常用无机盐溶于水的溶解热(18℃)
- 格式:docx
- 大小:26.27 KB
- 文档页数:4
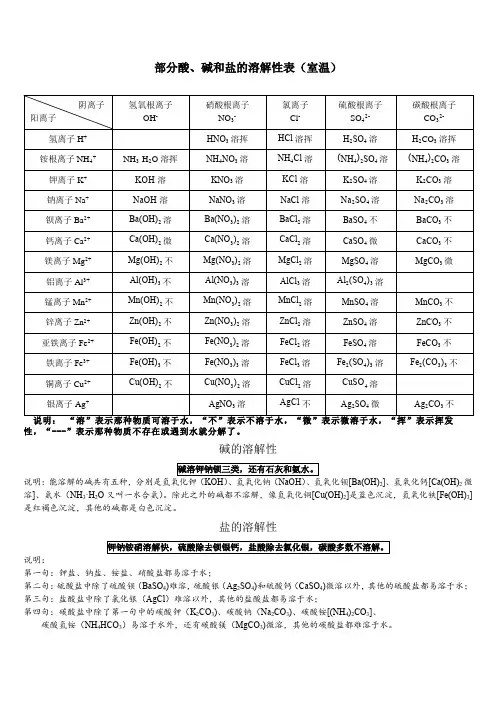
部分酸、碱和盐的溶解性表(室温)
性,“---”表示那种物质不存在或遇到水就分解了。
碱的溶解性
说明:能溶解的碱共有五种,分别是氢氧化钾(KOH)、氢氧化钠(NaOH)、氢氧化钡[Ba(OH)2]、氢氧化钙[Ca(OH)2微溶]、氨水(NH3·H2O又叫一水合氨)。
除此之外的碱都不溶解,像氢氧化铜[Cu(OH)2]是蓝色沉淀,氢氧化铁[Fe(OH)3]是红褐色沉淀,其他的碱都是白色沉淀。
盐的溶解性
说明:
第一句:钾盐、钠盐、铵盐、硝酸盐都易溶于水;
第二句:硫酸盐中除了硫酸钡(BaSO4)难溶,硫酸银(Ag2SO4)和硫酸钙(CaSO4)微溶以外,其他的硫酸盐都易溶于水;第三句:盐酸盐中除了氯化银(AgCl)难溶以外,其他的盐酸盐都易溶于水;
第四句:碳酸盐中除了第一句中的碳酸钾(K2CO3)、碳酸钠(Na2CO3)、碳酸铵[(NH4)2CO3]、碳酸氢铵(NH4HCO3)易溶于水外,还有碳酸镁(MgCO3)微溶,其他的碳酸盐都难溶于水。
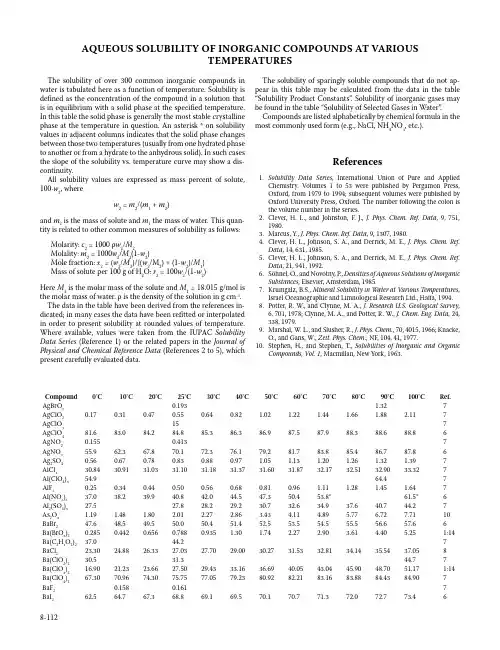
AQUEOUS SOLUBILITY OF INORGANIC COMPOUNDS AT VARIOUSTEMPERATURESThe solubility of over 300 common inorganic compounds in water is tabulated here as a function of temperature. Solubility is defined as the concentration of the compound in a solution that is in equilibrium with a solid phase at the specified temperature. In this table the solid phase is generally the most stable crystalline phase at the temperature in question. An asterisk * on solubility values in adjacent columns indicates that the solid phase changes between those two temperatures (usually from one hydrated phase to another or from a hydrate to the anhydrous solid). In such cases the slope of the solubility vs. temperature curve may show a dis-continuity.All solubility values are expressed as mass percent of solute, 100⋅w2, wherew2 = m2/(m1 + m2)and m2 is the mass of solute and m1 the mass of water. This quan-tity is related to other common measures of solubility as follows: Molarity: c2 = 1000 ρw2/M2Molality: m2 = 1000w2/M2(1-w2)Mole fraction: x2 = (w2/M2)/{(w2/M2) + (1-w2)/M1}Mass of solute per 100 g of H2O: r2 = 100w2/(1-w2)Here M2 is the molar mass of the solute and M1 = 18.015 g/mol is the molar mass of water. ρ is the density of the solution in g cm-3. The data in the table have been derived from the references in-dicated; in many cases the data have been refitted or interpolated in order to present solubility at rounded values of temperature. Where available, values were taken from the IUPAC Solubility Data Series (Reference 1) or the related papers in the Journal of Physical and Chemical Reference Data (References 2 to 5), which present carefully evaluated data.The solubility of sparingly soluble compounds that do not ap-pear in this table may be calculated from the data in the table “Solubility Product Constants”. Solubility of inorganic gases may be found in the table “Solubility of Selected Gases in Water”. Compounds are listed alphabetically by chemical formula in the most commonly used form (e.g., NaCl, NH4NO3, etc.).References1. Solubility Data Series, International Union of Pure and AppliedChemistry. Volumes 1 to 53 were published by Pergamon Press, Oxford, from 1979 to 1994; subsequent volumes were published by Oxford University Press, Oxford. The number following the colon is the volume number in the series.2. Clever, H. L., and Johnston, F. J., J. Phys. Chem. Ref. Data, 9, 751,1980.3. Marcus, Y., J. Phys. Chem. Ref. Data, 9, 1307, 1980.4. Clever, H. L., Johnson, S. A., and Derrick, M. E., J. Phys. Chem. Ref.Data, 14, 631, 1985.5. Clever, H. L., Johnson, S. A., and Derrick, M. E., J. Phys. Chem. Ref.Data, 21, 941, 1992.6. Söhnel, O., and Novotny, P., Densities of Aqueous Solutions of InorganicSubstances, Elsevier, Amsterdam, 1985.7. Krumgalz, B.S., Mineral Solubility in Water at Various Temperatures,Israel Oceanographic and Limnological Research Ltd., Haifa, 1994.8. Potter, R. W., and Clynne, M. A., J. Research U.S. Geological Survey,6, 701, 1978; Clynne, M. A., and Potter, R. W., J. Chem. Eng. Data, 24, 338, 1979.9. Marshal, W. L., and Slusher, R., J. Phys. Chem., 70, 4015, 1966; Knacke,O., and Gans, W., Zeit. Phys. Chem., NF, 104, 41, 1977.10. Stephen, H., and Stephen, T., Solubilities of Inorganic and OrganicCompounds, Vol. 1, Macmillan, New York, 1963.Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. AgBrO30.193 1.327 AgClO20.170.310.470.550.640.82 1.02 1.22 1.44 1.66 1.88 2.117 AgClO3157 AgClO481.683.084.284.885.386.386.987.587.988.388.688.86 AgNO20.1550.4137 AgNO355.962.367.870.172.376.179.281.783.885.486.787.86 Ag2SO40.560.670.780.830.880.97 1.05 1.13 1.20 1.26 1.32 1.397 AlCl330.8430.9131.0331.1031.1831.3731.6031.8732.1732.5132.9033.327 Al(ClO4)354.964.47 AlF30.250.340.440.500.560.680.810.96 1.11 1.28 1.45 1.647 Al(NO3)337.038.239.940.842.044.547.350.453.8*61.5*6 Al2(SO4)327.527.828.229.230.732.634.937.640.744.27 As2O3 1.19 1.48 1.80 2.01 2.27 2.86 3.43 4.11 4.89 5.77 6.727.7110 BaBr247.648.549.550.050.451.452.553.554.555.556.657.66 Ba(BrO3)20.2850.4420.6560.7880.935 1.30 1.74 2.27 2.90 3.61 4.40 5.251:14 Ba(C2H3O2)237.044.27 BaCl223.3024.8826.3327.0327.7029.0030.2731.5332.8134.1435.5437.058 Ba(ClO2)230.531.344.77 Ba(ClO3)216.9021.2323.6627.5029.4333.1636.6940.0543.0445.9048.7051.171:14 Ba(ClO4)267.3070.9674.3075.7577.0579.2380.9282.2183.1683.8884.4384.907 BaF20.1580.1617 