2014年大陆DRAM消化102亿美金全球份额20%
- 格式:doc
- 大小:2.88 KB
- 文档页数:1
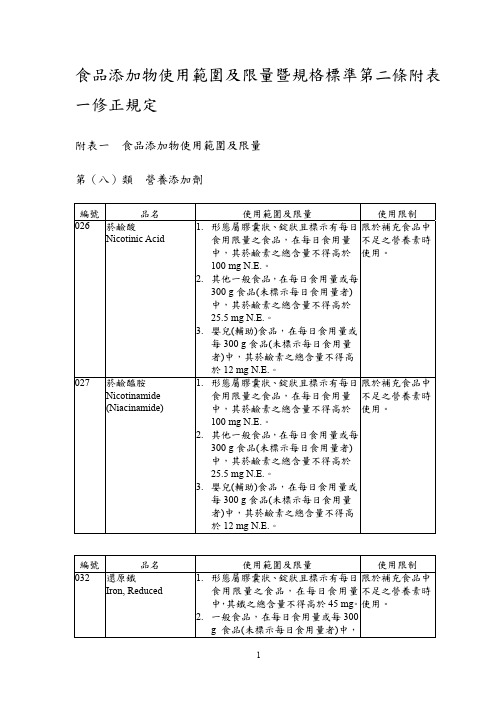


川楝素结构式
川楝素(Taxifolin)是一种天然的化合物,具有丰富的药理活性和保健功能。
它的结构式可以用化学式(C15H12O7)表示,由15个碳原子、12个氢原子和7个氧原子组成。
川楝素的结构中,有三个苯环相互连接,形成一个稳定的分子结构。
这种结构使得川楝素具有很强的抗氧化活性,能有效清除人体内的自由基,减缓细胞组织的氧化损伤。
因此,川楝素常被用作天然抗氧化剂,有助于预防和治疗多种慢性疾病。
川楝素还具有抗炎活性,可抑制炎症介质的释放和炎症反应的发生。
研究表明,川楝素能够抑制一系列炎症相关分子的表达,如肿瘤坏死因子α、白细胞介素-1β等。
对于一些炎症性疾病,如类风湿性关节炎和炎症性肠病,川楝素具有显著的治疗作用。
此外,川楝素还展现出抗肿瘤活性。
研究发现,川楝素能够抑制肿瘤细胞的生长和扩散,并诱导肿瘤细胞凋亡。
它还能够抑制血管生成,阻止肿瘤细胞的血液供应,因此具有潜在的抗肿瘤作用。
最近的研究还发现,川楝素对心血管健康有益。
川楝素能够降低胆固醇水平,保护心脏免受动脉粥样硬化的损害。
它还具有抗血小板聚集和降压的作用,有助于预防心脑血管疾病。
总的来说,川楝素是一种天然的化合物,具有多种药理活性和保健功能。
它具有抗氧化、抗炎、抗肿瘤和心血管保护作用。
因此,川
楝素被广泛应用于药物研发和保健品制造,对于人类健康具有重要的指导意义。
未来的研究还需进一步深入挖掘川楝素的潜力,发现更多的应用领域,为人们提供更好的健康保障。


离子通道病所致的心源性猝死与死后基因检测技术官大威;赵锐【摘要】心脏疾病引起的猝死占人类各类疾病所致猝死的首位.多数心源性猝死案例通过尸体解剖、病理组织学检验可以明确死因为心源性疾病,但尚有少数案例虽经过详细检验并高度怀疑为心源性猝死,但仍不能明确检测到可说明死因的心脏疾病.随着现代分子生物学技术的进步,发现此类猝死者中相当一部分属于先天性心肌细胞离子通道疾病所致,主要包括Brugada综合征、长QT综合征、儿茶酚胺敏感性多形性室性心动过速、短QT综合征等.本文对此类疾病的分子遗传学、心电图所见、临床表现和猝死机制以及死后基因检测技术在死因鉴定中的作用进行了详细的阐述,以期为法医学实践中先天性心肌细胞离子通道疾病所致猝死原因的鉴定提供指导.【期刊名称】《法医学杂志》【年(卷),期】2010(026)002【总页数】8页(P120-127)【关键词】法医病理学;猝死;心脏;综述[文献类型];离子通道病;基因检测【作者】官大威;赵锐【作者单位】中国医科大学,法医学院法医病理学教研室,辽宁,沈阳,110001;中国医科大学,法医学院法医病理学教研室,辽宁,沈阳,110001【正文语种】中文【中图分类】DF795.1心源性猝死(sudden cardiac death,SCD)是指因心脏自身疾病而导致的猝死,是猝死案例中,同时也是心血管疾病猝死案例中最常见的一种类型。
其中以缺血性心脏病,尤其冠状动脉粥样硬化性心脏病(冠心病)导致的猝死占大多数,其他较为常见的原因还包括心肌病、心肌炎等。
近年研究表明,在发达国家,如美国每年SCD的发生率为30/10万~200/10万,每年死亡人数约33.5~35万人[1-2]。
我国目前尚无较为系统的SCD调查统计研究报告,但个别调查显示,在我国冠心病猝死发生率,男性为10.5/10万,女性为3/10万~6/10万。
随着人民生活水平的不断提高,冠心病的发病率不断增高,而且年轻人发生冠心病的比例有所增加,但冠心病所致的死亡仍主要见于中老年人群。
Title Originator Highest Dev Status 111In-capromab pendetide Cytogen Corp Launched111In-imciromab pentetate Janssen Biotech Inc Launched131I-chTNT-1/B Peregrine Pharmaceuticals Inc Launched131I-metuximab Fourth Military Medical University PLA Launchedbrentuximab vedotin Seattle Genetics Inc Launchedgemtuzumab Wyeth Research Launchedibritumomab tiuxetan IDEC Pharmaceuticals Corp Launchedtrastuzumab emtansine Genentech Inc LaunchedATL-101, ATLAB Cornell University Phase 3 Clinical inotuzumab ozogamicin Wyeth Research Phase 3 Clinical oportuzumab monatox (intratumoral, head and neck cancer), Viventia University of Zurich Phase 3 ClinicalRIGS CC49Navidea Biopharmaceuticals Inc Phase 3 ClinicalABT-414Abbott Laboratories Phase 2 ClinicalCDX-1401Celldex Therapeutics Inc (pre-merger)Phase 2 Clinical glembatumumab vedotin CuraGen Corp Phase 2 ClinicalLMB-2National Cancer Institute Phase 2 Clinical lorvotuzumab mertansine ImmunoGen Inc Phase 2 Clinical moxetumomab pasudotox National Cancer Institute Phase 2 Clinical oportuzumab monatox (intravesicular, bladder cancer), Viventia University of Zurich Phase 2 ClinicalPSMA-ADC Cytogen Corp Phase 2 ClinicalRG-7593Genentech Inc Phase 2 ClinicalRG-7596Genentech Inc Phase 2 ClinicalSAR-3419ImmunoGen Inc Phase 2 Clinical212-Pb-TCMC-trastuzumab National Cancer Institute Phase 1 ClinicalActimab-A PDL BioPharma Inc Phase 1 ClinicalAGS-16M8F Agensys Inc Phase 1 Clinicalanti-CD3/anti-CD20 bispecific antibody-armed activated T-cells (non-Hodgkin's lymphoma), Wayne State University/Barbara Ann KarmanosCancer Institute Barbara Ann Karmanos Cancer Institute Phase 1 ClinicalASG-5ME Agensys Inc Phase 1 ClinicalBAY-79-4620MorphoSys AG Phase 1 Clinical citatuzumab bogatox Viventia Biotech Inc Phase 1 Clinical doxorubicin-loaded anti-EGFR immunoliposomes (solid tumors), UniversityHospital Basel University Hospital of Basel Phase 1 ClinicalHuM195/rGel (intravenous infusion, AML/CML/meylodisplastic syndrome),Targa Therapeutics Memorial Sloan-Kettering Cancer Center Phase 1 ClinicalIMGN-529ImmunoGen Inc Phase 1 ClinicalIMGN-853ImmunoGen Inc Phase 1 ClinicalIMMU-132Immunomedics Inc Phase 1 Clinical labetuzumab-SN-38Immunomedics Inc Phase 1 ClinicalNHS-IL-12National Cancer Institute Phase 1 ClinicalRG-7450Genentech Inc Phase 1 ClinicalRG-7458Genentech Inc Phase 1 Clinical RG-7598Genentech Inc Phase 1 Clinical RG-7599Genentech Inc Phase 1 Clinical RG-7600Genentech Inc Phase 1 Clinical RG-7636Genentech Inc Phase 1 Clinical SAR-566658ImmunoGen Inc Phase 1 Clinical T-Guard University