英文原版教材班“材料科学与工程基础”考试试题
- 格式:doc
- 大小:393.00 KB
- 文档页数:6

《材料科学与工程基础》习题和思考题及答案第二章2-1.按照能级写出N、O、Si、Fe、Cu、Br原子的电子排布(用方框图表示)。
2-2.的镁原子有13个中子,11.17%的镁原子有14个中子,试计算镁原子的原子量。
2-3.试计算N壳层内的最大电子数。
若K、L、M、N壳层中所有能级都被电子填满时,该原子的原子序数是多少?2-4.计算O壳层内的最大电子数。
并定出K、L、M、N、O壳层中所有能级都被电子填满时该原子的原子序数。
2-5.将离子键、共价键和金属键按有方向性和无方向性分类,简单说明理由。
2-6.按照杂化轨道理论,说明下列的键合形式:(1)CO2的分子键合(2)甲烷CH4的分子键合(3)乙烯C2H4的分子键合(4)水H2O的分子键合(5)苯环的分子键合(6)羰基中C、O间的原子键合2-7.影响离子化合物和共价化合物配位数的因素有那些?2-8.试解释表2-3-1中,原子键型与物性的关系?2-9.0℃时,水和冰的密度分别是1.0005 g/cm3和0.95g/cm3,如何解释这一现象?2-10.当CN=6时,K+离子的半径为0.133nm(a)当CN=4时,半径是多少?(b)CN=8时,半径是多少?2-11.(a)利用附录的资料算出一个金原子的质量?(b)每mm3的金有多少个原子?(c)根据金的密度,某颗含有1021个原子的金粒,体积是多少?(d)假设金原子是球形(r Au=0.1441nm),并忽略金原子之间的空隙,则1021个原子占多少体积?(e)这些金原子体积占总体积的多少百分比?2-12.一个CaO的立方体晶胞含有4个Ca2+离子和4个O2-离子,每边的边长是0.478nm,则CaO的密度是多少?2-13.硬球模式广泛的适用于金属原子和离子,但是为何不适用于分子?2-14.计算(a)面心立方金属的原子致密度;(b)面心立方化合物NaCl的离子致密度(离子半径r Na+=0.097,r Cl-=0.181);(C)由计算结果,可以引出什么结论?2-15.铁的单位晶胞为立方体,晶格常数a=0.287nm,请由铁的密度算出每个单位晶胞所含的原子个数。

《材料科学与工程基础》英文习题及思考题及答案第二章习题和思考题Questions and Problems2.6 Allowed values for the quantum numbers ofelectrons are as follows:The relationships between n and the shell designationsare noted in Table 2.1.Relative tothe subshells,l =0 corresponds to an s subshelll =1 corresponds to a p subshelll =2 corresponds to a d subshelll =3 corresponds to an f subshellFor the K shell, the four quantum numbersfor each of the two electrons in the 1s state, inthe order of nlmlms , are 100(1/2 ) and 100(-1/2 ).Write the four quantum numbers for allof the electrons inthe L and M shells, and notewhich correspond to the s, p, and d subshells.2.7 Give the electron configurations for the followingions: Fe2+, Fe3+, Cu+, Ba2+,Br-, andS2-.2.17 (a) Briefly cite the main differences betweenionic, covalent, and metallicbonding.(b) State the Pauli exclusion principle.2.18 Offer an explanation as to why covalently bonded materials are generally lessdense than ionically or metallically bonded ones.2.19 Compute the percents ionic character of the interatomic bonds for the followingcompounds: TiO2 , ZnTe, CsCl, InSb, and MgCl2 .2.21 Using Table 2.2, determine the number of covalent bonds that are possible foratoms of the following elements: germanium, phosphorus, selenium, and chlorine.2.24 On the basis of the hydrogen bond, explain the anomalous behavior of waterwhen it freezes. That is, why is there volume expansion upon solidification?3.1 What is the difference between atomic structure and crystal structure?3.2 What is the difference between a crystal structure and a crystal system?3.4Show for the body-centered cubic crystal structure that the unit cell edge lengtha and the atomic radius R are related through a =4R/√3.3.6 Show that the atomic packing factor for BCC is 0.68. .3.27* Show that the minimum cation-to-anion radius ratio for a coordinationnumber of 6 is 0.414. Hint: Use the NaCl crystal structure (Figure 3.5), and assume that anions and cations are just touching along cube edges and across face diagonals.3.48 Draw an orthorhombic unit cell, and within that cell a [121] direction and a(210) plane.3.50 Here are unit cells for two hypothetical metals:(a)What are the indices for the directions indicated by the two vectors in sketch(a)?(b) What are the indices for the two planes drawn in sketch (b)?3.51* Within a cubic unit cell, sketch the following directions:.3.53 Determine the indices for the directions shown in the following cubic unit cell:3.57 Determine the Miller indices for the planesshown in the following unit cell:3.58Determine the Miller indices for the planes shown in the following unit cell: 3.61* Sketch within a cubic unit cell the following planes:3.62 Sketch the atomic packing of (a) the (100)plane for the FCC crystal structure, and (b) the (111) plane for the BCC crystal structure (similar to Figures 3.24b and 3.25b).3.77 Explain why the properties of polycrystalline materials are most oftenisotropic.5.1 Calculate the fraction of atom sites that are vacant for lead at its meltingtemperature of 327_C. Assume an energy for vacancy formation of 0.55eV/atom.5.7 If cupric oxide (CuO) is exposed to reducing atmospheres at elevatedtemperatures, some of the Cu2_ ions will become Cu_.(a) Under these conditions, name one crystalline defect that you would expect toform in order to maintain charge neutrality.(b) How many Cu_ ions are required for the creation of each defect?5.8 Below, atomic radius, crystal structure, electronegativity, and the most commonvalence are tabulated, for several elements; for those that are nonmetals, only atomic radii are indicated.Which of these elements would you expect to form the following with copper:(a) A substitutional solid solution having complete solubility?(b) A substitutional solid solution of incomplete solubility?(c) An interstitial solid solution?5.9 For both FCC and BCC crystal structures, there are two different types ofinterstitial sites. In each case, one site is larger than the other, which site isnormally occupied by impurity atoms. For FCC, this larger one is located at the center of each edge of the unit cell; it is termed an octahedral interstitial site. On the other hand, with BCC the larger site type is found at 0, __, __ positions—that is, lying on _100_ faces, and situated midway between two unit cell edges on this face and one-quarter of the distance between the other two unit cell edges; it is termed a tetrahedral interstitial site. For both FCC and BCC crystalstructures, compute the radius r of an impurity atom that will just fit into one of these sites in terms of the atomic radius R of the host atom.5.10 (a) Suppose that Li2O is added as an impurity to CaO. If the Li_ substitutes forCa2_, what kind of vacancies would you expect to form? How many of thesevacancies are created for every Li_ added?(b) Suppose that CaCl2 is added as an impurity to CaO. If the Cl_ substitutes forO2_, what kind of vacancies would you expect to form? How many of thevacancies are created for every Cl_ added?5.28 Copper and platinum both have the FCC crystal structure and Cu forms asubstitutional solid solution for concentrations up to approximately 6 wt% Cu at room temperature. Compute the unit cell edge length for a 95 wt% Pt-5 wt% Cu alloy.5.29 Cite the relative Burgers vector–dislocation line orientations for edge, screw, andmixed dislocations.6.1 Briefly explain the difference between selfdiffusion and interdiffusion.6.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion.(b) Cite two reasons why interstitial diffusion is normally more rapid thanvacancy diffusion.6.4 Briefly explain the concept of steady state as it applies to diffusion.6.5 (a) Briefly explain the concept of a driving force.(b) What is the driving force for steadystate diffusion?6.6Compute the number of kilograms of hydrogen that pass per hour through a5-mm thick sheet of palladium having an area of 0.20 m2at 500℃. Assume a diffusion coefficient of 1.0×10- 8 m2/s, that the concentrations at the high- and low-pressure sides of the plate are 2.4 and 0.6 kg of hydrogen per cubic meter of palladium, and that steady-state conditions have been attained.6.7 A sheet of steel 1.5 mm thick has nitrogen atmospheres on both sides at 1200℃and is permitted to achieve a steady-state diffusion condition. The diffusion coefficient for nitrogen in steel at this temperature is 6×10-11m2/s, and the diffusion flux is found to be 1.2×10- 7kg/m2-s. Also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 4 kg/m3. How far into the sheet from this high-pressure side will the concentration be 2.0 kg/m3?Assume a linear concentration profile.6.24. Carbon is allowed to diffuse through a steel plate 15 mm thick. Theconcentrations of carbon at the two faces are 0.65 and 0.30 kg C/m3 Fe, whichare maintained constant. If the preexponential and activation energy are 6.2 _10_7 m2/s and 80,000 J/mol, respectively, compute the temperature at which the diffusion flux is 1.43 _ 10_9 kg/m2-s.6.25 The steady-state diffusion flux through a metal plate is 5.4_10_10 kg/m2-s at atemperature of 727_C (1000 K) and when the concentration gradient is _350kg/m4. Calculate the diffusion flux at 1027_C (1300 K) for the sameconcentration gradient and assuming an activation energy for diffusion of125,000 J/mol.10.2 What thermodynamic condition must be met for a state of equilibrium to exist? 