BaI262.564.767.368.869.169.570.170.771.372.072.773.468-112Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. Ba(IO3)20.01820.02620.03420.03960.045*0.058*0.0730.0900.1090.1310.1560.1821:14 Ba(NO2)231.136.641.844.346.851.656.260.564.668.572.175.610 Ba(NO3)2 4.7 6.38.29.310.212.414.717.019.321.523.525.56 Ba(OH)2 1.67 4.688.4193352741007 BaS 2.79 4.78 6.978.219.5812.6716.1820.0524.1928.5533.0437.617 Ba(SCN)262.67 BaSO30.00111:26 BeCl240.541.77 Be(ClO4)259.57 BeSO426.6927.5828.6129.2229.9031.5133.3935.5037.7840.2142.7245.287 CaBr2555659616368717310 CaCl236.7039.1942.1344.83*49.12*52.85*56.05*56.7357.4458.2159.0459.948 Ca(ClO3)263.264.265.566.367.269.071.073.275.5*77.4*77.778.01:14 Ca(ClO4)265.37 CaF20.00130.001610 CaI264.666.067.668.369.070.872.474.076.078.079.681.07 Ca(IO3)20.0820.1550.2430.3050.384*0.517*0.5900.6520.811*0.665*0.6681:14 Ca(NO2)238.639.544.548.67 Ca(NO3)250.153.156.759.060.965.477.878.178.278.378.478.56 CaSO30.00590.00540.00490.00410.00350.00300.00260.00230.00200.00191:26 CaSO40.1740.1910.2020.2050.2080.2100.2070.2010.1930.1840.1730.1639 CdBr236.043.049.953.456.460.3*60.3*60.560.760.961.361.66 CdC2O40.00605 CdCl247.250.153.254.656.3*57.3*57.557.858.158.5158.9859.56 Cd(ClO4)258.766.97 CdF2 5.82 4.65 4.18 3.765 CdI244.144.945.846.346.847.949.050.251.552.754.155.46 Cd(IO3)20.0915 Cd(NO3)255.457.159.661.062.866.570.686.186.586.887.187.46 CdSO443.143.143.243.443.644.143.542.541.440.238.536.76 CdSeO442.0440.5939.0238.1837.2935.3533.1530.6527.8424.6921.2417.495 Ce(NO3)357.9959.8061.8963.0564.31*67.0*68.671.1*74.9*79.280.983.11:13 CoCl230.3032.6034.8735.9937.1039.2741.3843.4645.5047.5149.5151.507 Co(ClO4)250.053.07 CoF2 1.47 CoI258.0061.7865.3566.9968.5171.1773.4175.2976.8978.2879.5280.707 Co(NO2)20.0760.497 Co(NO3)245.547.049.450.852.456.060.162.664.967.76 CoSO419.923.026.127.729.232.334.435.935.533.230.627.86 Co(SCN)250.77 CrO362.262.362.662.863.063.564.164.765.566.267.167.96 CsBr55.27 CsBrO3 1.16 1.93 3.01 3.69 4.46 6.328.6011.3214.4517.9621.8325.981:30 CsCl61.8363.4864.9665.6466.2967.5068.6069.6170.5471.4072.2172.961:47 CsClO3 2.40 3.87 5.947.228.6912.1516.3321.1426.4532.1037.8943.421:30 CsClO40.79 1.01 1.51 1.96 2.57 4.28 6.559.2912.4115.8019.3923.077 CsI30.937.243.245.948.653.357.360.763.665.967.769.26 CsIO3 1.08 1.58 2.21 2.59 3.02 3.96 5.06 6.297.709.2010.7912.451:30 CsNO38.4613.018.621.825.132.039.045.751.957.362.166.26 CsOH757 Cs2SO462.663.464.164.564.865.566.166.767.367.868.368.86 CuBr255.87 CuCl240.841.742.643.143.744.846.047.248.549.951.352.76 Cu(ClO4)254.359.37 CuF20.0757 Cu(NO3)245.249.856.359.261.162.063.164.565.967.569.271.06 CuSO412.414.416.718.019.322.225.428.832.436.340.343.56 CuSeO410.616.07 Dy(NO3)358.7959.9961.4962.3563.2965.4368.0471.581:13 Er(NO3)361.5863.1564.8465.7566.6968.7070.9673.6477.751:13 Eu(NO3)355.256.758.559.460.462.564.61:13Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. FeBr254.664.8*7 FeCl233.2*39.4*48.7*7 FeCl342.744.947.947.751.674.876.784.684.384.384.484.76 Fe(ClO4)263.3967.767 FeF3 5.597 Fe(NO3)340.1546.577 Fe(NO3)241.4446.677 FeSO413.517.020.822.824.828.832.835.533.630.427.124.06 Gd(NO3)356.357.759.260.161.062.965.267.971.51:13 HIO373.4574.1074.9875.4876.0377.2078.4679.7881.1382.4883.8285.141:30 H3BO3 2.61 3.57 4.77 5.48 6.278.1010.312.915.919.323.127.36 HgBr20.260.370.520.610.720.96 1.26 1.63 2.08 2.61 3.23 3.954 Hg(CN)2 6.577.839.3310.211.113.115.518.221.224.628.332.36 HgCl2 4.24 5.05 6.17 6.817.629.5312.0215.1819.1624.0629.9036.624 HgI20.00410.00550.00720.01220.01994 Hg(SCN)20.0704 Hg2Cl20.00043 Hg2(ClO4)273.879.8*85.3*7 Hg2SO40.0380.0430.0480.0510.0540.0590.0650.0700.0760.0820.0880.0934 Ho(NO3)363.81:13 KBF40.280.340.450.550.75 1.38 2.09 2.82 3.58 4.34 5.12 5.9010 KBr35.037.339.440.441.443.244.846.247.648.849.850.86 KBrO3 2.97 4.48 6.427.558.7911.5714.7118.1421.7925.5729.4233.281:30 KC2H3O268.4070.2972.0972.9273.7075.0876.2777.3178.2279.0479.8080.557 KCl21.7423.6125.3926.2227.0428.5930.0431.4032.6633.8634.9936.051:47 KClO3 3.03 4.67 6.747.939.2112.0615.2618.7822.6526.8831.5336.651:30 KClO40.70 1.10 1.67 2.04 2.47 3.54 4.94 6.748.9911.7114.9418.676 KF30.9039.847.350.4153.260.07 KHCO318.6221.7324.9226.628.1331.3234.4637.5140.456 KHSO427.129.732.333.635.037.840.543.446.249.0251.8254.66 KH2PO411.7414.9118.2519.9721.7725.2828.9532.7636.7540.9645.4150.121:31 KI56.057.659.059.760.461.662.863.864.865.766.667.46 KIO3 4.53 5.967.578.449.3411.0913.2215.2917.4119.5821.7824.031:30 KIO40.160.220.370.510.70 1.24 1.96 2.83 3.82 4.89 6.027.177 KMnO4 2.74 4.12 5.967.068.2811.1114.4218.166 KNO273.774.675.375.776.076.777.478.078.579.179.680.16 KNO312.017.624.227.731.338.645.752.258.063.067.370.86 KOH48.750.853.254.756.157.958.659.560.661.863.164.66 KSCN63.866.469.170.471.674.176.578.981.183.385.387.36 K2CO351.351.752.352.753.154.054.956.057.258.459.661.06 K2CrO437.138.138.939.439.840.541.341.942.643.243.844.36 K2Cr2O7 4.307.1210.913.115.520.826.331.736.941.545.548.96 K2HAsO448.5*63.6*79.8*7 K2HPO457.059.161.562.764.167.7*72.7*1:31 K2MoO464.766.57 K2SO351.3051.3951.4951.5551.6251.7651.9352.1152.3252.5452.7953.061:26 