朗道质控和校准明细
- 格式:xls
- 大小:44.00 KB
- 文档页数:10

HIGH SENSITIVITY CRP CALIBRATOR SERIES(hsCRP CAL)(HIGH SENSITIVITY CRP CALIBRANT)CAT. NO. CP 2478SIZE: 6 x 2 mlINTENDED USEThis product is intended for in vitro diagnostic use in the calibration of CRP on clinical chemistry systems.SAFETY PRECAUTIONS AND WARNINGSThe calibrator contains human CRP in a stabilised protein matrix. Human source material from which the product has been derivedhas been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), Hepatitis C Virus (HCV) antibody, HBV DNA, HCV RNA and HIV DNA and found to be NON-REACTIVE. FDA approved methodshave been used to conduct these tests.However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samplesshould be handled as though capable of transmitting infectious diseases and disposed of accordingly.For in vitro diagnostic use only, do not pipette by mouth, exercise the normal precautions required for handling laboratory reagents.This material contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flushaffected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush withlarge volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Dispose of this material according to local regulations.STORAGE AND STABILITYThe CRP calibrators are supplied ready for use and are stable up to the expiry date when capped and stored at +2°C to +8°C in the absence of contamination. Only the required amount of product should be removed. After use, any residual product should NOT BE RETURNED to the original vial.MATERIALS PROVIDEDCRP CalibratorsLevel 1 (1 x 2 ml) Level 4 (1 x 2 ml)Level 2 (1 x 2 ml) Level 5 (1 x 2 ml)Level 3 (1 x 2 ml) Level 6 (1 x 2 ml)MATERIALS REQUIRED BUT NOT PROVIDEDNoneVALUE ASSIGNMENTCalibration of CRP Calibrators has been preformed at Randox by latex-enhanced immunoturbidimetry with reference to material standardised against an appropriate International Reference Preparation. The assigned values for the batch are listed below.LOT NO. CRP (mg/l) CRP (mg/dl) EXPIRY DATE1986CP 0.00 0.00 2013-031981CP 0.54 0.054 2013-031982CP 1.07 0.107 2013-031983CP 1.61 0.161 2013-031984CP 5.35 0.535 2013-031985CP 10.69 1.069 2013-0314 Sep ’11 neLOT NOS.1981CP -1986CP THIS PAGE IS INTENTIONALLY BLANK。

0843PAGE 1 OF 24LIQUID ASSAYED CHEMISTRY CONTROL PREMIUM PLUS - LEVEL 1 (LIQ CHEM ASY PREMIUM PLUS 1)Cat. No . LAL 4213 Lot No . 153UL Size : 12 x 5 ml Expiry : 2015-02INTENDED USEThis product is intended for in vitro diagnostic use, in the quality control of diagnostic assays. The Liquid Assayed Chemistry Control Premium Plus is for the control of accuracy.DEVICE DESCRIPTIONThe Liquid Assayed Chemistry Control Premium Plus is supplied at 3 levels, level 1, 2 and 3. Target values and ranges are supplied for the analytes listed in the values section at all three levels.SAFETY PRECAUTIONS AND WARNINGSFor in vitro diagnostic use only. Do not pipette by mouth. Exercise the normal precautions required for handling laboratory reagents.Human source material from which this product has been derived has been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), and Hepatitis C Virus (HCV) antibody and found to be NON-REACTIVE. FDA approved methods have been used to conduct these tests.However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samples should be handled as though capable of transmitting infectious diseases and disposed of accordingly.Health and Safety Data Sheets are available on request.STORAGE AND STABILITY OPENED: Store refrigerated (+2ºC to + 8ºC). Thawed serum is stable for 7 days at +2ºC to +8ºC, with the followingexceptions: Troponin T is stable for 3 days at +2ºC to +8ºC. Only the required amount of product should be removed. After use, any residual product should NOT BE RETURNED to the original vial.UNOPENED: Store frozen at -20ºC to -70ºC. Stable to expiration date printed on individual vials (see Limitations).LIMITATIONSFor Total Acid Phosphatase, the material