Medical Center St Radboud Phase 1 Clinical vorsetuzumab mafodotin Seattle Genetics Inc Phase 1 Clinical 131I-catuximab (colorectal cancer), Pacific Meinuoke Fourth Military Medical University PLA Discovery177Lu-tetraxetan-tetulomab (non-Hodgkin's lymphoma), Nordic Nanovector Nordic Nanovector AS Discovery227Th-epratuzumab (hematological cancer), Algeta Algeta ASA Discovery227Th-rituximab (cancer), Algeta Algeta ASA Discovery227Th-trastuzumab (cancer), Algeta Algeta ASA Discovery4s3-0014s3 Bioscience Inc Discovery4s3-0024s3 Bioscience Inc Discovery64Cu-NOTA-ALT-836Altor BioScience Corp DiscoveryAA-A225Actinium Pharmaceuticals Inc Discovery AbGn-107AbGenomics Corp Discovery Actimab-B Fred Hutchinson Cancer Research Center Discovery Actimab-C Actinium Pharmaceuticals Inc Discovery Actimab-P Actinium Pharmaceuticals Inc Discovery adalimumab + anti-Ang2 Zybody (rheumatoid arthritis/inflammatory boweldisease), Zyngenia Zyngenia Inc DiscoveryAGS-15E ADC Agensys Inc DiscoveryAGT-160ArmaGen Technologies Inc DiscoveryAGT-185ArmaGen Technologies Inc DiscoveryAGT-190ArmaGen Technologies Inc Discovery amanitin-trastuzumab conjugate (cancer), Heidelberg Pharma Heidelberg Pharma Holding Ltd Discoveryanti-CD133-immunotoxin conjugates (photochemical internalization, cancer),PCI Biotech PCI Biotech Holding ASA Discoveryanti-ET8R-MC-vc-PAB-MMAE Genentech Inc Discoveryanti-NaPi3b antibody-drug conjugate (cancer), Genentech/Roche Genentech Inc Discovery antibody drug conjugates (cancer), Sanofi Sanofi Discovery antibody-drug conjugates (cancer), ADC Therapeutics ADC Therapeutics Sarl Discovery antibody-drug conjugates (cancer), Seattle Genetics/Oxford BioTherapeutics Seattle Genetics Inc Discovery antibody-drug conjugates (TAP, cancer), Lilly Eli Lilly & Co Discovery antibody-IFN lambda conjugates (cancer), Immunomedics Immunomedics Inc Discovery anticancer therapy (TAP technology), Amgen Amgen Inc DiscoveryAPH-0912Aphios Corp Discovery Aurixin BioIntegrator DiscoveryAZ-05Allozyne Inc Discovery BIOO-1BIOO Therapeutics DiscoveryBIOO-2BIOO Therapeutics Discovery BIOO-3BIOO Therapeutics Discovery BIOO-4BIOO Therapeutics Discovery BIOO-5BIOO Therapeutics Discovery BIOO-6BIOO Therapeutics Discovery BIOO-7BIOO Therapeutics Discovery botulinum toxin B inhibitor (injectable, heteropolymer mAbs, botulism),Immunome Immunome Inc Discovery BT-2111biOasis Technologies Inc Discovery C2-2b-2b Immunomedics Inc Discovery CDX-014CuraGen Corp Discovery chiHEA-125-Ama Heidelberg Pharma Holding Ltd Discovery CK-22-(20)-(20)Immunomedics Inc Discovery complement factor H-derived short consensus repeat-antibody constructs(infection), LysoVac University of Innsbruck Discovery Cymac-001Cytoguide ApS Discovery CYP-Ab Cytune Pharma Discovery D2C7-based immunotoxins (glioma), Duke University Duke University Discovery EGFR modulators (antibody conjugates, PIT, cancer), Aspyrian Aspyrian Therapeutics Inc Discovery engineered cysteine drug conjugates mAbs (cancer), Seattle Genetics Seattle Genetics Inc Discovery epratuzumab-SN-38Immunomedics Inc Discovery ETBs (cancer), Molecular Templates/ Imclone Molecular Templates Inc Discovery Fluorescent-labeled bevacizumab (imaging, ocular disease), Mivenion mivenion Gmbh Discovery gemcitabine + paclitaxel (prodrug, nanomAb, cancer), ImmunePharmaceuticals Immune Pharmaceuticals Corp Discovery Herceptin:Endostatin-P125A University of Miami Discovery hLL1-CL2A-SN-38Immunomedics Inc Discovery hPAM4-CL2A-SN-38Immunomedics Inc Discovery human monoclonal antibody-toxin conjugates (myocardial infarction), Celdara Celdara Medical LLC Discovery HuMax-TF-ADC Genmab A/S Discovery IFNalpha-fused mAbs (HBV infection), Roche Roche Holding AG Discovery IL-13 receptor alpha 2 inhibitors (iv, cancer), Pfizer Pfizer Inc Discovery IMGN-289ImmunoGen Inc Discovery intracellular antibodies (Intraphilin, inflammatory diseases/infectiousdiseases/ophthalmic diseases), Permeon Biologics Permeon Biologics Inc Discovery mapp-66Mapp Biopharmaceutical Inc Discovery MB-2003Mapp Biopharmaceutical Inc Discovery monoclonal antibody-drug conjugates, Chirogenix/ImmunoGen/Celltrion Chirogenix