梅特勒-托利多 STAR软件之DSC--简明操作手册(中文版)
- 格式:pdf
- 大小:268.72 KB
- 文档页数:32

发酵控制系统在线pH电极的维护与使用1.每次使用前进行电极的检查和维护1.1 发酵工艺控制中pH电极的使用寿命一般为50个消毒批次(6个月有效寿命);1.2将电极连接,观察变送器上电极零点(Zero),可用范围在6.50~7.50PH或-30mV~+30 mV。
1.3观察斜率(Slope),可用范围在53 mV~65 mV/PH或90%~110%。
斜率公式S=2.303RT/F(R:气体常数8.314;T:开尔文温度K,摄氏度值+273;F:法拉第常数,96493)例子:在25℃计算斜率S=2.303*8.314*(273+25)/96493=59.13mv/PH 或者表示为:S=0.1984*(273+____℃)1.4观察膜阻抗(Glass lmp),可用范围在10M~1000M欧姆。
1.5检查电极是否被污染,如电极出现污染,需严格按照清洗规程进行清洗。
1.5.1 对于一般污染,使用水、0.1MNaOH或0.1MHCL清洗电极十分钟左右;1.5.2 油脂或有机物的污染,用丙酮或乙醇清洗电极数秒钟。
1.5.3 蛋白质污染(隔膜变黄),将电极用9891清洗液处理1.5.4 硫化物污染(隔膜变黑),将电极用9892清洗液处理1.5.5 如电极隔膜污染严重,用9895再生液浸泡,浸泡时间不能超过10分钟1.6检修拆下的电极必须用相应的电解液进行浸泡和保养,不能干放电极,不能放在蒸馏水中保存。
在确认电极无法使用时根据规定对报废的电极进行处理。
1.7对于465电极,发现电解液减少后,应及时加入电解液。
加入量不得超过最高标志线。
1.8 25℃时,变送器把输入信号-414mv~+414mv转化为0~14PH;2.现场常见故障及解决2.1 pH值的零点漂移,漂移指输入信号为0mv时的PH值(正常值为7)2.1.1被测介质的浸入造成参比电解质被污染,解决方法是保证压力仓的压力高于被测点绝压1Bar以上;2.1.2生化反应造成电极隔膜被污染,按照上述2.5条处理电极;2.1.3电解质溶液的蒸发,由于高温消毒且补偿压力与罐压相当造成,解决方法是更换电解质溶液,保证补偿压力足够大;2.1.4高温下内电解质释放碱性物质,参比电极的完全还原,连接部件的腐蚀,解决方法是更换电极;2.2 pH电极的斜率变小,2.2.1缓冲液被污染或选择不当,解决方法是选用标值准确恰当的缓冲液;2.2.2pH电极被污染,包括被测溶液的浸入和隔膜污染,解决方法参考3.1.1和3.1.2;2.2.3电极的老化,老化是指玻璃膜上胶层厚度增加,使膜阻变大,响应迟缓,解决方法是用电极再生液9895再生电极;2.2.4 电极损坏,解决方法是更换电极;2.3 pH值显示波动2.3.1 电极的老化或污染,造成膜阻增大,导致测量值变化无规律,解决方法参考3.2.2和3.2.3;2.3.2 电缆线接头松动或进水,解决方法是处理接头或吸干水份;2.3.3 电极内有气泡,解决方法是排出电极内的气泡;2.3.4电极保护仓压力低或为零,解决方法是打气保压;2.3.5电极损坏,解决方法是更换电极;2.3.6电缆线接头氧化或腐蚀及外界信号的干扰,解决方法是更换电缆或排除干扰;2.4 pH值不动2.4.1变送器损坏,解决方法是更换变送器2.4.2 电缆线短路或断路,解决方法是更换电缆;2.5 pH值显示迟缓2.5.1玻璃膜的水化不足(或者称活化),解决方法是1至2天即可稳定;2.5.2电极的老化,解决方法参考2.2.3;2.5.3PH电极的敏感膜受损,解决方法是更换电极;2.5.4电极被污染,解决方法参考2.2.2;3.pH显示表标定时用对应的电极和相应的标准缓冲液进行标定。



To ensure the future success of late -stage product development processes, itcontinuously strengthens its capabilities, and seeks to maximize the time it spendson the science through investment in emerging and state -of -the -art technologiesthat can reduce otherwise trivial manual labor. This mindset drove the productdevelopment team ’s search for an automated solutions preparation system that iscompliant with regulatory requirements in a GMP setting. For example, for themeasuring devices in the system the team needed to follow the requirementsstated in pharmacopeias (USP and Ph.Eur.). They recognized that the balances, pHprobes, conductivity probes, temperature sensors and volumetric glassware mustmeet the stringent requirements set by these pharmacopeias. In addition, it wasalso essential to comply with data integrity requirements set by the FDA ’s 21 CFRPart 11 and EU ’s Annex 11. The selected system needed to capture and log criticalaspects of record keeping such as audit trails, reports, recipe composition, userpermissions and dosing records.THE CHALLENGEA top 20 global pharmaceutical company with headquarters in Europe, has been