should be stabilised by adding 1 drop (25 µl – 30 µl) of 0.7M Acetic acid solution to 1ml of the serum after thawing. After stabilisation, Total Acid Phosphatase is stable for 7 days at +2ºC to +8ºC. Bilirubin in the serum is light sensitive and it is recommended that the serum is stored in the dark.ALT, Total Acid Phosphatase, Alkaline Phosphatase, Total and Direct Bilirubin values may gradually decrease during the products shelf life.Bacterial contamination of the thawed serum will cause reductions in the stability of many components. The control should not be used as a calibration material.PREPARATION1. Allow the frozen control to thaw at room temperature (+15ºC to +25ºC) until completely thawed. Swirl the contents to ensurehomogeneity.2. Refer to the Control section of the individual analyser application.3. Refrigerate any unused material. Prior to reuse, mix contents thoroughly.MATERIALS PROVIDEDLiquid Assayed Chemistry Control Premium Plus - Level 1 12 x 5 mlMATERIALS REQUIRED BUT NOT PROVIDED NoneASSIGNED VALUESEach lot of serum is submitted to a number of external laboratories. Values are assigned from a consensus of results obtained by these laboratories and internal testing conducted at Randox Laboratories Ltd. With each batch, a control range is provided for individual parameters and each parameter method.If an instrument specific value is not available, refer to the Mean of all Instruments section. If necessary, contact Randox Laboratories – Customer Technical Services, Northern Ireland, Tel: +44 (0) 28 9445 1070 or email Technical.Services@29 Nov 13 rwPage 2 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 3 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 4 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 5 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 6 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 7 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 8 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 9 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 10 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 11 of 2429/11/2013___________________________________________________________________________________________________Page 12 of 2429/11/2013___________________________________________________________________________________________________Page 13 of 2429/11/2013___________________________________________________________________________________________________Page 14 of 2429/11/2013___________________________________________________________________________________________________Page 15 of 2429/11/2013___________________________________________________________________________________________________Page 16 of 2429/11/2013___________________________________________________________________________________________________Page 17 of 2429/11/2013___________________________________________________________________________________________________Page 18 of 2429/11/2013___________________________________________________________________________________________________Page 19 of 2429/11/2013___________________________________________________________________________________________________Page 20 of 2429/11/2013___________________________________________________________________________________________________Page 21 of 2429/11/2013___________________________________________________________________________________________________Page 22 of 2429/11/2013___________________________________________________________________________________________________Page 23 of 2429/11/2013___________________________________________________________________________________________________Page 24 of 2429/11/2013___________________________________________________________________________________________________。
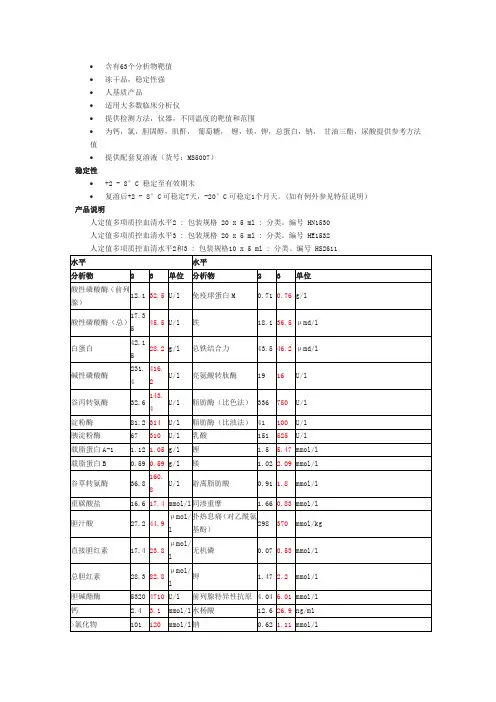
氨/乙醇质控水平1 : 包装规格 6 x 2 ml : 分类。
编号 EA1366氨/乙醇质控水平2 : 包装规格 6 x 2 ml : 分类。