Co Ltd Discovery MP-Ter-ADC MediaPharma Srl Discovery N01-OX2Intellect Neurosciences Inc Discovery PC-91ProCell Therapeutics Inc Discovery ProstaLite PhotoBiotics Ltd Discoveryrecombinant mAb-biocide fusion proteins (oral/Directed Biocide,cryptosporidium infection), ioGenetics ioGenetics Inc Discovery SGN-CD33A Seattle Genetics Inc Discovery SGN-LIV1A Seattle Genetics Inc Discovery SL-101Stemline Therapeutics Inc Discovery SYD-983Synthon Biopharmaceuticals Discovery T01-OX2Intellect Neurosciences Inc Discovery TBL-0306L Transgene Biotek Ltd Discovery TBL-0306M Transgene Biotek Ltd Discovery TBL-0805E Transgene Biotek Ltd Discovery thio-trastuzumab-mpeo-DM1Genentech Inc Discovery trastuzumab-PNU-159682 antibody-drug conjugate (cancer), Genentech Genentech Inc Discovery veltuzumab-IFN alpha 2b conjugate (cancer), IBC/Immunomedics IBC Pharmaceuticals Inc Discovery BIIB-015Biogen Inc Suspended Pretarget technology (gastrointestinal adenocarcinoma), NeoRx Poniard Pharmaceuticals Inc Suspended125I-AnnA1 IgG Sidney Kimmel Cancer Center No Development Reported131I-CC49-SCA Enzon Labs Inc No Development Reported177Lu-capromab pendetide Cytogen Corp No Development Reported90Y-capromab pendetide Cytogen Corp No Development Reported99mTC-BERH2Medac GmbH No Development Reportedanti-CD133-vcMMAF Seattle Genetics Inc No Development Reportedanti-CD22 antibody drug-conjugates, Medarex/BMS Medarex Inc No Development Reportedanti-PSMA antibody-drug conjugates (cancer), Medarex Medarex Inc No Development Reportedantibody-drug conjugates (solid tumors), Daiichi Sankyo Seattle Genetics Inc No Development ReportedAVE-9633ImmunoGen Inc No Development Reportedbectumomab Immunomedics Inc No Development ReportedCA125/MUC16-targeting antibody-drug conjugate (ovarian cancer),Genentech Genentech Inc No Development Reportedcathepsin B-sensitive prodrugs, BMS Bristol-Myers Squibb Pharmaceutical ResearchInstituteNo DevelopmentReportedCC49 humanized radioimmunoconjugates, National Cancer Institute,University of Alabama at Birmingham National Cancer Institute No Development ReportedCD4-BFFI Roche Holding AG No Development ReportedCHB-111ViRexx Medical Corp No Development ReportedCHT-25University College London No Development ReportedCMD-193Wyeth No Development ReportedCNTO-95 immunoconjugates (cancer), Centocor Janssen Biotech Inc No Development Reportedconjugated PEI/anti-CD133 mAb plasmid-based gene therapy (brain tumor),Discovery genomics Discovery Genomics Inc No Development ReportedcT84.66City of Hope No Development Reporteddelta 9-cadherin targeting antibody (gastric cancer), Actinium Helmholtz Zentrum München No Development Reporteddiphtheria toxin, RCT Research Corporation Technologies No Development Reporteddoxorubicin-C225 conjugate (STEALTH), SEQUUS SEQUUS Pharmaceuticals Inc No Development ReportedDTPA-BrE-3University of Colorado System No Development ReportedDXL-625InNexus Biotechnology Inc No Development ReportedG3.519-PAP-S Tanox Inc No Development ReportedhuHMFG1-caspase Antisoma plc No Development ReportedIMGN-007ImmunoGen Inc No Development ReportedIMGN-009ImmunoGen Inc No Development Reportedimmunoconjugate (cancer MN), Bayer Bayer AG No Development ReportedImmuRAID-AFP-99mTc, Immunomedics Immunomedics Inc No Development ReportedIMTOX 22-97A University of Texas Southwestern MedicalCenterNo DevelopmentReportedKSB-201KS Biomedix Holdings plc No Development ReportedLA22-radioimmunoconjugates (cancer), Welson/Peking University Welson Pharmaceuticals Inc No Development Reportedlabetuzumab Immunomedics Inc No Development ReportedLMB-1, NIH National Institutes of Health No Development ReportedLu-177-trastuzumab Tarbiat Modares University No Development ReportedLymphoScan Immunomedics Inc No Development ReportedMDX-11Medarex Inc No Development ReportedMDX-1203Medarex Inc No Development ReportedMDX-1206Medarex Inc No Development Reportedmonoclonal-porphyrins, Quadra Logic QLT Inc No Development Reportedmonoclonals, Quest Quest Biotechnology Inc No Development ReportedNogo receptor modulators, Biogen Idec Yale University No Development ReportedONS-1210Oncobiologics Inc