inoperation for a century. With a mission of developing medicines and treatmentsfor endocrine diseases, this company helps people live better every day. In orderto speed the development of medicines and treatments to market, it has adopteda culture of innovation where implementing cutting edge technology isencouraged, embraced and expected. It was this innovation culture that spurredthe concept of moving a research automation system into the GMP environmentand improve the efficiency of laboratory personnel and operations there.ABOUT OUR CUSTOMER"The flexibility the solution has provided for just -in -time preparation is a major benefit."AUTOMATED PREPARATION OFREAGENT SOLUTIONS FOR GMP LABS Case StudyAT A GLANCEC. SchwartzProject ManagerLabMinds collaborated with aTop 20 Global Pharmaceutical company and madeenhancements to Revo thatevolved the research productinto a GMP compliant solution.C. Schwartz, Project ManagerTHE SOLUTIONAfter an extensive review of available solutions,they selected LabMinds® Revo®,the benchtop automated solution preparation system that enables companies to automate the reagent preparation bMinds,upon the customer’s request,decided to collaborate and make enhancements to Revo that would evolve the research product into a GMP compliant solution.The project was launched in May of2019and they set out to find a dedicated and innovative technical Project Manager to drive this project.Schwartz was assigned as Project Manager.Schwartz is a specialist in laboratory automation and has a strong track record of leading teams and driving transformation.He accepted the challenge in a large part due to the fact that he had previous exposure to the Revo system from the2016SLAS conference when Revo won the innovation award.He recognized the potential to transform Revo into a GMP compliant system and accepted the challenge.REAGENT SUPPLY SCENARIOSThe above graphic demonstrates the need and the options available for reagents in a lab.By using the Revo, just in time reagent solutions are achieved without using lab personnel time.FORMING A TEAMSchwartz formed a dedicated team comprised of subject matter experts including a chemistry specialist and cross functional experts from other departments.This team combined forces with LabMinds and worked to deliver an automated solution preparation system that complied with GMP requirements.They knew that it needed to deliver a solution that provided the consistent quality,traceability and documentation required in the regulated pharmaceutical industry.The team also sought to solve three main challenges: reducing the time lab technicians spent on preparing reagents to allow them to focus on the science; limiting solution overproduction; and eliminating risk associated