编号 EA1367氨/乙醇质控水平3 : 包装规格 6 x 2 ml : 分类。
编号 EA1368水平分析物 1 2 3 单位氨 6.9 187 372 μmol/l乙醇0.55 1.55 2.73 g/l高值胆红素质控特征•l 总胆红素靶值接近290 μmol/l•用于监测儿科医学的胆红素水平•冻干品,稳定性强。
•牛基质•根据不同方法提供靶值和范围稳定性•未复溶+2 - 8°C 可稳定至有效期末•复溶后+2 - 8°C可稳定5天特征产品说明高值胆红素质控品 : 包装规格 10 x 3 ml : 分类。
货号BE454甘油质控品特征•冻干品,稳定性强•人基质产品稳定性•未复溶+2 - 8°C可稳定至有效期末•复溶后25°C时可稳定8小时,+2 - 8°C 可稳定7天,-20°C可稳定1个月产品说明甘油质控水平1 : 包装规格 3 x 5 ml : 分类。
货号 .GY1369葡萄糖-6-磷酸脱氢酶质控品特征•冻干品,稳定性强•人基质全血稳定性•+2 - 8°C 可稳定至有效期末•复溶后+2 - 8°C 可稳定5天产品说明葡萄糖-6-磷酸脱氢酶低含量质控品 : 包装规格 6 x 0.5 ml : 分类。
货号 .PD2617 葡萄糖-6-磷酸脱氢酶正常值质控品 : 包装规格 6 x 0.5 ml : 分类。
货号 .PD2618 脑脊液质控特征•包含10个分析物•冻干品,稳定性强•100%全人基质无动物添加成分•适用于大多数的临床分析仪•为常用仪器和方法提供靶值和参考范围稳定性•+2 - 8°C 稳定至有效期末•复溶后在+2 - 8°C可稳定5天,-20°C可稳定1个月。

R A N D X尿液生化多项检测用质控品货号: AU2352 包装: 12 x 10 ml批号: 792UC 效期: 2019-03产品用途该质控品用于临床生化体外诊断中尿液检测分析的质量控制。
产品描述朗道供应两种浓度水平的尿液生化多项检测用质控品(水平2:AU2352;水平3:AU2353)。
为以下分析项目提供了两个水平的靶值和范围:淀粉酶、钙、氯、铜、皮质醇(氢化可的松)、肌酐、多巴胺、肾上腺素、血糖、5-羟吲哚乙酸(5-HIAA)、镁、变肾上腺素、微量白蛋白、去甲肾上腺素、去甲变肾上腺素、同渗重摩、草酸盐、无机磷、钾、总蛋白、钠、尿素、尿酸和香草扁桃酸(VMA)。
安全预防措施和警告本产品仅用于体外诊断。
禁止用口吸,按照实验室常规预防措施对试剂进行处理。
该质控品采用人源性成分,对所有人源性成分均进行了HIV(HIV1、HIV2)抗体、肝炎B表面抗原(HbsAg)和肝炎C病毒(HCV)抗体的测试,发现均呈阴性。
所采用的方法均经FDA认证。
但既然没有一种方法能够完全保证其没有传染物质,因此该质控品和所有的病人样品均应当按照能够传播疾病的样品小心处理。
保存和稳定性开瓶后,2~8℃保存。
复溶后若原瓶,并且无污染,可在15~25℃稳定8小时,可在2~8℃稳定5天,可在-20℃稳定14天。
每次使用只吸取所需用量,剩余的样品不可返回原瓶。
儿茶酚胺、VMA、草酸盐的样品制备及稳定性:这些分析物在尿液样本中不稳定,完全复溶后15分钟,取一定量,按每1 mL加入8 μL HCl (6 M),2~8℃可稳定5天。
对草酸盐的测试,建议每10 mL尿液中加入5 mg EDTA,以此阻止形成草酸钙沉淀。
5-羟基吲哚乙酸(5-HIAA)的样品制备及稳定性:该分析物在尿液样本中不稳定,完全复溶后15分钟,取一定量,按每1 mL加入10 μL冰醋酸(17.4 M),2~8℃可稳定7天。
如使用亚硝基萘酚法,需往每毫升复溶尿液中加入12 μL HCl (6 M),2~8℃可稳定7天。

$%&quality management solutions 质控管理解决方案Why is it important for laboratories to use 247?--为什么要使用247?Importance for laboratories to use 247使用247的重要性1. Improper Quality Control leads to increased costs in the laboratory-质量控制做的不好会导致实验室成本增加–Due to possible repeated tests-可能导致重复检测–Improper patient treatment because of incorrect test results –因检验结果不准确导致的治疗不当Could be detected using 247 –可通过247发现问题Importance for laboratories to use 247使用247的重要性2. Result Validation-结果的确认–Data being entered can be validated using multi rule QC procedures-可通过多规则质控程序保证结果可靠–The advantages of Multirule QC procedures are that false rejections can be kept low while at the same time maintaining high error detection-使用多规则质控程序的好处在于可降低误拒绝率同时提高错误检出率Importance for laboratories to use 247使用247的重要性3.Turnaround time for reporting test result to clinicians can be reduced–Through minimising false rejections that requires repeat analysis of controls and re-runs of patient samples–误拒绝的结果需进行质控和样本的重新检测,通过最小化这种错误拒绝,可减少向临床报告检验结果的时间Importance for laboratories to use 247 4.Can be used as a trouble-shooting tool–If there are concerns that the controlmaterial is not performing well-如果担心质控物有问题–247 enables the lab to separate the effects of the method from suspected effects ofthe control by looking at the effect of thesame controls in other labs-可通过247观察其他实验室的数据迅速区分是质控物的问题还是分析系统的问题–Leading to more rapid and effectiveproblem solving –更加快速有效的解决问题Importance for laboratories to use 247 5. 247 provides means and SD’s for the material that are relevant because they reflect current testing conditions among labs 247软件可以提供相关质控物的均值和SD范围,反映实验室间的检测情况Importance for laboratories to use 247 6. Many managers ‘sign off’on the results without really investigating if those results are in fact accurate–247 provides an easy and fast way for laboratory managers to be confident that the results theysign for are correct.–实验室管理者在检验报告单上签字时往往并没有去考察结果是否可靠。


丙氨酸氨基转移酶测定试剂(ALT)(酶法)1. 检测原理本试剂采用国际临床化学联合会(IFCC)推荐方法:丙氨酸+α-酮戊二酸−−→−ALT丙酮酸+L-谷氨酸丙酮酸+NADH+H+−−→−LDH L-乳酸+NAD++H2ONADH氧化成NAD+引起340nm处吸光度的下降,下降速率与样品中ALT活性成正比。
2.标本采集与处理2.1 受检者的准备:病人空腹12h,不饮酒24h后采集血样。
体检对象抽血前应有两周的的正常状况记录。
注意有无应用影响测试项目的药物。
此外,对于体检者,采血的季节都应做相关记录,因为样本中各项目的含量有季节性变动,为了前后比较应在每年同一季节检验。
应嘱体检对象在抽血前24小时内不做剧烈运动。
2.2 静脉采血:除非是卧床的病人,一般在采血时取坐位。
体位影响水分在血管内外的分布,会影响测试项目的浓度。
在采血前至少应静坐5分钟,一般从肘静脉取血,使用止血带的时间不超过1分钟,穿刺成功后立即松开止血带。
2.3 抗凝剂:血浆使用肝素或EDTA(1mg/mL)作为抗凝剂2.4 标本的处理和储存:1血标本室温放置30min~45min后离心分离血清,置洁净试管加盖低温保存。