K2SO47.118.469.9510.711.412.914.215.516.717.718.619.36 K2S2O349.0*62.3*75.7*7 K2S2O522.126.731.133.135.239.042.646.049.152.054.61:26 K2SeO368.4*68.5*68.5*7 K2SeO452.7052.9353.1753.3053.4353.7053.9954.3054.6154.9455.2655.607 K3AsO451.5*55.6*73*7 K3Fe(CN)623.927.631.132.834.337.239.641.743.545.046.147.06 K3PO444.351.47 K4Fe(CN)612.517.322.023.925.629.232.535.538.240.641.443.16 LaCl349.048.548.648.949.350.552.154.056.358.961.76 La(NO3)355.056.958.960.061.163.666.369.9*74.1*1:13 LiBr58.460.162.764.465.967.868.369.069.870.771.772.86 LiBrO361.0362.6264.4465.4466.5168.9071.68*73.24*74.4375.6676.9378.321:30 LiC2H3O223.7626.4929.4231.0232.7236.4840.6545.1549.9354.9160.0465.267 LiCl40.4542.46*45.29*45.8146.2547.3048.4749.7851.2752.9854.98*56.34*1:47Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. LiClO373.275.6*80.8*82.183.485.9*87.1*88.289.691.393.495.71:30 LiClO430.132.635.537.038.641.945.549.253.257.261.371.46 LiF0.1200.1260.1310.1347 LiH2PO455.87 LiI59.460.561.762.363.064.365.867.368.881.381.782.66 LiIO343.81:30 LiNO24145495153566063666810 LiNO334.837.642.750.557.960.162.264.065.767.268.569.76 LiOH10.810.811.011.111.311.712.212.713.414.215.116.16 LiSCN54.57 Li2CO3 1.54 1.43 1.33 1.28 1.24 1.15 1.070.990.920.850.780.727 Li2C2O4 5.877 Li2HPO39.078.407.777.477.18 6.64 6.16 5.71 5.30 4.91 4.53 4.167 Li2SO426.325.925.625.525.325.024.824.524.324.023.823.66 Li3PO40.0271:31 Lu(NO3)371.11:13 MgBr249.349.850.350.650.951.552.152.853.554.255.055.76 Mg(BrO3)243.045.248.049.451.054.357.961.665.369.0*70.9*71.71:14 Mg(C2H3O2)236.1837.5538.9239.617 MgC2O40.0387 MgCl233.9634.8535.5835.9036.2036.7737.3437.9738.7139.6240.7542.158 Mg(ClO3)253.3554.4056.8158.6660.91*65.46*67.3369.2771.0172.4473.481:14 Mg(ClO4)247.848.749.650.150.551.352.16 MgCrO432.06*35.39*7 MgCr2O758.967.07 MgF20.0137 MgI254.756.158.259.460.863.965.065.065.065.065.165.26 Mg(IO3)2 3.19* 6.70*7.928.529.1110.4511.9913.715.617.619.61:14 Mg(NO2)2477 Mg(NO3)238.439.540.841.642.444.145.947.950.052.270.672.06 MgSO30.320.370.460.520.610.87*0.85*0.760.690.640.620.601:26 MgSO418.221.725.126.328.230.933.435.636.935.934.733.36 MgS2O330.734.17 MgSeO431.4*35.7*47*7 MnBr256.0057.7259.3960.1960.9662.4163.7565.0166.1967.3268.4269.507 MnCl238.740.642.543.644.747.049.454.154.755.255.756.16 MnF20.80* 1.01*0.487 Mn(IO3)20.270.347 Mn(NO3)250.561.77 MnSO434.637.338.638.938.937.736.334.632.830.828.826.76 NH4Br37.540.242.743.945.147.349.451.353.054.656.157.47 NH4Cl22.9225.1227.2728.3429.3931.4633.5035.4937.4639.4041.3343.241:47 NH4ClO410.814.117.819.721.725.829.833.637.340.743.846.66 NH4F41.743.244.745.546.347.849.350.952.554.17 NH4HCO310.613.717.619.922.427.934.241.449.358.167.678.07 NH4H2AsO425.229.032.734.536.339.743.146.249.352.255.07 NH4H2PO417.822.026.428.831.236.241.647.253.059.265.772.47 NH4I60.762.163.464.064.665.866.867.868.769.670.471.16 NH4IO3 3.70 4.20 5.647.631:30 NH4NO255.759.064.968.87 NH4NO354.060.165.568.070.374.377.780.883.485.888.290.36 NH4SCN64.481.17 (NH4)2C2O4 2.31 3.11 4.25 4.94 5.737.569.7312.215.118.321.825.77 (NH4)2HPO436.438.240.041.042.044.146.248.550.953.355.958.67 (NH4)2S2O565.567.969.870.571.372.372.973.11:26 (NH4)2S2O837.0040.4543.8445.4947.1150.2553.2856.2359.1362.007 (NH4)2SO332.234.937.739.140.643.747.050.654.558.91:26 (NH4)2SO441.342.142.943.343.844.745.646.647.548.549.550.56 (NH4)2SeO349.051.153.454.756.058.962.065.469.17 (NH4)2SeO454.027 (NH4)3PO415.57Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. NaBr44.445.947.748.649.651.653.754.154.354.554.754.96 NaBrO320.023.2226.6528.2829.8632.8335.5538.0540.3742.521:30 NaCHO230.837.945.748.750.652.053.555.06 NaC2H3O226.528.831.833.535.539.945.158.359.360.561.762.96 NaCl26.2826.3226.4126.4526.5226.6726.8427.0327.2527.5027.7828.051:47 NaClO22.744.47 NaClO297.0*95.3*7 NaClO344.2746.6749.350.151.253.655.557.058.560.563.367.11:30 NaClO461.964.166.267.268.370.472.574.174.775.476.176.76 NaF 3.52 3.72 3.89 3.97 4.05 4.20 4.34 4.46 4.57 4.66 4.75 4.826 NaHCO3 6.487.598.739.329.9111.1312.4013.7015.0216.3717.7319.107 NaHSO422.233.310 NaH2PO436.5441.0746.0048.6851.5457.89*61.7*62.3*65.968.71:31 NaI61.262.463.964.865.767.769.872.074.774.874.975.16 NaIO3 2.43 4.407.78*8.65*9.6011.6713.9916.5219.25*21.1*22.924.71:30 NaIO412.627 NaNO241.943.445.145.946.848.750.752.855.057.259.561.86 NaNO342.244.446.647.748.851.053.255.357.559.661.763.86 NaOH30394650535863677174767910 NaSCN52.957.160.262.763.564.265.065.966.967.969.06 Na2B4O7 1.23 1.71 2.50 3.07 3.82 6.029.714.917.119.923.528.06 Na2CO3 6.4410.817.923.528.732.832.231.731.331.130.930.96 Na2C2O4 2.62 2.95 3.30 3.48 3.65 4.00 4.36 4.71 5.06 5.41 5.75 6.086 Na2CrO422.632.344.646.746.948.951.053.455.355.555.856.16 Na2Cr2O762.163.164.465.266.168.070.172.374.677.079.680.76 Na2HAsO4 5.6*29.3*67*7 Na2HPO4 1.66 4.197.5110.5516.34*35.17*44.64*45.2046.8148.7850.5251.531:31 Na2MoO430.638.839.439.439.840.341.041.742.643.544.545.56 Na2S11.113.215.717.118.622.126.728.130.233.036.441.06 Na2SO312.016.120.923.526.3*27.3*25.924.823.722.822.121.51:26 