with manual documentation processes.After 18 months of intense dedication, the team achieved a huge milestone and Revo was approved internally for use in its GMP laboratory. The system approval was earned through a series of rigorous data integrity process reviews and hardware upgrades. They were then able to tackle the challenges they set out to solve. Just a few months after the launch, the team on -boarded many of its recipes and is already seeing positive impact.SYSTEM APPROVAL“I appreciate the opportunity to work for a company that iswilling to implement cutting edge technology. We willreap many benefits moving forward,” commentedSchwartz, the Project Manager. “The flexibility the solutionhas provided for just -in -time preparation is a majorbenefit. We have already reduced solution overproduction costs and leveraged the Revo system toreallocate our reagent prep team from redundant tasks towork that helps increase the speed of productdevelopment.”"I appreciate the opportunity to work for a company that is willing to implement cutting edge technology that will reap many benefits moving forward."C. Schwartz Project ManagerPrior to the implementation of GMP Revo, three laboratory technicians spent an entire daypreparing reagents for the week. Upon review of recipes required, many were found tohave an organic component. To overcome a current system limitation, LabMindsdeveloped a workflow process where the Revo produces the aqueous portion of thesolution with instructions for the organic component addition printed on the label. Eventhough organic components are still added manually it was decided that the newfoundflexibility of automating the manual weighing, pH verification and data registration madethe process worthwhile. The system now allows the team to order the reagents via theWeb or mobile interface, and reduces the FTE time needed to fulfill monotonous,procedural laboratory tasks by 66%. This allows these key team members to focus on thedata analysis and scientific work needed to produce results from sample analysis.POSITIVE RESULTSThis lab technician team also used to ensure that they did not run out of reagents during the week by creating an extra supply. At expiry, extra solutions were thrown away. Now the team has implemented just -in -time inventory best practices and produce the exact quantities of the solutions they need. They know that they have increased flexibility, and if they need more solutions they do not have to stop analysis to create them. They can simply reorder via the easy Web interface, and the solution will be ready within an hour. This flexibility allows the team to reduce the amount of inventory needed each week.Prior to the implementation, the team manually tracked preparation data