No Development ReportedOP-06 program (cancer), Onco-Pharmakon Onco-Pharmakon Inc No Development Reportedpaclitaxel analogs and immunoconjugates (cancer), Bioxel Bioxel Pharma Inc No Development ReportedPE38-conjugated anti-CD30 immunotoxin, NCI National Cancer Institute No Development Reportedprostate-specific MAb, NIH National Institutes of Health No Development ReportedR-1549The UK Imperial Cancer Research Fund No Development Reportedradiolabeled Tx3.833Beth Israel Deaconess Medical Center No Development Reportedscu-PA-59D8Bristol-Myers Squibb Co No Development ReportedSGN-17/19Seattle Genetics Inc No Development Reportedtaxane-monoclonal antibody conjugates, ImClone ImClone Systems Inc No Development Reportedtranscobalamin (vitamin B12) receptor-targeting mAbTCR23-saporinconjugate (cancer), Kyto Kyto Biopharma Inc No Development Reportedtrastuzumab-autophilic peptide conjugate (breast cancer), InNexusBiotechnology InNexus Biotechnology Inc No Development Reportedtrastuzumab-MC-vc-PAB-MMAF Genentech Inc No Development ReportedTRP-targeted antibody conjugate (Yttrium 90/MX-DTPA), SomantaPharmaceuticals Immunodex Inc No Development Reportedtucotuzumab celmoleukin EMD Lexigen Research Center Corp No Development ReportedVB4-011Viventia Biotech Inc No Development ReportedVB6-011Viventia Biotech Inc No Development ReportedVB6-050Viventia Biotech Inc No Development ReportedXomaZyme-791XOMA Corp No Development Reportednofetumomab Poniard Pharmaceuticals Inc Withdrawn 131I-81C6Duke University Discontinued 131I-ImmuRAIT-HCG, Immunomedics Immunomedics Inc Discontinued B-B4-DC1ImmunoGen Inc Discontinued CC49 radioimmunoconjugates, University of Alabama at Birmingham University of Alabama at Birmingham Discontinued CD5 monoclonals/RIPs, Italfarmaco Italfarmaco SpA Discontinued CVX-045CovX Pharmaceuticals Inc Discontinued CVX-060CovX Pharmaceuticals Inc Discontinued CVX-241CovX Pharmaceuticals Inc Discontinued CVX-343CovX Pharmaceuticals Inc Discontinued doxorubicin-BR96 conjugate, BMS Bristol-Myers Squibb Co Discontinued doxorubicin-CEA conjugate, Immunomedics Immunomedics Inc Discontinued doxorubicin-LL2 conjugate, Immunomedics Immunomedics Inc Discontinued FAP5-DM1Boehringer Ingelheim Corp Discontinued FGFR4-CovX-Body CovX Pharmaceuticals Inc Discontinued HuM-195-Bi-213PDL BioPharma Inc Discontinued human placental growth factor 1-CVX-2000 monoclonal antibody conjugatedtherapeutic (CovX-body, cancer), CovX CovX Pharmaceuticals Inc Discontinued huN901-CC-1065ImmunoGen Inc Discontinued huN901-DC1ImmunoGen Inc Discontinued ImmuRAID-HCG-99mTc, Immunomedics Immunomedics Inc Discontinued ImmuRAIT-CEA-rhenium-188, Immunomedics Immunomedics Inc Discontinued MDX-214Medarex Inc Discontinued MEDI-547MedImmune LLC Discontinued MLN-2704Cornell University Discontinued Oncolym Peregrine Pharmaceuticals Inc Discontinued Oncolysin B Dana-Farber Cancer Institute Inc DiscontinuedOncolysin CD6Dana-Farber Cancer Institute Inc Discontinued Oncolysin M Dana-Farber Cancer Institute Inc Discontinued Oncolysin S Dana-Farber Cancer Institute Inc Discontinued Oncopurge Poniard Pharmaceuticals Inc Discontinued rhenium-188-LL2, Immunomedics Immunomedics Inc Discontinued SMART ABL-364Novartis AG Discontinued targeted ranpirnase conjugates (cancer), Alfacell Tamir Biotechnology Inc DiscontinuedHighest Dev Status。
附录:常用免疫学名词解释Aabsorption吸收应用特异性抗原与溶液中的抗体结合,形成不溶性复合物而除去抗体,例如用此法处理血清,即称为吸收血清。
用作吸收的抗原称为吸收剂。
accessory cell辅佐细胞特异性免疫应答需要的细胞,但不是实际介导的,通常用于描述抗原呈递细胞(APC)。
acquired immunity获得性免疫机体在生活过程中所获得的免疫力,称为获得性免疫,它与先天性免疫或天然免疫相反。
获得性免疫可分为:自动免疫,被动免疫,体液免疫与细胞免疫。
参看适应性免疫(adaptive immunity),过继性免疫(adoptive immunity),免疫耐受(immune tolerance)。
acquired immunodeficiency Sydrom(AIDS)获得性免疫缺陷综合征(艾滋病)由人类免疫缺陷病毒(HIV)所致的免疫缺陷病。
HIV感染主要引起T淋巴细胞CD4亚群的极度减少。
患者表现为迟发型超敏反应降低或消失,对机会感染菌极其易感,易发生某些少见的,如Kaposi氏肉瘤或Burkitt氏淋巴瘤。
HIV也可引起B 淋巴细胞多克隆性扩增,导致高丙种球蛋白血症。
尽管血清中免疫球蛋白量明显增加,但对抗原不能发生免疫反应。
这种综合征发生于“危险”人群,包括同性恋的男子,滥用静脉药物者,血液或血液制品的接受者,以及某些来自中非或加勒比海的人群。
在“危险”人群的异性伙伴中和AIDS母亲的婴儿中也已发现了这种综合症。
active immunization主动免疫(作用)抗原进入机体起免疫应答(自动免疫)adaptation tolerance适应性耐受生物在长期进化过程,宿主与寄生物在相互反应中,宿主的防御能力选择性地被减弱。
adaptative immunity适应免疫机体与抗原接触而发生的免疫力(包括主动体液免疫与主动细胞免疫)。