2标本在2-8℃可稳定3天,避免溶血。
3.试剂3.1 试剂及配套品:3.1.1试剂:本科使用重庆中元生物技术有限公司ALT试剂盒,为液体双试剂,各组分如下:试剂R1:Tris缓冲液NADH 100mmol/L 0.3mmol/L试剂R2:Tris缓冲液100mmol/Lα-酮戊二酸85mmol/LL-丙氨酸 800mmol/LLDH ≥8.5kU/L不同批号试剂盒中各组份不能互换。
3.1.2配套品:无配套品。
3.2 试剂的稳定性:1.原包装试剂储存在2-8℃至标签所示失效日期。
试剂的有效期为12个月。
2.试剂开瓶后,在仪器中至少可保存30天4:校准4.1校准血清:朗道复合校准品4.2校准方式:使用去离子水与校准血清两点定标。

CALIBRATION SERUM LEVEL 2 (CAL 2)20 x 5mlCAT. NO. CAL 2350 SIZE:2014-01LOT NO. 746UN EXP:INTENDED USEFor use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised human serum. The concentrations and activities are suitable for calibration of clinical chemistry assays on a wide range of automatic analysers. Constituent concentrations are available at 2 levels.SAFETY PRECAUTIONS AND WARNINGSHuman source material from which this product has been derived has been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), and Hepatitis C Virus (HCV) antibody and found to be NON-REACTIVE. FDA approved methods have been used to conduct these tests. However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samples should be handled as though capable of transmitting infectious diseases and disposed of accordingly.For IN VITRO diagnostic use only.STORAGE AND STABILITYUnreconstituted serum is stable up to the expiry date shown on the side of each individual bottle. Once reconstituted, the components of the Calibration Sera are stable for 8 hours at +25°C, 7 days at +2°C to +8°C and 1 month at -20°C when frozen once (see limitations).PREPARATION FOR USESerum must only be reconstituted using the following procedure:1. Open the vial carefully, avoiding any loss of material.2. Reconstitute by pipetting exactly 5ml of distilled water at +20°C to +25°C, into the vial.3. Replace the rubber stopper and leave to stand for 30 minutes out of bright light before use.4. Swirl gently several times during the reconstitution period to ensure that the contents are completely dissolved.5. Prior to use, mix the contents by inverting the vial. Do not shake the vial as the formation of foam should be avoided. Ensure that no lyophilised material remains unreconstituted.6. The serum is then ready for use with either a manual test or with an automated instrument.MATERIALS PROVIDEDCalibration Serum - Level 2Cat No. CAL2350 20 x 5mlMATERIALS REQUIRED BUT NOT PROVIDEDCalibrated Pipette, double deionised water.LIMITATIONSAfter reconstitution, Bicarbonate is stable for 8 hours in the closed bottle and 1 hour in the open bottle.For Total and Prostatic Acid Phosphatase, the material should be stabilised by adding 1 drop (25H l - 30H l) of 0.7M Acetic acid solution to 1ml of the serum exactly 30 minutes after reconstitution. After stabilisation, Total & Prostatic Acid Phosphatase are stable for 2 hours at +25°C, 2 days at +2°C to +8°C and 1 month when frozen once at -20°C. Alkaline Phosphatase levels in the reconstituted serum will rise over the stability period. It is recommended that the reconstituted serum be allowed to stand for 1 hour at +25°C before measurement.Bilirubin in the serum is light sensitive and it is recommended that the serum be stored in the dark. Stored in the dark it is stable for 1 day at +2°C to +8°C. Do not store at +15°C to +25°C. Do not freeze.Bacterial contamination of the reconstituted serum will cause reductions in the stability of many components. Different lot numbers of this calibrator should not be interchanged as the values assigned to the calibrators vary from lot to lot.LOT NO. 746UNVALUE ASSIGNMENTEach batch of serum is distributed to approximately 3000 laboratories worldwide and values are assigned by a consensusof results obtained by these laboratories. The Calibration values for each instrument have been determined in at least 10 independent laboratories. Values are verified against a master lot of calibrator which is traceable to reference methods or reference materials. In some cases values may be assigned at Randox Laboratories in comparison to a master lot ofcalibrator which is traceable to reference methods or reference materials.If an instrument specific value is not available, refer to the Mean of all Instruments section. If necessary contact Randox Laboratories - Technical Support, Northern Ireland, tel: (028) 9442 2413 or email Technical.Support@NOTES ® All trademarks recognised.