Na2SO416.1321.9429.22*32.35*31.5530.9030.3930.0229.7929.678 Na2S2O333.136.340.643.345.952.062.365.768.869.470.171.06 Na2S2O538.439.540.040.641.843.044.245.546.848.149.51:26 Na2SeO347.3*45*7 Na2SeO411.736.9*42.1*7 Na2WO441.641.942.342.642.943.644.445.346.247.348.449.56 Na3PO4 4.287.3010.812.614.116.622.928.432.437.640.443.56 Na4P2O7 2.23 3.28 4.81 6.627.0010.1014.3820.0727.3136.0332.3730.676 NdCl349.049.349.750.050.451.252.253.354.555.857.158.56 Nd(NO3)355.7657.4959.3760.3861.4363.6966.2769.471:13 NiCl234.736.138.540.341.742.143.245.046.146.246.446.66 Ni(ClO4)251.152.87 NiF2 2.50 2.527 NiI255.4057.6859.7860.6961.5062.8063.7364.3864.8065.0965.307 Ni(NO3)244.146.048.449.851.354.658.361.063.165.667.969.06 NiSO421.424.427.428.830.3*32.0*34.135.837.739.942.344.86 Ni(SCN)235.487 NiSeO421.626.2*45.6*7 PbBr20.4490.6200.8410.966 1.118 1.46 1.892 PbCl20.660.810.98 1.07 1.17 1.39 1.64 1.93 2.24 2.60 2.99 3.422 Pb(ClO4)281.57 PbF20.06030.06490.06700.06932 PbI20.0410.0520.0670.0760.0860.1120.1440.1870.2430.3152 Pb(IO3)20.00257 Pb(NO3)228.4632.1335.6737.3839.0542.2245.1747.9050.4252.7254.8256.752 PbSO40.00330.00380.00420.00440.00470.00520.00582 PrCl348.048.148.649.049.550.852.354.156.158.36 Pr(NO3)357.5059.2061.1662.2463.40*65.7*67.870.273.41:13 RbBr47.450.152.653.854.957.058.860.662.163.564.865.96 RbBrO30.97 1.55 2.36 2.87 3.45 4.87 6.648.7811.2914.1517.3220.761:30 RbCl43.5845.6547.5348.4249.2750.8652.3453.6754.9256.0857.1658.151:47Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. RbClO3 2.10 3.38 5.14 6.227.4510.3513.8517.9322.5327.5732.9638.601:30 RbClO41 1.5177 RbF757 RbHCO353.77 RbI55.858.661.162.363.465.467.268.870.371.672.773.86 RbIO3 1.09 1.53 2.07 2.38 2.74 3.52 4.41 5.42 6.527.749.0010.361:30 RbNO316.425.034.639.444.253.160.867.272.276.179.081.26 RbOH63.47 Rb2CrO438.2743.267 Rb2SO427.330.032.533.734.836.938.740.341.843.044.144.96 SbCl385.790.87 SbF379.483.17 Sc(NO3)357.059.361.662.863.966.268.51:13 Sm(NO3)354.8356.3358.0859.0560.0862.3865.05*68.1*70.874.21:13 SmCl348.048.248.448.649.250.06 SnCl246647 SnI20.97 3.877 SrBr246.048.350.651.752.955.257.659.962.364.666.869.06 Sr(BrO3)218.5322.0025.3927.0228.5931.5534.2136.5738.64*40.2*40.841.01:14 SrCl231.9432.9334.4335.3736.4338.9341.9445.44*46.81*47.6948.7049.878 Sr(ClO2)213.013.614.114.314.514.915.315.615.97 Sr(ClO3)263.2963.4263.6463.7763.9364.2964.7065.1665.6566.1866.7467.311:14 Sr(ClO4)270.04*75.35*78.44*7 SrF20.0110.0217 SrI262.562.863.563.964.565.867.369.070.872.774.779.26 Sr(IO3)20.1020.1260.1520.1650.1790.2060.2330.2590.2840.3070.3280.3461:14 Sr(MnO4)2 2.57 Sr(NO2)241.944.358.67 Sr(NO3)228.234.641.044.547.047.447.948.448.949.550.150.76 Sr(OH)20.9 2.27 SrSO30.00151:26 SrSO40.01357 SrS2O38.813.217.720.022.226.87 Tb(NO3)360.661.021:13 Tl2SO4 2.65 3.56 4.61 5.19 5.807.098.469.8911.3312.7714.1815.536 Tm(NO3)367.91:13 UO2(NO3)249.5251.8254.4255.8557.5561.5967.071:55 Y(NO3)355.5756.9358.7559.8661.11*63.3*64.967.972.51:13 Yb(NO3)370.51:13 ZnBr279.380.181.883.084.185.685.886.186.386.686.887.16 ZnC2O40.00100.00190.00265 ZnCl276.679.080.381.481.882.483.083.784.485.286.06 Zn(ClO4)244.29*46.27*48.707 ZnF2 1.535 ZnI281.181.281.381.481.581.782.082.382.683.083.383.76 Zn(IO3)20.580.640.690.770.825 Zn(NO3)247.850.854.454.658.579.180.187.589.96 ZnSO30.17860.17900.17940.18030.18125 ZnSO429.132.035.036.638.241.343.042.141.039.938.837.66 ZnSeO433.0634.9837.3838.7940.345。

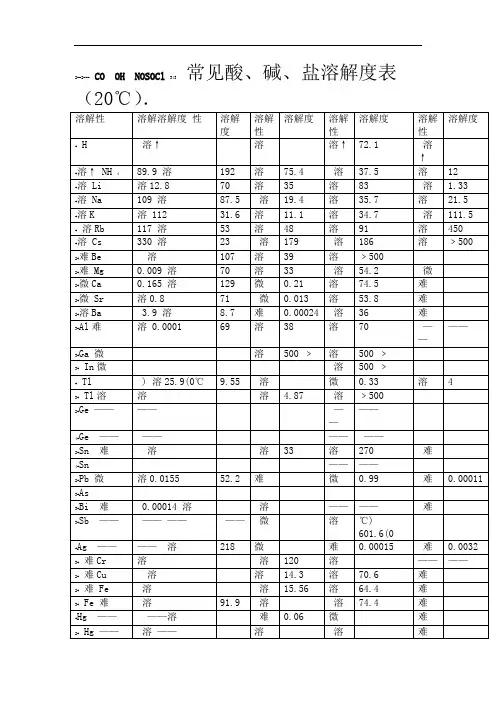
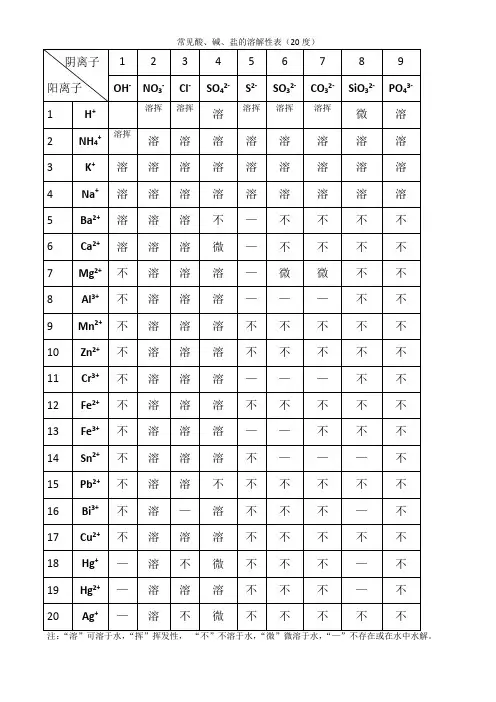
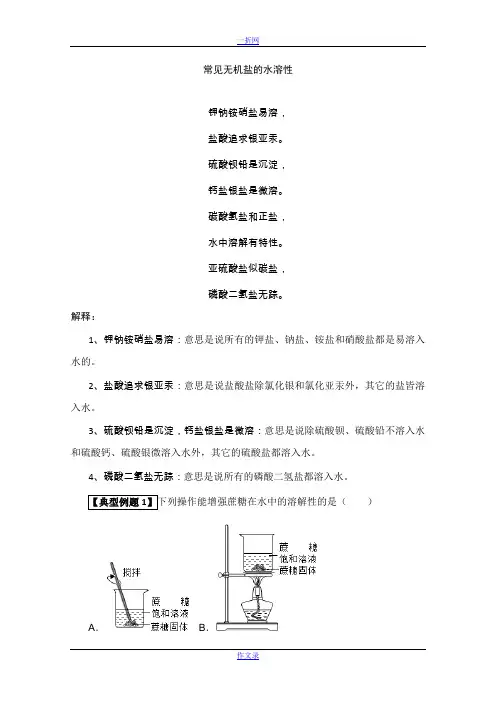
常见无机盐的水溶性钾钠铵硝盐易溶,盐酸追求银亚汞。
硫酸钡铅是沉淀,钙盐银盐是微溶。
碳酸氢盐和正盐,水中溶解有特性。
亚硫酸盐似碳盐,磷酸二氢盐无踪。
解释:1、钾钠铵硝盐易溶:意思是说所有的钾盐、钠盐、铵盐和硝酸盐都是易溶入水的。
2、盐酸追求银亚汞:意思是说盐酸盐除氯化银和氯化亚汞外,其它的盐皆溶入水。
3、硫酸钡铅是沉淀,钙盐银盐是微溶:意思是说除硫酸钡、硫酸铅不溶入水和硫酸钙、硫酸银微溶入水外,其它的硫酸盐都溶入水。
4、磷酸二氢盐无踪:意思是说所有的磷酸二氢盐都溶入水。
)A.B.C.D.【考点】物质的溶解性及影响溶解性的因素【分析】根据固体溶解度的概念和固体溶解度的影响因素解答本题.固体的溶解度受温度,溶质,溶剂的性质影响【解析】由于蔗糖的溶解度随温度的升高而增大,因此使蔗糖在水中的溶解性只能升高温度.饱和溶液不能再继续溶解这种溶质,不会增大蔗糖的溶解性,故选B 【点评】主要考查了固体的溶解度和温度之间的关系,并由此培养学生分析问题、解决问题的能力)A.AlCl3溶液加过量NaOH溶液B.NaAlO2溶液加过量盐酸C.NaAlO2溶液通入过量CO2D.石灰水通入过量CO2【考点】镁、铝的重要化合物【分析】A、氢氧化钠先和氯化铝反应生成氢氧化铝,氢氧化铝和氢氧化钠能反应生成偏铝酸钠;B、偏铝酸钠和盐酸反应先生成氢氧化铝,氢氧化铝和盐酸反应生成氯化铝;C、偏铝酸钠和二氧化碳反应生成氢氧化铝;D、氢氧化钙和二氧化碳反应生成碳酸钙,碳酸钙和二氧化碳、水反应生成碳酸氢钙。
根据生成物是溶解性判断即可。
【解析】A、氯化铝和氢氧化钠反应生成难溶性的氢氧化铝,当氢氧化钠过量时,氢氧化铝又和氢氧化钠反应生成可溶性的偏铝酸钠,所以最后没有沉淀生成,故A 错误;B、偏铝酸钠和盐酸反应生成难溶性的氢氧化铝,当盐酸过量时,氢氧化铝又和盐酸反应生成可溶性的氯化铝,所以最后没有沉淀生成,故B错误;C、无论二氧化碳是否过量,偏铝酸钠都和二氧化碳反应生成氢氧化铝沉淀,所以最后有沉淀生成,故C正确;D、氢氧化钙和二氧化碳反应生成难溶性的碳酸钙,碳酸钙和水、二氧化碳继续反应生成可溶性的碳酸氢钙,所以最后没有沉淀生成,故D 错误。
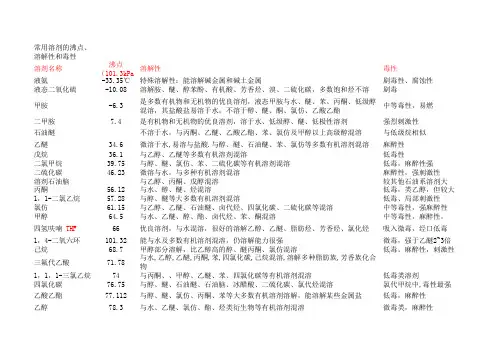
常用溶剂的沸点、溶解性和毒性溶剂名称沸点(101.3kPa溶解性毒性液氨-33.35℃特殊溶解性:能溶解碱金属和碱土金属剧毒性、腐蚀性液态二氧化硫-10.08溶解胺、醚、醇苯酚、有机酸、芳香烃、溴、二硫化碳,多数饱和烃不溶剧毒甲胺-6.3是多数有机物和无机物的优良溶剂,液态甲胺与水、醚、苯、丙酮、低级醇混溶,其盐酸盐易溶于水,不溶于醇、醚、酮、氯仿、乙酸乙酯中等毒性,易燃二甲胺7.4是有机物和无机物的优良溶剂,溶于水、低级醇、醚、低极性溶剂强烈刺激性石油醚不溶于水,与丙酮、乙醚、乙酸乙酯、苯、氯仿及甲醇以上高级醇混溶与低级烷相似乙醚34.6微溶于水,易溶与盐酸.与醇、醚、石油醚、苯、氯仿等多数有机溶剂混溶麻醉性戊烷36.1与乙醇、乙醚等多数有机溶剂混溶低毒性二氯甲烷39.75与醇、醚、氯仿、苯、二硫化碳等有机溶剂混溶低毒,麻醉性强二硫化碳46.23微溶与水,与多种有机溶剂混溶麻醉性,强刺激性溶剂石油脑与乙醇、丙酮、戊醇混溶较其他石油系溶剂大丙酮56.12与水、醇、醚、烃混溶低毒,类乙醇,但较大1,1-二氯乙烷57.28与醇、醚等大多数有机溶剂混溶低毒、局部刺激性氯仿61.15与乙醇、乙醚、石油醚、卤代烃、四氯化碳、二硫化碳等混溶中等毒性,强麻醉性甲醇64.5与水、乙醚、醇、酯、卤代烃、苯、酮混溶中等毒性,麻醉性,四氢呋喃 THF66优良溶剂,与水混溶,很好的溶解乙醇、乙醚、脂肪烃、芳香烃、氯化烃吸入微毒,经口低毒1,4-二氧六环101.32能与水及多数有机溶剂混溶,仍溶解能力很强微毒,强于乙醚2~3倍己烷68.7甲醇部分溶解,比乙醇高的醇、醚丙酮、氯仿混溶低毒。