adherent cell粘附细胞在体外能粘附于表面的细胞。
K07.006下颌骨增生K07.007下颌发育不全K07.008下颌角肥大K07.009下颌角肥大伴咬肌肥大K07.010小颌畸形K07.011小颏畸形K07.012唇腭裂术后颌骨发育不全K07.100颌-颅底关系异常K07.101偏颌畸形K07.102双突颌畸形K07.103上颌后缩K07.104上颌前突K07.105上下颌前突畸形K07.106下颌后缩K07.107下颌偏斜K07.108下颌前突K07.109颌后缩K07.110颌骨不对称K07.200牙弓关系异常K07.201覆咬合K07.202前牙开合K07.203牙弓中线偏离K07.204咬合异常K07.205反(牙合)K07.300牙位置异常K07.301牙错位K07.302牙列不齐K07.303牙体缺损K07.304牙拥挤K07.400错(牙合)K07.401咬合不正K07.500牙面功能异常K07.600颞下颌关节疾患K07.601陈旧性颞下颌关节脱位K07.602颞颌关节综合征K07.603颞颌关节骨关节病K07.604颞颌关节炎K07.605颞下颌关节-疼痛-功能紊乱综合征K07.800特指牙面畸形K07.900牙面畸形K07.901下颌畸形K07.902颌骨畸形K07.903颌骨先天畸形K08.000全身性疾病牙脱落K08.100意外事故、拔除或局部牙周病引起的牙缺失K08.101单颌牙列缺失K08.102外伤性牙齿缺失K08.103后天性牙齿缺失K08.104牙列部分缺失K08.200无牙牙槽嵴萎缩K08.201无牙牙槽突萎缩K08.202牙槽骨萎缩K08.203牙槽嵴萎缩K08.204牙槽突萎缩K08.300牙根滞留K08.301残留牙根K08.302残冠K08.800特指牙及支持结构疾患K08.801牙痛K08.802牙槽嵴裂K08.803牙槽突不齐K08.804牙槽嵴粘膜角化过度K08.805牙槽突裂K08.806牙槽出血K08.807牙槽隐性裂K08.808牙槽嵴增大K08.809牙槽骨缺损K08.900牙及支持结构疾患K09.000发育性牙源性囊肿K09.001颌骨角化囊肿K09.002颌骨牙源性囊肿K09.003牙龈囊肿K09.004颌骨含牙囊肿K09.005颌骨始基囊肿K09.100口区发育性(非牙源性)囊肿K09.101腭正中囊肿K09.102腭部皮脂腺囊肿K09.103鼻腭囊肿K09.104球上颌囊肿K09.105切牙管囊肿K09.106悬雍垂囊肿K09.107硬腭囊肿K09.200颌特指囊肿K09.201颌出血性囊肿K09.202颌动脉瘤性囊肿K09.203髁状突囊肿K09.204上颌骨囊肿K09.205下颌骨囊肿K09.800特指口区囊肿K09.801腮腺淋巴上皮囊肿K09.802鼻唇囊肿K09.803鼻唇沟囊肿K09.804颏部皮样囊肿K09.805颊囊肿K09.806口腔表皮样囊肿K09.807口腔皮样囊肿K09.808口腔粘液腺囊肿K09.809口腔淋巴上皮囊肿K09.900口腔囊肿K10.000颌发育性疾患K10.001下颌隆凸K10.002腭裂手术后畸形K10.100中心性巨细胞肉芽肿K10.101颌骨巨细胞修复性肉芽肿K10.102颌骨巨细胞肉芽肿K10.103颌肉芽肿K10.200颌炎性情况K10.201放射性颌骨坏死K10.202颌骨骨髓炎K10.203颌骨放射性骨髓炎K10.204颌骨炎性增生K10.205颌骨骨炎K10.206颌骨死骨K10.207化脓性颌骨髓炎K10.208髁状突炎K10.209慢性下颌骨边缘性骨髓炎K10.210慢性下颌骨中央性骨髓炎K10.211慢性颌骨炎K10.212下颌炎性窦道K10.213下颌骨局限坏死K10.214翼腭窝炎K10.300颌的牙槽炎K10.301牙槽骨骨炎K10.302干槽症K10.800特指颌疾病K10.801单侧髁状突肥大K10.802髁状突骨疣K10.803后天性腭畸形K10.804颌骨纤维结构发育不良K10.805颌外生性骨疣K10.806上颌骨骨质增生K10.807下颌骨骨质增生K10.808上腭穿孔K10.809颌部瘤样纤维组织增生K10.900颌疾病K10.901颌骨肿物K11.000涎腺萎缩K11.100涎腺增生K11.101腮腺肥大K11.102颌下腺肥大K11.200涎腺炎K11.201急性腮腺炎K11.202急性颌下腺炎K11.203急性舌下腺炎K11.204慢性腮腺炎K11.205慢性颌下腺炎K11.206慢性舌下腺炎K11.207腮腺炎性假瘤K11.208硬化性涎腺炎K11.209阻塞性颌下腺炎K11.210阻塞性腮腺炎K11.211化脓性腮腺炎K11.300涎腺脓肿K11.301腮腺脓肿K11.302颌下腺脓肿K11.400涎腺瘘K11.401腮腺瘘K11.402腮腺导管瘘K11.403腮腺腺体瘘K11.404颌下腺瘘K11.500涎石病K11.501腮腺导管结石K11.502涎腺导管阻塞K11.503颌下腺导管结石K11.600涎腺粘液囊肿K11.601腮腺囊肿K11.602腮腺涎液潴留K11.603舌下腺囊肿K11.604舌下囊肿K11.605颌下腺囊肿K11.606颌下腺粘液囊肿K11.700唾液分泌障碍K11.701口干燥症K11.800特指涎腺疾病K11.801涎腺管狭窄K11.802米库利奇病K11.803腮腺管扩张K11.804腮腺肉芽肿K11.805涎腺良性淋巴上皮损害K11.806涎腺管扩张K11.900涎腺病K11.901腮腺区肿物K11.902涎腺肿物K11.903颌下腺肿物K12.000复发性阿佛他溃疡K12.001复发性坏死性粘膜腺周炎K12.002口腔阿弗他溃疡K12.003疱疹样口炎K12.100特指形式的口炎K12.101创伤性口腔粘膜溃疡K12.102腭部溃疡K12.103腭溃疡穿孔K12.104腭粘膜炎K12.105腭部炎性假瘤K12.106变应性口炎K12.107溃疡性口炎K12.108义齿性口炎K12.109口腔粘膜溃疡K12.110口底炎性假瘤K12.111口腔感染K12.112口腔炎K12.113口炎K12.114小泡性口炎K12.115颊溃疡K12.116口腔炎性肿块K12.117上腭炎性肿物K12.118慢性颊粘膜下炎K12.200口腔蜂窝织炎和脓肿K12.201口腔脓肿K12.202颌下间隙感染K12.203颊部脓肿K12.204颊间隙感染K12.205颊瘘K12.206腭瘘K12.207颌下瘘管K12.208口腔瘘管K12.209口腔皮肤瘘K12.210眶下间隙感染K12.211颞下间隙感染K12.212舌下间隙感染K12.213咬肌间隙感染K12.214口蜂窝织炎K12.215翼下颌间隙感染K12.216牙源性面部皮肤瘘K12.217颌面间隙感染K12.218颊粘膜脓肿K13.000唇疾病K13.001唇瘘K13.002唇瘢痕K13.003唇肥厚K13.004唇畸形K13.005唇溃疡K13.006唇囊肿K13.007唇息肉K13.008唇外翻K13.009唇肉芽肿K13.010唇部肿物K13.011唇蜂窝织炎K13.012唇鳞状上皮增生K13.013唇炎K13.014口角炎K13.015唇皲裂K13.016唇疼K13.100唇咬伤K13.101颊咬伤K13.200口腔上皮(包括舌)白斑和特指障碍K13.201腭粘膜角化不良K13.202腭白斑K13.203口腔白斑K13.204口腔粘膜过度角化K13.205舌白斑K13.206舌白色水肿K13.207舌良性过度角化症K13.208牙龈白斑K13.209颊白斑K13.210舌鳞状上皮增生K13.300毛状白斑K13.400口腔粘膜肉芽肿和类肉芽肿损害K13.401口腔粘膜肉芽肿K13.402口腔粘膜嗜酸性肉芽肿K13.403口腔粘膜疣状黄瘤K13.500口腔粘膜下纤维化K13.600口腔粘膜刺激性增生K13.601口腔粘膜增生K13.602腭增生症K13.700口腔粘膜特指损害K13.701口腔粘膜损害K13.702口腔肿物K13.703口腔瘢痕K13.704口腔粘蛋白沉积症K13.705口腔出血K13.706后天性小口畸形K13.707颊部炎性假瘤K13.708颊部囊肿K13.709腭咽闭合不全K13.710悬雍垂(腭垂)肥大K13.711悬雍垂(腭垂)息肉K13.712腭粘膜息肉K13.713腭粘膜炎症K13.714软腭肿瘤放疗后畸形K13.715下颌前庭沟过浅K13.716颏部瘘管K14.000舌炎K14.001舌脓肿K14.002舌炎性肿块K14.003舌溃疡K14.004舌乳突炎K14.100地图样舌K14.102移行性舌炎K14.200正中菱形舌炎K14.300舌乳头肥大K14.301舌叶乳头增生K14.302黑毛舌K14.400舌乳头萎缩K14.401萎缩性舌炎K14.500裂缝舌K14.600舌痛K14.800舌特指疾病K14.801舌肉芽肿K14.802舌出血K14.803舌肥大K14.804舌萎缩K14.805舌囊肿K14.807舌肌阵挛K14.808舌瘢痕K14.809舌尖瘘管K14.900舌疾患K14.901舌肿物K20.x00食管炎K20.x01化学性食管炎K20.x02T66放射性食管炎K20.x03手术后食管炎K21.000胃-食管反流性疾病伴有食管炎K21.001反流性食管炎K21.900胃-食管反流病K21.901食管反流K21.902贲门松弛K21.903喉咽反流K22.000贲门弛缓不能K22.001贲门失弛缓症K22.100食管溃疡K22.101贲门糜烂K22.102贲门溃疡K22.103食管糜烂K22.200食管梗阻K22.201贲门梗阻K22.202贲门狭窄K22