(1) Values established by reference laboratories officially recognised by the Federal Chamber of Physicians inGermany.(2) DGKC : German Society for Clinical Chemistry(3) IFCC : International Federation of Clinical Chemistry(4) SCE : Scandinavian Committee on Enzymes14 Nov ’11 nePage 1 of 3Page 2 of 3Page 3 of 3Page 1 of 2Page 2 of 2Page 1 of 1Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 1Page 1 of 1Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 4Page 2 of 4Page 3 of 4Page 4 of 4Page 1 of 2Page 2 of 2Page 1 of 2Page 2 of 2Page 1 of 7Page 2 of 7Page 3 of 7Page 4 of 7Page 5 of 7Page 6 of 7Page 7 of 7Page 1 of 1Page 1 of 1Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 2Page 2 of 2Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 1Page 1 of 1Page 1 of 2Page 2 of 2Page 1 of 2Page 2 of 2。

氨/乙醇质控水平1 : 包装规格 6 x 2 ml : 分类。
编号 EA1366氨/乙醇质控水平2 : 包装规格 6 x 2 ml : 分类。
编号 EA1367氨/乙醇质控水平3 : 包装规格 6 x 2 ml : 分类。
编号 EA1368水平分析物 1 2 3 单位氨 6.9 187 372 μmol/l乙醇0.55 1.55 2.73 g/l高值胆红素质控特征•l 总胆红素靶值接近290 μmol/l•用于监测儿科医学的胆红素水平•冻干品,稳定性强。
•牛基质•根据不同方法提供靶值和范围稳定性•未复溶+2 - 8°C 可稳定至有效期末•复溶后+2 - 8°C可稳定5天特征产品说明高值胆红素质控品 : 包装规格 10 x 3 ml : 分类。
货号BE454甘油质控品特征•冻干品,稳定性强•人基质产品稳定性•未复溶+2 - 8°C可稳定至有效期末•复溶后25°C时可稳定8小时,+2 - 8°C 可稳定7天,-20°C可稳定1个月产品说明甘油质控水平1 : 包装规格 3 x 5 ml : 分类。
货号 .GY1369葡萄糖-6-磷酸脱氢酶质控品特征•冻干品,稳定性强•人基质全血稳定性•+2 - 8°C 可稳定至有效期末•复溶后+2 - 8°C 可稳定5天产品说明葡萄糖-6-磷酸脱氢酶低含量质控品 : 包装规格 6 x 0.5 ml : 分类。
货号 .PD2617 葡萄糖-6-磷酸脱氢酶正常值质控品 : 包装规格 6 x 0.5 ml : 分类。
货号 .PD2618 脑脊液质控特征•包含10个分析物•冻干品,稳定性强•100%全人基质无动物添加成分•适用于大多数的临床分析仪•为常用仪器和方法提供靶值和参考范围稳定性•+2 - 8°C 稳定至有效期末•复溶后在+2 - 8°C可稳定5天,-20°C可稳定1个月。

分析用人基质质控血清水平II货号:HN1530 批号:944UN 包装:20×5ml 效期:06/2018准确量取5ml蒸馏水,小心复溶一瓶冻干质控血清。
注释::注册商标(1). 只适用于德国。
根据德国内科医生联邦议院的方针制订范围(中文说明已略,如需请另行参见英文说明)。
(2). 德国内科医生联邦议院官方认可的参考实验室测定的浓度值。
(3). DGKC:德国临床化学协会(4). IFCC:国际临床化学联盟(5). SCE:斯堪的纳维亚酶委员会使用说明朗道的人基质质控血清为冻干品,用于临床准确性或者重复性质量控制。
朗道供应2种水平的质控血清。
赋值每一批质控血清都要送到参考实验室,根据国际参考标准进行赋值。
若没有国际参考标准,就使用参考方法。
朗道也将质控血清送到全世界3000多家实验室,然后将结果用独特的统计分析赋值。
各个批号的每个分析物,每个方法学都会提供一个质控范围,质控范围值是平均值±2S.D.。
该结果非常准确可靠,实验室尽可放心使用。
准备1. 小心打开瓶盖,避免内容物的任何损失。
2. 在15-25℃的室温下,准确量取5ml蒸馏水复溶1瓶质控血清。
3. 盖上橡皮塞,拧紧瓶盖,使用前避光放置30分钟。
4. 轻轻旋转,确保内容物完全溶解。
勿摇晃,避免形成泡沫。
5. 将小瓶倒置,确保所有的冻干物完全溶解。
复溶后的血清既可以用于手工测试,也可以用于全自动生化分析仪。
该血清只能按照上述步骤复溶。
稳定性该血清自生产之日起,在2-8℃下保存可以稳定4年。
效期标在试剂盒的侧面。
该血清一旦复溶,在15-25℃下可以稳定8小时,在2-8℃下可以稳定7天,在-20℃下稳定28天(只能冻融一次)(见受限情况)。
受限情况1、为保证总酸性磷酸酶和前列腺酸性磷酸酶的稳定性,复溶30分钟后,该血清每1ml应当加入1滴(25-30μl)0.7M的醋酸溶液。
其稳定作用可以使总酸性磷酸酶和前列腺酸性磷酸酶在15-25℃下稳定2小时,在2℃-8℃下稳定2天,在-20℃下稳定28天(只能冻融1次)。

试剂2REAGENTS 推进实验室生化检测23朗道易读和易装型试剂24POWERLINE25朗道 - 全球诊断解决方案提供商26联系我们3朗道试剂优势朗道致力于为全球各地的实验室提供第三方诊断试剂,凭借其卓越的品质和精准的测试结果获得国际高度认可,深受各大实验室信赖。
我们广泛的检测菜单包涵111个检测项目,涵盖逾114 种疾病标志物,包括特定蛋白、血脂、治疗药物监测、药物滥用、抗氧化剂、凝血、糖尿病和兽医学测试。
我们的试剂提供不同的形态,方法学和试剂盒规格,为您带来更大的灵活性和选择性。
我们也拥有全面的分析仪应用列表,结合朗道易读型和易装型试剂,可让您自由选择不同品牌的分析仪。
宽泛的检测菜单无需额外购买昂贵的仪器,即可扩展实验室的检测菜单。
朗道试剂可在大部分主流的生化分析仪上运行。
在实验室内完成更多检测朗道提供不同规格的试剂盒,伴随优异的稳定性(大部分待机稳定性在28天,实验室不必担心产生浪费,可在自身实验室内完成更多检测。
扩展常规检测为195种主流的生化分析仪提供特定的检测试剂,朗道帮助实验室扩展常规检测菜单。
减少人力朗道的液体即用,自动化方法(相较于如胱抑素C使用的传统的手工ELISA方法),易装型试剂,大大减少实验室人力成本投入。
降低成本朗道试剂优异的稳定性和高质量,减少重复检测的次数,多种规格的试剂盒,可帮助实验室有效降低成本。
降低错误发生率原材料的溯源性,极其严格的生产工艺,确保试剂批次的一致性。
所有的检验都经过金标准的验证,低CV和优异的精密度,确保实验室得到正确的检测结果。