10.4 What is the difference between the states of phase equilibrium and metastability?10.5 Cite the phases that are present and the phase compositions for the followingalloys:(a) 90 wt% Zn–10 wt% Cu at 400℃(b) 75 wt% Sn–25wt%Pb at 175℃(c) 55 wt% Ag–45 wt% Cu at 900℃(d) 30 wt% Pb–70 wt% Mg at 425℃(e) 2.12 kg Zn and 1.88 kg Cu at 500℃(f ) 37 lbm Pb and 6.5 lbm Mg at 400℃(g) 8.2 mol Ni and 4.3 mol Cu at 1250℃.(h) 4.5 mol Sn and 0.45 mol Pb at 200℃10.6 For an alloy of composition 74 wt% Zn–26 wt% Cu, cite the phases presentand their compositions at the following temperatures: 850℃, 750℃, 680℃, 600℃, and 500℃.10.7 Determine the relative amounts (in terms of mass fractions) of the phases forthe alloys and temperatures given inProblem 10.5.10.9 Determine the relative amounts (interms of volume fractions) of the phases forthe alloys and temperatures given inProblem 10.5a, b, and c. Below are given theapproximate densities of the various metalsat the alloy temperatures:10.18 Is it possible to have a copper–silveralloy that, at equilibrium, consists of a _ phase of composition 92 wt% Ag–8wt% Cu, and also a liquid phase of composition 76 wt% Ag–24 wt% Cu? If so, what will be the approximate temperature of the alloy? If this is not possible,explain why.10.20 A copper–nickel alloy of composition 70 wt% Ni–30 wt% Cu is slowly heatedfrom a temperature of 1300_C .(a) At what temperature does the first liquid phase form?(b) What is the composition of this liquid phase?(c) At what temperature does complete melting of the alloy occur?(d) What is the composition of the last solid remaining prior to completemelting?10.28 .Is it possible to have a copper–silver alloy of composition 50 wt% Ag–50 wt%Cu, which, at equilibrium, consists of _ and _ phases having mass fractions W_ _0.60 and W_ _ 0.40? If so, what will be the approximate temperature of the alloy?If such an alloy is not possible, explain why.10.30 At 700_C , what is the maximum solubility (a) of Cu in Ag? (b) Of Ag in Cu?第三章习题和思考题3.3If the atomic radius of aluminum is 0.143nm, calculate the volume of its unitcell in cubic meters.3.8 Iron has a BCC crystal structure, an atomic radius of 0.124 nm, and an atomicweight of 55.85 g/mol. Compute and compare its density with the experimental value found inside the front cover.3.9 Calculate the radius of an iridium atom given that Ir has an FCC crystal structure,a density of 22.4 g/cm3, and an atomic weight of 192.2 g/mol.3.13 Using atomic weight, crystal structure, and atomic radius data tabulated insidethe front cover, compute the theoretical densities of lead, chromium, copper, and cobalt, and then compare these values with the measured densities listed in this same table. The c/a ratio for cobalt is 1.623.3.15 Below are listed the atomic weight, density, and atomic radius for threehypothetical alloys. For each determine whether its crystal structure is FCC,BCC, or simple cubic and then justify your determination. A simple cubic unitcell is shown in Figure 3.40.3.21 This is a unit cell for a hypotheticalmetal:(a) To which crystal system doesthis unit cell belong?(b) What would this crystal structure be called?(c) Calculate the density of the material, given that its atomic weight is 141g/mol.3.25 For a ceramic compound, what are the two characteristics of the component ionsthat determine the crystal structure?3.29 On the basis of ionic charge and ionic radii, predict the crystal structures for thefollowing materials: (a) CsI, (b) NiO, (c) KI, and (d) NiS. Justify your selections.3.35 Magnesium oxide has the rock salt crystal structure and a density of 3.58 g/cm3.(a) Determine the unit cell edge length. (b) How does this result compare withthe edge length as determined from the radii in Table 3.4, assuming that theMg2_ and O2_ ions just touch each other along the edges?3.36 Compute the theoretical density of diamond given that the CUC distance andbond angle are 0.154 nm and 109.5°, respectively. How does this value compare with the measured density?3.38 Cadmium sulfide (CdS) has a cubic unit cell, and from x-ray diffraction data it isknown that the cell edge length is 0.582 nm. If the measured density is 4.82 g/cm3 , how many Cd 2+ and S 2—ions are there per unit cell?3.41 A hypothetical AX type of ceramic material is known to have a density of 2.65g/cm 3 and a unit cell of cubic symmetry with a cell edge length of 0.43 nm. The atomic weights of the A and X elements are 86.6 and 40.3 g/mol, respectively.On the basis of this information, which of the following crystal structures is (are) possible for this material: rock salt, cesium chloride, or zinc blende? Justify your choice(s).3.42 The unit cell for Mg Fe2O3 (MgO-Fe2O3) has cubic symmetry with a unit celledge length of 0.836 nm. If the density of this material is 4.52 g/cm 3 , compute its atomic packing factor. For this computation, you will need to use ionic radii listed in Table 3.4.3.44 Compute the atomic packing factor for the diamond cubic crystal structure(Figure 3.16). Assume that bonding atoms touch one another, that the angle between adjacent bonds is 109.5°, and that each atom internal to the unit cell is positioned a/4 of the distance away from the two nearest cell faces (a is the unit cell edge length).3.45 Compute the atomic packing factor for cesium chloride using the ionic radii inTable 3.4 and assuming that the ions touch along the cube diagonals.3.46 In terms of bonding, explain why silicate materials have relatively low densities.3.47 Determine the angle between covalent bonds in an SiO44—tetrahedron.3.63 For each of the following crystal structures, represent the indicated plane in themanner of Figures 3.24 and 3.25, showing both anions and cations: (a) (100)plane for the rock salt crystal structure, (b) (110) plane for the cesium chloride crystal structure, (c) (111) plane for the zinc blende crystal structure, and (d) (110) plane for the perovskite crystal structure.3.66 The zinc blende crystal structure is one that may be generated from close-packedplanes of anions.(a) Will the stacking sequence for this structure be FCC or HCP? Why?(b) Will cations fill tetrahedral or octahedral positions? Why?(c) What fraction of the positions will be occupied?3.81* The metal iridium has an FCC crystal structure. If the angle of diffraction forthe (220) set of planes occurs at 69.22°(first-order reflection) when monochromatic x-radiation having a wavelength of 0.1542 nm is used, compute(a) the interplanar spacing for this set of planes, and (b) the atomic radius for aniridium atom.4.10 What is the difference between configuration and conformation in relation topolymer chains? vinyl chloride).4.22 (a) Determine the ratio of butadiene to styrene mers in a copolymer having aweight-average molecular weight of 350,000 g/mol and weight-average degree of polymerization of 4425.