.203食管受压K22.204食管挛缩K22.205食管狭窄K22.206胡桃夹食管K22.207创伤性食管狭窄K22.208手术后食道狭窄K22.300食管穿孔K22.301食管破裂K22.400食管运动障碍K22.401食管痉挛K22.500后天性食管憩室K22.600胃-食管撕裂-出血综合征K22.601食管贲门粘膜撕裂综合征K22.700巴雷特食管K22.800食管特指疾病K22.801食管隆起性病变K22.802创伤后食管瘘K22.803食管白斑K22.804食管出血K22.805食管囊肿K22.806食管扩张K22.807食管息肉K22.808食管肠上皮化生K22.809食管黏膜剥脱症K22.811食管瘘K22.812食管粘膜不典型增生K22.813手术后食管瘘K22.900食管疾患K22.901食管肿物K22.902食道功能不全K25.000胃溃疡伴急性出血K25.001迪厄拉富瓦溃疡K25.100胃溃疡伴急性穿孔K25.200胃溃疡伴急性出血和穿孔K25.300急性胃溃疡K25.400胃溃疡伴出血K25.401幽门溃疡伴出血K25.500胃溃疡伴穿孔K25.501幽门穿孔K25.600慢性胃溃疡伴出血和穿孔K25.700慢性胃溃疡K25.900胃溃疡K25.901残胃溃疡K25.902胃小弯溃疡K25.903幽门管溃疡K26.000急性十二指肠溃疡伴出血K26.001急性十二指肠球部溃疡并出血K26.100急性十二指肠溃疡伴穿孔K26.200急性十二指肠溃疡伴出血和穿孔K26.300急性十二指肠溃疡K26.400十二指肠溃疡伴出血K26.401十二指肠球部溃疡伴出血K26.500十二指肠溃疡伴穿孔K26.501十二指肠球部溃疡伴穿孔K26.600十二指肠溃疡伴穿孔和出血K26.700慢性十二指肠球部溃疡K26.701慢性十二指肠溃疡K26.900十二指肠溃疡K27.000急性胃十二指肠溃疡伴出血K27.100急性消化性溃疡伴穿孔K27.200急性消化性溃疡伴出血和穿孔K27.300急性胃十二指肠溃疡K27.400胃十二指肠溃疡伴出血K27.401应激性溃疡伴出血K27.500胃十二指肠溃疡伴穿孔K27.501消化性溃疡伴穿孔K27.502应激性溃疡伴穿孔K27.503上消化道穿孔K27.504消化道穿孔K27.600消化性溃疡伴出血和穿孔K27.700慢性消化性溃疡K27.900复合性溃疡K27.901消化性溃疡K27.902应激性溃疡K27.904应激性胃溃疡K28.000急性胃空肠溃疡伴出血K28.100急性胃空肠溃疡伴穿孔K28.200急性胃空肠溃疡伴出血和穿孔K28.300急性胃空肠溃疡K28.400胃肠吻合口溃疡伴出血K28.401空肠溃疡伴出血K28.500胃肠吻合口溃疡伴穿孔K28.600慢性胃空肠溃疡伴出血和穿孔K28.700胃肠吻合口溃疡K28.900空肠溃疡K28.901胃空肠溃疡K29.000急性出血性胃炎K29.100特指急性胃炎K29.101急性糜烂性胃炎K29.200酒精性胃炎K29.300慢性浅表性胃炎K29.400慢性萎缩性胃炎K29.500慢性胃炎K29.501慢性胃窦炎K29.600特指胃炎K29.601变应性胃炎K29.602肥厚性胃炎K29.603糜烂性胃炎K29.604梅内特里耶病K29.605肉芽肿性胃炎K29.606胃粘膜肥厚K29.607疣状胃炎K29.608药物性胃炎K29.700胃炎K29.701残胃炎K29.800十二指肠炎K29.801十二指肠球炎K29.802十二指肠乳头炎K29.900胃十二指肠炎K30.x00消化不良K31.000急性胃扩张K31.100成人肥厚性幽门狭窄K31.101瘢痕性幽门梗阻K31.102幽门不全梗阻K31.103幽门肥大K31.104幽门狭窄K31.200胃沙漏状狭窄及缩窄K31.300幽门痉挛K31.400胃憩室K31.500十二指肠梗阻K31.501十二指肠狭窄K31.502十二指肠淤积K31.600胃和十二指肠瘘K31.601胃空肠结肠瘘K31.602胃结肠瘘K31.603胃腹壁瘘K31.604十二指肠瘘K31.605手术后食管胃瘘K31.606手术后胃瘘K31.607手术后胃小肠瘘K31.608手术后胃大肠瘘K31.609手术后十二指肠瘘K31.700胃息肉K31.701十二指肠息肉K31.702十二指肠球部息肉K31.800胃和十二指肠特指疾病K31.801胃粘膜肠上皮化生K31.802高张力胃K31.803沙漏状胃痉挛K31.804胃酸过多K31.805胃酸缺乏K31.806胃狭窄K31.807胃痉挛K31.808胃结石K31.809胃麻痹K31.810胃囊肿K31.811胃下垂K31.812胃扭转K31.813胃破裂K31.814胃穿孔K31.815胃黄色斑K31.816胃粘膜脱垂K31.817上消化道梗阻K31.818十二指肠球变形K31.819胃潴留K31.900胃和十二指肠疾病K31.901胃排空障碍K31.902胃肿物K31.903十二指肠肿物K31.904急性胃粘膜病变K31.905胃粘膜病变K31.906胃占位性病变K35.000急性阑尾炎伴弥漫性腹膜炎K35.001急性阑尾炎穿孔伴局限性腹膜炎K35.002急性阑尾炎伴穿孔K35.003急性化脓性阑尾炎伴穿孔K35.004急性坏疽性阑尾炎伴穿孔K35.100急性阑尾炎伴腹膜脓肿K35.101阑尾脓肿K35.102回盲部脓肿K35.103阑尾周围脓肿K35.104急性化脓性阑尾炎伴周围脓肿K35.900急性阑尾炎K35.901急性阑尾炎伴腹膜炎K35.902急性化脓性阑尾炎K35.903急性坏疽性阑尾炎K35.904急性阑尾炎伴局限性腹膜炎K35.905急性化脓性阑尾炎伴阑尾周围炎K35.906急性坏疽性阑尾炎伴阑尾周围炎K35.907慢性阑尾炎急性发作K36.x00特指阑尾炎K36.x01复发性阑尾炎K36.x02慢性阑尾炎K37.x00阑尾炎K38.000阑尾增生K38.100阑尾结石K38.200阑尾憩室K38.300阑尾瘘K38.800阑尾特指疾病K38.801阑尾粘液囊肿K38.802阑尾囊肿K38.900阑尾疾病K40.000双侧腹股沟疝伴肠梗阻K40.001双侧腹股沟斜疝伴肠梗阻K40.002双侧腹股沟直疝伴肠梗阻K40.100双侧腹股沟疝伴坏疽K40.101双侧腹股沟斜疝伴肠坏死K40.102双侧腹股沟直疝伴肠坏死K40.200双侧腹股沟疝K40.201双侧腹股沟斜疝K40.202双侧腹股沟直疝K40.203双侧滑动性腹股沟斜疝K40.204双侧腹股沟疝(一侧直疝一侧斜疝) K40.300单侧腹股沟疝伴梗阻K40.301单侧绞窄性腹股沟斜疝K40.302单侧绞窄性腹股沟直疝K40.303单侧难复性腹股沟直疝K40.304单侧难复性腹股沟斜疝K40.305单侧嵌顿性腹股沟直疝K40.306单侧嵌顿性腹股沟斜疝K40.307单侧嵌顿性腹股沟疝伴梗阻K40.308单侧滑动性腹股沟疝伴梗阻K40.309腹股沟嵌顿性滑疝K40.310绞窄性腹股沟疝K40.311难复性腹股沟疝K40.312嵌顿性腹股沟疝K40.313嵌顿性腹股沟疝伴梗阻K40.314嵌顿性腹股沟斜疝K40.315腹股沟直疝嵌顿K40.400单侧腹股沟疝伴坏疽K40.401单侧腹股沟斜疝伴坏疽K40.402单侧腹股沟直疝伴坏疽K40.900腹股沟疝K40.901腹股沟斜疝K40.902腹股沟直疝K40.903腹股沟滑动疝K40.904复发性腹股沟斜疝K40.905复发性腹股沟直疝K40.906复发性腹股沟疝K40.907阴囊疝K41.000双侧股疝伴梗阻K41.100双侧股疝伴坏疽K41.200双侧股疝K41.300单侧股疝伴梗阻K41.301绞窄性股疝K41.302嵌顿性股疝K41.400单侧股疝伴坏疽K41.900股疝K42.000脐疝伴梗阻K42.001嵌顿性脐疝K42.100坏疽性脐疝K42.900脐疝K42.901脐旁疝K42.902复发性脐疝K43.000腹疝伴肠梗阻K43.001腹壁嵌顿疝K43.002嵌顿性切口疝K43.003切口疝伴肠梗阻K43.100腹疝伴肠坏死K43.900腹白线疝K43.901腹壁切口疝K43.902腹壁疝K43.903复发性切口疝K44.000膈疝伴肠梗阻K44.100膈疝伴坏疽K44.900膈疝K44.901食管裂孔疝K45.000特指腹疝伴梗阻K45.001绞窄性造口疝K45.002嵌顿性闭孔疝K45.003绞窄性腹疝伴肠梗阻K45.100特指腹疝伴坏疽K45.800特指腹疝K45.801坐骨大孔疝K45.802闭孔疝K45.803肠造口旁疝K45.804绞窄性闭孔疝K45.805库珀疝K45.806人工肛门处疝K45.807腰疝K46.000腹内疝伴肠梗阻K46.001绞窄性小肠疝K46.002嵌顿性小肠疝K46.100腹内疝伴坏疽K46.101坏疽性小肠疝K46.900腹内疝K46.901肠系膜裂孔疝K46.902肠系膜内疝K46.903阑尾疝K46.904特赖茨窝上疝K46.905小肠疝K50.000小肠克罗恩病K50.001空肠克罗恩病K50.002回肠克罗恩病K50.100大肠克罗恩病K50.101肉芽肿性结肠炎K50.102结肠克罗恩病K50.103直肠克罗恩病K50.104肉芽肿性盲肠炎K50.800特指克罗恩病K50.801食管克罗恩病K50.900克罗恩病K50.901食管克罗恩病(Crohn)K50.902+M07.4*克罗恩病性关节病K51.000溃疡性全结肠炎K51.001溃疡性全结肠炎,轻度K51.002溃疡性全结肠炎,中度K51.003溃疡性全结肠炎,重度K51.004溃疡性小肠结肠炎K51.005溃疡性回肠结肠炎K51.200溃疡性(慢性)直肠炎K51.201溃疡