麻醉性,刺激性三氟代乙酸71.78与水,乙醇,乙醚,丙酮,苯,四氯化碳,己烷混溶,溶解多种脂肪族,芳香族化合物1,1,1-三氯乙烷74与丙酮、、甲醇、乙醚、苯、四氯化碳等有机溶剂混溶低毒类溶剂四氯化碳76.75与醇、醚、石油醚、石油脑、冰醋酸、二硫化碳、氯代烃混溶氯代甲烷中,毒性最强乙酸乙酯77.112与醇、醚、氯仿、丙酮、苯等大多数有机溶剂溶解,能溶解某些金属盐低毒,麻醉性乙醇78.3与水、乙醚、氯仿、酯、烃类衍生物等有机溶剂混溶微毒类,麻醉性丁酮79.64与丙酮相似,与醇、醚、苯等大多数有机溶剂混溶低毒,毒性强于丙酮苯80.1难溶于水,与甘油、乙二醇、乙醇、氯仿、乙醚、、四氯化碳、二硫化碳、丙酮、甲苯、二甲苯、冰醋酸、脂肪烃等大多有机物混溶强烈毒性环己烷80.72与乙醇、高级醇、醚、丙酮、烃、氯代烃、高级脂肪酸、胺类混溶低毒,中枢抑制作用乙睛81.6与水、甲醇、乙酸甲酯、乙酸乙酯、丙酮、醚、氯仿、四氯化碳、氯乙烯及各种不饱和烃混溶,但是不与饱和烃混溶中等毒性,大量吸入蒸气,引起急性中毒异丙醇82.4与乙醇、乙醚、氯仿、水混溶微毒,类似乙醇1,2-二氯乙烷83.48与乙醇、乙醚、氯仿、四氯化碳等多种有机溶剂混溶高毒性、致癌乙二醇二甲醚85.2溶于水,与醇、醚、酮、酯、烃、氯代烃等多种有机溶剂混溶。

AQUEOUS SOLUBILITY OF INORGANIC COMPOUNDS AT VARIOUSTEMPERATURESThe solubility of over 300 common inorganic compounds in water is tabulated here as a function of temperature. Solubility is defined as the concentration of the compound in a solution that is in equilibrium with a solid phase at the specified temperature. In this table the solid phase is generally the most stable crystalline phase at the temperature in question. An asterisk * on solubility values in adjacent columns indicates that the solid phase changes between those two temperatures (usually from one hydrated phase to another or from a hydrate to the anhydrous solid). In such cases the slope of the solubility vs. temperature curve may show a dis-continuity.All solubility values are expressed as mass percent of solute, 100⋅w2, wherew2 = m2/(m1 + m2)and m2 is the mass of solute and m1 the mass of water. This quan-tity is related to other common measures of solubility as follows: Molarity: c2 = 1000 ρw2/M2Molality: m2 = 1000w2/M2(1-w2)Mole fraction: x2 = (w2/M2)/{(w2/M2) + (1-w2)/M1}Mass of solute per 100 g of H2O: r2 = 100w2/(1-w2)Here M2 is the molar mass of the solute and M1 = 18.015 g/mol is the molar mass of water. ρ is the density of the solution in g cm-3. The data in the table have been derived from the references in-dicated; in many cases the data have been refitted or interpolated in order to present solubility at rounded values of temperature. Where available, values were taken from the IUPAC Solubility Data Series (Reference 1) or the related papers in the Journal of Physical and Chemical Reference Data (References 2 to 5), which present carefully evaluated data.The solubility of sparingly soluble compounds that do not ap-pear in this table may be calculated from the data in the table “Solubility Product Constants”. Solubility of inorganic gases may be found in the table “Solubility of Selected Gases in Water”. Compounds are listed alphabetically by chemical formula in the most commonly used form (e.g., NaCl, NH4NO3, etc.).References1. Solubility Data Series, International Union of Pure and AppliedChemistry. Volumes 1 to 53 were published by Pergamon Press, Oxford, from 1979 to 1994; subsequent volumes were published by Oxford University Press, Oxford. The number following the colon is the volume number in the series.2. Clever, H. L., and Johnston, F. J., J. Phys. Chem. Ref. Data, 9, 751,1980.3. Marcus, Y., J. Phys. Chem. Ref. Data, 9, 1307, 1980.4. Clever, H. L., Johnson, S. A., and Derrick, M. E., J. Phys. Chem. Ref.Data, 14, 631, 1985.5. Clever, H. L., Johnson, S. A., and Derrick, M. E., J. Phys. Chem. Ref.Data, 21, 941, 1992.6. Söhnel, O., and Novotny, P., Densities of Aqueous Solutions of InorganicSubstances, Elsevier, Amsterdam, 1985.7. Krumgalz, B.S., Mineral Solubility in Water at Various Temperatures,Israel Oceanographic and Limnological Research Ltd., Haifa, 1994.8. Potter, R. W., and Clynne, M. A., J. Research U.S. Geological Survey,6, 701, 1978; Clynne, M. A., and Potter, R. W., J. Chem. Eng. Data, 24, 338, 1979.9. Marshal, W. L., and Slusher, R., J. Phys. Chem., 70, 4015, 1966; Knacke,O., and Gans, W., Zeit. Phys. Chem., NF, 104, 41, 1977.10. Stephen, H., and Stephen, T., Solubilities of Inorganic and OrganicCompounds, Vol. 1, Macmillan, New York, 1963.Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. AgBrO30.193 1.327 AgClO20.170.310.470.550.640.82 1.02 1.22 1.44 1.66 1.88 2.117 AgClO3157 AgClO481.683.084.284.885.386.386.987.587.988.388.688.86 AgNO20.1550.4137 AgNO355.962.367.870.172.376.179.281.783.885.486.787.86 Ag2SO40.560.670.780.830.880.97 1.05 1.13 1.20 1.26 1.32 1.397 AlCl330.8430.9131.0331.1031.1831.3731.6031.8732.1732.5132.9033.327 Al(ClO4)354.964.47 AlF30.250.340.440.500.560.680.810.96 1.11 1.28 1.45 1.647 Al(NO3)337.038.239.940.842.044.547.350.453.8*61.5*6 Al2(SO4)327.527.828.229.230.732.634.937.640.744.27 As2O3 1.19 1.48 1.80 2.01 2.27 2.86 3.43 4.11 4.89 5.77 6.727.7110 BaBr247.648.549.550.050.451.452.553.554.555.556.657.66 Ba(BrO3)20.2850.4420.6560.7880.935 1.30 1.74 2.27 2.90 3.61 4.40 5.251:14 Ba(C2H3O2)237.044.27 BaCl223.3024.8826.3327.0327.7029.0030.2731.5332.8134.1435.5437.058 Ba(ClO2)230.531.344.77 Ba(ClO3)216.9021.2323.6627.5029.4333.1636.6940.0543.0445.9048.7051.171:14 