(b) Which type(s) of copolymer(s) will this copolymer be, considering thefollowing possibilities: random, alternating, graft, and block? Why?4.23 Crosslinked copolymers consisting of 60 wt% ethylene and 40 wt% propylenemay have elastic properties similar to those for natural rubber. For a copolymer of this composition, determine the fraction of both mer types.4.25 (a) Compare the crystalline state in metals and polymers.(b) Compare thenoncrystalline state as it applies to polymers and ceramic glasses.4.26 Explain briefly why the tendency of a polymer to crystallize decreases withincreasing molecular weight.4.27* For each of the following pairs of polymers, do the following: (1) state whetheror not it is possible to determine if one polymer is more likely to crystallize than the other; (2) if it is possible, note which is the more likely and then cite reason(s) for your choice; and (3) if it is not possible to decide, then state why.(a) Linear and syndiotactic polyvinyl chloride; linear and isotactic polystyrene.(b) Network phenol-formaldehyde; linear and heavily crosslinked ci s-isoprene.(c) Linear polyethylene; lightly branched isotactic polypropylene.(d) Alternating poly(styrene-ethylene) copolymer; randompoly(vinylchloride-tetrafluoroethylene) copolymer.4.28 Compute the density of totally crystalline polyethylene. The orthorhombic unitcell for polyethylene is shown in Figure 4.10; also, the equivalent of two ethylene mer units is contained within each unit cell.5.11 What point defects are possible for MgO as an impurity in Al2O3? How manyMg 2+ ions must be added to form each of these defects?5.13 What is the composition, in weight percent, of an alloy that consists of 6 at% Pband 94 at% Sn?5.14 Calculate the composition, in weight per-cent, of an alloy that contains 218.0 kgtitanium, 14.6 kg of aluminum, and 9.7 kg of vanadium.5.23 Gold forms a substitutional solid solution with silver. Compute the number ofgold atoms per cubic centimeter for a silver-gold alloy that contains 10 wt% Au and 90 wt% Ag. The densities of pure gold and silver are 19.32 and 10.49 g/cm3 , respectively.8.53 In terms of molecular structure, explain why phenol-formaldehyde (Bakelite)will not be an elastomer.10.50 Compute the mass fractions of αferrite and cementite in pearlite. assumingthat pressure is held constant.10.52 (a) What is the distinction between hypoeutectoid and hypereutectoid steels?(b) In a hypoeutectoid steel, both eutectoid and proeutectoid ferrite exist. Explainthe difference between them. What will be the carbon concentration in each?10.56 Consider 1.0 kg of austenite containing 1.15 wt% C, cooled to below 727_C(a) What is the proeutectoid phase?(b) How many kilograms each of total ferrite and cementite form?(c) How many kilograms each of pearlite and the proeutectoid phase form?(d) Schematically sketch and label the resulting microstructure.10.60 The mass fractions of total ferrite and total cementite in an iron–carbon alloyare 0.88 and 0.12, respectively. Is this a hypoeutectoid or hypereutectoid alloy?Why?10.64 Is it possible to have an iron–carbon alloy for which the mass fractions of totalferrite and proeutectoid cementite are 0.846 and 0.049, respectively? Why orwhy not?第四章习题和思考题7.3 A specimen of aluminum having a rectangular cross section 10 mm _ 12.7 mmis pulled in tension with 35,500 N force, producing only elastic deformation. 7.5 A steel bar 100 mm long and having a square cross section 20 mm on an edge ispulled in tension with a load of 89,000 N , and experiences an elongation of 0.10 mm . Assuming that the deformation is entirely elastic, calculate the elasticmodulus of the steel.7.7 For a bronze alloy, the stress at which plastic deformation begins is 275 MPa ,and the modulus of elasticity is 115 Gpa .(a) What is the maximum load that may be applied to a specimen with across-sectional area of 325mm, without plastic deformation?(b) If the original specimen length is 115 mm , what is the maximum length towhich it may be stretched without causing plastic deformation?7.8 A cylindrical rod of copper (E _ 110 GPa, Stress (MPa) ) having a yield strengthof 240Mpa is to be subjected to a load of 6660 N. If the length of the rod is 380 mm, what must be the diameter to allow an elongation of 0.50 mm?7.9 Consider a cylindrical specimen of a steel alloy (Figure 7.33) 10mm in diameterand 75 mm long that is pulled in tension. Determine its elongation when a load of 23,500 N is applied.7.16 A cylindrical specimen of some alloy 8 mm in diameter is stressed elasticallyin tension. A force of 15,700 N produces a reduction in specimen diameter of 5 _ 10_3 mm. Compute Poisson’s ratio for this material if its modulus of elasticity is 140 GPa .7.17 A cylindrical specimen of a hypothetical metal alloy is stressed in compression.If its original and final diameters are 20.000 and 20.025 mm, respectively, and its final length is 74.96 mm, compute its original length if the deformation is totally elastic. The elastic and shear moduli for this alloy are 105 Gpa and 39.7 GPa,respectively.7.19 A brass alloy is known to have a yield strength of 275 MPa, a tensile strength of380 MPa, and an elastic modulus of 103 GPa . A cylindrical specimen of thisalloy 12.7 mm in diameter and 250 mm long is stressed in tension and found to elongate 7.6 mm . On the basis of the information given, is it possible tocompute the magnitude of the load that is necessary to produce this change inlength? If so, calculate the load. If not, explain why.7.20A cylindrical metal specimen 15.0mmin diameter and 150mm long is to besubjected to a tensile stress of 50 Mpa; at this stress level the resulting deformation will be totally elastic.(a)If the elongation must be less than 0.072mm,which of the metals in Tabla7.1are suitable candidates? Why ?(b)If, in addition, the maximum permissible diameter decrease is 2.3×10-3mm,which of the metals in Table 7.1may be used ? Why?7.22 Cite the primary differences between elastic, anelastic, and plastic deformationbehaviors.7.23 diameter of 10.0 mm is to be deformed using a tensile load of 27,500 N. It mustnot experience either plastic deformation or a diameter reduction of more than7.5×10-3 mm. Of the materials listed as follows, which are possible candidates?Justify your choice(s).7.24 A cylindrical rod 380 mm long, having a diameter of 10.0 mm, is to besubjected to a tensile load. If the rod is to experience neither plastic deformationnor an elongation of more than 0.9 mm when the applied load is 24,500 N,which of the four metals or alloys listed below are possible candidates?7.25 Figure 7.33 shows the tensile engineering stress–strain behavior for a steel alloy.(a) What is the modulus of elasticity?(b) What is the proportional limit?(c) What is the yield strength at a strain offset of 0.002?(d) What is the tensile strength?7.27 A load of 44,500 N is applied to a cylindrical specimen of steel (displaying thestress–strain behavior shown in Figure 7.33) that has a cross-sectional diameter of 10 mm .(a) Will the specimen experience elastic or plastic deformation? Why?(b) If the original specimen length is 500 mm), how much will it increase inlength when t his load is applied?7.29 A cylindrical specimen of aluminumhaving a diameter of 12.8 mm and a gaugelength of 50.800 mm is pulled in tension. Usethe load–elongation characteristics tabulatedbelow to complete problems a through f.(a)Plot the data as engineering stressversusengineering strain.(b) Compute the modulus of elasticity.