 2012-2799356Source: Can. Pat. Appl., 45pp., Patent, 2014, CODEN: CPXXEBSciFinder®Accession Number: 2014:1031537, CAN 161:155937, CAPLUSLanguage: EnglishAbstractPhosphoranimide-metal catalysts and their role in C-O bond hydrogenolysis and hydrodeoxygenation are disclosed. The catalysts comprise of first row transition metals such as nickel, cobalt and iron. The catalysts have a metal to anionic phosphoranimide ratio of 1:1 and catalyze C-O bond hydrogenolysis of a range of oxygen-contg. org. compds. under lower temp. and pressure conditions than those commonly used in industrial hydrodeoxygenation. For example, styrene oxide nickel-catalyzed underwent hydrodeoxygenation to give styrene.Patent InformationPatent No. Kind Date Application No. DateCA 2799356 A1 Jun 21, 2014 CA 2012-2799356 Dec 21, 2012IndexingBenzene, Its Derivatives, and Condensed Benzenoid Compounds (Section 25-2)Section cross-reference(s):67, 78Substances1429325-02-9P1429325-04-1P1613520-28-7P1614254-20-4Pprepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds. Catalyst use; Synthetic preparation; Preparation; Uses60-29-7Ether, reactions93-04-92-Methoxynaphthalene96-09-3Styrene oxide101-84-8Diphenyl Ether132-64-9Dibenzofuran271-89-6Benzofuran286-99-7Cyclododecene Oxide613-37-64-Methoxybiphenyl937-30-4946-80-5Benzyl phenyl Ether6485-40-1R-Carvone7111-67-313118-91-71,3-Bis(4-methoxyphenoxy)benzene24771-62-83,4-Diphenyl-2-cyclopentenone69249-21-4Lithium tri-tert-butylphosphinimide1429325-01-8prepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds. Reactant; Reactant or reagent1333-74-0Hydrogen, reactions7646-79-9Cobalt dichloride, reactions28923-39-999611-53-7Dibromo(1,2-dimethoxyethane)ironprepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds. Reagent; Reactant or reagent71-43-2P Benzene, preparation90-43-7P Biphenyl-2-ol91-20-3P Naphthalene, preparation92-52-4P Biphenyl, preparation92-69-3P Biphenyl-4-ol100-42-5P Styrene, preparation100-66-3P Methoxybenzene, preparation105-05-5P1,4-Diethylbenzene108-95-2P Phenol, preparation135-19-3P2-Naphthalenol, preparation138-86-3P4-Isopropenyl-1-methylcyclohex-1-ene150-76-5P4-Methoxyphenol792-68-7P1,2-Dibenzylbenzene1501-82-2P Cyclododecene1655-69-2P4-Methoxydiphenyl ether3454-07-7P4-Ethylstyrene20357-70-4P24018-98-2P1-(4-Methoxyphenoxy)-3-phenoxybenzene35115-46-9P1,5-Diphenyl-1-cyclopentene74753-01-8P1,5-Diphenylcyclopentadiene173370-68-8P2,3-Di(4-ethylphenyl)butane1616251-16-1Pprepn. of transition