on paper to ensure they could obtain the necessary information for upcoming audits. Now, the team utilizes a QR code printed on the bottle label that contains all information describing the preparation and scans this data directly into the LIMS system where a record is created for each bottle.C. Schwartz and the Revo systemhttps:///labmindsinchttps:///labmindsinchttps:///company/labminds/“After 6 months in operation I get continued feedback from the users on how happy they are to be relieved from the tight planning of manual preparation they used to have. Even though organic components are still added manually it is by far overshadowed by the newfound flexibility in the fact that all manual weighing, pH verification and data registration are completely removed from the process.”C. SchwartzProject Manager© 2021 LabMinds. . All Rights Reserved. LabMinds and Revo are registered trademarks and the property of LabMinds.Unauthorized use is strictly prohibited.Scanning of QR code imports solutions data into LIMSCHALLENGESBENEFITS Reduce FTE time spent on preparing reagents Reagent prep FTE time decreased by 66%Limit solution waste Just -in -time inventory best practices reduced solutionoverproduction costsEliminate risk with manual documentationprocessesDocumentation completed with full transparency andregulatory compliance provides peace of mind All samples, solutions and controls are registered in the LIMS, and thisdata aligns with existing records to populate the documentation ofthe analysis and ensures audit readiness. This process not onlyreduces the chance of human error, but also frees up time for the labtechnicians to focus on other tasks. Our customer now has peace ofmind knowing that documentation is completed with fulltransparency and regulatory compliance.。

托利多电子秤设置方法对于一台新条码秤,需要做以下工作。
一、在秤上操作1、格式化开机后依次按“代码”→“24681357”→“*”,(屏幕显示SERVICE-MODE),输入“15”→“*”(屏幕显示WORK CONFIG),→1(屏幕显示CONFIG 0-1)→“*”,条码秤初始化后自动重启即可。
注:此操作将条码秤中的数据、格式、配置数据恢复到出厂值。
2、设置秤号开机后依次按“代码”→“24681357”→“*”,(屏幕显示SERVICE-MODE),输入“08”(屏幕显示PRIMARY)→“*”(屏幕显示SCALE NUMBER),输入秤号如“10”→“*”。
注:秤号和IP地址是两个概念,与寺岗秤有本质的区别。
秤号是秤的称号或是一种标识,IP号只是秤的IP地址。
3、设置IP地址依次按“代码”→“24681357”→“*”,(屏幕显示SERVICE-MODE),输入“21”(屏幕显示NETWORKCONFIG)→“*”(屏幕显示IP ADDRESS)然后屏幕出现一串0,直接输入IP 地址,如192.168.0.10,直接输入192168000010,输入完毕后按“↓”键(屏幕显示NET MASK)然后屏幕又出现一串0,直接输入子网掩码“255255255000”,输入完毕后一直按“↓”键,直到秤自动重启后IP设置完毕。
4、设置打印强度及倒转功能依次按“代码”→“24681357”→“*”,(屏幕显示SERVICE-MODE),输入“25”→“*”(屏幕显示RESIST 600-999默认850)→“*”(屏幕显示SPEED 85mm/S默认0-5,打印速度设置)→“*”(屏幕显示ENERGY -4-+4默认1,打印强度设置)将这里设置成3→“*”→“*”(屏幕显示REWIND NO默认0,倒转功能设置)将这里设置成1→连续按“*”键直到屏幕显示25 LABLE PRINTER,按“代码”键退出。

梅特勒—托利多电子秤操作方法及注意事项(一)基本操作一、开机:先取掉秤盘上的任何物品,然后打开电源开关,等待秤内系统自检,显示屏全部显示为零时便可操作。