(c) Determine the yield strength at astrainoffset of 0.002.(d) Determine the tensile strength of thisalloy.(e) What is the approximate ductility, in percent elongation?(f ) Compute the modulus of resilience.7.35 (a) Make a schematic plot showing the tensile true stress–strain behavior for atypical metal alloy.(b) Superimpose on this plot a schematic curve for the compressive truestress–strain behavior for the same alloy. Explain any difference between thiscurve and the one in part a.(c) Now superimpose a schematic curve for the compressive engineeringstress–strain behavior for this same alloy, and explain any difference between this curve and the one in part b.7.39 A tensile test is performed on a metal specimen, and it is found that a true plasticstrain of 0.20 is produced when a true stress of 575 MPa is applied; for the same metal, the value of K in Equation 7.19 is 860 MPa. Calculate the true strain that results from the application of a true stress of 600 Mpa.7.40 For some metal alloy, a true stress of 415 MPa produces a plastic true strain of0.475. How much will a specimen of this material elongate when a true stress of325 MPa is applied if the original length is 300 mm ? Assume a value of 0.25 for the strain-hardening exponent n.7.43 Find the toughness (or energy to cause fracture) for a metal that experiences bothelastic and plastic deformation. Assume Equation 7.5 for elastic deformation,that the modulus of elasticity is 172 GPa , and that elastic deformation terminates at a strain of 0.01. For plastic deformation, assume that the relationship between stress and strain is described by Equation 7.19, in which the values for K and n are 6900 Mpa and 0.30, respectively. Furthermore, plastic deformation occurs between strain values of 0.01 and 0.75, at which point fracture occurs.7.47 A steel specimen having a rectangular cross section of dimensions 19 mm×3.2mm (0.75in×0.125in.) has the stress–strain behavior shown in Figure 7.33. If this specimen is subjected to a tensile force of 33,400 N (7,500lbf ), then(a) Determine the elastic and plastic strain values.(b) If its original length is 460 mm (18 in.), what will be its final length after theload in part a is applied and then released?7.50 A three-point bending test was performed on an aluminum oxide specimenhaving a circular cross section of radius 3.5 mm; the specimen fractured at a load of 950 N when the distance between the support points was 50 mm . Another test is to be performed on a specimen of this same material, but one that has a square cross section of 12 mm length on each edge. At what load would you expect this specimen to fracture if the support point separation is 40 mm ?7.51 (a) A three-point transverse bending test is conducted on a cylindrical specimenof aluminum oxide having a reported flexural strength of 390 MPa . If the speci- men radius is 2.5 mm and the support point separation distance is 30 mm ,predict whether or not you would expect the specimen to fracture when a load of 620 N is applied. Justify your prediction.(b) Would you be 100% certain of the prediction in part a? Why or why not?7.57 When citing the ductility as percent elongation for semicrystalline polymers, it isnot necessary to specify the specimen gauge length, as is the case with metals.Why is this so?7.66 Using the data represented in Figure 7.31, specify equations relating tensilestrength and Brinell hardness for brass and nodular cast iron, similar toEquations 7.25a and 7.25b for steels.8.4 For each of edge, screw, and mixed dislocations, cite the relationship between thedirection of the applied shear stress and the direction of dislocation line motion.8.5 (a) Define a slip system.(b) Do all metals have the same slip system? Why or why not?8.7. One slip system for theBCCcrystal structure is _110__111_. In a manner similarto Figure 8.6b sketch a _110_-type plane for the BCC structure, representingatom positions with circles. Now, using arrows, indicate two different _111_ slip directions within this plane.8.15* List four major differences between deformation by twinning and deformationby slip relative to mechanism, conditions of occurrence, and final result.8.18 Describe in your own words the three strengthening mechanisms discussed inthis chapter (i.e., grain size reduction, solid solution strengthening, and strainhardening). Be sure to explain how dislocations are involved in each of thestrengthening techniques.8.19 (a) From the plot of yield strength versus (grain diameter)_1/2 for a 70 Cu–30 Zncartridge brass, Figure 8.15, determine values for the constants _0 and ky inEquation 8.5.(b) Now predict the yield strength of this alloy when the average grain diameteris 1.0 _ 10_3 mm.8.20. The lower yield point for an iron that has an average grain diameter of 5 _ 10_2mm is 135 MPa . At a grain diameter of 8 _ 10_3 mm, the yield point increases to 260MPa. At what grain diameter will the lower yield point be 205 Mpa ?8.24 (a) Show, for a tensile test, thatif there is no change in specimen volume during the deformation process (i.e., A0 l0 _Ad ld).(b) Using the result of part a, compute the percent cold work experienced bynaval brass (the stress–strain behavior of which is shown in Figure 7.12) when a stress of 400 MPa is applied.8.25 Two previously undeformed cylindrical specimens of an alloy are to be strainhardened by reducing their cross-sectional areas (while maintaining their circular cross sections). For one specimen, the initial and deformed radii are 16 mm and11 mm, respectively. The second specimen, with an initial radius of 12 mm, musthave the same deformed hardness as the first specimen; compute the secondspecimen’s radius after deformation.8.26 Two previously undeformed specimens of the same metal are to be plasticallydeformed by reducing their cross-sectional areas. One has a circular cross section, and the other is rectangular is to remain as such. Their original and deformeddimensions are as follows:Which of these specimens will be the hardest after plastic deformation, and why?8.27 A cylindrical specimen of cold-worked copper has a ductility (%EL) of 25%. Ifits coldworked radius is 10 mm (0.40 in.), what was its radius beforedeformation?8.28 (a) What is the approximate ductility (%EL) of a brass that has a yield strengthof 275 MPa ?(b) What is the approximate Brinell hardness of a 1040 steel having a yieldstrength of 690 MPa?8.41 In your own words, describe the mechanisms by which semicrystalline polymers(a) elasticallydeform and (b) plastically deform, and (c) by which elastomerselastically deform.8.42 Briefly explain how each of the following influences the tensile modulus of asemicrystallinepolymer and why:(a) molecular weight;(b) degree of crystallinity;(c) deformation by drawing;(d) annealing of an undeformed material;(e) annealing of a drawn material.8.43* Briefly explain how each of the following influences the tensile or yieldstrength of a semicrystalline polymer and why:(a) molecular weight;。