性直肠炎,轻度K51.202溃疡性直肠炎,中度K51.203溃疡性直肠炎,重度K51.300溃疡性(慢性)直肠乙状结肠炎K51.301溃疡性直肠乙状结肠炎,轻度K51.302溃疡性直肠乙状结肠炎,中度K51.303溃疡性直肠乙状结肠炎,重度K51.400炎性息肉K51.401结肠炎性息肉K51.500左侧结肠炎K51.800特指溃疡性结肠炎K51.900溃疡性结肠炎K51.901溃疡性结肠炎,轻度K51.902溃疡性结肠炎,中度K51.903溃疡性结肠炎,重度K51.904M07.5*溃疡性结肠炎性关节病K52.000放射性胃肠炎K52.001放射性结肠炎K52.100中毒性胃肠炎和结肠炎K52.101中毒性胃肠炎K52.102中毒性肠炎K52.103中毒性腹泻K52.200变应性及饮食性胃肠炎和结肠炎K52.201过敏性腹泻K52.202过敏性结肠炎K52.203过敏性肠炎K52.204饮食性腹泻K52.300未定型结肠炎K52.800特指非感染性胃肠炎和结肠炎K52.801胶原性结肠炎K52.802淋巴细胞性结肠炎K52.803嗜酸粒细胞性胃炎K52.804嗜酸细胞性胃肠炎K52.900非感染性胃肠炎和结肠炎K52.901非感染性胃肠炎K52.902非感染性腹泻K52.903回盲部炎症K52.904急性肠炎K52.905急性胃肠炎K52.906急性结肠炎K52.907慢性肠炎K52.908慢性腹泻K52.909慢性胃肠炎K52.910慢性结肠炎K52.911盲肠炎K52.912乙状结肠炎K52.913M07.6*肠病性关节炎K52.914肠炎性包块K52.915肠炎K52.916腹泻K52.917小儿肠炎K52.918婴儿肠炎K52.919幼儿腹泻(夏季腹泻)K55.000肠急性血管疾患K55.001急性肠血管梗塞K55.002急性缺血性肠坏死K55.003出血性肠梗塞K55.004肠坏死K55.005肠系膜坏疽K55.006肠系膜动脉栓塞K55.007肠系膜静脉血栓形成伴肠坏死K55.008肠系膜动脉血栓形成K55.009肠系膜动脉栓塞伴肠坏死K55.010肠系膜静脉血栓形成K55.011肠系膜静脉栓塞K55.012肠系膜梗死K55.013大网膜坏死K55.100肠慢性血管疾患K55.101肠系膜动脉狭窄K55.102肠系膜上动脉狭窄K55.103肠系膜动脉硬化K55.104肠系膜动脉供血不足K55.105肠系膜上动脉压迫综合征K55.106慢性缺血性小肠炎K55.200结肠血管发育不良K55.201结肠血管扩张症K55.800特指肠血管疾患K55.801肠系膜动脉炎K55.802小肠毛细血管扩张K55.900肠血管疾患K55.901缺血性小肠炎K55.902缺血性肠病K56.000麻痹性肠梗阻K56.001神经源性肠梗阻K56.100肠套叠K56.101结肠套叠K56.102直肠套叠K56.200肠扭转K56.201绞窄性肠梗阻K56.202肠绞窄K56.203结肠扭转K56.300胆石性肠梗阻K56.400肠特指嵌塞K56.401肠结石K56.500肠粘连伴梗阻K56.501腹膜粘连伴肠梗阻K56.502肠梗阻伴粘连K56.503肠粘连性狭窄K56.600特指肠梗阻K56.601肠狭窄K56.602乙状结肠狭窄K56.603肠梗阻伴坏死K56.604机械性肠梗阻K56.700肠梗阻K56.701不完全性肠梗阻K56.702结肠梗阻K57.000小肠憩室伴穿孔K57.001小肠憩室伴脓肿K57.002小肠憩室病伴腹膜炎K57.003十二指肠憩室伴穿孔K57.100小肠憩室病K57.101十二指肠憩室梗阻性黄疸综合征K57.102回盲部憩室K57.103空肠憩室K57.104十二指肠憩室K57.105回肠憩室K57.106小肠憩室炎K57.107空肠憩室炎K57.108十二指肠憩室炎K57.200大肠憩室伴穿孔K57.201大肠憩室病伴有脓肿K57.202结肠憩室伴腹膜炎K57.300大肠憩室K57.301盲肠憩室K57.302直肠憩室K57.303结肠憩室K57.304结肠憩室炎K57.305盲肠憩室炎K57.400小肠和大肠憩室病伴穿孔和脓肿K57.401小肠和大肠憩室病伴腹膜炎K57.500小肠和大肠憩室病K57.800肠憩室病伴穿孔和脓肿K57.801肠憩室病伴腹膜炎K57.900肠憩室病K58.000肠易激综合征伴腹泻K58.900肠易激综合征K58.901肠痉挛K58.902过敏性结肠K59.000便秘K59.001盆底痉挛综合征K59.002粪便潴留K59.100功能性腹泻K59.101肠道菌群失调K59.200神经源性肠K59.300巨结肠K59.301结肠扩张K59.302后天性巨结肠K59.303中毒性巨结肠K59.400肛门痉挛K59.401盆底肌痉挛综合征K59.800特指功能性肠疾患K59.801脾区综合征K59.900肠功能紊乱K60.000急性肛裂K60.100慢性肛裂K60.200肛裂K60.300肛瘘K60.301高位肛瘘K60.302低位肛瘘K60.303复杂性肛瘘K60.400直肠瘘K60.401直肠会阴瘘K60.402直肠皮肤瘘K60.403直肠阴囊皮肤瘘K60.500肛门直肠瘘K61.000肛门脓肿K61.001肛周脓肿K61.002肛门蜂窝织炎K61.100直肠脓肿K61.101直肠周围脓肿K61.200肛门直肠脓肿K61.300坐骨直肠窝脓肿K61.400括约肌内脓肿K62.000肛门息肉K62.001肛管息肉K62.100直肠息肉K62.200脱肛K62.201肛管脱垂K62.202肛门括约肌脱垂K62.300直肠脱垂K62.301直肠粘膜脱垂K62.400直肠狭窄K62.401肛门狭窄K62.402直肠梗阻K62.500直肠出血K62.501肛门出血K62.600直肠溃疡K62.601肛管溃疡K62.602肛门周围溃疡K62.700放射性直肠炎K62.800肛门和直肠特指疾病K62.801肛窦炎K62.802肛管炎K62.803巨直肠K62.804直肠吻合口瘢痕K62.805直肠不典型增生K62.806直肠纤维钙化K62.807直肠吻合口炎K62.808直肠肉芽肿K62.809直肠瘢痕K62.810直肠囊肿K62.811直肠炎K62.812直肠痛K62.813肛门括约肌松弛K62.814肛管炎性肿物K62.815肛门周围感染K62.816肛乳头肥大K62.817肛门白斑K62.818肛管囊肿K62.819肛门囊肿K62.820肛门痛K62.821肛门炎K62.822慢性肛管直肠炎K62.900肛门和直肠疾患K62.901肛旁肿物K62.902肛管肿物K62.903直肠肿物K63.000肠脓肿K63.001小肠脓肿K63.100肠穿孔(非创伤性)K63.101空肠穿孔K63.102回肠穿孔K63.103结肠穿孔K63.104回盲部溃疡伴穿孔K63.105乙状结肠穿孔K63.106直肠穿孔K63.107肠破裂K63.108小肠穿孔K63.200肠瘘K63.201腹壁肠瘘K63.202腹壁盲肠瘘K63.203腹壁窦道K63.204结肠瘘K63.205手术后结肠瘘K63.206手术后盲肠瘘K63.207手术后空肠瘘K63.208手术后回肠瘘K63.209手术后肠腹壁瘘K63.210手术后肠吻合口瘘K63.211手术后小肠结肠瘘K63.212手术后结肠直肠瘘K63.213手术后肠瘘K63.214腹壁瘘K63.215手术后大肠瘘K63.216手术后小肠瘘K63.300肠溃疡K63.301回肠溃疡K63.302小肠溃疡K63.303小肠粘膜糜烂K63.304原发性小肠溃疡K63.305结肠溃疡K63.306盲肠溃疡K63.307直肠乙状结肠溃疡K63.308肠糜烂K63.400肠下垂K63.401结肠下垂K63.402回肠粘膜脱垂K63.403内脏下垂K63.500结肠息肉K63.501升结肠息肉K63.502降结肠息肉K63.503乙状结肠息肉K63.504多发性结肠息肉K63.800肠特指疾病K63.801肠脂肪垂K63.802小肠囊肿K63.803小肠息肉K63.804小肠肿物K63.805小肠肉芽肿K63.806小肠不典型增生K63.807十二指肠囊肿K63.808后天性小肠畸形K63.809结肠积气K63.810结肠囊肿K63.811结肠肿物K63.812结肠黑变病K63.813回盲部息肉K63.814回盲部肉芽肿K63.815回盲部粘液性囊肿K63.816盲肠息肉K63.817直肠乙状结肠炎K63.818大肠不典型增生K63.819肠肉芽肿K63.900肠疾患K63.901回盲部肿物K63.902功能性肠病K65.000急性腹膜炎K65.001急性化脓性弥漫性腹膜炎K65.002急性化脓性腹膜炎K65.003急性弥漫性腹膜炎K65.005腹腔脓肿K65.006腹膜后脓肿K65.007肝下脓肿K65.008肝周脓肿K65.009膈下脓肿K65.010网膜脓肿K65.011盲肠后脓肿K65.012肠系膜脓肿K65.013男性盆腔脓肿K65.014男性盆腔炎K65.015男性盆腔炎性包块K65.016细菌性腹膜炎K65.017继发性腹膜炎K65.800特指腹膜炎K65.801肠系膜脂肪坏死K65.802慢性腹膜炎K65.803胆汁性腹膜炎K65.804硬化性腹膜炎K65.805多浆膜炎K65.806多浆膜腔积液K65.807嗜酸粒细胞性腹膜炎K65.900腹膜炎K65.901局限性腹膜炎K65.902自发性腹膜炎K65.903腹腔感染K65.904腹膜后感染K65.905大网膜炎K65.906原发性腹膜炎K66.000腹膜粘连K66.001回盲部粘连K66.002肠粘连K66.003胃粘连K66.004肠系膜粘连K66.005膈肌粘连K66.006大网膜粘连K66.007腹腔粘连K66.008十二指肠粘连K66.100腹腔积血K66.101腹膜出血K66.102腹膜后血肿K66.103肠系膜出血K66.800腹膜特指疾患K66.801网膜囊肿K66.802肠系膜囊肿K66.803肠系膜钙化K66.804腹茧症K66.805腹膜后囊肿K66.806腹壁肉芽肿K66.807腹腔囊肿K66.808腹膜囊肿K66.810腹膜后肉芽肿K66.811膈下囊肿K66.812骶前囊肿K66.900腹膜疾患K66.901腹膜后肿物K70.000酒精性脂肪肝K70.001齐夫综合征K70.100酒精性肝炎K70.200酒精性肝纤维化和肝硬化K70.201酒精性肝纤维化K70.300酒精性肝硬变K70.400酒精性肝衰竭K70.401急性酒精性肝衰竭K70.402慢性酒精性肝衰竭K70.403酒精性肝衰竭伴肝昏迷K70.900酒精性肝病K70.901酒精性肝损害K71.000中毒性肝病伴胆汁淤积K71.001药物性肝炎伴胆汁淤积K71.100中毒性肝病伴肝坏死 