123白蛋白肌酐胱抑素 CD-3 羟基丁酸 (Ranbut)果糖胺葡萄糖HbA1c 微量白蛋白游离脂肪酸(NEFA)糖尿病载脂蛋白 A-I 载脂蛋白 B 载脂蛋白 A-II 载脂蛋白 C-II 载脂蛋白 C-III 载脂蛋白 E高密度脂蛋白胆固醇 (HDL)低密度脂蛋白胆固醇 (LDL)胆固醇 (总)脂蛋白 (a)甘油三酯血脂抗链球菌溶血素 O 载脂蛋白 A-I 载脂蛋白 A-II 载脂蛋白 B 载脂蛋白 C-II 载脂蛋白 C-III 载脂蛋白 E CRP 胱抑素 C铁蛋白HbA1c 脂蛋白 (a)微量白蛋白肌红蛋白类风湿因子转铁蛋白甲状腺素运载蛋白 (前白蛋白)特定蛋白谷氨酸转氨酶 (ALT)醛缩酶碱性磷酸酶氨淀粉酶淀粉酶 (胰腺)天冬氨酸转氨酶 (AST)胆汁酸胆红素钙氯化物胆碱酯酶CK-MB CK-NAC 二氧化碳总量铜γ-谷氨酰转移酶 (γGT)谷氨酸脱氢酶 (GLDH)甘油铁乳酸乳酸脱氢酶脂肪酶镁磷钾钠总蛋白尿蛋白尿素尿酸锌4卡马西平锂苯妥英丙戊酸治疗药物监测胆红素 (总)铁蛋白谷胱甘肽过氧化物酶 (Ransel)谷胱甘肽还原酶超氧化物歧化酶 (Ransod)总抗氧化状态 (TAS)转铁蛋白尿酸抗氧化剂谷氨酸转氨酶 (ALT)白蛋白醛缩酶碱性磷酸酶氨淀粉酶天冬氨酸转氨酶 (AST)胆汁酸胆红素钙氯化物高密度脂蛋白胆固醇 (HDL)低密度脂蛋白胆固醇 (LDL)胆固醇 (总)胆碱酯酶CK-NAC 二氧化碳总量铜肌酐CRP CRP (犬)D-3 羟基丁酸 (Ranbut)铁蛋白果糖胺γ-谷氨酰转移酶 (γGT)谷氨酸脱氢酶 (GLDH)葡萄糖谷胱甘肽过氧化物酶 (Ransel)谷胱甘肽还原酶乳酸乳酸脱氢酶脂肪酶微量白蛋白游离脂肪酸(NEFA)磷钾钠超氧化物歧化酶 (Ransod)总抗氧化状态 (TAS)总蛋白甘油三酸酯尿素尿酸尿蛋白锌兽医学抗链球菌溶血素 O CRP类风湿因子快速测试/血清学超敏CRP心脏型脂肪酸结合蛋白 (H-FABP)同型半胱氨酸 (酶法)谷氨酸转氨酶 (ALT) 6 白蛋白 6醛缩酶 6碱性磷酸酶 7 氨 7淀粉酶 7抗链球菌溶血素 O 8 载脂蛋白 A-I 8载脂蛋白 A-II 8载脂蛋白 B 8载脂蛋白 C-II 9 载脂蛋白 C-III 9载脂蛋白 E 9天冬氨酸转氨酶 9胆汁酸 10 胆红素 10钙 10卡马西平 11 氯化物 11高密度脂蛋白胆固醇 (HDL) 11低密度脂蛋白胆固醇 (LDL) 11胆固醇(总) 12 胆碱酯酶 12 CK-MB 12 CK-NAC12谷胱甘肽过氧化物酶 (Ransel) 15谷胱甘肽还原酶 16 甘油 16 HbA1c 16心脏型脂肪酸结合蛋白 (H-FABP) 16同型半胱氨酸 16 D-3-羟基丁酸 17 铁 17 乳酸 17 乳酸脱氢酶 18 脂肪酶 18脂蛋白 (a) 18锂 18镁 18 微量白蛋白 19肌红蛋白 19游离脂肪酸 19苯妥英 19磷 19 钾 20类风湿因子 20钠 20超氧化物歧化酶 (Ransod) 20 总抗氧化状态 (TAS) 20总蛋白 21转铁蛋白 21 甲状腺素运载蛋白(前白蛋白) 21甘油三酯 21尿素 22尿酸 22尿蛋白 22丙戊酸 22锌 22A-Z 试剂索引二氧化碳总量 13 铜 13肌酐 13 CRP 13超敏CRP 14 胱抑素 C 14铁蛋白 14果糖胺 14γ-谷氨酰转移酶 15 谷氨酸脱氢酶 (GLDH) 15葡萄糖 1556白蛋白 (BCG)目录编号: AB38009 x 51ml 20172406853*AB80004 x 68ml 20152403589*醛缩酶 (UV 法)目录编号: AD1895 x 20ml 20172400289**CFDA谷氨酸转氨酶 (ALT)(UV) (IFCC)目录编号:AL7930 R1 7 x 100ml 20172400776*R2 3 x 60mlAL3801 R1 6 x 51ml 20152400339* R2 6 x 14mlAL8006 R1 6 x 56ml 20152400339* R2 6 x 20ml (Mod IFCC)AL8304 R1 4 x 20ml 20152400339* R2 4 x 7ml(Mod IFCC)碱性磷酸酶(DEA 法)(DGKC)目录编号: AP30710 x 10ml 20152402791*AP3803 R1 6 x 51ml 20152402791* R2 6 x 14mlAP9764 R1 7 x 38ml 20152402791*R2 7 x 11.7ml• 提供液体和冻干粉试剂• 当储存于 +2 至 +8o C 时,可在有效期内一直保持稳定• 测量范围:16.3 - 2183U/l • 专用Acusera质控和校准品,可供采购7碱性磷酸酶(AMP 法)目录编号: AP3802 R1 6 x 51ml 20152402789* R2 6 x 14mlAP3877 6 x 21ml 20152402789*AP7927 R1 5 x 100ml 20152402789* R2 5 x 20mlAP8002 R1 7 x 20ml 20152402789* R2 7 x 8ml*CFDA氨(酶 UV 法)目录编号: AM101510 x 5ml (C) 20162400248* AM1054 20 x 3ml (S) 20162400248* AM3979 R1 4 x 20ml 20162400248* R2 2 x 10ml• 冻干粉试剂• 在 +2 至 +8o C 的环境下可保持稳定 3 周,在 +15 至 +25°C 的环境下可保持稳定 5 天• 测量范围:16.9 - 1180μmol/l淀粉酶(亚乙基 PNPG7)目录编号: AY3805R1 4 x 16ml 20172407015*R2 4 x 5mlAY7931 R1 6 x 50ml 20172400284* R2 4 x 18mlAY1580 20 x 5ml 20162401964*AY8335 R1 4 x 20ml 20172407015*R2 4 x 7ml• 提供液体和冻干粉试剂 • 在 +2 至 +8o C 的环境下可在有效期内一直保持稳定• 测量范围:7.3 - 1245U/l • 专用Acusera质控和校准品,可供采购淀粉酶(胰腺)(亚乙基 PNPG7)目录编号:AY7934 R1 6 x 20ml 20172400765* R2 3 x 10ml(乳胶增强免疫比浊法)目录编号: LO3998 R1 2 x 9ml 20162401084*R2 2 x 14mlLO8015 R1 2 x 8.7ml 20162401084*R2 2 x 12mlLO8305 R1 1 x 7.7ml 20162401084*R2 1 x 11.2ml载脂蛋白 A-I (免疫比浊法)目录编号: LP2116 R1 4 x 40ml (C) 20162404617*R2 4 x 17mlLP3838 R1 4 x 30ml 20172407200*R2 4 x 12ml载脂蛋白 A-II(免疫比浊法)目录编号: LP3867 R1 2 x 11ml 20162400257*R2 2 x 5ml目录编号: LP2117载脂蛋白 C-II(免疫比浊法)目录编号: LP3866 R1 2 x 11ml 20192401677*R2 2 x 5ml • 液体即用型试剂• 当储存于 +2 至 +8o C 时,可在有效期内一直保持稳定• 测量范围:1.48 - 9.7 mg/dl• 专用Acusera质控和校准品,可供采购载脂蛋白 C-III(免疫比浊法)目录编号: LP3865 R1 2 x 11ml 20162400258*R2 2 x 5ml载脂蛋白 E(免疫比浊法)目录编号: LP3864 R1 2 x 11ml 20192401679*R2 2 x 5ml天冬氨酸转氨酶 (AST)(UV) (Mod.IFCC)目录编号:AS3804 R1 6 x 51ml 20152402855*R2 6 x 14mlAS8005 R1 6 x 56ml 20152402855*R2 6 x 20mlAS8306 R1 4 x 20ml 20152402855*R2 4 x 7mlAS1204 10 x 10ml 20152402855*胆汁酸(比色法)目录编号: BI3863 R1 2 x 18ml 20172407224*R2 2 x 8ml (第五代)• 液体即用型试剂• 当储存于 +2 至 +8o C 时,可在有效期内一直保持稳定• 测量范围:1.47 - 150μmol/l • 专用Acusera质控和校准品,可供采购胆红素(总)(经改进的 Jendrassik 法)目录编号: BR23612 x 250ml 20172400766*BR243 R1 1 x 100ml (DCA)R2 2 x 100mlBR411 1 x 225ml 20162401722*总和直接胆红素钙 (CPC/AMP)目录编号: CA590 R1 1 x 100ml (S) 20162401702* R2 1 x 100mlCA7941 R1 6 x 50ml 20172400767* R2 3 x 50ml• 液体即用型试剂 • 21 天开瓶稳定性• 测量范围:0.075 - 5.67mmol/l • 专用Acusera质控和校准品,可供采购钙 (Arsenazo)目录编号: CA38719 x 51ml 20172407199* CA8021 4 x 68ml 20172407199*卡马西平(乳胶增强免疫比浊法)目录编号: TD3416 R1 2 x 12ml 20172406860*R2 2 x 5ml氯化物(水银 (II) -硫氰酸盐)目录编号: CL16456 x 500ml 20192402027*高密度脂蛋白胆固醇 (HDL)(直接清除法)目录编号: CH2652R1 6 x 30ml 20172407198*R2 3 x 20mlCH2655 R1 6 x 78ml 20172407198* R2 3 x 52mlCH3811 R1 3 x 51ml 20172407198 R2 3 x 20mlCH8033 R1 4 x 38.2ml 20172407198R2 4 x 18.2ml低密度脂蛋白胆固醇 (LDL)(直接清除法)目录编号: CH2656R1 6 x 78ml 20172406854*R2 3 x 52mlCH3841 R1 3 x 51ml 20172406854* R2 3 x 20mlCH8032 R1 4 x 19.2ml 20172406854* R2 4 x 10.1mlCH8312 R1 4 x 20ml 20172406854R2 4 x 9ml胆固醇(总)(CHOD-PAP)目录编号: CH8019 4 x 68ml 20172407005*• 液体即用型试剂 • 当储存于 +2 至 +8o C 时,可在有效期内一直保持稳定• 测量范围:0.22-21.7 mmol/l • 专用Acusera质控和校准品,可供采购胆碱酯酶(丁酰)(比色法)目录编号: CE190R1 5 x 30ml 20142405709*R2 5 x 1mlCK-MB (免疫抑制法)(UV)目录编号: CK129619 x 2.5ml 20162400876*CK7946 R1 6 x 20ml 20162404621* R2 3 x 10mlCK4043 R1 4 x 20ml 20162400918*R2 4 x 6ml• 提供液体和冻干粉试剂• 当储存于 +2 至 +8o C 时,可在有效期内一直保持稳定• 测量范围:9.1 - 2216U/l • 专用Acusera质控和校准品,可供采购CK-NAC (UV DGKC)目录编号: CK3892R1 4 x 16.5ml 20152403484*R2 4 x 6.2ml (IFCC)• 液体即用型试剂• 当储存于 +2 至 +8o C 时,可在有效期内 一直保持稳定• 测量范围:9.16 - 2886U/l • 专用Acusera质控和校准品,可供采购二氧化碳总量(酶法)目录编号: CD127 10 x 10ml (C) 20153403591*• 冻干粉试剂• 当储存于 +2 至 +8o C 时,可在有效期内一直保持稳定 • 开瓶稳定性:+10o C 下 14 天• 测量范围:0.004 - 50 mmol/l• 专用Acusera质控和校准品,可供采购铜(比色法)目录编号: CU2340 R1 5 x 20 ml (S) 20162404594*R2 1 x 30 ml • 冻干粉试剂• 当储存于 +2 至 +8o C 时,可在 2 周内保持稳定• 测量范围:6.6 - 86 µmol/l肌酐(酶 UV 法)目录编号: CR2336 R1 4 x 50ml (S) 20162404060*R2 4 x 10mlCR2337 R1 4 x 100ml (S) 20162404060*R2 4 x 20ml目录编号: CP7950 R1 7 x 20ml 20162404618*R2 2 x 12mlCRP(免疫比浊法)CRP(全范围)(乳胶增强免疫比浊法)目录编号: CP3847 R1 2 x 11ml 20152400348*R2 2 x 11mlCP3849 R1 4 x 50ml 20152400348*R2 4 x 50mlCP8315 R1 4 x 10ml 20152400348*R2 4 x 10ml超敏CRP(乳胶增强免疫比浊法)目录编号: CP3885 R1 2 x 11ml 20152403485*R2 2 x 11ml胱抑素 C(乳胶增强免疫比浊法)目录编号: CYS4004 R1 2 x 17.6ml 20152402863*R2 2 x 6.1ml铁蛋白(乳胶增强免疫比浊法)目录编号: FN3452 R1 1 x 40ml 20192401678*R2 1 x 20mlFN3453 R1 4 x 40ml 20192401678*R2 4 x 20mlFN3888 R1 3 x 20ml 20192401678*R2 3 x 11mlFN8346 R1 1 x 12.2mlR2 1 x 7.4ml 20192401678*果糖胺(酶法)目录编号: FR3133 R1 5 x 25ml 20152403689*R2 5 x 6.3mlFR4030 R1 4 x 19.8ml 20152403689*R2 4 x 6.9mlγ-谷氨酰转移酶(比色法)目录编号: GT52310 x 10ml20152400927*• 液体即用型试剂 • 当储存于 +2 至 +8o C 时,可在有效期内一直保持稳定 • 测量范围:3.9 - 1285U/l • 专用Acusera质控和校准品,可供采购GLDH (UV) (DGKC)目录编号: GL4418 x 6ml 20172400775*GL4425 x 100ml 20172400775*葡萄糖(GOD-PAP 法和己糖激酶法)目录编号: GL16114 x 100ml (己糖激酶法)(S) 20162402092*GL3881 4 x 50ml (己糖激酶法)20162402092* GL8038 4 x 68ml 20162400420* GL3981 4 x 20ml 20162400420*GL2614 2 x 500ml (S) 20162400420* GL2623 6 x 100ml (S) 20162400420* GL3815 9 x 51ml 20162400420* GL3816 R1 4 x 51ml 20152402802*R2 3 x 20ml (己糖激酶法)• 液体和冻干粉试剂• 在 +2 至 +8o C 的环境下可在有效期内一直保持稳定• 测量范围:0.200 - 35.5mmol/l • 专用Acusera质控和校准品,可供采购谷胱甘肽过氧化物酶 (Ransel)(酶法)目录编号: RS5048 x 6.5ml 20172401191*RS5058 x 10ml 20172401191GT3817 R1 6 x 51ml 20152400927* R2 6 x 14mlGT38746 x 21ml 20152400927*谷胱甘肽还原酶 (UV)目录编号: GR2368 R1 5 x 5ml 20172400300*R2 5 x 3ml甘油(GPO-PAP 法)目录编号: GY105 6 x 15ml (S) 20162400260*HbA1c (Indirect)(乳胶增强免疫比浊法)目录编号: HA3830 R1 3 x 14ml 20162404632*R2 3 x 14ml心脏型脂肪酸结合蛋白 (H-FABP)(免疫比浊法)目录编号: FB4025 R1 1 x 19ml 20172407021*R2 1 x 7ml同型半胱氨酸 (酶法)目录编号: HY4036 R1 2 x 21.7ml (C)R2 2 x 4.6ml铁(Ferene 法)目录编号: SI2572 x 100ml (S) 20162400245*• 冻干粉试剂• 测量范围:0.964 - 150μmol/l • 专用Acusera质控和校准品,可供采购铁(亚铁嗪法)目录编号: SI3821 R1 6 x 20ml 20152402853*R2 3 x 11mlD-3-羟基丁酸 (Ranbut)(酶法)目录编号: RB1007 10 x 10ml (S) 20162404142*RB100810 x 50ml (S) 20162404142*• 冻干粉试剂• 测量范围:0.100 - 5.75 mmol/l(血清)• 专用Acusera质控和校准品,可供采购(S )试剂盒内包含标准品,且该标准品仅可供手工和半自动使用乳酸(比色法)目录编号: LC238916 x 6ml (S) 20162404061乳酸脱氢酶 L - P (LDH) (NAD)目录编号: LD3842 R1 6 x 20ml 20152402843* R2 3 x 18ml乳酸脱氢酶 P - L (UV)目录编号: LD401 20 x 3ml 20162400247*LD3818 R1 6 x 20ml 20162400247*R2 3 x 11ml • 液体和冻干粉试剂• 在 +2 至 +8o C 的环境下可在有效期内一直保持稳定 • 测量范围:42.3 - 1191U/l• 专用Acusera质控和校准品,可供采购脂肪酶(比色法)目录编号: LI3837 R1 3 x 9ml 20162404632*R2 3 x 6ml脂蛋白 (a)(免疫比浊法)目录编号: LP2757 R1 1 x 30ml 20172400769*R2 1 x 15mlLP3403 R1 1 x 10ml 20172400769R2 1 x 6ml锂 (比色法)目录编号: LM4005 R1 2 x 18.3mlR2 2 x 6.5ml20162401522*镁(二甲苯胺蓝法)目录编号: MG3880 6 x 20ml 20182400422*MG8326 2 x 16ml 20182400422*1819微量白蛋白(免疫比浊法)目录编号: MA2426 R1 1 x 60ml (C) 20162404591* R2 1 x 7mlMA3828 R1 6 x 20ml 20162401401* R2 3 x 8ml肌红蛋白(乳胶增强免疫比浊法)目录编号: MY2127 R1 1 x 9.5ml 20172400294*R2 1 x 4.5ml游离脂肪酸(NEFA)(比色法)目录编号: FA115R1 3 x 10ml (C)20162404622*R2 3 x 20ml苯妥英(乳胶增强免疫比浊法)目录编号: TD3409 R1 2 x 17ml 20172406849*R2 2 x 6ml磷(无机)(UV)目录编号: PH1016300ml (S) 20192402028* PH3872 6 x 20ml 20192402028*PH8048 R1 6 x 68ml 20192402028*R2 6 x 40.3ml• 液体即用型试剂• 当储存于 +15 至 +25o C 时,可在有效期内一直保持稳定 • 测量范围:0.065 - 10mmol/l(血清/血浆)• 专用Acusera质控和校准品,可供采购*CFDA20钾 (UV)目录编号: PT3852 R1 3 x 20ml 20172400781*R2 3 x 9mlPT8329 R1 4 x 10ml 20172400781*R2 4 x 6ml• 冻干粉试剂 • 在 +2 至 +8 o C 的环境下可保持稳定 7 天• 测量范围:0.8 - 10mmol/l • 专用Acusera质控和校准品,可供采购类风湿因子(乳胶增强免疫比浊法)目录编号: RF3836 R1 2 x 20ml 20162402303*R2 2 x 8mlRF7980 R1 2 x 15ml 20162404062* R2 1 x 10mlRF8063 R1 2 x 8.7ml 20162404062*R2 2 x 4.5ml RF8345 R1 1 x 11.7mlR2 1 x 5.7ml 20162402303*钠(酶法)目录编号: NA3851R1 3 x 20ml 20172400770*R2 3 x 9mlNA8327 R1 4 x 10ml 20172400770*R2 4 x 6ml总抗氧化状态 (TAS)(比色法)目录编号: NX23325 x 10ml (S) 20172400764*超氧化物歧化酶 (Ransod)(比色法)目录编号: SD1255 x 20ml (S) 20172400278*• 冻干粉试剂• 在 +2 至 +8o C 的环境下可保持稳定 10 天 • 专用Acusera质控和校准品,可供采购(S) 试剂盒内包含标准品,且该标准品仅可供手动和半自动使用。
5.方茴说:“那时候我们不说爱,爱是多么遥远、多么沉重的字眼啊。
我们只说喜欢,就算喜欢也是偷偷摸摸的。
”6.方茴说:“我觉得之所以说相见不如怀念,是因为相见只能让人在现实面前无奈地哀悼伤痛,而怀念却可以把已经注定的谎言变成童话。
”7.在村头有一截巨大的雷击木,直径十几米,此时主干上唯一的柳条已经在朝霞中掩去了莹光,变得普普通通了。
8.这些孩子都很活泼与好动,即便吃饭时也都不太老实,不少人抱着陶碗从自家出来,凑到了一起。
9.石村周围草木丰茂,猛兽众多,可守着大山,村人的食物相对来说却算不上丰盛,只是一些粗麦饼、野果以及孩子们碗中少量的肉食。
1.“噢,居然有土龙肉,给我一块!”2.老人们都笑了,自巨石上起身。
而那些身材健壮如虎的成年人则是一阵笑骂,数落着自己的孩子,拎着骨棒与阔剑也快步向自家中走去。
∙ 含有63个分析物靶值 ∙ 冻干品,稳定性强 ∙ 人基质产品∙ 适用大多数临床分析仪∙ 提供检测方法,仪器,不同温度的靶值和范围∙ 为钙,氯,胆固醇,肌酐, 葡萄糖, 锂,镁,钾,总蛋白,钠, 甘油三酯,尿酸提供参考方法值 ∙ 提供配套复溶液(货号:MS5007) 稳定性∙ +2 - 8°C 稳定至有效期末∙复溶后+2 - 8°C 可稳定7天,-20°C 可稳定1个月天。
(如有例外参见特征说明) 产品说明人定值多项质控血清水平2 : 包装规格 20 x 5 ml : 分类。
编号 HN1530 人定值多项质控血清水平3 : 包装规格 20 x 5 ml : 分类。
编号 HE1532 人定值多项质控血清水平2和3 : 包装规格10 x 5 ml : 分类。
编号 HS2611 水平 水平分析物23单位 分析物23单位酸性磷酸酶(前列腺)12.1 32.5 U/l 免疫球蛋白M0.71 0.76 g/l酸性磷酸酶(总) 17.35 45.5 U/l铁 18.1 36.5 μmd/l白蛋白42.1528.2 g/l总铁结合力 43.5 46.2 μmd/l碱性磷酸酶231.4 416.2 U/l 亮氨酸转肽酶 19 16 U/l谷丙转氨酶 32.6143.4U/l 脂肪酶(比色法) 336 750 U/l 淀粉酶 81.2 314 U/l 脂肪酶(比浊法) 41 100 U/l胰淀粉酶 67310 U/l乳酸 151 525 U/l 载脂蛋白A-1 1.12 1.05 g/l 锂 1.5 5.47 mmol/l 载脂蛋白B 0.59 0.59 g/l 镁 1.02 2.09 mmol/l 谷草转氨酶 36.8160.8U/l游离脂肪酸0.91 1.8 mmol/l 重碳酸盐 16.6 17.4 mmol/l 同渗重摩 1.66 0.83 mmol/l 胆汁酸27.2 44.9μmol/l 扑热息痛(对乙酰氨基酚) 298 370 mmol/kg直接胆红素 17.4 23.8 μmol/l无机磷0.07 0.53 mmol/l 总胆红素28.3 82.8 μmol/钾1.472.2 mmol/l5.方茴说:“那时候我们不说爱,爱是多么遥远、多么沉重的字眼啊。
朗道质控血清1. 什么是朗道质控血清?朗道质控血清是一种用于质量控制的生物制品,主要用于检测和评估实验室在临床诊断、药物研发和科学研究等领域中的分析准确性和可靠性。
它是由一定数量的已知浓度的特定分析目标物质溶解在人或动物血清中制成的。
朗道质控血清通常包含多个不同浓度水平,用于构建标准曲线和评估实验室检测方法的准确性。
2. 为什么需要朗道质控血清?在医学诊断、药物研发和科学研究过程中,准确可靠的检测结果至关重要。
朗道质控血清可以帮助实验室监测其分析方法的稳定性和准确性,并评估其在不同浓度水平下的检测能力。
通过使用朗道质控血清,实验室可以及时发现并纠正可能导致误差和偏差的问题,提高实验结果的可靠性。
3. 朗道质控血清的制备过程朗道质控血清的制备过程主要包括以下几个步骤:3.1 选择合适的分析目标物质根据实验需求,选择具有代表性的分析目标物质。
这些目标物质可以是生化指标、药物成分、病原体抗原等。
3.2 准备血清样本和稀释液采集足够量的人或动物血清样本,并进行初步处理,如离心、去除杂质等。
同时,准备稀释液,一般使用无菌生理盐水或其他适当的缓冲液。
3.3 将分析目标物质溶解于稀释液中将选定的分析目标物质按照一定比例溶解于稀释液中,得到不同浓度水平的溶液。
通常会制备多个浓度水平,以覆盖实验室检测方法的动态范围。
3.4 进行灭菌处理为确保朗道质控血清的无菌性和安全性,需要对溶液进行灭菌处理。
常用的灭菌方法包括高温灭菌、紫外线灭菌和过滤灭菌等。
3.5 分装和储存将制备好的朗道质控血清按照一定容量分装到无菌密封的容器中,并进行储存。
储存条件应符合要求,如低温、避光、避免冻结等。
4. 朗道质控血清的应用朗道质控血清广泛应用于临床诊断、药物研发和科学研究等领域。
其主要应用包括以下几个方面:4.1 实验室质量控制实验室通过使用朗道质控血清来监测其分析方法的稳定性和准确性。
通过比对实验室检测结果与已知浓度的朗道质控血清结果,可以评估实验室的检测能力,并及时发现并纠正可能导致误差和偏差的问题。
朗道混合生化校准品1 控制品用途RANDOX 校准品用于临床检验分析系统的校准。
2 控制品说明RANDOX提供两种水平的校准。
分为二水平和三水平,均为冻干粉。
3 控制品储存不论是否打开控制品,都必须储存在2~8℃。
4 控制品稳定性4.1 未开瓶:在 2~8℃避光、密封的储存条件下可稳定至失效期。
4.2 开瓶:该校准复溶后(未污染、原瓶内、加盖条件下),在 15℃~25℃环境下至少可以稳定 8 小时,在 2℃~8℃环境下至少可以稳定 7 天,在-18℃~-24℃环境下(仅冻融一次)至少可以稳定 28 天(参阅“产品的局限性”说明)。
5 控制品注意事项仅用于体外诊断用途。
不要用嘴吸取移液器。
采取处理实验室试剂要求的常规预防措施。
可索要健康和安全数据表。
该质控品采用人基质,对所有捐献者的血清均进行了艾滋 HIV(HIV1、HIV2)抗体、乙型肝炎表面抗原(HbsAg)和丙型肝炎病毒(HCV)抗体的测试,发现均呈阴性。
测试所采用的方法均通过美国 FDA 认证。
然而,目前没有一种方法能够完全确保该血清无传染物质,因此质控品与所有的病人样品均应当按照可能传播疾病的样品小心处理。
6 控制品准备本控制品为干粉。
使用蒸馏水后复溶后使用。
7 控制品使用方法:7.1 小心打开瓶盖,避免固体内容物的任何损失。
7.2在 20℃~25℃的室温下,用精密量具准确量取瓶签所标毫升数的蒸馏水复溶 1 瓶血清。
7.3盖上橡皮塞,拧紧瓶盖。
7.4轻轻旋转小瓶,确保内容物完全溶解。
7.5将小瓶倒置,确保所有的冻干物完全溶解。
7.6为避免产生气泡,勿摇晃小瓶,使用前避光放置 30 分钟。
取出的血清不应放回原始瓶中,避免交叉污染。
8 定值和范围每批生产的校准品将提交给若干第三方实验室,由这些实验室得到可靠的结果后完成赋值。