《材料科学与⼯程基础》英⽂影印版习题及思考题及答案《材料科学与⼯程基础》英⽂习题及思考题及答案第⼆章习题和思考题Questions and Problems2.6 Allowed values for the quantum numbers ofelectrons are as follows:The relationships between n and the shell designationsare noted in Table 2.1.Relative tothe subshells,l =0 corresponds to an s subshelll =1 corresponds to a p subshelll =2 corresponds to a d subshelll =3 corresponds to an f subshellFor the K shell, the four quantum numbersfor each of the two electrons in the 1s state, inthe order of nlmlms , are 100(1/2 ) and 100(-1/2 ).Write the four quantum numbers for allof the electrons inthe L and M shells, and notewhich correspond to the s, p, and d subshells.2.7 Give the electron configurations for the followingions: Fe2+, Fe3+, Cu+, Ba2+,Br-, andS2-.2.17 (a) Briefly cite the main differences betweenionic, covalent, and metallicbonding.(b) State the Pauli exclusion principle.2.18 Offer an explanation as to why covalently bonded materials are generally lessdense than ionically or metallically bonded ones.2.19 Compute the percents ionic character of the interatomic bonds for the followingcompounds: TiO2 , ZnTe, CsCl, InSb, and MgCl2 .2.21 Using Table 2.2, determine the number of covalent bonds that are possible foratoms of the following elements: germanium, phosphorus, selenium, and chlorine.2.24 On the basis of the hydrogen bond, explain the anomalous behavior of waterwhen it freezes. That is, why is there volume expansion upon solidification?3.1 What is the difference between atomic structure and crystal structure?3.2 What is the difference between a crystal structure and a crystal system?3.4Show for the body-centered cubic crystal structure that the unit cell edge lengtha and the atomic radius R are related through a =4R/√3.3.6 Show that the atomic packing factor for BCC is 0.68. .3.27* Show that the minimum cation-to-anion radius ratio for a coordinationnumber of 6 is 0.414. Hint: Use the NaCl crystal structure (Figure 3.5), and assume that anions and cations are just touching along cube edges and across face diagonals.3.48 Draw an orthorhombic unit cell, and within that cell a [121] direction and a(210) plane.3.50 Here are unit cells for two hypothetical metals:(a)What are the indices for the directions indicated by the two vectors in sketch(a)?(b) What are the indices for the two planes drawn in sketch (b)?3.51* Within a cubic unit cell, sketch the following directions:.3.53 Determine the indices for the directions shown in the following cubic unit cell:3.57 Determine the Miller indices for the planesshown in the following unit cell:3.58Determine the Miller indices for the planes shown in the following unit cell:3.61* Sketch within a cubic unit cell the following planes:3.62 Sketch the atomic packing of (a) the (100)plane for the FCC crystal structure, and (b) the (111) plane for the BCC crystal structure (similar to Figures 3.24b and 3.25b).3.77 Explain why the properties of polycrystalline materials are most oftenisotropic.5.1 Calculate the fraction of atom sites that are vacant for lead at its meltingtemperature of 327_C. Assume an energy for vacancy formation of 0.55eV/atom.5.7 If cupric oxide (CuO) is exposed to reducing atmospheres at elevatedtemperatures, some of the Cu2_ ions will become Cu_.(a) Under these conditions, name one crystalline defect that you would expect toform in order to maintain charge neutrality.(b) How many Cu_ ions are required for the creation of each defect?5.8 Below, atomic radius, crystal structure, electronegativity, and the most commonvalence are tabulated, for several elements; for those that are nonmetals, only atomic radii are indicated.Which of these elements would you expect to form the following with copper:(a) A substitutional solid solution having complete solubility?(b) A substitutional solid solution of incomplete solubility?(c) An interstitial solid solution?5.9 For both FCC and BCC crystal structures, there are two different types ofinterstitial sites. In each case, one site is larger than the other, which site isnormally occupied by impurity atoms. For FCC, this larger one is located at the center of each edge of the unit cell; it is termed an octahedral interstitial site. On the other hand, with BCC the larger site type is found at 0, __, __ positions—that is, lying on _100_ faces, and situated midway between two unit cell edges on this face and one-quarter of the distance between the other two unit cell edges; it is termed a tetrahedral interstitial site. For both FCC and BCC crystalstructures, compute the radius r of an impurity atom that will just fit into one of these sites in terms of the atomic radius R of the host atom.5.10 (a) Suppose that Li2O is added as an impurity to CaO. If the Li_ substitutes forCa2_, what kind of vacancies would you expect to form? How many of thesevacancies are created for every Li_ added?(b) Suppose that CaCl2 is added as an impurity to CaO. If the Cl_ substitutes forO2_, what kind of vacancies would you expect to form? How many of thevacancies are created for every Cl_ added?5.28 Copper and platinum both have the FCC crystal structure and Cu forms asubstitutional solid solution for concentrations up to approximately 6 wt% Cu at room temperature. Compute the unit cell edge length for a 95 wt% Pt-5 wt% Cu alloy.5.29 Cite the relative Burgers vector–dislocation line orientations for edge, screw, andmixed dislocations.6.1 Briefly explain the difference between selfdiffusion and interdiffusion.6.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion.(b) Cite two reasons why interstitial diffusion is normally more rapid thanvacancy diffusion.6.4 Briefly explain the concept of steady state as it applies to diffusion.6.5 (a) Briefly explain the concept of a driving force.(b) What is the driving force for steadystate diffusion?6.6Compute the number of kilograms of hydrogen that pass per hour through a5-mm thick sheet of palladium having an area of 0.20 m2at 500℃. Assume a diffusion coefficient of 1.0×10- 8 m2/s, that the concentrations at the high- and low-pressure sides of the plate are 2.4 and 0.6 kg of hydrogen per cubic meter of palladium, and that steady-state conditions have been attained.6.7 A sheet of steel 1.5 mm thick has nitrogen atmospheres on both sides at 1200℃and is permitted to achieve a steady-state diffusion condition. The diffusion coefficient for nitrogen in steel at this temperature is 6×10-11m2/s, and the diffusion flux is found to be 1.2×10- 7kg/m2-s. Also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 4 kg/m3. How far into the sheet from this high-pressure side will the concentration be 2.0 kg/m3?Assume a linear concentration profile.6.24. Carbon is allowed to diffuse through a steel plate 15 mm thick. Theconcentrations of carbon at the two faces are 0.65 and 0.30 kg C/m3 Fe, whichare maintained constant. If the preexponential and activation energy are 6.2 _10_7 m2/s and 80,000 J/mol, respectively, compute the temperature at which the diffusion flux is 1.43 _ 10_9 kg/m2-s.6.25 The steady-state diffusion flux through a metal plate is 5.4_10_10 kg/m2-s at atemperature of 727_C (1000 K) and when the concentration gradient is _350kg/m4. Calculate the diffusion flux at 1027_C (1300 K) for the sameconcentration gradient and assuming an activation energy for diffusion of125,000 J/mol.10.2 What thermodynamic condition must be met for a state of equilibrium to exist? 10.4 What is the difference between the states of phase equilibrium and metastability?10.5 Cite the phases that are present and the phase compositions for the followingalloys:(a) 90 wt% Zn–10 wt% Cu at 400℃(b) 75 wt% Sn–25wt%Pb at 175℃(c) 55 wt% Ag–45 wt% Cu at 900℃(d) 30 wt% Pb–70 wt% Mg at 425℃(e) 2.12 kg Zn and 1.88 kg Cu at 500℃(f ) 37 lbm Pb and 6.5 lbm Mg at 400℃(g) 8.2 mol Ni and 4.3 mol Cu at 1250℃.(h) 4.5 mol Sn and 0.45 mol Pb at 200℃10.6 For an alloy of composition 74 wt% Zn–26 wt% Cu, cite the phases presentand their compositions at the following temperatures: 850℃, 750℃, 680℃, 600℃, and 500℃.10.7 Determine the relative amounts (in terms of mass fractions) of the phases forthe alloys and temperatures given inProblem 10.5.10.9 Determine the relative amounts (interms of volume fractions) of the phases forthe alloys and temperatures given inProblem 10.5a, b, and c. Below are given theapproximate densities of the various metalsat the alloy temperatures:10.18 Is it possible to have a copper–silveralloy that, at equilibrium, consists of a _ phase of composition 92 wt% Ag–8。