二、服务模式下打印标签(手动打印)1、计重商品: 秤盘上放商品按P LU号~V1(打印键)2、计数商品: 按P LU号( ×商品数量)~V1(打印键)三、预包装模式下打印标签(自动打印)按“模式”键两次,显示屏左下角显示“P P”即可,操作完后再按“模式”键两次退回到手动打印。
1、计重商品:按P LU号,秤盘上放商品,等待重量显示稳定后自动打印出标签2、计数商品:按PL U号(×商品数量)按* 键~输入打印标签份数~按*键四、通过预置键打印标签: 按键选择上层L1和下层L2~按预置键(或连续按预置键2次选择)~按V1(打印键)即可。
五、修改单价:(在允许修改的情况下)按P LU号~按#键~输入新价格~按V1键。
(二)注意事项1)电子秤最大称量为15K G,严禁超载,以免损坏传感器。
2)电子秤必须使用独立、有接地电源。
3)清洁打印头时先关电源并只能用清洁笔或者棉签沾高浓度工业酒精清洁。
严禁用小刀、指甲等硬器接触打印头。
4)换纸时先看打印头旁边的胶轮和间距检测器中间以及打印头上是否粘贴有标签纸(如果有会连续出纸或打印空白条码纸等),要先取掉,然后再换标签纸。
换纸时标签纸沿打印机上所示走纸方向安装,完成后多按“走纸”键几次,等出纸正常后便可操作。
5)输入P LU号和操作错误时按“清除”键清除。
秤盘上无商品显示屏重量位显示为正负数时按“清零”键清除。
6)键盘上的“代码”键和“模式”键严禁随意按动`,以免改变秤内参数致使电子秤不能正常使用。
7)按键盘上的按键请用指腹,严禁用指甲按键盘上的按键。
8)电子秤必须做好防水和防虫处理。
9)如发现问题,请及时与电脑员或本公司联系!托利多电子秤操作手册编制PLU:按“代码”——8*——02*——输入新PLU号——输入货号(条形码)——输入商品名称(查字表,每输4个字符后按空格键)——输入单价(公斤价)——输入保质天数(多少天)——输入计价方式(0:计重1:计数)—(输入完成并返回到下一个PLU),如果继续输入新商品,请接至前面“输入新PLU号”的位置开始,如果不输请按“代码”两次退出。


(完整版)梅特勒DSC1仪器操作规程
差示扫描量热仪(DSC)操作规程
一、开机:先打开主机及制冷机电源,打开氮气钢瓶总阀,将干燥气流量阀打开。
打开计算机,再打开工作软件。
二、关机:首先关闭软件,关闭氮气钢瓶总阀,然后关闭主机及制冷机电源,最后关闭计算机。
三、一般操作:
(一)样品准备
将样品放入坩锅在天平称重,然后盖上锅盖(锅盖根据需要可打若干小孔)用压盖机压封,然后放入主机炉内左侧的传感器上,盖上炉盖。
(二)工作软件操作程序:
点击STARe软件图标,在USER NAME对话框内输入密码,点击确认
在主菜单上下拉SESSION,点击INSTALL WINDOW
在弹出的窗口中选中DSC1/500/…….,点击ACTIVE
在弹出的窗口点击ROUTINE EDITOR
选中METHOD中的NEW
选击ADD DYN或ADD ISO
输入测试参数
点击SEND EXPERIMENT
选中ON MODULE
点击OK
主机指示灯由绿转红,测试开始。
TC45: 干燥气流量60~80; 反应气流量40~60
温度:-35 ---500 ℃
线性升温速率: 0.01…300℃/min;
四、突发情况如何处理
任何危及仪器的事件发生时,应迅速切断电源和气源,待情况正
常后,重新测试。
五、特别注意事项
坩锅必须加盖,除非有特殊的测试要求。
炉内必须保持清洁,放置和取出样品时避免硬器碰及炉底。
仪器出现异常,应及时与供应商联系,不得擅自处理。
请确保仪器工作环境
放在室内稳定工作台上水平
具有足够距离(> 15cm)
照明充足
强烈气流温度波动
电子天平使用注意事项良好的称量管理规范
1. 准备操作
2. 注意事项
调节水平
在使用前,必须查看水平指示器中的气泡是否在黑圈内。
如气泡不在黑圈内,请利用水平调节脚将气泡调至黑圈中央位置。
此时,天平就处于完全水平状态。
预热天平
处于断电状态的天平,在说明书所指定的使用环境状况小时;分析天平2 天平应放置于具有防磁表面的稳定工作台上,应避免震动、气流及阳光的照射。
天平是精密仪器,使用时动作要轻缓。
称量时,请勿按压工作台。
3. 更多天平知识
除了基础称量,天平还有密度称量、配方称量等许多
其他应用。
关于应用的详细介绍,请查阅具体型号的说明书。
获取更多最新的天平信息,敬请访问我们的网站:
请勿用手或样品冲击秤盘,尽量避免将超出量程范围的样品放置在秤盘上,以降低不慎操作损坏天平的可能性。
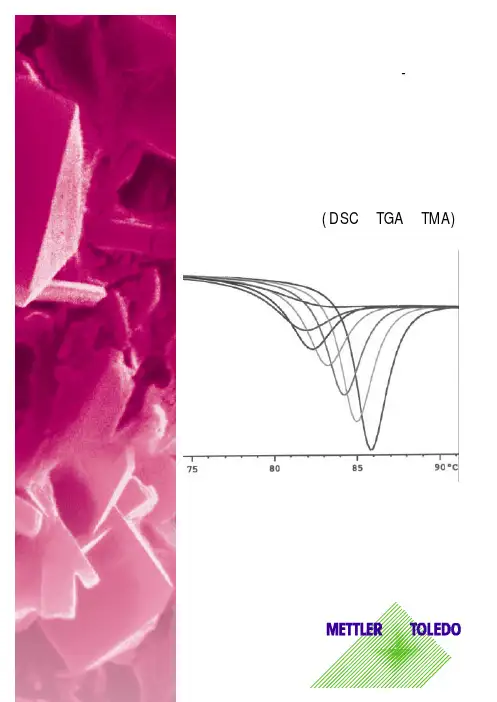
DSC操作规程1. 工作原理该台差示扫描量热仪(DSC)属于功率补偿型,即通过内加热的方式使样品不管在吸热还是放热状态都具有与参比物相同的温度。
DSC测定的就是维持样品与参比物处于相同温度时所需要的能量差。
2. 仪器测试条件DSC仪器:Mettler-Toledo公司操作系统:STAR e操作系统坩埚:标准40ul铝坩埚最高测定温度:600℃3. 操作步骤3.1 系统启动3.1.1 按如下顺序打开下述设备:打印机→计算机→显示器→DSC仪器3.1.2 双击桌面上的STAR e图标以打开操作系统软件。
3.1.3 在弹出窗口中,键入用户名METTLER,并按OK确认,就可以打开仪器的主菜单栏。
3.1.4 选择保存路径:单击Database→Select,在Select对话框中选择所需数据库,然后单击OK确定。
3.2 创建方法3.2.1 单击绿色的日常操作窗口图标。
3.2.2 点击左边的Routine Editor,在右面的实验编辑区域中,Method选项下选择按钮New。
方法编辑窗口出现。
3.2.3 设定程序升温:通过单击按钮Add Dyn,在弹出窗口中输入起始温度、结束温度和加热速率。
3.2.4 设定固定温度:选择Add Iso,在弹出的窗口中输入相应的温度和时间,并按OK确认。
3.2.5 单击按钮Save As,对该方法进行保存。
3.3 实验操作3.3.1 样品准备:称量样品,记录下样品的质量,然后用压片机把样品盘密封。
3.3.2 选择测定方法:在日常操作窗口中,单击左边的“Routine Editor”,在“Method”下单击“Open”选择合适的实验方法。
然后,在相应的区域输入样品名、样品的质量等信息。
最后,单击按钮Send Experiment。
此时,在左边一栏Experiment-On module 的列表中,就出现了该实验的信息。
3.3.3 进样:当添加样品的温度到达后,屏幕下方的绿色状态条里会指示“Waiting forsample insertion”。