metal catalysts for C-O hydrogenolysis and hydrodeoxygenation of oxygen-contg. org. compds. Synthetic preparation; PreparationSupplementary Termsether hydrodeoxygenation nickel cobalt; cycloalkene arom hydrocarbon prepn; nickel cobalt hydrodeoxygenation catalystCopyright © 2015 American Chemical Society (ACS). All Rights Reserved.61. Reactor for carrying out an autothermal gas-phase dehydrogenationBy: Olbert, Gerhard; Tellaeche Herranz, Carlos; Asprion, Norbert; Weck, Alexander; Dahlhoff, EllenAssignee: BASF SE, GermanyPatent Information: Jun 19, 2014, US 20140171709, A1。

图书馆开通试用SCI、EI、ASCE及ASME等四个西文数据库应广大读者要求,图书馆积极联系数据库商。
自9月23日起逐步开通了SCI、EI、ASCE 及ASME四个西文数据库。
1.Web of Science(SCI/SSCI)数据库试用期2014年9月23日——10月23日。
Web of Science是获取全球学术信息的重要数据库,它收录了全球12,400多种权威的、高影响力的学术期刊,内容涵盖自然科学、工程技术、生物医学、社会科学、艺术与人文等领域。
Web of Science拥有严格的筛选机制,其依据文献计量学中的布拉德福定律,只收录各学科领域中的重要学术期刊。
2.美国工程索引Ei试用期2014年9月23日——。
Ei Compendex(1884年-):对应的印刷版检索刊为《工程索引》,是目前最常用的文摘数据库之一,侧重于工程技术领域的文献的报道,涉及核技术、生物工程、交通运输、化学和工艺工程、照明和光学技术、农业工程和食品技术、计算机和数据处理、应用物理、电子和通信、控制工程、土木工程、机械工程、材料工程、石油、宇航、汽车工程以及这些领域的子学科。
其数据来源于5100种工程类期刊、会议论文集和技术报告。
每周更新。
3. ASCE(美国土木工程师协会)数据库试用期:2014年10月21日——11月21日ASCE(美国土木工程师协会)数据库:侧重于土木工程领域的文献的报道,涉及交通运输、地质工程、工程管理、建筑材料、城市规划,适用于建筑工程学院(结构工程、岩土工程、防护工程、给排水)能源与动力工程学院(供热、供燃气、通风及空调工程)。
其期刊34种,1983年至今(其中26种被JCR收录)会议录380多卷,1996年至今共约9万篇文献。
4. ASME(美国机械工程师协会)数据库试用期:2014年10月21日——11月21日ASCE(美国土木工程师协会)数据库:侧重于力学、热力学、机械工程领域的文献的报道,涉及动力工程、仪器设备、信息处理、材料技术、生物机械工程,适用于自动化工程学院(控制工程)能源与动力工程学院(动力工程和工程热物理)。
文献检索报告一、课题名称:PLC二、检索分析:可编程逻辑控制器采用一类可编程的存储器,用于其内部存储程序,执行逻辑运算、顺序控制、定时、计数与算术操作等面向用户的指令,并通过数字或模拟式输入/输出控制各种类型的机械或生产过程。
三、检索词:中文:可编程逻辑控制器,可编程逻辑控制器,可编程控制器件外文:Programmable Logic Controller,PLC四、资源检索1、查找本校纸本图书馆藏目录(1)检索途径:关键词(2)检索式:关键词=PLC(3)检中数量:378条(4)检中结果列举:①张凤珊.电气控制及可编程序控制器.2版 [M].北京: 中国轻工业出版社,2003.②刘增良,刘国亭.电气工程CAD [M].北京: 中国水利水电出版社,2002.③史国生.电气控制与可编程控制器技术 [M].北京: 化学工业出版社,2003.④马志溪.电气工程设计 [M].北京: 机械工业出版社,2002.2、查找中文数据库(1)检索数据库名称:万方数字化系统(2)检索途径:关键词(3)检索式:关键词=PLC(4)检中数量:95084条(5)检中结果列举:①郭昌荣.FX系列PLC的链接通信及VB图形监控[M].北京:北京航空航天大学出版社,2008.②魏艳君.多功能屋面SP板切割机 [J].机电一体化,2002.③三菱微型可编程控制器手册 [M].MITSUBISHI SOCIO-TECH,2003.4. 查找外文数据库(1)检索数据库名称:ASCE(美国土木工程师协会)(2)检索途径:关键词(3)检索式:关键词=PLC(4)检中数量:292条(5)检中结果列举:①Rizzi E,Hahner P.[J].International Journal of Plasticity,2004.121.②Cottrell A H. Doloeatiorns and Plastic Flow in Crystals[M].Oxford:Oxford University Press,1953.134.③Pink E,Grinberg A.[J].Acta Metallurgica,1982.2153.五、文献综述在工业生产过程中,大量的开关量顺序控制,它按照逻辑条件进行顺序动作,并按照逻辑关系进行连锁保护动作的控制离散量的数据采集。