复习题1、On the basis of crystal structure, using the following equation and values of parameters, compute the theoretical density for sodium chloride (NaCI). How does this compare with its measured density? Measured density of NaCI is 2.16 g/cm3.Theoretical density formula:n' = the number of formula units1 within the unit cellSX C = the sum of the atomic weights of all cations in the formula unitSX A = the sum of the atomic weights of all anions in the formula unitV c = the unit cell volumeN A = Avogadro's number, 6.023 X 1023 formula units/mol4Na = 22.99 g/molA C} = 35.45 g/molr Na+ =0.102nm r c f= 0.18 InmONa+ O Cl-Solution:According to the structure of NaCI, each unit cell consist of 4 NaCI formula units. Therefore,n =4.Furthermore, since the unit cell of NaCI is cubic, therefore, V c=a3, a being the edge length of the unit cell. Again with referred to the structure of NaCI,a=2r Na++2r c fThus,V c=(2r Na++2r C i_)3Using the theoretical density formula,_ 〃'(4卬+ 4q)P = (2『3 + 2,C1 )3'A_ 4(22.99 + 35.45)~ [2(0.102 X 10~7) + 2(0.181 X 10-7)]3(6.023 X 1023)=2.14 g/cm3This value agrees fairly well with the experimental value.2、Calculate the number of vacancies per cubic meter in gold at 900°C. The energy for vacancy formation is 1.602xl0'19 J/atom. Furthermore, the density and atomic weight for Au are 18.63 g/cm3 (at 900°C) and 196.9 g/mol, respectively.N〃 = B (-新N v: number of equilibrium vacanciesN: total number of atomic sitesQ v: formation energy of a vacancyk: Boltzmann constant (1.38xlO'23J/K)T: temperature in KelvinSolution:In order to calculate the number of vacancies per cubic meter in gold at 900°C, it has to be known the total number of atomic sites per cubmic meter in gold first. N can be computed as,N=(18.63xl06g/m3/196.9 g/mol)x6.02xl023=5.7xl028Substituting the calculated N and other parameters into the equation, we have- ( 1.602 x 10T9 \N v = 5.7 x 1028 exp —~~——~——~~—— = 2.87 X 1024v 1.38 x 10-23 x (900 + 273)J3、Explain the relative orientation between Burgers vector and dislocation line for edge dislocation, screw dislocation, and mixed dislocation.Solution:For edge dislocation, Burgers vector is perpendicular to the dislocation line.For screw dislocation, Burgers vector is parallel to the dislocation line.For mixed dislocation, the angle between Burgers vector and dislocation is higher than 0° but lower than 90°.4、For both FCC and BCC crystal structures, there are two different types of interstitial sites. In each case, one site is larger than the other; and is normally occupied by impurity atoms. For FCC, this larger one is located at the center of each edge of the unit cell; it is termed an octahedral interstitial site. On the other hand, with BCC the larger site type is found at positions 0 % %; it is termed a tetrahedral interstitial site. For both FCC and BCC crystal structures, compute the radius r of an impurity atom that will just fit into one of these sites in terms of the atomic radius R of the host atom.Solution:Assuming the lattice parameter of both FCC and BCC crystal structures is a.Then, for FCC structure, a 2 + a 2 = (4R)2For octahedral interstitial position, 1, L 、r 0 = -(2V2R - 2R )= 0.414RFor tetragonal interstitial position, I a o a~ a~~「T =北产 + 0 + (J = °・225RFor BCC structure, a 2 + a 2 + a 2 = (4R)2For octahedral interstitial position, 1/4V3 \r 0 - 2(~R-2R ) = 0・155RFor tetragonal interstitial position,2V3 , V3 o (—R)2 + (—R)2 - R = 0.291R O sites T sitesBCCA copper-nickel alloy of composition 70 wt% Ni-30 wt% Cu is slowly heated from a temperature of 1300°C (2370°F).(a) At what temperature does the first liquid phase form?(b) What is the composition of this liquid phase?(c) At what temperature does complete melting of the alloy occur?(d) What is the composition of the last solid remaining prior to complete melting?Composition (at% Ni)(Cu) Composition (wt% Ni) (Ni)Is it possible to have a copper-nickel alloy that, at equilibrium, consists of an a phase of composition 37 wt% Ni-63 wt% Cu, and also a liquid phase of composition 20 wt% Ni-80 wt% Cu? If so, what will be the approximate temperature of the alloy? If this is not possible, explain why.Composition (at% Ni)(Cu) Composition (wt% Ni) (Ni)Solution:No, it is not possible to have such an alloy. From the phase diagram, it can be seen that if the composition of a phase is 37wt.%Ni-63wt.%Cu, at equilibrium, the corresponding composition of liquid phase should be 22wt.%Ni-78wt.%Cu.7、(a) Using the NaCl-H?。
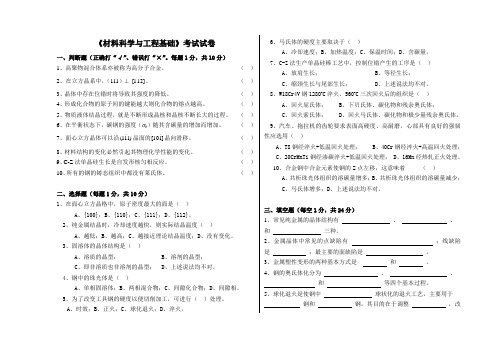
《材料科学与工程基础》考试试卷一、判断题(正确打“√”、错误打“×”。
每题1分,共10分)1、高聚物混合体系亦被称为高分子合金。
( )2、在立方晶系中,(111)⊥ ]211[-。
( ) 3、晶体中存在位错时将导致其强度的降低。
( ) 4、形成化合物的原子间的键能越大则化合物的熔点越高。
( ) 5、物质液体结晶过程,就是不断形成晶核和晶核不断长大的过程。
( ) 6、在平衡状态下,碳钢的强度(σb )随其含碳量的增加而增加。
( ) 7、面心立方晶体可以沿)111(-晶面的]110[-晶向滑移。
( ) 8、材料结构的变化必然引起其物理化学性能的变化。
( ) 9、C-Z 法单晶硅生长是自发形核匀相反应。
( )10、所有的钢的铸态组织中都没有莱氏体。
( )二、选择题(每题1分,共10分)1、在面心立方晶格中,原子密度最大的面是( ) A 、{100};B 、{110};C 、{111};D 、{112}。
2、纯金属结晶时,冷却速度越快,则实际结晶温度( ) A 、越低;B 、越高;C 、越接近理论结晶温度;D 、没有变化。
3、固溶体的晶体结构是( )A 、溶质的晶型;B 、溶剂的晶型;C 、即非溶质也非溶剂的晶型;D 、上述说法均不对。
4、钢中的珠光体是( )A 、单相固溶体;B 、两相混合物;C 、间隙化合物;D 、间隙相。
5、为了改变工具钢的硬度以便切削加工,可进行( )处理。
A 、时效;B 、正火;C 、球化退火;D 、淬火。
6、马氏体的硬度主要取决于( )A 、冷却速度;B 、加热温度;C 、保温时间;D 、含碳量。
7、C-Z 法生产单晶硅棒工艺中,控制位错产生的工序是( ) A 、放肩生长; B 、等径生长; C 、缩颈生长与尾部生长; D 、上述说法均不对。
8、W18Cr4V 钢1280o C 淬火、560o C 三次回火后的组织是( ) A 、回火屈氏体; B 、下贝氏体、碳化物和残余奥氏体; C 、回火索氏体; D 、回火马氏体、碳化物和极少量残余奥氏体。

《材料科学与工程基础》题集大题一:选择题1.下列哪一项是材料的基本属性?A. 密度B. 颜色C. 形状D. 体积2.材料的力学性能主要包括哪一项?A. 导电性B. 耐腐蚀性C. 强度D. 透明度3.下列哪一项不是金属材料的常见类型?A. 钢铁B. 铝合金C. 陶瓷D. 铜合金4.材料的硬度是指其抵抗什么的能力?A. 拉伸B. 压缩C. 弯曲D. 刻划5.下列哪一项是热塑性材料的特性?A. 在加热后不能变形B. 在加热后可以永久变形C. 在冷却后可以恢复原形D. 在任何温度下都不易变形6.材料的韧性是指其在受力时什么的能力?A. 易碎B. 易弯曲C. 吸收能量而不破裂D. 迅速恢复原形7.下列哪一项是陶瓷材料的主要成分?A. 金属B. 塑料C. 无机非金属D. 有机物8.复合材料是由哪两种或多种材料组合而成的?A. 同一种材料的不同形态B. 不同性质的材料C. 相同性质的材料D. 任意两种材料9.下列哪一项不是高分子材料的特性?A. 高强度B. 高韧性C. 低密度D. 低耐温性10.材料的疲劳是指其在什么条件下性能逐渐降低的现象?A. 持续受力B. 持续加热C. 持续冷却D. 持续暴露在潮湿环境中大题二:填空题1.材料的密度是指单位体积内材料的______。
2.材料的导电性是指材料传导______的能力。
3.金属材料的晶体结构常见的有______、体心立方和面心立方。
4.陶瓷材料因其______、高硬度和高耐温性而被广泛应用于高温和腐蚀环境。
5.复合材料的优点包括高强度、高刚性和良好的______。
6.高分子材料的分子结构特点是具有长链状的______结构。
7.材料的疲劳强度是指材料在______作用下抵抗破坏的能力。
大题三:判断题1.材料的力学性能只包括强度和硬度。
()2.金属材料都是良好的导体。
()3.陶瓷材料的主要成分是金属。
()4.复合材料的性能总是优于其单一组分的性能。
()5.高分子材料的耐温性一般较低。
第一组材料的刚性越大,材料就越脆。
F按受力方式,材料的弹性模量分为三种类型,以下哪一种是错误的:DA. 正弹性模量(E)B. 切弹性模量(G)C. 体积弹性模量(G)D. 弯曲弹性模量(W)滞弹性是无机固体和金属的与时间有关的弹性,它与下列哪个因素无关BA 温度;B 形状和大小;C 载荷频率高弹性有机聚合物的弹性模量随温度的升高而AA. 上升;B. 降低;C. 不变。
金属材料的弹性模量随温度的升高而BA. 上升;B. 降低;C. 不变。
弹性模量和泊松比之间有一定的换算关系,以下换算关系中正确的是DA. K=E /[3(1+2)];B. E=2G (1-);C. K=E /[3(1-)];D. E=3K (1-2);E. E=2G (1-2)。
7.Viscoelasticity”的意义是BA 弹性;B粘弹性; C 粘性8.均弹性摸量的表达式是AA、E=σ/εB、G=τ/rC、K=σ。
/(△V/V)9.金属、无机非金属和高分子材料的弹性摸量一般在以下数量级范围内C GPaA.10-102、<10,10-102B.<10、10-102、10-102C.10-102、10-102、<1010.体心立方晶胞的金属材料比面心立方晶胞的同类金属材料具有更高的摸量。
T11.虎克弹性体的力学特点是BA、小形变、不可回复B、小形变、可回复C、大形变、不可回复D、大形变、可回复13、金属晶体、离子晶体、共价晶体等材料的变形通常表现为,高分子材料则通常表现为和。
AA 普弹行、高弹性、粘弹性B 纯弹行、高弹性、粘弹性C 普弹行、高弹性、滞弹性14、泊松比为拉伸应力作用下,材料横向收缩应变与纵向伸长应变的比值υ=ey/ex F第二组1.对各向同性材料,以下哪一种应变不属于应变的三种基本类型CA. 简单拉伸;B. 简单剪切;C. 扭转;D. 均匀压缩2.对各向同性材料,以下哪三种应变属于应变的基本类型ABDA. 简单拉伸;B. 简单剪切;C. 弯曲;D. 均匀压缩3.“Tension”的意义是AA 拉伸;B 剪切;C 压缩4.“Compress”的意义是CA 拉伸;B剪切; C 压缩5.陶瓷、多数玻璃和结晶态聚合物的应力-应变曲线一般表现为纯弹性行为T6.Stress”and “strain”的意义分别是AA 应力和应变;B应变和应力;C应力和变形7.对各向同性材料,以下哪三种应变属于应变的三种基本类型ACDA. tension;B. torsional deformation;C. shear;D. compression8.对各向同性材料,以下哪一种应变不属于应变的三种基本类型CA. tension;B. shear;C. Flexural deformation;D. compression9.对各向同性材料,应变的三种基本类型是AA tension, shear and compression;B tension, shear and torsional deformation;C. tension, shear and flexural deformation10.非金属态聚合物的三种力学状态是AA、玻璃态、高弹态、粘流态。