Ba(ClO4)267.3070.9674.3075.7577.0579.2380.9282.2183.1683.8884.4384.907 BaF20.1580.1617 BaI262.564.767.368.869.169.570.170.771.372.072.773.468-112Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. Ba(IO3)20.01820.02620.03420.03960.045*0.058*0.0730.0900.1090.1310.1560.1821:14 Ba(NO2)231.136.641.844.346.851.656.260.564.668.572.175.610 Ba(NO3)2 4.7 6.38.29.310.212.414.717.019.321.523.525.56 Ba(OH)2 1.67 4.688.4193352741007 BaS 2.79 4.78 6.978.219.5812.6716.1820.0524.1928.5533.0437.617 Ba(SCN)262.67 BaSO30.00111:26 BeCl240.541.77 Be(ClO4)259.57 BeSO426.6927.5828.6129.2229.9031.5133.3935.5037.7840.2142.7245.287 CaBr2555659616368717310 CaCl236.7039.1942.1344.83*49.12*52.85*56.05*56.7357.4458.2159.0459.948 Ca(ClO3)263.264.265.566.367.269.071.073.275.5*77.4*77.778.01:14 Ca(ClO4)265.37 CaF20.00130.001610 CaI264.666.067.668.369.070.872.474.076.078.079.681.07 Ca(IO3)20.0820.1550.2430.3050.384*0.517*0.5900.6520.811*0.665*0.6681:14 Ca(NO2)238.639.544.548.67 Ca(NO3)250.153.156.759.060.965.477.878.178.278.378.478.56 CaSO30.00590.00540.00490.00410.00350.00300.00260.00230.00200.00191:26 CaSO40.1740.1910.2020.2050.2080.2100.2070.2010.1930.1840.1730.1639 CdBr236.043.049.953.456.460.3*60.3*60.560.760.961.361.66 CdC2O40.00605 CdCl247.250.153.254.656.3*57.3*57.557.858.158.5158.9859.56 Cd(ClO4)258.766.97 CdF2 5.82 4.65 4.18 3.765 CdI244.144.945.846.346.847.949.050.251.552.754.155.46 Cd(IO3)20.0915 Cd(NO3)255.457.159.661.062.866.570.686.186.586.887.187.46 CdSO443.143.143.243.443.644.143.542.541.440.238.536.76 CdSeO442.0440.5939.0238.1837.2935.3533.1530.6527.8424.6921.2417.495 Ce(NO3)357.9959.8061.8963.0564.31*67.0*68.671.1*74.9*79.280.983.11:13 CoCl230.3032.6034.8735.9937.1039.2741.3843.4645.5047.5149.5151.507 Co(ClO4)250.053.07 CoF2 1.47 CoI258.0061.7865.3566.9968.5171.1773.4175.2976.8978.2879.5280.707 Co(NO2)20.0760.497 Co(NO3)245.547.049.450.852.456.060.162.664.967.76 CoSO419.923.026.127.729.232.334.435.935.533.230.627.86 Co(SCN)250.77 CrO362.262.362.662.863.063.564.164.765.566.267.167.96 CsBr55.27 CsBrO3 1.16 1.93 3.01 3.69 4.46 6.328.6011.3214.4517.9621.8325.981:30 CsCl61.8363.4864.9665.6466.2967.5068.6069.6170.5471.4072.2172.961:47 CsClO3 2.40 3.87 5.947.228.6912.1516.3321.1426.4532.1037.8943.421:30 CsClO40.79 1.01 1.51 1.96 2.57 4.28 6.559.2912.4115.8019.3923.077 CsI30.937.243.245.948.653.357.360.763.665.967.769.26 CsIO3 1.08 1.58 2.21 2.59 3.02 3.96 5.06 6.297.709.2010.7912.451:30 CsNO38.4613.018.621.825.132.039.045.751.957.362.166.26 CsOH757 Cs2SO462.663.464.164.564.865.566.166.767.367.868.368.86 CuBr255.87 CuCl240.841.742.643.143.744.846.047.248.549.951.352.76 Cu(ClO4)254.359.37 CuF20.0757 Cu(NO3)245.249.856.359.261.162.063.164.565.967.569.271.06 CuSO412.414.416.718.019.322.225.428.832.436.340.343.56 CuSeO410.616.07 Dy(NO3)358.7959.9961.4962.3563.2965.4368.0471.581:13 Er(NO3)361.5863.1564.8465.7566.6968.7070.9673.6477.751:13 Eu(NO3)355.256.758.559.460.462.564.61:13Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. FeBr254.664.8*7 FeCl233.2*39.4*48.7*7 FeCl342.744.947.947.751.674.876.784.684.384.384.484.76 Fe(ClO4)263.3967.767 FeF3 5.597 Fe(NO3)340.1546.577 Fe(NO3)241.4446.677 FeSO413.517.020.822.824.828.832.835.533.630.427.124.06 Gd(NO3)356.357.759.260.161.062.965.267.971.51:13 HIO373.4574.1074.9875.4876.0377.2078.4679.7881.1382.4883.8285.141:30 H3BO3 2.61 3.57 4.77 5.48 6.278.1010.312.915.919.323.127.36 HgBr20.260.370.520.610.720.96 1.26 1.63 2.08 2.61 3.23 3.954 Hg(CN)2 6.577.839.3310.211.113.115.518.221.224.628.332.36 HgCl2 4.24 5.05 6.17 6.817.629.5312.0215.1819.1624.0629.9036.624 HgI20.00410.00550.00720.01220.01994 Hg(SCN)20.0704 Hg2Cl20.00043 Hg2(ClO4)273.879.8*85.3*7 Hg2SO40.0380.0430.0480.0510.0540.0590.0650.0700.0760.0820.0880.0934 Ho(NO3)363.81:13 KBF40.280.340.450.550.75 1.38 2.09 2.82 3.58 4.34 5.12 5.9010 KBr35.037.339.440.441.443.244.846.247.648.849.850.86 KBrO3 2.97 4.48 6.427.558.7911.5714.7118.1421.7925.5729.4233.281:30 KC2H3O268.4070.2972.0972.9273.7075.0876.2777.3178.2279.0479.8080.557 KCl21.7423.6125.3926.2227.0428.5930.0431.4032.6633.8634.9936.051:47 KClO3 3.03 4.67 6.747.939.2112.0615.2618.7822.6526.8831.5336.651:30 KClO40.70 1.10 1.67 2.04 2.47 3.54 4.94 6.748.9911.7114.9418.676 KF30.9039.847.350.4153.260.07 KHCO318.6221.7324.9226.628.1331.3234.4637.5140.456 KHSO427.129.732.333.635.037.840.543.446.249.0251.8254.66 KH2PO411.7414.9118.2519.9721.7725.2828.9532.7636.7540.9645.4150.121:31 KI56.057.659.059.760.461.662.863.864.865.766.667.46 KIO3 4.53 5.967.578.449.3411.0913.2215.2917.4119.5821.7824.031:30 KIO40.160.220.370.510.70 1.24 1.96 2.83 3.82 4.89 6.027.177 KMnO4 2.74 4.12 5.967.068.2811.1114.4218.166 KNO273.774.675.375.776.076.777.478.078.579.179.680.16 KNO312.017.624.227.731.338.645.752.258.063.067.370.86 KOH48.750.853.254.756.157.958.659.560.661.863.164.66 KSCN63.866.469.170.471.674.176.578.981.183.385.387.36 K2CO351.351.752.352.753.154.054.956.057.258.459.661.06 K2CrO437.138.138.939.439.840.541.341.942.643.243.844.36 K2Cr2O7 4.307.1210.913.115.520.826.331.736.941.545.548.96 