K71.101药物性肝炎伴肝功能衰竭K71.102急性药物性肝衰竭K71.103中毒性肝衰竭K71.104亚急性药物性肝衰竭K71.200中毒性肝病伴急性肝炎K71.300中毒性肝病伴慢性迁延性肝炎K71.400中毒性肝病伴慢性小叶性肝炎K71.500中毒性肝病伴慢性活动性肝炎K71.600中毒性肝炎K71.601药物性肝炎K71.700中毒性肝纤维化K71.701药物性肝硬化K71.702中毒性肝硬化K71.800中毒性肝病伴肝特指疾患K71.900中毒性肝病K71.901药物性肝损害K72.000急性肝衰竭K72.001亚急性肝衰竭K72.002急性黄色肝萎缩K72.003急性肝功能衰竭K72.004重症肝炎K72.100慢性肝功能衰竭K72.900肝衰竭K72.901亚急性肝坏死K72.902肝萎缩K72.903肝坏死K72.904肝性脑病K72.905肝功能不全K73.000慢性迁延性肝炎K73.100慢性小叶性肝炎K73.200慢性活动性肝炎K73.800特指慢性肝炎K73.900慢性肝炎K73.901慢性重型肝炎K74.000肝纤维化K74.200肝纤维化伴肝硬化K74.300原发性胆汁性肝硬变K74.400继发性胆汁性肝硬变K74.500胆汁性肝硬变K74.600肝硬化K74.601特指肝硬化K74.602乙型肝炎后肝硬化失代偿期K74.603丙型肝炎后肝硬化失代偿期K74.604自身免疫性肝炎后肝硬化失代偿期K74.605肝炎后肝硬化失代偿期K74.606混合型肝硬化失代偿期K74.607肝硬化失代偿期K74.608肝炎后肝硬化K74.609坏死性肝硬化K74.610结节性肝硬化K74.611门脉性肝硬化K74.612混合型肝硬化K74.613隐源性肝硬化K74.614自身免疫性肝硬化K74.615+I98.3*肝硬化伴食管静脉曲张破裂出血K74.616+I98.2*肝硬化伴食管静脉曲张K74.617+I98.3*肝硬化伴食管胃底静脉曲张破裂出血K74.618+I98.3*肝硬化伴胃底静脉曲张破裂出血K74.619+I98.2*肝硬化伴食管胃底静脉曲张K74.620+I98.2*肝硬化伴胃底静脉曲张K74.621慢性间质性肝炎K74.622血吸虫性肝硬化K75.000肝脓肿K75.001胆源性肝脓肿K75.002血源性肝脓肿K75.003细菌性肝脓肿K75.100门静脉炎K75.200非特异性反应性肝炎K75.300肉芽肿性肝炎K75.301肝炎性假瘤K75.400自身免疫性肝炎K75.800特指炎性肝脏疾病K75.801肝旁炎性肿物K75.802肝内胆管缺失综合征K75.803营养性肝炎K75.804胆汁淤积性肝炎K75.805肝胆管炎K75.806急性隐源性肝炎K75.807淤血性肝炎K75.809肝内胆管炎K75.900炎性肝脏疾病K75.901肝炎K76.000脂肪肝K76.100肝慢性阻性充血K76.101心源性肝硬化K76.102慢性淤血性肝损害K76.200肝中心性出血性坏死K76.300肝梗死K76.400紫癜样肝病K76.401肝血管瘤病K76.500肝静脉梗阻症K76.600门静脉高压K76.601胰源性门脉高压K76.602特发性门脉高压K76.603班蒂综合征K76.700肝肾综合征K76.800特指肝病K76.801自发性肝破裂出血K76.802急性淤血性肝损害K76.803肝出血K76.804肝脏结节K76.805肝肺综合征K76.806多发性肝囊肿K76.807肝囊肿K76.808肝结节性局灶性增生K76.809肝下垂K76.810缺血性肝病K76.811肝血肿K76.812肝结节性增生K76.813肝内钙化点K76.814肝癌破裂出血K76.815肝管息肉K76.816肝细胞性黄疸K76.900肝病K76.901肝肿物K80.000胆囊结石伴急性胆囊炎K80.001胆囊结石伴坏疽性胆囊炎K80.002胆囊结石伴急性化脓性胆囊炎K80.100胆囊结石伴特指胆囊炎K80.101胆囊结石伴慢性胆囊炎K80.200胆囊结石K80.201胆囊管结石K80.202胆囊绞痛K80.203胆囊结石嵌顿K80.300胆管结石伴胆管炎K80.301胆总管结石伴急性化脓性胆管炎K80.302胆总管结石伴胆管炎K80.303肝胆管结石伴胆管炎K80.304胆总管结石伴急性胆管炎K80.305肝内胆管结石伴胆管炎K80.306肝外胆管结石伴胆管炎K80.400胆管结石伴胆囊炎K80.401胆管结石伴急性胆囊炎K80.402胆总管结石伴急性胆囊炎K80.403胆管结石伴慢性胆囊炎K80.404胆总管结石伴慢性胆囊炎K80.405肝胆管结石伴胆囊炎K80.406肝管结石伴慢性胆囊炎K80.500胆管结石K80.501胆总管结石K80.502胆绞痛K80.503肝内胆管结石K80.504肝胆管结石K80.505胆总管残余结石K80.506胆肠吻合口结石K80.507肝管结石K80.800特指胆石症K80.801Mirizzi综合征K81.000急性胆囊炎K81.001胆囊脓肿K81.002急性化脓性胆囊炎K81.003急性坏疽性胆囊炎K81.004胆囊周围脓肿K81.005胆囊坏死K81.006慢性胆囊炎急性发作K81.007急性梗阻性化脓性胆囊炎K81.008胆囊坏疽K81.100慢性胆囊炎K81.101慢性残余胆囊炎K81.800特指胆囊炎K81.801胆囊周炎K81.900胆囊炎K81.901黄色肉芽肿性胆囊炎K82.000胆囊梗阻K82.001胆囊管梗阻K82.100胆囊积液K82.101胆囊粘液囊肿K82.200胆囊穿孔K82.300胆囊瘘K82.301胆囊肠瘘K82.302胆囊胃瘘K82.303胆囊十二指肠瘘K82.304胆囊结肠瘘K82.305手术后胆囊瘘K82.306胆囊腹壁瘘K82.400胆囊胆固醇沉着症K82.800胆囊特指疾病K82.801胆囊肿大K82.802胆囊息肉K82.803胆囊腺肌症K82.804胆囊管扩张K82.805胆囊肥大K82.806胆囊钙化。
2014年大陆DRAM消化102亿美金全球份额20%
中国工信部主导的投资基金持续展开收购,此次收购利基型DRAM及SRAM厂矽成,被视为是为了打造更完整的物联网系统作准备。
2014年各DRAM产品在陆市占
大陆工信部领衔成立的国家积体电路产业投资基金,持续在全球展开收购,继日前决定出手收购CMOS影像感测器大厂豪威(OmniVision)之后,由中国武岳峰资本主导的基金,也宣布将以每股19.25美元价格,收购美国记忆体设计厂矽成(ISSI)。
业界认为,大陆打造自己的半导体生产链,当然不会放过DRAM及Flash,而此次出手收购利基型DRAM及SRAM厂矽成,就是要建立更完整的供应链。
而由近期的并购动作来看,大陆官方是想打造更完整的物联网生态系统。
法人表示,此次大陆官方出手并购,等于开始着手布建利基型DRAM及SRAM等记忆体设计或生产能力,台湾业者中,南亚科及华邦电因为拥有12寸厂,压力不大,但晶豪科、钰创、力积等记忆体设计业者未来可能得面临严重的竞争威胁。
中国武岳峰资本宣布以每股19.25美元、合计约6.395亿美元资金,提案收购美商矽成积体电路。
此并购案虽为中国武岳峰资本主导,但尚包含eTown MemTek Ltd.、清芯华创、华清基业,同时也与具官方色彩的上海市创业引导基金有合作,其中清芯华创背后代表北京集成电路产业基金。
集邦科技指出,中国大陆市场半导体包括了处理器、手机晶片、DRAM、Flash,进口额已超过原油进口额,进口替代将是未来重点国家政策。
手机晶片部分则在清华紫光整合展讯、RDA、英特尔资源后,后续成效将陆续浮现。
处理器、DRAM、Flash三大项目占进口金额比例不小,中国大陆政府也与台湾DRAM业者洽谈合作,希望结合中国市场规模与台湾生产技术,大幅降低每年中国市场DRAM的入超现状。
集邦科技统计,2014年中国大陆市场的DRAM消化量金额为102亿美金,占全球营业额约20%。
其中,行动式记忆体(Mobile DRAM)在中国大陆的营业额更达到56亿美元,占中国大陆市场总金额的55%;标准型与伺服器DRAM也都分占中国大陆DRAM的19%与11%。
而中国大陆笔记型电脑出货达2,500万台,占全球14%;桌上型电脑亦有3,200万台规模,占全球25%,显示出中国大陆市场强劲的消费潜力。
大陆半导体产业中唯一一家在DRAM技术上有明显突破的,就是当年收购奇梦达矽智财相关资产的西安华芯,而此次拟收购的美商矽成,是由旅美台湾人李学勉、韩光宇等创立,有近9成营收来自于利基型DRAM及SRAM,NOR Flash营收占比略低于1成,主要代工厂为南亚科及力晶。