《材料科学与工程基础》考试试卷一、名词解释(20分)1.相2.刃型位错3.淬硬性4.滑移5.合金二、 Fe-Fe3C相图基本知识(共30分)1.写出Fe-Fe3C相图中三条水平线相变反应过程及名称;(6分)2.根据Fe-Fe3C相图计算室温下二次渗碳体的最大量;(6分)3.计算40钢在室温下的相组成物的相对量;(4分)4.计算60钢在室温下的组织组成物的相对量;(4分)5.某铁碳合金平衡组织由珠光体和铁素体组成,其中铁素体占50%,求该合金的含碳量;(4分)6. 计算20钢室温时共析渗碳体、共析铁素体以及先共析铁素体的相对量。
(6分)三、 回答下列问题:(共50分)1.在立方晶格中确定下列图示晶面(阴影部分)的晶面指数。
(6分)2.从所给出的材料中选定零件适用的钢材,并说明其热处理方法或使用状态: Q345(16Mn ),40Cr ,W6Mo5Cr4V2,GCr15,2Cr13,T12,40Cr ,60Si2Mn 。
(10分)3.主轴选用20Cr 钢制作,其工艺路线如下:下料→锻造→正火①→粗加工→渗碳淬火+低温回火②→磨削。
请回答上述工艺路线中①②热处理工艺的目的以及热处理后相应的组织。
(6)4.在共析钢的TTT 曲线中,写出各条冷却曲线下获得的组织。
(10分)① ② ③ ④ ⑤5.写出三种材料强化的方式并说明之。
(8)YY(a)(b)温 度 A M M6.一块凹模,材料为Cr12MoV钢。
因材料库下料时搞错了,下成了T12A。
试分析:(10分)(1)如果按Cr12MoV钢进行预备热处理和最终热处理,凹模会产生哪些缺陷?(2)如果预备热处理按Cr12MoV钢进行,而最终热处理的淬火按T12A 钢进行,则又会产生哪些缺陷?(1)T12A组织中的渗碳体有一部分会转变为奥氏体,在冷却时再转变为珠光体,如果再按Cr12MoV进行最终热处理的话,由于处理温度高达1030-1080度,组织会很粗大且硬度较低,只能是废品。
材料科学:材料科学与工程考试卷及答案 考试时间:120分钟 考试总分:100分遵守考场纪律,维护知识尊严,杜绝违纪行为,确保考试结果公正。
1、问答题 说明斯宾那多分解相变和成核-生长相变的主要区别? 本题答案: 2、判断题 高分子原子量大,所以它的结构很复杂。
本题答案: 3、问答题 简要介绍玻璃的生成工艺流程。
本题答案: 4、单项选择题 如何增加歧化终止比例( )A.升高温度 B.降低温度 C.升高压力 D.降低压力 本题答案: 5、填空题 约束超导现象的三大条件是( )、( )和( )。
本题答案: 6、单项选择题 属于多孔催化剂载体的是( )A 、刚玉粉 B 、碳化硅姓名:________________ 班级:________________ 学号:________________--------------------密----------------------------------封 ----------------------------------------------线----------------------C、磨细的玻璃D、多孔金属本题答案:7、单项选择题滞弹性是无机固体和金属的与时间有关的弹性,它与下列哪个因素无关()A.温度;B.形状和大小;C.载荷频率本题答案:8、问答题高分子有哪三种力学状态?各有什么特点?本题答案:9、多项选择题在自由基聚合反应的工业实施方法有()。
A.本体聚合B.悬浮聚合C.乳液聚合D.溶剂聚合本题答案:10、问答题.在三元系统中,无变量点有三种,分别是什么?本题答案:11、名词解释晶界能本题答案:12、单项选择题测定PE.PP相对分子质量方法有().A.稀溶液黏度法B.熔体流动速率法(MFR)C.光散射法D.气相色谱法本题答案:13、名词解释空间点阵本题答案:14、单项选择题鞣制后的动物毛皮称为()A.生皮B.裘皮C.皮革D.绒面革本题答案:15、单项选择题下列官能度体系中,能生成体型产物的是()A.1-1B.1-3C.2-2D.2-3本题答案:16、判断题基体与增强体间界面的模量比增强体和基体高,则复合材料的弹性模量也越高。
材料科学与工程基础(英文)_南京航空航天大学中国大学mooc课后章节答案期末考试题库2023年1.The driving force for steady-state diffusion is the __________.答案:concentration gradient2.Diffusion coefficient is with the increasing diffusion temperature.答案:exponentially increased;3.Due to , alloys are usually than pure metals of the solvent.答案:solid solution strengthening, stronger;4.The finer the grains, the larger the , and .答案:strength, hardness, toughness;5.With plastic deformation,the increase of dislocationdensity will result in .答案:higher strength;6.In general, Brinell Hardness test is to measure thematerial’s hardness.答案:relatively softer7.Yield strength is corresponding to the occurrenceof deformation.答案:noticeable plastic8.Strain Hardening is also named as .答案:work hardening9.Vacancy diffusion is usually interstitial one.答案:slower than10.Edge and screw dislocations differ in what way?答案:angle between Burgers vector and line direction.11. A ____ may form when impurity atoms are added to a solid, in which case theoriginal crystal structure is retained and no new phases are formed.答案:solid solution12.One explanation for why graphite powder acts so well as a “solid lubricant”is .答案:carbon atoms in graphite are covalently bonded within planar layers but have weaker secondary bonds between layers13.Substitutional atom (impurity) is an example of ______.答案:point defect14.Interstitial solid solution belongs to .答案:finite solid solution;15.The atomic packing factor for FCC is .答案:0.7416.The coordination number of BCC crystal structure is .答案:817.The crystal structure of Cu is ?答案:FCC18.How many atoms does the face centered cubic unit cell contain?答案:Four19.If the electron configuration of Fe is 1s2 2s2 2p6 3s2 3p6 3d6 4s2, then theelectron configurations for the Fe3+ is 1s2 2s2 2p6 3s2 _____.答案:3p6 3d520.Bonds in most metals are referred to as ______.答案:Non-directional21.Covalent bonding occurs as a result of _________ sharing.答案:electron22.Which of the following is NOT an example of primary bonding?答案:Van der Waals23.Atomic weight (A) of an element corresponds to the weighted average of theatomic masses of the atom’s naturally occurring ___________.答案:isotopes24.The point on a phase diagram where the maximum number of allowablephases are in equilibrium is .答案:eutectic point25.Sterling silver (92.5%Ag/7.5%Cu) is an example of ___________.答案:Solid solution26.Engineering stress-strain curve and true stress-strain curve are equal up to .答案:Yeild point27.Among thefollowingtypical transformations of austenite in steels,____________transformation is diffusionless.答案:martensitic28.The heat-treatable aluminum alloy can be strengthened by .答案:Both of above29.In the as-quenched state, martensite is very hard and so brittle that a heattreatment known as must be accomplished sequently.答案:tempering30.During heat treatment of steel, austenite transforms into martensite by .答案:quenching31.Which of the following plane has the highest planar density for fcc.答案:(111)32.Which of the following describes recrystallization?答案:Diffusion dependent with no change in phase composition33.Heating the cold-worked metal progresses in three stages: .答案:recovery, recrystallization, grain growth;34.Strength is increased by making dislocation motion .答案:difficult35.The boundary above which only liquid phase exist is called _________.答案:liquidus36.We have an annealed carbon steel which has hardness of 150HBS. Supposewe know the hardness of Pearlite is 200HBS and the hardness of Ferrite is 80HBS, determine the carbon amount of this steel.答案:0.45%37.The maximum solubility of C in γ-austenite - solid solution is .答案:2.1438.In a plain steel that contains 0.2 percentage carbon, we should expect: .答案:a 25% pearlite and 75% pro-eutectoid ferrite39. A copper-nickel alloy is high-temperature heat treated; the diffusion of Cuinto Ni and Ni into Cu regions is referred to as _____________________.答案:Inter-diffusion40.The phase diagram of Sn-Pb alloy is called .答案:Eutectic phase diagram。
英文原版教材班“材料科学与工程基础”考试试题Examination problems of the course of “fundament of materials science”(注:第1、2、3、5题为必做题;第4、6、7题为选择题,必须二选一。
共100分)姓名:班级:记分:1. Glossary (2 points for each)1) crystal structure:2) basis (or motif):3) packing fractor:4) slip system:5) critical size:6) homogeneous nucleation:7) coherent precipitate:8) precipitation hardening:9) diffusion coefficient:10) uphill diffusion:2. Determine the indices for the planes in the cubic unit cell shown in Figure 1. (5 points)Fig. 13. Determine the crystal structure for the following: (a) a metal with a0 =4.9489 Å, r = 1.75 Å and one atom per lattice point; (b) a metal with a0 = 0.42906 nm, r = 0.1858 nm and one atom per lattice point. (10 points)4-1. What is the characteristic of brinell hardness test, rockwell hardness test and Vickers hardness test? What are the effects of strain rate and temperature on the mechanical properties of metallic materials? (15 points)4-2. What are the effects of cold-work on metallic materials? How to eliminate those effects? And what is micro-mechanism for the eliminating cold-work effects? (15 points)5-1. Based on the Pb-Sn-Bi ternary diagram as shown in Fig. 2, try to(1)Show the vertical section of 40wt.