K2HAsO448.5*63.6*79.8*7 K2HPO457.059.161.562.764.167.7*72.7*1:31 K2MoO464.766.57 K2SO351.3051.3951.4951.5551.6251.7651.9352.1152.3252.5452.7953.061:26 K2SO47.118.469.9510.711.412.914.215.516.717.718.619.36 K2S2O349.0*62.3*75.7*7 K2S2O522.126.731.133.135.239.042.646.049.152.054.61:26 K2SeO368.4*68.5*68.5*7 K2SeO452.7052.9353.1753.3053.4353.7053.9954.3054.6154.9455.2655.607 K3AsO451.5*55.6*73*7 K3Fe(CN)623.927.631.132.834.337.239.641.743.545.046.147.06 K3PO444.351.47 K4Fe(CN)612.517.322.023.925.629.232.535.538.240.641.443.16 LaCl349.048.548.648.949.350.552.154.056.358.961.76 La(NO3)355.056.958.960.061.163.666.369.9*74.1*1:13 LiBr58.460.162.764.465.967.868.369.069.870.771.772.86 LiBrO361.0362.6264.4465.4466.5168.9071.68*73.24*74.4375.6676.9378.321:30 LiC2H3O223.7626.4929.4231.0232.7236.4840.6545.1549.9354.9160.0465.267 LiCl40.4542.46*45.29*45.8146.2547.3048.4749.7851.2752.9854.98*56.34*1:47Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. LiClO373.275.6*80.8*82.183.485.9*87.1*88.289.691.393.495.71:30 LiClO430.132.635.537.038.641.945.549.253.257.261.371.46 LiF0.1200.1260.1310.1347 LiH2PO455.87 LiI59.460.561.762.363.064.365.867.368.881.381.782.66 LiIO343.81:30 LiNO24145495153566063666810 LiNO334.837.642.750.557.960.162.264.065.767.268.569.76 LiOH10.810.811.011.111.311.712.212.713.414.215.116.16 LiSCN54.57 Li2CO3 1.54 1.43 1.33 1.28 1.24 1.15 1.070.990.920.850.780.727 Li2C2O4 5.877 Li2HPO39.078.407.777.477.18 6.64 6.16 5.71 5.30 4.91 4.53 4.167 Li2SO426.325.925.625.525.325.024.824.524.324.023.823.66 Li3PO40.0271:31 Lu(NO3)371.11:13 MgBr249.349.850.350.650.951.552.152.853.554.255.055.76 Mg(BrO3)243.045.248.049.451.054.357.961.665.369.0*70.9*71.71:14 Mg(C2H3O2)236.1837.5538.9239.617 MgC2O40.0387 MgCl233.9634.8535.5835.9036.2036.7737.3437.9738.7139.6240.7542.158 Mg(ClO3)253.3554.4056.8158.6660.91*65.46*67.3369.2771.0172.4473.481:14 Mg(ClO4)247.848.749.650.150.551.352.16 MgCrO432.06*35.39*7 MgCr2O758.967.07 MgF20.0137 MgI254.756.158.259.460.863.965.065.065.065.065.165.26 Mg(IO3)2 3.19* 6.70*7.928.529.1110.4511.9913.715.617.619.61:14 Mg(NO2)2477 Mg(NO3)238.439.540.841.642.444.145.947.950.052.270.672.06 MgSO30.320.370.460.520.610.87*0.85*0.760.690.640.620.601:26 MgSO418.221.725.126.328.230.933.435.636.935.934.733.36 MgS2O330.734.17 MgSeO431.4*35.7*47*7 MnBr256.0057.7259.3960.1960.9662.4163.7565.0166.1967.3268.4269.507 MnCl238.740.642.543.644.747.049.454.154.755.255.756.16 MnF20.80* 1.01*0.487 Mn(IO3)20.270.347 Mn(NO3)250.561.77 MnSO434.637.338.638.938.937.736.334.632.830.828.826.76 NH4Br37.540.242.743.945.147.349.451.353.054.656.157.47 NH4Cl22.9225.1227.2728.3429.3931.4633.5035.4937.4639.4041.3343.241:47 NH4ClO410.814.117.819.721.725.829.833.637.340.743.846.66 NH4F41.743.244.745.546.347.849.350.952.554.17 NH4HCO310.613.717.619.922.427.934.241.449.358.167.678.07 NH4H2AsO425.229.032.734.536.339.743.146.249.352.255.07 NH4H2PO417.822.026.428.831.236.241.647.253.059.265.772.47 NH4I60.762.163.464.064.665.866.867.868.769.670.471.16 NH4IO3 3.70 4.20 5.647.631:30 NH4NO255.759.064.968.87 NH4NO354.060.165.568.070.374.377.780.883.485.888.290.36 NH4SCN64.481.17 (NH4)2C2O4 2.31 3.11 4.25 4.94 5.737.569.7312.215.118.321.825.77 (NH4)2HPO436.438.240.041.042.044.146.248.550.953.355.958.67 (NH4)2S2O565.567.969.870.571.372.372.973.11:26 (NH4)2S2O837.0040.4543.8445.4947.1150.2553.2856.2359.1362.007 (NH4)2SO332.234.937.739.140.643.747.050.654.558.91:26 (NH4)2SO441.342.142.943.343.844.745.646.647.548.549.550.56 (NH4)2SeO349.051.153.454.756.058.962.065.469.17 (NH4)2SeO454.027 (NH4)3PO415.57Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. NaBr44.445.947.748.649.651.653.754.154.354.554.754.96 NaBrO320.023.2226.6528.2829.8632.8335.5538.0540.3742.521:30 NaCHO230.837.945.748.750.652.053.555.06 NaC2H3O226.528.831.833.535.539.945.158.359.360.561.762.96 NaCl26.2826.3226.4126.4526.5226.6726.8427.0327.2527.5027.7828.051:47 NaClO22.744.47 NaClO297.0*95.3*7 NaClO344.2746.6749.350.151.253.655.557.058.560.563.367.11:30 NaClO461.964.166.267.268.370.472.574.174.775.476.176.76 NaF 3.52 3.72 3.89 3.97 4.05 4.20 4.34 4.46 4.57 4.66 4.75 4.826 NaHCO3 6.487.598.739.329.9111.1312.4013.7015.0216.3717.7319.107 NaHSO422.233.310 NaH2PO436.5441.0746.0048.6851.5457.89*61.7*62.3*65.968.71:31 NaI61.262.463.964.865.767.769.872.074.774.874.975.16 NaIO3 2.43 4.407.78*8.65*9.6011.6713.9916.5219.25*21.1*22.924.71:30 NaIO412.627 NaNO241.943.445.145.946.848.750.752.855.057.259.561.86 NaNO342.244.446.647.748.851.053.255.357.559.661.763.86 NaOH30394650535863677174767910 NaSCN52.957.160.262.763.564.265.065.966.967.969.06 Na2B4O7 1.23 1.71 2.50 3.07 3.82 6.029.714.917.119.923.528.06 Na2CO3 6.4410.817.923.528.732.832.231.731.331.130.930.96 Na2C2O4 2.62 2.95 3.30 3.48 3.65 4.00 4.36 4.71 5.06 5.41 5.75 6.086 Na2CrO422.632.344.646.746.948.951.053.455.355.555.856.16 Na2Cr2O762.163.164.465.266.168.070.172.374.677.079.680.76 Na2HAsO4 5.6*29.3*67*7 Na2HPO4 1.66 4.197.5110.5516.34*35.17*44.64*45.2046.8148.7850.5251.531:31 