%Sn; (4 points)(2) Describe the solidification process of the alloy 2# with very low cooling speed (includingphase and microstructure changes); (4 points)(3)Plot the isothermal section at 150o C. (7 points)5-2. A 1mm sheet of FCC iron is used to contain N 2 in a heated exchanger at 1200o C. The concentration of N at one surface is 0.04 atomic percent and the concentration at the second surface is 0.005 atomic percent. At 1000 o C, if same N concentration is demanded at the second surface and the flux of N becomes to half of that at 1200o C, then what is the thickness of sheet? (15 points)6-1. Supposed that a certain liquid metal is undercooled until homogeneous nucleation occurs. (15 points)(1) How to calculate the critical radius of the nucleus required? Please give the deductionprocess.(2) For the Metal Ni, the Freezing Temperature is 1453︒C, the Latent Heat of Fusion is 2756J/cm 3, and the Solid-liquid Interfacial Energy is 255⨯10-7 J/cm 2. Please calculate the critical radius at 1353︒C. (Assume that the liquid Ni is not solidified.)6-2. Fig.3 is a portion of the Mg-Al phase diagram. (15 points)(1) If the solidification is too rapid, please describe the solidification process of Mg-10wt%Alalloy.(2) Please describe the equilibrium solidification process of Mg-20wt%Al alloy, and calculate theamount of each phase at 300︒C.Fig. 3Fig. 27-1. Figure 4 shows us the Al-Cu binary diagram and some microstructures found in a cooling process for an Al-4%Cu alloy. Please answer following questions according to this figure. (20 points)Fig. 4(1)What are precipitate, matrix and microconstituent? Please point them out in the in the figure and explain.(2)Why is need-like precipitate not good for dispersion strengthening? The typical microstructure shown in the figure is good or not? why?(3)Please tell us how to obtain the ideal microstructure shown in this figure.(4)Can dispersion strengthened materials be used at high temperature? Please give the reasons (comparing with cold working strengthening)7-2. Please answer following questions according to the time-temperature-transformation (TTT) diagram as shown in Fig. 5. (20 points)(1)What steel is this TTT diagram for? And what means P, B, and M in the figure?(2)Why dose the TTT diagram exhibits a ‘C’ shape?(3)Point out what microconstituent will be obtained after austenite is cooled according to the curves I, II, III and IV .(4)What is microstructural difference between the curve I and the curve II?(5)How to obtain the steel with the structure of(a) P+B(b) P+M+A (residual)(c) P+B+M+A (residual)(d) Full tempered martensiteIf you can, please draw the relative cooling curve or the flow chart of heat treatment.Fig. 5Examination of the course of “fundament of materials science”“材料科学与工程基础”考试试题 – 英文原版教材班(注:第1、2、3题为必做题;第4、5、6、7题为选择题,必须二选一。
共100分)1. Glossary (2 points for each)III III IV1) crystal structure: The arrangement of the atoms in a material into a repeatable lattice.2) basis (or motif): A group of atoms associated with a lattice.3) packing fractor: The fraction of space in a unit cell occupied by atoms.4) slip system: The combination of the slip plane and the slip direction.5) critical size: The minimum size that must be formed by atoms clustering together in theliquid before the solid particle is stable and begins to grow.6) homogeneous nucleation: Formation of a critically sized solid from the liquid by theclustering together of a large number of atoms at a high undercooling (without an external interface).7) coherent precipitate:A precipitate whose crystal structure and atomic arrangement havea continuous relationship with matrix from which precipitate is formed.8) precipitation hardening: A strengthening mechanism that relies on a sequence of solidstate phase transformations in a dispersion of ultrafine precipitates of a 2nd phase. This is same as age hardening. It is a form of dispersion strengthening.9) diffusion coefficient:A temperature-dependent coefficient related to the rate at whichatom, ion, or other species diffusion. The DC depends on temperature, the composition and microstructure of the host material and also concentration of the diffusion species.10) uphill diffusion: A diffusion process in which species move from regions of lowerconcentration to that of higher concentration.2. Determine the indices for the planes in the cubic unit cell shown in Figure 1. (5 points)Fig. 1Solution: A(-364), B(-340), C(346).3. Determine the crystal structure for the following: (a) a metal with a0 =4.9489 Å, r = 1.75 Å and one atom per lattice point; (b) a metal with a0 = 0.42906 nm, r = 0.1858 nm and one atom per lattice point. (10 points)Solution: (a)fcc; (b) bcc.4-1. What is the characteristic of brinell hardness test, rockwell hardness test and Vickers hardness test? What are the effects of strain rate and temperature on the mechanical properties of metallic materials? (15 points)4-2. What are the effects of cold-work on metallic materials? How to eliminate those effects? And what is micro-mechanism for the eliminating cold-work effects? (15 points)5-1. Based on the Pb-Sn-Bi ternary diagram as shown in Fig. 2, try to(1)Show the vertical section of 40wt.%Sn; (5 points)(2) Describe the solidification process of the alloy 2# with very low cooling speed (includingphase and microstructure changes); (5 points)(3)Plot the isothermal section at 150o C. (5 points)5-2. A 1mm sheet of FCC iron is used to contain N 2 in a heated exchanger at 1200o C. The concentration of N at one surface is 0.04 atomic percent and the concentration at the second surface is 0.005 atomic percent. At 1000 o C, if same N concentration is demanded at the second surface and the flux of N becomes to half of that at 1200o C, then what is the thickness of sheet? (15 points)6-1. Supposed that a certain liquid metal is undercooled until homogeneous nucleation occurs. (15 points)(3) How to calculate the critical radius of the nucleus required? Please give the deductionprocess.(4) For the Metal Ni, the Freezing Temperature is 1453︒C, the Latent Heat of Fusion is 2756J/cm 3, and the Solid-liquid Interfacial Energy is 255⨯10-7 J/cm 2. Please calculate the critical radius at 1353︒C. (Assume that the liquid Ni is not solidified.)6-2. Fig.3 is a portion of the Mg-Al phase diagram. (15 points)(3) If the solidification is too rapid, please describe the solidification process of Mg-10wt%Alalloy.(4) Please describe the equilibrium solidification process of Mg-20wt%Al alloy, and calculate theamount of each phase at 300︒C.Fig. 3Fig. 27-1. Figure 4 shows us the Al-Cu binary diagram and some microstructures found in a cooling process for an Al-4%Cu alloy. Please answer following questions according to this figure. (20 points)Fig. 4(1)What are precipitate, matrix and microconstituent? Please point them out in the in the figure and explain.(2)Why is need-like precipitate not good for dispersion strengthening? The typical microstructure shown in the figure is good or not? why?(3)Please tell us how to obtain the ideal microstructure shown in this figure.(4)Can dispersion strengthened materials be used at high temperature? Please give the reasons (comparing with cold working strengthening)7-2. Please answer following questions according to the time-temperature-transformation (TTT) diagram as shown in Fig. 5. (20 points)(1)What steel is this TTT diagram for? And what means P, B, and M in the figure?(2)Why dose the TTT diagram exhibits a ‘C’ shape?(3)Point out what microconstituent will be obtained after austenite is cooled according to the curves I, II, III and IV .(4)What is microstructural difference between the curve I and the curve II?(5)How to obtain the steel with the structure of(a) P+B(b) P+M+A (residual)(c) P+B+M+A (residual)(d) Full tempered martensiteIf you can, please draw the relative cooling curve or the flow chart of heat treatment.Fig. 5 III III IV。