Na2MoO430.638.839.439.439.840.341.041.742.643.544.545.56 Na2S11.113.215.717.118.622.126.728.130.233.036.441.06 Na2SO312.016.120.923.526.3*27.3*25.924.823.722.822.121.51:26 Na2SO416.1321.9429.22*32.35*31.5530.9030.3930.0229.7929.678 Na2S2O333.136.340.643.345.952.062.365.768.869.470.171.06 Na2S2O538.439.540.040.641.843.044.245.546.848.149.51:26 Na2SeO347.3*45*7 Na2SeO411.736.9*42.1*7 Na2WO441.641.942.342.642.943.644.445.346.247.348.449.56 Na3PO4 4.287.3010.812.614.116.622.928.432.437.640.443.56 Na4P2O7 2.23 3.28 4.81 6.627.0010.1014.3820.0727.3136.0332.3730.676 NdCl349.049.349.750.050.451.252.253.354.555.857.158.56 Nd(NO3)355.7657.4959.3760.3861.4363.6966.2769.471:13 NiCl234.736.138.540.341.742.143.245.046.146.246.446.66 Ni(ClO4)251.152.87 NiF2 2.50 2.527 NiI255.4057.6859.7860.6961.5062.8063.7364.3864.8065.0965.307 Ni(NO3)244.146.048.449.851.354.658.361.063.165.667.969.06 NiSO421.424.427.428.830.3*32.0*34.135.837.739.942.344.86 Ni(SCN)235.487 NiSeO421.626.2*45.6*7 PbBr20.4490.6200.8410.966 1.118 1.46 1.892 PbCl20.660.810.98 1.07 1.17 1.39 1.64 1.93 2.24 2.60 2.99 3.422 Pb(ClO4)281.57 PbF20.06030.06490.06700.06932 PbI20.0410.0520.0670.0760.0860.1120.1440.1870.2430.3152 Pb(IO3)20.00257 Pb(NO3)228.4632.1335.6737.3839.0542.2245.1747.9050.4252.7254.8256.752 PbSO40.00330.00380.00420.00440.00470.00520.00582 PrCl348.048.148.649.049.550.852.354.156.158.36 Pr(NO3)357.5059.2061.1662.2463.40*65.7*67.870.273.41:13 RbBr47.450.152.653.854.957.058.860.662.163.564.865.96 RbBrO30.97 1.55 2.36 2.87 3.45 4.87 6.648.7811.2914.1517.3220.761:30 RbCl43.5845.6547.5348.4249.2750.8652.3453.6754.9256.0857.1658.151:47Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. RbClO3 2.10 3.38 5.14 6.227.4510.3513.8517.9322.5327.5732.9638.601:30 RbClO41 1.5177 RbF757 RbHCO353.77 RbI55.858.661.162.363.465.467.268.870.371.672.773.86 RbIO3 1.09 1.53 2.07 2.38 2.74 3.52 4.41 5.42 6.527.749.0010.361:30 RbNO316.425.034.639.444.253.160.867.272.276.179.081.26 RbOH63.47 Rb2CrO438.2743.267 Rb2SO427.330.032.533.734.836.938.740.341.843.044.144.96 SbCl385.790.87 SbF379.483.17 Sc(NO3)357.059.361.662.863.966.268.51:13 Sm(NO3)354.8356.3358.0859.0560.0862.3865.05*68.1*70.874.21:13 SmCl348.048.248.448.649.250.06 SnCl246647 SnI20.97 3.877 SrBr246.048.350.651.752.955.257.659.962.364.666.869.06 Sr(BrO3)218.5322.0025.3927.0228.5931.5534.2136.5738.64*40.2*40.841.01:14 SrCl231.9432.9334.4335.3736.4338.9341.9445.44*46.81*47.6948.7049.878 Sr(ClO2)213.013.614.114.314.514.915.315.615.97 Sr(ClO3)263.2963.4263.6463.7763.9364.2964.7065.1665.6566.1866.7467.311:14 Sr(ClO4)270.04*75.35*78.44*7 SrF20.0110.0217 SrI262.562.863.563.964.565.867.369.070.872.774.779.26 Sr(IO3)20.1020.1260.1520.1650.1790.2060.2330.2590.2840.3070.3280.3461:14 Sr(MnO4)2 2.57 Sr(NO2)241.944.358.67 Sr(NO3)228.234.641.044.547.047.447.948.448.949.550.150.76 Sr(OH)20.9 2.27 SrSO30.00151:26 SrSO40.01357 SrS2O38.813.217.720.022.226.87 Tb(NO3)360.661.021:13 Tl2SO4 2.65 3.56 4.61 5.19 5.807.098.469.8911.3312.7714.1815.536 Tm(NO3)367.91:13 UO2(NO3)249.5251.8254.4255.8557.5561.5967.071:55 Y(NO3)355.5756.9358.7559.8661.11*63.3*64.967.972.51:13 Yb(NO3)370.51:13 ZnBr279.380.181.883.084.185.685.886.186.386.686.887.16 ZnC2O40.00100.00190.00265 ZnCl276.679.080.381.481.882.483.083.784.485.286.06 Zn(ClO4)244.29*46.27*48.707 ZnF2 1.535 ZnI281.181.281.381.481.581.782.082.382.683.083.383.76 Zn(IO3)20.580.640.690.770.825 Zn(NO3)247.850.854.454.658.579.180.187.589.96 ZnSO30.17860.17900.17940.18030.18125 ZnSO429.132.035.036.638.241.343.042.141.039.938.837.66 ZnSeO433.0634.9837.3838.7940.345。

计算机联用测定无机盐溶解热实验报告
实验目的:
本次实验的目的是通过计算机联用的方法测定无机盐在水中的溶解热,并掌握实验方法及数据处理方法。
实验原理:
盐在水中溶解时会放出或吸收热量,这个热量变化可以用实验方法测量出来。
在这个实验中,我们使用的是计算机联用的方法来测定无机盐溶解热。
实验步骤:
1. 进行实验前准备工作,包括清洗实验仪器和准备实验用水。
2. 在实验室控制温度下,将预先称量好的盐溶解于水中。
3. 使用热测量仪器对溶解过程中温度变化进行监测。
4. 将实验数据通过计算机联用的方法处理,并获得溶解热值。
实验结果分析:
通过本次实验,我们掌握了以计算机联用的方法来测定无机盐在水中的溶解热的实验方法。
在实验过程中,我们发现不同的无机盐的溶解热值是不同的,这是因为每种无机盐的分子结构和化学键有所不同,所以溶解热的数值也会有所不同。
同时,本次实验还有一些注意事项,如在实验过程中控制实验室温度的稳定性,以及溶解过程中避免液体的蒸发等等。
这些都需要我们在实际操作中加以注意。
结论:
综上所述,本次实验成功地利用了计算机联用的方法测定了不同无机盐在水中的溶解热,并掌握了实验方法及数据处理方法。
通过实验,我们深入了解了无机盐的分子结构及化学键对其溶解
热的影响,同时也了解了实验操作上的一些细节问题,对我们今后的实验操作具有很大的帮助。
计算机联用测定无机盐溶解热一 实验目的1. 用量热计测定KCl 的积分溶解热。
2. 掌握量热实验中温差校正方法以及与计算机联用测量溶解过程动态曲线的方法。
二 实验原理盐类的溶解过程通常包含着两个同时进行的过程:晶格的破坏和离子的溶剂化。
前者为吸热过程,后者为放热过程。
溶解热是这两种热效应的总和。
因此,盐溶解过程最终是吸热或放热,是由这两个热效应的相对大小所决定的。
常用的积分溶解热是指等温等压下,将1摩尔溶质溶解于一定量溶剂中形成一定浓度溶液的热效应。
溶解热的测定可以在具有良好绝热层的量热计中进行的。
在恒压条件下,由于量热计为绝热系统,溶解过程所吸收的热或放出的热全部由系统温度的变化反映出来。
为求KCl 溶解过程的热效应,进而求得积分溶解热(即焓变∆H ),可以根据盖斯(Γecc )定律将实际溶解过程设计成两步进行,如图2-1。
图2-1 KCl 溶解过程的图解由图可知,恒压下焓变∆H 为两个过程焓变∆H 1和∆H 2 之和,即:(2-1)21H H H ∆+∆=∆因为量热计为绝热系统,1H Q P ∆=所以在t 1温度下溶解的恒压热效应∆H 为 :(2-2) )()(12212t t K t t K H H −−=−=∆=∆式中K 是量热计与KCl 水溶液所组成的系统的总热容量,(t 2-t 1 )为KCl 溶解过程系统的温度变化值。
溶解t ∆设将质量为m 的KCl 溶解于一定体积的水中,KCl 的摩尔质量为M ,则在此浓度下KCl 的积分溶解热为: 溶解t mKM m M H H ∆−=⋅∆=∆m sol (2-3) K 值可由电热法求取。
即在同一实验中用电加热提供一定的热量Q ,测得温升为,则。
若电热丝电阻为R ,电流强度为I ,通电时间为加热t ∆Q t K =∆⋅加热 τ,则:(2-4) τR I t K 2=∆⋅加热所以 加热t R I K ∆=τ2 (2-5) 由于实验中搅拌操作提供了一定热量,而且系统也并不是严格绝热的,因此在盐溶解的过程或电加热过程中都会引入微小的额外温差。